Ultrasound-Assisted Deep Eutectic Solvent Extraction of Phenolic Compounds from Thinned Young Kiwifruits and Their Beneficial Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Ultrasound-Assisted Deep Eutectic Solvent Extraction (UADE) of Phenolic Compounds from Thinned Young Kiwifruits
2.2.1. Preparation of Deep Eutectic Solvent
2.2.2. Optimization of UADE Conditions
2.3. Ultrasound-Assisted Ethanol Extraction (UAEE) of Phenolic Compounds from Thinned Young Kiwifruits
2.4. Conventional Organic Solvent Extraction (CSE) of Phenolic Compounds from Thinned Young Kiwifruits
2.5. Determination of Total Polyphenolics in the Thinned Young Kiwifruit Extracts
2.6. Identification of Phenolic Compounds in the Thinned Young Kiwifruit Extracts by UPLC-MS/MS
2.7. Quantification of Major Phenolic Compounds in the Thinned Young Kiwifruit Extracts
2.8. Evaluation of Antioxidant Capacities of the Thinned Young Kiwifruit Extracts
2.9. Evaluation of Inhibitory Effects of the Thinned Young Kiwifruit Extracts against α-Glucosidase and Pancreatic Lipase
2.10. Evaluation of In Vitro Anti-Inflammatory Activities of the Thinned Young Kiwifruit Extracts
2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimal Conditions of UADE for the Extraction of Phenolic Compounds from Thinned Young Kiwifruits
3.1.1. Optimal Conditions from the Single-Factor Experiment
3.1.2. Optimal Conditions from the Box-Behnken Design
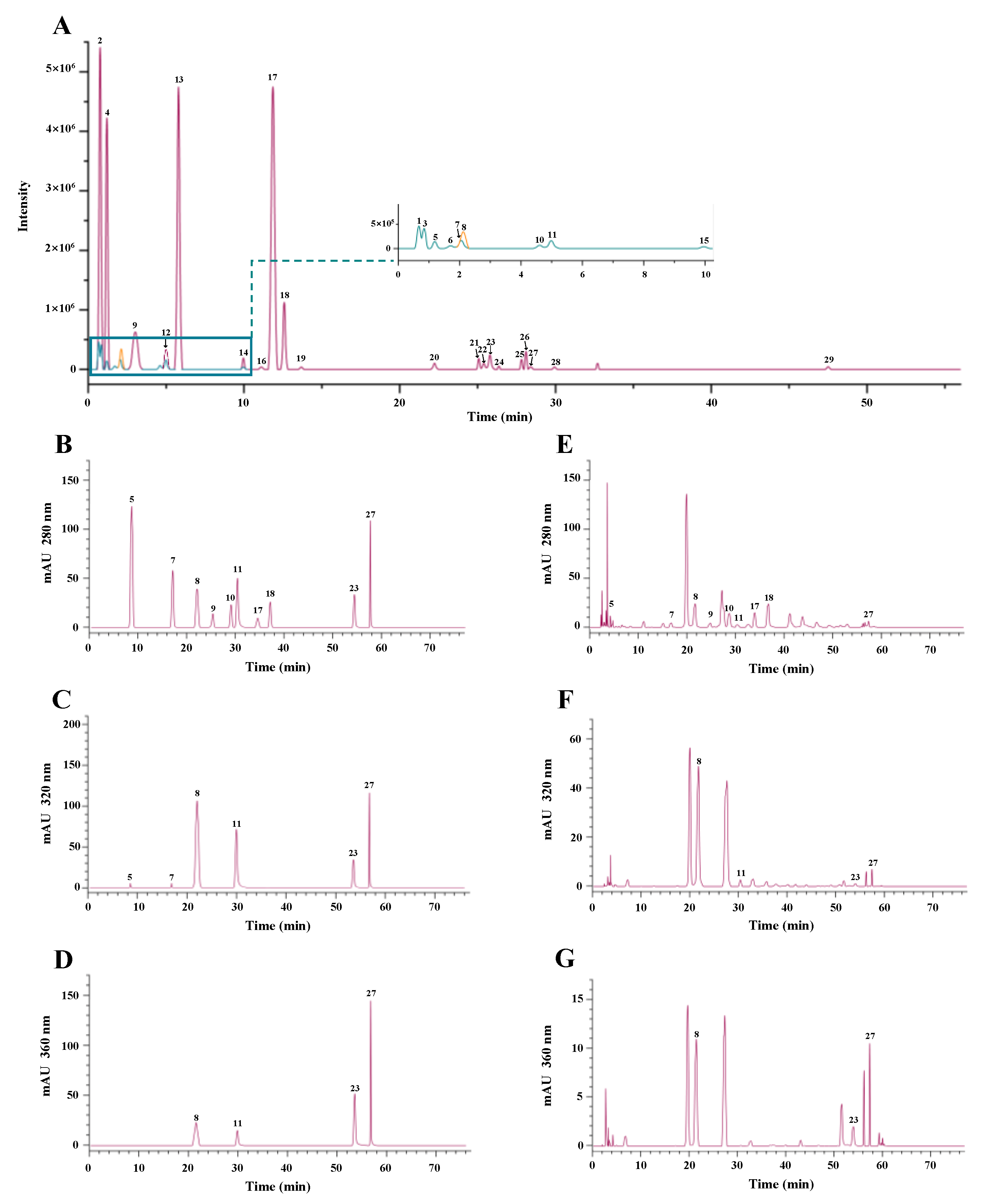
3.2. Identification and Quantification of Major Phenolic Compounds in the Thinned Young Kiwifruit Extracts
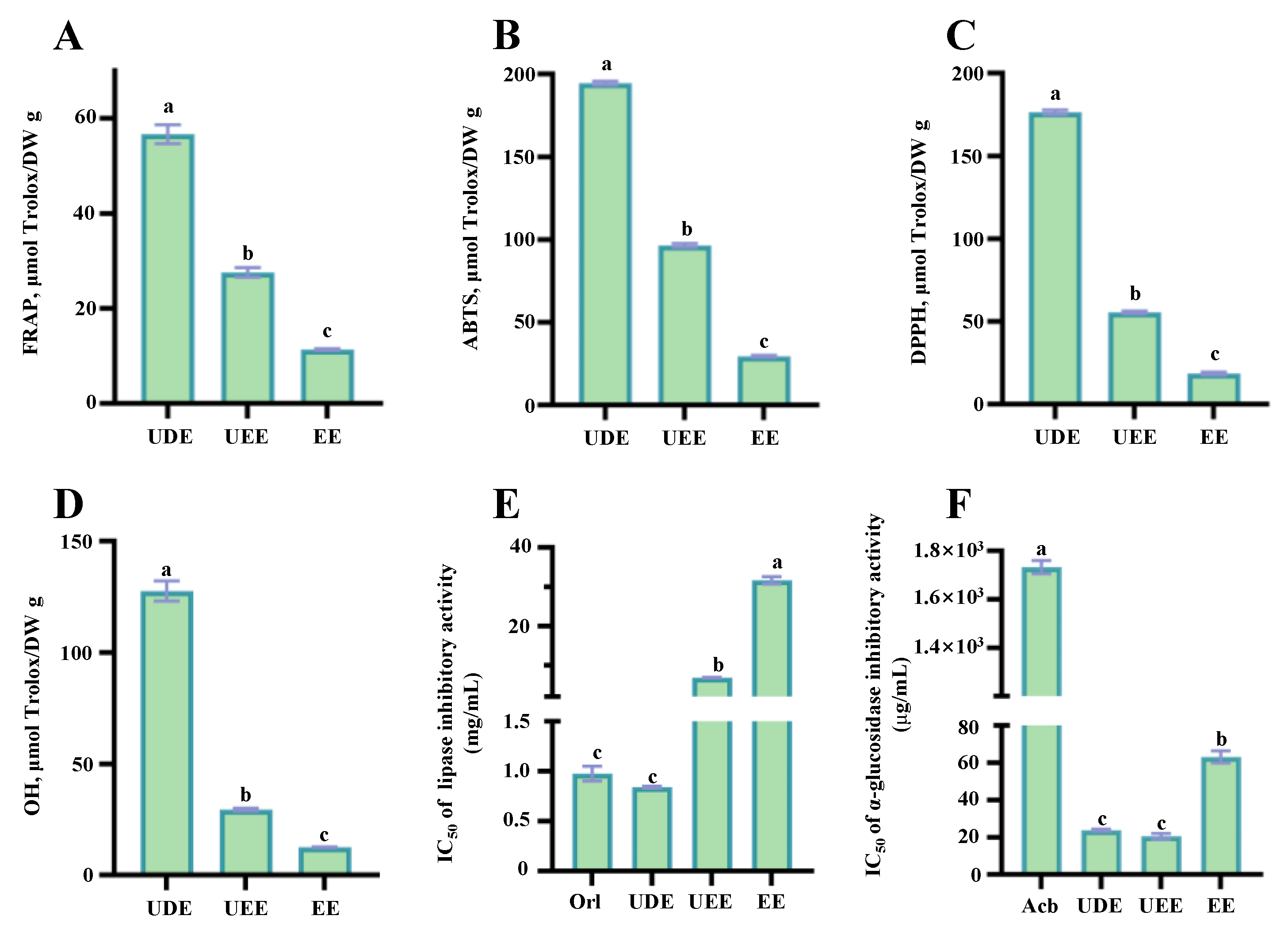
3.3. Comparison of Antioxidant Capacities of the Thinned Young Kiwifruit Extracts
3.4. Comparison of Inhibitory Effects on Digestive Enzymes of the Thinned Young Kiwifruit Extracts
3.5. Comparison of Anti-Inflammatory Effects of the Thinned Young Kiwifruit Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Li, H.Y.; Yuan, Q.; Yang, Y.L.; Han, Q.H.; He, J.L.; Zhao, L.; Zhang, Q.; Liu, S.X.; Lin, D.R.; Wu, D.T.; et al. Phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of different kiwifruits. Molecules 2018, 23, 2957. [Google Scholar] [CrossRef] [Green Version]
- Mai, Y.H.; Zhuang, Q.G.; Li, Q.H.; Du, K.; Wu, D.T.; Li, H.B.; Xia, Y.; Zhu, F.; Gan, R.Y. Ultrasound-assisted extraction, identification, and quantification of antioxidants from ‘Jinfeng’ kiwifruit. Foods 2022, 11, 827. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef]
- Li, K.; Liu, L.; McClements, D.J.; Liu, Z.D.; Liu, X.B.; Liu, F.G. A review of the bioactive compounds of kiwifruit: Bioactivity, extraction, processing and challenges. Food Res. Int. 2023, in press. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, Y.; Chen, X.; He, H.; Liu, Z.; Zhang, Z.; Ren, Y.; Ren, X. Phenolic compounds and antioxidant activity in red- and in green-fleshed kiwifruits. Food Res. Int. 2019, 116, 291–301. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Chen, X.; Zhao, Z.; Li, Y.; Meng, Y.; Huang, L. Actinidia chinensis Planch.: A review of chemistry and pharmacology. Front. Pharmacol. 2019, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Chen, D.; Fan, M.; Young Quek, S. UPLC-QqQ-MS/MS-based phenolic quantification and antioxidant activity assessment for thinned young kiwifruits. Food Chem. 2019, 281, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Quek, S.Y.; Gu, M.; Guo, Y.; Liu, Y. Polyphenols from thinned young kiwifruit as natural antioxidant: Protective effects on beef oxidation, physicochemical and sensory properties during storage. Food Control 2020, 108, 106870. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S.; et al. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef]
- Silva, A.M.; Pinto, D.; Moreira, M.M.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Valorization of kiwiberry leaves recovered by ultrasound-assisted extraction for skin application: A response surface methodology approach. Antioxidants 2022, 11, 763. [Google Scholar] [CrossRef]
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process. 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Cai, C.; Li, F.; Liu, L.; Tan, Z. Deep eutectic solvents used as the green media for the efficient extraction of caffeine from Chinese dark tea. Sep. Purif. Technol. 2019, 227, 115723. [Google Scholar] [CrossRef]
- Li, J.; Han, Z.; Zou, Y.; Yu, B. Efficient extraction of major catechins in Camellia sinensis leaves using green choline chloride-based deep eutectic solvents. RSC Adv. 2015, 5, 93937–93944. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Gan, R.Y. Green extraction of antioxidant polyphenols from green tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.-Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D.-T. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Chen, L.; Liu, R.; Chang, M.; Wang, X. Identification and in vitro anti-inflammatory activity of different forms of phenolic compounds in Camellia oleifera oil. Food Chem. 2021, 344, 128660. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.Y.; Zhou, R.R.; Fang, L.Z.; Zhao, D.; Cai, P.; Yu, R.; Zhang, S.H.; Huang, J.H. The extraction of phenolic acids and polysaccharides from Lilium lancifolium Thunb. using a deep eutectic solvent. Anal. Methods 2021, 13, 1226–1231. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: Oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trend. Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Wu, D.T.; Feng, K.L.; Huang, L.; Gan, R.Y.; Hu, Y.C.; Zou, L. Deep eutectic solvent-assisted extraction, partially structural characterization, and bioactivities of acidic polysaccharides from lotus leaves. Foods 2021, 10, 2330. [Google Scholar] [CrossRef]
- Wu, D.-T.; Fu, M.-X.; Guo, H.; Hu, Y.-C.; Zheng, X.-Q.; Gan, R.-Y.; Zou, L. Microwave-assisted deep eutectic solvent extraction, structural characteristics, and biological functions of polysaccharides from sweet tea (Lithocarpus litseifolius) leaves. Antioxidants 2022, 11, 1578. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.A.; Bouska, L. Optimization of the ultrasound-assisted extraction of polyphenols from Aronia and grapes. Food Chem. 2022, 386, 132703. [Google Scholar] [CrossRef]
- Garcia-Roldan, A.; Piriou, L.; Jauregi, P. Natural deep eutectic solvents as a green extraction of polyphenols from spent coffee ground with enhanced bioactivities. Front. Plant Sci. 2022, 13, 1072592. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-R.; Li, H.-Y.; Wei, S.-Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Li, S.-Q.; Qin, W.; Wu, D.-T. Changes of phenolic compounds, antioxidant capacities, and inhibitory effects on digestive enzymes of kiwifruits (Actinidia chinensis) during maturation. J. Food Meas. Charact. 2020, 14, 1765–1774. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Oliveras, M.J.; Quesada, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res. Int. 2018, 105, 461–472. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.E.; Jang, H.W.; Lim, T.G.; Yoo, M.; Nam, T.G. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Li, J.; Wang, A.; Li, G.; Ren, X.; Yin, W. Ultrasound-assisted deep eutectic solvent extraction of echinacoside and oleuropein from Syringa pubescens Turcz. Ind. Crops Prod. 2020, 151, 112442. [Google Scholar] [CrossRef]
- George, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.C.; Greenwood, J.; Zhang, J.L.; Skinner, M.A. Antioxidant and ‘Natural Protective’ properties of kiwifruit. Curr. Top. Med. Chem. 2011, 11, 1811–1820. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, X.; Shi, Q.; Lu, Y.; Yan, J.; Wu, D.T.; Qin, W. Changes in the fruit quality, phenolic compounds, and antioxidant potential of red-fleshed kiwifruit during postharvest ripening. Foods 2023, 12, 1509. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, E.H.; Lee, O.K.; Park, S.Y.; Lee, B.; Kim, S.H.; Park, I.; Chung, I.M. Variation and correlation analysis of phenolic compounds in mungbean (Vigna radiata L.) varieties. Food Chem. 2013, 141, 2988–2997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on alpha-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef]
- Podsedek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziolkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Gondoin, A.; Grussu, D.; Stewart, D.; McDougall, G.J. White and green tea polyphenols inhibit pancreatic lipase in vitro. Food Res. Int. 2010, 43, 1537–1544. [Google Scholar] [CrossRef]
- Azuma, T.; Kayano, S.I.; Matsumura, Y.; Konishi, Y.; Tanaka, Y.; Kikuzaki, H. Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011, 125, 471–475. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as alpha-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Lian, L.; Zhang, S.; Yu, Z.; Ge, H.; Qi, S.; Zhang, X.; Long, L.; Xiong, X.; Chu, D.; Ma, X.; et al. The dietary freeze-dried fruit powder of Actinidia arguta ameliorates dextran sulphate sodium-induced ulcerative colitis in mice by inhibiting the activation of MAPKs. Food Funct. 2019, 10, 5768–5778. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Pannucci, E.; Bernini, R.; Campo, M.; Romani, A.; Santi, L.; Velotti, F. In vitro studies on anti-inflammatory activities of kiwifruit peel extract in human THP-1 monocytes. J. Ethnopharmacol. 2019, 233, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.H.; Park, Y.; Jo, Y.H.; Kim, S.B.; Yeon, S.W.; Kim, J.G.; Turk, A.; Song, J.Y.; Kim, Y.; Hwang, B.Y.; et al. Organic acid conjugated phenolic compounds of hardy kiwifruit (Actinidia arguta) and their NF-κB inhibitory activity. Food Chem. 2020, 308, 125666. [Google Scholar] [CrossRef]
- Dias, M.; Caleja, C.; Pereira, C.; Calhelha, R.C.; Kostic, M.; Sokovic, M.; Tavares, D.; Baraldi, I.J.; Barros, L.; Ferreira, I. Chemical composition and bioactive properties of byproducts from two different kiwi varieties. Food Res. Int. 2020, 127, 108753. [Google Scholar] [CrossRef]
- Ye, C.; Jin, M.; Jin, C.; Jin, L.; Sun, J.; Ma, Y.J.; Zhou, W.; Li, G. Inhibitory effects of chemical constituents from Actinidia kolomikta on LPS-induced inflammatory responses. Rev. Bras. Farm. 2020, 30, 127–131. [Google Scholar] [CrossRef]

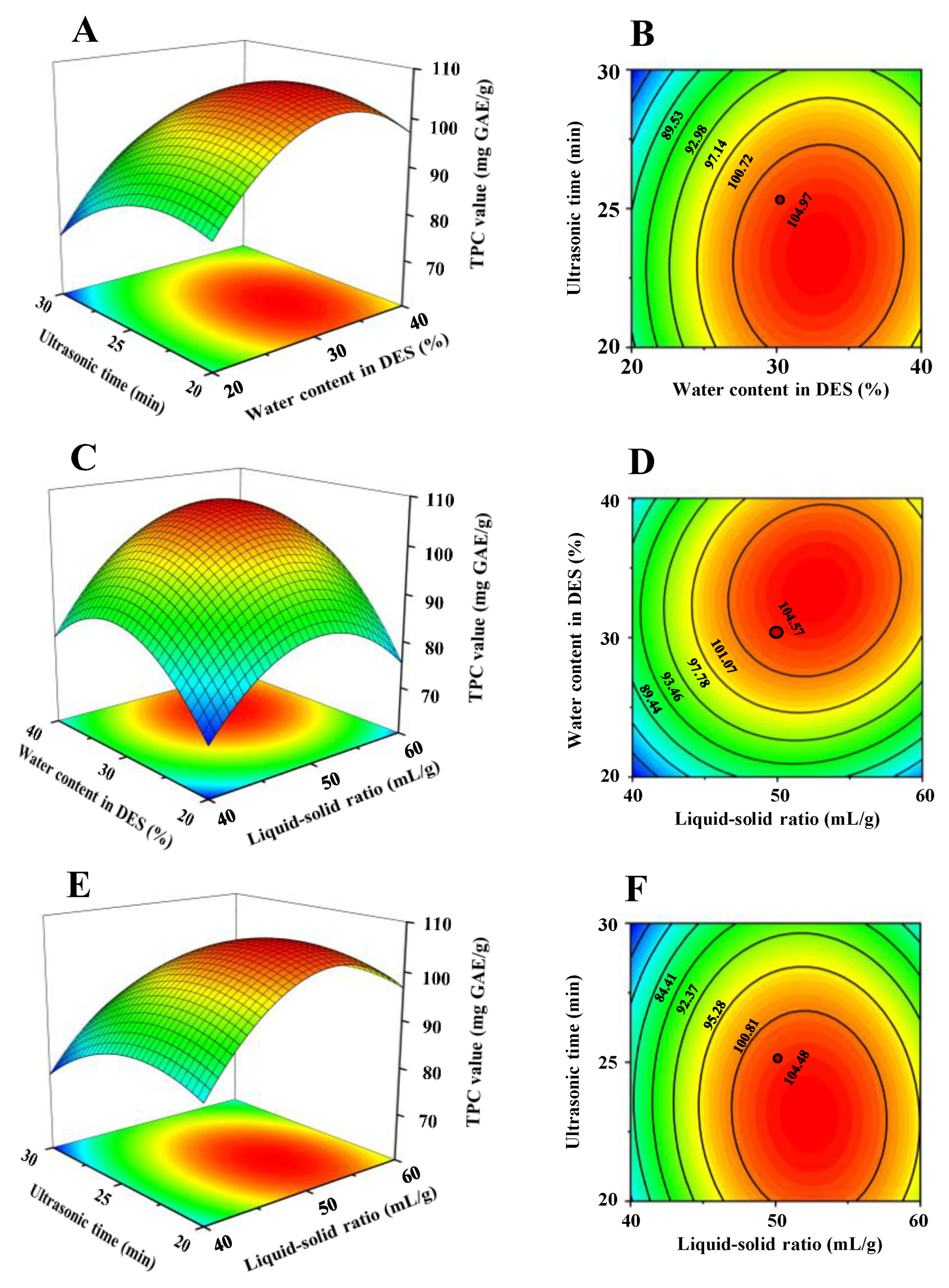


| Runs | Levels of Independent Factors | Extraction Yields (mg GAE/g DW) | ||
|---|---|---|---|---|
| X1 (%) | X2 (mL/g) | X3 (min) | ||
| 1 | 1 (40) | −1 (40) | 0 (25) | 78.21 |
| 2 | −1 (20) | −1 (40) | 0 (25) | 68.74 |
| 3 | −1 (20) | 0 (50) | 1 (30) | 71.52 |
| 4 | 0 (30) | 0 (50) | 1 (30) | 103.79 |
| 5 | −1 (20) | 0 (50) | −1 (20) | 84.91 |
| 6 | 1 (40) | 1 (60) | 0 (25) | 97.56 |
| 7 | −1 (20) | 1 (60) | 0 (25) | 71.86 |
| 8 | 0 (30) | 1 (60) | 1 (30) | 83.42 |
| 9 | 1 (40) | 0 (50) | 1 (30) | 90.15 |
| 10 | 0 (30) | 1 (60) | −1 (20) | 96.07 |
| 11 | 0 (30) | −1 (40) | 1 (30) | 77.06 |
| 12 | 0 (30) | 0 (50) | 0 (25) | 104.16 |
| 13 | 0 (30) | 0 (50) | 0 (25) | 105.86 |
| 14 | 0 (30) | −1 (40) | −1 (20) | 85.18 |
| 15 | 1 (40) | 0 (50) | −1 (20) | 95.98 |
| 16 | 0 (30) | 0 (50) | 0 (25) | 103.07 |
| 17 | 0 (30) | 0 (50) | 0 (25) | 106.57 |
| Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|
| Model | 2720.72 | 9 | 302.30 | 131.72 | <0.0001 ** |
| X1 | 526.01 | 1 | 526.01 | 229.20 | <0.0001 ** |
| X2 | 197.21 | 1 | 197.21 | 85.93 | <0.0001 ** |
| X3 | 199.90 | 1 | 199.90 | 28.69 | <0.0001 ** |
| X1X2 | 65.85 | 1 | 65.85 | 6.23 | 0.0011 * |
| X1X3 | 14.29 | 1 | 14.29 | 1.76 | 0.0413 * |
| X2X3 | 5.13 | 1 | 5.13 | 2.24 | 0.1785 |
| X12 | 678.58 | 1 | 678.58 | 295.68 | <0.0001 ** |
| X22 | 700.95 | 1 | 700.95 | 305.42 | <0.0001 ** |
| X32 | 170.05 | 1 | 170.05 | 74.09 | 0.0002 ** |
| Residual | 16.07 | 7 | 2.30 | ||
| Lack of Fit | 7.45 | 3 | 2.48 | 1.15 | 0.4305 |
| Pure error | 8.62 | 4 | 2.15 | ||
| Corrected total | 2736.78 | 16 | |||
| R² | 0.9941 | ||||
| Adjusted R² | 0.9866 |
| NO. | Formula | Retention Time (min) | Calculated [M-H]− | Observed [M-H]− | Error (ppm) | Identified Compounds |
|---|---|---|---|---|---|---|
| 1 | C15H14O7 | 0.67 | 305.06668 | 305.06729 | 2.00 | Gallocatechin a |
| 2 | C7H12O6 | 0.77 | 191.05611 | 191.05518 | −4.87 | Quinic acid ab |
| 3 | C6H8O6 | 0.80 | 175.02481 | 175.02376 | −4.74 | Ascorbic acid ab |
| 4 | C6H8O7 | 1.19 | 191.01973 | 191.01894 | −4.14 | Citric acid ab |
| 5 | C7H6O5 | 1.49 | 169.01425 | 169.0106 | −2.07 | Gallic acid abc |
| 6 | C11H12O5 | 1.70 | 223.0612 | 223.06038 | −3.68 | Sinapic acid a |
| 7 | C13H16O9 | 2.05 | 315.07216 | 315.07217 | 0.03 | Protocatechuic acid-O-hexoside ab |
| 8 | C16H18O9 | 2.11 | 353.08781 | 353.0878 | −0.03 | Neochlorogenic acid abc |
| 9 | C30H26O12 | 2.98 | 577.13515 | 577.13519 | 0.07 | Procyanidin B1 abc |
| 10 | C15H14O6 | 4.90 | 289.07176 | 289.07172 | −0.14 | Catechin abc |
| 11 | C16H18O9 | 4.98 | 353.08781 | 353.0878 | −0.03 | Chlorogenic acid abc |
| 12 | C9H10O5 | 5.05 | 197.04555 | 197.04498 | −2.89 | Syringic acid ab |
| 13 | C8H8O4 | 5.78 | 167.03498 | 167.03415 | −4.97 | Vanillic acid ab |
| 14 | C16H18O8 | 9.93 | 337.092829 | 337.09283 | 0.00 | 3-p-Coumaroylquinic acid ab |
| 15 | C15H18O9 | 9.95 | 341.08781 | 341.08771 | −0.29 | Caffeic acid-O-hexoside ab |
| 16 | C16H18O10 | 11.68 | 369.08272 | 369.08273 | 0.03 | Fraxin a |
| 17 | C30H26O12 | 11.74 | 577.13515 | 577.13513 | −0.03 | Procyanidin B2 abc |
| 18 | C15H14O6 | 12.14 | 289.07176 | 289.07172 | −0.14 | Epicatechin abc |
| 19 | C21H22O11 | 13.67 | 449.10893 | 449.10889 | −0.09 | Astilbin a |
| 20 | C45H38O18 | 22.33 | 865.19854 | 865.19854 | 0.00 | Procyanidin trimer C1 ab |
| 21 | C21H20O12 | 25.39 | 463.0882 | 463.0882 | 0.00 | Hyperoside ab |
| 22 | C27H30O16 | 25.42 | 609.14611 | 609.14618 | 0.11 | Rutin ab |
| 23 | C21H20O12 | 25.79 | 463.0882 | 463.0882 | 0.00 | Quercetin 3-O-glucoside abc |
| 24 | C15H10O6 | 26.27 | 285.04046 | 285.04135 | 3.12 | Kaempferol ab |
| 25 | C16H14O4 | 27.81 | 269.08193 | 269.08218 | 0.93 | Pinostrobin a |
| 26 | C15H10O5 | 28.01 | 269.04555 | 269.04523 | −1.19 | Apigenin ab |
| 27 | C21H20O11 | 28.3 | 447.09328 | 447.09323 | −0.11 | Quercetin 3-O-rhamnoside abc |
| 28 | C28H34O15 | 29.93 | 609.18249 | 609.1825 | 0.02 | Hesperidin a |
| 29 | C12H16O7 | 47.50 | 271.08233 | 271.08295 | 2.29 | Arbutin a |
| Compounds | Regression Equation | R2 | Linear Range (μg/mL) | Retention Time (min) | UDE (mg/g DW) | UEE (mg/g DW) | EE (mg/g DW) |
|---|---|---|---|---|---|---|---|
| GA | y = 72.203x + 645.05 | 0.9962 | 2.4–38.5 | 8.32 | 0.951 ± 0.042 a | 0.287 ± 0.019 b | 0.108 ± 0.034 c |
| PA | y = 27.56x + 604.29 | 0.9965 | 4.9–76.9 | 16.55 | 1.226 ± 0.022 a | 0.384 ± 0.053 b | 0.026 ± 0.037 c |
| PB1 | y = 1.6993x + 49.478 | 0.9984 | 4.9–76.9 | 21.63 | 1.947 ± 0.134 a | 0.855 ± 0.062 b | 0.276 ± 0.012 c |
| Ca | y = 7.0168x + 48.739 | 0.9994 | 4.9–76.9 | 24.93 | 0.914 ± 0.102 a | 0.259 ± 0.079 b | 0.086 ± 0.011c |
| PB2 | y = 6.4073x + 350.22 | 0.9971 | 4.9–76.9 | 28.76 | 4.799 ± 0.128 a | 1.635 ± 0.081 b | 0.644 ± 0.057 c |
| EC | y = 10.786x + 121.29 | 0.9978 | 4.9–76.9 | 30.4 | 1.583 ± 0.164 a | 1.264 ± 0.139 b | 0.470 ± 0.063 c |
| NCHL | y = 45.262x + 970.83 | 0.9969 | 4.9–76.9 | 34.09 | 1.512 ± 0.112 a | 0.732 ± 0.097 b | 0.280 ± 0.047 c |
| CHL | y = 35.003x + 425.98 | 0.9988 | 4.9–76.9 | 36.76 | 0.732 ± 0.093 a | 0.207 ± 0.062 b | 0.069 ± 0.013 c |
| QGlu | y = 23.737x + 319.63 | 0.9962 | 4.9–76.9 | 54.12 | 0.708 ± 0.064 a | 0.222 ± 0.034 b | 0.074 ± 0.018 c |
| QRha | y = 18.549x + 227.08 | 0.9961 | 4.9–76.9 | 57.45 | 0.696 ± 0.051 a | 0.271 ± 0.077 b | 0.088 ± 0.024 c |
| Total content (mg/g DW) | 15.067 ± 1.143 a | 6.122 ± 0.074 b | 2.218 ± 0.271 c | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.-T.; Deng, W.; Li, J.; Geng, J.-L.; Hu, Y.-C.; Zou, L.; Liu, Y.; Liu, H.-Y.; Gan, R.-Y. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Phenolic Compounds from Thinned Young Kiwifruits and Their Beneficial Effects. Antioxidants 2023, 12, 1475. https://doi.org/10.3390/antiox12071475
Wu D-T, Deng W, Li J, Geng J-L, Hu Y-C, Zou L, Liu Y, Liu H-Y, Gan R-Y. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Phenolic Compounds from Thinned Young Kiwifruits and Their Beneficial Effects. Antioxidants. 2023; 12(7):1475. https://doi.org/10.3390/antiox12071475
Chicago/Turabian StyleWu, Ding-Tao, Wen Deng, Jie Li, Jin-Lei Geng, Yi-Chen Hu, Liang Zou, Yi Liu, Hong-Yan Liu, and Ren-You Gan. 2023. "Ultrasound-Assisted Deep Eutectic Solvent Extraction of Phenolic Compounds from Thinned Young Kiwifruits and Their Beneficial Effects" Antioxidants 12, no. 7: 1475. https://doi.org/10.3390/antiox12071475






