Chemical Constituents, Antioxidant, and α-Glucosidase Inhibitory Activities of Different Fermented Gynostemma Pentaphyllum Leaves and Untargeted Metabolomic Measurement of the Metabolite Variation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fermentation and Sample Preparation
2.3. Fermentation pH
2.4. Determination of Total Phenolic Content
2.5. Determination of Total Flavonoid Content
2.6. Determination of DPPH· Scavenging Capacity
2.7. Determination of ABTS·+ Scavenging Capacity
2.8. Determination of Ferric Reducing Antioxidant Power
2.9. Determination of α-Glucosidase Inhibitory Activity
2.10. HPLC Analysis
2.11. Untargeted Metabolomics Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Fermentation pH
3.2. Total Phenolic Content and Total Flavonoid Content
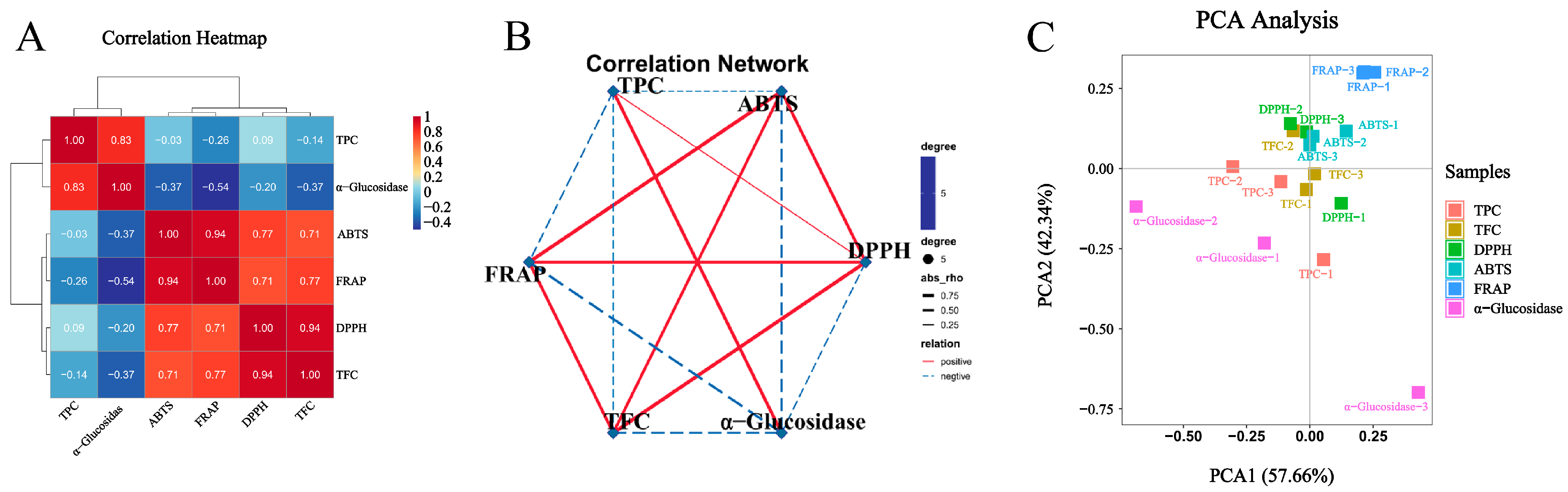
3.3. Antioxidant Capacity
3.4. α-Glucosidase Inhibitory Capacity
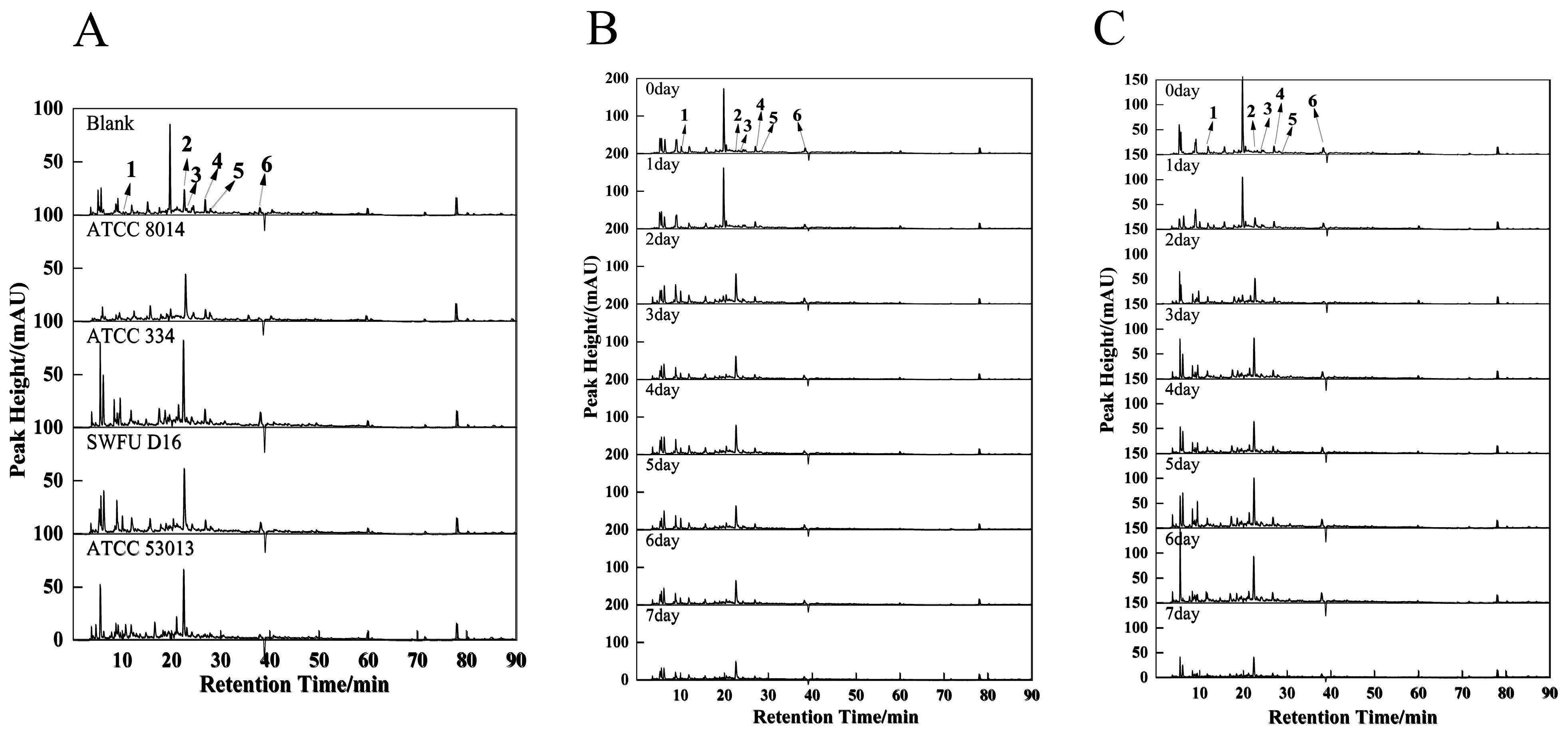
3.5. HPLC Analyses
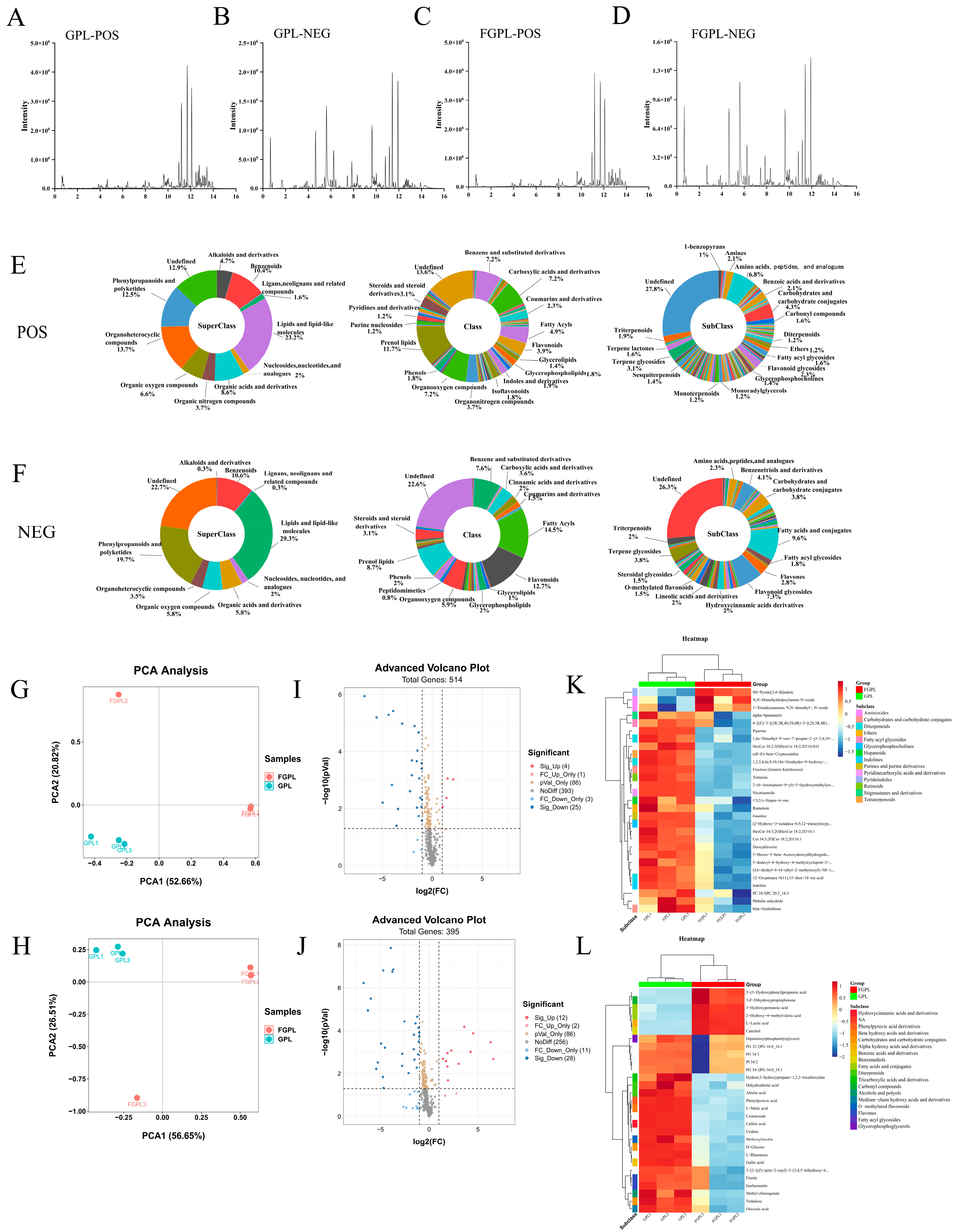
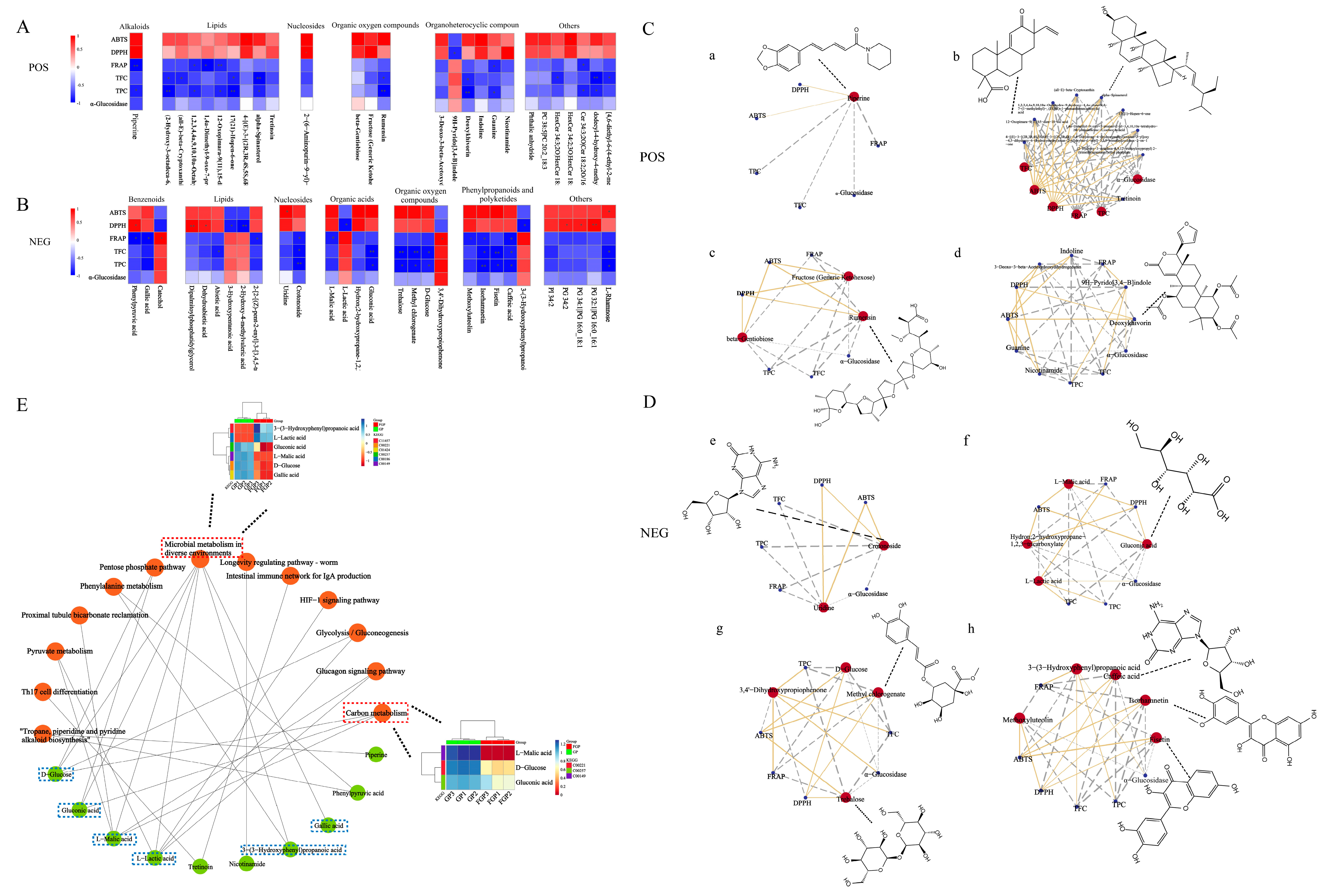
3.6. Metabolomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shen, Y.; Guo, X. Isolation, Structures, and Bioactivities of the Polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino: A Review. BioMed Res. Int. 2018, 2018, 6285134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Ip, F.C.; Fu, G.; Pang, H.; Ye, W.; Ip, N.Y. Dammarane saponins from Gynostemma pentaphyllum. Phytochemistry 2010, 71, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Chao, Y.; Zhang, Y.; Lu, C.; Xu, C.; Niu, W. Immunomodulatory and Antioxidant Effects of Polysaccharides from Gynostemma pentaphyllum Makino in Immunosuppressed Mice. Molecules 2016, 21, 1085. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Yang, X. Protective effects of Gynostemma pentaphyllum polysaccharides on PC12 cells impaired by MPP+. Int. J. Biol. Macromol. 2014, 69, 171–175. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, H.; Zhao, Y.; Shi, H.; Wang, S.; Wang, T.T.; Chen, P.; Yu, L.L. Chemical composition and anti-proliferative and anti-inflammatory effects of the leaf and whole-plant samples of diploid and tetraploid Gynostemma pentaphyllum (Thunb.) Makino. Food Chem. 2012, 132, 125–133. [Google Scholar] [CrossRef]
- Yan, W.; Niu, Y.; Lv, J.; Xie, Z.; Jin, L.; Yao, W.; Gao, X.; Yu, L.L. Characterization of a heteropolysaccharide isolated from diploid Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2013, 92, 2111–2117. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wang, M.; Jiao, L. Characterization and antioxidant activities of acidic polysaccharides from Gynostemma pentaphyllum (Thunb.) Markino. Carbohydr. Polym. 2015, 127, 209–214. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, X.; Zhao, Y.; Ruan, Y.; Yang, Y.; Wang, Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Potential of the Probiotic Lactobacillus Plantarum ATCC 14917 Strain to Produce Functional Fermented Pomegranate Juice. Foods 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Jiang, J.; Yue, Y.; Feng, Z.; Chen, J.; Ye, X. Influence of mixed probiotics on the the bioactive composition, antioxidant activity and appearance of fermented red bayberry pomace. LWT 2020, 133, 110076. [Google Scholar] [CrossRef]
- Gowd, V.; Bao, T.; Wang, L.; Huang, Y.; Chen, S.; Zheng, X.; Cui, S.; Chen, W. Antioxidant and antidiabetic activity of blackberry after gastrointestinal digestion and human gut microbiota fermentation. Food Chem. 2018, 269, 618–627. [Google Scholar] [CrossRef]
- Grover, S.; Rashmi, H.M.; Srivastava, A.K.; Batish, V.K. Probiotics for human health—New innovations and emerging trends. Gut Pathog. 2012, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Tavera-Quiroz, M.J.; Romano, N.; Mobili, P.; Pinotti, A.; Gómez-Zavaglia, A.; Bertola, N. Green apple baked snacks functionalized with edible coatings of methylcellulose containing Lactobacillus plantarum. J. Funct. Foods 2015, 16, 164–173. [Google Scholar] [CrossRef]
- Tugizimana, F.; Piater, L.; Dubery, I. Plant metabolomics: A new frontier in phytochemical analysis. S. Afr. J. Sci. 2013, 109, 11. [Google Scholar] [CrossRef] [Green Version]
- Arkorful, E.; Yu, Y.; Chen, C.; Lu, L.; Hu, S.; Yu, H.; Ma, Q.; Thangaraj, K.; Periakaruppan, R.; Jeyaraj, A.; et al. Untargeted metabolomic analysis using UPLC-MS/MS identifies metabolites involved in shoot growth and development in pruned tea plants (Camellia sinensis (L.) O. Kuntz). Sci. Hortic. 2020, 264, 109164. [Google Scholar] [CrossRef]
- Ru, Y.R.; Wang, Z.X.; Li, Y.J.; Kan, H.; Kong, K.W.; Zhang, X.C. The influence of probiotic fermentation on the active compounds and bioactivities of walnut flowers. J. Food Biochem. 2022, 46, e13887. [Google Scholar] [CrossRef]
- He, S.; Cui, X.; Khan, A.; Liu, Y.; Wang, Y.; Cui, Q.; Zhao, T.; Cao, J.; Cheng, G. Activity Guided Isolation of Phenolic Compositions from Anneslea fragrans Wall. and Their Cytoprotective Effect against Hydrogen Peroxide Induced Oxidative Stress in HepG2 Cells. Molecules 2021, 26, 3690. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, Y.; Zhou, W.; He, S.; Yang, M.; Xue, Q.; Wang, Y.; Zhao, T.; Cao, J.; Khan, A.; et al. Phenolic composition, antioxidant and cytoprotective effects of aqueous-methanol extract from Anneslea fragrans leaves as affected by drying methods. Int. J. Food Sci. Tech. 2021, 56, 4807–4819. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I. Optimization and stabilization of the antioxidant properties from Alkanet (Alkanna tinctoria) with natural deep eutectic solvents. Arab. J. Chem. 2020, 13, 6437–6450. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, M.; Kong, L.; Xue, Q.; Wang, Y.; Wang, Y.; Khan, A.; Cao, J.; Cheng, G. Bioactivity-Guided Isolation of Phytochemicals from Vaccinium dunalianum Wight and Their Antioxidant and Enzyme Inhibitory Activities. Molecules 2021, 26, 2075. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.Q.; Wang, Y.M.; Yang, Y.L.; Zeng, Y.; Mei, L.J.; Shi, Y.P.; Tao, Y.D. Antioxidants and alpha-glucosidase inhibitors from “Liucha” (young leaves and shoots of Sibiraea laevigata). Food Chem. 2017, 230, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-R.; Xing, S.-F.; Lin, M.; Gu, Y.-L.; Piao, X.-L. Determination of flavonoids from Gynostemma pentaphyllum using ultra-performance liquid chromatography with triple quadrupole tandem mass spectrometry and an evaluation of their antioxidant activity in vitro. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 437–444. [Google Scholar] [CrossRef]
- Wang; Jin, X.; Zhang, X.; Xie, X.; Tu, Z.; He, X. From Function to Metabolome: Metabolomic Analysis Reveals the Effect of Probiotic Fermentation on the Chemical Compositions and Biological Activities of Perilla frutescens Leaves. Front. Nutr. 2022, 9, 933193. [Google Scholar] [CrossRef]
- Lee, T.K.; Nguyen, T.T.H.; Park, N.; Kwak, S.H.; Kim, J.; Jin, S.; Son, G.M.; Hur, J.; Choi, J.I.; Kim, D. The use of fermented buckwheat to produce L-carnitine enriched oyster mushroom. AMB Express 2018, 8, 138. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.J.; Lee, J.J. Coating rice with mulberry leaves rich in deoxynojirimycin ameliorates hyperglycemia and dyslipidemia in C57BL/KsJ db/db mice. Nutr. Res. Pract. 2018, 12, 469–478. [Google Scholar] [CrossRef]
- Banerjee, A.; Dasgupta, N.; De, B. In vitro study of antioxidant activity of fruit. Food Chem. 2005, 90, 727–733. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.Y.; Kang, H.R.; Cho, S.K. Changes Over the Fermentation Period in Phenolic Compounds and Antioxidant and Anticancer Activities of Blueberries Fermented by Lactobacillus plantarum. J. Food Sci. 2019, 84, 2347–2356. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, F.; Chai, Z.; Liu, M.; Battino, M.; Meng, X. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Boscolo, M.; da Silva, R.; Ferreira, H.; Gomes, E. Purification and characterization of the alpha-glucosidase produced by thermophilic fungus Thermoascus aurantiacus CBMAI 756. J. Microbiol. 2010, 48, 452–459. [Google Scholar] [CrossRef]
- Zhong, H.; Abdullah; Zhao, M.; Tang, J.; Deng, L.; Feng, F. Probiotics-fermented blueberry juices as potential antidiabetic product: Antioxidant, antimicrobial and antidiabetic potentials. J. Sci. Food Agric. 2021, 101, 4420–4427. [Google Scholar] [CrossRef]
- Wongsa, P.; Chaiwarit, J.; Zamaludien, A. In vitro screening of phenolic compounds, potential inhibition against α-amylase and α-glucosidase of culinary herbs in Thailand. Food Chem. 2012, 131, 964–971. [Google Scholar] [CrossRef]
- Khan, S.A.; Liu, L.; Lai, T.; Zhang, R.; Wei, Z.; Xiao, J.; Deng, Y.; Zhang, M. Phenolic profile, free amino acids composition and antioxidant potential of dried longan fermented by lactic acid bacteria. J. Food Sci. Technol. 2018, 55, 4782–4791. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Z.; Ma, L.; Huang, Q. Advances in Nanodelivery of Green Tea Catechins to Enhance the Anticancer Activity. Molecules 2021, 26, 3301. [Google Scholar] [CrossRef]
- Puertas-Bartolome, M.; Benito-Garzon, L.; Fung, S.; Kohn, J.; Vazquez-Lasa, B.; San Roman, J. Bioadhesive functional hydrogels: Controlled release of catechol species with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110040. [Google Scholar] [CrossRef]
- Lafayette, E.A.; de Almeida, S.M.V.; Cavalcanti Santos, R.V.; de Oliveira, J.F.; Amorim, C.; da Silva, R.M.F.; Pitta, M.; Pitta, I.D.R.; de Moura, R.O.; de Carvalho Junior, L.B.; et al. Synthesis of novel indole derivatives as promising DNA-binding agents and evaluation of antitumor and antitopoisomerase I activities. Eur. J. Med. Chem. 2017, 136, 511–522. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef]
- Biemelt, S.; Sonnewald, U. Plant–microbe interactions to probe regulation of plant carbon metabolism. J. Plant Physiol. 2006, 163, 307–318. [Google Scholar] [CrossRef]

| Fermentation Time (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | Probiotics | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| gallic acid | Blank | ND | ND | ND | ND | ND | ND | ND | ND |
| ATCC 8014 | ND | ND | 0.46 ± 0.06 a | 0.21 ± 0.0374 b | 0.09 ± 0.0395 c | ND | ND | ND | |
| ATCC 334 | 0.27 ± 0.0302 abc | 0.16 ± 0.0489 bc | 0.17 ± 0.0509 bc | 0.2 ± 0.0369 ab | 0.18 ± 0.0332 abc | 0.24 ± 0.0443 a | 0.17 ± 0.019 c | ND | |
| SWFU D16 | 0.65 ± 0.0575 b | 0.14 ± 0.0124 f | 0.78 ± 0.069 a | 0.54 ± 0.0478 b | 0.41 ± 0.0363 c | 0.36 ± 0.0319 cd | 0.29 ± 0.0257 de | 0.24 ± 0.0212 ef | |
| ATCC 53013 | ND | ND | ND | ND | ND | ND | ND | ND | |
| catechin | Blank | 0.147 ± 0.026 ab | 0.149 ± 0.0087 ab | 0.1671 ± 0.037 ab | 0.1675 ± 0.042 ab | 0.1711 ± 0.03 ab | 0.1691 ± 0.01 ab | 0.1699 ± 0.038 a | 0.146 ± 0.0082 b |
| ATCC 8014 | 0.98 ± 0.397 b | 0.21 ± 0.009 b | 0.23 ± 0.0327 b | 3.82 ± 0.278 a | 0.37 ± 0.081 b | 3.63 ± 0.296 a | 0.18 ± 0.036 b | 0.21 ± 0.058 b | |
| ATCC 334 | ND | 0.09 ± 0.0165 c | 0.39 ± 0.0716 b | 0.65 ± 0.119 a | 0.41 ± 0.0753 b | 0.59 ± 0.108 ab | 0.48 ± 0.0881 ab | 0.05 ± 0.009 c | |
| SWFU D16 | 0.32 ± 0.345 c | 0.32 ± 0.0285 c | 6.69 ± 0.437 a | 5.25 ± 0.343 b | 6.09 ± 0.397 a | 5.57 ± 0.363 ab | 5.53 ± 0.361 ab | 4.29 ± 0.28 ab | |
| ATCC 53013 | 0.53 ± 0.047 b | 0.37 ± 0.033 c | 0.51 ± 0.045 b | 0.66 ± 0.058 a | 0.33 ± 0.0229 c | 0.42 ± 0.037 bc | 0.33 ± 0.029 c | 0.31 ± 0.027 c | |
| chlorogenic acid | Blank | 0.0057 ± 0.0026 b | 0.0061 ± 0.001 b | 0.0199 ± 0.0042 a | 0.0196 ± 0.0064 a | 0.0211 ± 0.0054 a | 0.0217 ± 0.0023 a | 0.0185 ± 0.0047 a | 0.0039 ± 0.0007 b |
| ATCC 8014 | 0.59 ± 0.0884 a | 0.041 ± 0.00614 b | 0.08 ± 0.012 b | 0.04 ± 0.006 b | 0.05 ± 0.0075 b | 0.08 ± 0.012 b | 0.11 ± 0.0165 b | 0.09 ± 0.0135 b | |
| ATCC 334 | 0.1 ± 0.0148 b | 0.09 ± 0.0133 b | 0.09 ± 0.004 b | 0.11 ± 0.0163 b | 0.08 ± 0.0119 b | 0.16 ± 0.0237 a | 0.18 ± 0.0267 a | 0.03 ± 0.0046 c | |
| SWFU D16 | 0.11 ± 0.0259 ab | 0.11 ± 0.0244 ab | 0.16 ± 0.0378 a | 0.08 ± 0.0189 b | 0.12 ± 0.0284 ab | 0.1 ± 0.0236 b | 0.09 ± 0.0213 b | 0.076 ± 0.018 b | |
| ATCC 53013 | 0.11 ± 0.0072 a | 0.093 ± 0.0061 bc | 0.103 ± 0.0067 ab | 0.084 ± 0.0055 c | 0.056 ± 0.0037 d | 0.09 ± 0.0059 bc | 0.059 ± 0.39 d | 0.064 ± 0.0042 d | |
| epicatechin | Blank | 0.0023 ± 0.00054 b | 0.0068 ± 0.0016 b | 0.102 ± 0.024 a | 0.0089 ± 0.0021 b | 0.0094 ± 0.0022 b | ND | ND | ND |
| ATCC 8014 | 0.08 ± 0.0391 b | 0.04 ± 0.339 b | 0.22 ± 0.0105 b | 0.57 ± 0.0753 a | 0.03 ± 0.0138 b | ND | 0.008 ± 0.0731 b | 0.004 ± 0.005 b | |
| ATCC 334 | 0.78 ± 0.123 a | 0.73 ± 0.115 ab | 0.58 ± 0.0911 b | 0.066 ± 0.0104 c | 0.03 ± 0.0047 c | 0.06 ± 0.0094 c | 0.05 ± 0.078 c | ND | |
| SWFU D16 | 0.91 ± 0.0727 ab | 0.94 ± 0.0751 a | 0.84 ± 0.0671 ab | 0.51 ± 0.0407 cd | 0.78 ± 0.0623 b | 0.77 ± 0.0615 b | 0.59 ± 0.0471 c | 0.39 ± 0.0311 d | |
| ATCC 53013 | 0.025 ± 0.0059 c | 0.033 ± 0.0078 c | 0.03 ± 0.0071 c | 0.022 ± 0.0052 c | 0.078 ± 0.018 c | 0.003 ± 0.0007 c | 0.28 ± 0.066 b | 0.56 ± 0.132 a | |
| dihydromy- ricetin | Blank | ND | 0.0088 ± 0.0007 b | 0.0075 ± 0.0006 b | 0.0064 ± 0.00051 b | 0.143 ± 0.0114 a | 0.0086 ± 0.0007 b | ND | ND |
| ATCC 8014 | 0.07 ± 0.0099 bc | 0.51 ± 0.114 a | 0.13 ± 0.051 b | 0.05 ± 0.021 c | ND | 0.007 ± 0.0041 c | 0.07 ± 0.0217 bc | 0.06 ± 0.0103 bc | |
| ATCC 334 | 0.02 ± 0.00481 bc | 0.008 ± 0.00192 c | 0.17 ± 0.0409 a | 0.04 ± 0.00626 bc | ND | 0.04 ± 0.00498 bc | 0.06 ± 0.0693 b | ND | |
| SWFU D16 | 0.05 ± 0.0076 a | 0.034 ± 0.0057 bc | 0.029 ± 0.0088 bc | 0.026 ± 0.0054 c | 0.046 ± 0.0023 ab | ND | 0.008 ± 0.0004 d | ND | |
| ATCC 53013 | 0.027 ± 0.0022 b | 0.032 ± 0.0026 ab | 0.033 ± 0.0026 ab | 0.034 ± 0.0027 a | 0.019 ± 0.0015 c | 0.031 ± 0.0025 ab | ND | ND | |
| epicatechin gallate | Blank | ND | 0.146 ± 0.0074 a | 0.156 ± 0.0079 a | 0.102 ± 0.0051 b | 0.0055 ± 0.0003 c | ND | ND | ND |
| ATCC 8014 | ND | 0.88 ± 0.246 a | ND | ND | ND | 0.76 ± 0.107 a | 0.07 ± 0.018 b | 0.07 ± 0.016 b | |
| ATCC 334 | 0.14 ± 0.0427 cd | 0.12 ± 0.0366 cd | 0.08 ± 0.0244 d | 0.33 ± 0.101 ab | 0.22 ± 0.067 bc | 0.36 ± 0.109 a | 0.12 ± 0.0366 cd | 0.05 ± 0.0153 d | |
| SWFU D16 | 0.15 ± 0.0128 bc | 0.17 ± 0.0291 ab | 0.28 ± 0.0482 a | 0.17 ± 0.0359 a | 0.12 ± 0.0173 c | 0.19 ± 0.0246 ab | 0.14 ± 0.01 bc | 0.08 ± 0.0247 c | |
| ATCC 53013 | ND | 0.26 ± 0.013 a | 0.18 ± 0.0091 b | ND | ND | 0.049 ± 0.0025 c | ND | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, S.; Zhang, Z.; Kong, K.W.; Wang, Z.; He, X. Chemical Constituents, Antioxidant, and α-Glucosidase Inhibitory Activities of Different Fermented Gynostemma Pentaphyllum Leaves and Untargeted Metabolomic Measurement of the Metabolite Variation. Antioxidants 2023, 12, 1505. https://doi.org/10.3390/antiox12081505
Zhang X, Li S, Zhang Z, Kong KW, Wang Z, He X. Chemical Constituents, Antioxidant, and α-Glucosidase Inhibitory Activities of Different Fermented Gynostemma Pentaphyllum Leaves and Untargeted Metabolomic Measurement of the Metabolite Variation. Antioxidants. 2023; 12(8):1505. https://doi.org/10.3390/antiox12081505
Chicago/Turabian StyleZhang, Xuechun, Shi Li, Zhibin Zhang, Kin Weng Kong, Zhenxing Wang, and Xiahong He. 2023. "Chemical Constituents, Antioxidant, and α-Glucosidase Inhibitory Activities of Different Fermented Gynostemma Pentaphyllum Leaves and Untargeted Metabolomic Measurement of the Metabolite Variation" Antioxidants 12, no. 8: 1505. https://doi.org/10.3390/antiox12081505
APA StyleZhang, X., Li, S., Zhang, Z., Kong, K. W., Wang, Z., & He, X. (2023). Chemical Constituents, Antioxidant, and α-Glucosidase Inhibitory Activities of Different Fermented Gynostemma Pentaphyllum Leaves and Untargeted Metabolomic Measurement of the Metabolite Variation. Antioxidants, 12(8), 1505. https://doi.org/10.3390/antiox12081505







