Ameliorative Effects of Lactobacillus paracasei L14 on Oxidative Stress and Gut Microbiota in Type 2 Diabetes Mellitus Rats
Abstract
:1. Introduction
2. Materials and Methods
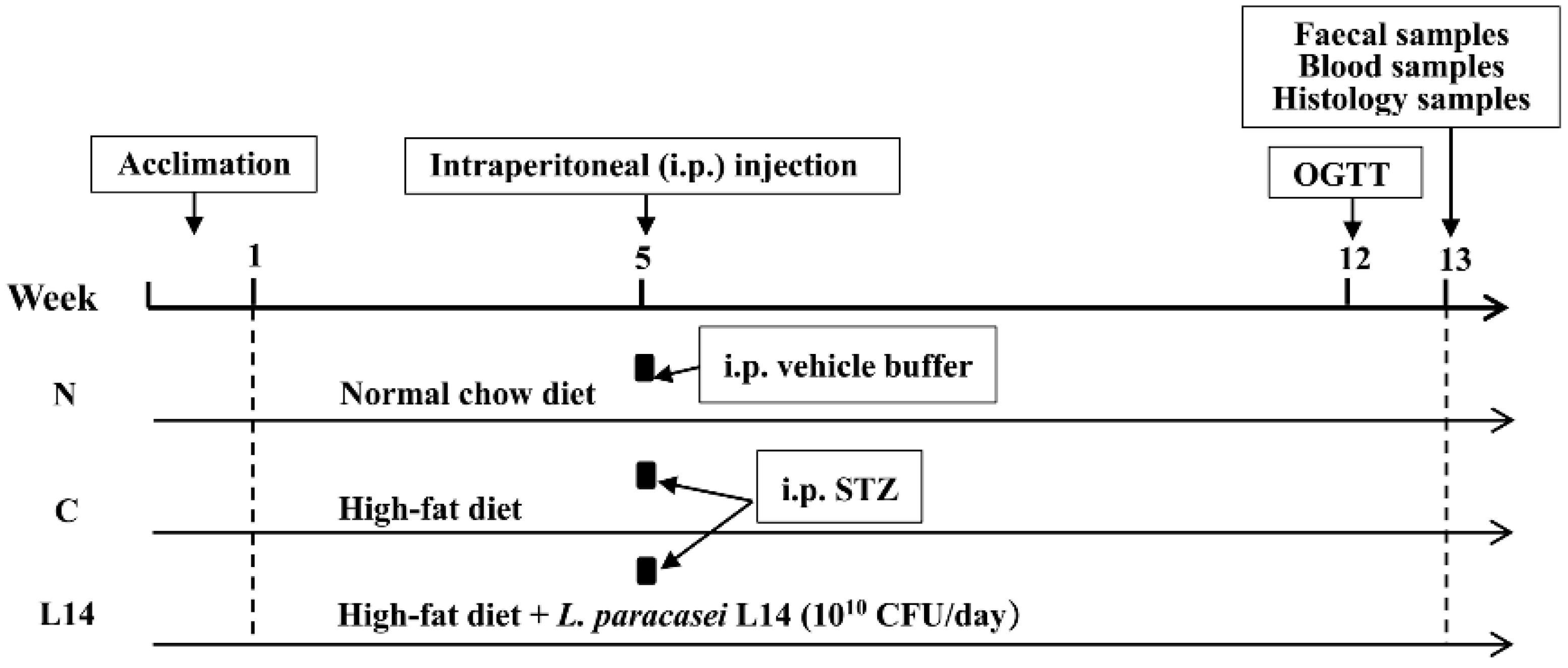
2.1. The T2DM Rat Model Induced by HFD/STZ
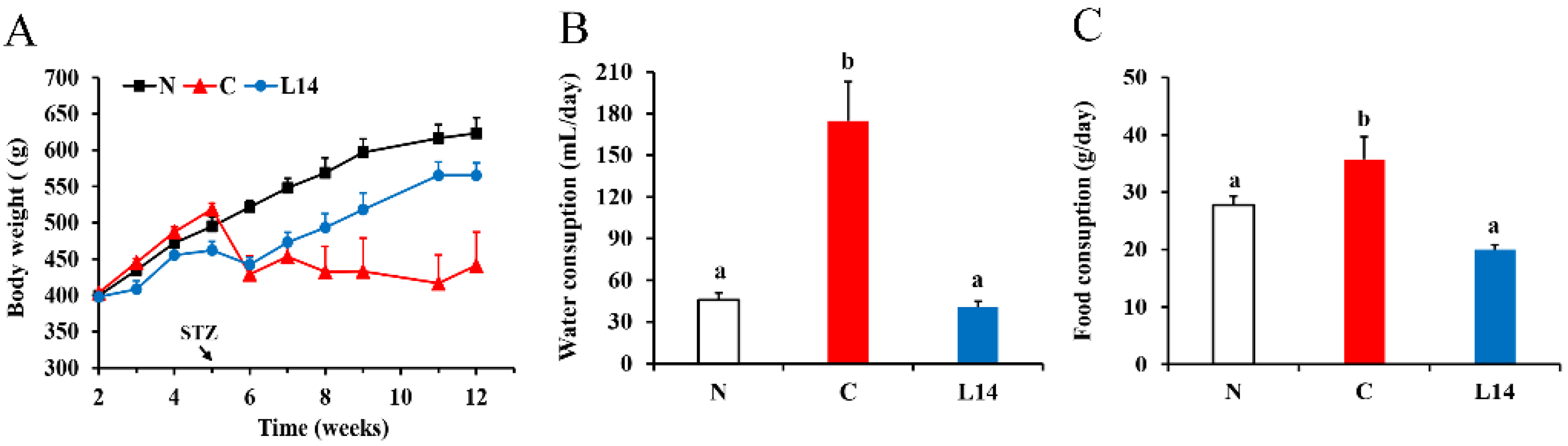
2.2. The FBG, BW, and Water and Food Consumption
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Biochemical Parameters
2.5. Histopathological Examination of the Pancreas and Liver Tissues
2.6. Gut Microbiota Analysis
2.7. Whole-Genome Sequencing of L. paracasei L14
2.8. Statistical Analysis
3. Results and Discussion
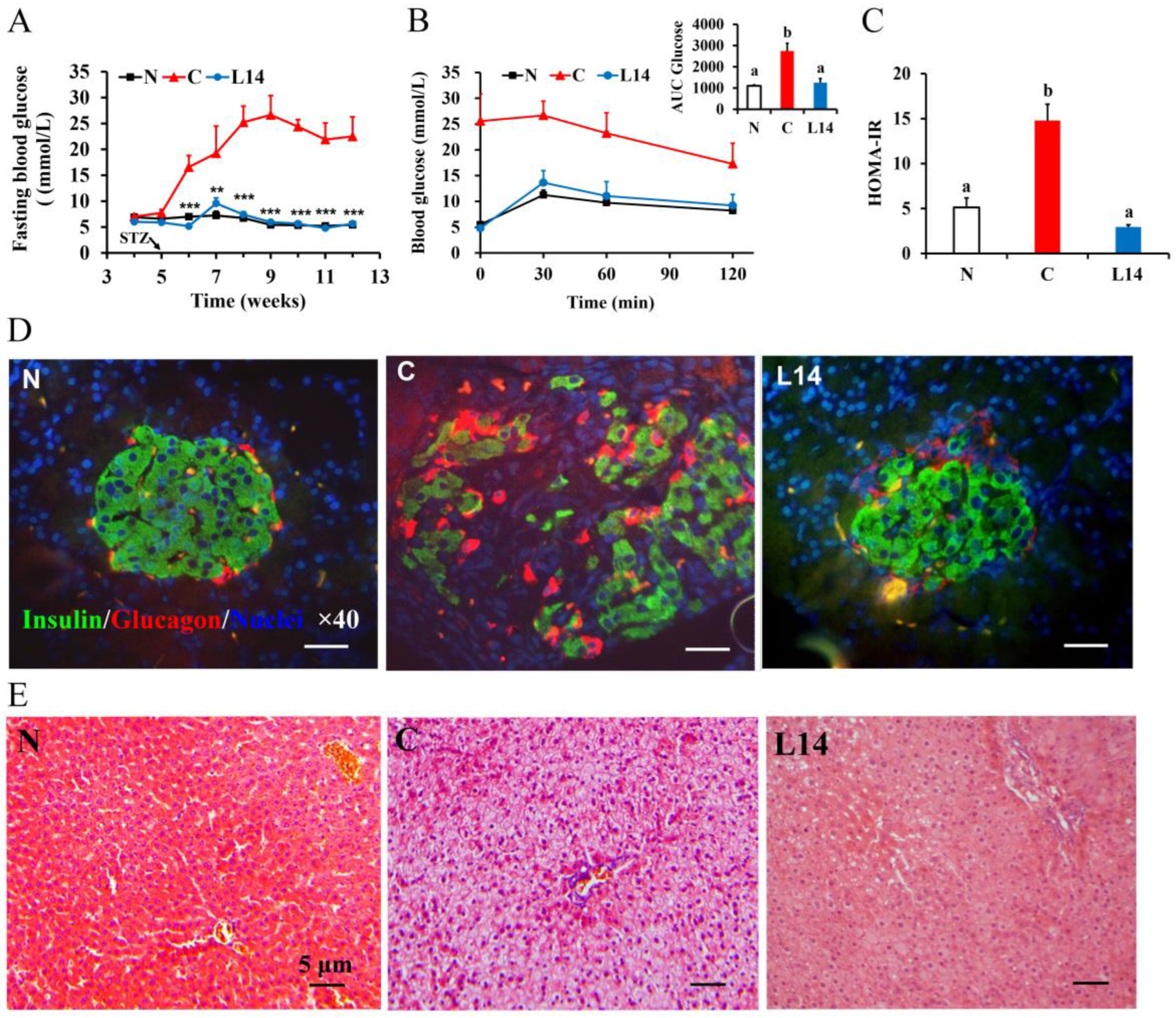
3.1. L. paracasei L14 Reduces Hyperglycemia, Improves IR, and Protects Pancreatic Beta-Cell Function in the T2DM Rat Model Induced by HFD/STZ
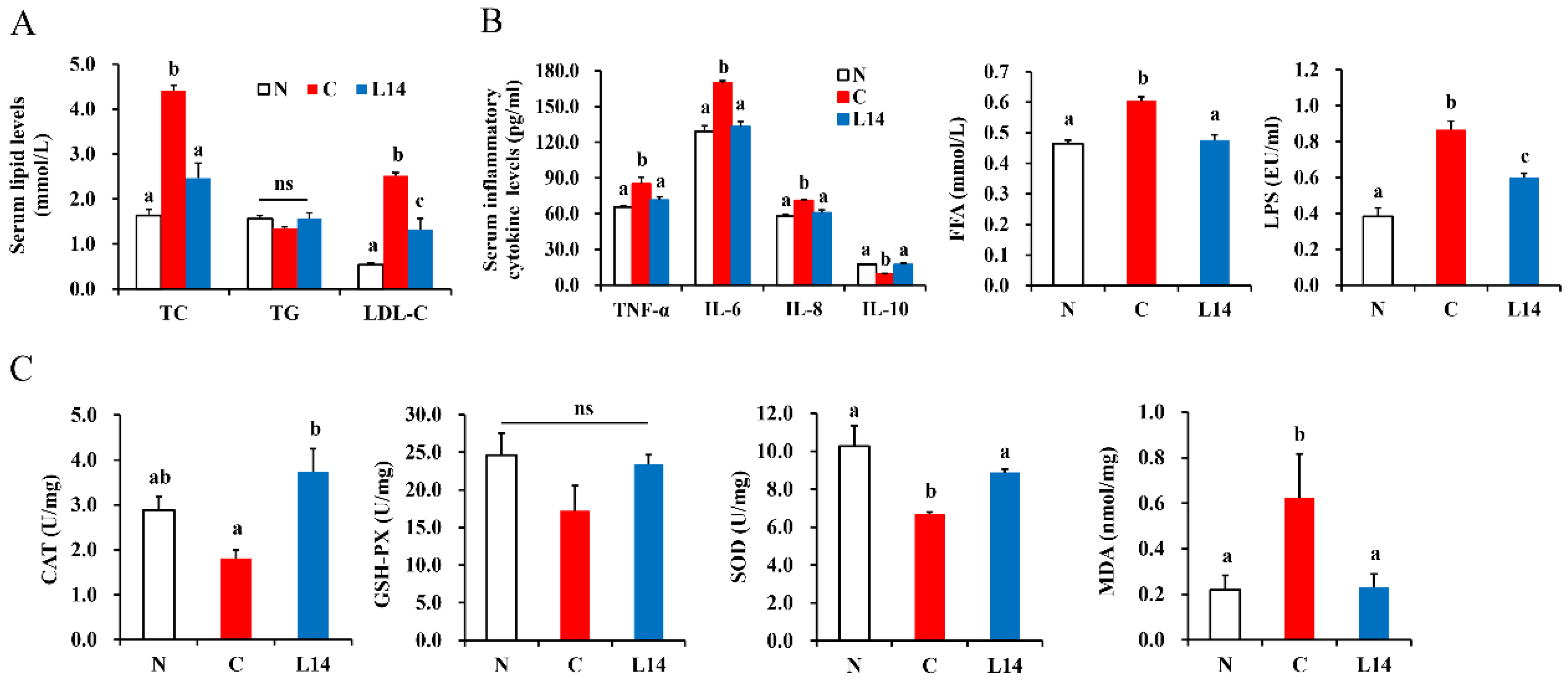
3.2. L. paracasei L14 Ameliorates the Hyperlipidemia Status and Improves the Inflammatory and Antioxidant Status of the T2DM Rats Exposed to HFD/STZ
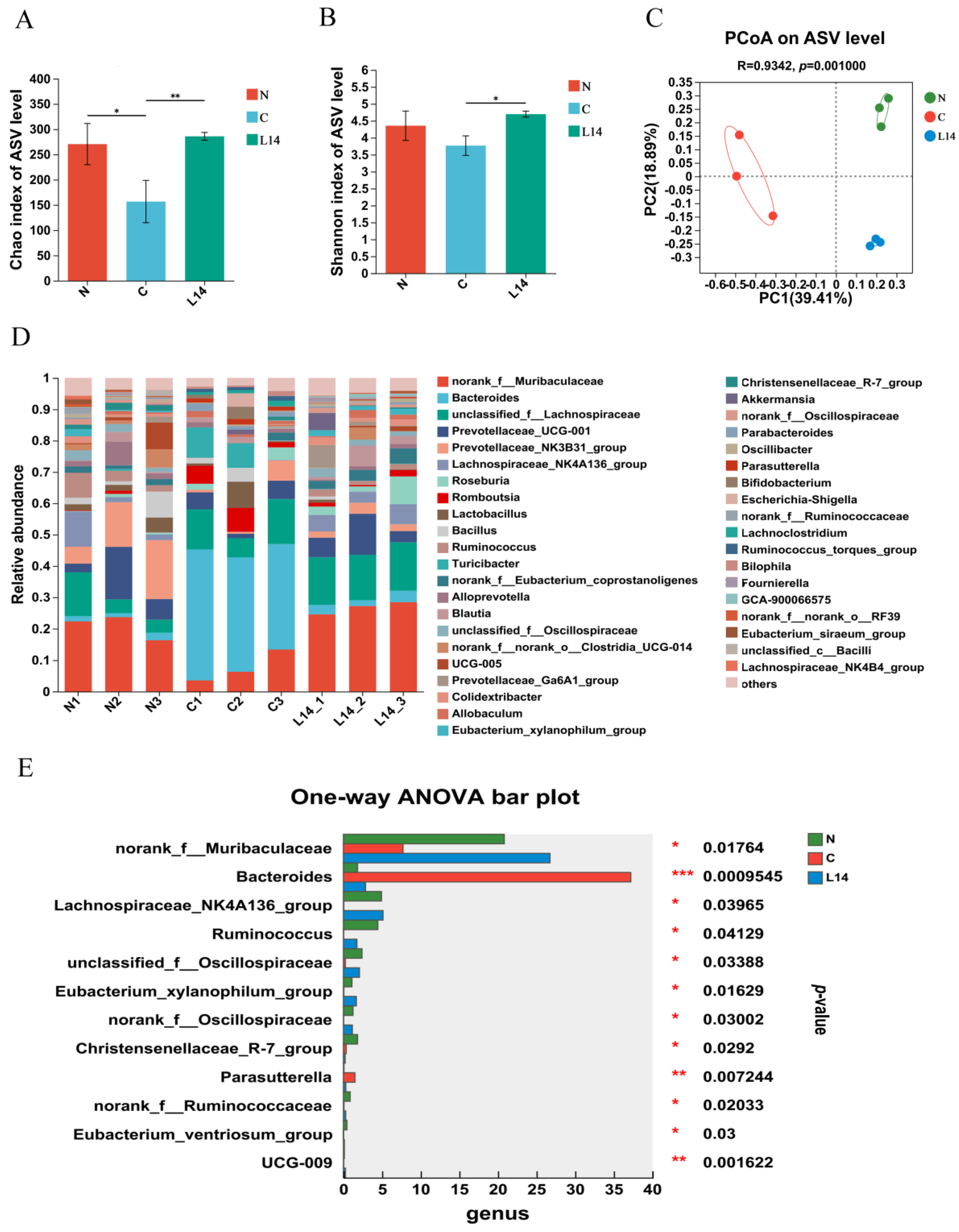
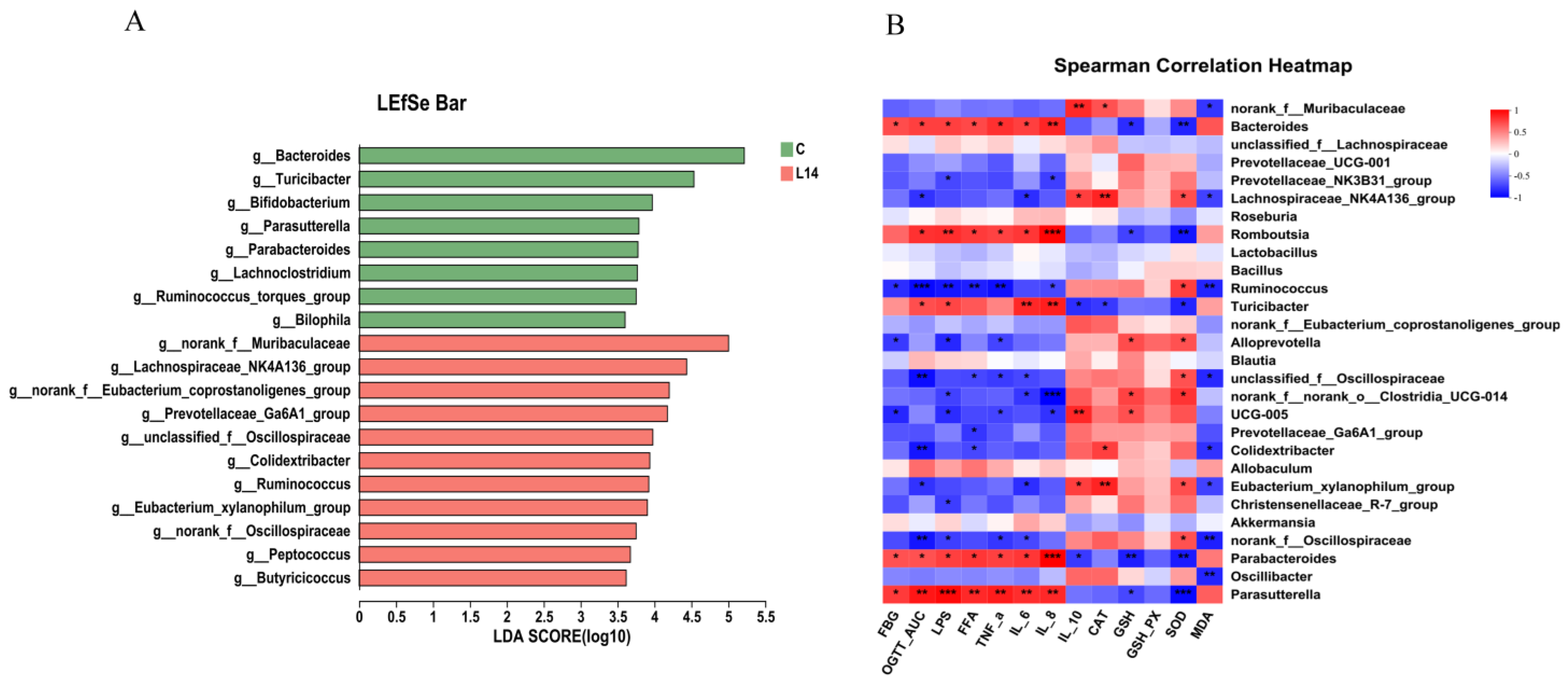
3.3. L. paracasei L14 Modulates the Intestinal Microbiota to Alleviate HFD/STZ-Induced Dysbiosis
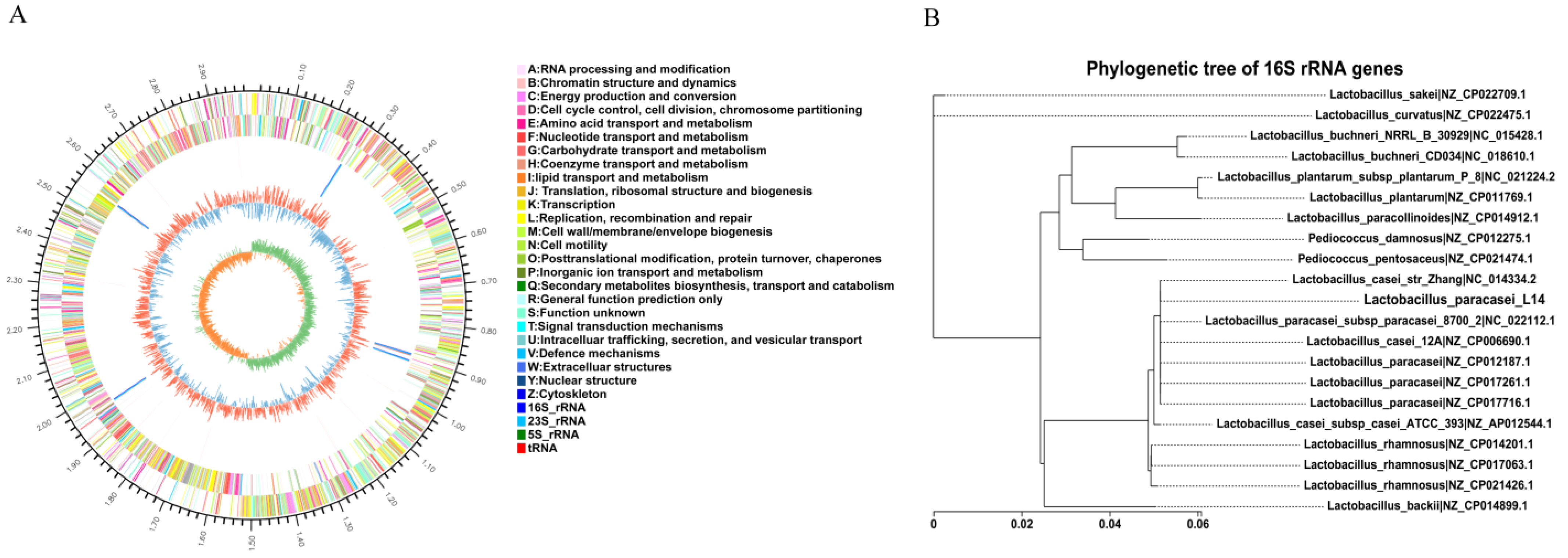
3.4. Whole-Genome Analysis of L. paracasei L14
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas 10th Edition. 2021. Available online: https://diabetesatlas.org/resources/ (accessed on 16 May 2023).
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Kyriienko, D.; Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab. Syndr. 2018, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Bielka, W.; Przezak, A.; Pawlik, A. The role of the gut microbiota in the pathogenesis of diabetes. Int. J. Mol. Sci. 2022, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Hernandez, A.; Garcia-Amezquita, L.E.; Requena, T.; Garcia-Cayuela, T. Probiotics; prebiotics, and synbiotics added to dairy products: Uses and applications to manage type 2 diabetes. Food Res. Int. 2021, 142, 110208. [Google Scholar] [CrossRef]
- Toejing, P.; Khat-Udomkiri, N.; Intakhad, J.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Putative mechanisms responsible for the antihyperglycemic action of Lactobacillus paracasei HII01 in experimental type 2 diabetic rats. Nutrients 2020, 12, 3015. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Song, J.; Huang, Y.; Li, X.; Wang, H.; Zhang, Y.; Suo, H. Lactobacillus plantarum SHY130 isolated from yak yogurt attenuates hyperglycemia in C57BL/6J mice by regulating the enteroinsular axis. Food Funct. 2022, 13, 675–687. [Google Scholar] [CrossRef]
- Zeng, Z.; Luo, J.Y.; Zuo, F.L.; Zhang, Y.; Ma, H.Q.; Chen, S.W. Screening for potential novel probiotic Lactobacillus strains based on high dipeptidyl peptidase IV and α-glucosidase inhibitory activity. J. Funct. Foods 2016, 20, 486–495. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, Y.L.; Chen, S.W. Screening for potential probiotic lactic acid bacteria strains based on α-glucosidase inhibitory activity. Trans. Chin. Soc. Agric. Mach. 2020, 51, 350–356. [Google Scholar]
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S.S. Ameliorative effects of probiotic Lactobacillus paracasei NL41 on insulin sensitivity, oxidative stress, and beta-cell function in a type 2 diabetes mellitus rat model. Mol. Nutr. Food Res. 2019, 63, e1900457. [Google Scholar] [CrossRef]
- Zeng, Z.; Guo, X.; Zhang, J.; Yuan, Q.; Chen, S. Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct. 2021, 12, 6809–6820. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PloS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.Q.; Liu, J.; Xia, X.Y.; Ai, L.Z. Probiotics-based interventions for diabetes mellitus: A review. Food Biosci. 2021, 43, 101172. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Madry, E. High-Fat, western-style diet, systemic inflammation, gut microbiota: A narrative review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Wood, L.G.; Li, Q.; Berthon, B.S.; Gibson, P.G.; Baines, K.J. Saturated fatty acids, but not n-6 polyunsaturated fatty acids or carbohydrates, increase airway inflammation in non-obese asthmatics. Respirology 2017, 22, 64. [Google Scholar]
- Lipke, K.; Kubis-Kubiak, A.; Piwowar, A. Molecular mechanism of lipotoxicity as an interesting aspect in the development of pathological states-current view of knowledge. Cells 2022, 11, 844. [Google Scholar] [CrossRef]
- Riedel, S.; Pheiffer, C.; Johnson, R.; Louw, J.; Muller, C.J.F. Intestinal barrier function and immune homeostasis are missing links in obesity and type 2 diabetes development. Front. Endocrinol. 2022, 12, 833544. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhang, J.H.; Cheng, Y.; Zhu, M.; Xiao, Z.F.; Ruan, G.C.; Wei, Y.L. Gut microbiota: A new target for T2DM prevention and treatment. Front. Endocrinol. 2022, 13, 958218. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Yu, D.S.; Zhu, C.S. Gut microbiota: An important player in type 2 diabetes mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Liu, Z.; Zhu, Y.; Wang, H.; Dai, Z.; Yang, X.; Ren, X.; Xue, Y.; Shen, Q. Cooked Adzuki Bean reduces high-fat diet- induced body weight gain, ameliorates inflammation, and modulates intestinal homeostasis in mice. Front. Nutr. 2022, 9, 918696. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Hu, J.; Chen, H.; Geng, F.; Nie, S. Arabinoxylan ameliorates type 2 diabetes by regulating the gut microbiota and metabolites. Food Chem. 2022, 371, 131106. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caparros-Martin, J.A.; Lareu, R.R.; Ramsay, J.P.; Peplies, J.; Reen, F.J.; Headlam, H.A.; Ward, N.C.; Croft, K.D.; Newsholme, P.; Hughes, J.D.; et al. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome 2017, 5, 95. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Xu, H.Y.; Zhan, L.B.; Lu, X.G.; Zhang, L.J. Dynamic development of fecal microbiome during the progression of diabetes mellitus in Zucker diabetic fatty rats. Front. Microbiol. 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmel-Harel, O.; Storz, G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 2000, 54, 439–461. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Zeng, Z.; Zuo, F.L.; Marcotte, H. Putative adhesion factors in vaginal Lactobacillus gasseri DSM 14869: Functional characterization. Appl. Environ. Microbiol. 2019, 85, e00800-19. [Google Scholar] [CrossRef] [Green Version]
- Pourjafar, H.; Ansari, F.; Sadeghi, A.; Samakkhah, S.A.; Jafari, S.M. Functional and health-promoting properties of probiotics’ exopolysaccharides; isolation, characterization, and applications in the food industry. Crit. Rev. Food Sci. Nutr. 2022, in press. [Google Scholar] [CrossRef]
| No. | Organism | Strain | Assembly Accession | Genome Size (Mb) | Scaffold No. | Chromosome No. | G + C (%) | Protein-Coding Sequences |
|---|---|---|---|---|---|---|---|---|
| 1 | L. paracasei | Zhang | GCF_000019245.4 | 2.86 | 2 | 2 | 46 | 2641 |
| 2 | L. paracasei | 8700 | GCF_000155515.2 | 2.94 | 3 | 3 | 46 | 2803 |
| 3 | L. casei | 12A | GCF_000359565.2 | 2.91 | 1 | 1 | 46 | 2682 |
| 4 | L. paracasei | CAUH35 | GCF_001191565.1 | 2.77 | 5 | 5 | 46 | 2690 |
| 5 | L. paracasei | FAM18149 | GCF_002442835.1 | 2.71 | 6 | 6 | 46 | 2730 |
| 6 | L. paracasei | TK1501 | GCF_002257625.1 | 2.94 | 1 | 1 | 46.5 | 2665 |
| Locus Tag(s) | Accession No. | Gene | Predicted Encoded Function | Protein Size |
|---|---|---|---|---|
| PUK88_12395 | WP003567571.1 | ahpC | Alkyl hydroperoxide reductase C | 187aa |
| PUK88_04685 | WP003601666.1 | gpx | Glutathione peroxidase | 157aa |
| PUK88_07930 | WP005685874.1 | nrdH | Glutaredoxin-like protein | 76aa |
| PUK88_09810 | WP012491719.1 | Superoxide dismutase [Mn] | 205aa | |
| PUK88_00185 | WP011673942.1 | mntH | Nramp family divalent metal transporter | 458aa |
| PUK88_04200 | WP010493758.1 | trxA | Thioredoxin | 103aa |
| PUK88_01345 | WP003568841.1 | Thioredoxin family protein | 104aa | |
| PUK88_04960 | WP003564228.1 | trxB | Thioredoxin–disulfide reductase | 315aa |
| PUK88_06640 | WP003590482.1 | msrA | peptide–methionine (S)-S-oxide reductase | 276aa |
| PUK88_08180 | WP003565681.1 | msrB | peptide–methionine (R)-S-oxide reductase | 150aa |
| PUK88_06850 | WP003574830.1 | GAF domain-containing protein | 160aa | |
| PUK88_11715 | WP003595937.1 | NAD(P)/FAD-dependent oxidoreductase | 632aa | |
| PUK88_02000 | WP016370227.1 | sorbitol-6-phosphate dehydrogenase subunit | 266aa | |
| PUK88_02065 | WP019862015.1 | srlD | SDR family oxidoreductase | 267aa |
| PUK88_02200 | WP003577685.1 | sorbitol-6-phosphate dehydrogenase subunit | 266aa | |
| PUK88_13525 | WP019862015.1 | SDR family oxidoreductase | 266aa |
| Locus Tag(s) | Predicted Encoded Function | Protein Size | Domain |
|---|---|---|---|
| PUK88_10270 | polysaccharide deacetylase | 332aa | Polysaccharide deacetylase (PF01522) |
| PUK88_10275 | sulfatase-like hydrolase/transferase | 774aa | Sulfatase (PF00884) |
| PUK88_10280 | EpsG family protein | 349aa | EpsG family (PF14897) |
| PUK88_10285 | CpsD/CapB family tyrosine–protein kinase | 211aa | AAA domain (PF13614); NUBPL iron-transfer P-loop NTPase (PF10609); CobQ/CobB/MinD/ParA nucleotide-binding domain (PF01656) |
| PUK88_10290 | Wzz/FepE/Etk N-terminal domain-containing protein | 230aa | Chain-length-determinant protein (PF02706) |
| PUK88_10295 | O-unit flippase-like protein | 479aa | - |
| PUK88_10300 | glycosyltransferase | 378aa | Glycosyl transferase group 1 (PF00534) |
| PUK88_10305 | glycosyltransferase family 2 protein | 321aa | Glycosyl transferase family 2 (PF00535); Glycosyltransferase-like family 2 (PF13641); Glycosyltransferase-like family 2 (PF10111) |
| PUK88_10310 | hypothetical protein | 558aa | - |
| PUK88_10315 | hypothetical protein | 579aa | - |
| PUK88_10320 | GW dipeptide domain-containing protein | 693aa | GW (Gly-Tryp) dipeptide domain (PF01183); Glycosyl hydrolases family 25 (PF13457) |
| PUK88_10325 | glycosyltransferase family 39 protein | 495aa | Dolichyl-phosphate-mannose-protein mannosyltransferase (PF13231) |
| PUK88_10330 | acyltransferase | 346aa | Acyltransferase family (PF01757) |
| PUK88_10335 | glycosyltransferase family 2 protein | 330aa | Glycosyl transferase family 2 (PF00535) |
| PUK88_10340 | UDP-N-acetylglucosamine 2-epimerase, wecB | 380aa | UDP-N-acetylglucosamine 2-epimerase (PF02350) |
| PUK88_10345 | hypothetical protein | 288aa | - |
| PUK88_10350 | sugar transferase | 466aa | Bacterial sugar transferase (PF13727); CoA-binding domain (PF02397) |
| PUK88_10355 | Glycosyl transferase | 246aa | Glycosyltransferase sugar-binding region containing DXD motif (PF04488) |
| PUK88_10360 | glycosyltransferase family 2 protein | 252aa | Glycosyl transferase family 2 (PF00535) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Yang, Y.; Zhong, X.; Dai, F.; Chen, S.; Tong, X. Ameliorative Effects of Lactobacillus paracasei L14 on Oxidative Stress and Gut Microbiota in Type 2 Diabetes Mellitus Rats. Antioxidants 2023, 12, 1515. https://doi.org/10.3390/antiox12081515
Zeng Z, Yang Y, Zhong X, Dai F, Chen S, Tong X. Ameliorative Effects of Lactobacillus paracasei L14 on Oxidative Stress and Gut Microbiota in Type 2 Diabetes Mellitus Rats. Antioxidants. 2023; 12(8):1515. https://doi.org/10.3390/antiox12081515
Chicago/Turabian StyleZeng, Zhu, Yi Yang, Xinxin Zhong, Fangyin Dai, Shangwu Chen, and Xiaoling Tong. 2023. "Ameliorative Effects of Lactobacillus paracasei L14 on Oxidative Stress and Gut Microbiota in Type 2 Diabetes Mellitus Rats" Antioxidants 12, no. 8: 1515. https://doi.org/10.3390/antiox12081515






