Quinones as Neuroprotective Agents
Abstract
:1. Introduction
1.1. General Features of Neurodegenerative Diseases
1.2. General Features of Quinone Chemistry Relevant to Neuroprotection
- (a)
- Quinones are redox active molecules that can form semiquinone radicals by a reversible one-electron transfer process that in the presence of oxygen generates superoxide, a reactive oxygen species in the reverse reaction (Figure 2). Thus, quinones can cause oxidative stress leading to the oxidation of lipids, proteins and DNA.
- (b)
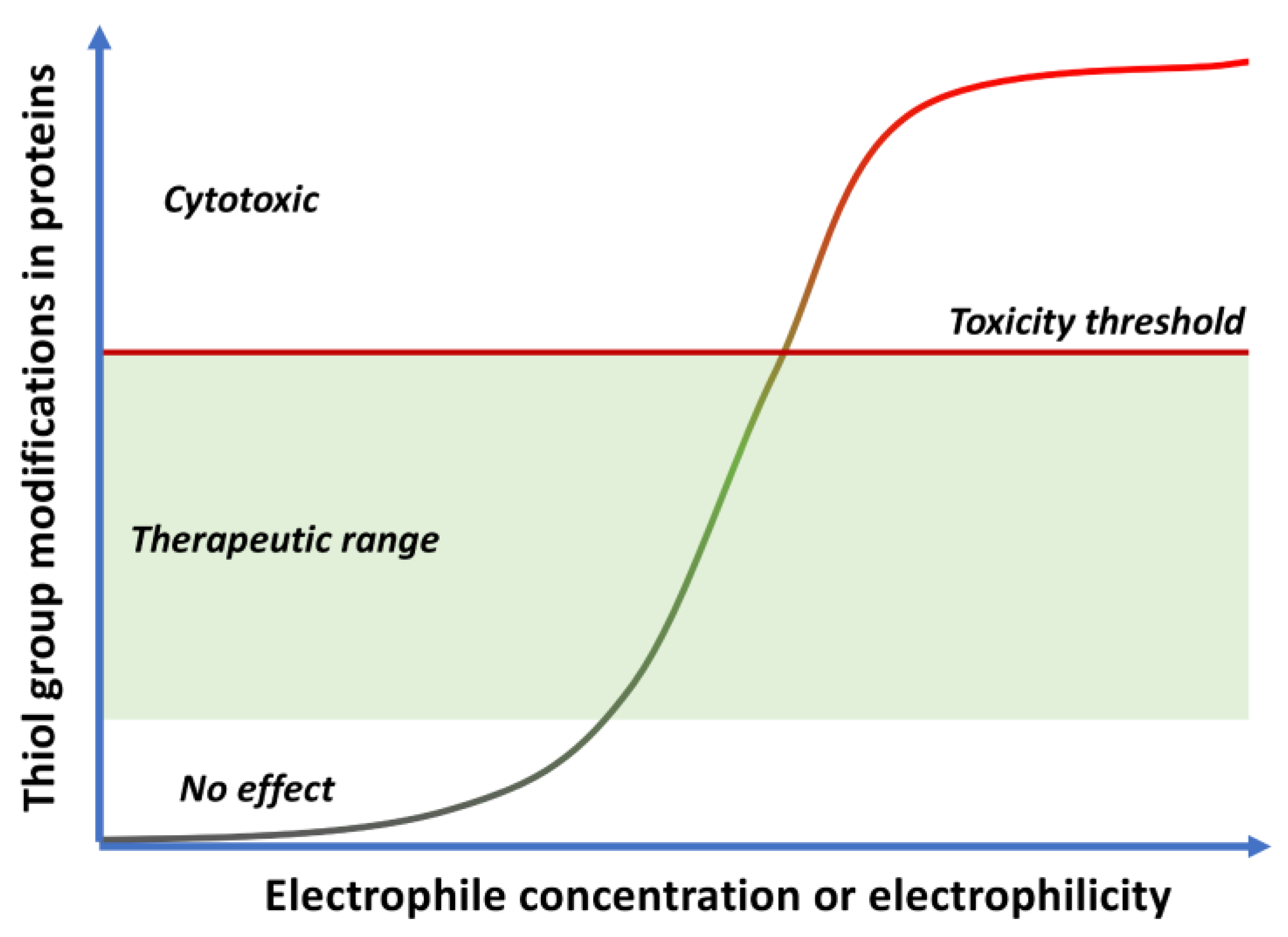
- Quinones are Michael acceptors and thus can cause cellular damage by alkylating cellular proteins, especially at cysteine residues, or DNA (Figure 2).
- (c)
- Due to their planar structure and their ability to form hydrogen bonds, many quinones are able to bind to amyloid proteins, preventing their aggregation and allowing the visualization of diffuse and dense-core amyloid plaques [13]. Molecular dynamics simulations have shown that anthraquinone, used as a model simple quinone, interferes with the early phase of aggregation by intercalation into the β-sheet of the hydrophobic H14QKLVFF20 sequence that promotes Aβ self-assembly. The amyloid-quinone complex is formed via polar interactions with the peptide backbone, which destabilize interstrand hydrogen bonds [14].
1.3. Quinones as Redox Modifiers of Biomolecules
1.4. Quinones as Covalent Modifiers of Biomolecules
2. Endogenous Quinones and Their Analogues
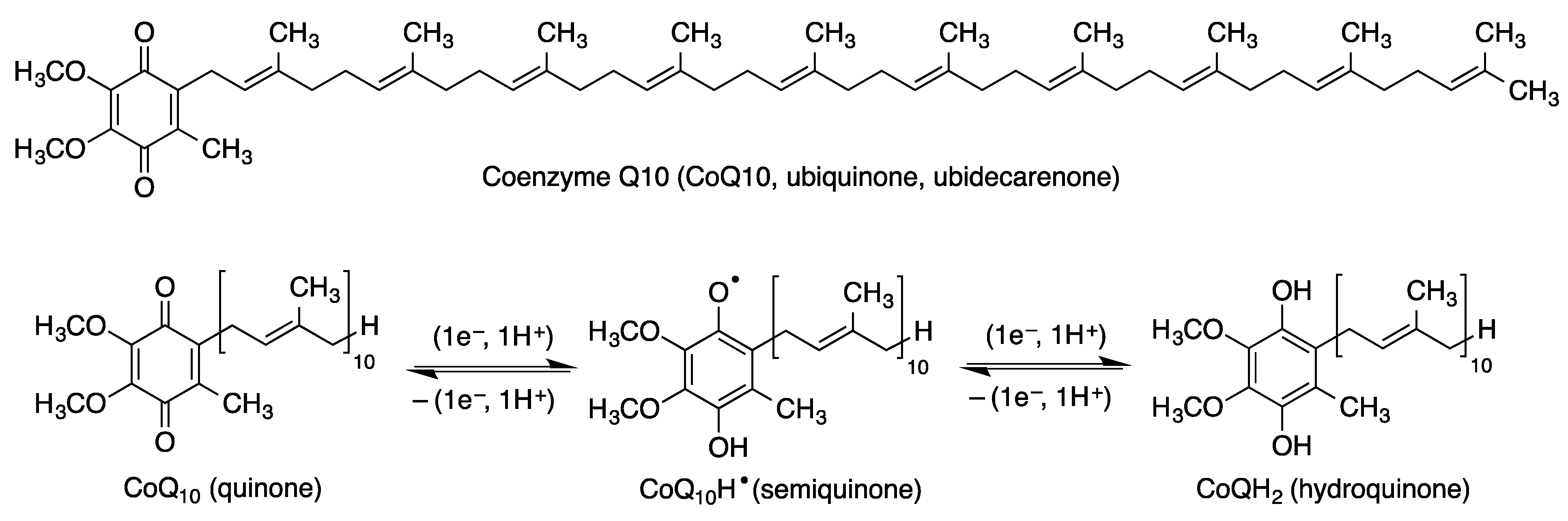
2.1. Coenzyme Q10 (CoQ, Ubiquinone)
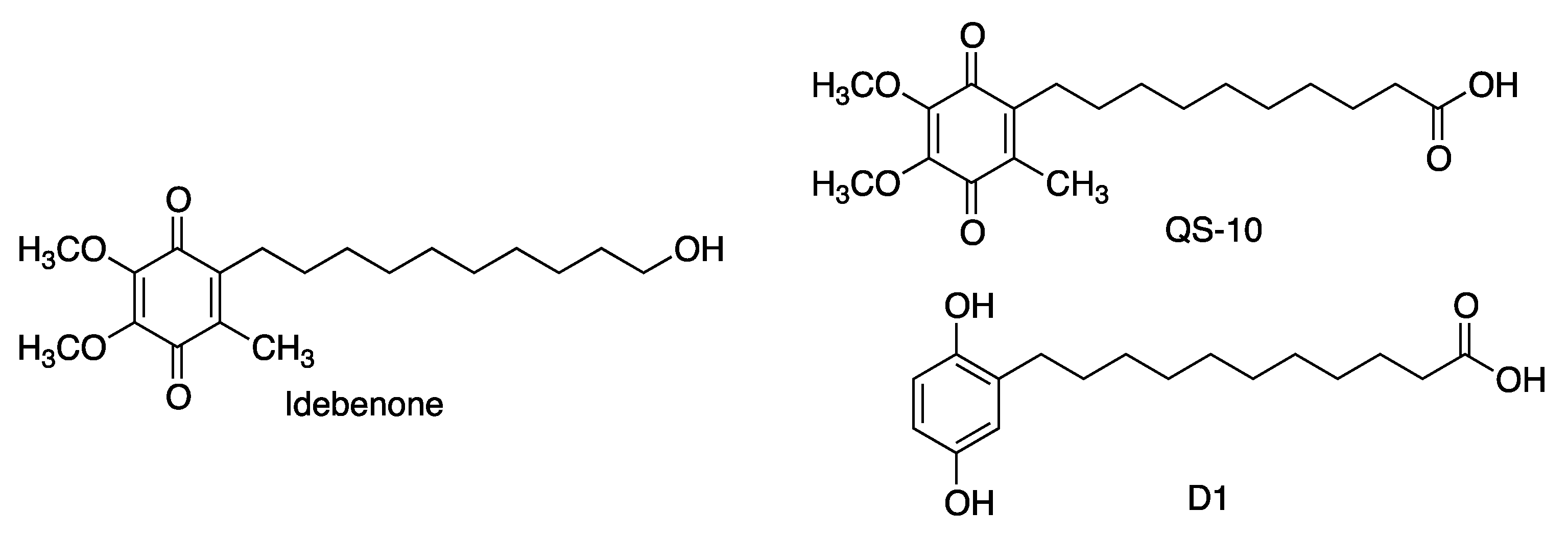
2.2. Coenzyme Q10 Analogues
2.2.1. Idebenone
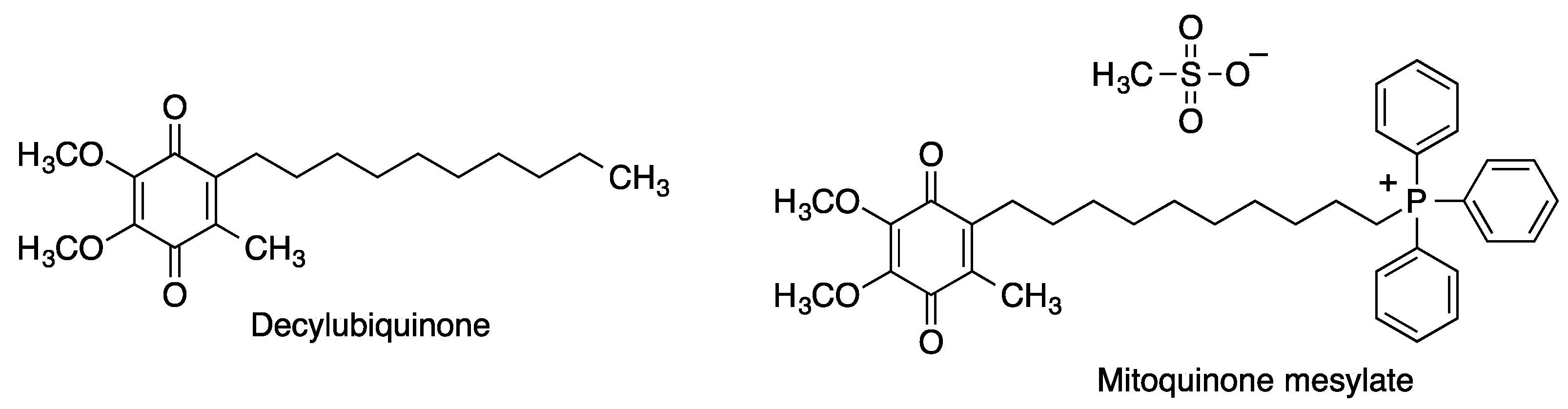
2.2.2. Decylubiquinone and Mitoquinone
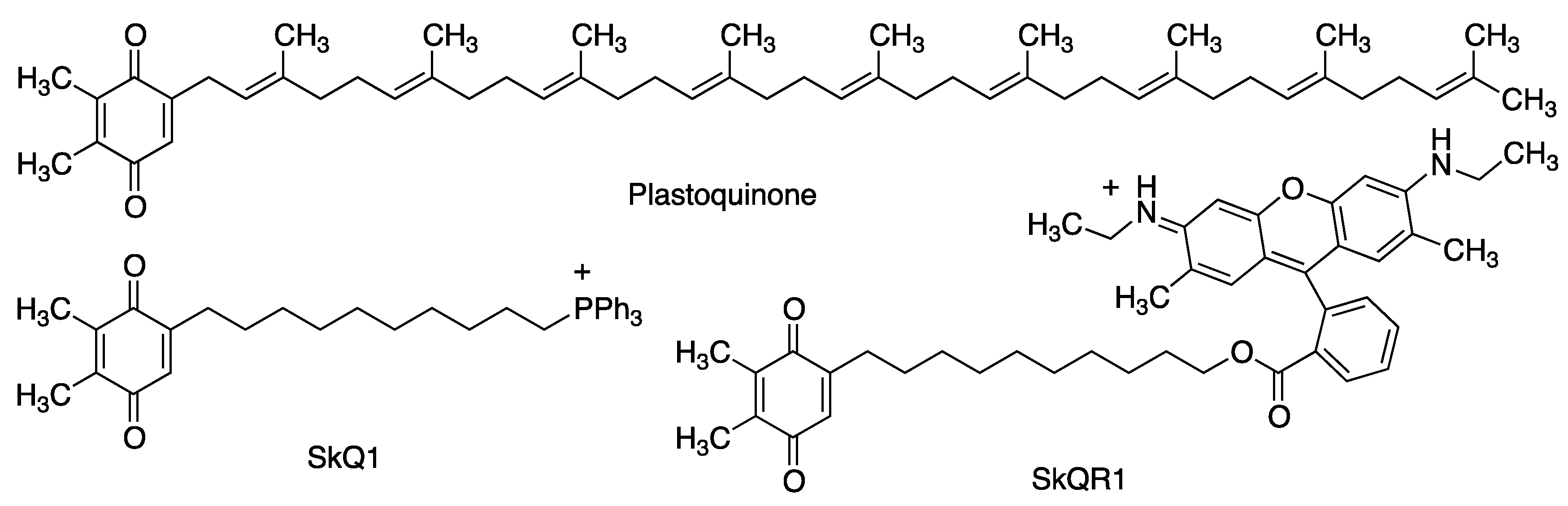
2.2.3. Plastoquinone and Its Analogues
2.3. Tocopherol-Derived Quinones
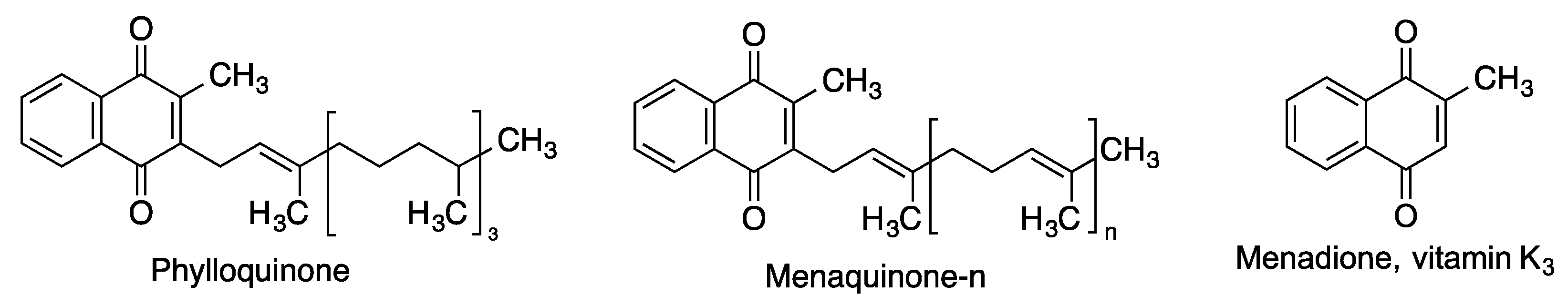
2.4. Vitamin K and Related Quinones
3. Non-Endogenous Quinones
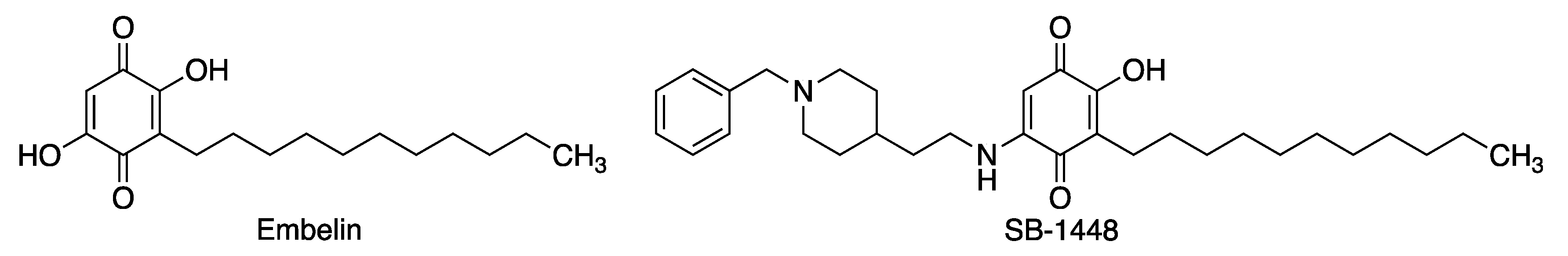
3.1. Embelin
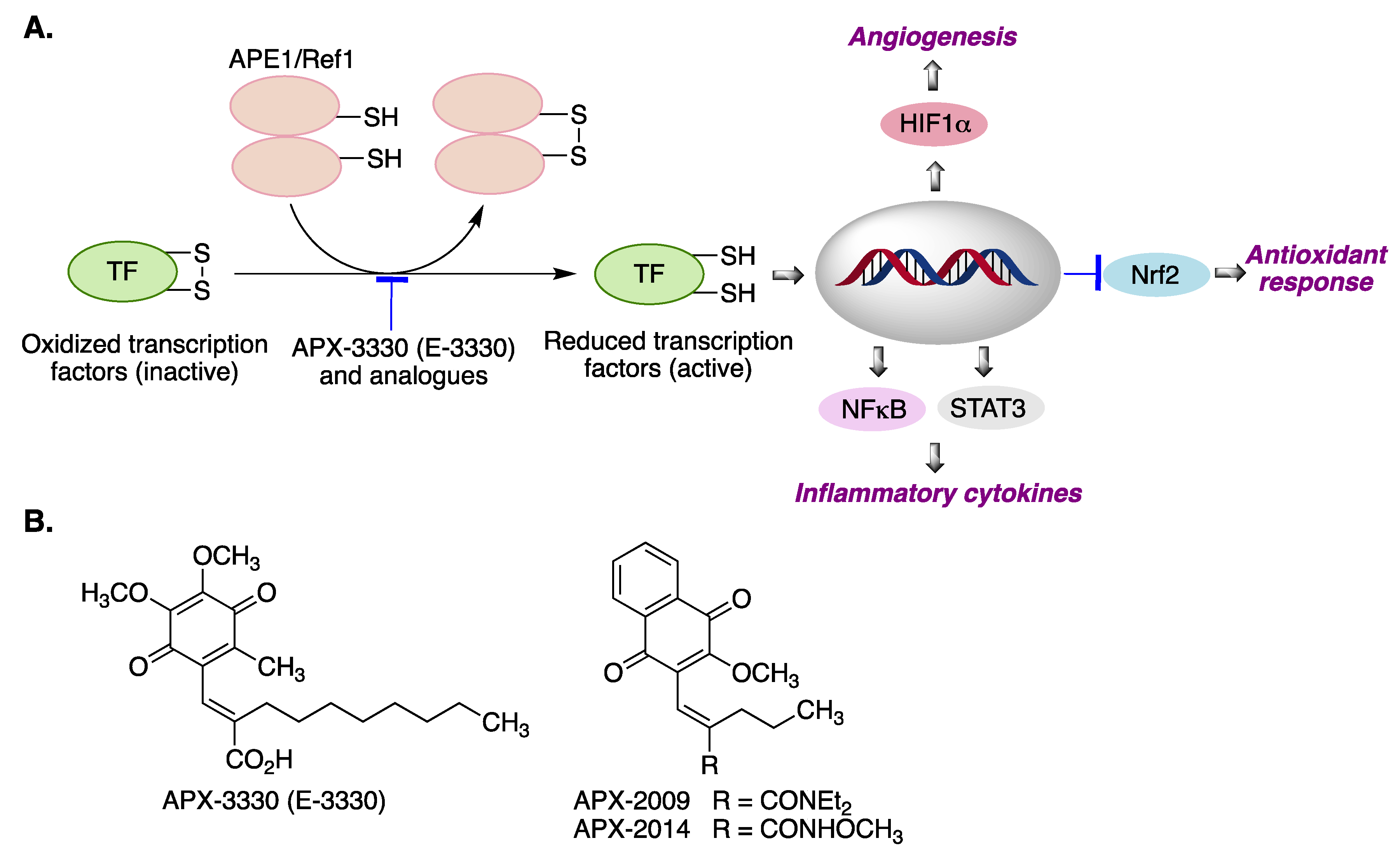
3.2. APX-3330 (E-3300)
3.3. Cannabinoid-Derived Quinones
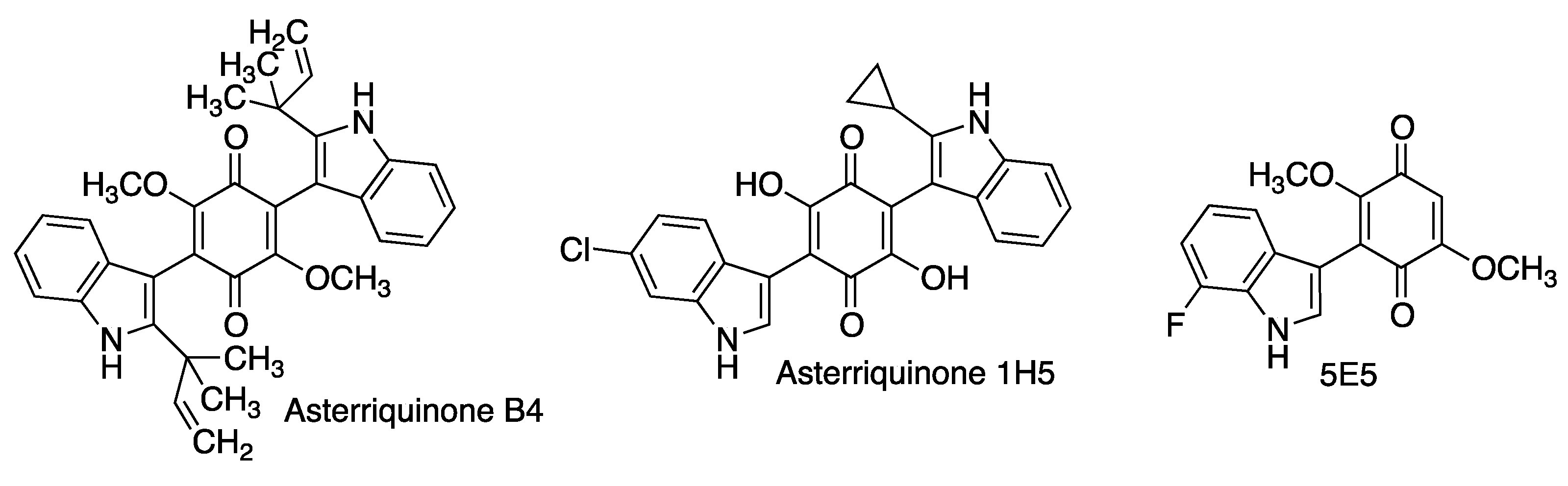
3.4. Asterriquinones and Other Indolylquinones
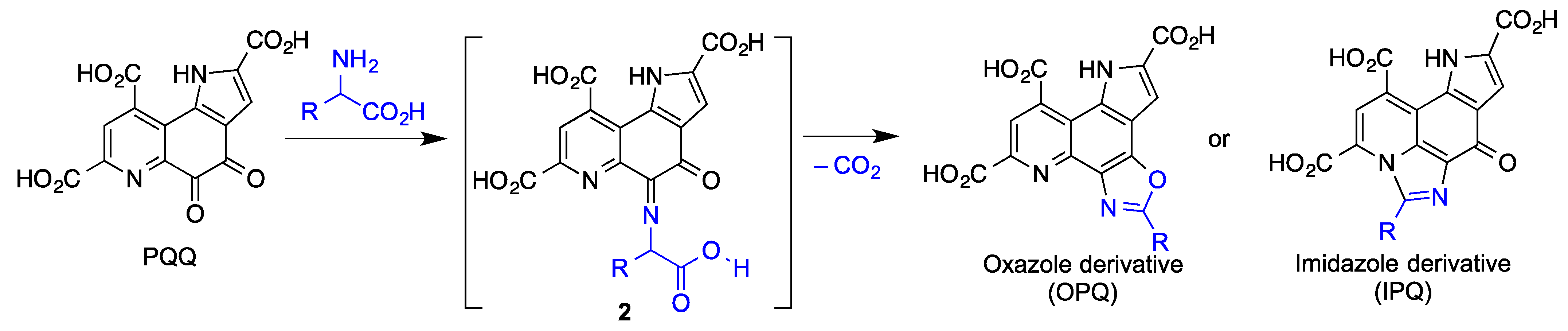
3.5. Pyrroloquinolinequinone (PQQ)
3.6. Geldanamycin and Its Analogues
3.7. Rifampicin and Its Quinone
3.8. Miscellaneous Carbocyclic Quinones
3.8.1. Benzoquinones
3.8.2. Naphthoquinones
3.8.3. Anthraquinones
3.9. Miscellaneous Heterocyclic Quinones
4. Hybrid Compounds Based on Quinone Moieties
4.1. Memoquin
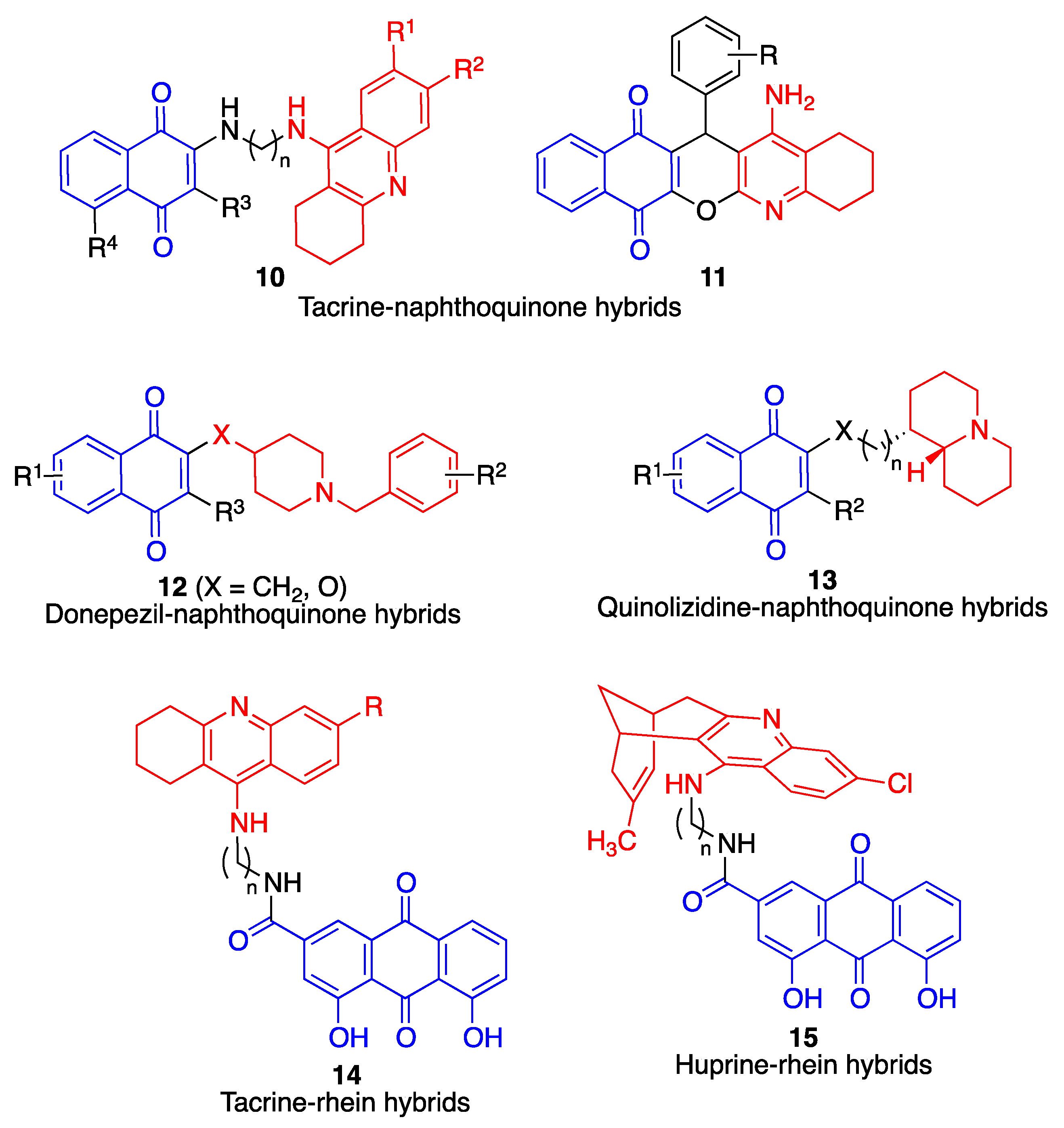
4.2. Quinone-Cholinesterase Inhibitor Hybrids
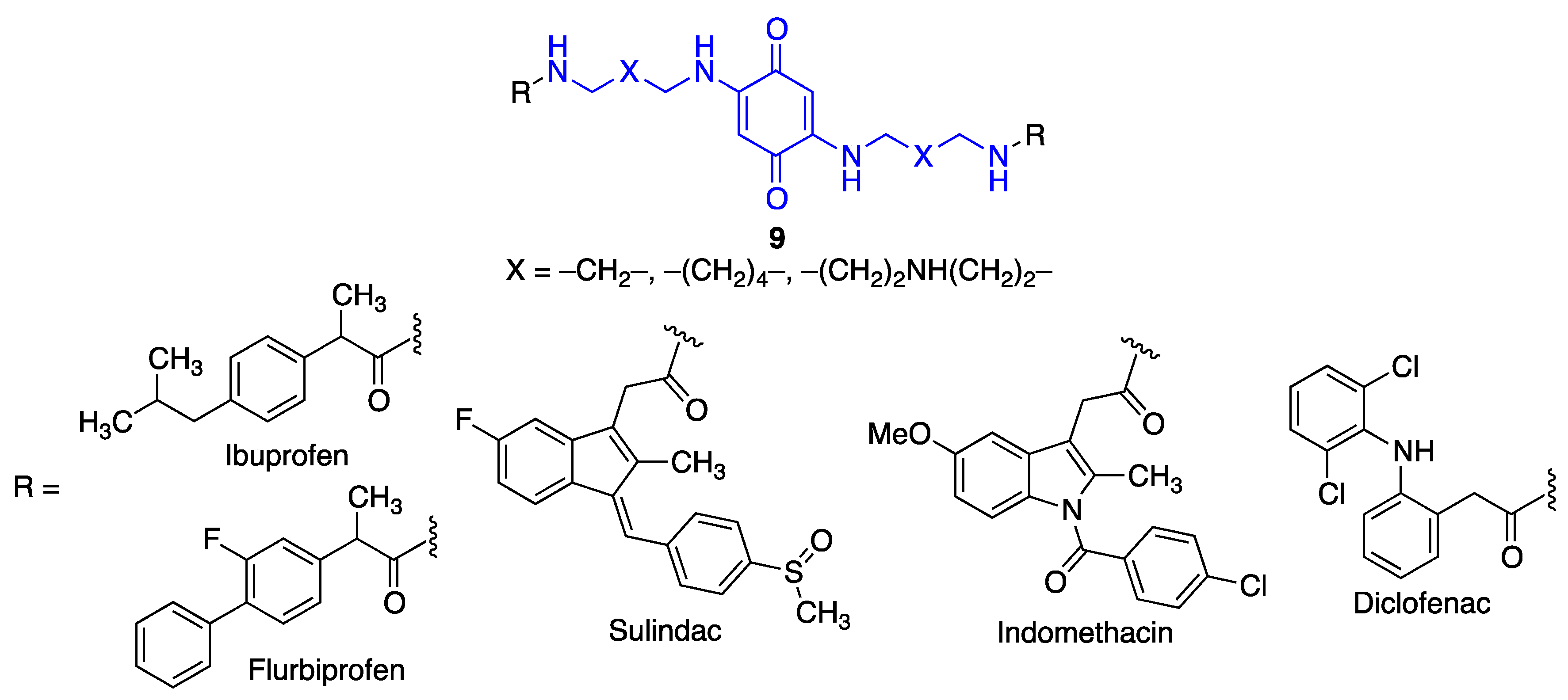
4.3. Quinone-Amino Acid Hybrids
5. Quinone Structural Fragments in the Design of Neuroprotective Prodrugs
6. Summary
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokholyan, N.V.; Mohs, R.C.; Bateman, R.J. Challenges and progress in research, diagnostics, and therapeutics in Alzheimer’s disease and related dementias. Alzheimer’s Dement. Transl. Res. Clin. 2022, 8, e12330. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R. What causes neurodegenerative disease? Folia Neuropathol. 2020, 58, 93–112. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376. [Google Scholar]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, 18. [Google Scholar] [CrossRef]
- Cummings, J. Disease modification and neuroprotection in neurodegenerative disorders. Transl. Neurodegener. 2017, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Dahlem, M.A.; Nguema Edzang, R.W.; Catto, A.L.; Raimundo, J.-M. Quinones as an efficient molecular scaffold in the antibacterial/antifungal or antitumoral arsenal. Int. J. Mol. Sci. 2022, 23, 14108. [Google Scholar] [CrossRef]
- Subhasmita Sahoo, P.M.; Behera, S.; Behura, R.; Acharya, A.; Biswal, D.; Kumar Suna, S.; Shaoo, R.; Soren, R.C.; Jali, B.R. A brief review: Antibacterial activity of quinone derivates. Biointerface Res. Appl. Chem. 2022, 12, 3247–3258. [Google Scholar]
- Ferreira, V.F.; de Carvalho, A.S.; Ferreira, P.G.; Lima, C.G.; Silva, F.D.C.D. Quinone-based drugs: An important class of molecules in medicinal chemistry. Med. Chem. 2021, 17, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Campora, M.; Francesconi, V.; Schenone, S.; Tasso, B.; Tonelli, M. Journey on naphthoquinone and anthraquinone derivatives: New insights in Alzheimer’s disease. Pharmaceuticals 2021, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.N.; Jeon, H.; Jung, Y.; Baek, S.; Lee, S.; Yoo, H.C.; Bae, G.H.; Park, K.; Yang, S.-H.; Han, J.M.; et al. Fluorescent 1,4-naphthoquinones to visualize diffuse and dense-core amyloid plaques in APP/PS1 transgenic mouse brains. ACS Chem. Neurosci. 2019, 10, 3031–3044. [Google Scholar] [CrossRef]
- Convertino, M.; Pellarin, R.; Catto, M.; Carotti, A.; Caflisch, A. 9,10-Anthraquinone hinders β-aggregation: How does a small molecule interfere with Aβ-peptide amyloid fibrillation? Prot. Sci. 2009, 18, 792–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
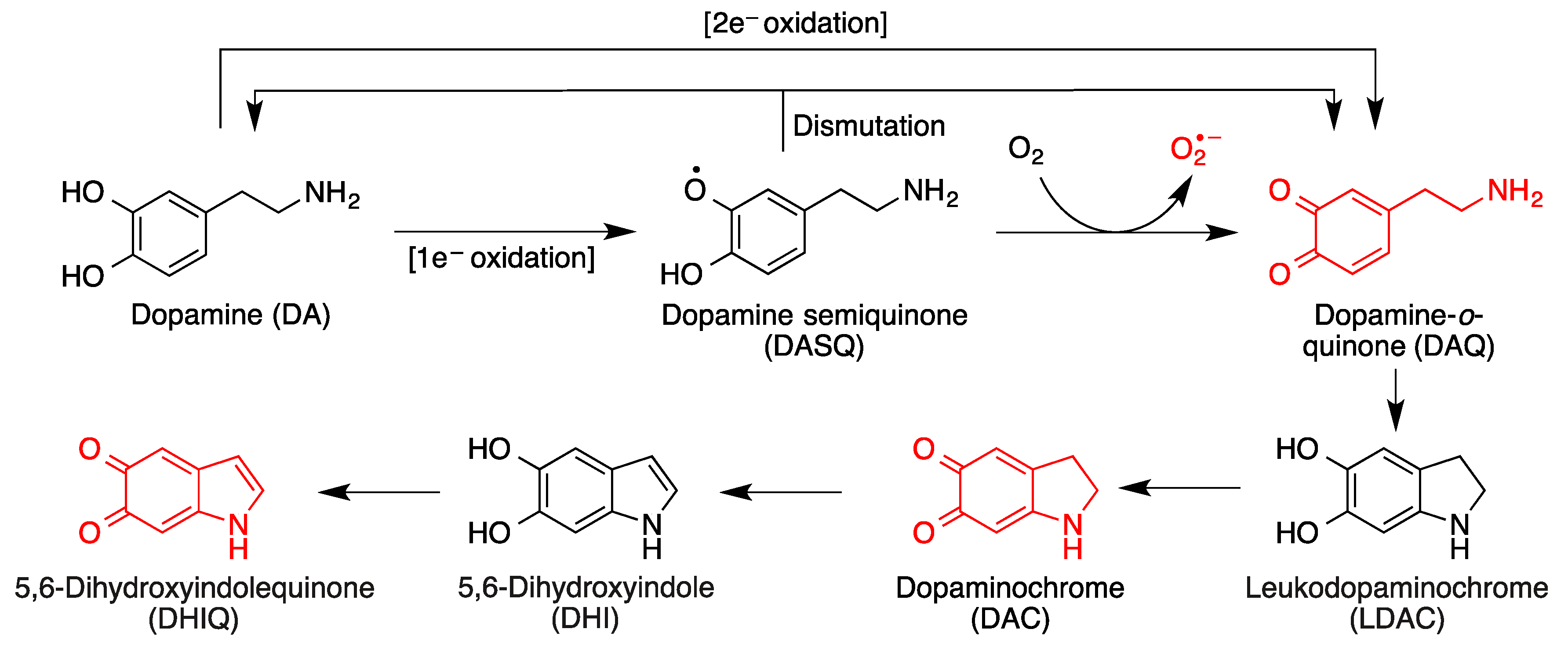
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, oxidative stress and protein–quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int. Ed. 2019, 58, 6512–6527. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, M.; Repič, M.; Vianello, R.; Mavri, J. The Chemistry of neurodegeneration: Kinetic data and their implications. Mol. Neurobiol. 2016, 53, 3400–3415. [Google Scholar] [CrossRef] [PubMed]
- Biosa, A.; Arduini, I.; Soriano, M.E.; Giorgio, V.; Bernardi, P.; Bisaglia, M.; Bubacco, L. Dopamine oxidation products as mitochondrial endotoxins, a potential molecular mechanism for preferential neurodegeneration in Parkinson’s disease. ACS Chem. Neurosci. 2018, 9, 2849–2858. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Wang, G. Impact of dopamine oxidation on dopaminergic neurodegeneration. ACS Chem. Neurosci. 2019, 10, 945–953. [Google Scholar] [CrossRef]
- Liu, J.; Song, E.; Liu, L.; Ma, X.; Tian, X.; Dong, H.; Song, Y. Polychlorinated biphenyl quinone metabolites lead to oxidative stress in HepG2 cells and the protective role of dihydrolipoic acid. Toxicol. In Vitro 2012, 26, 841–848. [Google Scholar] [CrossRef]
- Li, L.; Dong, H.; Song, E.; Xu, X.; Liu, L.; Song, Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact. 2014, 209, 56–67. [Google Scholar] [CrossRef]
- Avendaño, C.; Menéndez, J.C. Anticancer strategies involving radical species. In Medicinal Chemistry of Anticancer Drugs, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 4. [Google Scholar]
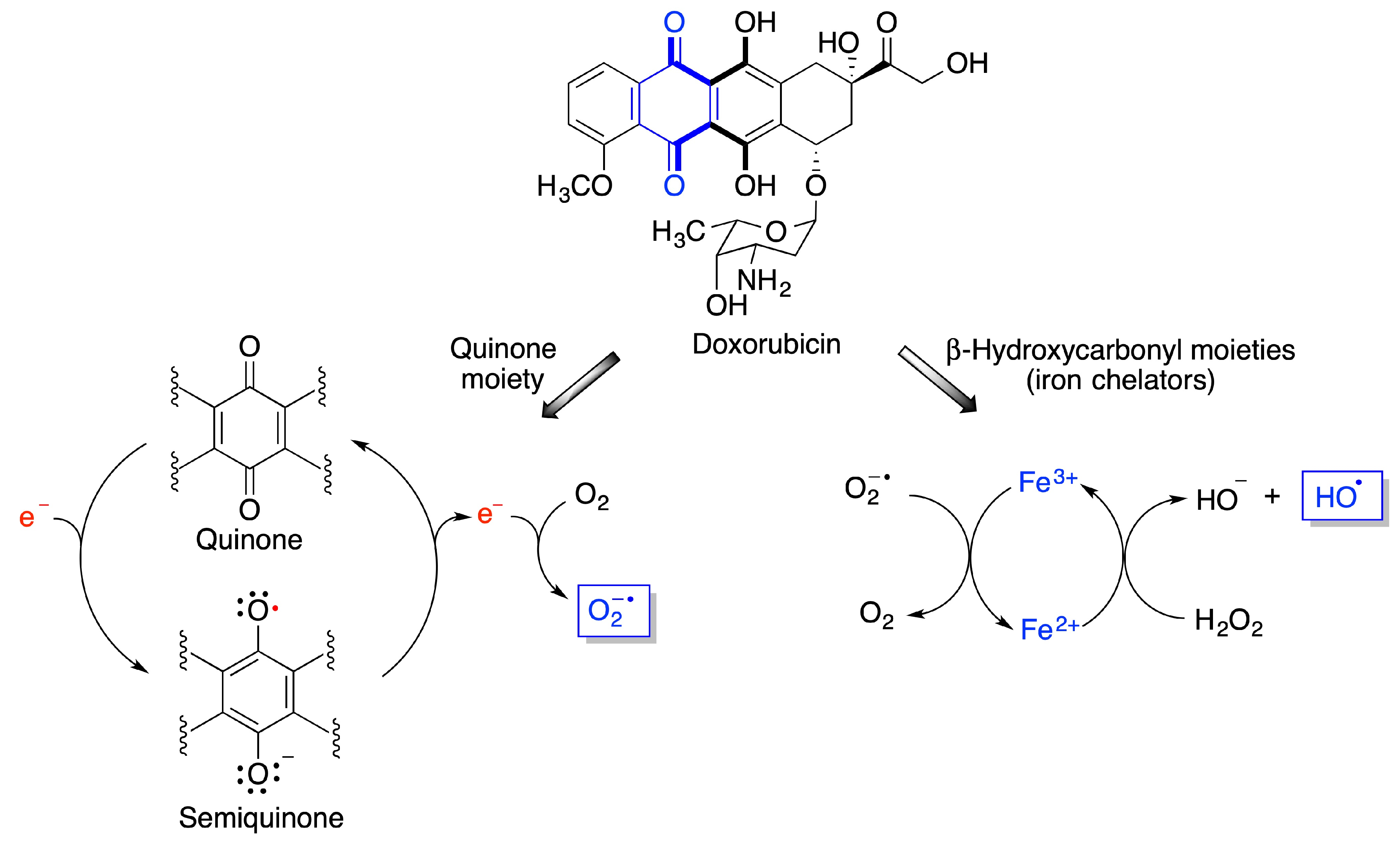
- Cole, M.P.; Chaiswing, L.; Oberley, T.D.; Edelmann, S.E.; Piascik, M.T.; Lin, S.M.; Kiningham, K.K.; St Clair, D.K. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc. Res. 2006, 69, 186–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
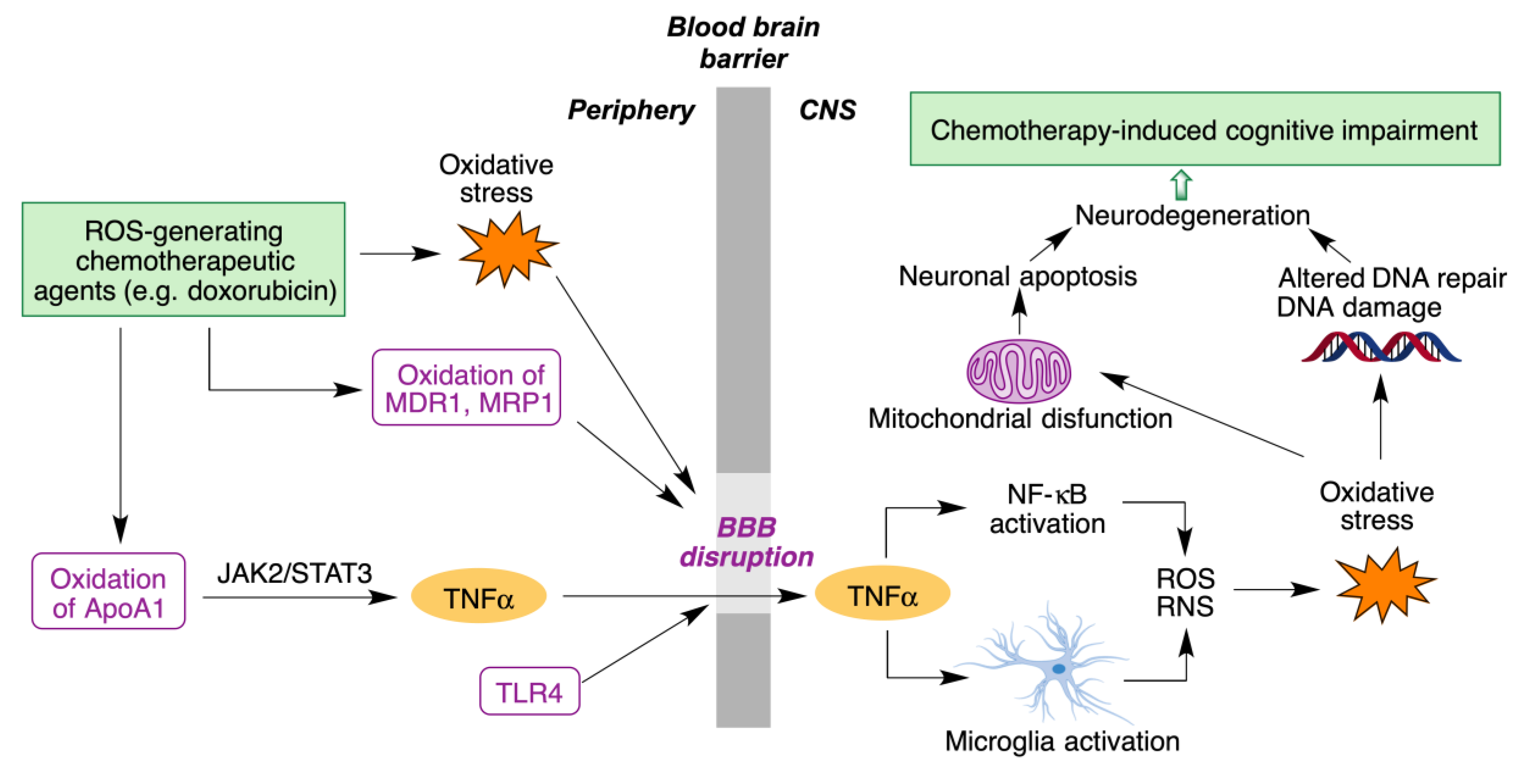
- Rena, X.; Boriero, D.; Chaiswing, L.; Bondada, S.; St. Clair, D.K.; Butterfield, D.A. Plausible biochemical mechanisms of chemotherapy-induced cognitive T impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1088–1097. [Google Scholar] [CrossRef]
- Singh, J. The ascension of targeted covalent inhibitors. J. Med. Chem. 2022, 65, 5886–5901. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Dunlap, T.L. Formation and biological targets of quinones: Cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Dunlap, T.L.; Dietz, B.M. Formation and biological targets of botanical o-quinones. Food Chem. Toxicol. 2018, 120, 700–707. [Google Scholar] [CrossRef]
- Birringer, M. Hormetics: Dietary triggers of an adaptive stress response. Pharm. Res. 2012, 28, 2680–2694. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Cores, A.; Piquero, M.; Villacampa, M.; León, R.; Menéndez, J.C. NRF2 regulation processes as a source of potential drug targets against neurodegenerative diseases. Biomolecules 2020, 10, 904. [Google Scholar] [CrossRef]
- Satoh, T.; Rezaie, T.; Seki, M.; Sunico, C.R.; Tabuchi, T.; Kitagawa, T.; Yanagitai, M.; Senzaki, M.; Kosegawa, C.; Taira, H.; et al. Dual neuroprotective pathways of a pro-electrophilic compound via HSF-1-activated heat-shock proteins and Nrf2-activated phase 2 antioxidant response enzymes. J. Neurochem. 2011, 119, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Baell, J.B. Feeling Nature’s PAINS: Natural products, natural product drugs, and pan assay interference compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Capuzzi, S.J.; Muratov, E.N.; Tropsha, A. Phantom PAINS: Problems with the utility of alerts for Pan-Assay INterference Compounds. J. Chem. Inf. Model. 2017, 57, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Gutiérrez, A.; González-García, P.; Díaz-Casado, M.E.; Barriocanal-Casado, E.; López-Herrador, S.; Quinzii, C.M.; López, L.C. Metabolic targets of coenzyme Q10 in mitochondria. Antioxidants 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Rodick, T.C.; Seibels, D.R.; Babu, J.R.; Huggins, K.W.; Ren, G.; Mathews, S.T. Potential role of coenzyme Q10 in health and disease conditions. Nutr. Diet. Suppl. 2018, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chokchaiwong, S.; Kuo, Y.T.; Lin, S.H.; Hsu, Y.C.; Hsu, S.P.; Liu, Y.T.; Chou, A.J.; Kao, S.H. Coenzyme Q10 serves to couple mitochondrial oxidative phosphorylation and fatty acid beta-oxidation, and attenuates NLRP3 inflammasome activation. Free Radic. Res. 2018, 52, 1445–1455. [Google Scholar] [CrossRef]
- Liang, S.; Ping, Z.; Ge, J. Coenzyme Q10 regulates antioxidative stress and autophagy in acute myocardial ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2017, 2017, 9863181. [Google Scholar] [CrossRef]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Du, J.; Lian, Y.; Zhang, Y.; Li, X.; Liu, Y.; Zou, L.; Wu, T. Protective effects of coenzyme Q10 against hydrogen peroxide-induced oxidative stress in PC12 cell: The role of Nrf2 and antioxidant enzymes. Mol. Neurobiol. 2016, 36, 103–111. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Soliman, D.; Kassab, R.B.; Metwally, D.M.; Abdel Moneim, A.E.; El-Khadragy, M.F. Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front. Physiol. 2020, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Park, H.-H.; Koh, S.H.; Choi, N.Y.; Yu, H.J.; Park, J.; Lee, J.Y.; Lee, K.-Y. Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. NeuroToxicology 2012, 33, 85–90. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.-J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G. Coenzyme Q10 and dementia: A systematic review. Antioxidants 2023, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, Y. Serum coenzyme Q10 levels as a predictor of dementia in a Japanese general population. Atherosclerosis 2014, 237, 433–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, H.N.; Maklad, Y.A. Neuroprotective effects of coenzyme Q10 on paraquat-induced Parkinson’s disease in experimental animals. Behav. Pharmacol. 2018, 29, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ghasemloo, E.; Mostafavi, H.; Josseini, M.; Forouzandeh, M.; Eskandari, M.; Mousavi, S.S. Neuroprotective effects of coenzyme Q10 in Parkinson’s model via a novel Q10/miR-149-5p/MMPs pathway. Metab. Brain. Dis. 2021, 36, 2089–2100. [Google Scholar] [CrossRef]
- Park, H.W.; Park, C.G.; Park, M.; Lee, S.H.; Park, H.R.; Lim, J.; Paek, S.H.; Choy, Y.B. Intrastriatal administration of coenzyme Q10 enhances neuroprotection in a Parkinson’s disease rat model. Sci. Rep. 2020, 10, 9572. [Google Scholar]
- Muthukumaran, K.; Kanwar, A.; Vegh, C.; Marginean, A.; Elliott, A.; Guilbeault, N.; Badour, A.; Sikorska, M.; Cohen, J.; Pandey, S. Ubisol-Q10 (a nanomicellar water-soluble formulation of CoQ10) treatment inhibits Alzheimer-type behavioral and pathological symptoms in a double transgenic mouse (tgAPEswe, PSEN1dE9) model of Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 61, 221–236. [Google Scholar] [CrossRef]
- Wear, D.; Vegh, C.; Sandhu, J.K.; Sikorska, M.; Cohen, J.; Pandey, S. Ubisol-Q10, a nanomicellar and water-dispersible formulation of coenzyme-Q10 as a potential treatment for Alzheimer’s and Parkinson’s disease. Antioxidants 2021, 10, 764. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 analogues: Benefits and challenges for therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef]
- Giorgio, V.; Schiavone, M.; Galber, C.; Carini, M.; Da Ros, T.; Petronilli, V.; Argenton, F.; Carelli, V.; Lopez, M.J.A.; Salviati, L.; et al. The idebenone metabolite QS10 restores electron transfer in complex I and coenzyme Q defects. Biophys. Acta Bioenerg. 2018, 1859, 901–908. [Google Scholar] [CrossRef]
- Abdelzaher, W.Y.; Mostafa-Hedeab, G.; AboBakr Ali, A.H.S.; Fawzy, M.A.; Ahmed, A.F.; Bahaa El-deen, M.A.; Welson, N.N.; Aly Labib, D.A. Idebenone regulates sirt1/Nrf2/TNF-α pathway with inhibition of oxidative stress, inflammation, and apoptosis in testicular torsion/detorsion in juvenile rats. Hum. Exptl. Toxicol. 2022, 41, 1–11. [Google Scholar] [CrossRef]
- Jaber, J.; Polster, B.M. Idebenone and neuroprotection: Antioxidant, pro-oxidant, or electron carrier? J. Bioenerg. Biomembr. 2015, 47, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyseng-Williamson, K.A. Idebenone: A review in Leber’s hereditary optic neuropathy. Drugs 2016, 76, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Klopstock, T.; Metz, G.; Yu-Wai-Man, P.; Büchner, B.; Gallenmüller, C.; Bailie, M.; Nwali, N.; Griffiths, P.G.; von Livonius, B.; Reznicek, L.; et al. Persistence of the treatment effect of idebenone in Leber’s hereditary optic neuropathy. Brain 2013, 136, e230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, D.R.; Perlman, S.L.; Meier, T. A Phase 3, double-blind, placebo-controlled trial of idebenone in Friedreich ataxia. Arch. Neurol. 2010, 67, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Kosa, P.; Wu, T.; Phillips, J.; Leinonen, M.; Masvekar, R.; Komori, M.; Wichman, A.; Sandford, M.; Bielekova, B. Idebenone does not inhibit disability progression in primary progressive MS. Mult. Scler. Relat. Disord. 2020, 45, 102434. [Google Scholar] [CrossRef] [PubMed]
- Idebenone Treatment of Early Parkinson’s Disease Symptoms (ITEP). ClinicalTrials.gov Identifier: NCT03727295. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03727295 (accessed on 7 June 2023).
- Duarte-Jurado, A.P.; Gopar-Cuevas, Y.; Saucedo-Cardenas, O.; Loera-Arias, M.d.J.; Montes-de-Oca-Luna, R.; García-García, A.; Rodríguez-Rocha, H. Antioxidant therapeutics in Parkinson’s disease: Current challenges and opportunities. Antioxidants 2021, 10, 453. [Google Scholar] [CrossRef]
- MitoQ for Fatigue in Multiple Sclerosis (MS). ClinicalTrials.gov Identifier: NCT03166800. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03166800 (accessed on 7 June 2023).
- Havaux, M. Plastoquinone in and beyond photosynthesis. Trends Plant Sci. 2020, 25, 1252–1265. [Google Scholar] [CrossRef]
- Silachev, D.N.; Plotnikov, E.Y.; Zorova, L.D.; Pevzner, I.B.; Sumbatyan, N.V.; Korshunova, G.A.; Gulyaev, M.V.; Pirogov, Y.A.; Skulachev, V.P.; Zorov, D.B. Neuroprotective effects of mitochondria-targeted plastoquinone and thymoquinone in a rat model of brain ischemia/reperfusion injury. Molecules 2015, 20, 14487–14503. [Google Scholar] [CrossRef] [Green Version]
- Kolosova, N.G.; Tyumentsev, M.A.; Muraleva, N.A.; Kiseleva, E.; Vitovtov, A.O.; Stefanova, N.A. Antioxidant SkQ1 alleviates signs of Alzheimer’s disease-like pathology in old OXYS rats by reversing mitochondrial deterioration. Curr. Alzheimer Res. 2017, 14, 1283–1292. [Google Scholar] [CrossRef]
- Mustacich, D.J.; Bruno, R.S.; Traber, M.G. Vitamin E. Vitam. Horm. 2007, 76, 1–21. [Google Scholar]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory redox interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Shrader, W.D.; Amagata, A.; Barnes, A.; Hinman, A.; Jankowski, O.; Lee, E.; Kheifets, V.; Komatsuzaki, R.; Mollard, P.; Murase, K.; et al. Towards a modern definition of vitamin E-evidence for a quinone hypothesis. Bioorg. Med. Chem. Lett. 2012, 22, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Shrader, W.D.; Amagata, A.; Barnes, A.; Enns, G.M.; Hinman, A.; Jankowski, O.; Kheifets, V.; Komatsuzaki, R.; Lee, E.; Mollard, P.; et al. α-Tocotrienol quinone modulates oxidative stress response and the biochemistry of aging. Bioorg. Med. Chem. Lett. 2011, 21, 3693–3698. [Google Scholar] [CrossRef]
- Hinman, A.; Holst, C.R.; Latham, J.C.; Bruegger, K.J.; Ulas, G.; McCusker, K.P.; Amagata, A.; David, D.; Hoff, K.G.; Kahn-Kirby, A.H.; et al. Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PLoS ONE 2018, 13, e0201369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell. Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Oliana Reichert, C.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; de Lima Barros, P.; Levy, D.; Bydlowski, S.P. Ferroptosis mechanisms involved in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef]
- Enns, G.M.; Cohen, B.H. Clinical trials in mitochondrial disease: An update on EPI-743 and RP103. J. Inborn Errors Metab. Screen. 2017, 5, 1–7. [Google Scholar] [CrossRef]
- Perlman, S. Emerging therapies in Friedreich’s ataxia: A review. touchREVIEWS Neurol. 2022, 18, 32–37. [Google Scholar] [CrossRef]
- Ferland, G. Revisiting the therapeutic potential of tocotrienol. BioFactors 2021, 38, 151–157. [Google Scholar] [CrossRef]
- Alisi, L.; Cao, R.; De Angelis, C.; Cafolla, A.; Caramia, F.; Cartocci, G.; Librando, A.; Fiorelli, M. The relationships between vitamin K and cognition: A review of current evidence. Front Neurol. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaue, M.; Mori, N.; Okazaki, M.; Kadowaki, E.; Kaneko, T.; Hemmi, N.; Sekiguchi, H.; Maki, T.; Ozawa, A.; Hara, S.; et al. Vitamin K has the potential to protect neurons from methylmercury-induced cell death in vitro. J. Neurosci. Res. 2011, 89, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Hirota, Y.; Sawada, N.; Yuge, N.; Watanabe, M.; Uchino, Y.; Okuda, N.; Shimomura, Y.; Suhara, Y.; Okano, T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 2010, 468, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, H.H.; Drittij-Reijnders, M.J. Vitamin K distribution in rat tissues: Dietary phylloquinone is a source of tissue menaquinone-4. Br. J. Nutr. 1994, 72, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrié, I.; Portoukalian, J.; Vicaretti, R.; Rochford, J.; Potvin, S.; Ferland, G. Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J. Nutr. 2004, 134, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Posse de Chaves, E.; Sipione, S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2009, 584, 1748–1759. [Google Scholar] [CrossRef] [Green Version]
- Jana, A.; Hogan, E.L.; Pahan, K. Ceramide and neurodegeneration: Susceptibility of neurons and oligodendrocytes to cell damage and death. J. Neurol. Sci. 2009, 278, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef] [Green Version]
- Alam, P.; Chatuvedi, S.K.; Siddiqi, M.K.; Rajpoot, R.K.; Ajmal, M.R.; Zaman, M.; Khan, R.H. Vitamin K3 inhibits protein aggregation: Implication in the treatment of amyloid diseases. Sci. Rep. 2016, 6, 26759. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, E.C.; Netz, P.A.; Diniz, C.; Do Canto, V.P.; Follmer, C. Molecular insights into human monoamine oxidase (MAO) inhibition by 1,4-naphthoquinone: Evidences for menadione (vitamin K3) acting as a competitive and reversible inhibitor of MAO. Bioorg. Med. Chem. 2011, 19, 7416–7424. [Google Scholar] [CrossRef] [Green Version]
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative stress in age-related neurodegenerative diseases: An overview of recent tools and findings. Antioxidants 2023, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, S.-J.; Yu, X.-A.; Li, J.; Gao, X.-M.; He, J.; Chang, Y.-X. Pharmacokinetic and bioavailability studies of embelin after intravenous and oral administration to rats. Evid.-Based Complement. Altern. Med. 2019, 2019, 9682495. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Ge, S.; Gao, M.; Jian, R.; Chen, X.; Xu, X.; Li, D.; Zhang, K.; Chen, W.H. Synthesis and biological activity of embelin and its derivatives: An overview. Mini Rev. Med. Chem. 2020, 20, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Basha, N.J.; Basavarajaiah, S.M.; Baskaran, S.; Kumar, P. A comprehensive insight on the biological potential of embelin and its derivatives. Nat. Prod. Res. 2022, 36, 3054–3068. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanendran, S.; Hanapi, N.A.; Ahemad, N.; Othman, I.; Yusof, S.R.; Shaikh, M.F. Embelin, a potent molecule for Alzheimer’s disease: A proof of concept from blood-brain barrier permeability, acetylcholinesterase inhibition and molecular docking studies. Front. Neurosci. 2021, 13, 495. [Google Scholar] [CrossRef] [Green Version]
- Nuthakki, V.K.; Sharma, A.; Kumar, A.; Bharate, S.B. Identification of embelin, a 3-undecyl-1,4-benzoquinone from Embelia ribes as a multitargeted anti-Alzheimer agent. Drug Dev. Res. 2019, 80, 655–665. [Google Scholar] [CrossRef]
- Bhuvanendran, S.; Kumari, Y.; Othman, I.; Shaikh, M.F. Amelioration of cognitive deficit by embelin in a scopolamine-induced Alzheimer’s disease-like condition in a rat model. Front. Pharmacol. 2018, 9, 665. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Kaur, S.; Garcia-Villar, E.; Molasky, N.; Belli, S.; Sitek, J.D.; Gionfra, F.; Pedersen, J.Z.; Incerpi, S. Antioxidant properties of embelin in cell culture. Electrochemistry and theoretical mechanism of scavenging. Potential scavenging of superoxide radical through the cell membrane. Antioxidants 2020, 9, 382. [Google Scholar] [CrossRef]
- Mahendran, S.; Badami, S.; Ravi, S.; Thippeswamy, B.S.; Veerapur, V.P. Synthesis and evaluation of analgesic and anti-inflammatory activities of most active free radical scavenging derivatives of embelin—A structure–activity relationship. Chem. Pharm. Bull. 2011, 59, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Nuthakki, V.K.; Choudhary, S.; Reddy, C.N.; Bhatt, S.; Jamwal, A.; Jotshi, A.; Raghuvanshi, R.; Sharma, A.; Thakur, S.; Jadhav, H.R.; et al. Design, synthesis, and pharmacological evaluation of embelin–aryl/alkyl amine hybrids as orally bioavailable blood–brain barrier permeable multitargeted agents with therapeutic potential in Alzheimer’s disease: Discovery of SB-1448. ACS Chem. Neurosci. 2023, 14, 1193–1219. [Google Scholar] [CrossRef]
- Choi, S.; Joo, H.K.; Jeon, B.H. Dynamic regulation of APE1/Ref-1 as a therapeutic target protein. Chonnam Med. J. 2016, 52, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tell, G.; Quadrifoglio, F.; Tiribelli, C.; Kelley, M.R. The many functions of APE1/Ref-1: Not only a DNA repair enzyme. Antiox. Redox. Signal. 2009, 11, 601–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcon, G.; Tell, G.; Perrone, L.; Garbelli, R.; Quadrifoglio, F.; Tagliavini, F.; Giaccone, G. APE1/Ref-1 in Alzheimer’s disease: An immunohistochemical study. Neurosci. Lett. 2009, 466, 124–127. [Google Scholar] [CrossRef]
- Teixeira Oliveira, T.; Gomes Coutinho, L.; Ohana Alves de Oliveira, L.; de Souza, A.R.; Cavalcanti Farias, G.; Fasarella Agnez-Lina, L. APE1/Ref-1 role in inflammation and immune response. Front. Immunol. 2022, 13, 793096. [Google Scholar] [CrossRef]
- Hartman, G.D.; Lambert-Cheatham, N.A.; Kelley, M.R.; Corson, T.W. Inhibition of APE1/Ref-1 for neovascular eye diseases: From biology to therapy. Int. J. Mol. Sci. 2021, 22, 10279. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef]
- Carrillo-Salinas, F.J.; Navarrete, C.; Mecha, M.; Feliú, A.; Collado, J.A.; Cantarero, I.; Bellido, M.L.; Muñoz, E.; Guaza, C. A cannabigerol derivative suppresses immune responses and protects mice from experimental autoimmune encephalomyelitis. PLoS ONE 2014, 9, e94733. [Google Scholar] [CrossRef]
- Díaz-Alonso, J.; Paraíso-Luna, J.; Navarrete, C.; del Río, C.; Cantarero, I.; Palomares, B.; Aguareles, J.; Fernández-Ruiz, J.; Bellido, M.L.; Pollastro, F.; et al. VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease. Sci. Rep. 2016, 6, 29789. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Cueto, C.; Santos-García, I.; García-Toscano, L.; Espejo-Porras, F.; Bellido, M.L.; Fernández-Ruiza, J.; Muñoz, E.; de Lago, E. Neuroprotective effects of the cannabigerol quinone derivative VCE-003.2 in T SOD1G93A transgenic mice, an experimental model of amyotrophic lateral sclerosis. Biochem. Pharmacol. 2018, 157, 217–226. [Google Scholar] [CrossRef]
- Kogan, N.M.; Peters, M.; Mechoulam, R. Cannabinoid quinones—A review and novel observations. Molecules 2021, 26, 1761. [Google Scholar] [CrossRef]
- Casares, L.; Unciti-Broceta, J.D.; Prados, M.E.; Caprioglio, D.; Mattoteia, D.; Higgins, M.; Apendino, G.; Dinkova-Kostova, A.T.; Muñoz, E.; de la Vega, L. Isomeric O-methyl cannabidiolquinones with dual BACH1/NRF2 activity. Redox Biol. 2020, 37, 101689. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Bellido, M.L.; Muñoz, E. Novel Cannabidiol Quinone Derivatives. European Patent EP 3131874B1, 25 July 2018. [Google Scholar]
- Girich, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Hoai Trinh, P.T.; Duy Ngoc, N.T.; Pivkin, M.V.; Popov, R.S.; Pislyagin, E.A.; Menchinskaya, E.S.; Chingizova, E.A.; et al. Neuroprotective metabolites from Vietnamese marine derived fungi of Aspergillus and Penicillium genera. Mar. Drugs 2020, 18, 608. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Pirrung, M.C.; Deng, L.; Li, Z.; Liu, Y.; Webste, N.J.G. Neuroprotection by small molecule activators of the nerve growth factor receptor. J. Pharmacol. Exp. Ther. 2007, 322, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, K.; Toyama, H.; Yamada, M.; Adachi, O. Quinoproteins: Structure, function, and biotechnological applications. Appl. Microbiol. Biotechnol. 2002, 58, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, T.; Seno, H.; Urakami, T.; Matsumoto, T.; Suzuki, O. Trace levels of pyrroloquinoline quinone in human and rat samples detected by gas chromatography/mass spectrometry. Biochim. Biophys. Acta 1992, 1156, 62–66. [Google Scholar] [CrossRef]
- Mitchell, A.E.; Jones, A.D.; Mercer, R.S.; Rucker, R.B. Characterization of pyrroloquinoline quinone amino acid derivatives by electrospray ionization mass spectrometry and detection in human milk. Anal. Biochem. 1999, 269, 317–325. [Google Scholar] [CrossRef]
- Akagawa, M.; Nakano, M.; Ikemoto, K. Recent progress in studies on the health benefits of pyrroloquinoline quinone. Biosci. Biotechnol. Biochem. 2016, 80, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Noji, N.; Nakamura, T.; Kitahata, N.; Taguchi, K.; Kudo, T.; Yoshida, S.; Tsujimoto, M.; Sugiyama, T.; Asami, T. Simple and sensitive method for pyrroloquinoline quinone (PQQ) analysis in various foods using liquid chromatography/electrospray-ionization tandem mass spectrometry. J. Agric. Food Chem. 2007, 55, 7258–7263. [Google Scholar] [CrossRef]
- Stites, T.E.; Mitchell, A.E.; Rucker, R.B. Physiological importance of quinoenzymes and the o-quinone family of cofactors. J. Nutr. 2000, 130, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Hara, H.; Hiramatsu, H.; Adachi, T. Pyrroloquinoline quinone is a potent neuroprotective nutrient against 6-hydroxydopamine-induced neurotoxicity. Neurochem. Res. 2007, 32, 489–495. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, Y.; Zhang, Y.; Tian, D.; Jiang, S.; Tang, Y. Hepatoprotective effect of pyrroloquinoline quinone against alcoholic liver injury through activating Nrf2-mediated antioxidant and inhibiting TLR4-mediated inflammation responses. Process Biochem. 2020, 92, 303–312. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, M.; Ding, M.; Shen, D.; Ding, F. The neuroprotective action of pyrroloquinoline quinone against glutamate-induced apoptosis in hippocampal neurons is mediated through the activation of PI3K/Akt pathway. Toxicol. Appl. Pharmacol. 2011, 252, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Yao, Z.-W.; Peng, Y.; Mao, S.-S.; Xu, D.; Qin, X.-F.; Zhang, R.-J. PQQ ameliorates D-galactose induced cognitive impairments by reducing glutamate neurotoxicity via the GSK-3β/Akt signaling pathway in mouse. Sci. Rep. 2018, 8, 8894. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, Q.; Yu, S.; Gao, X.; Zhang, J.; Dong, X.; Ji, J.; Zhang, Y.; Zhou, L.; Zhang, Q.; et al. Pyrroloquinoline quinone-conferred neuroprotection in rotenone models of Parkinson’s disease. Toxicol. Lett. 2015, 238, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Katsuno, M.; Waza, M.; Minamiyama, M.; Tanaka, F.; Sobue, G. Heat shock proteins in neurodegenerative diseases: Pathogenic roles and therapeutic implications. Int. J. Hyperth. 2009, 25, 647–654. [Google Scholar] [CrossRef]
- Sajjad, M.U.; Samson, B.; Wyttenbach, A. Heat shock proteins: Therapeutic drug targets for chronic neurodegeneration? Curr. Pharm. Biotechnol. 2010, 11, 198–215. [Google Scholar] [CrossRef]
- Campanella, C.; Pace, A.; Caruso-Bavisotto, C.; Marzullo, P.; Gammazza, A.M.; Buscemi, S.; Palumbo-Piccionello, A. Heat shock proteins in Alzheimer’s disease: Role and targeting. Int. J. Mol. Sci. 2018, 19, 2603. [Google Scholar] [CrossRef] [Green Version]
- Hurtado-Lorenzo, A.; Anand, V.S. Heat shock protein 90 modulates LRRK2 stability: Potential implications for Parkinson’s disease treatment. J. Neurosci. 2008, 28, 6757–6759. [Google Scholar] [CrossRef]
- Shen, H.-Y.; He, J.-C.; Wang, Y.; Huang, Q.-Y.; Chen, J.F. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J. Biol. Chem. 2005, 280, 39962–39969. [Google Scholar] [CrossRef] [Green Version]
- McFarland, N.R.; Dimant, H.; Kibuuka, L.; Ebrahimi-Fakhari, D.; Desjardins, C.A.; Danzer, K.M.; Danzer, M.; Fan, Z.; Schwarzschild, M.A.; Hirst, W.; et al. Chronic treatment with novel small molecule HSP-90 inhibitors rescues striatal dopamine levels but not α-synuclein-induced neuronal cell loss. PLoS ONE 2014, 9, e86048. [Google Scholar] [CrossRef]
- Zare, N.; Motamedi, F.; Digaleh, H.; Khodagholi, F.; Maghsoudi, N. Collaboration of geldanamycin-activated P70S6K and Hsp70 against beta-amyloid-induced hippocampal apoptosis: An approach to long-term memory and learning. Cell Stress Chaperones 2015, 20, 309–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opattova, A.; Filipcik, P.; Cente, M.; Novak, M. Intracellular degradation of misfolded tau protein induced by geldanamycin is associated with activation of proteasome. J. Alzheimer’s Dis. 2013, 33, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Manaenko, A.; Fathali, N.; Williams, S.; Lekic, T.; Zhang, J.H.; Tang, J. Geldanamycin reduced brain injury in mouse model of intracerebral hemorrhage. Acta Neurochir. Suppl. 2011, 111, 161–165. [Google Scholar] [PubMed] [Green Version]
- Chen, Y.; Wang, B.; Liu, D.; Li, J.J.; Xue, Y.; Sakata, K.; Zhu, L.Q.; Heldt, S.A.; Xu, H.; Liao, F.-F. Hsp90 chaperone inhibitor 17-AAG attenuates Aβ-induced synaptic toxicity and memory impairment. J. Neurosci. 2014, 34, 2464–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokui, K.; Adachi, H.; Waza, M.; Katsuno, M.; Minamiyama, M.; Doi, H.; Tanaka, K.; Hamazaki, J.; Murata, S.; Tanaka, F.; et al. 17-DMAG ameliorates polyglutamine-mediated motor neuron degeneration through well-preserved proteasome function in an SBMA model mouse. Hum. Mol. Genet. 2009, 18, 898–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Fernandes, A.; Duarte-Silva, S.; Neves-Carvalho, A.; Amorim, M.; Soares-Cunha, C.; Oliveira, P.; Thirstrup, K.; Teixeira-Castro, A.; Maciel, P. Chronic treatment with 17-DMAG improves balance and coordination in a new mouse model of Machado-Joseph disease. Neurotherapeutics 2014, 11, 433–449. [Google Scholar] [CrossRef] [Green Version]
- Kitson, R.R.A.; Chang, C.-H.; Xiong, R.; Williams, H.E.L.; Davis, A.L.; Lewis, W.; Dehn, D.L.; Siegel, D.; Roe, S.M.; Prodromou, C.; et al. Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90. Nat. Chem. 2013, 5, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Mitchison, D.A. Understanding the chemotherapy of tuberculosis–current problems. J. Antimicrob. Chemother. 1992, 29, 477–493. [Google Scholar] [CrossRef]
- Sensi, P. History of the development of rifampin. Rev. Infect. Dis. 1983, 5, S402–S406. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; Rajamani, S.; Uversky, V.N.; Fink, A.L. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem. Biol. 2004, 11, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- Chui, D.H.; Tabira, T.; Izumi, S.; Koya, G.; Ogata, J. Decreased beta-amyloid and increased abnormal Tau deposition in the brain of aged patients with leprosy. Am. J. Pathol. 1994, 145, 771–775. [Google Scholar] [PubMed]
- Acuña, L.; Corbalán, N.S.; Raisman-Vozari, R. Rifampicin quinone pretreatment improves neuronal survival by modulating microglia inflammation induced by α-synuclein. Neural Regen. Res. 2020, 15, 1473–1474. [Google Scholar] [CrossRef] [PubMed]
- Acuña, L.; Hamadat, S.; Corbalán, N.S.; González-Lizárraga, F.; Dos Santos-Pereira, M.; Rocca, J.; Sepúlveda-Díaz, J.; Del-Bel, E.; Papy-García, D.; Chehín, R.N.; et al. Rifampicin and its derivative rifampicin quinone reduce microglial inflammatory responses and neurodegeneration induced in vitro by α-synuclein fibrillary aggregates. Cells 2019, 8, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konrad, P.; Stenberg, P. Rifampicin quinone is an immunosuppressant, but not rifampicin itself. Clin. Immunol. Immunopathol. 1988, 46, 162–166. [Google Scholar] [CrossRef]
- Bi, W.; Zhu, L.; Jing, X.; Liang, Y.; Tao, E. Rifampicin and Parkinson’s disease. Neurol. Sci. 2013, 34, 137–141. [Google Scholar] [CrossRef]
- Umeda, T.; Ono, K.; Sakai, A.; Yamashita, M.; Mizuguchi, M.; Klein, W.L.; Yamada, M.; Mori, H.; Tomiyama, T. Rifampicin is a candidate preventive medicine against amyloid-β and tau oligomers. Brain 2016, 139, 1568–1586. [Google Scholar] [CrossRef] [Green Version]
- Tomiyama, T.; Shoji, A.; Kataoka, K.-I.; Suwa, Y.; Asano, S.; Kaneko, H.; Endo, N. Inhibition of amyloid β protein aggregation and neurotoxicity by rifampicin. J. Biol. Chem. 1996, 271, 6839–6844. [Google Scholar] [CrossRef] [Green Version]
- Elmaci, I.; Altinoz, M.A. Thymoquinone: An edible redox-active quinone for the pharmacotherapy of neurodegenerative conditions and glial brain tumors. A short review. Biomed. Pharmacother. 2016, 83, 635–640. [Google Scholar] [CrossRef]
- Bermejo-Bescós, P.; Martín-Aragón, S.; Jiménez-Aliaga, K.L.; Ortega, A.; Molina, M.T.; Buxaderas, E.; Orellana, G.; Csákÿ, A.G. In vitro antiamyloidogenic properties of 1,4-naphthoquinones. Biochem. Biophys. Res. Commun. 2010, 400, 169. [Google Scholar] [CrossRef]
- Chao, S.-H.; Greenleaf, A.L.; Price, D.H. Juglone, an inhibitor of the peptidyl-prolyl isomerase Pin1, also directly blocks transcription. Nucleic Acids Res. 2001, 29, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Galas, M.C.; Dourlen, P.; Bégard, S.; Ando, K.; Blum, D.; Hamdane, M.; Buee, L. The peptidylprolyl cis/trans-isomerase Pin1 modulates stress-induced dephosphorylation of Tau in neurons: Implication in a pathological mechanism related to Alzheimer disease. J. Biol. Chem. 2006, 281, 19296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chobot, V.; Hadacek, F. Milieu-dependent pro-and antioxidant activity of juglone may explain linear and nonlinear effects on seedling development. J. Chem. Ecol. 2009, 35, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamafo Fouegue, A.D.; Ghogomu, J.N.; Bikélé Mama, D.; Nkungli, N.K.; Younang, E. Structural and antioxidant properties of compounds obtained from Fe2+ chelation by juglone and two of its derivatives: DFT, QTAIM, and NBO studies. Bioinorg. Chem. Appl. 2016, 2016, 8636409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, T.G.; Camandola, S.; Arumugam, T.V.; Cutler, R.G.; Telljohann, R.S.; Mughal, M.R.; Moore, T.A.; Yu, Q.-S.; Johnson, D.A.; Johnson, J.A.; et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J. Neurochem. 2010, 112, 1316. [Google Scholar] [CrossRef] [Green Version]
- Nakhate, K.T.; Bharne, A.P.; Verma, V.S.; Aru, D.N.; Kokare, D.M. Plumbagin ameliorates memory dysfunction in streptozotocin induced Alzheimer’s disease via activation of Nrf2/ARE pathway and inhibition of β-secretase. Biomed. Pharmacother. 2018, 101, 379. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, J.G.; Wu, L. Plumbagin inhibits neuronal apoptosis, intimal hyperplasia and also suppresses TNF-α/NF-κB pathway induced inflammation and matrix metalloproteinase-2/9 expression in rat cerebral ischemia. Saudi J. Biol. Sci. 2018, 25, 1033. [Google Scholar] [CrossRef]
- Tan, M.; Liu, Y.; Luo, X.; Chen, Z.; Liang, H. Antioxidant activities of plumbagin and its Cu (II) complex. Bioinorg. Chem. Appl. 2011, 2011, 898726. [Google Scholar] [CrossRef] [Green Version]
- Campora, M.; Canale, C.; Gatta, E.; Tasso, B.; Laurini, E.; Relini, A.; Pricl, S.; Catto, M.; Tonelli, M. Multitarget biological profiling of new naphthoquinone and anthraquinone-based derivatives for the treatment of Alzheimer’s disease. ACS Chem. Neurosci. 2021, 12, 447–461. [Google Scholar] [CrossRef]
- Viswanathan, G.K.; Shwartz, D.; Losev, Y.; Arad, E.; Shemesh, C.; Pichinuk, E.; Engel, H.; Raveh, A.; Jelinek, R.; Cooper, I.; et al. Purpurin modulates Tau-derived VQIVYK fibrillization and ameliorates Alzheimer’s disease-like symptoms in animal model. Cell. Mol. Life Sci. 2020, 77, 2795. [Google Scholar] [CrossRef]
- Kim, W.; Kwon, H.J.; Jung, H.Y.; Hahn, K.R.; Yoon, Y.S.; Hwang, I.K.; Choi, S.Y.; Kim, D.W. Neuroprotective effects of purpurin against ischemic damage via MAPKs, Bax, and oxidative stress cascades in the gerbil hippocampus. Mol. Neurobiol. 2022, 59, 2580. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hahn, K.R.; Nam, S.M.; Yoon, Y.S.; Moon, S.M.; Hwang, I.K.; Kim, D.W. Purpurin ameliorates D-galactose-induced aging phenotypes in mouse hippocampus by reducing inflammatory responses. Neurochem. Int. 2023, 167, 105552. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Ali, M.Y.; Jung, H.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against β-secretase and cholinesterases. J. Ethnopharmacol. 2016, 191, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Anjum, J.; Muni, M.; Das, R.; Rauf, A.; Islam, F.; Emran, T.B.; Semwal, P.; Hemeg, H.A.; Alhumaydhi, F.A.; et al. Exploring the journey of emodin as a potential neuroprotective agent: Novel therapeutic insights with molecular mechanism of action. Biomed. Pharmacother. 2022, 149, 112877. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lv, H.; Xia, Z.; Fan, R.; Zhang, C.; Wang, Y.; Wang, D. Rhein exhibits antioxidative effects similar to Rhubarb in a rat model of traumatic brain injury. BMC Complement. Altern. Med. 2017, 17, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Fan, X.; Tang, T.; Fan, R.; Zhang, C.; Huang, Z.; Peng, W.; Gan, P.; Xiong, X.; Huang, W.; et al. Rhein and rhubarb similarly protect the blood-brain barrier after experimental traumatic brain injury via gp91phox subunit of NADPH oxidase/ROS/ERK/MMP-9 signaling pathway. Sci. Rep. 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, F.; Ma, H.; Ji, C.; Chang, C.; Liu, W.; Xie, K. Rhein protects against neurological deficits after traumatic brain injury in mice via inhibiting neuronal pyroptosis. Front. Pharmacol. 2020, 11, 564367. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Chu, M.T.; Hsu, C.Y.; Wu, Y.J.J.; Lee, J.Y.; Chen, T.J.; Chung, W.-H.; Chen, D.-Y.; Hung, S.I. Rhein, an anthraquinone drug, suppresses the NLRP3 inflammasome and macrophage activation in urate crystal-induced gouty inflammation. Am. J. Chin. Med. 2019, 47, 135. [Google Scholar] [CrossRef]
- Palomo, V.; Tosat-Bitrian, C.; Nozal, V.; Nagaraj, S.; Martín-Requero, A.; Martínez, A. TDP-43: A key therapeutic target beyond amyotrophic lateral sclerosis. ACS Chem. Neurosci. 2019, 10, 1183–1196. [Google Scholar] [CrossRef] [Green Version]
- Moujalled, D.; James, J.L.; Parker, S.J.; Lidgerwood, G.E.; Duncan, C.; Meyerowitz, J.; Nonaka, T.; Hasegawa, M.; Kanninen, K.M.; Grubman, A.; et al. Kinase inhibitor screening identifies cyclin-dependent kinases and glycogen synthase kinase 3 as potential modulators of TDP-43 cytosolic accumulation during cell stress. PLoS ONE 2013, 8, e67433. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, W.; Qin, J.; Wang, L.; Wei, S.; Tang, H. Assessment of novel azaanthraquinone derivatives as potent multi-target inhibitors of inflammation and amyloid-β aggregation in Alzheimer’s disease. Bioorg. Chem. 2019, 83, 477. [Google Scholar] [CrossRef]
- McNaughton-Smith, G.; Jiménez-Alonso, S.; Gutiérrez-Ravelo, A.D.; Fernández-Pérez, L.; Díaz-Chico, B.-N. Fused Quinonic Compounds. European Patent EP2877455A1, 17 August 2016. [Google Scholar]
- Ceyzériat, K.; Abjean, L.; Carrillo-De Sauvage, M.A.; Ben Haim, L.; Escartin, C. The complex STATes of astrocyte reactivity: How are they controlled by the JAK-STAT3 pathway? Neuroscience 2016, 330, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Gibson, S.A.; Buckley, J.A.; Qin, H.; Benveniste, E.N. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol. 2018, 189, 4–13. [Google Scholar] [CrossRef]
- Wan, J.; Fu, A.K.Y.; Ip, F.C.P.; Ng, H.-K.; Hugon, J.; Page, G.; Wang, J.H.; Lai, K.-O.; Wu, Z.; Ip, N.Y. Tyk2/STAT3 signaling mediates β-amyloid-induced neuronal cell death: Implications in Alzheimer’s disease. J. Neurosci. 2010, 30, 6873–6881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lia, X.; Wang, L.; Cykowskic, M.; He, T.; Liu, T.; Chakranarayana, J.; Rivera, A.; Zhao, H.; Powell, S.; Xia, W.; et al. OCIAD1 contributes to neurodegeneration in Alzheimer’s disease by inducing mitochondria dysfunction, neuronal vulnerability and synaptic damages. EBioMedicine 2020, 51, 102569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertini, C.; Salerno, A.; de Sena Murteira Pinheiro, P.; Bolognesi, M.L. From combinations to multitarget-directed ligands: A continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2021, 41, 2606–2633. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-X.; Dai, C.-L.; Liu, F.; Iqbal, K. Multi-targets: An unconventional drug development strategy for Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 837649. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.L.; Tiew, J.K.; Fong, Y.H.; Leong, H.W.; Chan, Y.M.; Chan, Z.L.; Kong, E.W.J. Current pharmacotherapy and multi-target approaches for Alzheimer’s disease. Pharmaceuticals 2022, 15, 1560. [Google Scholar] [CrossRef] [PubMed]
- Makletsova, M.G.; Rikhireva, G.T.; Kirichenko, Y.E.; Trinitatsky, M.Y.; Vakulenko, M.Y.; Ermakov, A.M. The role of polyamines in the mechanisms of cognitive impairment. Neurochem. J. 2022, 16, 283–294. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Cavalli, A.; Melchiorre, C. Memoquin: A multi-target-directed ligand as an innovative therapeutic opportunity for Alzheimer’s disease. Neurotherapeutics 2009, 6, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Bolognesi, M.L.; Chiriano, G.P.; Bartolini, M.; Mancini, F.; Bottegoni, G.; Maestri, V.; Czvitkovich, S.; Windisch, M.; Cavalli, A.; Minarini, A.; et al. Synthesis of monomeric derivatives to probe memoquin’s bivalent interactions. J. Med. Chem. 2011, 54, 8299–8304. [Google Scholar] [CrossRef]
- Wu, J.M.; Oraee, A.; Doonan, B.B.; Pinto, J.T.; Hsieh, T.-C. Activation of NQO1 in NQO1*2 polymorphic human leukemic HL-60 cells by diet-derived sulforaphane. Exp. Hematol. Oncol. 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolognesi, M.L.; Cavalli, A.; Bergamini, C.; Fato, R.; Lenaz, G.; Rosini, M.; Bartolini, M.; Andrisano, V.; Melchiorre, C. Toward a rational design of multitarget-directed antioxidants: Merging memoquin and lipoic acid molecular frameworks. J. Med. Chem. 2009, 52, 7883–7886. [Google Scholar] [CrossRef]
- Prati, F.; Bartolini, M.; Simoni, E.; De Simone, A.; Pinto, A.; Andrisano, V.; Bolognesi, M.L. Quinones bearing non-steroidal anti-inflammatory fragments as multitarget ligands for Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2013, 23, 6254–6258. [Google Scholar] [CrossRef] [PubMed]
- Nepovimova, E.; Uliassi, E.; Korabecny, J.; Peña-Altamira, L.E.; Samez, S.; Pesaresi, A.; García, G.E.; Bartolini, M.; Andrisano, V.; Bergamini, C.; et al. Multitarget drug design strategy: Quinone-tacrine hybrids designed to block amyloid-β aggregation and to exert anticholinesterase and antioxidant effects. J. Med. Chem. 2014, 57, 8576–8589. [Google Scholar] [CrossRef] [PubMed]
- Chioua, M.; Serrano, E.; Dgachi, Y.; Martin, H.; Jun, D.; Janockova, J.; Sepsova, V.; Soukup, O.; Moraleda, I.; Chabchoub, F.; et al. Synthesis, biological assessment and molecular modeling of racemic quinopyranotacrines for Alzheimer’s disease therapy. ChemistrySelect 2018, 3, 461–466. [Google Scholar] [CrossRef]
- Perone, R.; Albertini, C.; Uliassi, E.; Di Pietri, F.; de Sena Murteira Pinheiro, P.; Petralla, S.; Rizzardi, N.; Fato, R.; Pulkrabkova, L.; Soukup, O.; et al. Turning donepezil into a multi-target-directed ligand through a merging strategy. ChemMedChem 2021, 16, 187–198. [Google Scholar] [CrossRef]
- Tonelli, M.; Catto, M.; Tasso, B.; Novelli, F.; Canu, C.; Iusco, G.; Pisani, L.; De Stradis, A.; Denora, N.; Sparatore, A.; et al. Multitarget therapeutic leads for Alzheimer’s disease: Quinolizidinyl derivatives of bi- and tricyclic systems as dual inhibitors of cholinesterases and β-amyloid (Aβ) aggregation. ChemMedChem 2015, 10, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Jiang, N.; Xie, S.S.; Wang, K.D.G.; Wang, X.B.; Kong, L.Y. Design, synthesis and evaluation of novel tacrine-rhein hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Org. Biomol. Chem. 2014, 12, 801–814. [Google Scholar] [CrossRef]
- Camps, P.; Cusack, B.; Mallender, W.D.; El Achab, R.E.; Morral, J.; Muñoz-Torrero, D.; Rosenberry, T.L. Huprine X is a novel high-affinity inhibitor of acetylcholinesterase that is of interest for treatment of Alzheimer’s disease. Mol. Pharmacol. 2000, 57, 409–417. [Google Scholar]
- Viayna, E.; Sola, I.; Bartolini, M.; De Simone, A.; Tapia-Rojas, C.; Serrano, F.G.; Sabaté, R.; Juárez-Jiménez, J.; Pérez, B.; Luque, F.J.; et al. Synthesis and multitarget biological profiling of a novel family of rhein derivatives as disease-modifying anti-Alzheimer agents. J. Med. Chem. 2014, 57, 2549–2567. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Areales, F.J.; Betari, N.; Viayna, A.; Pont, C.; Espargaró, A.; Bartolini, M.; De Simone, A.; Rinaldi Alvarenga, J.F.; Pérez, B.; Sabaté, R.; et al. Design, synthesis and multitarget biological profiling of second-generation anti-Alzheimer rhein-huprine hybrids. Future Med. Chem. 2017, 9, 965–981. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, G.K.; Paul, A.; Gazit, E.; Segal, D. Naphthoquinone tryptophan hybrids: A promising small molecule scaffold for mitigating aggregation of amyloidogenic proteins and peptides. Front. Cell Dev. Biol. 2019, 7, 242. [Google Scholar] [CrossRef] [Green Version]
- Scherzer-Attali, R.; Pellarin, R.; Convertino, M.; Frydman-Marom, A.; Egoz-Matia, N.; Peled, S.; Levy-Sakin, M.; Shalev, D.E.; Caflisch, A.; Gazit, E.; et al. Complete phenotypic recovery of an Alzheimer’s disease model by a quinone-tryptophan hybrid aggregation inhibitor. PLoS ONE 2010, 5, e11101. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Huber, A.; Rand, D.; Gosselet, F.; Cooper, I.; Gazit, E.; Segal, D. Naphthoquinone–dopamine hybrids inhibit α-synuclein aggregation, disrupt preformed fibrils, and attenuate aggregate-induced toxicity. Chem. Eur. J. 2020, 26, 16486–16496. [Google Scholar] [CrossRef] [PubMed]
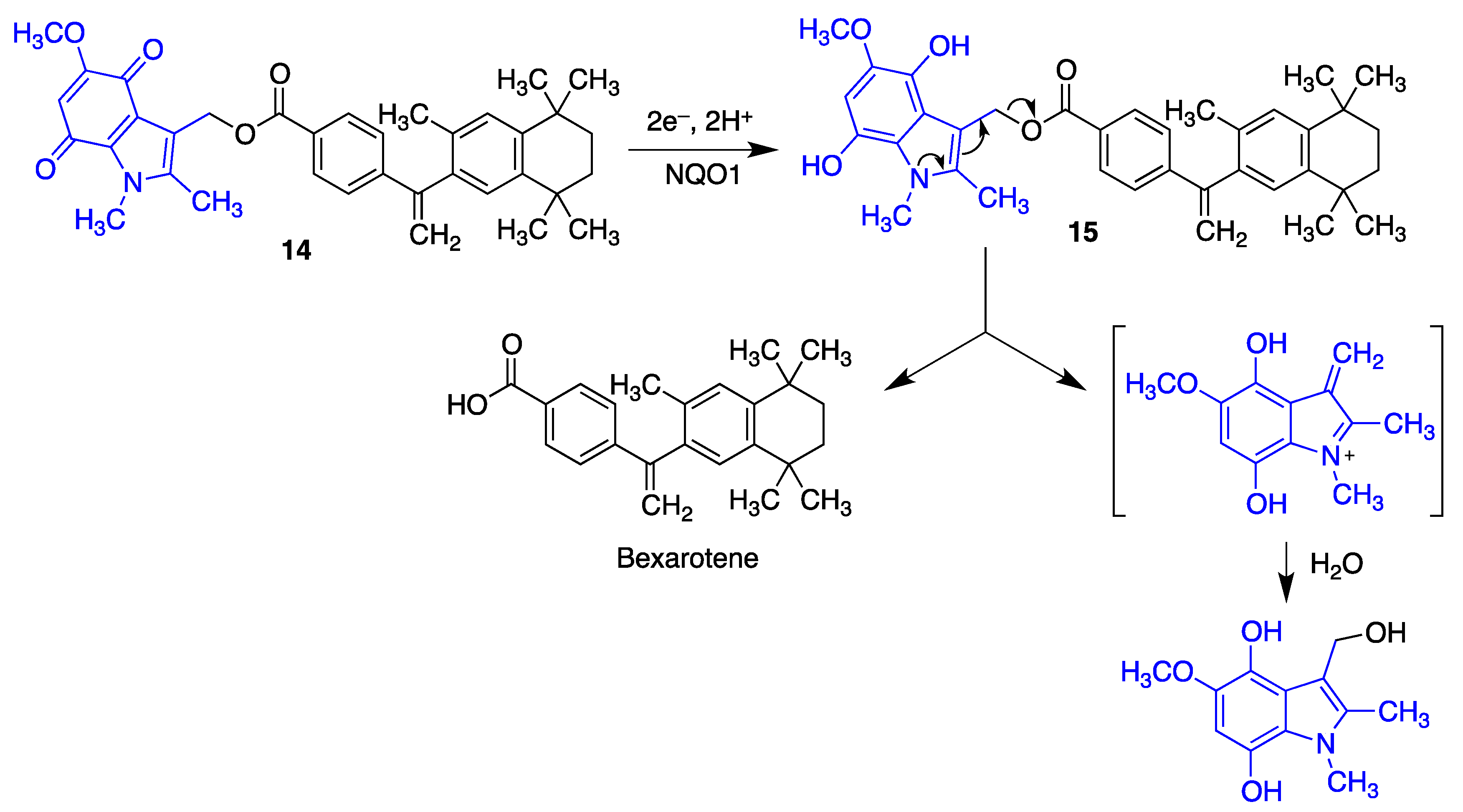
- Tousi, B. The emerging role of bexarotene in the treatment of Alzheimer’s disease: Current evidence. Neuropsychiatr. Dis. Treat. 2015, 11, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S. Toward neuroprotective treatments of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 3795–3797. [Google Scholar] [CrossRef]
- Riancho, J.; Ruiz-Soto, M.; Berciano, M.T.; Berciano, J.; Lafarga, M. Neuroprotective effect of bexarotene in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2015, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Liu, H.; Zhong, J.; Guo, Z.; Wu, J.; Zhang, H.; Huang, Z.; Jiang, L.; Li, H.; Zhang, Z.; et al. Bexarotene protects against neurotoxicity partially through a PPARγ-dependent mechanism in mice following traumatic brain injury. Neurobiol. Dis. 2018, 117, 114–124. [Google Scholar] [CrossRef]
- Schäfer, A.; Burstein, E.S.; Olsson, R. Bexarotene prodrugs: Targeting through cleavage by NQO1 (DT-diaphorase). Bioorg. Med. Chem. Lett. 2014, 24, 1944–1947. [Google Scholar] [CrossRef]












| Quinone | Properties | Refs. |
|---|---|---|
| Coenzyme Q10 | ROS scavenger in the mitochondria, Nrf2 inductor [38,39]. Anti-inflammatory effects, decreasing the protein and mRNA levels of pro-inflammatory cytokines. Anti-apoptotic properties [40]. Increases the levels of tyrosine hydroxylase [45]. | [38,39,40,45] |
| QS-10 | Activation of the Keap1/Nrf2 pathway. Activation of the heat-shock transcription factor-1 (HSF-1). | [51] |
| Idebenone | Treatment for complex I-related diseases: Leber’s hereditary optic neuropathy (LHON) Leigh syndrome, mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS), Duchene muscular dystrophy and glaucoma. Under clinical assay for Friedreich ataxia (FRDA) [55], multiple sclerosis [56] and Parkinson’s disease [57]. | [53,54,55,56,57] |
| Mitoquinone | Treatment of Parkinson’s disease [58] and multiple sclerosis [59]. | [58,59] |
| SkQ1 | Neuroprotective properties in a model of middle cerebral artery occlusion, when introduced immediately after reperfusion [61]. Neuroprotective properties in models of Alzheimer’s disease [62]. | [61,62] |
| Vatiquinone (EPI-743, α-tocotrienol quinone) | Modulates the oxidative stress response [67]. Prevents ferroptotic cell death in some neurodegenerative diseases, including Alzheimer’s, Parkinson’s and Huntington’s diseases [68,70] Treatment of Leigh syndrome and other inherited mitochondrial diseases [71]. FDA orphan drug status for the treatment of Friedrich’s ataxia [72]. | [67,68,69,70,71,72] |
| MK-4 | Prevents the depletion of glutathione mediated by free radical-mediated oxidative injury [81]. | [81] |
| Menadione (vitamin K3) | Protects neurons from methylmercury-induced cell death [75]. Inhibits the aggregation of the Aβ1–42 protein, and it is also neuroprotective against Aβ1–42 insult in human neuroblastoma cells (SH-SY5Y) [82]. Competitive inhibitor of monoamino oxidases (MAO), with some selectivity for MAO-B [83]. | [75,82,83] |
| Embelin | Inhibits beta-secretase 1 (BACE-1), acetylcholinesterase and butyryl cholinesterase, with a balanced multitarget profile [88]. Reduces cognitive deficit in a scopolamine-induced Alzheimer’s disease-like condition in a rat model [90]. Potent radical scavenger [91]. Anti-inflammatory properties [92]. | [88,90,91,92] |
| SB-1448 | Inhibits acetylcholinesterase and butyryl cholinesterase [93]. Inhibits Aβ self-aggregation [93]. Reduces scopolamine-induced cognitive impairments in mouse models [93]. | [93] |
| APX-3330 (E-3330) | Treatment of diabetic retinopathy by APE1/Ref-1 inhibition [98]. | [98] |
| APX-2009 APX-2014 | Targets neovascular eye diseases by APE1/Ref-1 inhibition [98]. | [98] |
| VCE-003 | Alleviates neuroinflammation in a chronic model of multiple sclerosis by PPARγ activation [99]. | [99] |
| VCE-003.2 | Enhances neuronal progenitor cell survival and to also provide a symptomatic relief in murine models of Huntington’s disease [101]. Showed protective effects in SOD1G93A transgenic mice, a model of amyotrophic lateral sclerosis [102]. | [101,102] |
| HU-331 | Anticancer properties [103]. Activation of the Keap1-Nrf2 pathway and BACH1 inhibitors [104]. | [103,104] |
| Quinones 1 | Neuroprotective activity [105]. | [105] |
| Asterriquinone B4 | Neuroprotective activity in several cellular using oxidative stress models of Parkinson’s disease [106]. | [106] |
| Asterriquinone 1H5 Indolylquinone 5E5 | Promote neuronal differentiation of PC12 cells [107]. | [107] |
| PQQ | Potent radical scavenger [113]. Reduces oxidative stress by increasing the expression of Nrf2 [115]. Neuroprotection of primary cultured hippocampal neurons against glutamate-induced cell damage by activation of the PI3K/Akt pathway [116]. Reduced indirectly the activity of GSK-3β [117]. Exerted effective protection in SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity [113,114]. Reduce oxidative stress and improve mitochondrial functions and dopamine redistribution [118]. | [113,114,115,116,117,118] |
| Geldanamycin | Binds to Hsp90 a target in neurodegeneration [119,120,121]. Protects against MPTP-induced dopaminergic neurotoxicity [123]. Rescues striatal dopamine levels [124]. Suppresses memory deficits in β-amyloid-injected rats [125]. Induces the degradation of misfolded tau protein [126]. Reduces brain injury in a mouse model of intracerebral hemorrhage and improves the neurological outcome of these mice [127]. | [119,120,121,123,124,125,126,127] |
| Tanespimycin | Reduces polyglutamine-mediated motor neuron degeneration through proteasomal degradation of the androgen receptor in a mouse model [129]. Improves balance and coordination in a mouse model of the Machado-Joseph disease (spinocerebellar ataxia type 3) [130]. | [129,130] |
| Rifampicin quinone | Inhibits fibrillation and disaggregates formed fibrils of β-amyloid [134]. Ameliorates the neurotoxic effects mediated by α-synuclein in microglial cells [136]. Protects PC12 cells against apoptosis induced by N-methyl-4-phenylpyridinium (MPP+). Suppresses the expression of α-synuclein multimers [139]. | [134,136,139] |
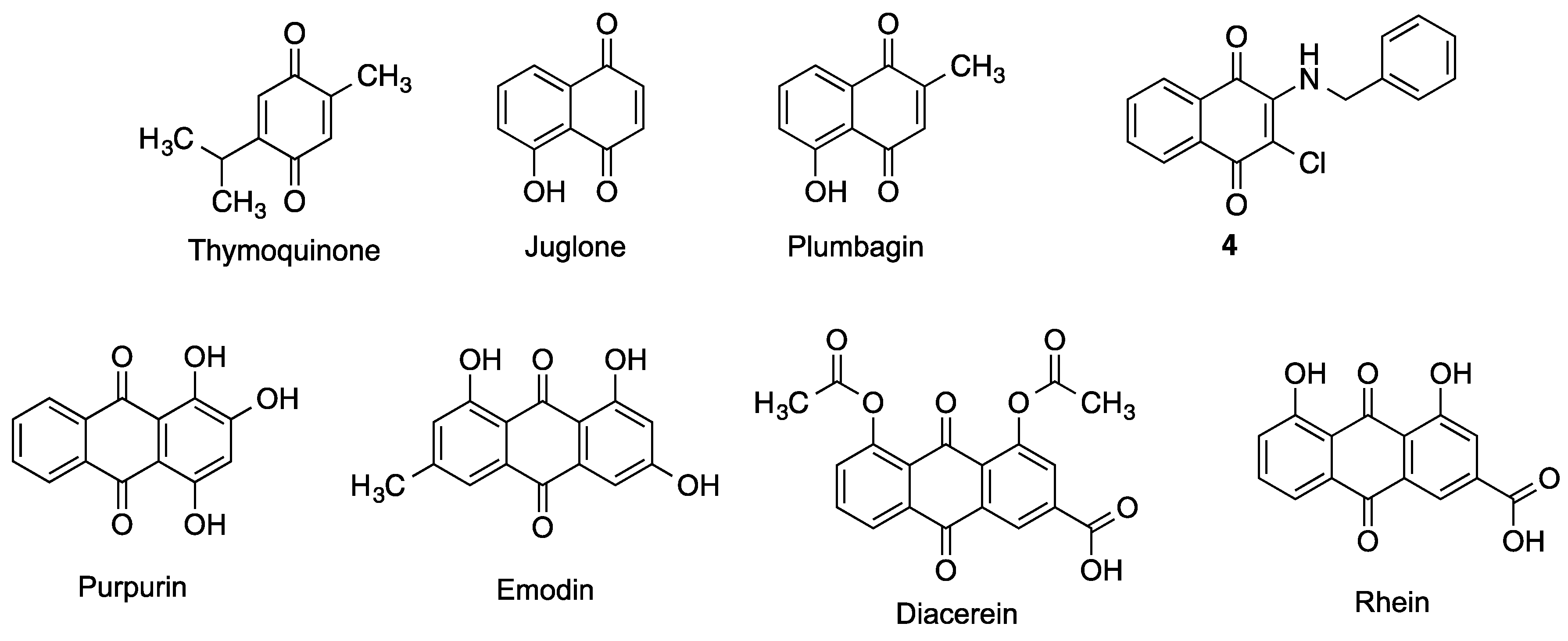
| Thymoquinone | Protects against ischemic brain damage and reduces oxidative damage associated to epileptic seizures [142]. | [142] |
| Juglone | Inhibits BACE and Aβ aggregation and induce Aβ fibril disaggregation [143]. Reverses the Thr-231 dephosphorylation of Tau induced by oxidative or heat stress in primary cortical cultures [145]. Neuroprotective potential by redox cycling [146] acting as a chelating agent [147] | [143,145,146,147] |
| Plumbagin | Reduces the extent of brain damage and neurological deficit associated to middle cerebral artery occlusion/reperfusion in mice [148]. Activates the Nrf2-ARE pathway, inhibits BACE-1 and attenuates astrocyte hyperactivation [149]. Inhibits the NF-kB pathway [150] and displays antioxidant and chelating activities [151]. | [148,149,150,151] |
| Naphthoquinone 4 | Inhibits the aggregation of Aβ40 and the PHF6 tau fragment [152]. Inhibits AChE and MAO B [152]. | [152] |
| Purpurin | Hampers the aggregation of PHF-6 and diassambles pre-formed PHF-6 fibrils, reducing Tau phosphorylation and accumulation [153]. Inhibits NF-κB activation [154]. Decreases oxidative stress and inflammation markers [155]. | [153,154,155] |
| Emodin | Radical scavenger and chelating agent. Nrf2 inducer. Inhibits Aβ42 and Tau aggregation and toxicity. Inhibits AChE and BACE [152]. | [152] |
| Rhein | Treatment of traumatic brain injury (TBI) by attenuating inflammation markers, pyroptosis and neurological dysfunction [158,160]. NADPH oxidase inhibition, avoiding the increase of ROS levels [159]. Modulation of inflammatory response by NF-kB-pathway and NLRP-3 inflammasome inhibition [161]. Moderate inhibition of AChE [183]. | [158,159,160,161,183] |
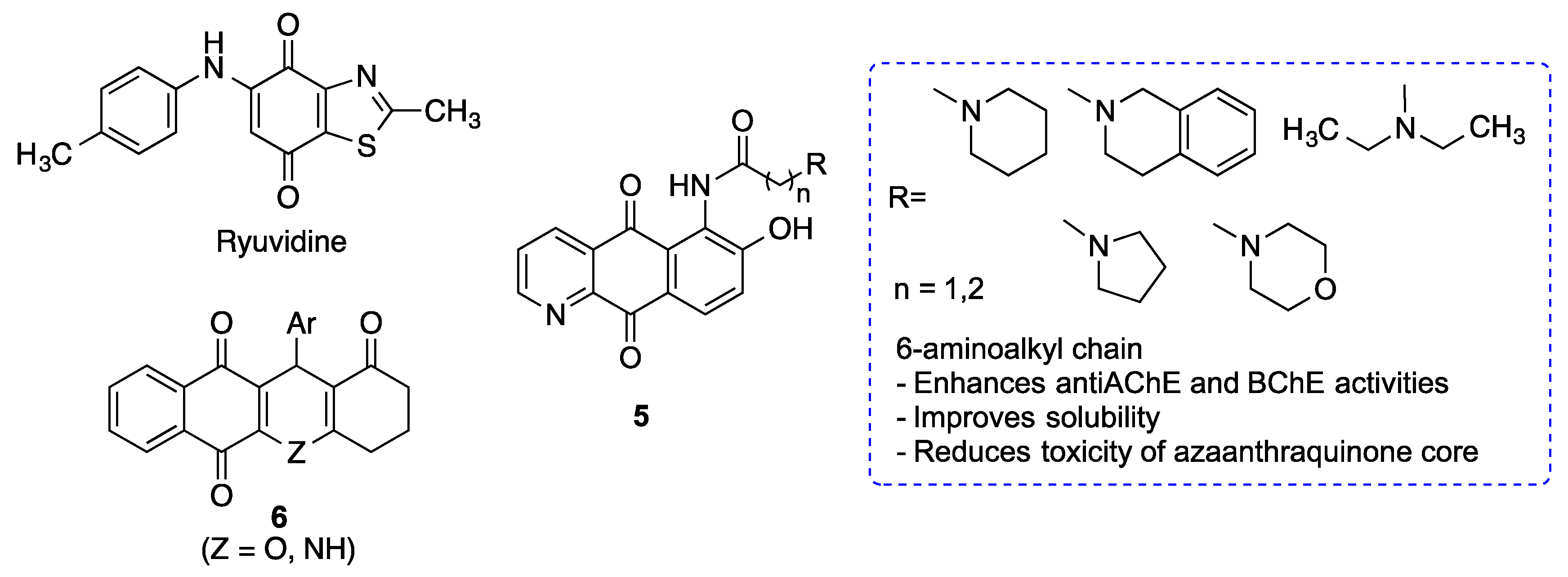
| Compounds 5 | Neuroprotective activity against H2O2-induced oxidative stress. Reduces the levels of inflammatory cytokines and NO produced by macrophages exposed to LPS. Inhibits the production and aggregation of Aβ. Moderate AChE and BChE inhibition [164]. | [164] |
| Quinones 6 | Inhibits the JAK-STAT pathway [165]. | [165] |
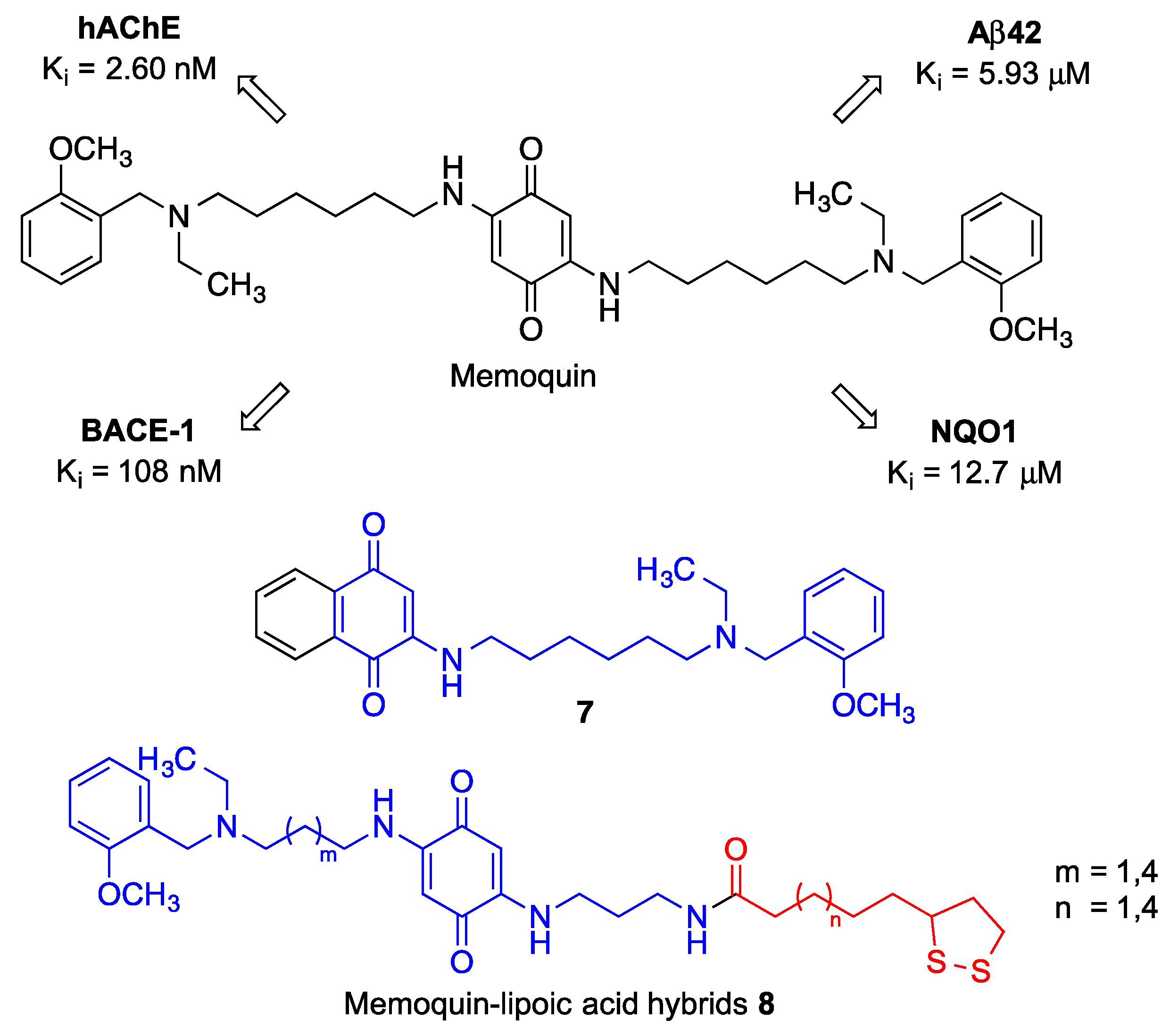
| Memoquin | Scavenges ROS. Inhibitis β-amyloid aggregation, BACE-1 and AChE [174]. Prevents cognitive impairment [173]. | [173,174] |
| Compound 7 | Inhibits BACE [175]. | [175] |
| Compounds 8 | Inhibit ROS production and ROS-induced cytotoxicity. Induce Nrf2, thereby increasing the levels of the NQO1 enzyme [176]. Reduce Aβ42 self-aggregation. Inhibit AChE and BChE [177]. | [176,177] |
| Compounds 9 | Inhibit β-amyloid aggregation Inhibit AChE [178]. | [178] |
| Compounds 10 | Inhibit AChE. Inhibit spontaneous amyloid aggregation [179]. | [179] |
| Compounds 11 | Inhibit AChE. Radical scavengers [180]. | [180] |
| Compounds 12 | Selectively inhibit butyrylcholinesterase. Antioxidant and amyloid antiaggregating properties [181]. | [181] |
| Compounds 13 | Inhibit both AChE and BChE. Inhibit β-amyloid aggregation [182]. | [182] |
| Compounds 14 | Inhibit AChE [183]. | [183] |
| Compounds 15 | Inhibit AChE and BChE. Inhibit BACE-1. Show dual Aβ42 and tau antiaggregating activity [185]. | [185] |
| NQTrp | Reduces the accumulation of oligomeric Aβ species in an Alzheimer’s disease model of Drosophila flies [188]. Inhibits the aggregation of α-synuclein. | [188] |
| NQDA | Inhibits α-synuclein aggregation and disrupts preformed fibrils. Reduces aggregate-induced toxicity [189] | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cores, Á.; Carmona-Zafra, N.; Clerigué, J.; Villacampa, M.; Menéndez, J.C. Quinones as Neuroprotective Agents. Antioxidants 2023, 12, 1464. https://doi.org/10.3390/antiox12071464
Cores Á, Carmona-Zafra N, Clerigué J, Villacampa M, Menéndez JC. Quinones as Neuroprotective Agents. Antioxidants. 2023; 12(7):1464. https://doi.org/10.3390/antiox12071464
Chicago/Turabian StyleCores, Ángel, Noelia Carmona-Zafra, José Clerigué, Mercedes Villacampa, and J. Carlos Menéndez. 2023. "Quinones as Neuroprotective Agents" Antioxidants 12, no. 7: 1464. https://doi.org/10.3390/antiox12071464
APA StyleCores, Á., Carmona-Zafra, N., Clerigué, J., Villacampa, M., & Menéndez, J. C. (2023). Quinones as Neuroprotective Agents. Antioxidants, 12(7), 1464. https://doi.org/10.3390/antiox12071464









