Chemical Profile and Bioactivity Evaluation of Salvia Species from Eastern Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extraction
2.3. Total Phenolics Quantification
2.4. LC-HRMS/MS Analysis
2.5. Antimicrobial Assay
2.5.1. Microbial Strains
2.5.2. Minimum Inhibitory Concentration (MIC)
2.6. Antioxidant Assays
2.6.1. DPPH Radical-Scavenging Assay
2.6.2. ABTS Radical-Cation-Scavenging Assay
2.6.3. Ferric Ion Reducing Antioxidant Power Assay (FRAP)
2.7. Cell Viability Assay
2.7.1. Cell Lines
2.7.2. MTT Assay
2.8. Data Analysis
3. Results and Discussion
3.1. Total Phenolic Content
3.2. Metabolite Profiling Using LC-HRMS/MS
3.3. Assessment of the Antimicrobial Activity
3.4. Assessment of Antioxidant Activity
3.5. Assessment of the Cytotoxic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mervić, M.; Bival Štefan, M.; Kindl, M.; Blažeković, B.; Marijan, M.; Vladimir-Knežević, S. Comparative antioxidant, anti-acetylcholinesterase and anti-α-glucosidase activities of mediterranean Salvia species. Plants 2022, 11, 625. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.; Ferreira, I.C.; Cardoso, S.M. Phytochemical composition and bioactive effects of Salvia africana, Salvia officinalis ‘Icterina’and Salvia mexicana aqueous extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency (EMA). Assessment report on Salvia officinalis L., folium and Salvia officinalis L., aetheroleum. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-salvia-officinalis-l-folium-salvia-officinalis-l-aetheroleum-revision-1_en.pdf (accessed on 12 June 2023).
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.; Leal, A.L.A.B. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food. Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Trad. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Grdiša, M.; Jug-Dujaković, M.; Lončarić, M.; Carović-Stanko, K.; Ninčević, T.; Liber, Z.; Radosavljević, I.; Šatović, Z. Dalmatian sage (Salvia officinalis L.): A review of biochemical contents, medical properties and genetic diversity. Agric. Conspec. Sci. 2015, 80, 69–78. [Google Scholar]
- Balmus, Z.; Gonceariuc, M.; Cotelea, L.; Butnaras, V. Parfum perfect the new early variety of Salvia sclarea L. (clary sage). In Proceedings of the International Congress of Geneticists and Breeders from the Republic of Moldova, Chisinau, Moldova, 15–16 June 2021; p. 70. [Google Scholar]
- Ciocarlan, N. Family Lamiaceae: Main important spontaneous medicinal and aromatic species in the Republic of Moldova. Rev. Bot. 2016, 12, 86–91. [Google Scholar]
- Gonceariuc, M. Medicinal and aromatic plant varieties developed in the Republic of Moldova. Olten. Stud. Si Comun. Stiint. Nat. 2014, 30, 29–34. [Google Scholar]
- Hanganu, D.; Olah, N.K.; Pop, C.E.; Vlase, L.; Oniga, I.; Ciocarlan, N.; Matei, A.; Puşcaş, C.M.; Silaghi-Dumitrescu, R.; Benedec, D. Evaluation of polyphenolic profile and antioxidant activity for some Salvia species. Farmacia 2019, 67, 801–805. [Google Scholar] [CrossRef] [Green Version]
- Aprotosoaie, A.C.; Mihai, C.T.; Vochita, G.; Rotinberg, P.; Trifan, A.; Luca, S.V.; Petreus, T.; Gille, E.; Miron, A. Antigenotoxic and antioxidant activities of a polyphenolic extract from European Dracocephalum moldavica L. Ind. Crops Prod. 2016, 79, 248–257. [Google Scholar] [CrossRef]
- Grădinariu, V.; Cioancă, O.; Gille, E.; Aprotosoaie, A.; Hriţcu, L.; Hăncianu, M. The chemical profile of basil biovarieties and its implication on the biological activity. Farmacia 2013, 61, 632–639. [Google Scholar]
- M02-A10; Performance Standards for Antimicrobial Disk Susceptibility Tests (10th ed.), Approved Standard. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009.
- Grădinaru, A.; Trifan, A.; Şpac, A.; Brebu, M.; Miron, A.; Aprotosoaie, A. Antibacterial activity of traditional spices against lower respiratory tract pathogens: Combinatorial effects of Trachyspermum ammi essential oil with conventional antibiotics. Lett. Appl. Microb. 2018, 67, 449–457. [Google Scholar] [CrossRef]
- Malterud, K.E.; Farbrot, T.L.; Huse, A.E.; Sund, R.B. Antioxidant and radical scavenging effects of anthraquinones and anthrones. Pharmacology 1993, 47, 77–85. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immun. Met. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Sengul, M.; Ozer, H.; Polat, T.; Ozturk, E. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol. Res. 2009, 42, 175–181. [Google Scholar] [CrossRef]
- Srećković, N.; Mišić, D.; Gašić, U.; Matić, S.L.; Stanković, J.S.K.; Mihailović, N.R.; Monti, D.M.; D’Elia, L.; Mihailović, V. Meadow sage (Salvia pratensis L.): A neglected sage species with valuable phenolic compounds and biological potential. Ind. Crops Prod. 2022, 189, 115841. [Google Scholar] [CrossRef]
- Asadi, S.; Ahmadiani, A.; Esmaeili, M.A.; Sonboli, A.; Ansari, N.; Khodagholi, F. In vitro antioxidant activities and an investigation of neuroprotection by six Salvia species from Iran: A comparative study. Food Chem. Toxicol 2010, 48, 1341–1349. [Google Scholar] [CrossRef]
- Mocan, A.; Babotă, M.; Pop, A.; Fizeșan, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R. Chemical constituents and biologic activities of sage species: A comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex griseb. & schenk) schur. Antioxidants 2020, 9, 480. [Google Scholar]
- Mekinić, I.G.; Ljubenkov, I.; Možina, S.S.; Abramović, H.; Šimat, V.; Katalinić, A.; Novak, T.; Skroza, D. Abiotic factors during a one-year vegetation period affect sage phenolic metabolites, antioxidants and antimicrobials. Ind. Crops Prod. 2019, 141, 111741. [Google Scholar] [CrossRef]
- Gericke, S.; Lübken, T.; Wolf, D.; Kaiser, M.; Hannig, C.; Speer, K. Identification of new compounds from sage flowers (Salvia officinalis L.) as markers for quality control and the influence of the manufacturing technology on the chemical composition and antibacterial activity of sage flower extracts. J. Agric. Food Chem. 2018, 66, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Piasecka, A.; Gryszczyńska, A.; Sawikowska, A.; Pietrowiak, A.; Opala, B.; Mikołajczak, P.; Kujawski, R.; Kachlicki, P.; Buchwald, W. Determination of phenolic compounds and diterpenes in roots of Salvia miltiorrhiza and Salvia przewalskii by two LC–MS tools: Multi-stage and high resolution tandem mass spectrometry with assessment of antioxidant capacity. Phytochem. Lett. 2017, 20, 331–338. [Google Scholar] [CrossRef]
- Yang, S.; Wu, X.; Rui, W.; Guo, J.; Feng, Y. UPLC/Q-TOF-MS analysis for identification of hydrophilic phenolics and lipophilic diterpenoids from Radix Salviae Miltiorrhizae. Acta Chromatogr. 2015, 27, 711–728. [Google Scholar] [CrossRef] [Green Version]
- Shojaeifard, Z.; Hemmateenejad, B.; Jassbi, A.R. Chemometrics-based LC-UV-ESIMS analyses of 50 Salvia species for detecting their antioxidant constituents. J. Pharm. Biomed. Anal. 2021, 193, 113745. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Bahadori, M.B.; Mocan, A.; Locatelli, M.; Aktumsek, A. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crops Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Haq, F.U.; Ali, A.; Akhtar, N.; Aziz, N.; Khan, M.N.; Ahmad, M.; Musharraf, S.G. A high-throughput method for dereplication and assessment of metabolite distribution in Salvia species using LC-MS/MS. J. Adv. Res. 2020, 24, 79–90. [Google Scholar] [CrossRef]
- Yener, I. Determination of antioxidant, cytotoxic, anticholinesterase, antiurease, antityrosinase, and antielastase activities and aroma, essential oil, fatty acid, phenolic, and terpenoid-phytosterol contents of Salvia poculata. Ind. Crops Prod. 2020, 155, 112712. [Google Scholar] [CrossRef]
- Toplan, G.G.; Kurkcuoglu, M.; Goger, F.; İşcan, G.; Ağalar, H.G.; Mat, A.; Baser, K.H.C.; Koyuncu, M.; Sarıyar, G. Composition and biological activities of Salvia veneris Hedge growing in Cyprus. Ind. Crops Prod. 2017, 97, 41–48. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Kılıç, Ö.; Taslimi, P.; Gören, A.C.; Gülçin, İ. Phytochemical content, antioxidant activity, and enzyme inhibition effect of Salvia eriophora Boiss. & Kotschy against acetylcholinesterase, α-amylase, butyrylcholinesterase, and α-glycosidase enzymes. J. Food Biochem. 2019, 43, e12776. [Google Scholar]
- Uysal, S.; Zengin, G.; Sinan, K.I.; Ak, G.; Ceylan, R.; Mahomoodally, M.F.; Uysal, A.; Sadeer, N.B.; Jekő, J.; Cziáky, Z. Chemical characterization, cytotoxic, antioxidant, antimicrobial, and enzyme inhibitory effects of different extracts from one sage (Salvia ceratophylla L.) from Turkey: Open a new window on industrial purposes. RSC Adv. 2021, 11, 5295–5310. [Google Scholar] [CrossRef]
- Koutsoulas, A.; Čarnecká, M.; Slanina, J.; Tóth, J.; Slaninová, I. Characterization of phenolic compounds and antiproliferative effects of Salvia pomifera and Salvia fruticosa extracts. Molecules 2019, 24, 2921. [Google Scholar] [CrossRef] [Green Version]
- Sarrou, E.; Martens, S.; Chatzopoulou, P. Metabolite profiling and antioxidative activity of Sage (Salvia fruticosa Mill.) under the influence of genotype and harvesting period. Ind. Crops Prod. 2016, 94, 240–250. [Google Scholar] [CrossRef]
- Piątczak, E.; Owczarek, A.; Lisiecki, P.; Gonciarz, W.; Kozłowska, W.; Szemraj, M.; Chmiela, M.; Kiss, A.K.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Identification and quantification of phenolic compounds in Salvia cadmica Boiss. and their biological potential. Ind. Crops Prod. 2021, 160, 113113. [Google Scholar] [CrossRef]
- Bielecka, M.; Pencakowski, B.; Stafiniak, M.; Jakubowski, K.; Rahimmalek, M.; Gharibi, S.; Matkowski, A.; Ślusarczyk, S. Metabolomics and DNA-based authentication of two traditional Asian medicinal and aromatic species of Salvia subg. Perovskia. Cells 2021, 10, 112. [Google Scholar] [CrossRef]
- KNApSAcK. Available online: https://www.knapsackfamily.com/knapsack_core/result.php?sname=all&word=Salvia (accessed on 12 June 2023).
- Pacifico, S.; Piccolella, S.; Lettieri, A.; Nocera, P.; Bollino, F.; Catauro, M. A metabolic profiling approach to an Italian sage leaf extract (SoA541) defines its antioxidant and anti-acetylcholinesterase properties. J. Funct. Foods 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Gök, H.N.; Luca, S.V.; Ay, S.T.; Komsta, Ł.; Salmas, R.E.; Orhan, I.E.; Skalicka-Woźniak, K. Profiling the annual change of the neurobiological and antioxidant effects of five Origanum species in correlation with their phytochemical composition. Food Chem. 2022, 368, 130775. [Google Scholar] [CrossRef]
- Bujor, A.; Miron, A.; Luca, S.V.; Skalicka-Wozniak, K.; Silion, M.; Trifan, A.; Girard, C.; Demougeot, C.; Totoson, P. Vasorelaxant effects of Crataegus pentagyna: Links with arginase inhibition and phenolic profile. J. Ethnopharmacol. 2020, 252, 112559. [Google Scholar] [CrossRef]
- Velamuri, R.; Sharma, Y.; Fagan, J.; Schaefer, J. Application of UHPLC-ESI-QTOF-MS in phytochemical profiling of sage (Salvia officinalis) and rosemary (Rosmarinus officinalis). Planta Med. Int. Open 2020, 7, e133–e144. [Google Scholar] [CrossRef]
- Farimani, M.M.; Mazarei, Z. Sesterterpenoids and other constituents from Salvia lachnocalyx Hedge. Fitoterapia 2014, 98, 234–240. [Google Scholar] [CrossRef]
- Tohma, H.; Köksal, E.; Kılıç, Ö.; Alan, Y.; Yılmaz, M.A.; Gülçin, İ.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS analysis of the phenolic compounds, antioxidant and antimicrobial activities of Salvia L. species. Antioxidants 2016, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Stanković, J.S.K.; Srećković, N.; Mišić, D.; Gašić, U.; Imbimbo, P.; Monti, D.M.; Mihailović, V. Bioactivity, biocompatibility and phytochemical assessment of lilac sage, Salvia verticillata L. (Lamiaceae)-A plant rich in rosmarinic acid. Ind. Crops Prod. 2020, 143, 111932. [Google Scholar] [CrossRef]
- Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chem. 2017, 224, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P. Evaluation of antioxidant activity, toxicity, and phenolic profile of aqueous extracts of chamomile (Matricaria chamomilla L.) and sage (Salvia officinalis L.) prepared at different temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef] [Green Version]
- Nourbakhsh, F.; Lotfalizadeh, M.; Badpeyma, M.; Shakeri, A.; Soheili, V. From plants to antimicrobials: Natural products against bacterial membranes. Phytother. Res. 2022, 36, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Miri, R.; Asadollahi, M.; Eslami, S.; Jassbi, A.R. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven Salvia species from Iran. Iran J. Pharm. Res. 2013, 12, 801–810. [Google Scholar] [PubMed]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of phenolic acid antimicrobial and antioxidant structure–property relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health. 2018, 15, 2321. [Google Scholar] [CrossRef] [Green Version]
- Mahizan, N.A.; Yang, S.-K.; Moo, C.-L.; Song, A.A.-L.; Chong, C.-M.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.E.; Lai, K.-S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [Green Version]
- Oniga, I.; Vlase, L.; Hanganu, D.; Toiu, A.; Benedec, D. Comparative assessment of phenolic profile and antioxidant activity of some indigenous Salvia species. Hop Med. Plants 2018, 26, 76–83. [Google Scholar]
- Matkowski, A.; Zielińska, S.; Oszmiański, J.; Lamer-Zarawska, E. Antioxidant activity of extracts from leaves and roots of Salvia miltiorrhiza Bunge, S. przewalskii Maxim., and S. verticillata L. Biores. Technol. 2008, 99, 7892–7896. [Google Scholar] [CrossRef]
- Onder, A.; Izgi, M.N.; Cinar, A.S.; Zengin, G.; Yilmaz, M.A. The characterization of phenolic compounds via LC-ESI-MS/MS, antioxidant, enzyme inhibitory activities of Salvia absconditiflora, Salvia sclarea, and Salvia palaestina: A comparative analysis. S. Afr. J. Bot. 2022, 150, 313–322. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Ilea, M.; Dias, M.I.; Calhelha, R.C.; Gavrilaș, L.; Rocchetti, G.; Crișan, G.; Mocan, A.; Barros, L. Potential therapeutic applications of infusions and hydroalcoholic extracts of Romanian glutinous sage (Salvia glutinosa L.). Front. Pharmacol. 2022, 13, 975800. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A comprehensive review of rosmarinic acid: From phytochemistry to pharmacology and its new insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Muruganathan, N.; Dhanapal, A.R.; Baskar, V.; Muthuramalingam, P.; Selvaraj, D.; Aara, H.; Shiek Abdullah, M.Z.; Sivanesan, I. Recent updates on source, biosynthesis, and therapeutic potential of natural flavonoid luteolin: A review. Metabolites 2022, 12, 1145. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsalahat, I.; Daoud, S.; Abutayeh, R.F.; Mahmod, A.I. Plant-derived natural products in cancer research: Extraction, mechanism of action, and drug formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef]
- Camarillo, I.G.; Xiao, F.; Madhivanan, S.; Salameh, T.; Nichols, M.; Reece, L.M.; Leary, J.F.; Otto, K.; Natarajan, A.; Ramesh, A. Low and high voltage electrochemotherapy for breast cancer: An in vitro model study. In Electroporation-Based Therapies for Cancer; Elsevier: Amsterdam, The Netherlands, 2014; pp. 55–102. [Google Scholar]
- Ghoderao, P.; Sahare, S.; Kulkarni, A.A.; Bhave, T. Paclitaxel conjugated magnetic carbon nanotubes induce apoptosis in breast cancer cells and breast cancer stem cells in vitro. In Paclitaxel; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–331. [Google Scholar]
- Çebi, A.; Akgün, E.; Çelikler, S.; Firat, M.; Özel, M.Z.; Ulukaya, E.; Ari, F. Cytotoxic and genotoxic effects of an endemic plant of Turkey Salvia kronenburgii on breast cancer cell lines. J. Cancer Res. Ther. 2019, 15, 1080–1086. [Google Scholar] [CrossRef]
- Ezema, C.A.; Ezeorba, T.P.C.; Aguchem, R.N.; Okagu, I.U. Therapeutic benefits of Salvia species: A focus on cancer and viral infection. Heliyon 2022, 8, e08763. [Google Scholar] [CrossRef]
- Zhao, H.; Han, B.; Li, X.; Sun, C.; Zhai, Y.; Li, M.; Jiang, M.; Zhang, W.; Liang, Y.; Kai, G. Salvia miltiorrhiza in breast cancer treatment: A review of its phytochemistry, derivatives, nanoparticles, and potential mechanisms. Front. Pharmacol. 2022, 13, 872085. [Google Scholar] [CrossRef]


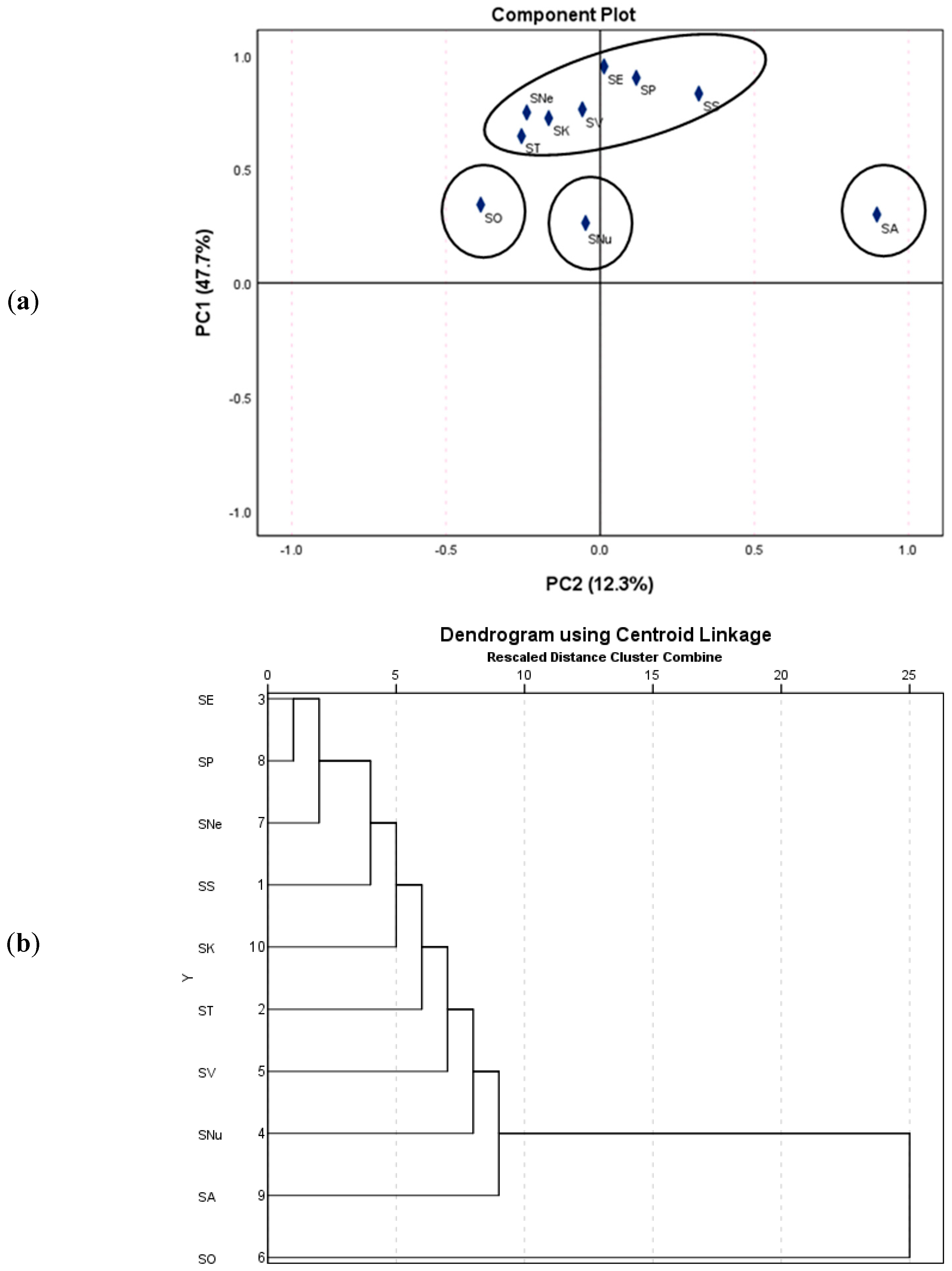


| No. | Species | Voucher | Code | Extraction Yield (%) | TPC (mg GAE/g Extract) |
|---|---|---|---|---|---|
| 1 | Salvia sclarea L. | SS/2019 | SS | 53.35 | 110.90 ± 0.26 |
| 2 | Salvia tesquicola Klok. and Pobed. | ST/2019 | ST | 51.39 | 71.47 ± 0.16 |
| 3 | Salvia aethiopis L. | SE/2019 | SE | 47.71 | 81.43 ± 0.25 |
| 4 | Salvia nutans L. | SNu/2019 | SNu | 55.11 | 66.12 ± 0.15 |
| 5 | Salvia verticillata L. | SV/2019 | SV | 48.52 | 107.62 ± 0.08 |
| 6 | Salvia officinalis L. | SO/2019 | SO | 55.33 | 126.91 ± 0.56 |
| 7 | Salvia nemorosa L. | SNe/2019 | SNe | 47.56 | 98.42 ± 0.38 |
| 8 | Salvia pratensis L. | SP/2019 | SP | 52.35 | 81.70 ± 0.20 |
| 9 | Salvia austriaca Jacq. | SA/2019 | SA | 47.67 | 57.87 ± 0.33 |
| 10 | Salvia kopetdaghensis Kudr. | SK/2019 | SK | 48.67 | 107.63 ± 0.21 |
| No. | Proposed Identity | Class | TR (min) | Exp. (m/z) | Calcd. (m/z) | Δ (ppm) | MF | MS/MS (-) | Ref. | Samples § |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sucrose | Sugars | 1.8 | 341.1104 | 341.1089 | −4.28 | C12H22O11 | 179.0548, 119.0359 | [28] | SS, ST, SE, SNu, SV, SO, SNe, SP, SA, SK |
| 2 | Malic acid | Organic acid | 2.4 | 133.0145 | 133.0142 | −1.89 | C4H6O5 | 115.0093 | [41] | SE, SNu, SV, SO, SP, SA |
| 3 | Quinic acid | Organic acid | 4.7 | 191.0567 | 191.0561 | −3.06 | C7H12O6 | 145.0453, 129.0482, 115.0363, 101.0573 | [41] | SNu, SK |
| 4 | Danshensu/salvianic acid | Phenolic acid | 6.3 | 197.0456 | 197.0455 | −0.27 | C9H10O5 | 179.0395, 135.0461, 123.0456 | [25] | SS, ST, SE, SV, SO, SNe, SP, SK |
| 5 | Dihydroxybenzoic acid | Phenolic acid | 7.9 | 153.0124 | 153.0193 | 6.05 | C7H6O4 | 108.0225 | [41] | SNe |
| 6 | Hydroxybenzoic acid | Phenolic acid | 10.3 | 137.0249 | 137.0244 | −3.49 | C7H6O3 | 108.0185 | [30] | SS, ST, SE, SV, SO, SNe, SP, SK |
| 7 | Tuberonic acid-O-hexoside | Fatty acid | 15.2 | 387.1679 | 387.1661 | −4.75 | C18H8O9 | 207.0956, 163.1025, 101.0232 | [40] | ST, SNu, SNe |
| 8 | Caffeic acid * | Phenolic acid | 17.3 | 179.0359 | 179.0350 | −5.10 | C9H8O4 | 161.0434, 135.0470, 107.0488 | [25] | SS, ST, SE, SNu, SV, SO, SNe, SP, SA, SK |
| 9 | Caffeoylthreonic acid | Phenolic acid | 18.5 | 297.0645 | 297.0616 | 3.66 | C13H14O4 | 179.0326, 161.0227, 135.0323 | [36] | SNe, SP |
| 10 | Apigenin-O-pentoside-O-hexoside | Flavonoid | 21.1 | 563.1428 | 563.1406 | −3.85 | C26H28O14 | 473.1105, 383.0745, 353.0694, 297.0852, 269.0640 | [40] | SNu |
| 11 | Luteolin-O-hexoside-O-glucuronide | Flavonoid | 21.7 | 623.1243 | 623.1254 | 1.72 | C27H28O17 | 447.0639, 285.0218 | [28] | ST |
| 12 | Luteolin di-O-glucuronide | Flavonoid | 22.0 | 637.1065 | 637.1046 | −2.92 | C27H26O18 | 351.0358, 285.0122 | [28] | ST, SE, SNe, SA |
| 13 | Quercetin-O-hexoside | Flavonoid | 22.1 | 463.0902 | 463.0902 | −4.31 | C21H20O12 | 301.0424, 300.0286 | [29] | SO |
| 14 | Caffeic acid-O-hexoside | Phenolic acid | 22.4 | 341.0914 | 341.0878 | 1.18 | C15H18O9 | 223.0618, 179.0737, 135.0311 | [39] | SNu, SO |
| 15 | Luteolin-O-hexoside-O-rhamnoside | Flavonoid | 22.9 | 593.1525 | 593.1512 | −2.20 | C27H30O15 | 327.0822, 285.0445, 267.0343 | [28] | ST, SNu, SO |
| 16 | Quercetin-O-rhamnoside-O-glucoside | Flavonoid | 23.2 | 609.1473 | 609.1461 | −1.96 | C27H30O16 | 300.0167, 271.0154, 150.994 | [28] | SNu, SNe, SK |
| 17 | Luteolin−7-O-glucoside * | Flavonoid | 23.7 | 447.0945 | 447.0933 | −2.71 | C21H20O11 | 285.0434, 257.0504, 151.0031 | [28] | SS, ST, SE, SNu, SV, SO, SNe, SP, SA, SK |
| 18 | Feruloylmalic acid | Phenolic acid | 24.2 | 309.0612 | 309.0616 | 1.26 | C14H13O8 | 193.0522, 133.0381 | [38] | SE |
| 19 | 12-Deoxy-7,7-dimethoxy-6-ketoroyleanone | Diterpene | 24.3 | 373.2030 | 373.2020 | −2.55 | C22H30O5 | 358.1920, 343.1934, 283.1752 | [38] | ST, SNu, SNe |
| 20 | Luteolin-O-glucuronide I | Flavonoid | 24.9 | 461.0732 | 461.0725 | −1.41 | C21H18O12 | 357.0635, 285.0393, 175.0150 | [31] | SS, ST, SE, SV, SO, SNe, SP, SA, SK |
| 21 | Apigenin-7-O-glucoside * | Flavonoid | 25.5 | 431.1002 | 431.0984 | −4.23 | C21H20O10 | 269.0631, 151.0096 | [28] | SS, SNe, SA |
| 22 | Gallocatechin | Flavonoid | 25.6 | 305.0652 | 305.0667 | 4.82 | C15H14O7 | 225.1161 | [42] | SNu, SO, SNe, SA |
| 23 | Rosmarinic acid * | Phenolic acid | 26.5 | 359.0786 | 359.0772 | −3.77 | C18H16O8 | 197.0494, 179.0375, 161.0261, 135.0451 | [28] | SS, ST, SE, SNu, SV, SO, SNe, SP, SA, SK |
| 24 | Salvianolic acid B | Phenolic acid | 27.4 | 717.1492 | 717.1461 | −4.30 | C36H30O16 | 519.0998, 493.1205, 295.0849, 203.0513, 179.0488 | [28] | SS, ST, SO, SNe, SP, SK |
| 25 | Luteolin-O-glucuronide II | Flavonoid | 28.2 | 461.0742 | 461.0725 | −3.57 | C21H18O12 | 285.0589, 241.0651, 199.0513, 151.0129, 133.0326 | [31] | SS |
| 26 | Salvianolic acid K | Phenolic acid | 28.4 | 555.1153 | 555.1144 | −1.59 | C27H24O13 | 537.1068, 493.1148, 359.0808, 197.0465 | [28] | ST, SV, SO, SNe, SA, SK |
| 27 | Salvianolic acid H | Phenolic acid | 28.6 | 537.1073 | 537.1038 | −6.41 | C27H22O12 | 493.1205, 359.0827, 295.0642, 161.0271 | [25] | SP |
| 28 | Luteolin-O-acetylglucuronide | Flavonoid | 28.5 | 503.0901 | 503.0831 | −0.57 | C23H20O13 | 285.0435, 217.0504, 175.0343 | [31] | SNu, SV |
| 29 | Methylrosmarinate | Phenolic acid | 29.4 | 373.0909 | 373.0929 | 5.32 | C31H52O11 | 193.0584, 179.0347, 161.0173, 135.0479 | [28] | ST, SE, SV, SO, SNe, SP, SK |
| 30 | Chrysoeriol-O-acetylglucuronide I | Flavonoid | 30.6 | 517.1053 | 517.0988 | −2.96 | C24H22O13 | 299.0594, 217.0362, 175.0267 | [31] | SNu |
| 31 | Luteolin * | Flavonoid | 30.8 | 285.0407 | 285.0405 | −0.83 | C15H10O6 | 151.0060, 133.0314, 107.0130 | [28] | SS, ST, SO, SP, SA, SK |
| 32 | Trihydroxyoctadecadienoic acid | Fatty acid | 31.8 | 327.2187 | 327.2177 | −3.05 | C18H32O5 | 309.2011, 239.1382, 229.1456, 211.1312, 171.1039 | [28] | SS, ST, SE, SNu, SV, SO, SNe, SP, SA, SK |
| 33 | Chrysoeriol-O-acetylglucuronide II | Flavonoid | 32.6 | 517.1053 | 517.0988 | −2.96 | C24H22O13 | 457.0739, 299.0594, 284.0272, 217.0362, 175.0267 | [31] | SNu |
| 34 | Tricoumaroylspermidine | Phenolic acid | 33.1 | 582.2623 | 582.2610 | −2.3 | C34H37N3O6 | 462.2030, 342.1455, 316.1757, 145.0278, 119.0521 | [24] | SNe, SP, SA, SK |
| 35 | Trihydroxyoctadecenoic acid | Fatty acid | 33.5 | 329.2335 | 329.2333 | −0.76 | C18H34O5 | 229.1436, 211.1309, 171.1004 | [40] | ST, SE, SNu, SV, SO, SNe, SP, SK |
| 36 | Apigenin | Flavonoid | 33.7 | 329.0678 | 329.0667 | −3.40 | C17H14O7 | 314.0447, 299.0225, 285.0452, 271.0294, 243.0318, 227.0390 | [31] | SS, SA |
| 37 | Hydroxycarnosic acid I | Diterpene | 34.5 | 347.1858 | 347.1864 | 1.72 | C20H28O5 | 303.2015, 259.2180 | [40] | SS, ST |
| 38 | Dihydroxyhexadecanoic acid | Fatty acid | 34.9 | 287.2240 | 287.2228 | −4.22 | C16H32O4 | 171.1045 | [40] | SV, SO, SP, SA, SK |
| 39 | Hydroxyoxooctadecadienoic acid | Fatty acid | 36.0 | 309.2080 | 309.2071 | −2.79 | C18H30O4 | 291.1957, 251.1660, 171.1045 | [40] | SS, SNu, SNe, SP, SA |
| 40 | Rosmanol I | Diterpene | 36.4 | 345.1709 | 345.1707 | −0.44 | C20H26O5 | 330.1366, 315.1609, 301.1794, 283.1413 | [34] | ST, SO, SNe |
| 41 | Cirsimaritin | Flavonoid | 37.3 | 313.0716 | 313.0718 | 0.51 | C17H14O6 | 289.0486, 283.0281, 255.0338, 227.0375, 163.0053, 135.0085, 117.0363 | [27] | SS, ST, SE, SO, SNe, SP, SA |
| 42 | Rosmanol II | Diterpene | 37.6 | 345.1717 | 345.1707 | −2.75 | C20H26O5 | 330.1366, 315.1609, 301.1794, 283.1413 | [34] | SO |
| 43 | Lachnocalyxolide C | Sesterpene | 38.0 | 461.2562 | 461.2545 | −3.73 | C26H38O7 | 429.2174, 385.2302, 341.2410 | [43] | SNu |
| 44 | Salvimirzacolide I | Sesterpene | 38.5 | 417.2630 | 417.2646 | 3.94 | C25H38O5 | 373.2700, 235.1544, 205.1478, 137.0943 | [38] | SE |
| 45 | Rosmanol III | Diterpene | 38.7 | 345.1698 | 345.1707 | 2.74 | C20H26O5 | 330.1366, 315.1609, 301.1794, 283.1413 | [34] | SO, SP |
| 46 | Lachnocalyxolide C’ | Sesterpene | 39.3 | 461.2581 | 461.2545 | −7.84 | C26H38O7 | 385.2133, 341.2381 | [43] | SNu |
| 47 | Hydroxycarnosic acid II | Diterpene | 40.0 | 347.1858 | 347.1864 | 1.72 | C20H28O5 | 329.1832, 303.2018, 259.2078 | [40] | ST, SNe, SK |
| 48 | Genkwanin | Flavonoid | 40.6 | 283.0620 | 283.0612 | −2.83 | C16H12O5 | 268.0311, 240.03666, 239.0341, 211.0332, | [34] | SS, SP, SA |
| 49 | Hydroperoxyoctadecadienoic acid | Fatty acid | 40.7 | 311.2237 | 311.2228 | −2.94 | C18H32O4 | 293.2085, 253.1793, 223.1693 | [40] | ST, SNu, SV, SO, SP, SA |
| 50 | Lachnocalyxolide A | Sesterpene | 42.2 | 429.2305 | 429.2283 | −5.20 | C25H34O6 | 385.2405, 341.2499, 299.2342, 205.1180 | [43] | SNu |
| 51 | Acetylhorminone II | Diterpene | 42.7 | 373.1995 | 373.2020 | 5.74 | C22H30O5 | 313.1384, 193.1266 | [38] | SV |
| 52 | Carnosic acid | Diterpene | 42.8 | 331.1935 | 331.1915 | −4.87 | C20H28O4 | 287.2177, 259.2130 | [34] | ST, SO, SNe, SK |
| 53 | Hydroxydodecanoic acid | Fatty acid | 42.9 | 215.1639 | 215.1653 | 6.33 | C12H24O3 | 171.1045 | [40] | SA |
| 54 | Methoxycarnosol | Diterpene | 44.5 | 359.1856 | 359.1864 | 2.21 | C21H28O5 | 283.1678 | [31] | SO |
| 55 | Dihydroxycarnosic acid | Diterpene | 44.9 | 363.1834 | 363.1813 | −5.73 | C20H28O6 | 319.1970, 275.2060, 257.1916 | [31] | ST, SK |
| 56 | Rosmadial | Diterpene | 45.3 | 343.1543 | 343.1551 | 2.32 | C20H24O5 | 299.1693, 243.1035, 216.0784 | [34] | SO, SNe, SP, SA |
| 57 | Salvimirzacolide I | Sesterpene | 45.7 | 417.2654 | 417.2646 | −1.80 | C25H38O5 | 373.2784, 235.1751, 137.1009 | [38] | SE |
| 58 | Salvipiliferol | Diterpene | 46.1 | 303.1973 | 303.1966 | −2.41 | C19H28O3 | 205.1221 | [38] | SNe |
| 59 | Carnosol | Diterpene | 46.5 | 329.1761 | 329.1758 | −0.81 | C20H26O4 | 285.1871, 201.0936 | [34] | SO, SK |
| 60 | Hydroxyoctadecatrienoic acid | Fatty acid | 46.9 | 293.2125 | 293.2122 | −0.96 | C18H30O3 | 275.2143, 224.1450, 195.1416 | [40] | SS, ST, SE, SNu, SV, SNe, SP, SA, SK |
| 61 | ent-19-Acetoxy-15,16-epoxy-3,13(16),14-clerodatrien-6,18-diol | Diterpene | 48.2 | 375.2177 | 375.2177 | 5.31 | C22H32O5 | 315.2082, 285.2014 | [38] | SV, SK |
| 62 | Hydroxysalviol | Diterpene | 48.3 | 317.2114 | 317.2122 | 2.57 | C20H30O3 | 273.2682, 137.1239 | [40] | SNe |
| 63 | Oxooctadecatrienoic acid | Fatty acid | 48.4 | 291.1980 | 291.1966 | −2.16 | C18H28O3 | 211.1170, 109.1020 | [40] | SA |
| 64 | Acetylhorminone III | Diterpene | 48.7 | 373.2028 | 373.2020 | −2.01 | C22H30O5 | 313.1384, 193.1266 | [38] | SO |
| 65 | Rosmaridiphenol | Diterpene | 49.0 | 315.1958 | 315.1966 | 2.43 | C20H28O3 | 285.1877, 201.0888 | [31] | SO |
| 66 | Hydroxytetradecanoic acid | Fatty acid | 49.6 | 243.1974 | 243.1966 | −3.41 | C14H28O3 | 197.1966 | [40] | SA |
| 67 | Hydroxyoctadecadienoic acid | Fatty acid | 49.7 | 295.2269 | 295.2279 | 3.27 | C18H32O3 | 277.2134, 235.1680, 195.1328, 171.1023 | [40] | SS, ST, SNu, SV, SO, SNe, SP, SK |
| 68 | Methylcarnosic acid | Diterpene | 51.6 | 345.2084 | 345.2071 | −3.66 | C21H30O4 | 301.2190, 286.2012, 191.1768 | [34] | ST, SV, SO, SNe, SP |
| 69 | Hydroxyhexadecanoic acid I | Fatty acid | 52.7 | 271.2260 | 271.2279 | −0.12 | C16H32O3 | 225.2151 | [40] | SP, SA, SK |
| 70 | Salviol | Diterpene | 52.9 | 301.2095 | 301.2173 | −1.31 | C20H30O2 | 205.1268, 169.9510 | [24] | SNe |
| 71 | Hydroxyhexadecanoic acid II | Fatty acid | 53.4 | 271.2270 | 271.2279 | 3.19 | C16H32O3 | 225.2151 | [40] | SP, SK |
| 72 | Oleanolic acid | Triterpene | 54.1 | 455.3549 | 455.3531 | 1.25 | C30H48O3 | 407.3436 | [34] | ST, SNu, SV, SNe, SP, SA |
| 73 | Ursolic acid | Triterpene | 54.6 | 455.3551 | 455.3531 | −4.45 | C30H48O3 | 408.3315, 373.2988 | [34] | ST, SNu, SV, SO, SNe, SP |
| Sample § | SS | ST | SE | SNu | SV | SO | SNe | SP | SA | SK | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Microbial Strain | MIC (mg/mL) | ||||||||||
| S. aureus ATCC 25923 | 1.25 | 2.5 | 1.25 | 5 | 1.25 | 0.625 | 2.5 | 2.5 | 2.5 | 2.5 | |
| S. pneumoniae ATCC 49619 | 1.25 | 2.5 | 2.5 | 2.5 | 2.5 | 0.625 | 2.5 | 2.5 | 2.5 | 2.5 | |
| E. coli ATCC 25922 | 5 | 5 | 5 | 5 | 2.5 | 5 | 5 | 5 | 5 | 2.5 | |
| P. aeruginosa ATCC 27853 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | |
| C. albicans ATCC 10231 | 2.5 | 2.5 | 5 | 5 | 2.5 | 2.5 | 1.25 | 1.25 | 5 | 2.5 | |
| Sample § | DPPH | ABTS | FRAP | |
|---|---|---|---|---|
| Test | EC50 (μg/mL) | |||
| SS | 32.23 ± 0.35 a | 17.20 ± 0.10 a | 29.67 ± 0.02 a | |
| ST | 41.16 ± 0.15 b | 26.50 ± 0.20 b | 28.51 ± 0.22 b | |
| SE | 42.00 ± 0.10 b | 17.00 ± 0.10 a | 36.94 ± 0.18 c | |
| SNu | 178.90 ± 1.1 c | 50.83 ± 0.15 c | 52.08 ± 0.01 d | |
| SV | 27.36 ± 0.32 d | 13.40 ± 0.10 d | 19.75 ± 0.02 e | |
| SO | 25.33 ± 0.05 d | 8.13 ± 0.05 e | 21.01 ± 0.02 e | |
| SNe | 57.40 ± 0.40 e | 16.46 ± 0.15 f | 55.61 ± 0.33 f | |
| SP | 39.53 ± 0.15 f | 15.06 ± 0.05 g | 40.94 ± 0.07 g | |
| SA | 146.6 ± 1.1 c | 59.16 ± 0.05 h | 80.02 ± 0.05 h | |
| SK | 38.53 ± 0.25 f | 14.06 ± 0.05 i | 19.74 ± 0.09 e | |
| Gallic acid | 1.60 ± 0.01 g | 0.60 ± 0.01 j | 1.57 ± 0.01 i | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, S.V.; Skalicka-Woźniak, K.; Mihai, C.-T.; Gradinaru, A.C.; Mandici, A.; Ciocarlan, N.; Miron, A.; Aprotosoaie, A.C. Chemical Profile and Bioactivity Evaluation of Salvia Species from Eastern Europe. Antioxidants 2023, 12, 1514. https://doi.org/10.3390/antiox12081514
Luca SV, Skalicka-Woźniak K, Mihai C-T, Gradinaru AC, Mandici A, Ciocarlan N, Miron A, Aprotosoaie AC. Chemical Profile and Bioactivity Evaluation of Salvia Species from Eastern Europe. Antioxidants. 2023; 12(8):1514. https://doi.org/10.3390/antiox12081514
Chicago/Turabian StyleLuca, Simon Vlad, Krystyna Skalicka-Woźniak, Cosmin-Teodor Mihai, Adina Catinca Gradinaru, Alexandru Mandici, Nina Ciocarlan, Anca Miron, and Ana Clara Aprotosoaie. 2023. "Chemical Profile and Bioactivity Evaluation of Salvia Species from Eastern Europe" Antioxidants 12, no. 8: 1514. https://doi.org/10.3390/antiox12081514
APA StyleLuca, S. V., Skalicka-Woźniak, K., Mihai, C.-T., Gradinaru, A. C., Mandici, A., Ciocarlan, N., Miron, A., & Aprotosoaie, A. C. (2023). Chemical Profile and Bioactivity Evaluation of Salvia Species from Eastern Europe. Antioxidants, 12(8), 1514. https://doi.org/10.3390/antiox12081514







