Blb-NRF2-PON1 Cross-Talk in Abdominal Aortic Aneurysm Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.3. Statistical Analyses
3. Results
3.1. Comparisons
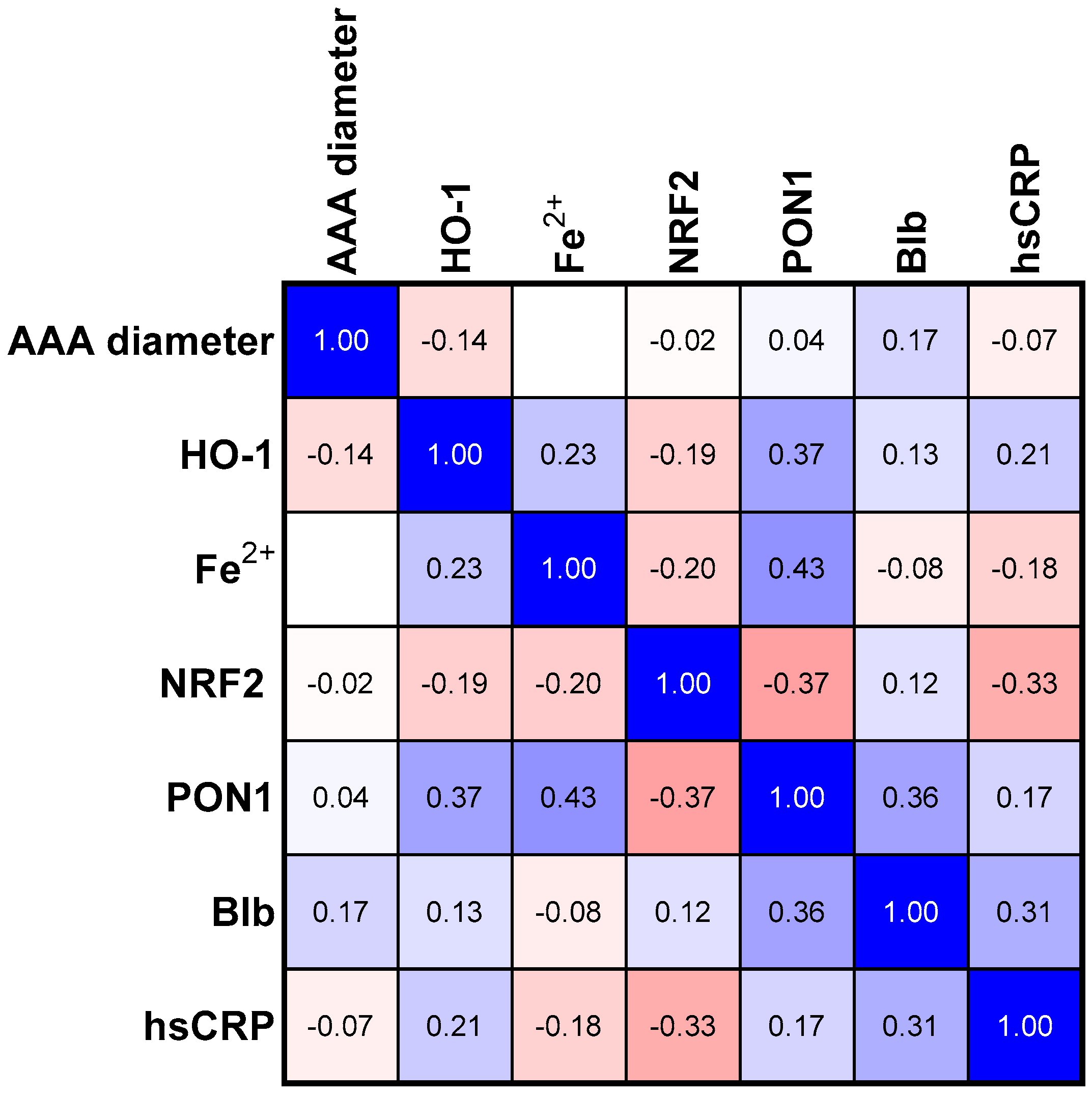
3.2. Correlations
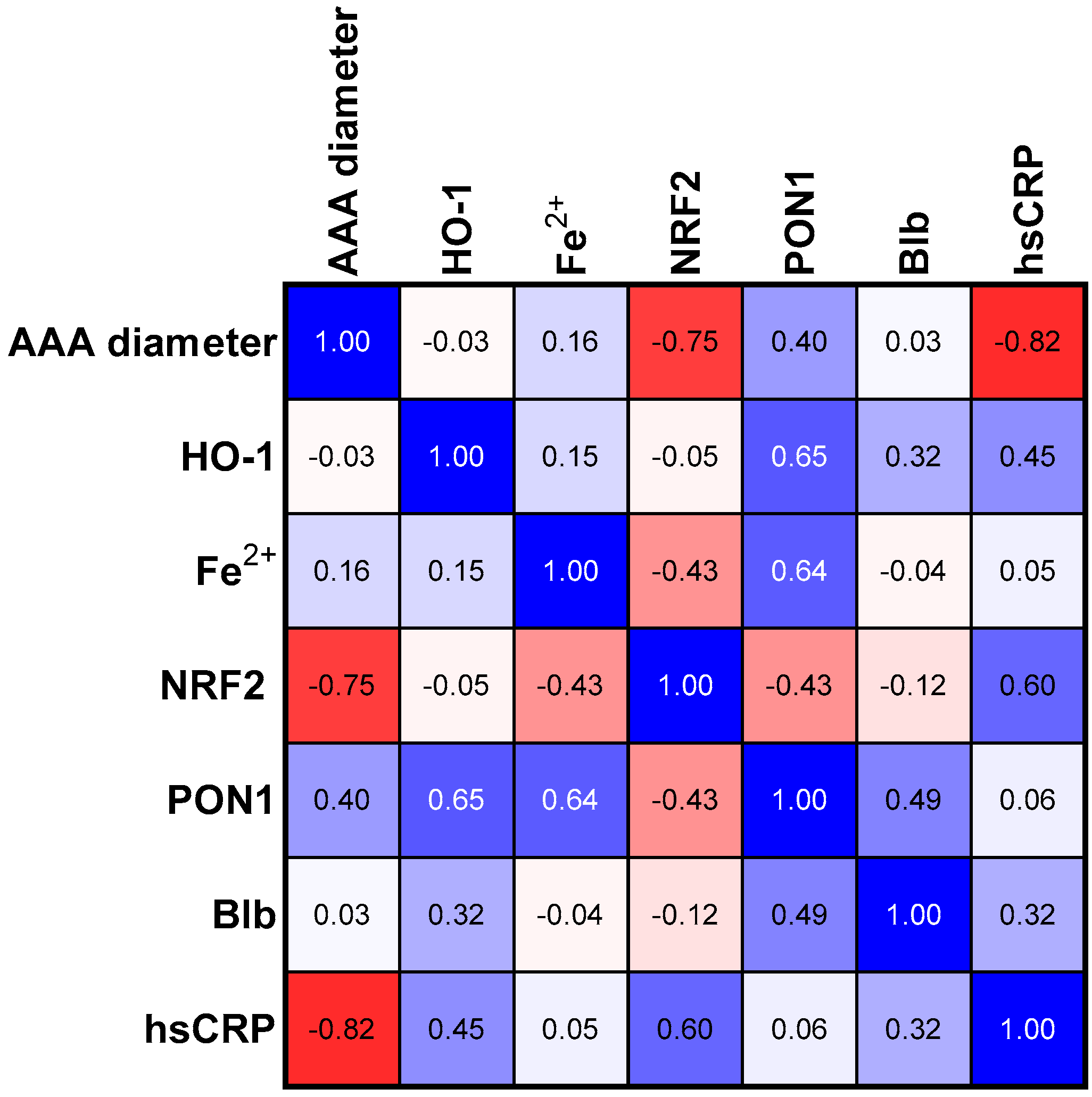
3.3. Correlations in SMALL Size Aneurism Group
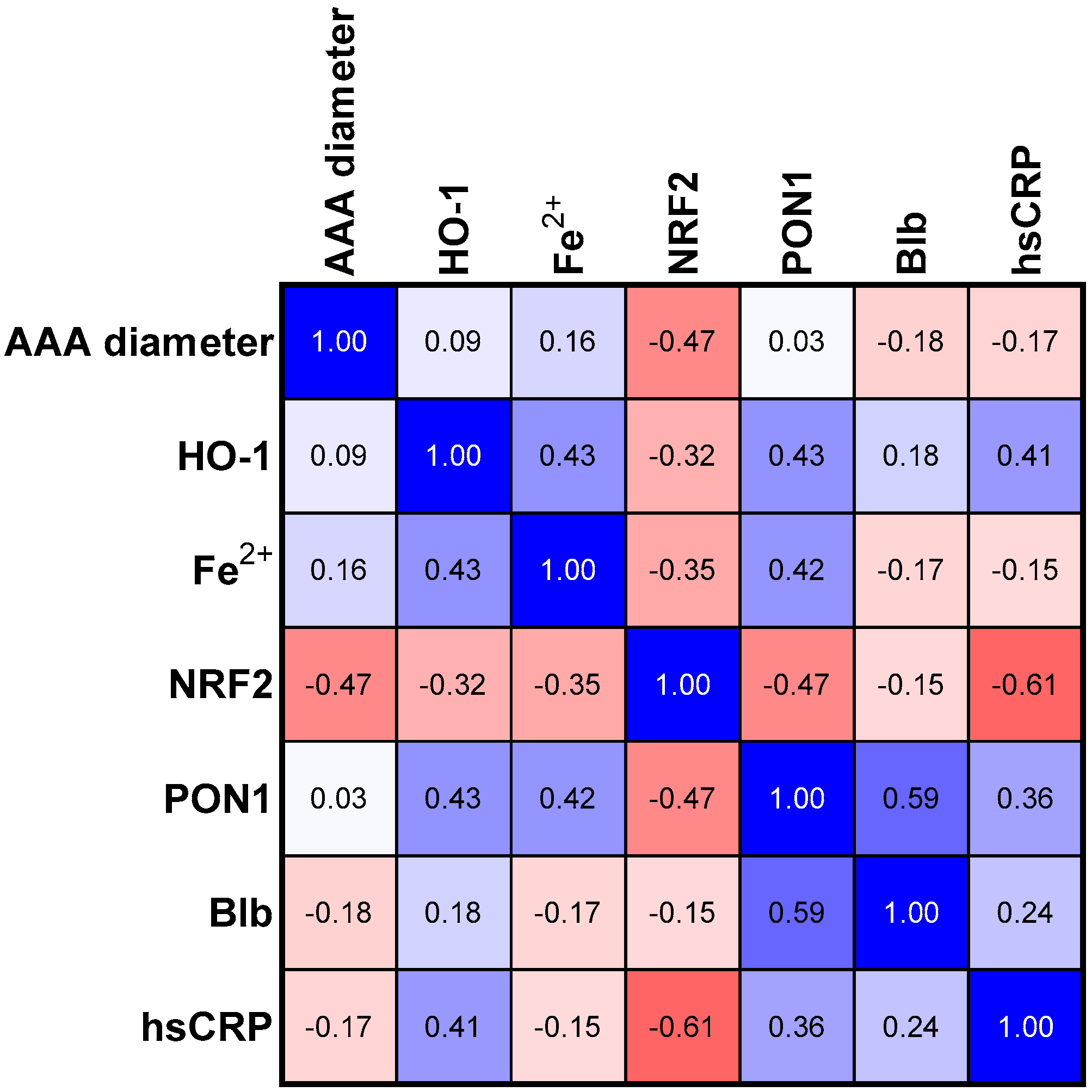
3.4. Correlations in Medium Size Aneurism Group
3.5. Correlations in Large Size Aneurism Group
3.6. Contingency Table for Bilirubin Cut-Off Values
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the Diagnosis and Treatment of Aortic Diseases: Document Covering Acute and Chronic Aortic Diseases of the Thoracic and Abdominal Aorta of the Adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, E.; Enea, I.; Zamboni, M.; Federici, M.; Bracale, U.M.; Sangiorgi, G.; Martelli, A.R.; Messina, T.; Settembrini, A.M. Focus on the Most Common Paucisymptomatic Vasculopathic Population, from Diagnosis to Secondary Prevention of Complications. Diagnostics 2023, 13, 2356. [Google Scholar] [CrossRef] [PubMed]
- Limet, R.; Sakalihassan, N.; Albert, A. Determination of the Expansion Rate and Incidence of Ruptur of Abdominal Aortic Aneurysms. J. Vasc. Surg. 1991, 14, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.; Lu, Y.; Wei, J.; Wu, J.; Yang, J.; Cai, Z. Abdominal Aortic Aneurysm: Roles of Inflammatory Cells. Front. Immunol. 2021, 11, 609161. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.; Suh, M.K.; Carson, J.S.; Dale, M.A.; Meisinger, T.M.; Fitzgerald, M.; Opperman, P.J.; Luo, J.; Pipinos, I.I.; Xiong, W.; et al. IL-1β (Interleukin-1β) and TNF-α (Tumor Necrosis Factor-α) Impact Abdominal Aortic Aneurysm Formation by Differential Effects on Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 457–463. [Google Scholar] [CrossRef]
- Sangiorgi, G.; Biondi-Zoccai, G.; Pizzuto, A.; Martelli, E. Commentary: Biochemical Markers for Diagnosis and Follow-up of Aortic Diseases: An Endless Search for the Holy Grail. J. Endovasc. Ther. 2019, 26, 836–842. [Google Scholar] [CrossRef]
- Gamberi, T.; Puglia, M.; Guidi, F.; Magherini, F.; Bini, L.; Marzocchini, R.; Modesti, A.; Modesti, P.A. A Proteomic Approach to Identify Plasma Proteins in Patients with Abdominal Aortic Aneurysm. Mol. BioSyst. 2011, 7, 2855. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Chen, Z.; Xu, F.; Zheng, Y. Proteomics Applications in Biomarker Discovery and Pathogenesis for Abdominal Aortic Aneurysm. Expert Rev. Proteom. 2021, 18, 305–314. [Google Scholar] [CrossRef]
- Serum Bilirubin Levels and the Presence and Progression of Abdominal Aortic Aneurysms-Ertan Vuruşkan, Erhan Saraçoğlu, İrfan Veysel Düzen. 2017. Available online: https://journals.sagepub.com/doi/abs/10.1177/0003319716660240 (accessed on 15 July 2023).
- Adin, C.A. Bilirubin as a Therapeutic Molecule: Challenges and Opportunities. Antioxidants 2021, 10, 1536. [Google Scholar] [CrossRef]
- Gazzin, S.; Vitek, L.; Watchko, J.; Shapiro, S.M.; Tiribelli, C. A Novel Perspective on the Biology of Bilirubin in Health and Disease. Trends Mol. Med. 2016, 22, 758–768. [Google Scholar] [CrossRef]
- Vítek, L.; Tiribelli, C. Bilirubin: The Yellow Hormone? J. Hepatol. 2021, 75, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Qaisiya, M.; Coda Zabetta, C.D.; Bellarosa, C.; Tiribelli, C. Bilirubin Mediated Oxidative Stress Involves Antioxidant Response Activation via Nrf2 Pathway. Cell. Signal. 2014, 26, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Sun, R.; Bao, S.; Chen, R.; Kou, L. Bilirubin Protects Transplanted Islets by Targeting Ferroptosis. Front. Pharmacol. 2020, 11, 907. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Chattopadhyay, A. Nrf2–ARE Signaling in Cellular Protection: Mechanism of Action and the Regulatory Mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/jcp.29219 (accessed on 24 January 2023). [CrossRef] [PubMed]
- Hofmann, A.; Müglich, M.; Wolk, S.; Khorzom, Y.; Sabarstinski, P.; Kopaliani, I.; Egorov, D.; Horn, F.; Brunssen, C.; Giebe, S.; et al. Induction of Heme Oxygenase-1 Is Linked to the Severity of Disease in Human Abdominal Aortic Aneurysm. J. Am. Heart Assoc. 2021, 10, e022747. [Google Scholar] [CrossRef]
- Smith, R.E.; Tran, K.; Smith, C.C.; McDonald, M.; Shejwalkar, P.; Hara, K. The Role of the Nrf2/ARE Antioxidant System in Preventing Cardiovascular Diseases. Diseases 2016, 4, 34. [Google Scholar] [CrossRef]
- Burillo, E.; Tarin, C.; Torres-Fonseca, M.-M.; Fernandez-García, C.-E.; Martinez-Pinna, R.; Martinez-Lopez, D.; Llamas-Granda, P.; Camafeita, E.; Lopez, J.A.; Vega de Ceniga, M.; et al. Paraoxonase-1 Overexpression Prevents Experimental Abdominal Aortic Aneurysm Progression. Clin. Sci. 2016, 130, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Cook, J.L. Transcriptional Regulation of the Heme Oxygenase-1 Gene Via the Stress Response Element Pathway. Curr. Pharm. Des. 2003, 9, 2499–2511. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Fernandez, P.L.; Mourao-Sa, D.S.; Porto, B.N.; Dutra, F.F.; Alves, L.S.; Oliveira, M.F.; Oliveira, P.L.; Graça-Souza, A.V.; Bozza, M.T. Characterization of Heme as Activator of Toll-like Receptor 4*. J. Biol. Chem. 2007, 282, 20221–20229. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, S.; Singh, U.; Jialal, I. The Evolving Role of C-Reactive Protein in Atherothrombosis. Clin. Chem. 2009, 55, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerşit, S.; Öcal, L.; Keskin, M.; Gürsoy, M.O.; Kalçik, M.; Bayam, E.; Karaduman, A.; Uysal, S.; Uslu, A.; Küp, A.; et al. Association of C-Reactive Protein-to-Albumin Ratio with the Presence and Progression of Abdominal Aortic Aneurysm. Angiology 2021, 72, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Matsumura, M.; Kasai, K. Vascular Smooth Muscle Cell Activation by C-Reactive Protein. Cardiovasc. Res. 2003, 58, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayan, V.; Wagener, F.A.D.T.G.; Immenschuh, S. The Macrophage Heme-Heme Oxygenase-1 System and Its Role in Inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.P.; Olasińska-Wiśniewska, A.; Gryszczyńska, B.; Budzyń, M.; Lesiak, M.; Trojnarska, O.; Iskra, M. Changes in the Nrf2/Keap1 Ratio and PON1 Concentration in Plasma of Patients Undergoing the Left Main Coronary Artery Stenting. Oxidative Med. Cell. Longev. 2020, 2020, e8249729. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]

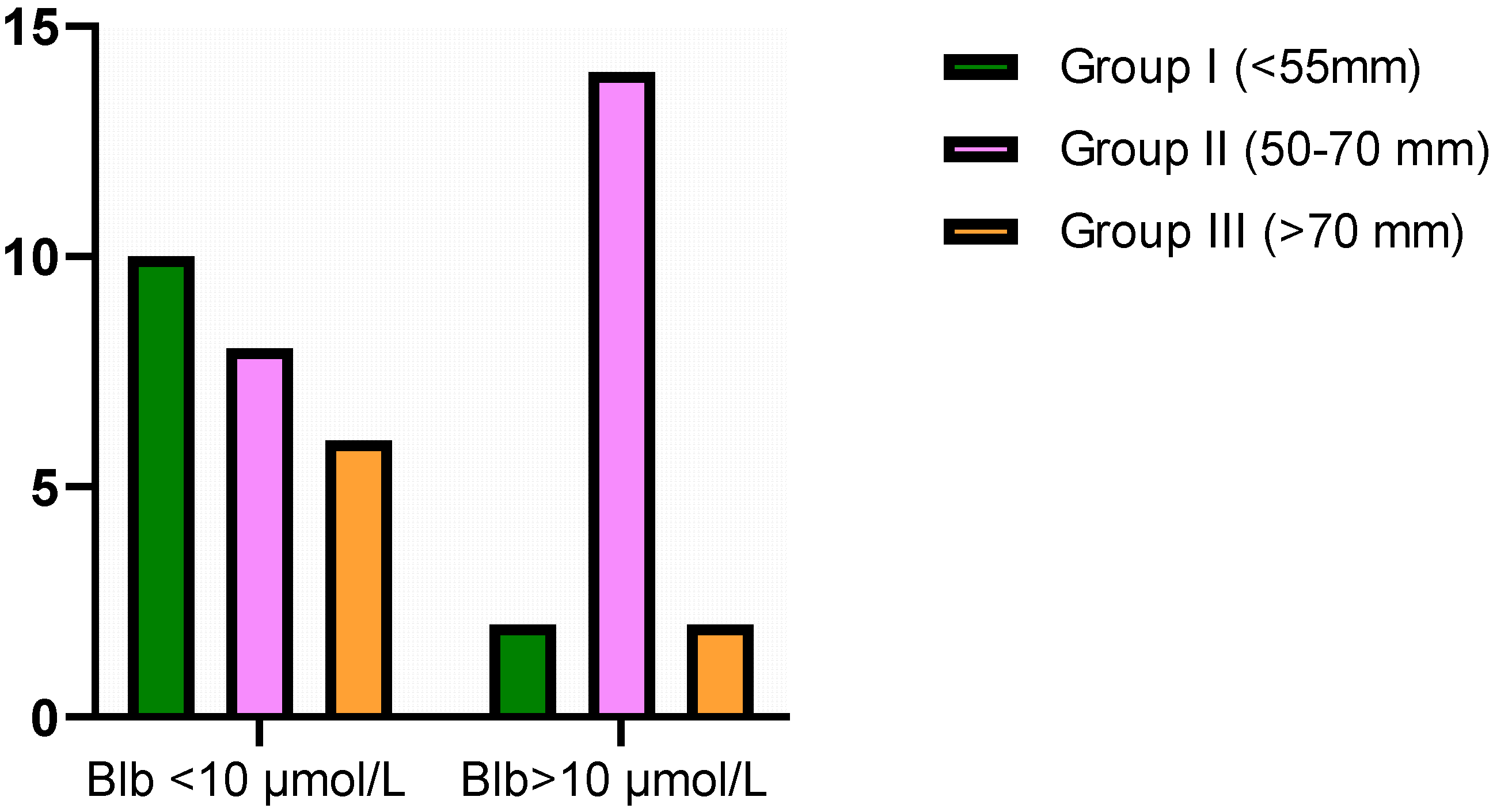
| Group I (n = 12) | Group II (n = 22) | Group III (n = 10) | ANOVA p-Value | |
|---|---|---|---|---|
| Aneurism size (mm) | 50.00 (34.00–55.00) | 66.00 (58.00–70.00) | 75.00 (74.00–85.00) | <0.0001 |
| Age | 72.17 ± 3.512 | 74.64 ± 8.18 | 62.25 ± 4.559 | <0.001 * |
| BMI | 29.93 ± 2.935 | 27.19 ± 4.988 | 25.12 ± 4.522 | ns |
| LDL cholesterol [mmol/L] | 2.57±2.08 | 1.982±0.8 | 2.775 ± 0.462 | ns |
| HDL cholesterol [mmol/L] | 1.751 ± 0.8 | 1.291 ± 0.43 | 1.305 (0.770–1.580) | 0.031 * |
| Total cholesterol [mmol/L] | 4.410 (2.280–10.23) | 3.878 ± 0.906 | 4.598 ± 0.369 | ns |
| TG [mmol/L] | 1.362 ± 0.65 | 1.286 ± 0.64 | 1.353 ± 0.432 | ns |
| Glucose [mmol/L] | 6.077 ± 1.39 | 6.206 ± 0.857 | 7.440 ± 1.483 | ns |
| hsCRP [mg/L] | 9.25 ± 6.86 | 10.37 ± 5.542 | 12.61 ± 9.891 | ns |
| WBC [109/L] | 6.486 ± 0.923 | 7.030 ± 2.751 | 9.555 ± 3.817 | 0.023 * |
| RBC [109/L] | 4.555 ± 0.29 | 4.540 (3.990–5.290) | 4.458 ± 0.149 | ns |
| HGB [mmol/L] | 8.162 ± 0.614 | 8.609 ± 1.081 | 8.350 ± 0.469 | ns |
| PLT [109/L] | 245.3 ± 60.68 | 204.5 ± 75.43 | 400.5 ± 175.1 | <0.001 * |
| GFR [ml/min/1.73 m2] | 73.50 ± 7.46 | 62.00 (16.00–72.00) | 76.65 ± 5.24 | <0.001 * |
| Creatinine [μmol/L] | 87.19 ± 18.74 | 102.4 (76.50–363.0) | 83.83 ± 12.30 | 0.043 * |
| Fibrinogen [mg/dL] | 281.0 (270.8–436.5) | 287.3 ± 81.61 | 394.9 ± 136.1 | 0.021 * |
| Urea [mmol/L] | 6.462 ± 2.467 | 7250 (3.950–21.12) | 4.255 ± 2.034 | 0.038 * |
| Group I (n = 12) | Group II (n = 22) | Group III (n = 10) | ANOVA p-Value | |
|---|---|---|---|---|
| HO-1 [ng/mL] | 3.64 ± 1.56 | 3.74 ± 1.65 | 2.469 ± 1.146 | ns |
| Fe2+ [μmol/L] | 10.01 ± 6.13 | 9.46 ± 3.57 | 9.867 ± 4.314 | ns |
| NRF2 [ng/mL] | 1.37 (0.67–2.2) | 3.48 (0.8404–8.58) | 2.299 (1.466–2.48) | 0.032 * |
| PON1 [ng/mL] | 328.84 ± 147.9 | 367.6 ± 321.8 | 409 ± 153.7 | ns |
| Blb [μmol/L] | 8.41 ± 1.89 | 12.29 ± 3.89 | 8.90 ± 4.351 | 0.007 * |
| p Values | Group I (<55 nm) | Group II (55–70 nm) | Group III (>70 nm) | AAA Group |
|---|---|---|---|---|
| AAA vs. NRF2 | 0.007 | 0.049 | <0.001 | ns |
| AAA vs. hsCRP | 0.001 | ns | 0.035 | ns |
| PON1 vs. HO-1 | 0.015 | 0.061 | ns | 0.018 |
| PON1 vs. NRF2 | ns | 0.049 | 0.079 | 0.023 |
| PON1 vs. Fe2+ | 0.018 | 0.066 | ns | 0.005 |
| PON1 vs. Blb | ns | 0.006 | ns | 0.023 |
| HO-1 vs. Blb | ns | ns | <0.001 | ns |
| HO-1 vs. Fe2+ | ns | 0.047 | ns | ns |
| hsCRP vs. Blb | ns | ns | ns | 0.046 |
| hsCRP vs. NRF2 | 0.05 | 0.008 | ns | 0.056 |
| Blb vs. NRF2 | ns | ns | <0.001 | ns |
| Variable | 95% CI | p-Value |
|---|---|---|
| Intercept | −621.1 to 302.0 | 0.485 |
| AAA diameter | −5.435 to 7.523 | 0.744 |
| HO-1 | −9.522 to 64.54 | 0.140 |
| Fe2+ | −5.510 to 31.61 | 0.161 |
| NRF2 | −29.27 to −1.934 | 0.027 * |
| Blb | 9.591 to 48.42 | 0.005 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak, M.P.; Gryszczyńska, B.; Olasińska-Wiśniewska, A.; Urbanowicz, T.; Jawień, A.; Krasiński, Z.; Formanowicz, D. Blb-NRF2-PON1 Cross-Talk in Abdominal Aortic Aneurysm Progression. Antioxidants 2023, 12, 1568. https://doi.org/10.3390/antiox12081568
Kasprzak MP, Gryszczyńska B, Olasińska-Wiśniewska A, Urbanowicz T, Jawień A, Krasiński Z, Formanowicz D. Blb-NRF2-PON1 Cross-Talk in Abdominal Aortic Aneurysm Progression. Antioxidants. 2023; 12(8):1568. https://doi.org/10.3390/antiox12081568
Chicago/Turabian StyleKasprzak, Magdalena P., Bogna Gryszczyńska, Anna Olasińska-Wiśniewska, Tomasz Urbanowicz, Andrzej Jawień, Zbigniew Krasiński, and Dorota Formanowicz. 2023. "Blb-NRF2-PON1 Cross-Talk in Abdominal Aortic Aneurysm Progression" Antioxidants 12, no. 8: 1568. https://doi.org/10.3390/antiox12081568







