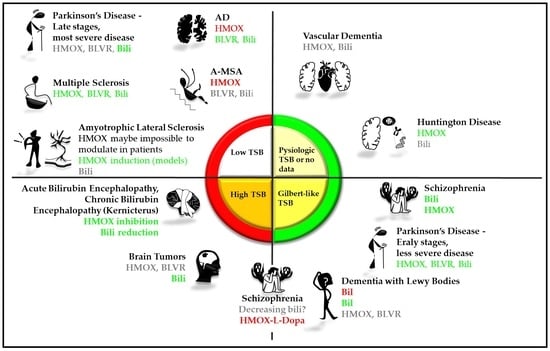
Bilirubin and Redox Stress in Age-Related Brain Diseases
Abstract
:1. Introduction
2. Oxidative Stress and the Role of Bilirubin in NCDs
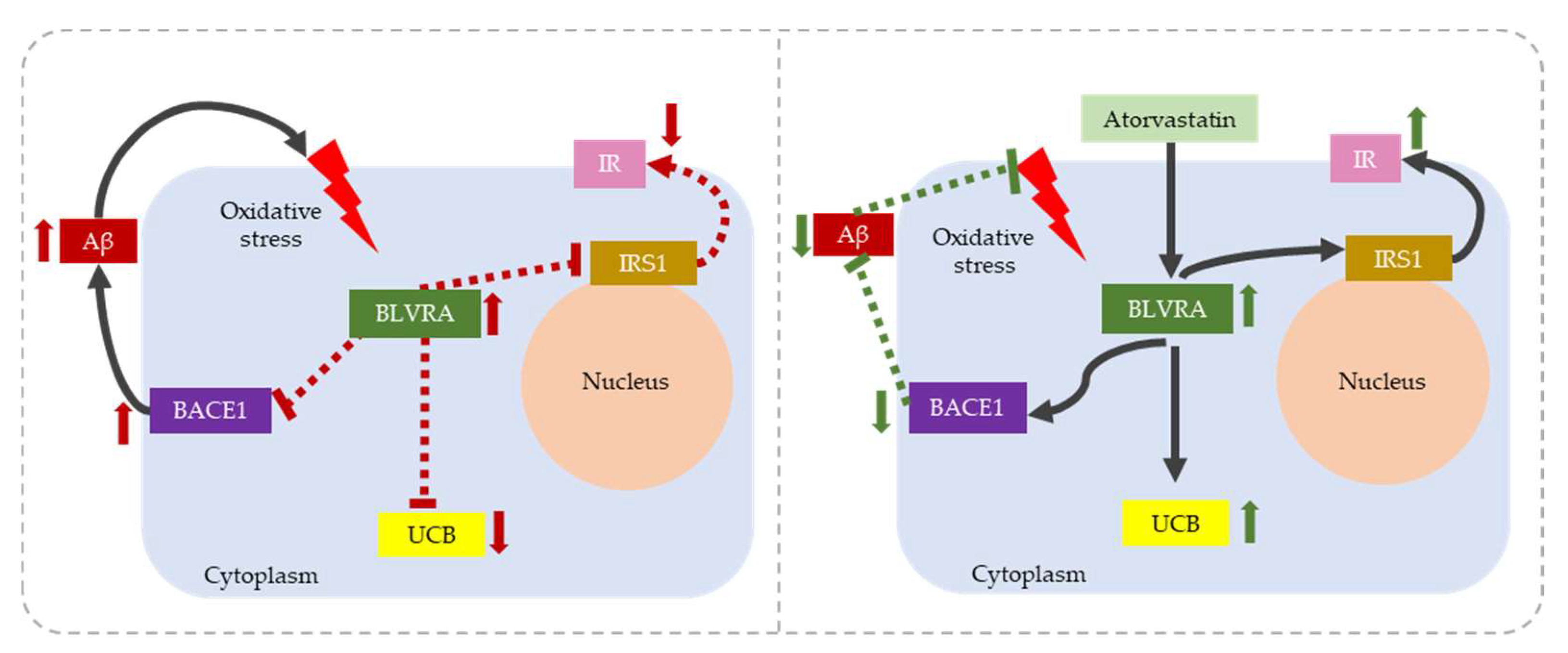
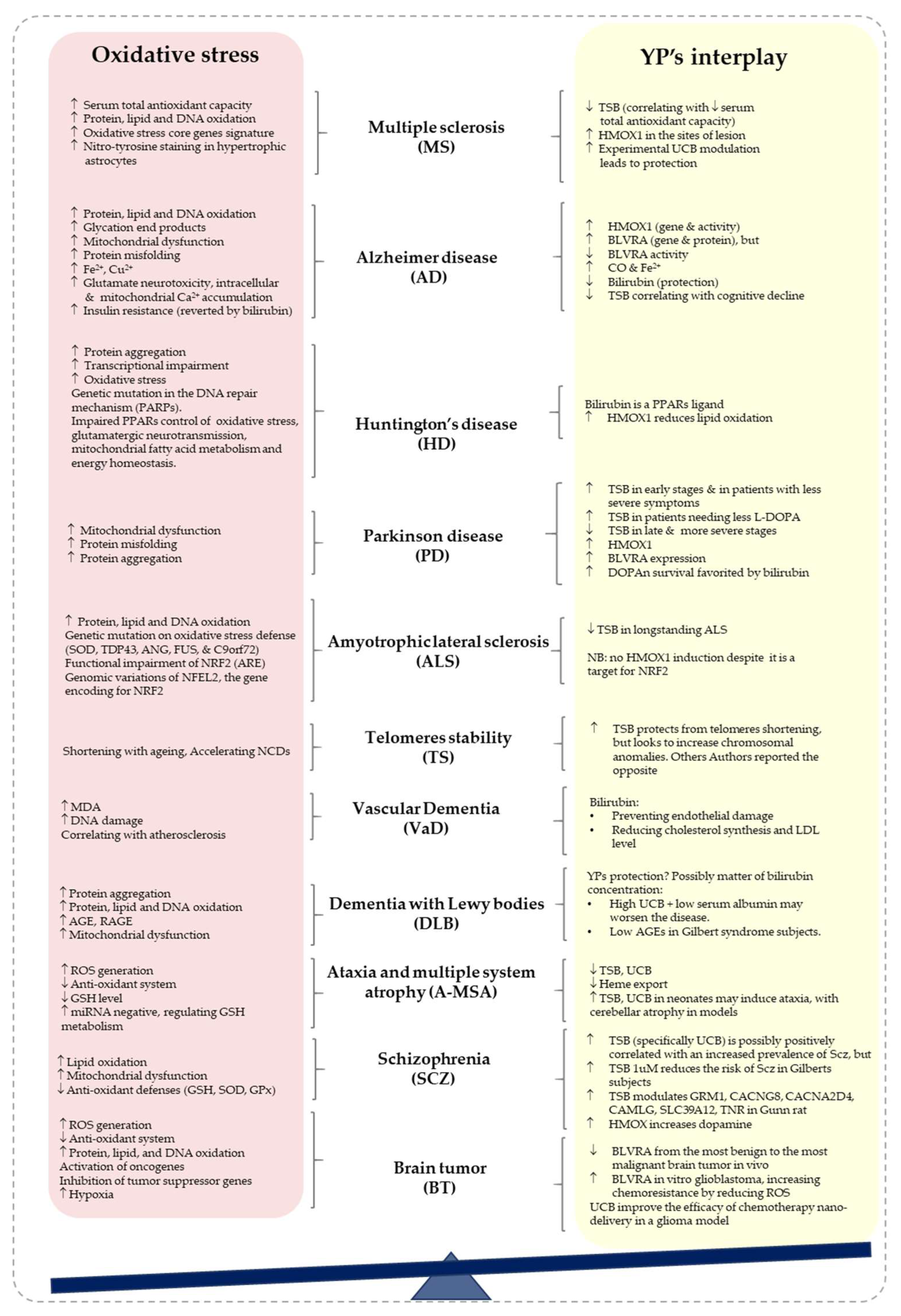
2.1. Alzheimer’s Disease (AD)
2.2. Parkinson’s Disease
2.3. Multiple Sclerosis (MS)
2.4. Amyotrophic Lateral Sclerosis (ALS)
2.5. Huntington’s Disease (HD)
2.6. Dementia with Lewy Bodies (DLB)
2.7. Vascular Dementia (VaD)
2.8. Schizophrenia (Scz)
2.9. Ataxia and Multiple System Atrophy (A-MSA)
2.10. Brain Tumors in the Elderly
2.11. Telomere Stability in Neurodegeneration
3. Bilirubin as a Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, A.; Yegla, B.; Foster, T.C. Redox Signaling in Neurotransmission and Cognition During Aging. Antioxid. Redox Signal. 2018, 28, 1724–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemma, C.; Vila, J. Oxidative Stress and the Aging Brain: From Theory to Prevention. In Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Schipper, H.M.; Song, W. The Sinister Face of Heme Oxygenase-1 in Brain Aging and Disease. Prog. Neurobiol. 2019, 172, 40–70. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Terracina, S.; Petrella, C. Antioxidant Intervention to Improve Cognition in the Aging Brain: The Example of Hydroxytyrosol and Resveratrol. Int. J. Mol. Sci. 2022, 23, 15674. [Google Scholar] [CrossRef]
- Sedlak, T.; Snyder, S. Bilirubin Benefits: Cellular Protection by a Biliverdin Reductase Antioxidant Cycle. Pediatrics 2004, 113, 1776–1782. [Google Scholar] [CrossRef]
- Sedlak, T.W.; Saleh, M. Bilirubin and Glutathione Have Complementary Antioxidant and Cytoprotective Roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef]
- Vasavda, C.; Kothari, R. Bilirubin Links Heme Metabolism to Neuroprotection by Scavenging Superoxide. Cell Chem. Biol. 2019, 26, 1450–1460.e7. [Google Scholar] [CrossRef]
- Gazzin, S.; Vitek, L. A Novel Perspective on the Biology of Bilirubin in Health and Disease. Trends Mol. Med. 2016, 22, 758–768. [Google Scholar] [CrossRef]
- Gazzin, S.; Masutti, F. The Molecular Basis of Jaundice: An Old Symptom Revisited. Liver Int. 2016, 37, 1094–1102. [Google Scholar] [CrossRef] [Green Version]
- Wagner, K.-H.; Wallner, M. Looking to the Horizon: The Role of Bilirubin in the Development and Prevention of Age-Related Chronic Diseases. Clin. Sci. 2015, 129, 1–25. [Google Scholar] [CrossRef]
- Creeden, J.F.; Gordon, D.M. Bilirubin as a Metabolic Hormone: The Physiological Relevance of Low Levels. Am. J. Physiol.-Endocrinol. Metab. 2020, 320, E191–E207. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Seyed Khoei, N. Oxidative Stress and Related Biomarkers in Gilbert’s Syndrome: A Secondary Analysis of Two Case-Control Studies. Antioxidants 2021, 10, 1474. [Google Scholar] [CrossRef]
- Chaudhari, H.; Goyal, S. Neonates with Sickle Cell Disease Are Vulnerable to Blue Light Phototherapy-Induced Oxidative Stress and Proinflammatory Cytokine Elevations. Med. Hypotheses 2016, 96, 78–82. [Google Scholar] [CrossRef]
- Ayyappan, S.; Philip, S. Antioxidant Status in Neonatal Jaundice before and after Phototherapy. J. Pharm. Bioallied. Sci. 2015, 7 (Suppl. 1), S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Sarici, D.; Gunes, T. Investigation on Malondialdehyde, S100B, and Advanced Oxidation Protein Product Levels in Significant Hyperbilirubinemia and the Effect of Intensive Phototherapy on These Parameters. Pediatr. Neonatol. 2015, 56, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.M. Chronic Bilirubin Encephalopathy: Diagnosis and Outcome. Semin. Fetal Neonatal Med. 2010, 15, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Le Pichon, J.-B.; Riordan, S.M. The Neurological Sequelae of Neonatal Hyperbilirubinemia: Definitions, Diagnosis and Treatment of the Kernicterus Spectrum Disorders (KSDs). Curr. Pediatr. Rev. 2017, 13, 199–209. [Google Scholar] [CrossRef]
- Jayanti, S.; Ghersi-Egea, J.-F. Severe Neonatal Hyperbilirubinemia and the Brain: The Old but Still Evolving Story. Pediatr. Med. 2021, 4, 37. [Google Scholar] [CrossRef]
- Rose, J.; Vassar, R. Movement Disorders Due to Bilirubin Toxicity. Semin. Fetal Neonatal Med. 2015, 20, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Baranano, D.E.; Rao, M. Biliverdin Reductase: A Major Physiologic Cytoprotectant. Proc. Natl. Acad. Sci. USA 2002, 99, 16093–16098. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y. Bilirubin Is an Antioxidant of Possible Physiological Importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Vítek, L.; Tiribelli, C. Bilirubin: The Yellow Hormone? J. Hepatol. 2021, 75, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.; Wang, H. Bilirubin Induces Pain Desensitization in Cholestasis by Activating 5-Hydroxytryptamine 3A Receptor in Spinal Cord. Front. Cell Dev. Biol. 2021, 9, 605855. [Google Scholar] [CrossRef]
- Vitek, L.; Ostrow, J.D. Bilirubin Chemistry and Metabolism; Harmful and Protective Aspects. Curr. Pharm. Des. 2009, 15, 2869–2883. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Hughes, M.N. Interaction of Bilirubin and Biliverdin with Reactive Nitrogen Species. FEBS Lett. 2003, 543, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, C.; Barone, E. Inhibition of Lipid Peroxidation and Protein Oxidation by Endogenous and Exogenous Antioxidants in Rat Brain Microsomes in Vitro. Neurosci. Lett. 2012, 518, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Datla, S.R.; Dusting, G.J. Induction of Heme Oxygenase-1 In Vivo Suppresses NADPH Oxidase–Derived Oxidative Stress. Hypertension 2007, 50, 636–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaisiya, M.; Coda Zabetta, C.D. Bilirubin Mediated Oxidative Stress Involves Antioxidant Response Activation via Nrf2 Pathway. Cell. Signal. 2014, 26, 512–520. [Google Scholar] [CrossRef]
- Dang, T.N.; Robinson, S.R. Uptake, Metabolism and Toxicity of Hemin in Cultured Neurons. Neurochem. Int. 2011, 58, 804–811. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y. Hemin Treatment Protects Neonatal Rats from Sevoflurane-Induced Neurotoxicity via the Phosphoinositide 3-Kinase/Akt Pathway. Life Sci. 2020, 242, 117151. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R. The Pathophysiology of Heme in the Brain. Curr. Alzheimer Res. 2016, 13, 174–184. Available online: https://www.eurekaselect.com/135089/article (accessed on 27 July 2020). [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V. Mechanisms of Cell Protection by Heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maines, M.D. New Insights into Biliverdin Reductase Functions: Linking Heme Metabolism to Cell Signaling. Physiology 2005, 20, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitti, M.; Piras, S. Heme Oxygenase 1 in the Nervous System: Does It Favor Neuronal Cell Survival or Induce Neurodegeneration? Int. J. Mol. Sci. 2018, 19, 2260. [Google Scholar] [CrossRef] [Green Version]
- Ryter, S.W.; Alam, J. Heme Oxygenase-1/Carbon Monoxide: From Basic Science to Therapeutic Applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Chiabrando, D.; Fiorito, V. Unraveling the Role of Heme in Neurodegeneration. Front. Neurosci. 2018, 12, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J. Heme Oxygenase in Neuroprotection: From Mechanisms to Therapeutic Implications. Rev. Neurosci. 2014, 25, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Kapitulnik, J.; Maines, M.D. Pleiotropic Functions of Biliverdin Reductase: Cellular Signaling and Generation of Cytoprotective and Cytotoxic Bilirubin. Trends Pharmacol. Sci. 2009, 30, 129–137. [Google Scholar] [CrossRef]
- Lerner-Marmarosh, N.; Shen, J. Human Biliverdin Reductase: A Member of the Insulin Receptor Substrate Family with Serine/Threonine/Tyrosine Kinase Activity. Proc. Natl. Acad. Sci. USA 2005, 102, 7109–7114. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M. Naturally Derived Heme-Oxygenase 1 Inducers and Their Therapeutic Application to Immune-Mediated Diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef]
- Luu Hoang, K.N.; Anstee, J.E. The Diverse Roles of Heme Oxygenase-1 in Tumor Progression. Front. Immunol. 2021, 12, 658315. [Google Scholar] [CrossRef]
- Medina, M.; Garrido, J.J. Modulation of GSK-3 as a Therapeutic Strategy on Tau Pathologies. Front. Mol. Neurosci. 2011, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Cheng, Q. Cytoprotective Role of Heme Oxygenase-1 in Cancer Chemoresistance: Focus on Antioxidant, Antiapoptotic, and Pro-Autophagy Properties. Antioxidants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, A.; Komatsu, M. Eel Green Fluorescent Protein Is Associated with Resistance to Oxidative Stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 181–182, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sheng, H. Bilirubin Stabilizes the Mitochondrial Membranes during NLRP3 Inflammasome Activation. Biochem. Pharmacol. 2022, 203, 115204. [Google Scholar] [CrossRef]
- Kumagai, A.; Ando, R. A Bilirubin-Inducible Fluorescent Protein from Eel Muscle. Cell 2013, 153, 1602–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayanti, S.; Vítek, L. The Role of Bilirubin and the Other “Yellow Players” in Neurodegenerative Diseases. Antioxidants 2020, 9, 900. [Google Scholar] [CrossRef]
- Gazzin, S.; Jayanti, S. Models of Bilirubin Neurological Damage: Lessons Learned and New Challenges. Pediatr. Res. 2023, 93, 1838–1845. [Google Scholar] [CrossRef]
- Watchko, J.F.; Tiribelli, C. Bilirubin-Induced Neurologic Damage—Mechanisms and Management Approaches. N. Engl. J. Med. 2013, 369, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Su, D. D-T7 Peptide-Modified PEGylated Bilirubin Nanoparticles Loaded with Cediranib and Paclitaxel for Antiangiogenesis and Chemotherapy of Glioma. ACS Appl. Mater. Interfaces 2019, 11, 176–186. [Google Scholar] [CrossRef]
- Halliwell, B.; Zhao, K. Nitric Oxide and Peroxynitrite. The Ugly, the Uglier and the Not so Good. Free Radic. Res. 1999, 31, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, F.; Scarfò, G. Oxidative Stress and Cognitive Decline: The Neuroprotective Role of Natural Antioxidants. Front. Neurosci. 2021, 15, 729757. [Google Scholar] [PubMed]
- Migliore, L.; Coppedè, F. Environmental-Induced Oxidative Stress in Neurodegenerative Disorders and Aging. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 674, 73–84. [Google Scholar] [CrossRef]
- Jové, M.; Pradas, I. Lipids and Lipoxidation in Human Brain Aging. Mitochondrial ATP-Synthase as a Key Lipoxidation Target. Redox Biol. 2019, 23, 101082. [Google Scholar] [CrossRef]
- Bruce, K.D.; Zsombok, A. Lipid Processing in the Brain: A Key Regulator of Systemic Metabolism. Front. Endocrinol. 2017, 8, 60. [Google Scholar]
- Butterfield, D.A. Brain Lipid Peroxidation and Alzheimer Disease: Synergy between the Butterfield and Mattson Laboratories. Ageing Res. Rev. 2020, 64, 101049. [Google Scholar] [CrossRef] [PubMed]
- Vitek, L.; Bellarosa, C. Induction of Mild Hyperbilirubinemia: Hype or Real Therapeutic Opportunity? Clin. Pharmacol. Ther. 2019, 106, 568–575. [Google Scholar] [CrossRef]
- Hinds, T.D.J.; Creeden, J.F. Bilirubin Nanoparticles Reduce Diet-Induced Hepatic Steatosis, Improve Fat Utilization, and Increase Plasma β-Hydroxybutyrate. Front. Pharmacol. 2020, 11, 594574. [Google Scholar] [CrossRef]
- Barone, E.; Di Domenico, F. The Janus Face of the Heme Oxygenase/Biliverdin Reductase System in Alzheimer Disease: It’s Time for Reconciliation. Neurobiol. Dis. 2014, 62, 144–159. [Google Scholar] [CrossRef] [Green Version]
- Duyckaerts, C.; Delatour, B. Classification and Basic Pathology of Alzheimer Disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Hall, N. Brain Regional Correspondence Between Alzheimer’s Disease Histopathology and Biomarkers of Protein Oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Adam, R.H.I. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G. RNA Oxidation Is a Prominent Feature of Vulnerable Neurons in Alzheimer’s Disease. J. Neurosci. 1999, 19, 1959–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero-Garcia, M.; Mahajani, S.U. Molecular Signatures Underlying Neurofibrillary Tangle Susceptibility in Alzheimer’s Disease. Neuron 2022, 110, 2929–2948.e8. [Google Scholar] [CrossRef]
- Rummel, N.G.; Butterfield, D.A. Altered Metabolism in Alzheimer Disease Brain: Role of Oxidative Stress. Antioxid. Redox Signal. 2022, 36, 1289–1305. [Google Scholar] [CrossRef]
- Barone, E.; Di Domenico, F. Biliverdin Reductase—A Protein Levels and Activity in the Brains of Subjects with Alzheimer Disease and Mild Cognitive Impairment. Biochim. Biophys. Acta 2011, 1812, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Triani, F.; Tramutola, A. Biliverdin Reductase-A Impairment Links Brain Insulin Resistance with Increased Aβ Production in an Animal Model of Aging: Implications for Alzheimer Disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 3181–3194. [Google Scholar] [CrossRef]
- Barone, E.; Mancuso, C. Biliverdin Reductase-A: A Novel Drug Target for Atorvastatin in a Dog Pre-Clinical Model of Alzheimer Disease. J. Neurochem. 2012, 120, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, H. Bilirubin Increases Insulin Sensitivity by Regulating Cholesterol Metabolism, Adipokines and PPARγ Levels. Sci. Rep. 2015, 5, 9886. [Google Scholar] [CrossRef] [Green Version]
- Jayanti, S.; Moretti, R. Bilirubin and Inflammation in Neurodegenerative and Other Neurological Diseases. Neuroimmunol. Neuroinflamm. 2020, 7, 92–108. [Google Scholar] [CrossRef]
- Di Domenico, F.; Barone, E. HO-1/BVR-a System Analysis in Plasma from Probable Alzheimer’s Disease and Mild Cognitive Impairment Subjects: A Potential Biochemical Marker for the Prediction of the Disease. J. Alzheimer’s Dis. 2012, 32, 277–289. [Google Scholar] [CrossRef]
- Feigin, V.L.; Abajobir, A.A. Global, Regional, and National Burden of Neurological Disorders during 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [Green Version]
- Mao, Q.; Qin, W. Recent Advances in Dopaminergic Strategies for the Treatment of Parkinson’s Disease. Acta Pharmacol. Sin. 2020, 41, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Oxidative Stress in Parkinson’s Disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar]
- Tanner, C.M.; Kamel, F. Rotenone, Paraquat, and Parkinson’s Disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schapira, A.H.; Cooper, J.M. Mitochondrial Complex I Deficiency in Parkinson’s Disease. Lancet 1989, 1, 1269. [Google Scholar] [CrossRef]
- Jayanti, S.; Moretti, R. Bilirubin: A Promising Therapy for Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 6223. [Google Scholar] [CrossRef]
- Yoo, M.S.; Chun, H.S. Oxidative Stress Regulated Genes in Nigral Dopaminergic Neuronal Cells: Correlation with the Known Pathology in Parkinson’s Disease. Mol. Brain Res. 2003, 110, 76–84. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L. Association of Serum Indirect Bilirubin Concentrations with Motor Subtypes of Parkinson’s Disease. Neurodegener. Dis. 2019, 19, 155–162. [Google Scholar] [CrossRef]
- Hatano, T.; Saiki, S. Identification of Novel Biomarkers for Parkinson’s Disease by Metabolomic Technologies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Scigliano, G.; Girotti, F. Increased Plasma Bilirubin in Parkinson Patients on L-Dopa: Evidence against the Free Radical Hypothesis? Ital. J. Neurol. Sci. 1997, 18, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Kataura, T.; Saiki, S. BRUP-1, an Intracellular Bilirubin Modulator, Exerts Neuroprotective Activity in a Cellular Parkinson’s Disease Model. J. Neurochem. 2020, 155, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Jayanti, S.; Moretti, R. Bilirubin Prevents the TH+ Dopaminergic Neuron Loss in a Parkinson’s Disease Model by Acting on TNF-α. Int. J. Mol. Sci. 2022, 23, 14276. [Google Scholar] [CrossRef]
- Gao, H.-M.; Zhou, H. Oxidative Stress, Neuroinflammation, and Neurodegeneration. In Neuroinflammation and Neurodegeneration; Peterson, P.K., Toborek, M., Eds.; Springer: New York, NY, USA, 2014; pp. 81–104. [Google Scholar] [CrossRef]
- Schipper, H.M.; Song, W. Heme Oxygenase-1 and Neurodegeneration: Expanding Frontiers of Engagement. J. Neurochem. 2009, 110, 469–485. [Google Scholar] [CrossRef]
- Schipper, H.M. Brain Iron Deposition and the Free Radical-Mitochondrial Theory of Ageing. Ageing Res. Rev. 2004, 3, 265–301. [Google Scholar] [CrossRef]
- Schipper, H.M. Heme Oxygenase-1: Role in Brain Aging and Neurodegeneration. Exp. Gerontol. 2000, 35, 821–830. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Pegoretti, V.; Swanson, K.A. Inflammation and Oxidative Stress in Multiple Sclerosis: Consequences for Therapy Development. Oxidative Med. Cell. Longev. 2020, 2020, e7191080. [Google Scholar] [CrossRef]
- van Horssen, J.; Schreibelt, G. Severe Oxidative Damage in Multiple Sclerosis Lesions Coincides with Enhanced Antioxidant Enzyme Expression. Free Radic. Biol. Med. 2008, 45, 1729–1737. [Google Scholar] [CrossRef]
- Haider, L.; Fischer, M.T. Oxidative Damage in Multiple Sclerosis Lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendiola, A.S.; Ryu, J.K. Transcriptional Profiling and Therapeutic Targeting of Oxidative Stress in Neuroinflammation. Nat. Immunol. 2020, 21, 513–524. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, B. Heme Oxygenase-1 Plays an Important Protective Role in Experimental Autoimmune Encephalomyelitis. Neuroreport 2001, 12, 1841–1845. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J. Biliverdin Reductase, a Major Physiologic Cytoprotectant, Suppresses Experimental Autoimmune Encephalomyelitis. Free Radic. Biol. Med. 2006, 40, 960–967. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, B. Bilirubin as a Potent Antioxidant Suppresses Experimental Autoimmune Encephalomyelitis: Implications for the Role of Oxidative Stress in the Development of Multiple Sclerosis. J. Neuroimmunol. 2003, 139, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Armon-Omer, A.; Waldman, C. New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients. Nutrients 2019, 11, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljubisavljevic, S.; Stojanovic, I. Association of Serum Bilirubin and Uric Acid Levels Changes during Neuroinflammation in Patients with Initial and Relapsed Demyelination Attacks. Metab. Brain Dis. 2013, 28, 629–638. [Google Scholar] [CrossRef]
- van Es, M.A.; Hardiman, O. Amyotrophic Lateral Sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A. Amyotrophic Lateral Sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Goutman, S.A.; Hardiman, O. Recent Advances in the Diagnosis and Prognosis of Amyotrophic Lateral Sclerosis. Lancet Neurol. 2022, 21, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Motataianu, A.; Serban, G. Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors. Int. J. Mol. Sci. 2022, 23, 9339. [Google Scholar] [CrossRef] [PubMed]
- Calingasan, N.Y.; Chen, J. β-Amyloid 42 Accumulation in the Lumbar Spinal Cord Motor Neurons of Amyotrophic Lateral Sclerosis Patients. Neurobiol. Dis. 2005, 19, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Browne, S.E. Evidence of Increased Oxidative Damage in Both Sporadic and Familial Amyotrophic Lateral Sclerosis. J. Neurochem. 1997, 69, 2064–2074. [Google Scholar] [CrossRef]
- Smith, R.G.; Henry, Y.K. Presence of 4-Hydroxynonenal in Cerebrospinal Fluid of Patients with Sporadic Amyotrophic Lateral Sclerosis. Ann. Neurol. 1998, 44, 696–699. [Google Scholar] [CrossRef]
- Ihara, Y.; Nobukuni, K. Oxidative Stress and Metal Content in Blood and Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients with and without a Cu, Zn-Superoxide Dismutase Mutation. Neurol. Res. 2005, 27, 105–108. [Google Scholar] [CrossRef]
- Mitsumoto, H.; Santella, R.M. Oxidative Stress Biomarkers in Sporadic ALS. Amyotroph. Lateral Scler. 2008, 9, 177–183. [Google Scholar] [CrossRef]
- Zuo, X.; Zhou, J. TDP-43 Aggregation Induced by Oxidative Stress Causes Global Mitochondrial Imbalance in ALS. Nat. Struct. Mol. Biol. 2021, 28, 132–142. [Google Scholar] [CrossRef]
- Hoang, T.T.; Johnson, D.A. Angiogenin Activates the Astrocytic Nrf2/Antioxidant-Response Element Pathway and Thereby Protects Murine Neurons from Oxidative Stress. J. Biol. Chem. 2019, 294, 15095–15103. [Google Scholar] [CrossRef]
- Sheng, J.; Xu, Z. Three Decades of Research on Angiogenin: A Review and Perspective. Acta Biochim. Biophys. Sin. 2016, 48, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, C.L.; Boggio, K.J. A Loss of FUS/TLS Function Leads to Impaired Cellular Proliferation. Cell Death Dis. 2014, 5, e1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Guo, W. Mutant FUS Causes DNA Ligation Defects to Inhibit Oxidative Damage Repair in Amyotrophic Lateral Sclerosis. Nat. Commun. 2018, 9, 3683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Villegas, J.; Kirby, J. Dipeptide Repeat Pathology in C9orf72-ALS Is Associated with Redox, Mitochondrial and NRF2 Pathway Imbalance. Antioxidants 2022, 11, 1897. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Resch, J.M. Activation of the Nrf2–ARE Pathway in Muscle and Spinal Cord during ALS-like Pathology in Mice Expressing Mutant SOD1. Exp. Neurol. 2007, 207, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Velde, C.V.; McDonald, K.K. Misfolded SOD1 Associated with Motor Neuron Mitochondria Alters Mitochondrial Shape and Distribution Prior to Clinical Onset. PLoS ONE 2011, 6, e22031. [Google Scholar] [CrossRef]
- Bergström, P.; von Otter, M. Association of NFE2L2 and KEAP1 Haplotypes with Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 130–137. [Google Scholar] [CrossRef]
- Iłżecka, J.; Stelmasiak, Z. Serum Bilirubin Concentration in Patients with Amyotrophic Lateral Sclerosis. Clin. Neurol. Neurosurg. 2003, 105, 237–240. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; de Lago, E. New Statement about NRF2 in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Biomolecules 2022, 12, 1200. [Google Scholar] [CrossRef]
- Dwyer, B.E.; Lu, S.-Y. Heme Oxygenase in the Experimental ALS Mouse. Exp. Neurol. 1998, 150, 206–212. [Google Scholar] [CrossRef]
- Minj, E.; Upadhayay, S. Nrf2/HO-1 Signaling Activator Acetyl-11-Keto-Beta Boswellic Acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-Induced Experimental Model of ALS. Neurochem. Res. 2021, 46, 2867–2884. [Google Scholar] [CrossRef] [PubMed]
- Wyant, K.J.; Ridder, A.J. Huntington’s Disease—Update on Treatments. Curr. Neurol. Neurosci. Rep. 2017, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Lalonde, K. New Avenues for the Treatment of Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 8363. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Kumar, P. Antidepressants for Neuroprotection in Huntington’s Disease: A Review. Eur. J. Pharmacol. 2015, 769, 33–42. [Google Scholar] [CrossRef]
- Maiuri, T.; Suart, C.E. DNA Damage Repair in Huntington’s Disease and Other Neurodegenerative Diseases. Neurotherapeutics 2019, 16, 948–956. [Google Scholar] [CrossRef]
- Park, H.; Kam, T.-I. Poly (ADP-Ribose) (PAR)-Dependent Cell Death in Neurodegenerative Diseases. Int. Rev. Cell Mol. Biol. 2020, 353, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Dickey, A.S.; Pineda, V.V. PPAR-δ Is Repressed in Huntington’s Disease, Is Required for Normal Neuronal Function and Can Be Targeted Therapeutically. Nat. Med. 2016, 22, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Albertz, J. Neuroprotective Effects of PPAR-γ Agonist Rosiglitazone in N171-82Q Mouse Model of Huntington’s Disease. J. Neurochem. 2013, 125, 410–419. [Google Scholar] [CrossRef]
- Corona, J.C.; Duchen, M.R. PPARγ as a Therapeutic Target to Rescue Mitochondrial Function in Neurological Disease. Free Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Kiaei, M. Peroxisome Proliferator-Activated Receptor-gamma in Amyotrophic Lateral Sclerosis and Huntington’s Disease. PPAR Res. 2008, 2008, e418765. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-T.; Liao, C.-K. Ligands of Peroxisome Proliferator-Activated Receptor-Alpha Promote Glutamate Transporter-1 Endocytosis in Astrocytes. Int. J. Biochem. Cell Biol. 2017, 86, 42–53. [Google Scholar] [CrossRef]
- Wójtowicz, S.; Strosznajder, A.K. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.M.; Neifer, K.L. Bilirubin Remodels Murine White Adipose Tissue by Reshaping Mitochondrial Activity and the Coregulator Profile of Peroxisome Proliferator-Activated Receptor α. J. Biol. Chem. 2020, 295, 9804–9822. [Google Scholar] [CrossRef]
- Vítek, L. Bilirubin as a Signaling Molecule. Med. Res. Rev. 2020, 40, 1335–1351. [Google Scholar] [CrossRef]
- Khan, A.; Jamwal, S. Neuroprotective Effect of Hemeoxygenase-1/Glycogen Synthase Kinase-3β Modulators in 3-Nitropropionic Acid-Induced Neurotoxicity in Rats. Neuroscience 2015, 287, 66–77. [Google Scholar] [CrossRef]
- Chin, K.S.; Teodorczuk, A. Dementia with Lewy Bodies: Challenges in the Diagnosis and Management. Aust. N. Z. J. Psychiatry 2019, 53, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Mukaetova-Ladinska, E.B.; Monteith, R. Cerebrospinal Fluid Biomarkers for Dementia with Lewy Bodies. Int. J. Alzheimer’s Dis. 2010, 2010, 536538. [Google Scholar] [CrossRef] [Green Version]
- McKeith, I.G.; Burn, D.J. Dementia with Lewy Bodies. Semin. Clin. Neuropsychiatry 2003, 8, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lyras, L.; Perry, R.H. Oxidative Damage to Proteins, Lipids, and DNA in Cortical Brain Regions from Patients with Dementia with Lewy Bodies. J. Neurochem. 1998, 71, 302–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalfó, E.; Portero-Otín, M. Evidence of Oxidative Stress in the Neocortex in Incidental Lewy Body Disease. J. Neuropathol. Exp. Neurol. 2005, 64, 816–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, A.; Boveris, A. Human Brain Cortex: Mitochondrial Oxidative Damage and Adaptive Response in Parkinson Disease and in Dementia with Lewy Bodies. Free Radic. Biol. Med. 2009, 46, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liao, Y. Abnormal Serum Bilirubin/Albumin Concentrations in Dementia Patients With Aβ Deposition and the Benefit of Intravenous Albumin Infusion for Alzheimer’s Disease Treatment. Front. Neurosci. 2020, 14, 859. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Novotny, L. Decreased Levels of Advanced Glycation End-Products in Patients with Gilbert Syndrome. Cell. Mol. Biol. 2005, 51, 387–392. [Google Scholar] [PubMed]
- Grinberg, L.T.; Heinsen, H. Toward a Pathological Definition of Vascular Dementia. J. Neurol. Sci. 2010, 299, 136–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, J.T.; Thomas, A. Vascular Dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabir, O.; Berwick, J. Neurovascular Dysfunction in Vascular Dementia, Alzheimer’s and Atherosclerosis. BMC Neurosci. 2018, 19, 62. [Google Scholar] [CrossRef] [Green Version]
- Casado, Á.; López-Fernández, M.E. Lipid Peroxidation and Antioxidant Enzyme Activities in Vascular and Alzheimer Dementias. Neurochem. Res. 2008, 33, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Gackowski, D.; Rozalski, R. Oxidative Stress and Oxidative DNA Damage Is Characteristic for Mixed Alzheimer Disease/Vascular Dementia. J. Neurol. Sci. 2008, 266, 57–62. [Google Scholar] [CrossRef]
- Gustavsson, A.-M.; van Westen, D. Midlife Atherosclerosis and Development of Alzheimer or Vascular Dementia. Ann. Neurol. 2020, 87, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Vítek, L.; Jirsa, M. Gilbert Syndrome and Ischemic Heart Disease: A Protective Effect of Elevated Bilirubin Levels. Atherosclerosis 2002, 160, 449–456. [Google Scholar] [CrossRef]
- Boon, A.-C.; Hawkins, C.L. Reduced Circulating Oxidized LDL Is Associated with Hypocholesterolemia and Enhanced Thiol Status in Gilbert Syndrome. Free Radic. Biol. Med. 2012, 52, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Pjanic, M. TCF21 and the Environmental Sensor Aryl-Hydrocarbon Receptor Cooperate to Activate a pro-Inflammatory Gene Expression Program in Coronary Artery Smooth Muscle Cells. PLoS Genet. 2017, 13, e1006750. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Meng, Q. Aryl Hydrocarbon Receptor Pathway: Role, Regulation and Intervention in Atherosclerosis Therapy (Review). Mol. Med. Rep. 2019, 20, 4763–4773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelan, D.; Winter, G.M. Activation of the Ah Receptor Signal Transduction Pathway by Bilirubin and Biliverdin. Arch. Biochem. Biophys. 1998, 357, 155–163. [Google Scholar] [CrossRef]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Longhi, M.S.; Vuerich, M. Bilirubin Suppresses Th17 Immunity in Colitis by Upregulating CD39. JCI Insight 2017, 2, e92791. [Google Scholar] [CrossRef] [Green Version]
- Charlson, F.J.; Ferrari, A.J. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef]
- Garrison, J.R.; Fernandez-Egea, E. Reality Monitoring Impairment in Schizophrenia Reflects Specific Prefrontal Cortex Dysfunction. Neuroimage Clin. 2017, 14, 260–268. [Google Scholar] [CrossRef]
- Krishnan, R.R.; Kraus, M.S. Comprehensive Model of How Reality Distortion and Symptoms Occur in Schizophrenia: Could Impairment in Learning-Dependent Predictive Perception Account for the Manifestations of Schizophrenia? Psychiatry Clin. Neurosci. 2011, 65, 305–317. [Google Scholar] [CrossRef]
- Lin, C.-H.; Huang, C.-L. Clinical Symptoms, Mainly Negative Symptoms, Mediate the Influence of Neurocognition and Social Cognition on Functional Outcome of Schizophrenia. Schizophr. Res. 2013, 146, 231–237. [Google Scholar] [CrossRef]
- Li, S.-B.; Liu, C. Revisiting the Latent Structure of Negative Symptoms in Schizophrenia: Evidence from Two Second-Generation Clinical Assessments. Schizophr. Res. 2022, 248, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Rogers, J.C. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry 2021, 12, 703452. [Google Scholar]
- Ermakov, E.A.; Dmitrieva, E.M. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 8881770. [Google Scholar] [CrossRef] [PubMed]
- Maas, D.A.; Vallès, A. Oxidative Stress, Prefrontal Cortex Hypomyelination and Cognitive Symptoms in Schizophrenia. Transl. Psychiatry 2017, 7, e1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okusaga, O.O. Accelerated Aging in Schizophrenia Patients: The Potential Role of Oxidative Stress. Aging Dis. 2014, 5, 256–262. [Google Scholar] [CrossRef]
- Bitanihirwe, B.K.Y.; Woo, T.-U.W. Oxidative Stress in Schizophrenia: An Integrated Approach. Neurosci. Biobehav. Rev. 2011, 35, 878–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flatow, J.; Buckley, P. Meta-Analysis of Oxidative Stress in Schizophrenia. Biol. Psychiatry 2013, 74, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Więdłocha, M.; Zborowska, N. Oxidative Stress Biomarkers among Schizophrenia Inpatients. Brain Sci. 2023, 13, 490. [Google Scholar] [CrossRef]
- Bošković, M.; Vovk, T. Oxidative Stress in Schizophrenia. Curr. Neuropharmacol. 2011, 9, 301–312. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, P. Cortical Interneurons, Immune Factors and Oxidative Stress as Early Targets for Schizophrenia. Eur. J. Neurosci. 2012, 35, 1866–1870. [Google Scholar] [CrossRef]
- Dornelles, E.P.; Marques, J.G. Unconjugated Bilirubin and Schizophrenia: A Systematic Review. CNS Spectr. 2019, 24, 577–588. [Google Scholar] [CrossRef]
- Becklén, M.; Orhan, F. Plasma Bilirubin Levels Are Reduced in First-Episode Psychosis Patients and Associates to Working Memory and Duration of Untreated Psychosis. Sci. Rep. 2021, 11, 7527. [Google Scholar] [CrossRef] [PubMed]
- Gama Marques, J.; Ouakinin, S. Clinical Profile in Schizophrenia and Schizoaffective Spectrum: Relation with Unconjugated Bilirubin in a Prospective and Controlled Study with Psychopathological and Psychosocial Variables. CNS Spectr. 2020, 25, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, T.; Seno, H. Schizophrenia-Associated Idiopathic Unconjugated Hyperbilirubinemia (Gilbert’s Syndrome). J. Clin. Psychiatry 2000, 61, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Vítek, L.; Novotná, M. Serum Bilirubin Levels and UGT1A1 Promoter Variations in Patients with Schizophrenia. Psychiatry Res. 2010, 178, 449–450. [Google Scholar] [CrossRef]
- Rodrigues, C.M.P.; Solá, S. Perturbation of Membrane Dynamics in Nerve Cells as an Early Event during Bilirubin-Induced Apoptosis. J. Lipid Res. 2002, 43, 885–894. [Google Scholar] [CrossRef]
- Brito, M.A.; Brites, D. A Link between Hyperbilirubinemia, Oxidative Stress and Injury to Neocortical Synaptosomes. Brain Res. 2004, 1026, 33–43. [Google Scholar] [CrossRef]
- Rodrigues, C.M.P.; Solá, S. Bilirubin Directly Disrupts Membrane Lipid Polarity and Fluidity, Protein Order, and Redox Status in Rat Mitochondria. J. Hepatol. 2002, 36, 335–341. [Google Scholar] [CrossRef]
- Ercan, I.; Cilaker Micili, S. Bilirubin Induces Microglial NLRP3 Inflammasome Activation in Vitro and in Vivo. Mol. Cell. Neurosci. 2023, 125, 103850. [Google Scholar] [CrossRef]
- Li, Y.; Huang, B. Physiological Concentrations of Bilirubin Control Inflammatory Response by Inhibiting NF-ΚB and Inflammasome Activation. Int. Immunopharmacol. 2020, 84, 106520. [Google Scholar] [CrossRef]
- Bora, E.; Murray, R.M. Meta-Analysis of Cognitive Deficits in Ultra-High Risk to Psychosis and First-Episode Psychosis: Do the Cognitive Deficits Progress over, or after, the Onset of Psychosis? Schizophr. Bull. 2014, 40, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, J.L.; Giedd, J.N. Neurodevelopmental Model of Schizophrenia: Update 2012. Mol. Psychiatry 2012, 17, 1228–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maimburg, R.D.; Væth, M. Neonatal Jaundice: A Risk Factor for Infantile Autism? Paediatr. Perinat. Epidemiol. 2008, 22, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, L.; Launes, J. Adult Neurobehavioral Outcome of Hyperbilirubinemia in Full Term Neonates—A 30 Year Prospective Follow-up Study. PeerJ 2014, 2, e294. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, S.T.; Finne, P.H. Males with Neonatal Hyperbilirubinemia Examined at 18 Years of Age. Acta Paediatr. 1984, 73, 176–180. [Google Scholar] [CrossRef]
- Ozmert, E.; Erdem, G. Long-Term Follow-up of Indirect Hyperbilirubinemia in Full-Term Turkish Infants. Acta Paediatr. 1996, 85, 1440–1444. [Google Scholar] [CrossRef]
- Amin, S.B.; Smith, T. Developmental Influence of Unconjugated Hyperbilirubinemia and Neurobehavioral Disorders. Pediatr. Res. 2019, 85, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Gunn, C.H. HEREDITARY ACHOLURIC JAUNDICE in a New Mutant Strain of Rats. J. Hered. 1938, 29, 137–139. [Google Scholar] [CrossRef]
- Hayashida, M.; Miyaoka, T. Hyperbilirubinemia-Related Behavioral and Neuropathological Changes in Rats: A Possible Schizophrenia Animal Model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 581–588. [Google Scholar] [CrossRef]
- Liaury, K.; Miyaoka, T. Morphological Features of Microglial Cells in the Hippocampal Dentate Gyrus of Gunn Rat: A Possible Schizophrenia Animal Model. J. Neuroinflamm. 2012, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Llido, J.P.; Fioriti, E. Bilirubin-Induced Transcriptomic Imprinting in Neonatal Hyperbilirubinemia. Biology 2023, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Reddy-Thootkur, M.; Kraguljac, N.V. The Role of Glutamate and GABA in Cognitive Dysfunction in Schizophrenia and Mood Disorders—A Systematic Review of Magnetic Resonance Spectroscopy Studies. Schizophr. Res. 2022, 249, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.C.; McCollum, L.A. Ultrastructural Evidence for Glutamatergic Dysregulation in Schizophrenia. Schizophr. Res. 2022, 249, 4–15. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Shapiro, S.M. Role of Glutamate Receptor-Mediated Excitotoxicity in Bilirubin-Induced Brain Injury in the Gunn Rat Model. Exp. Neurol. 1998, 150, 21–29. [Google Scholar] [CrossRef]
- Cayabyab, R.; Ramanathan, R. High Unbound Bilirubin for Age: A Neurotoxin with Major Effects on the Developing Brain. Pediatr. Res. 2019, 85, 183–190. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N. Excitotoxicity, Calcium and Mitochondria: A Triad in Synaptic Neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef]
- Ashizawa, T.; Xia, G. Ataxia. Continuum 2016, 22, 1208–1226. [Google Scholar] [CrossRef]
- de Silva, R.N.; Vallortigara, J. Diagnosis and Management of Progressive Ataxia in Adults. Pract. Neurol. 2019, 19, 196–207. [Google Scholar] [CrossRef]
- Kinoshita, C.; Kubota, N. Glutathione Depletion and MicroRNA Dysregulation in Multiple System Atrophy: A Review. Int. J. Mol. Sci. 2022, 23, 15076. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, Y. Oxidative Stress and Environmental Exposures Are Associated with Multiple System Atrophy in Chinese Patients. Can. J. Neurol. Sci. 2016, 43, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Parsons, D.W.; Jones, S. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef] [Green Version]
- McLendon, R.; Friedman, A. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Jha, S.K. Antioxidants in Brain Tumors: Current Therapeutic Significance and Future Prospects. Mol. Cancer 2022, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.B.; Christmann, M. Alterations in Molecular Profiles Affecting Glioblastoma Resistance to Radiochemotherapy: Where Does the Good Go? Cancers 2022, 14, 2416. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Expósito, M.J.; Martínez-Martos, J.M. The Delicate Equilibrium between Oxidants and Antioxidants in Brain Glioma. Curr. Neuropharmacol. 2019, 17, 342–351. [Google Scholar] [CrossRef]
- Conti, A.; Gulì, C. Role of Inflammation and Oxidative Stress Mediators in Gliomas. Cancers 2010, 2, 693–712. [Google Scholar] [CrossRef] [Green Version]
- Alghamri, M.S.; McClellan, B.L. Targeting Neuroinflammation in Brain Cancer: Uncovering Mechanisms, Pharmacological Targets, and Neuropharmaceutical Developments. Front. Pharmacol. 2021, 12, 680021. [Google Scholar]
- Atukeren, P.; Oner, S. Oxidant and Anti-Oxidant Status in Common Brain Tumors: Correlation to TP53 and Human Biliverdin Reductase. Clin. Neurol. Neurosurg. 2017, 158, 72–76. [Google Scholar] [CrossRef]
- Kim, S.S.; Seong, S. Biliverdin Reductase Plays a Crucial Role in Hypoxia-Induced Chemoresistance in Human Glioblastoma. Biochem. Biophys. Res. Commun. 2013, 440, 658–663. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Abate, C. Validation of Thiosemicarbazone Compounds as P-Glycoprotein Inhibitors in Human Primary Brain–Blood Barrier and Glioblastoma Stem Cells. Mol. Pharm. 2019, 16, 3361–3373. [Google Scholar] [CrossRef]
- Alves, A.L.V.; Gomes, I.N.F. Role of Glioblastoma Stem Cells in Cancer Therapeutic Resistance: A Perspective on Antineoplastic Agents from Natural Sources and Chemical Derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef]
- Gazzin, S.; Berengeno, A.L. Modulation of Mrp1 (ABCc1) and Pgp (ABCb1) by Bilirubin at the Blood-CSF and Blood-Brain Barriers in the Gunn Rat. PLoS ONE 2011, 6, e16165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Ling, Z. Unconjugated Bilirubin Elevation Impairs the Function and Expression of Breast Cancer Resistance Protein (BCRP) at the Blood-Brain Barrier in Bile Duct-Ligated Rats. Acta Pharmacol. Sin. 2016, 37, 1129–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Yu, X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-Related Diseases. Stem Cell Rev. Rep. 2022, 18, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D. Telomere Dysfunction in Ageing and Age-Related Diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chen, Q. Association of Serum Total Bilirubin Concentration with Telomere Length: The National Health and Nutrition Examination Survey. Oxid. Med. Cell. Longev. 2021, 2021, 4688900. [Google Scholar] [CrossRef]
- Tosevska, A.; Moelzer, C. Longer Telomeres in Chronic, Moderate, Unconjugated Hyperbilirubinaemia: Insights from a Human Study on Gilbert’s Syndrome. Sci. Rep. 2016, 6, 22300. [Google Scholar] [CrossRef] [Green Version]
- Wallner, M.; Blassnigg, S.M. Effects of Unconjugated Bilirubin on Chromosomal Damage in Individuals with Gilbert`s Syndrome Measured with the Micronucleus Cytome Assay. Mutagenesis 2012, 27, 731–735. [Google Scholar] [CrossRef] [Green Version]
- Croft, K.D.; Zhang, D. Structural Requirements of Flavonoids to Induce Heme Oxygenase-1 Expression. Free Radic. Biol. Med. 2017, 113, 165–175. [Google Scholar] [CrossRef]
- Smith, A.; Alam, J. Regulation of Heme Oxygenase and Metallothionein Gene Expression by the Heme Analogs, Cobalt-, and Tin-Protoporphyrin. J. Biol. Chem. 1993, 268, 7365–7371. [Google Scholar] [CrossRef]
- Mancuso, C.; Barone, E. The Heme Oxygenase/Biliverdin Reductase Pathway in Drug Research and Development. Curr. Drug Metab. 2009, 10, 579–594. Available online: https://www.eurekaselect.com/70167/article (accessed on 27 July 2020). [CrossRef]
- Kim, J.S.; Yoon, T.-J. Toxicity and Tissue Distribution of Magnetic Nanoparticles in Mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Petters, C.; Irrsack, E. Uptake and Metabolism of Iron Oxide Nanoparticles in Brain Cells. Neurochem. Res. 2014, 39, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.M.; Tarini, D. Nanoparticle-Based Technology Approaches to the Management of Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 6070. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, Y. PEGylated Bilirubin Nanoparticle as an Anti-Oxidative and Anti-Inflammatory Demulcent in Pancreatic Islet Xenotransplantation. Biomaterials 2017, 133, 242–252. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Thomsen, M.S. Targeted Drug Delivery to the Brain Using Magnetic Nanoparticles. Ther. Deliv. 2015, 6, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Ficiarà, E.; Ansari, S.A. Beyond Oncological Hyperthermia: Physically Drivable Magnetic Nanobubbles as Novel Multipurpose Theranostic Carriers in the Central Nervous System. Molecules 2020, 25, 2104. [Google Scholar] [CrossRef]
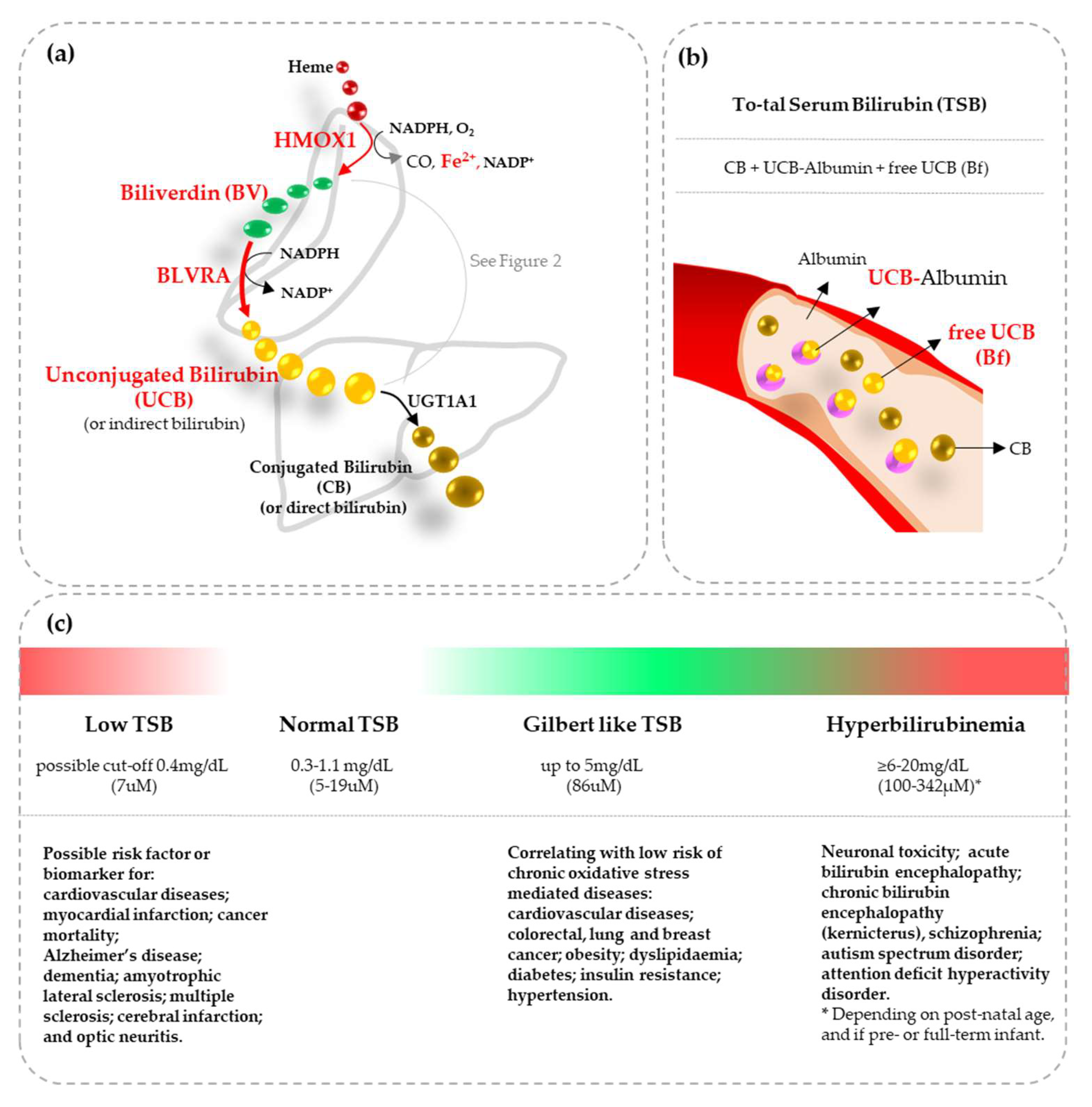
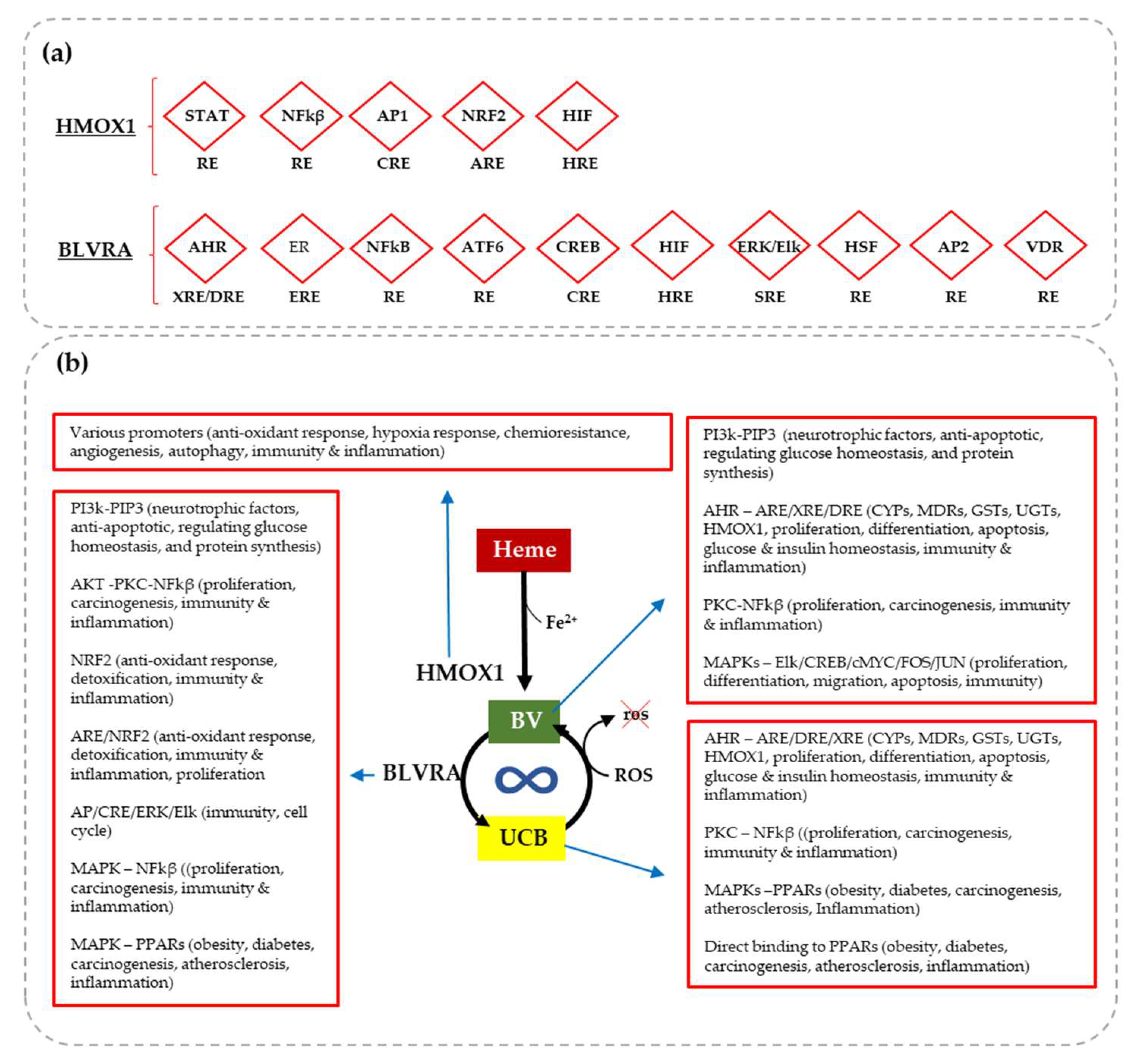
| Heme | HMOX | Fe2+ | BV | BLVR | UCB | |
|---|---|---|---|---|---|---|
| Changes During Disease | Accumulating in the site of lesion | Usually induced (chemical induction, inhibition, and Ko models frequently used to assess its biologic and pathologic functions) | Increased as part of BBB breakdown, hemorrhage and HMXO1 induction. | Rarely quantified. Suddenly added to model of diseases to assess its functions. | Usually induced (with possible induction of defects in its enzymatic activity in high redox stress environment). Fewer chemical inducers/inhibitors are available to assess its functions. KO models are seldom used for this purpose. | TSB: both increased and decreased. Supposed to be increased if HMXO and BLVR induced Seldom added to models of diseases to assess its functions. |
| Target and Effect | Protective Reducing apoptosis and inducing SOD and HMOX1, mitochondrial functions and cytochrome C release, and ferritin production [30,31,137]. Enhancing redox stress and heme release, protein and lipid oxidation, metalloproteinases release and tissue damage, inhibiting the antioxidant response through NRF2, and impairing the proteasome and unfolded protein response, inducing mitochondrial dysfunctions and mitophagy and apoptosis (Frederic ataxia, posterior column ataxia, neurodegenerative diseases) [32,37]. | Protective Reducing redox stress, increasing survival, inducing the transcription of the stress response genes, reducing lipid peroxidation [89] and inducing the synthesis and release of GSH [137]. Promoting proliferation and neuronal survival via PI3K/Akt/BDNF signaling, even migrating into the nuclei and acting as a transcription factor [9,11] (AD, PD, ischemia, HD [38]). Improving glutamate neurotoxicity, mitochondrial damage [137]. Antioxidant (by producing BV, UCB, and acting as a transcriptional factor [9,11]). Potentially dangerous if excessively induced (AD, PD, SCZ, Stroke, trauma [3,60]). Increasing cholesterol and products of cholesterol oxidation [99]. Increasing Fe2+ production in turn enhancing DNA damage, cell bioenergetic failure, mitophagy and autophagy, oxidizing catecholamine [3,60]. | Damaging Worsening redox stress, enhancing protein and lipid oxidation, and DNA damage. Reducing SOD activity, inducing a cell bioenergetic failure, apoptosis, neuronal autophagy, damaging the BBB (via NFkβ, AP1) [32,89]. | Protective Levering DNA damage (possibly by scavenging ROS directly or after conversion into UCB [27]), inducing BLVR translocation into nucleus [9,11], with multiple anti-inflammatory actions [9,11]. | Protective Protective in meningioma and glioma [220], and EAE [98]. Modulating Tau deposition [43]; enhancing neuronal and synaptic plasticity (MAPK/PI3k) [60], Reducing apoptosis (MAPK/Akt [9,11]) Activating the stress responses gene (including HMOX) [29], ameliorating insulin brain resistance [70]. Inducing chemoresistance [211]. Missed Protection Missed protection in AD (gene up, activity down [60,68,73]). | Protective Protective (EAE, PD, stroke, ischemia, traumatic brain injury, cerebral atherosclerosis, glioma, etc. [11,51,86,99]). Activating the antioxidant response (NRF2 [29]); boosting survival and repair (AKT/CREB/BDNF [9,11]); increasing mitochondrial respiration, AMPA and Ca channels [11]; enhancing the transcription of the detoxification system (CYPs, UGT, by MAPK/NRF2) [11,156], inhibiting NMDA excitotoxicity and related neuronal death [28] Damaging Responsible for acute and chronic bilirubin encephalopathy (kernicterus), and suggested increasing the risk of ADHD, SCZ, autism [194], by inducing a plethora of mechanism (among them oxidative stress, apoptosis, glutamate neurotoxicity, inflammation, epigenetic alterations of brain development, reduced myelinating, cell death, ca imbalance, etc. [49,194]). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llido, J.P.; Jayanti, S.; Tiribelli, C.; Gazzin, S. Bilirubin and Redox Stress in Age-Related Brain Diseases. Antioxidants 2023, 12, 1525. https://doi.org/10.3390/antiox12081525
Llido JP, Jayanti S, Tiribelli C, Gazzin S. Bilirubin and Redox Stress in Age-Related Brain Diseases. Antioxidants. 2023; 12(8):1525. https://doi.org/10.3390/antiox12081525
Chicago/Turabian StyleLlido, John Paul, Sri Jayanti, Claudio Tiribelli, and Silvia Gazzin. 2023. "Bilirubin and Redox Stress in Age-Related Brain Diseases" Antioxidants 12, no. 8: 1525. https://doi.org/10.3390/antiox12081525
APA StyleLlido, J. P., Jayanti, S., Tiribelli, C., & Gazzin, S. (2023). Bilirubin and Redox Stress in Age-Related Brain Diseases. Antioxidants, 12(8), 1525. https://doi.org/10.3390/antiox12081525