Comprehensive Comparison of Effects of Antioxidant (Astaxanthin) Supplementation from Different Sources in Haliotis discus hannai Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Feeding Experiment
2.3. Sampling and Analysis
2.3.1. Proximate Composition Analysis of the Diets
2.3.2. Sampling
2.3.3. Serum Immune Index and Hepatopancreas Antioxidant Enzyme Activity Analysis
2.4. Heat-Resistance Evaluation of the Experiment
2.5. Disease Resistance Evaluation
2.5.1. Bacterial Challenge Experiment
2.5.2. Hemolymph Immune Response Determination
2.6. Intestinal Microbiome Analysis
2.7. Statistical Analysis
3. Results
3.1. Survival and Growth Performance
3.2. Serum Immune Index and Hepatopancreas Antioxidant Enzyme Activity
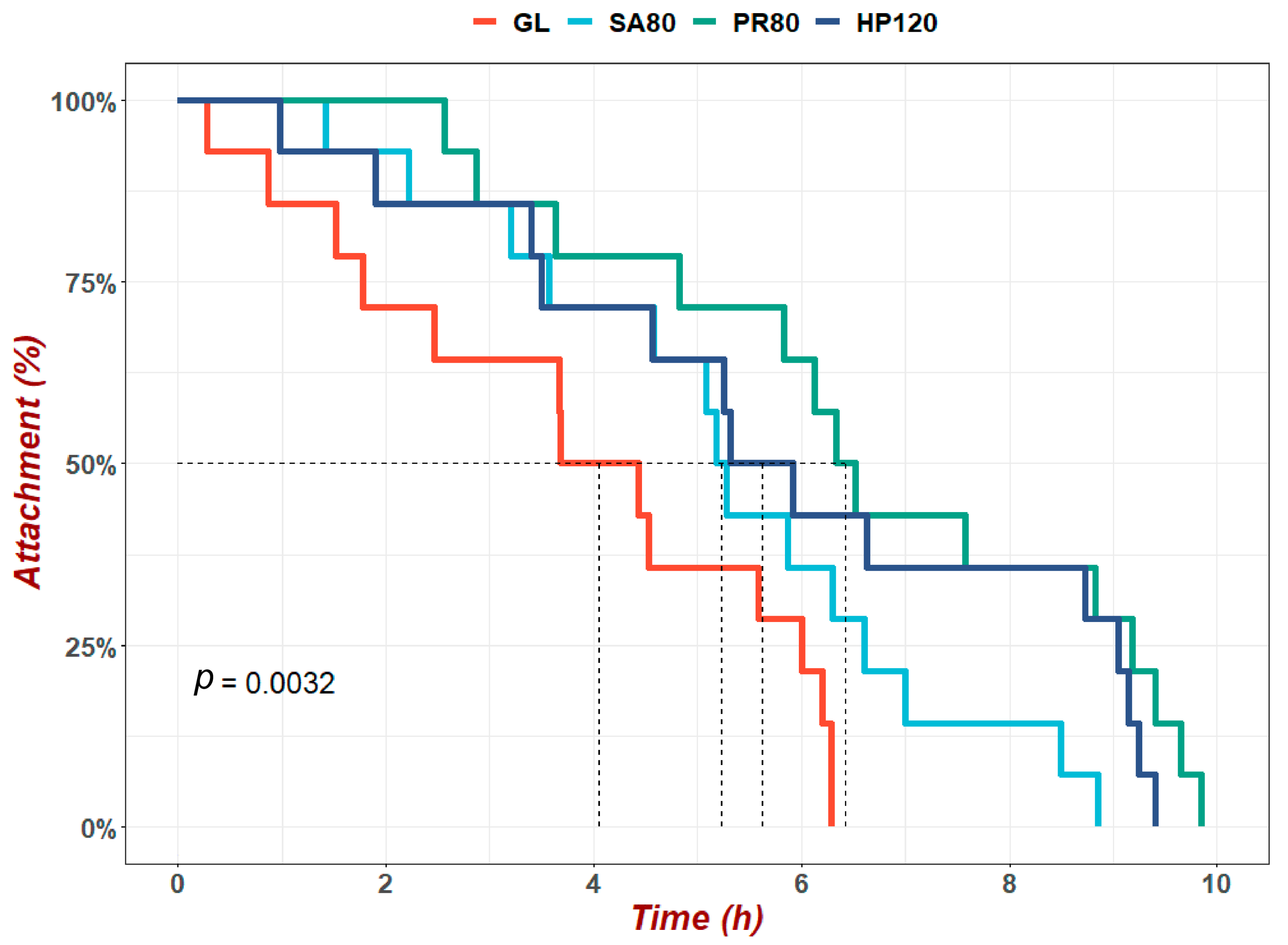
3.3. Heat-Resistance Evaluation in the Experiment
3.4. Bacterial Challenge Experiment
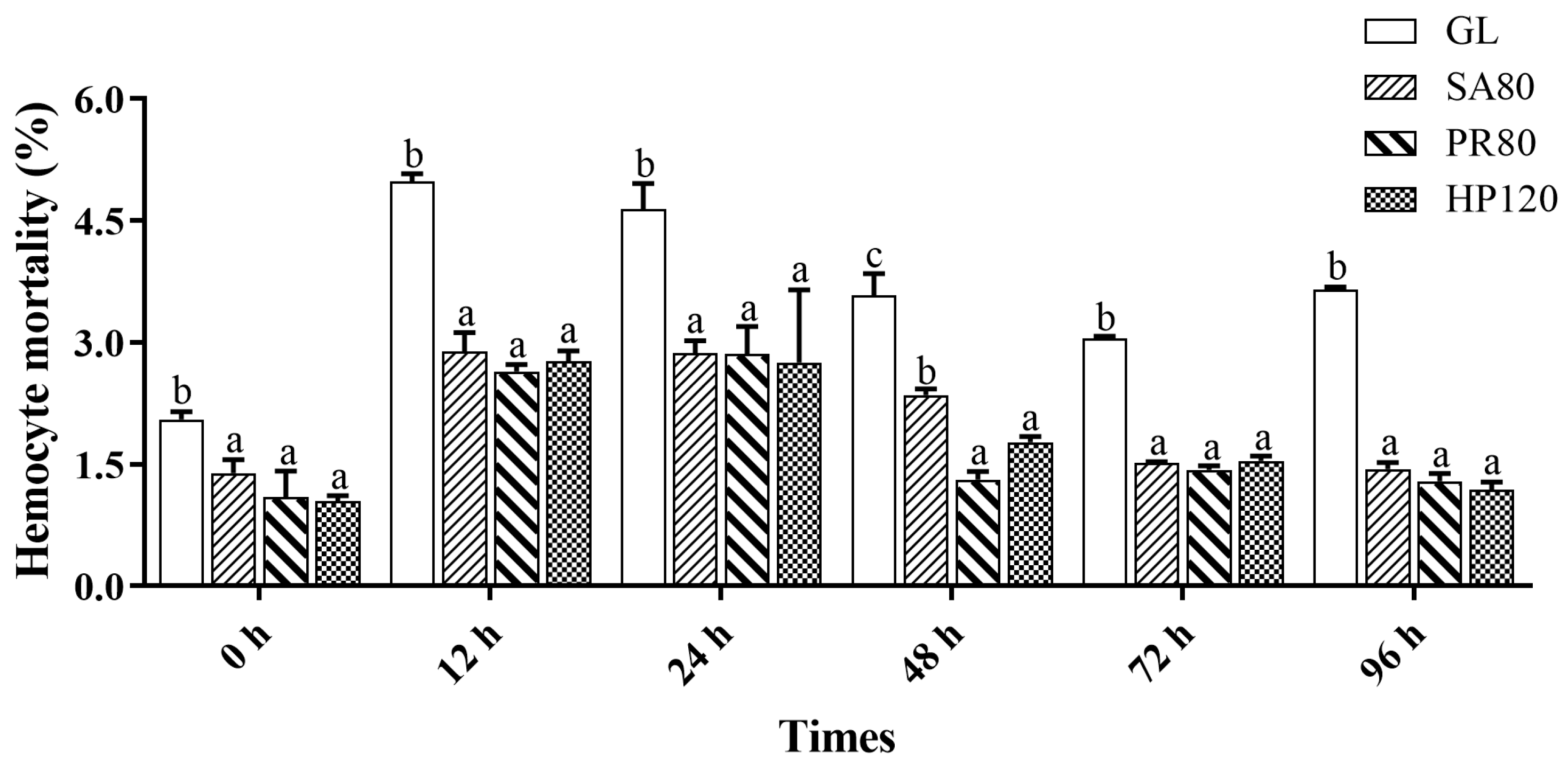
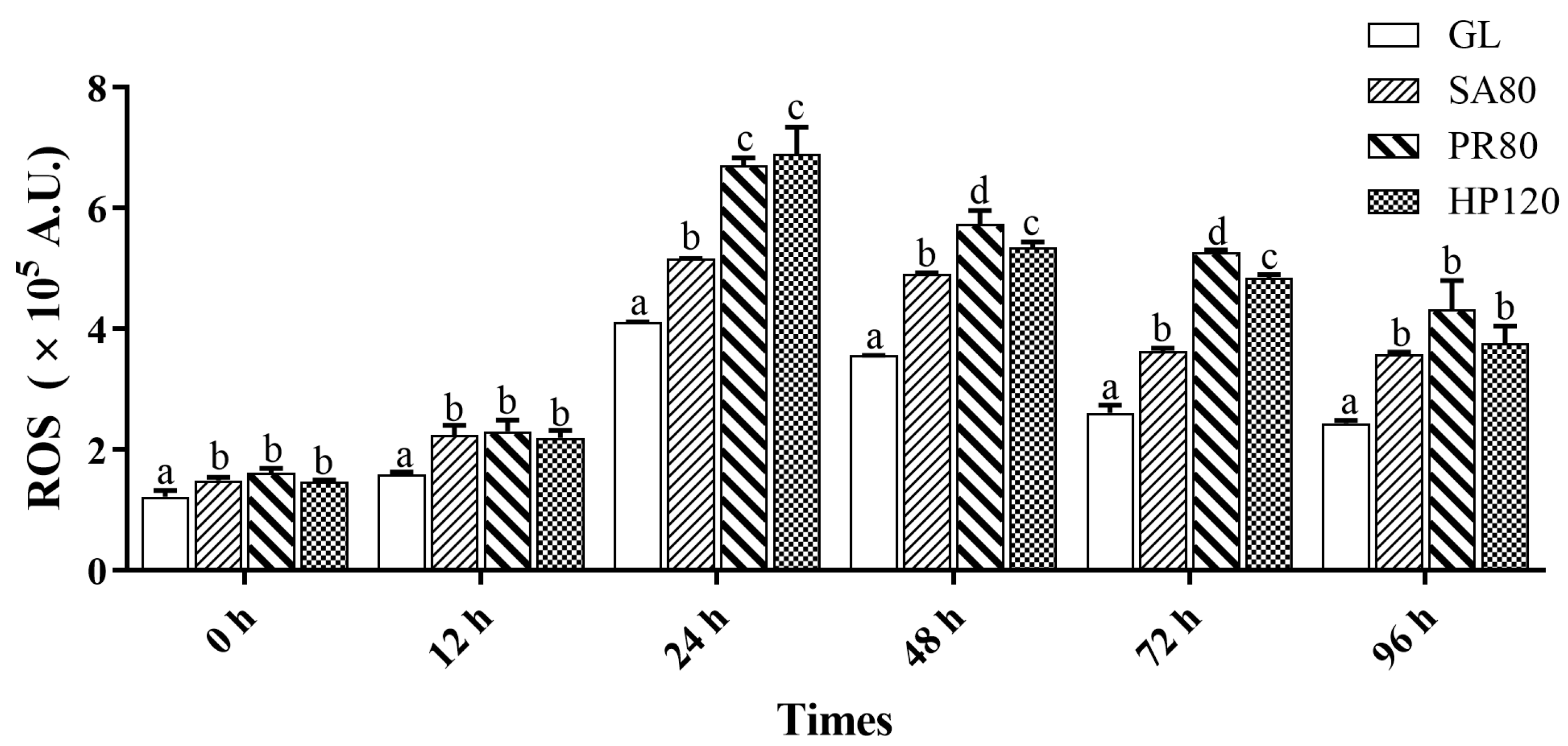
3.5. Hemolymph Immune Responses
3.6. Intestinal Microbiome
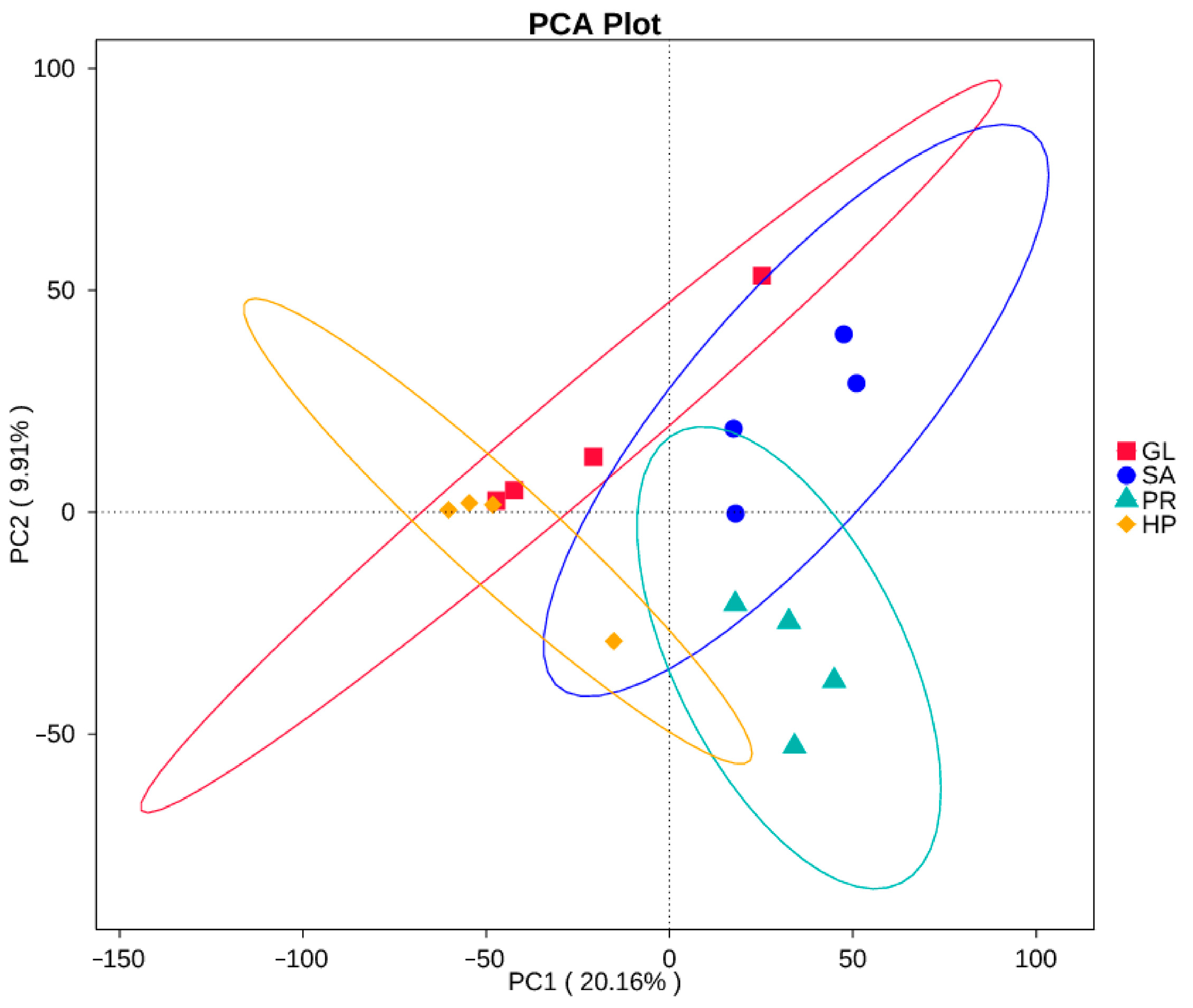
3.6.1. Intestinal Microbiota Diversity and Richness
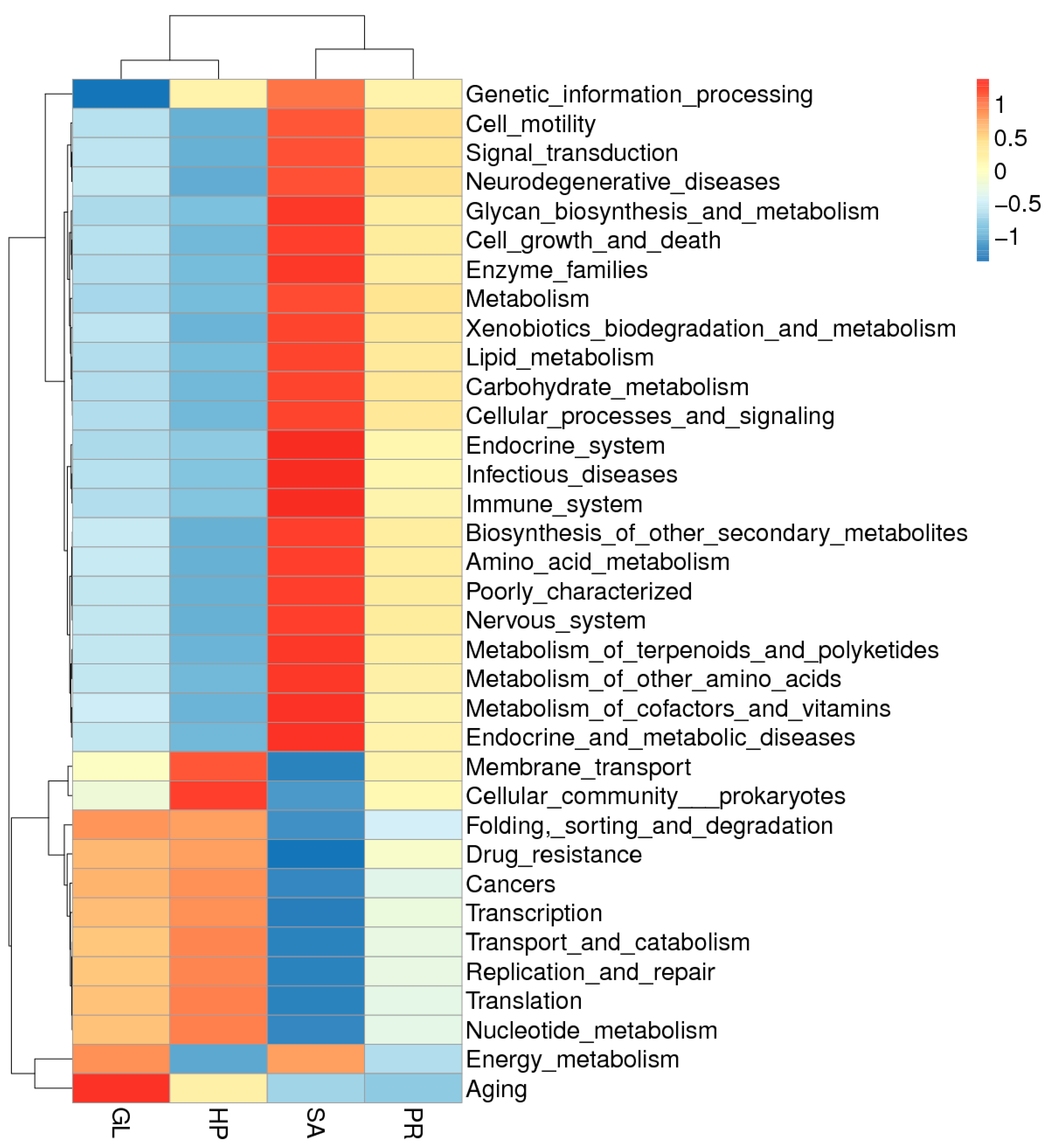
3.6.2. LefSe Analysis and Function Clustering Heat Map of Intestinal Microbiota
3.6.3. Significantly Different Species among Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Fu, Z.; Hu, J.; Zhou, S.; Yu, G.; Ma, Z. Dietary Curcumin Supplementation Enhanced Ammonia Nitrogen Stress Tolerance in Greater Amberjack (Seriola dumerili): Growth, Serum Biochemistry and Expression of Stress-Related Genes. J. Mar. Sci. Eng. 2022, 10, 1796. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.-N.; Wang, A.-L.; Wang, J.-M.; Sun, R.-Y. Effects of dietary vitamin E supplementation on antioxidant enzyme activities in Litopenaeus vannamei (Boone, 1931) exposed to acute salinity changes. Aquaculture 2007, 265, 351–358. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Zaineldin, A.I.; Van Doan, H.; Ahmed, H.A.; Elsabagh, M.; Abdel-Daim, M.M. An evaluation of dietary selenium nanoparticles for red sea bream (Pagrus major) aquaculture: Growth, tissue bioaccumulation, and antioxidative responses. Environ. Sci. Pollut. Res. 2019, 26, 30876–30884. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.; Lin, Z.; Chen, X.; Bu, X.; Liu, S.; Lei, Y.; Shi, Q.; Qin, J.; Chen, L. Effects of dietary Zn on growth, antioxidant capacity, immunity and tolerance to lipopolysaccharide challenge in juvenile Chinese mitten crab Eriocheir sinensis. Aquac. Res. 2022, 53, 1110–1120. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Melendez-Martinez, A.J.; Olmedilla-Alonso, B.; Palou, A. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Hien, T.T.T.; Loc, T.V.; Tu, T.L.C.; Phu, T.M.; Duc, P.M.; Nhan, H.T.; Liem, P.T. Dietary effects of carotenoid on growth performance and pigmentation in bighead catfish (Clarias macrocephalus Günther, 1864). Fishes 2022, 7, 37. [Google Scholar] [CrossRef]
- Nakano, T.; Wiegertjes, G. Properties of carotenoids in fish fitness: A review. Mar. Drugs 2020, 18, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Wang, J.; Liu, T. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour. Technol. 2014, 158, 329–335. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Dong, S.; Huang, Y.; Zhang, R.; Wang, S.; Liu, Y. Four different methods comparison for extraction of astaxanthin from green alga Haematococcus pluvialis. Sci. World J. 2014, 2014, 694305. [Google Scholar] [CrossRef]
- Lu, Q.; Li, H.; Zou, Y.; Liu, H.; Yang, L. Astaxanthin as a microalgal metabolite for aquaculture: A review on the synthetic mechanisms, production techniques, and practical application. Algal Res. 2021, 54, 102178. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Yamashita, E. Astaxanthin as a medical food. Funct. Foods Health Dis. 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, C.; Rao, W.; Chen, P.; Lei, K.; Mai, K.; Zhang, W. Dietary phospholipids improve growth performance and change the lipid composition and volatile flavor compound profiles in the muscle of abalone Haliotis discus hannai by affecting the glycerophospholipid metabolism. Aquac. Rep. 2023, 30, 101567. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Y.; Pan, M.; Li, X.; Huang, D.; Liu, Y.; Wu, C.; Zhang, W.; Mai, K. Functions of forkhead box O on glucose metabolism in abalone Haliotis discus hannai and its responses to high levels of dietary lipid. Genes 2021, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- China Fisheries Bureau. China Fisheries Statistics Yearbook in 2021; Agricultural Press of China: Beijing, China, 2021. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Xu, F.; Gao, T.; Liu, X. Metabolomics adaptation of juvenile pacific abalone Haliotis discus hannai to heat stress. Sci. Rep. 2020, 10, 6353. [Google Scholar] [CrossRef]
- Ding, J.; Li, L.; Wu, F.; Zhang, G. Effect of chronic temperature exposure on the immunity of abalone, Haliotis discus hannai. Aquac. Res. 2016, 47, 2861–2873. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yang, M.-H.; Choe, M.-K.; Han, S.-J.; Yeo, I.-K. Physiological studies on acute water-temperature stress of juvenile abalone, Haliotis discus hannai. J. Aquac. 2005, 18, 7–12. [Google Scholar]
- You, W.; Guo, Q.; Fan, F.; Ren, P.; Luo, X.; Ke, C. Experimental hybridization and genetic identification of Pacific abalone Haliotis discus hannai and green abalone H. fulgens. Aquaculture 2015, 448, 243–249. [Google Scholar] [CrossRef]
- Kang, H.Y.; Lee, Y.-J.; Song, W.-Y.; Kim, T.-I.; Lee, W.-C.; Kim, T.Y.; Kang, C.-K. Physiological responses of the abalone Haliotis discus hannai to daily and seasonal temperature variations. Sci. Rep. 2019, 9, 8019. [Google Scholar] [CrossRef] [PubMed]
- Morash, A.J.; Alter, K. Effects of environmental and farm stress on abalone physiology: Perspectives for abalone aquaculture in the face of global climate change. Rev. Aquac. 2016, 8, 342–368. [Google Scholar] [CrossRef]
- Lim, T.-J.; Lee, S.-M. Effect of dietary pigment sources on the growth and shell color of abalone (Haliotis discus hannai). Korean J. Fish. Aquat. Sci. 2003, 36, 601–605. [Google Scholar]
- Ma, S.; Li, X.; Huang, D.; Guo, Y.; Deng, J.; Zhou, W.; Zhang, W.; Mai, K. Effects of dietary chromium yeast and astaxanthin on the growth performance, anti-oxidative capacity, and resistance to heat stress of abalone Haliotis discus hannai. Aquac. Int. 2021, 29, 911–924. [Google Scholar] [CrossRef]
- Butler, T.; Golan, Y. Astaxanthin Production from Microalgae. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 175–242. [Google Scholar]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists, (AOAC). Official Methods of Association of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Yu, F.; Wu, Y.; Shen, Y.; Peng, W.; Liu, J.; Lin, W.; Huang, Z.; Gan, Y.; Xiao, Q.; Chen, N. Heat adhesion duration: A new high-throughput abalone thermal tolerance assessment method. Aquaculture 2021, 545, 737226. [Google Scholar] [CrossRef]
- Zou, W.; Ma, Y.; Ai, C.; Yu, W.; Gao, X.; Liu, S.; Luo, X.; You, W.; Ke, C. Dietary curcumin influence on growth, antioxidant status, immunity, gut flora and resistance to Vibrio harveyi AP37 in Haliotis discus hannai. Aquac. Rep. 2022, 26, 101336. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Schmitt, I.; Meyer, F.; Krahn, I.; Henke, N.A.; Peters-Wendisch, P.; Wendisch, V.F. From Aquaculture to Aquaculture: Production of the Fish Feed Additive Astaxanthin by Corynebacterium glutamicum Using Aquaculture Sidestream. Molecules 2023, 28, 1996. [Google Scholar] [CrossRef]
- Pereira da Costa, D.; Campos Miranda-Filho, K. The use of carotenoid pigments as food additives for aquatic organisms and their functional roles. Rev. Aquac. 2020, 12, 1567–1578. [Google Scholar] [CrossRef]
- Ritu, J.R.; Ambati, R.R.; Ravishankar, G.A.; Shahjahan, M.; Khan, S. Utilization of astaxanthin from microalgae and carotenoid rich algal biomass as a feed supplement in aquaculture and poultry industry: An overview. J. Appl. Phycol. 2023, 35, 145–171. [Google Scholar] [CrossRef]
- Liu, F.; Shi, H.-Z.; Guo, Q.-S.; Yu, Y.-B.; Wang, A.-M.; Lv, F.; Shen, W.-B. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2016, 51, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, E.P.; Shekarabi, P.H.; Yadollahi, F.; Soltani, M.; Najafi, E.; von Hellens, J.; Flores, C.L.; Salehi, K.; Faggio, C. Red yeast (Phaffia rhodozyma) and its effect on growth, antioxidant activity and color pigmentation of rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2022, 23, 101082. [Google Scholar] [CrossRef]
- Fawzy, S.; Wang, W.; Wu, M.; Yi, G.; Huang, X. Effects of dietary different canthaxanthin levels on growth performance, antioxidant capacity, biochemical and immune-physiological parameters of white shrimp (Litopenaeus Vannamei). Aquaculture 2022, 556, 738276. [Google Scholar] [CrossRef]
- Su, F.; Yu, W.; Liu, J. Comparison of effect of dietary supplementation with Haematococcus pluvialis powder and synthetic astaxanthin on carotenoid composition, concentration, esterification degree and astaxanthin isomers in ovaries, hepatopancreas, carapace, epithelium of adult female Chinese mitten crab (Eriocheir sinensis). Aquaculture 2020, 523, 735146. [Google Scholar]
- Xie, J.; Fang, H.; He, X.; Liao, S.; Liu, Y.; Tian, L.; Niu, J. Study on mechanism of synthetic astaxanthin and Haematococcus pluvialis improving the growth performance and antioxidant capacity under acute hypoxia stress of golden pompano (Trachinotus ovatus) and enhancing anti-inflammatory by activating Nrf2-ARE pathway to antagonize the NF-κB pathway. Aquaculture 2020, 518, 734657. [Google Scholar]
- Fan, L.; Chen, J.; Meng, S.; Song, C.; Qiu, L.; Hu, G.; Xu, P. Characterization of microbial communities in intensive GIFT tilapia (Oreochromis niloticus) pond systems during the peak period of breeding. Aquac. Res. 2017, 48, 459–472. [Google Scholar] [CrossRef]
- Qiao, F.; Liu, Y.; Sun, Y.; Wang, X.; Chen, K.; Li, T.; Li, E.; Zhang, M. Influence of different dietary carbohydrate sources on the growth and intestinal microbiota of Litopenaeus vannamei at low salinity. Aquac. Nutr. 2017, 23, 444–452. [Google Scholar] [CrossRef]
- Shui, Y.; Guan, Z.-B.; Liu, G.-F.; Fan, L.-M. Gut microbiota of red swamp crayfish Procambarus clarkii in integrated crayfish-rice cultivation model. AMB Express 2020, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Elolimy, A.; Alharthi, A.; Zeineldin, M.; Parys, C.; Loor, J.J. Residual feed intake divergence during the preweaning period is associated with unique hindgut microbiome and metabolome profiles in neonatal Holstein heifer calves. J. Anim. Sci. Biotechnol. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, D.L. The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 2002, 39, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lu, Y.; Shen, Y.; Liu, J.; Gong, S.; Yu, F.; Huang, Z.; Zou, W.; Zhou, M.; Luo, X. Exploring the intestinal microbiota and metabolome profiles associated with feed efficiency in Pacific abalone (Haliotis discus hannai). Front. Microbiol. 2022, 13, 852460. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Song, C.; Gao, Q.; Zhou, Q.; Sun, C.; Zhang, H.; Liu, M.; Tadese, D.A. Maternal, and environmental microbes dominate offspring microbial colonization in the giant freshwater prawn Macrobrachium rosenbergii. Sci. Total Environ. 2021, 790, 148062. [Google Scholar] [CrossRef] [PubMed]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Ochieng, J.B.; Boisen, N.; Lindsay, B.; Santiago, A.; Ouma, C.; Ombok, M.; Fields, B.; Stine, O.C.; Nataro, J.P. Serratia marcescens is injurious to intestinal epithelial cells. Gut Microbes 2014, 5, 729–736. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, K.; Yin, Y.; Zhang, X.; Zhang, Q.; Kong, X.; Tang, L.; Zhang, R.; Zhang, Z. Serratia marcescens in the intestine of housefly larvae inhibits host growth by interfering with gut microbiota. Parasites Vectors 2023, 16, 196. [Google Scholar] [CrossRef]
- Newport, M.T.; John, J.F.; Michel, Y.M.; Levkoff, A.H. Endemic Serratia marcescens infection in a neonatal intensive care nursery associated with gastrointestinal colonization. Pediatr. Infect. Dis. J. 1985, 4, 160–167. [Google Scholar] [CrossRef]
- Bai, L.; Wang, L.; Vega-Rodríguez, J.; Wang, G.; Wang, S. A gut symbiotic bacterium Serratia marcescens renders mosquito resistance to Plasmodium infection through activation of mosquito immune responses. Front. Microbiol. 2019, 10, 1580. [Google Scholar] [CrossRef]
- Nie, H.; Li, Y.; Lu, X.-L.; Yan, J.; Liu, X.-R.; Yin, Q. Prodigiosin derived from chromium-resistant Serratia sp. prevents inflammation and modulates gut microbiota homeostasis in DSS-induced colitis mice. Int. Immunopharmacol. 2023, 116, 109800. [Google Scholar] [CrossRef]
- Hagen, K.S. Dependence of the olive fly, Dacus oleae, larvae on symbiosis with Pseudomonas savastanoi for the utilization of olive. Nature 1966, 209, 423–424. [Google Scholar] [CrossRef]
- Santos, O.C.; Pontes, P.V.; Santos, J.F.; Muricy, G.; Giambiagi-deMarval, M.; Laport, M.S. Isolation, characterization and phylogeny of sponge-associated bacteria with antimicrobial activities from Brazil. Res. Microbiol. 2010, 161, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Rajasabapathy, R.; Ghadi, S.C.; Manikandan, B.; Mohandass, C.; Surendran, A.; Dastager, S.G.; Meena, R.M.; James, R.A. Antimicrobial profiling of coral reef and sponge associated bacteria from southeast coast of India. Microb. Pathog. 2020, 141, 103972. [Google Scholar] [CrossRef] [PubMed]
- Hooda, S.; Boler, B.M.V.; Serao, M.C.R.; Brulc, J.M.; Staeger, M.A.; Boileau, T.W.; Dowd, S.E.; Fahey, G.C., Jr.; Swanson, K.S. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J. Nutr. 2012, 142, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, L.f.; Dai, T.y.; Qi, X.; Wang, Y.; Zheng, T.z.; Gao, X.y.; Zhang, Y.j.; Ai, Y.; Ma, L. Short-Chain Fatty Acids Produced by Ruminococcaceae Mediate α-Linolenic Acid Promote Intestinal Stem Cells Proliferation. Mol. Nutr. Food Res. 2022, 66, 2100408. [Google Scholar] [CrossRef]
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.; Kuwahara, A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol 2008, 59, 251–262. [Google Scholar]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, 6617. [Google Scholar] [CrossRef]
- Diao, H.; Jiao, A.; Yu, B.; Mao, X.; Chen, D. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.; Murphy, K.; Brooks, L.; Bewick, G.; Hanyaloglu, A.; Ghatei, M.; Bloom, S.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wang, L.; Mulati, A.; Liu, Y.; Liu, Z.; Liu, X. Methionine restriction improves gut barrier function by reshaping diurnal rhythms of inflammation-related microbes in aged mice. Front. Nutr. 2021, 8, 746592. [Google Scholar] [CrossRef]
- Luo, M.; Xin, R.-J.; Hu, F.-R.; Yao, L.; Hu, S.-J.; Bai, F.-H. Role of gut microbiota in the pathogenesis and therapeutics of minimal hepatic encephalopathy via the gut-liver-brain axis. World J. Gastroenterol. 2023, 29, 144. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Xiong, B.; Zhang, C.; Kang, B.; Gao, Y.; Li, Z.; Ge, W.; Cheng, S.; Hao, Y. Microbiota from alginate oligosaccharide-dosed mice successfully mitigated small intestinal mucositis. Microbiome 2020, 8, 112. [Google Scholar] [CrossRef]
- Huyben, D.; Nyman, A.; Vidaković, A.; Passoth, V.; Moccia, R.; Kiessling, A.; Dicksved, J.; Lundh, T. Effects of dietary inclusion of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus on gut microbiota of rainbow trout. Aquaculture 2017, 473, 528–537. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, C.; Tang, C.; Zhang, Y.; Li, N.; Li, J. Fish oil enhances recovery of intestinal microbiota and epithelial integrity in chronic rejection of intestinal transplant. PLoS ONE 2011, 6, e20460. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Ambati, R.R.; Siew Moi, P.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Gong, X.-D.; Chen, F. Separation and analysis of carotenoids and chlorophylls in Haematococcus lacustris by high-performance liquid chromatography photodiode array detection. J. Agric. Food Chem. 1997, 45, 1952–1956. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.-H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Moretti, V.M.; Mentasti, T.; Bellagamba, F.; Luzzana, U.; Caprino, F.; Turchini, G.M.; Giani, I.; Valfrè, F. Determination of astaxanthin stereoisomers and color attributes in flesh of rainbow trout (Oncorhynchus mykiss) as a tool to distinguish the dietary pigmentation source. Food Addit. Contam. 2006, 23, 1056–1063. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]







| Parameters | Experimental Diets | |||
|---|---|---|---|---|
| GL | SA80 | PR80 | HP120 | |
| Fish meal a | 150 | 150 | 150 | |
| Wheat gluten a | 120 | 120 | 120 | |
| Soybean meal a | 130 | 130 | 130 | |
| High-gluten flour a | 129.2 | 110 | 126 | |
| Kelp powder a | 250 | 250 | 250 | |
| Dextrin b | 50 | 50 | 50 | |
| α-starch b | 50 | 50 | 50 | |
| Sodium alginate c | 30 | 30 | 30 | |
| Soybean lecithin d | 20 | 20 | 20 | |
| Cholesterol e | 5 | 5 | 5 | |
| Vitamin premix f | 20 | 20 | 20 | |
| Mineral premix g | 30 | 30 | 30 | |
| Choline chloride | 5 | 5 | 5 | |
| Monocalcium phosphate | 5 | 5 | 5 | |
| Astaxanthin h | 0.8 | |||
| Phaffia rhodozyma i | 20 | |||
| Haematococcus pluvialis j | 4 | |||
| Proximate composition (%) | ||||
| Moisture | 82.48 ± 0.55 | 5.26 ± 0.57 | 5.40 ± 0.30 | 6.40 ± 0.13 |
| Crude protein | 16.37 ± 0.28 | 31.80 ± 0.20 | 32.97 ± 0.76 | 32.10 ± 0.38 |
| Crude lipid | 1.86 ± 0.17 | 3.83 ± 0.40 | 3.67 ± 0.19 | 3.73 ± 0.15 |
| Ash | 21.13 ± 0.17 | 18.64 ± 0.26 | 17.97 ± 0.22 | 18.15 ± 0.03 |
| Parameters | Experimental Diets | |||
|---|---|---|---|---|
| GL | SA80 | PR80 | HP120 | |
| SR (%) | 91.67 ± 1.67 a | 92.50 ± 1.12 a | 93.33 ± 1.05 a | 93.33 ± 2.47 a |
| FSL (cm) | 64.60 ± 0.93 a | 73.47 ± 0.49 b | 74.17 ± 0.83 b | 74.16 ± 0.78 b |
| FW (g) | 35.62 ± 0.92 a | 46.56 ± 0.90 b | 48.92 ± 0.57 c | 48.81 ± 0.33 c |
| DISL (µm/day) | 57.71 ± 9.48 a | 135.01 ± 3.93 b | 141.79 ± 9.05 b | 146.00 ± 8.36 b |
| WGR (%) | 46.25 ± 3.65 a | 85.38 ± 3.82 b | 97.62 ± 3.35 c | 95.98 ± 1.77 c |
| SGR (%) | 0.42 ± 0.03 a | 0.68 ± 0.02 b | 0.76 ± 0.02 c | 0.75 ± 0.01 c |
| VIS (%) | 15.85 ± 0.52 a | 17.20 ± 0.27 ab | 18.11 ± 0.56 b | 18.17 ± 0.59 b |
| Parameters | Experimental Diets | |||
|---|---|---|---|---|
| GL | SA80 | PR80 | HP120 | |
| Serum | ||||
| GLU (mmol/L) | 0.23 ± 0.02 b | 0.19 ± 0.01 ab | 0.16 ± 0.01 a | 0.15 ± 0.01 a |
| COR (ng/mL) | 40.11 ± 1.47 b | 32.49 ± 1.15 a | 33.36 ± 2.02 a | 31.5 ± 0.96 a |
| AKP (U/L) | 224.9 ± 3.42 a | 241.67 ± 4.97 b | 245.88 ± 3.87 b | 243.52 ± 1.59 b |
| LZM (µg/mL) | 3.96 ± 0.28 a | 5.36 ± 0.17 b | 5.44 ± 0.31 b | 5.33 ± 0.18 b |
| Hepatopancreas | ||||
| T-AOC (U/mg prot) | 0.39 ± 0.03 a | 0.61 ± 0.04 b | 0.68 ± 0.03 b | 0.68 ± 0.05 b |
| CAT (U/mg prot) | 81.86 ± 1.91 a | 117.99 ± 3.90 b | 124.70 ± 1.66 b | 126.34 ± 4.84 b |
| SOD (U/mg prot) | 16.18 ± 0.94 a | 20.15 ± 0.32 b | 20.42 ± 0.21 b | 20.74 ± 0.90 b |
| GSH-PX (U/mg prot) | 85.99 ± 6.02 a | 106.86 ± 4.53 b | 122.67 ± 3.93 b | 117.70 ± 6.16 b |
| MDA (nmol/mg prot) | 8.43 ± 0.43 b | 4.40 ± 0.21 a | 3.83 ± 0.10 a | 3.82 ± 0.25 a |
| Groups | Goods Coverage | PD Whole Tree | Shannon | Simpson | Chao1 | ACE |
|---|---|---|---|---|---|---|
| GL | 0.993 | 171.907 | 4.741 | 0.751 | 1975.896 | 2041.389 |
| SA80 | 0.99 | 253.411 | 7.811 | 0.972 | 3236.198 | 3305.304 |
| PR80 | 0.989 | 266.931 | 7.779 | 0.969 | 3432.391 | 3487.38 |
| HP120 | 0.996 | 115.323 | 4.354 | 0.869 | 1173.165 | 1210.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Hong, J.; Yu, W.; Ma, Y.; Gan, J.; Liu, Y.; Luo, X.; You, W.; Ke, C. Comprehensive Comparison of Effects of Antioxidant (Astaxanthin) Supplementation from Different Sources in Haliotis discus hannai Diet. Antioxidants 2023, 12, 1641. https://doi.org/10.3390/antiox12081641
Zou W, Hong J, Yu W, Ma Y, Gan J, Liu Y, Luo X, You W, Ke C. Comprehensive Comparison of Effects of Antioxidant (Astaxanthin) Supplementation from Different Sources in Haliotis discus hannai Diet. Antioxidants. 2023; 12(8):1641. https://doi.org/10.3390/antiox12081641
Chicago/Turabian StyleZou, Weiguang, Jiawei Hong, Wenchao Yu, Yaobin Ma, Jiacheng Gan, Yanbo Liu, Xuan Luo, Weiwei You, and Caihuan Ke. 2023. "Comprehensive Comparison of Effects of Antioxidant (Astaxanthin) Supplementation from Different Sources in Haliotis discus hannai Diet" Antioxidants 12, no. 8: 1641. https://doi.org/10.3390/antiox12081641
APA StyleZou, W., Hong, J., Yu, W., Ma, Y., Gan, J., Liu, Y., Luo, X., You, W., & Ke, C. (2023). Comprehensive Comparison of Effects of Antioxidant (Astaxanthin) Supplementation from Different Sources in Haliotis discus hannai Diet. Antioxidants, 12(8), 1641. https://doi.org/10.3390/antiox12081641






