Lactate: A Theranostic Biomarker for Metabolic Psychiatry?
Abstract
1. Introduction
1.1. The Role of Mitochondria in the Pathogenesis of Psychiatric Disorders
1.2. Potential of Cerebral Lactate as a Neurometabolic Biomarker
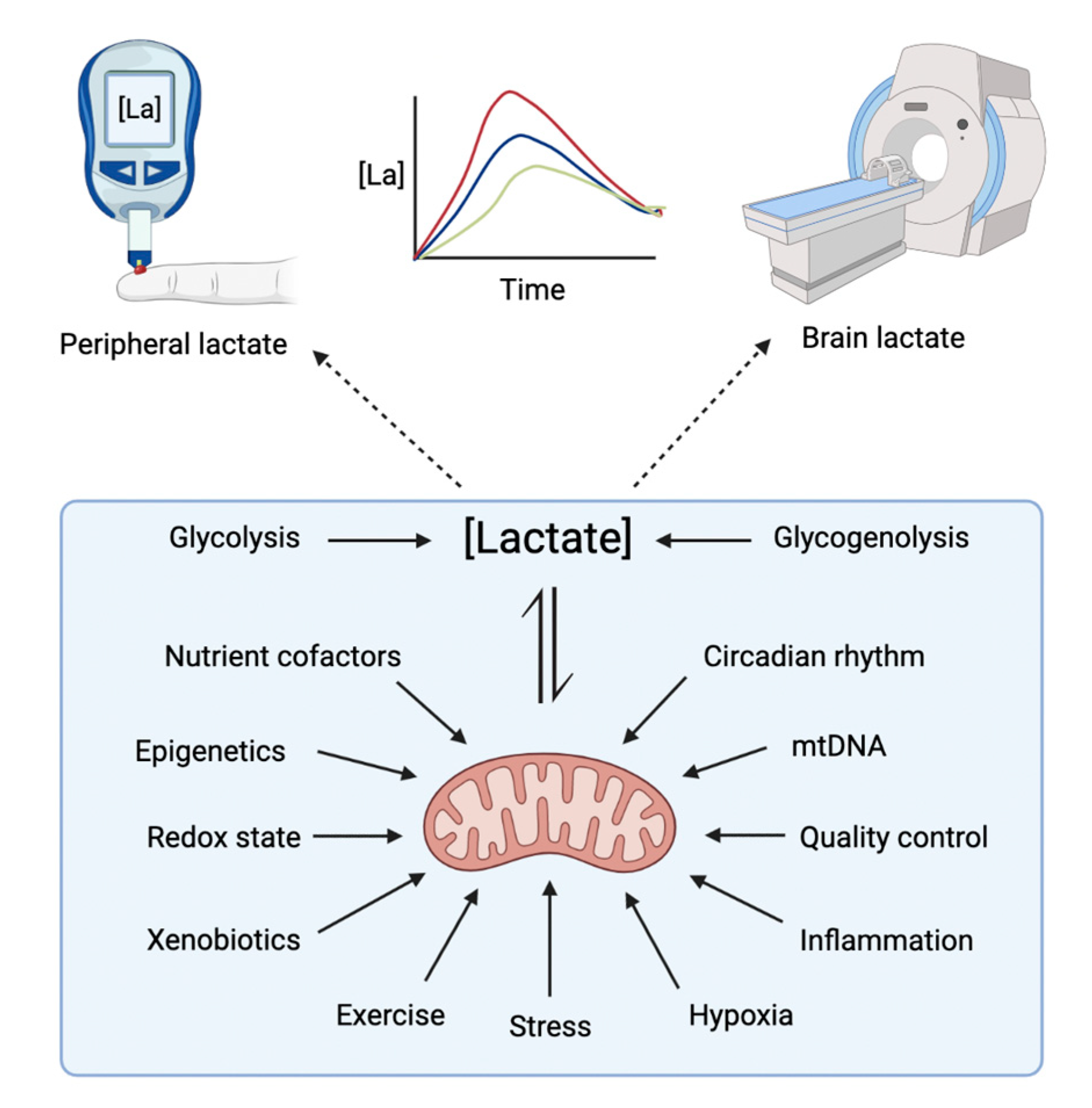
1.3. Aim of the Review
2. Lactate Neurophysiology
3. Lactate Signalling in the Brain
4. Brain Lactate Dynamics as a Putative Therapeutic Biomarker
Therapeutic Interventions Targeting Brain Mitochondria and Lactate Dynamics
- Physical exercise
- Pharmacological enhancement of mitochondrial function
- Nutritional and dietary intervention
- Stress, hypoxia, and inflammation
- Circadian rhythm
- Other examples of lactate manipulation
5. Brain Lactate Measurement with Functional Magnetic Resonance Spectroscopy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Zhao, W.; Lu, Y.; Zhao, Y.; Zhang, Y.; Dai, M.; Hai, S.; Ge, N.; Zhang, S.; Huang, M.; et al. Systematic metabolic characterization of mental disorders reveals age-related metabolic disturbances as potential risk factors for depression in older adults. Medcomm 2022, 3, e165. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-López, B.A.; Moreno-Altamirano, M.M.B.; Dockrell, H.M.; Duchen, M.R.; Sánchez-García, F.J. Mitochondria: An Integrative Hub Coordinating Circadian Rhythms, Metabolism, the Microbiome, and Immunity. Front. Cell Dev. Biol. 2020, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Kann, O. The interneuron energy hypothesis: Implications for brain disease. Neurobiol. Dis. 2016, 90, 75–85. [Google Scholar] [CrossRef]
- Colasanti, A.; Bugiardini, E.; Amawi, S.; Poole, O.V.; Skorupinska, I.; Skorupinska, M.; Germain, L.; Kozyra, D.; Holmes, S.; James, N.; et al. Primary mitochondrial diseases increase susceptibility to bipolar affective disorder. J. Neurol. Neurosurg. Psychiatry 2020, 91, 892–894. [Google Scholar] [CrossRef]
- Onyango, I.G.; Lu, J.; Rodova, M.; Lezi, E.; Crafter, A.B.; Swerdlow, R.H. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2010, 1802, 228–234. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Systematic Review. Psychosom. Med. 2018, 80, 141–153. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Nierenberg, A.A. Mitochondrial Dysfunction: At the Core of Psychiatric Disorders? Biol. Psychiatry 2018, 83, 718–719. [Google Scholar] [CrossRef]
- Giménez-Palomo, A.; Dodd, S.; Anmella, G.; Carvalho, A.F.; Scaini, G.; Quevedo, J.; Pacchiarotti, I.; Vieta, E.; Berk, M. The Role of Mitochondria in Mood Disorders: From Physiology to Pathophysiology and to Treatment. Front. Psychiatry 2021, 12, 546801. [Google Scholar] [CrossRef]
- Pinna, A.; Colasanti, A. The Neurometabolic Basis of Mood Instability: The Parvalbumin Interneuron Link—A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 689473. [Google Scholar] [CrossRef]
- Gong, Y.; Chai, Y.; Ding, J.-H.; Sun, X.-L.; Hu, G. Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neurosci. Lett. 2011, 488, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Detka, J.; Kurek, A.; Kucharczyk, M.; Głombik, K.; Basta-Kaim, A.; Kubera, M.; Lasoń, W.; Budziszewska, B. Brain glucose metabolism in an animal model of depression. Neuroscience 2015, 295, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Hock, A.; Henning, A.; Seifritz, E.; Boeker, H.; Grimm, S. Increased pregenual anterior cingulate glucose and lactate concentrations in major depressive disorder. Mol. Psychiatry 2016, 22, 113–119. [Google Scholar] [CrossRef]
- Bradley, K.; Mao, X.; Case, J.; Kang, G.; Shungu, D.; Gabbay, V. Increased ventricular cerebrospinal fluid lactate in depressed adolescents. Eur. Psychiatry 2016, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Regenold, W.T.; Phatak, P.; Marano, C.M.; Sassan, A.; Conley, R.R.; Kling, M.A. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: Implications for the mitochondrial dysfunction hypothesis. Biol. Psychiatry 2009, 65, 489–494. [Google Scholar] [CrossRef]
- Millar, J.K.; Christie, S.; Anderson, S.; Lawson, D.; Loh, D.; Devon, R.S.; Arveiler, B.; Muir, W.J.; Blackwood, D.H.; Porteous, D.J. Genomic structure and localisation within a linkage hotspot of Disrupted In Schizophrenia 1, a gene disrupted by a translocation segregating with schizophrenia. Mol. Psychiatry 2001, 6, 173–178. [Google Scholar] [CrossRef]
- Jouroukhin, Y.; Kageyama, Y.; Misheneva, V.; Shevelkin, A.; Andrabi, S.; Prandovszky, E.; Yolken, R.H.; Dawson, V.L.; Dawson, T.M.; Aja, S.; et al. DISC1 regulates lactate metabolism in astrocytes: Implications for psychiatric disorders. Transl. Psychiatry 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Sullivan, C.R.; O’donovan, S.M.; McCullumsmith, R.E.; Ramsey, A. Defects in Bioenergetic Coupling in Schizophrenia. Biol. Psychiatry 2018, 83, 739–750. [Google Scholar] [CrossRef]
- Roberts, R.; Barksdale, K.; Roche, J.; Lahti, A. Decreased synaptic and mitochondrial density in the postmortem anterior cingulate cortex in schizophrenia. Schizophr. Res. 2015, 168, 543–553. [Google Scholar] [CrossRef]
- Du, F.; Cooper, A.J.; Thida, T.; Sehovic, S.; Lukas, S.E.; Cohen, B.M.; Zhang, X.; Öngür, D. In Vivo Evidence for Cerebral Bioenergetic Abnormalities in Schizophrenia Measured Using31P Magnetization Transfer Spectroscopy. JAMA Psychiatry 2014, 71, 19–27. [Google Scholar] [CrossRef]
- Rowland, L.M.; Pradhan, S.; Korenic, S.; Wijtenburg, S.A.; Hong, L.E.; Edden, R.A.; Barker, P.B. Elevated brain lactate in schizophrenia: A 7 T magnetic resonance spectroscopy study. Transl. Psychiatry 2016, 6, e967. [Google Scholar] [CrossRef]
- Fizíková, I.; Dragašek, J.; Račay, P. Mitochondrial Dysfunction, Altered Mitochondrial Oxygen, and Energy Metabolism Associated with the Pathogenesis of Schizophrenia. Int. J. Mol. Sci. 2023, 24, 7991. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, A.M.; McPhie, D.L.; Lange, N.T.; Punzell, S.; Elmiligy, S.; Ye, N.Z.; Froimowitz, M.P.; Hassinger, L.C.; Menesale, E.B.; Sargent, L.W.; et al. Abnormalities in Mitochondrial Structure in Cells from Patients with Bipolar Disorder. Am. J. Pathol. 2010, 177, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, A.C.; Shao, L.; Wang, J.-F.; Young, L.T. Mitochondrial Complex I Activity and Oxidative Damage to Mitochondrial Proteins in the Prefrontal Cortex of Patients With Bipolar Disorder. Arch. Gen. Psychiatry 2010, 67, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, A.C.; Wang, J.-F.; Salmasi, F.; Shao, L.; Young, L.T. Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J. Neurochem. 2013, 127, 552–561. [Google Scholar] [CrossRef]
- CKonradi, C.; Eaton, M.; MacDonald, M.L.; Walsh, J.; Benes, F.M.; Heckers, S. Molecular Evidence for Mitochondrial Dysfunction in Bipolar Disorder. Arch. Gen. Psychiatry 2004, 61, 300–308. [Google Scholar] [CrossRef]
- Bodenstein, D.F.; Kim, H.K.; Brown, N.C.; Navaid, B.; Young, L.T.; Andreazza, A.C. Mitochondrial DNA content and oxidation in bipolar disorder and its role across brain regions. NPJ Schizophr. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Kato, T.; Takahashi, S.; Shioiri, T.; Murashita, J.; Hamakawa, H.; Inubushi, T. Reduction of brain phosphocreatine in bipolar II disorder detected by phosphorus-31 magnetic resonance spectroscopy. J. Affect. Disord. 1994, 31, 125–133. [Google Scholar] [CrossRef]
- Du, F.; Yuksel, C.; Chouinard, V.-A.; Huynh, P.; Ryan, K.; Cohen, B.M.; Öngür, D. Abnormalities in High-Energy Phosphate Metabolism in First-Episode Bipolar Disorder Measured Using 31P-Magnetic Resonance Spectroscopy. Biol. Psychiatry 2017, 84, 797–802. [Google Scholar] [CrossRef]
- Yuksel, C.; Du, F.; Ravichandran, C.; Goldbach, J.R.; Thida, T.; Lin, P.; Dora, B.; Gelda, J.; O’Connor, L.; Sehovic, S.; et al. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol. Psychiatry 2015, 20, 1079–1084. [Google Scholar] [CrossRef]
- Kuang, H.; Duong, A.; Jeong, H.; Zachos, K.; Andreazza, A.C. Lactate in bipolar disorder: A systematic review and meta-analysis. Psychiatry Clin. Neurosci. 2018, 72, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Stork, C.; Renshaw, P.F. Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol. Psychiatry 2005, 10, 900–919. [Google Scholar] [CrossRef] [PubMed]
- BCuthbert, B.N. The RDoC framework: Facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 2014, 13, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Definitions of the RDoC Domains and Constructs. National Institute of Mental Health (NIMH). Available online: https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/definitions-of-the-rdoc-domains-and-constructs (accessed on 18 May 2023).
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Rossiter, H.B.; Brooks, G.A.; Gladden, L.B. The anaerobic threshold: 50+ years of controversy. J. Physiol. 2020, 599, 737–767. [Google Scholar]
- Cohen, M.E.; White, P.D. Life Situations, Emotions, and Neurocirculatory Asthenia (Anxiety Neurosis, Neurasthenia, Effort Syndrome). Psychosom. Med. 1951, 13, 335–357. [Google Scholar] [CrossRef]
- Holmgren, A.; Strom, G. Blood lactate concentration in relation to absolute and relative work load in normal men, and in mitral stenosis, atrial septal defect and vasoregulatory asthenia. Acta Med. Scand. 1959, 163, 185–193. [Google Scholar] [CrossRef]
- Jones, M.; Mellersh, V.; Musgrave, M. A Comparison of the Exercise Response in Anxiety States and Normal Controls. Psychosom. Med. 1946, 8, 180–187. [Google Scholar] [CrossRef]
- Linko, E. Lactic acid response to muscular exercise in neurocirculatory asthenia. Ann. Med. Intern. Fenn. 1950, 39, 161–176. [Google Scholar]
- Pitts, F.N.; McClure, J.N. Lactate Metabolism in Anxiety Neurosis. N. Engl. J. Med. 1967, 277, 1329–1336. [Google Scholar] [CrossRef]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragnière, L.; Meylan, E.M.; Petit, J.M.; Fiumelli, H.; Magistretti, P.J.; Martin, J.L. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 2018, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A. From rags to riches: Lactate ascension as a pivotal metabolite in neuroenergetics. Front. Neurosci. 2023, 17, 1145358. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in contemporary biology: A phoenix risen. J. Physiol. 2022, 600, 1229–1251. [Google Scholar]
- Aryaman, J.; Johnston, I.G.; Jones, N.S. Mitochondrial Heterogeneity. Front. Genet. 2019, 9, 718. [Google Scholar]
- Wallace, D.C.; Chalkia, D. Mitochondrial DNA Genetics and the Heteroplasmy Conundrum in Evolution and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a021220. [Google Scholar] [CrossRef]
- Maddock, R.J.; Casazza, G.A.; Buonocore, M.H.; Tanase, C. Vigorous exercise increases brain lactate and Glx (glutamate+glutamine): A dynamic 1H-MRS study. NeuroImage 2011, 57, 1324–1330. [Google Scholar] [CrossRef]
- Shirbandi, K.B.; Rikhtegar, R.; Khalafi, M.; Attari, M.M.A.; Rahmani, F.; Javanmardi, P.B.; Iraji, S.M.; Aghdam, Z.B.; Rashnoudi, A.M.R. Functional Magnetic Resonance Spectroscopy of Lactate in Alzheimer Disease: A Comprehensive Review of Alzheimer Disease Pathology and the Role of Lactate. Top. Magn. Reson. Imaging 2023, 32, 15–26. [Google Scholar]
- Gladden, L.B. Lactate metabolism: A new paradigm for the third millennium. J. Physiol. 2004, 558 Pt 1, 5–30. [Google Scholar]
- Gibbs, M.E.; Hertz, L. Inhibition of astrocytic energy metabolism by d-lactate exposure impairs memory. Neurochem. Int. 2008, 52, 1012–1018. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xu, L.; Wang, A.; Zou, Y.; Li, T.; Huang, L.; Chen, W.; Liu, S.; Jiang, K.; et al. Ultrasensitive sensors reveal the spatiotemporal landscape of lactate metabolism in physiology and disease. Cell Metab. 2023, 35, 200–211.e9. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Hui, S.; Cowan, A.J.; Zeng, X.; Yang, L.; TeSlaa, T.; Li, X.; Bartman, C.; Zhang, Z.; Jang, C.; Wang, L.; et al. Quantitative Fluxomics of Circulating Metabolites. Cell Metab. 2020, 32, 676–688.e4. [Google Scholar] [CrossRef]
- Parikh, S.; Goldstein, A.; Koenig, M.K.; Scaglia, F.; Enns, G.M.; Saneto, R.; Anselm, I.; Cohen, B.H.; Falk, M.J.; Greene, C.; et al. Diagnosis and management of mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Anesth. Analg. 2015, 17, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.E.; Pories, W.J.; Houmard, J.A.; Tanner, C.J.; Zheng, D.; Zou, K.; Coen, P.M.; Goodpaster, B.H.; Kraus, W.E.; Dohm, G.L. Plasma lactate as a marker of metabolic health: Implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surgery 2019, 166, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Wolfel, E.E.; Butterfield, G.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A.; Sun, S.; Li, H.; Chen, J.; et al. Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 1999, 87, 1684–1696. [Google Scholar] [CrossRef]
- Donovan, C.M.; Brooks, G.A. Endurance training affects lactate clearance, not lactate production. Am. J. Physiol.-Endocrinol. Metab. 1983, 244, E83–E92. [Google Scholar] [CrossRef]
- Wallace, N.K.; Pollard, F.; Savenkova, M.; Karatsoreos, I.N. Effect of aging on daily rhythms in lactate metabolism in the medial prefrontal cortex of male mice. Neuroscience 2020, 448, 300–310. [Google Scholar] [CrossRef]
- Shram, N.; Netchiporouk, L.; Cespuglio, R. Lactate in the brain of the freely moving rat: Voltammetric monitoring of the changes related to the sleep-wake states. Eur. J. Neurosci. 2002, 16, 461–466. [Google Scholar] [CrossRef]
- Lundgaard, I.; Lu, M.L.; Yang, E.; Peng, W.; Mestre, H.; Hitomi, E.; Deane, R.; Nedergaard, M. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J. Cereb. Blood Flow Metab. 2016, 37, 2112–2124. [Google Scholar] [CrossRef]
- Noort, S.V.D.; Brine, K. Effect of sleep on brain labile phosphates and metabolic rate. Am. J. Physiol. Content 1970, 218, 1434–1439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wisor, J.P.; Rempe, M.J.; Schmidt, M.A.; Moore, M.E.; Clegern, W.C. Sleep Slow-Wave Activity Regulates Cerebral Glycolytic Metabolism. Cereb. Cortex 2012, 23, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Hadjihambi, A.; Karagiannis, A.; Theparambil, S.M.; Ackland, G.L.; Gourine, A.V. The effect of general anaesthetics on brain lactate release. Eur. J. Pharmacol. 2020, 881, 173188. [Google Scholar] [CrossRef] [PubMed]
- Koenigsberg, H.W.; Pollak, C.P.; Fine, J.; Kakuma, T. Lactate sensitivity in sleeping panic disorder patients and healthy controls. Biol. Psychiatry 1992, 32, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.; Aillon, D.V.; Barrett, B.S.; Wilson, G.S.; Johnson, D.A.; Johnson, D.A.; Harmon, H.P.; Gabbert, S.; Petillo, P.A. Lactate as a Biomarker for Sleep. Sleep 2012, 35, 1209–1222. [Google Scholar] [CrossRef]
- Ramanathan, L.; Hu, S.; Frautschy, S.A.; Siegel, J.M. Short-term total sleep deprivation in the rat increases antioxidant responses in multiple brain regions without impairing spontaneous alternation behavior. Behav. Brain Res. 2010, 207, 305–309. [Google Scholar] [CrossRef]
- Boumezbeur, F.; Petersen, K.F.; Cline, G.W.; Mason, G.F.; Behar, K.L.; Shulman, G.I.; Rothman, D.L. The Contribution of Blood Lactate to Brain Energy Metabolism in Humans Measured by Dynamic13C Nuclear Magnetic Resonance Spectroscopy. J. Neurosci. 2010, 30, 13983–13991. [Google Scholar] [CrossRef]
- Díaz-García, C.M.; Yellen, G. Neurons rely on glucose rather than astrocytic lactate during stimulation. J. Neurosci. Res. 2018, 97, 883–889. [Google Scholar] [CrossRef]
- Brown, A.M.; Ransom, B.R. Astrocyte glycogen and brain energy metabolism. Glia 2007, 55, 1263–1271. [Google Scholar] [CrossRef]
- Hu, Y.; Wilson, G.S. A Temporary Local Energy Pool Coupled to Neuronal Activity: Fluctuations of Extracellular Lactate Levels in Rat Brain Monitored with Rapid-Response Enzyme-Based Sensor. J. Neurochem. 1997, 69, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [PubMed]
- Prichard, J.; Rothman, D.; Novotny, E.; Petroff, O.; Kuwabara, T.; Avison, M.; Howseman, A.; Hanstock, C.; Shulman, R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc. Natl. Acad. Sci. USA 1991, 88, 5829–5831. [Google Scholar] [CrossRef] [PubMed]
- Vanzetta, I.; Grinvald, A. Increased Cortical Oxidative Metabolism Due to Sensory Stimulation: Implications for Functional Brain Imaging. Science 1999, 286, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.-L.; Stella, N.; Magistretti, P.J. Evidence Supporting the Existence of an Activity-Dependent Astrocyte-Neuron Lactate Shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Schurr, A.; Miller, J.J.; Payne, R.S.; Rigor, B.M. An Increase in Lactate Output by Brain Tissue Serves to Meet the Energy Needs of Glutamate-Activated Neurons. J. Neurosci. 1999, 19, 34–39. [Google Scholar] [CrossRef]
- Zuend, M.; Saab, A.S.; Wyss, M.T.; Ferrari, K.D.; Hösli, L.; Looser, Z.J.; Stobart, J.L.; Duran, J.; Guinovart, J.J.; Barros, L.F.; et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat. Metab. 2020, 2, 179–191. [Google Scholar] [CrossRef]
- Elekesa, O.; Venema, K.; Postema, F.; Dringen, R.; Hamprecht, B.; Korf, J. Evidence that stress activates glial lactate formation in vivo assessed with rat hippocampus lactography. Neurosci. Lett. 1996, 208, 69–72. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef]
- Newman, L.A.; Korol, D.L.; Gold, P.E. Lactate Produced by Glycogenolysis in Astrocytes Regulates Memory Processing. PLoS ONE 2011, 6, e28427. [Google Scholar] [CrossRef]
- Murphy-Royal, C.; Johnston, A.D.; Boyce, A.K.J.; Diaz-Castro, B.; Institoris, A.; Peringod, G.; Zhang, O.; Stout, R.F.; Spray, D.C.; Thompson, R.J.; et al. Stress gates an astrocytic energy reservoir to impair synaptic plasticity. Nat. Commun. 2020, 11, 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Q.; Shao, X.; Ouyang, L.; Wang, X.; Zhu, K.; Chen, L. Decreased Glycogen Content Might Contribute to Chronic Stress-Induced Atrophy of Hippocampal Astrocyte volume and Depression-like Behavior in Rats. Sci. Rep. 2017, 7, 43192. [Google Scholar] [CrossRef] [PubMed]
- Quistorff, B.; Secher, N.H.; Van Lieshout, J.J. Lactate fuels the human brain during exercise. FASEB J. 2008, 22, 3443–3449. [Google Scholar] [CrossRef]
- Kemppainen, J.; Aalto, S.; Fujimoto, T.; Kalliokoski, K.K.; Långsjö, J.; Oikonen, V.; Rinne, J.; Nuutila, P.; Knuuti, J. High intensity exercise decreases global brain glucose uptake in humans. J. Physiol. 2005, 568, 323–332. [Google Scholar] [CrossRef]
- Van Hall, G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 2010, 199, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.F. Metabolic signaling by lactate in the brain. Trends Neurosci. 2013, 36, 396–404. [Google Scholar] [CrossRef]
- Maddock, R.J.; Buonocore, M.H.; Copeland, L.E.; Richards, A.L. Elevated brain lactate responses to neural activation in panic disorder: A dynamic 1H-MRS study. Mol. Psychiatry 2008, 14, 537–545. [Google Scholar] [CrossRef]
- Dager, S.R.; Yim, J.B.; Khalil, G.E.; Artru, A.A.; Bowden, D.M.; Kenny, M.A. Application of a Novel Fiber-Optic Biosensor In Situ to Investigate the Metabolic Effect of Lactate Infusion. Neuropsychopharmacology 1995, 12, 307–313. [Google Scholar] [CrossRef]
- Garcia, C.K.; Goldstein, J.L.; Pathak, R.K.; Anderson, R.G.W.; Brown, M.S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: Implications for the Cori cycle. Cell 1994, 76, 865–873. [Google Scholar] [CrossRef]
- Sinning, A.; Hübner, C.A. Minireview: pH and synaptic transmission. FEBS Lett. 2013, 587, 1923–1928. [Google Scholar] [CrossRef]
- Waldmann, R.; Champigny, G.; Bassilana, F.; Heurteaux, C.; Lazdunski, M. A proton-gated cation channel involved in acid-sensing. Nature 1997, 386, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, G.; Schruers, K.R.; Maddock, R.J.; Colasanti, A.; Griez, E.J. Acids in the brain: A factor in panic? J. Psychopharmacol. 2010, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Milbank, E.; López, M. Orexins/Hypocretins: Key Regulators of Energy Homeostasis. Front. Endocrinol. 2019, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Ueta, Y.; Yamashita, H.; Yamaguchi, H.; Matsukura, S.; Kangawa, K.; Sakurai, T.; Yanagisawa, M.; Nakazato, M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems’. Proc. Natl. Acad. Sci. USA 1999, 96, 748–753. [Google Scholar] [CrossRef]
- Severson, C.A.; Wang, W.; Pieribone, V.A.; Dohle, C.I.; Richerson, G.B. Midbrain serotonergic neurons are central pH chemoreceptors. Nat. Neurosci. 2003, 6, 1139–1140. [Google Scholar] [CrossRef]
- Gargaglioni, L.H.; Hartzler, L.K.; Putnam, R.W. The locus coeruleus and central chemosensitivity. Respir. Physiol. Neurobiol. 2010, 173, 264–273. [Google Scholar] [CrossRef]
- Lauritzen, K.H.; Morland, C.; Puchades, M.; Holm-Hansen, S.; Hagelin, E.M.; Lauritzen, F.; Attramadal, H.; Storm-Mathisen, J.; Gjedde, A.; Bergersen, L.H. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb. Cortex 2014, 24, 2784–2795. [Google Scholar] [CrossRef]
- Morland, C.; Lauritzen, K.H.; Puchades, M.; Holm-Hansen, S.; Andersson, K.; Gjedde, A.; Attramadal, H.; Storm-Mathisen, J.; Bergersen, L.H. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J. Neurosci. Res. 2015, 93, 1045–1055. [Google Scholar] [CrossRef]
- De Castro Abrantes, H.; Briquet, M.; Schmuziger, C.; Restivo, L.; Puyal, J.; Rosenberg, N.; Rocher, A.B.; Offermanns, S.; Chatton, J.Y. The Lactate Receptor HCAR1 Modulates Neuronal Network Activity through the Activation of Gα and Gβγ Subunits. J. Neurosci. 2019, 39, 4422–4433. [Google Scholar] [CrossRef]
- Gilbert, E.; Tang, J.M.; Ludvig, N.; Bergold, P.J. Elevated lactate suppresses neuronal firing in vivo and inhibits glucose metabolism in hippocampal slice cultures. Brain Res. 2006, 1117, 213–223. [Google Scholar] [CrossRef]
- Coco, M.; Alagona, G.; Rapisarda, G.; Costanzo, E.; Calogero, R.A.; Perciavalle, V.; Perciavalle, V. Md Elevated blood lactate is associated with increased motor cortex excitability. Somatosens. Mot. Res. 2010, 27, 1–8. [Google Scholar] [CrossRef]
- Hollnagel, J.-O.; Cesetti, T.; Schneider, J.; Vazetdinova, A.; Valiullina-Rakhmatullina, F.; Lewen, A.; Rozov, A.; Kann, O. Lactate Attenuates Synaptic Transmission and Affects Brain Rhythms Featuring High Energy Expenditure. iScience 2020, 23, 101316. [Google Scholar] [CrossRef] [PubMed]
- Scavuzzo, C.J.; Rakotovao, I.; Dickson, C.T. Differential effects of L- and D-lactate on memory encoding and consolidation: Potential role of HCAR1 signaling. Neurobiol. Learn. Mem. 2020, 168, 107151. [Google Scholar] [CrossRef]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef]
- Lambertus, M.; Øverberg, L.T.; Andersson, K.A.; Hjelden, M.S.; Hadzic, A.; Haugen, Ø.P.; Storm-Mathisen, J.; Bergersen, L.H.; Geiseler, S.; Morland, C. L-lactate induces neurogenesis in the mouse ventricular-subventricular zone via the lactate receptor HCA1. Acta Physiol. 2021, 231, e13587. [Google Scholar]
- Lambertus, M.; Øverberg, L.T.; Andersson, K.A.; Hjelden, M.S.; Hadzic, A.; Haugen, Ø.P.; Storm-Mathisen, J.; Bergersen, L.H.; Geiseler, S.; Morland, C. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar]
- Vardjan, N.; Chowdhury, H.H.; Horvat, A.; Velebit, J.; Malnar, M.; Muhič, M.; Kreft, M.; Krivec, Š.G.; Bobnar, S.T.; Miš, K.; et al. Enhancement of Astroglial Aerobic Glycolysis by Extracellular Lactate-Mediated Increase in cAMP. Front. Mol. Neurosci. 2018, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Lane, S.; Korsak, A.; Paton, J.F.; Gourine, A.V.; Kasparov, S.; Teschemacher, A.G. Lactate-mediated glia-neuronal signalling in the mamalian brain. Nat. Commun. 2014, 5, 3284. [Google Scholar] [CrossRef]
- Shaif, N.A.; Jang, D.; Cho, D.; Kim, S.; Seo, D.B.; Shim, I. The Antidepressant-Like Effect of Lactate in an Animal Model of Menopausal Depression. Biomedicines 2018, 6, 108. [Google Scholar] [CrossRef]
- Gordon, G.R.J.; Choi, H.B.; Rungta, R.L.; Ellis-Davies, G.C.R.; MacVicar, B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008, 456, 745–749. [Google Scholar] [CrossRef]
- Mintun, M.A.; Vlassenko, A.G.; Rundle, M.M.; Raichle, M.E. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc. Natl. Acad. Sci. USA 2004, 101, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Holstein, J.D.; Upadhyay, G.; Lin, D.-T.; Conway, S.; Muller, E.; Lechleiter, J.D. Purinergic Receptor-Stimulated IP3-Mediated Ca2+ Release Enhances Neuroprotection by Increasing Astrocyte Mitochondrial Metabolism during Aging. J. Neurosci. 2007, 27, 6510–6520. [Google Scholar] [CrossRef] [PubMed]
- Requardt, R.P.; Hirrlinger, P.G.; Wilhelm, F.; Winkler, U.; Besser, S.; Hirrlinger, J. Ca2+ signals of astrocytes are modulated by the NAD+/NADH redox state. J. Neurochem. 2012, 120, 1014–1025. [Google Scholar] [CrossRef]
- Parsons, M.P.; Hirasawa, M. ATP-Sensitive Potassium Channel-Mediated Lactate Effect on Orexin Neurons: Implications for Brain Energetics during Arousal. J. Neurosci. 2010, 30, 8061–8070. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, Y.; Wang, H.; Zhang, T.; Duan, F.; Wu, K.; Yang, S.; Xu, K.; Jiang, X.; Sun, X. Lactate and Lactylation in the Brain: Current Progress and Perspectives. Cell Mol. Neurobiol. 2023, 43, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, W.; Zhou, X. Lactylation, an emerging hallmark of metabolic reprogramming: Current progress and open challenges. Front. Cell Dev. Biol. 2022, 10, 972020. [Google Scholar] [CrossRef]
- Hagihara, H.; Shoji, H.; Otabi, H.; Toyoda, A.; Katoh, K.; Namihira, M.; Miyakawa, T. Protein lactylation induced by neural excitation. Cell Rep. 2021, 37, 109820. [Google Scholar] [CrossRef]
- San-Millan, I.; Sparagna, G.C.; Chapman, H.L.; Warkins, V.L.; Chatfield, K.C.; Shuff, S.R.; Martinez, J.L.; Brooks, G.A. Chronic Lactate Exposure Decreases Mitochondrial Function by Inhibition of Fatty Acid Uptake and Cardiolipin Alterations in Neonatal Rat Cardiomyocytes. Front. Nutr. 2022, 9, 809485. [Google Scholar] [CrossRef]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef]
- Parikh, S.; The Mitochondrial Medicine Society; Saneto, R.; Falk, M.J.; Anselm, I.; Cohen, B.H.; Haas, R. A modern approach to the treatment of mitochondrial disease. Curr. Treat. Options Neurol. 2009, 11, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Zanetti, M.V.; Otaduy, M.C.; De Sousa, R.T.; Soeiro-de-Souza, M.G.; Costa, A.C.; Carvalho, A.F.; Leite, C.C.; Busatto, G.F.; Zarate, C.A., Jr.; et al. Increased Brain Lactate During Depressive Episodes and Reversal Effects by Lithium Monotherapy in Drug-Naive Bipolar Disorder: A 3-T 1H-MRS Study. J. Clin. Psychopharmacol. 2017, 37, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lyoo, I.K.; Yoon, S.J.; Choi, T.; Lee, B.; Kim, J.E.; Lee, J.S.; Renshaw, P.F. Clinical response of quetiapine in rapid cycling manic bipolar patients and lactate level changes in proton magnetic resonance spectroscopy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Brivio, P.; Audano, M.; Gallo, M.T.; Miceli, E.; Gruca, P.; Lason, M.; Litwa, E.; Fumagalli, F.; Papp, M.; Mitro, N.; et al. Venlafaxine’s effect on resilience to stress is associated with a shift in the balance between glucose and fatty acid utilization. Neuropsychopharmacology 2023, 48, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Forester, B.P.M.; Harper, D.G.; Georgakas, J.; Ravichandran, C.; Madurai, N.; Cohen, B.M. Antidepressant Effects of Open Label Treatment With Coenzyme Q10 in Geriatric Bipolar Depression. J. Clin. Psychopharmacol. 2015, 35, 338–340. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, E.; Park, J.; Kim, J.; Tanaka, M.; Choi, J. Physiological significance of elevated levels of lactate by exercise training in the brain and body. J. Biosci. Bioeng. 2023, 135, 167–175. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Mikami, T. Exercise-Induced Lactate Release Mediates Mitochondrial Biogenesis in the Hippocampus of Mice via Monocarboxylate Transporters. Front. Physiol. 2021, 12, 736905. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Steiner, J.L.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Davis, J.M. Exercise training increases mitochondrial biogenesis in the brain. J. Appl. Physiol. 2011, 111, 1066–1071. [Google Scholar] [CrossRef]
- Millán, I.S. The Use Of Individualized Exercise Prescription To Target Oxidative Metabolism In A Stage Iv Colorectal, Metastatic Cancer Patient: 581 May 29 1:20 PM–1:40 PM. Med. Sci. Sports Exerc. 2019, 51, 151. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. The art of mitochondrial maintenance. Curr. Biol. CB 2018, 28, R170–R185. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Walder, K.R.; Berk, M.; Marx, W.; Walker, A.J.; Maes, M.; Puri, B.K. The interplay between oxidative stress and bioenergetic failure in neuropsychiatric illnesses: Can we explain it and can we treat it? Mol. Biol. Rep. 2020, 47, 5587–5620. [Google Scholar] [CrossRef]
- Corona, J.C.; Duchen, M.R. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Sadovnikova, I.S.; Starkov, N.N.; Starkov, A.A.; Popov, V.N. p62-Nrf2-p62 Mitophagy Regulatory Loop as a Target for Preventive Therapy of Neurodegenerative Diseases. Brain Sci. 2020, 10, 847. [Google Scholar] [CrossRef]
- Koroshetz, W.J.; Jenkins, B.G.; Rosen, B.R.; Beal, M.F. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann. Neurol. 1997, 41, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, Z.; Zhu, J.; Yang, J.; He, J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology 2011, 60, 252–258. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Ye, X.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Martinez-Larranaga, M.R.; Wang, X.; Anadón, A.; Martinez, M.A. Mitochondria as an important target of metformin: The mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol. Res. 2022, 177, 106114. [Google Scholar] [CrossRef]
- Rudic, J.S.; Poropat, G.; Krstic, M.N.; Bjelakovic, G.; Gluud, C. Bezafibrate for primary biliary cirrhosis. Cochrane Database Syst. Rev. 2012, 1, CD009145. [Google Scholar] [CrossRef]
- Jakob, T.; Nordmann, A.J.; Schandelmaier, S.; Ferreira-González, I.; Briel, M. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst. Rev. 2016, 2017, CD009753. [Google Scholar] [CrossRef]
- Douiev, L.; Sheffer, R.; Horvath, G.; Saada, A. Bezafibrate Improves Mitochondrial Fission and Function in DNM1L-Deficient Patient Cells. Cells 2020, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Zhou, D.; Cui, X.-S. Bezafibrate prevents aging in in vitro-matured porcine oocytes. J. Anim. Sci. Technol. 2021, 63, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Yang, Z.; Hua, L.; Chen, Y.; Zhou, Y.; Ou, Y.; Chen, X.; Yue, H.; Yang, X.; Wu, X.; et al. Ciclopirox inhibits NLRP3 inflammasome activation via protecting mitochondria and ameliorates imiquimod-induced psoriatic inflammation in mice. Eur. J. Pharmacol. 2022, 930, 175156. [Google Scholar] [CrossRef] [PubMed]
- Minden, M.D.; Hogge, D.E.; Weir, S.J.; Kasper, J.; Webster, D.A.; Patton, L.; Jitkova, Y.; Hurren, R.; Gronda, M.; Goard, C.A.; et al. Oral ciclopirox olamine displays biological activity in a phase I study in patients with advanced hematologic malignancies. Am. J. Hematol. 2014, 89, 363–368. [Google Scholar] [CrossRef]
- Wang, W.-W.; Han, R.; He, H.-J.; Wang, Z.; Luan, X.-Q.; Li, J.; Feng, L.; Chen, S.-Y.; Aman, Y.; Xie, C.-L. Delineating the Role of Mitophagy Inducers for Alzheimer Disease Patients. Aging Dis. 2021, 12, 852–867. [Google Scholar] [CrossRef]
- Feigin, A.; Kieburtz, K.; Como, P.; Hickey, C.; Abwender, D.; Zimmerman, C.; Steinberg, K.; Shoulson, I. Assessment of coenzyme q10 tolerability in huntington’s disease. Mov. Disord. 1996, 11, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Shults, C.W.; Haas, R.H.; Passov, D.; Beal, M.F. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann. Neurol. 1997, 42, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Stokes, K.; Mahngar, K.; Domazet-Damjanov, D.; Sikorska, M.; Pandey, S. Inhibition of stress induced premature senescence in presenilin-1 mutated cells with water soluble Coenzyme Q10. Mitochondrion 2014, 17, 106–115. [Google Scholar] [CrossRef]
- Devos, D.; Moreau, C.; Devedjian, J.C.; Kluza, J.; Petrault, M.; Laloux, C.; Jonneaux, A.; Ryckewaert, G.; Garçon, G.; Rouaix, N.; et al. Targeting Chelatable Iron as a Therapeutic Modality in Parkinson’s Disease. Antioxid. Redox Signal. 2014, 21, 195–210. [Google Scholar] [CrossRef]
- Bollig, C.; Schell, L.K.; Rücker, G.; Allert, R.; Motschall, E.; Niemeyer, C.M.; Bassler, D.; Meerpohl, J.J. Deferasirox for managing iron overload in people with thalassaemia. Cochrane Database Syst. Rev. 2017, 2017, CD007476. [Google Scholar] [CrossRef]
- Meerpohl, J.J.; Schell, L.K.; Rücker, G.; Motschall, E.; Fleeman, N.; Niemeyer, C.M.; Bassler, D. Deferasirox for managing transfusional iron overload in people with sickle cell disease. Cochrane Database Syst. Rev. 2014, 2017, CD007477. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, M.; Arpa, J.; Delatycki, M.B.; Le Quan Sang, K.H.; Mariotti, C.; Munnich, A.; Sanz-Gallego, I.; Tai, G.; Tarnopolsky, M.A.; Taroni, F.; et al. Deferiprone in Friedreich Ataxia: A 6-Month Randomized Controlled Trial. Ann. Neurol. 2014, 76, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Deferiprone to Delay Dementia (The 3D Study)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03234686 (accessed on 29 June 2023).
- Saxena, D.; Spino, M.; Tricta, F.; Connelly, J.; Cracchiolo, B.M.; Hanauske, A.R.; D’Alliessi Gandolfi, D.; Mathews, M.B.; Karn, J.; Holland, B.; et al. Drug-Based Lead Discovery: The Novel Ablative Antiretroviral Profile of Deferiprone in HIV-1-Infected Cells and in HIV-Infected Treatment-Naive Subjects of a Double-Blind, Placebo-Controlled, Randomized Exploratory Trial. PLoS ONE 2016, 11, e0154842. [Google Scholar] [CrossRef]
- Yin, X.; Manczak, M.; Reddy, P.H. Mitochondria-targeted molecules MitoQ and SS31 reduce mutant huntingtin-induced mitochondrial toxicity and synaptic damage in Huntington’s disease. Hum. Mol. Genet. 2016, 25, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Calkins, M.J.; Manczak, M.; Mao, P.; Shirendeb, U.; Reddy, P.H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2011, 20, 4515–4529. [Google Scholar] [CrossRef] [PubMed]
- Daubert, M.A.; Yow, E.; Dunn, G.; Marchev, S.; Barnhart, H.; Douglas, P.S.; O’Connor, C.; Goldstein, S.; Udelson, J.E.; Sabbah, H.N. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ. Heart Fail. 2017, 10, e004389. [Google Scholar] [CrossRef]
- Hortmann, M.; Robinson, S.; Mohr, M.; Mauler, M.; Stallmann, D.; Reinöhl, J.; Duerschmied, D.; Peter, K.; Carr, J.; Gibson, C.M.; et al. The mitochondria-targeting peptide elamipretide diminishes circulating HtrA2 in ST-segment elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2017, 8, 695–702. [Google Scholar] [CrossRef]
- Mettu, P.S.; Allingham, M.J.; Cousins, S.W. Phase 1 Clinical Trial of Elamipretide in Dry Age-Related Macular Degeneration and Noncentral Geographic Atrophy. Ophthalmol. Sci. 2022, 2, 100086. [Google Scholar] [CrossRef]
- Karaa, A.; Bertini, E.; Carelli, V.; Cohen, B.H.; Enns, G.M.; Falk, M.J.; Goldstein, A.; Gorman, G.S.; Haas, R.; Hirano, M.; et al. Efficacy and Safety of Elamipretide in Individuals With Primary Mitochondrial Myopathy: The MMPOWER-3 Randomized Clinical Trial. Neurology 2023, 101, e238–e252. [Google Scholar] [CrossRef]
- Farsad-Naeimi, A.; Alizadeh, M.; Esfahani, A.; Aminabad, E.D. Effect of fisetin supplementation on inflammatory factors and matrix metalloproteinase enzymes in colorectal cancer patients. Food Funct. 2018, 9, 2025–2031. [Google Scholar] [CrossRef]
- Jia, S.; Xu, X.; Zhou, S.; Chen, Y.; Ding, G.; Cao, L. Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis. 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Prem, P.N.; Kurian, G.A. Fisetin attenuates renal ischemia/reperfusion injury by improving mitochondrial quality, reducing apoptosis and oxidative stress. Naunyn. Schmiedebergs Arch. Pharmacol. 2022, 395, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, K.; Ravindran, S.; Kurian, G.A.; Rajesh, M. Fisetin Confers Cardioprotection against Myocardial Ischemia Reperfusion Injury by Suppressing Mitochondrial Oxidative Stress and Mitochondrial Dysfunction and Inhibiting Glycogen Synthase Kinase 3β Activity. Oxidative Med. Cell. Longev. 2018, 2018, 9173436. [Google Scholar] [CrossRef] [PubMed]
- Verdoorn, B.P.; Evans, T.K.; Hanson, G.J.; Zhu, Y.; Prata, L.G.P.L.; Pignolo, R.J.; Atkinson, E.J.; Wissler-Gerdes, E.O.; Kuchel, G.A.; Mannick, J.B.; et al. Fisetin for COVID-19 in skilled nursing facilities: Senolytic trials in the COVID era. J. Am. Geriatr. Soc. 2021, 69, 3023–3033. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, D.; Wu, H.; Jia, H.; Yang, C.; Zhang, L. Fisetin Prolongs Therapy Window of Brain Ischemic Stroke Using Tissue Plasminogen Activator: A Double-Blind Randomized Placebo-Controlled Clinical Trial. Clin. Appl. Thromb. 2019, 25, 1076029619871359. [Google Scholar] [CrossRef] [PubMed]
- Bader, T.; Fazili, J.; Madhoun, M.; Aston, C.; Hughes, D.; Rizvi, S.; Seres, K.; Hasan, M. Fluvastatin Inhibits Hepatitis C Replication in Humans. Am. J. Gastroenterol. 2008, 103, 1383–1389. [Google Scholar] [CrossRef]
- Buemi, M.; Allegra, A.; Corica, F.; Aloisi, C.; Giacobbe, M.; Pettinato, G.; Corsonello, A.; Senatore, M.; Frisina, N. Effect of fluvastatin on proteinuria in patients with immunoglobulin A nephropathy. Clin. Pharmacol. Ther. 2000, 67, 427–431. [Google Scholar] [CrossRef]
- Paez, P.A.; Kolawole, M.; Taruselli, M.T.; Ajith, S.; Dailey, J.M.; Kee, S.A.; Haque, T.T.; Barnstein, B.O.; McLeod, J.J.A.; Caslin, H.L.; et al. Fluvastatin Induces Apoptosis in Primary and Transformed Mast Cells. Experiment 2020, 374, 104–112. [Google Scholar] [CrossRef]
- Ruiz-Limon, P.; Barbarroja, N.; Perez-Sanchez, C.; Aguirre, M.A.; Bertolaccini, M.L.; Khamashta, M.A.; Rodriguez-Ariza, A.; Almadén, Y.; Segui, P.; Khraiwesh, H.; et al. Atherosclerosis and cardiovascular disease in systemic lupus erythematosus: Effects of in vivo statin treatment. Ann. Rheum. Dis. 2014, 74, 1450–1458. [Google Scholar] [CrossRef]
- Winkler, K.; Ablethauser, C.; Gimpelewicz, C.; Bortolini, M.; Isaacsohn, J. Risk reduction and tolerability of fluvastatin in patients with the metabolic syndrome: A pooled analysis of thirty clinical trials. Clin. Ther. 2007, 29, 1987–2000. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, L.; Marshall, I.; Wolfe, C.; Wang, Y. Statin Therapy for Preventing Recurrent Stroke in Patients with Ischemic Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Observational Cohort Studies. Neuroepidemiology 2022, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Man, W.; Shen, M.; Zhang, M.; Lin, J.; Wang, T.; Duan, Y.; Li, C.; Zhang, R.; Gao, E.; et al. Luteolin alleviates post-infarction cardiac dysfunction by up-regulating autophagy through Mst1 inhibition. J. Cell. Mol. Med. 2016, 20, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An Open-Label Pilot Study of a Formulation Containing the Anti-Inflammatory Flavonoid Luteolin and Its Effects on Behavior in Children With Autism Spectrum Disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Versace, V.; Ortelli, P.; Dezi, S.; Ferrazzoli, D.; Alibardi, A.; Bonini, I.; Engl, M.; Maestri, R.; Assogna, M.; Ajello, V.; et al. Co-ultramicronized palmitoylethanolamide/luteolin normalizes GABAB-ergic activity and cortical plasticity in long COVID-19 syndrome. Clin. Neurophysiol. 2023, 145, 81–88. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Yue, Y.; Zhang, Y.; Wu, A. Luteoloside Prevents Sevoflurane-induced Cognitive Dysfunction in Aged Rats via Maintaining Mitochondrial Function and Dynamics in Hippocampal Neurons. Neuroscience 2023, 516, 42–53. [Google Scholar] [CrossRef]
- Calkin, C.V.; Chengappa, K.R.; Cairns, K.; Cookey, J.; Gannon, J.; Alda, M.; O’Donovan, C.; Reardon, C.; Sanches, M.; Růzicková, M. Treating Insulin Resistance With Metformin as a Strategy to Improve Clinical Outcomes in Treatment-Resistant Bipolar Depression (the TRIO-BD Study): A Randomized, Quadruple-Masked, Placebo-Controlled Clinical Trial. J. Clin. Psychiatry 2022, 83, 21m14022. [Google Scholar] [CrossRef]
- Hebrani, P.; Manteghi, A.A.; Behdani, F.; Hessami, E.; Rezayat, K.A.; Marvast, M.N.; Rezayat, A.A. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2015, 20, 364–371. [Google Scholar]
- Ma, Z.; Liu, Z.; Li, X.; Zhang, H.; Han, D.; Xiong, W.; Zhou, H.; Yang, X.; Zeng, Q.; Ren, H.; et al. Metformin Collaborates with PINK1/Mfn2 Overexpression to Prevent Cardiac Injury by Improving Mitochondrial Function. Biology 2023, 12, 582. [Google Scholar] [CrossRef]
- Rizvi, F.; Sheikh, A.; Ahmed, H.; Fahmi, S.; Ikramuddin, Z.; Asif, M. Miracle medicine for prevention of migraine attack: Metformin. Prof. Med. J. 2020, 27, 812–819. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Wang, T.; Sun, G.-F.; Xiao, J.-X.; Jiang, L.-P.; Tou, F.-F.; Qu, X.-H.; Han, X.-J. Metformin protects against retinal ischemia/reperfusion injury through AMPK-mediated mitochondrial fusion. Free Radic. Biol. Med. 2023, 205, 47–61. [Google Scholar] [CrossRef]
- Zhao, M.; Li, X.W.; Chen, D.Z.; Hao, F.; Tao, S.X.; Yu, H.Y.; Cheng, R.; Liu, H. Neuro-Protective Role of Metformin in Patients with Acute Stroke and Type 2 Diabetes Mellitus via AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Pathway and Oxidative Stress. Experiment 2019, 25, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, R.; Li, S.; Zhu, X.; Wang, T.; Wu, J.; Zhang, J. Methylene blue reduces incidence of early postoperative cognitive disorders in elderly patients undergoing major non-cardiac surgery: An open–label randomized controlled clinical trial. J. Clin. Anesthesia 2020, 68, 110108. [Google Scholar] [CrossRef]
- Singh, N.; MacNicol, E.; DiPasquale, O.; Randall, K.; Lythgoe, D.; Mazibuko, N.; Simmons, C.; Selvaggi, P.; Stephenson, S.; Turkheimer, F.E.; et al. The effects of acute Methylene Blue administration on cerebral blood flow and metabolism in humans and rats. J. Cereb. Blood Flow Metab. 2023. OnlineFirst. [Google Scholar] [CrossRef] [PubMed]
- Shiells, H.; Schelter, B.O.; Bentham, P.; Baddeley, T.C.; Rubino, C.M.; Ganesan, H.; Hammel, J.; Vuksanovic, V.; Staff, R.T.; Murray, A.D.; et al. Concentration-Dependent Activity of Hydromethylthionine on Clinical Decline and Brain Atrophy in a Randomized Controlled Trial in Behavioral Variant Frontotemporal Dementia. J. Alzheimer’s Dis. 2020, 75, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Liang, P.; Zhuo, X.; Su, C.; Zong, X.; Guo, B.; Han, D.; Yan, X.; Hu, S.; Zhang, Q.; et al. After Treatment with Methylene Blue is Effective against Delayed Encephalopathy after Acute Carbon Monoxide Poisoning. Basic Clin. Pharmacol. Toxicol. 2017, 122, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Zoellner, L.A.; Telch, M.; Foa, E.B.; Farach, F.J.; McLean, C.P.; Gallop, R.; Bluett, E.J.; Cobb, A.; Gonzalez-Lima, F. Enhancing Extinction Learning in Posttraumatic Stress Disorder With Brief Daily Imaginal Exposure and Methylene Blue: A Randomized Controlled Trial. J. Clin. Psychiatry 2017, 78, e782–e789. [Google Scholar] [CrossRef]
- Sadovnikova, I.S.; Gureev, A.P.; Ignatyeva, D.A.; Gryaznova, M.V.; Chernyshova, E.V.; Krutskikh, E.P.; Novikova, A.G.; Popov, V.N. Nrf2/ARE Activators Improve Memory in Aged Mice via Maintaining of Mitochondrial Quality Control of Brain and the Modulation of Gut Microbiome. Pharmaceuticals 2021, 14, 607. [Google Scholar] [CrossRef]
- Wischik, C.M.; Staff, R.T.; Wischik, D.J.; Bentham, P.; Murray, A.D.; Storey, J.M.; Kook, K.A.; Harrington, C.R. Tau Aggregation Inhibitor Therapy: An Exploratory Phase 2 Study in Mild or Moderate Alzheimer’s Disease. J. Alzheimers Dis. 2015, 44, 705–720. [Google Scholar] [CrossRef]
- Kshirsagar, S.; Sawant, N.; Morton, H.; Reddy, A.P.; Reddy, P.H. Protective effects of mitophagy enhancers against amyloid beta-induced mitochondrial and synaptic toxicities in Alzheimer disease. Hum. Mol. Genet. 2021, 31, 423–439. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef]
- Gale, E.A.M.; Bingley, P.J.; Emmett, C.L.; Collier, T.; European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. European Nicotinamide Diabetes Intervention Trial (ENDIT): A randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004, 363, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Libri, V.; Yandim, C.; Athanasopoulos, S.; Loyse, N.; Natisvili, T.; Law, P.P.; Chan, P.K.; Mohammad, T.; Mauri, M.; Tam, K.T.; et al. Epigenetic and neurological effects and safety of high-dose nicotinamide in patients with Friedreich’s ataxia: An exploratory, open-label, dose-escalation study. Lancet 2014, 384, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.; Wakade, C.; Seamon, M.; Giri, B.; Morgan, J.; Purohit, S. Niacin Enhancement for Parkinson’s Disease: An Effectiveness Trial. Front. Aging Neurosci. 2021, 13, 667032. [Google Scholar] [CrossRef] [PubMed]
- Tully, L.; Humiston, J.; Cash, A. Oxaloacetate reduces emotional symptoms in premenstrual syndrome (PMS): Results of a placebo-controlled, cross-over clinical trial. Obstet. Gynecol. Sci. 2020, 63, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Kaufman, D.L. Oxaloacetate Treatment For Mental And Physical Fatigue In Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Long-COVID fatigue patients: A non-randomized controlled clinical trial. J. Transl. Med. 2022, 20, 295. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Koppel, S.; Carl, S.M.; Ramanujan, S.; Weidling, I.; Michaelis, M.L.; Michaelis, E.K.; Swerdlow, R.H. Oxaloacetate enhances neuronal cell bioenergetic fluxes and infrastructure. J. Neurochem. 2016, 137, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H.; Lyons, K.E.; Khosla, S.K.; Nashatizadeh, M.; Pahwa, R. A Pilot Study of Oxaloacetate 100 mg Capsules in Parkinson’s Disease Patients. J. Park. Dis. Alzheimers Dis. 2016, 3, 4. [Google Scholar]
- Flicker, L.; Evans, J.G. Piracetam for dementia or cognitive impairment. Cochrane Database Syst. Rev. 2001, CD001011. [Google Scholar] [CrossRef]
- Hofmeyr, G.J.; Kulier, R. Piracetam for fetal distress in labour. Cochrane Database Syst. Rev. 2012, 2012, CD001064. [Google Scholar] [CrossRef]
- Ricci, S.; Celani, M.G.; Cantisani, T.A.; Righetti, E. Piracetam for acute ischaemic stroke. Cochrane Database Syst. Rev. 2012, 2012, CD000419. [Google Scholar] [CrossRef]
- Sivalingam, K.; Samikkannu, T. Neuroprotective Effect of Piracetam against Cocaine-Induced Neuro Epigenetic Modification of DNA Methylation in Astrocytes. Brain Sci. 2020, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Stockburger, C.; Kurz, C.; Koch, K.A.; Eckert, S.H.; Leuner, K.; Müller, W.E. Improvement of mitochondrial function and dynamics by the metabolic enhancer piracetam. Biochem. Soc. Trans. 2013, 41, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Nagalla, S.; Ballas, S.K. Drugs for preventing red blood cell dehydration in people with sickle cell disease. Cochrane Database Syst. Rev. 2018, 10, CD003426. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhao, G.; Wei, S.; Guo, C.; Wu, X.; Zhao, R.C.; Di, G. Pterostilbene alleviates liver ischemia/reperfusion injury via PINK1-mediated mitophagy. J. Pharmacol. Sci. 2021, 148, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.B.; Dollerup, O.L.; Møller, A.B.; Billeskov, T.B.; Dalbram, E.; Chubanava, S.; Damgaard, M.V.; Dellinger, R.W.; Trošt, K.; Moritz, T.; et al. A randomized placebo-controlled trial of nicotinamide riboside and pterostilbene supplementation in experimental muscle injury in elderly individuals. J. Clin. Investig. 2022, 7, e158314. [Google Scholar] [CrossRef] [PubMed]
- Simic, P.; Parada, X.F.V.; Parikh, S.M.; Dellinger, R.; Guarente, L.P.; Rhee, E.P. Nicotinamide riboside with pterostilbene (NRPT) increases NAD+ in patients with acute kidney injury (AKI): A randomized, double-blind, placebo-controlled, stepwise safety study of escalating doses of NRPT in patients with AKI. BMC Nephrol. 2020, 21, 342. [Google Scholar] [CrossRef]
- Steiner, A.Z.; Hansen, K.R.; Barnhart, K.T.; Cedars, M.I.; Legro, R.S.; Diamond, M.P.; Krawetz, S.A.; Usadi, R.; Baker, V.L.; Coward, R.M.; et al. The effect of antioxidants on male factor infertility: The Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertil. Steril. 2020, 113, 552–560.e3. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, W.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Mi, M.; Zhu, J. Pterostilbene Enhances Endurance Capacity via Promoting Skeletal Muscle Adaptations to Exercise Training in Rats. Molecules 2020, 25, 186. [Google Scholar] [CrossRef]
- De la Rubia, J.E.; Drehmer, E.; Platero, J.L.; Benlloch, M.; Caplliure-Llopis, J.; Villaron-Casales, C.; de Bernardo, N.; AlarcÓn, J.; Fuente, C.; Carrera, S.; et al. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: A randomized, double-blind, placebo-controlled human pilot study. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 115–122. [Google Scholar] [CrossRef]
- MJeyaraman, M.M.; Al-Yousif, N.S.H.; Mann, A.S.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 2020, CD011919. [Google Scholar]
- McCreary, M.R.; Schnell, P.M.; Rhoda, D.A. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Sci. Rep. 2022, 12, 10978. [Google Scholar] [CrossRef] [PubMed]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Bai, Y.; Sun, X.; Fu, R.; Liu, L.; Liu, M.; Li, Z.; Huang, X. Resveratrol Reestablishes Mitochondrial Quality Control in Myocardial Ischemia/Reperfusion Injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1α Pathway. Molecules 2022, 27, 5545. [Google Scholar] [CrossRef] [PubMed]
- Zortea, K.; Franco, V.C.; Francesconi, L.P.; Cereser, K.M.M.; Lobato, M.I.R.; Belmonte-De-Abreu, P.S. Resveratrol Supplementation in Schizophrenia Patients: A Randomized Clinical Trial Evaluating Serum Glucose and Cardiovascular Risk Factors. Nutrients 2016, 8, 73. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Tapia, E.; Sánchez-Lozada, L.G.; García-Arroyo, F.E.; Amador-Martínez, I.; Orozco-Ibarra, M.; Fernández-Valverde, F.; Pedraza-Chaverri, J. Sulforaphane Protects against Unilateral Ureteral Obstruction-Induced Renal Damage in Rats by Alleviating Mitochondrial and Lipid Metabolism Impairment. Antioxidants 2022, 11, 1854. [Google Scholar] [CrossRef]
- Ghazizadeh-Hashemi, F.; Bagheri, S.; Ashraf-Ganjouei, A.; Moradi, K.; Shahmansouri, N.; Mehrpooya, M.; Noorbala, A.A.; Akhondzadeh, S. Efficacy and safety of sulforaphane for treatment of mild to moderate depression in patients with history of cardiac interventions: A randomized, double-blind, placebo-controlled clinical trial. Psychiatry Clin. Neurosci. 2021, 75, 250–255. [Google Scholar] [CrossRef]
- Shiina, A.; Kanahara, N.; Sasaki, T.; Oda, Y.; Hashimoto, T.; Hasegawa, T.; Yoshida, T.; Iyo, M.; Hashimoto, K. An Open Study of Sulforaphane-rich Broccoli Sprout Extract in Patients with Schizophrenia. Clin. Psychopharmacol. Neurosci. 2015, 13, 62–67. [Google Scholar] [CrossRef]
- Zimmerman, A.W.; Singh, K.; Connors, S.L.; Liu, H.; Panjwani, A.A.; Lee, L.C.; Diggins, E.; Foley, A.; Melnyk, S.; Singh, I.N.; et al. Randomized controlled trial of sulforaphane and metabolite discovery in children with Autism Spectrum Disorder. Mol. Autism 2021, 12, 38. [Google Scholar] [CrossRef]
- Savencu, C.E.; Linţa, A.; Farcaş, G.; Bînă, A.M.; Creţu, O.M.; Maliţa, D.C.; Muntean, D.M.; Sturza, A. Impact of Dietary Restriction Regimens on Mitochondria, Heart, and Endothelial Function: A Brief Overview. Front. Physiol. 2021, 12, 768383. [Google Scholar] [CrossRef]
- López-Torres, M.; Gredilla, R.; Sanz, A.; Barja, G. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Radic. Biol. Med. 2002, 32, 882–889. [Google Scholar] [CrossRef]
- Pan, J.W.; Rothman, D.L.; Behar, K.L.; Stein, D.T.; Hetherington, H.P. Human Brain β-Hydroxybutyrate and Lactate Increase in Fasting-Induced Ketosis. J. Cereb. Blood Flow Metab. 2000, 20, 1502–1507. [Google Scholar] [CrossRef]
- Pan, J.W.; Telang, F.W.; Lee, J.H.; De Graaf, R.A.; Rothman, D.L.; Stein, D.T.; Hetherington, H.P. Measurement of β-hydroxybutyrate in acute hyperketonemia in human brain. J. Neurochem. 2001, 79, 539–544. [Google Scholar] [CrossRef]
- Bough, K.J.; Wetherington, J.; Hassel, B.; Pare, J.F.; Gawryluk, J.W.; Greene, J.G.; Shaw, R.; Smith, Y.; Geiger, J.D.; Dingledine, R.J. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.; Ruskin, D.N.; Masino, S.A.; Sacchetti, P. Ketone-Based Metabolic Therapy: Is Increased NAD+ a Primary Mechanism? Front. Mol. Neurosci. 2017, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef]
- Manosalva, C.; Quiroga, J.; Hidalgo, A.I.; Alarcón, P.; Anseoleaga, N.; Hidalgo, M.A.; Burgos, R.A. Role of Lactate in Inflammatory Processes: Friend or Foe. Front. Immunol. 2022, 12, 808799. [Google Scholar] [CrossRef]
- Tauffenberger, A.; Fiumelli, H.; Almustafa, S.; Magistretti, P.J. Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Price, N.L.; Ling, A.J.Y.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD+ Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef]
- Bosco, G.; Yang, Z.; Nandi, J.; Wang, J.; Chen, C.; Camporesi, E.M. Effects of Hyperbaric Oxygen on Glucose, Lactate, Glycerol and Anti-Oxidant Enzymes in the Skeletal Muscle of Rats During Ischaemia and Reperfusion. Clin. Exp. Pharmacol. Physiol. 2007, 34, 70–76. [Google Scholar] [CrossRef]
- Corbett, J.; Tipton, M.J.; Perissiou, M.; James, T.; Young, J.S.; Newman, A.; Cummings, M.; Montgomery, H.; Grocott, M.P.W.; Shepherd, A.I. Effect of different levels of acute hypoxia on subsequent oral glucose tolerance in males with overweight: A balanced cross-over pilot feasibility study. Physiol. Rep. 2023, 11, e15623. [Google Scholar] [CrossRef]
- Reiter, R.J.; Ma, Q.; Sharma, R. Melatonin in Mitochondria: Mitigating Clear and Present Dangers. Physiology 2020, 35, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.L.; Davidson, A.R.; Brown, A.M. Universal Glia to Neurone Lactate Transfer in the Nervous System: Physiological Functions and Pathological Consequences. Biosensors 2020, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Bonn, J.A.; Harrison, J.; Rees, W.L. Lactate-Induced Anxiety: Therapeutic Application. Br. J. Psychiatry 1971, 119, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Auriacombe, M.; Rénéric, J.P.; Usandizaga, D.; Gomez, F.; Combourieu, I.; Tignol, J. Post-ECT agitation and plasma lactate concentrations. J. ECT 2000, 16, 263–267. [Google Scholar] [CrossRef]
- Trangmar, S.J.; Chiesa, S.T.; Kalsi, K.K.; Secher, N.H.; González-Alonso, J. Whole body hyperthermia, but not skin hyperthermia, accelerates brain and locomotor limb circulatory strain and impairs exercise capacity in humans. Physiol. Rep. 2017, 5, e13108. [Google Scholar] [CrossRef]
- Koush, Y.; de Graaf, R.A.; Jiang, L.; Rothman, D.L.; Hyder, F. Functional MRS with J-edited lactate in human motor cortex at 4 T. Neuroimage 2018, 184, 101–108. [Google Scholar] [CrossRef]
- Koush, Y.; Rothman, D.L.; Behar, K.L.; de Graaf, R.A.; Hyder, F. Human brain functional MRS reveals interplay of metabolites implicated in neurotransmission and neuroenergetics. J. Cereb. Blood Flow Metab. 2022, 42, 911–934. [Google Scholar] [CrossRef]
- Crémillieux, Y.; Dumont, U.; Mazuel, L.; Salvati, R.; Zhendre, V.; Rizzitelli, S.; Blanc, J.; Roumes, H.; Pinaud, N.; Bouzier-Sore, A.-K. Online Quantification of Lactate Concentration in Microdialysate During Cerebral Activation Using 1H-MRS and Sensitive NMR Microcoil. Front. Cell Neurosci. 2019, 13, 89. [Google Scholar] [CrossRef]
- Dogan, A.E.; Yuksel, C.; Du, F.; Chouinard, V.-A.; Öngür, D. Brain lactate and pH in schizophrenia and bipolar disorder: A systematic review of findings from magnetic resonance studies. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018, 43, 1681–1690. [Google Scholar] [CrossRef]
- Maddock, R.J.; Buonocore, M.H. MR Spectroscopic Studies of the Brain in Psychiatric Disorders. In Brain Imaging in Behavioral Neuroscience; Carter, C.S., Dalley, J.W., Eds.; In Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–251. [Google Scholar]
- Corrigan, N.M.; Richards, T.L.; Friedman, S.D.; Petropoulos, H.; Dager, S.R. Improving 1H MRSI measurement of cerebral lactate for clinical applications. Psychiatry Res. Neuroimaging 2010, 182, 40–47. [Google Scholar] [CrossRef][Green Version]
- Maddock, R.J.; Buonocore, M.H.; Miller, A.R.; Yoon, J.H.; Soosman, S.K.; Unruh, A.M. Abnormal Activity-Dependent Brain Lactate and Glutamate+Glutamine Responses in Panic Disorder. Biol. Psychiatry 2013, 73, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S.; et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Tsukamoto, H.; Takenaka, S.; Olesen, N.D.; Petersen, L.G.; Sørensen, H.; Nielsen, H.B.; Secher, N.H.; Ogoh, S. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. 2018, 32, 1417–1427. [Google Scholar] [CrossRef] [PubMed]

| Compound | Target | Mechanism | Clinical Trials | References |
|---|---|---|---|---|
| Bezafibrate | PPARα | ↓Fission ↑Mitophagy ↑Biogenesis | Primary Biliary Cirrhosis Cardiovascular Disease | [140,141,142,143] |
| Ciclopirox olamine | PGC-1α Metal Chelator | ↑Mitophagy ↑Biogenesis | Anti-tumour | [144,145,146] |
| Coenzyme Q10 | Electron Acceptor | ↑Mitophagy | Huntington’s Disease Parkinson’s Disease | [147,148,149] |
| Deferiprone | Iron Chelator | ↑Mitophagy | Parkinson’s Disease Friedrich’s Ataxia Alzheimer’s Disease HIV Thalassaemia Sickle Cell Disease | [150,151,152,153,154,155] |
| Elamipretide | Cardiolipin Stabiliser | ↓Fission ↑Fusion ↑Mitophagy ↑Biogenesis | 1° Mitochondrial Myopathy Macular Degeneration Myocardial Infarction Heart Failure | [156,157,158,159,160,161] |
| Fisetin | Antioxidant SIRT1/AMPK PPARγ | ↓Fission ↑Fusion ↑Mitophagy ↑Biogenesis | COVID-19 Cancer Stroke | [162,163,164,165,166,167] |
| Fluvastatin | HMG-CoA Reductase | ↑Mitophagy ↑Biogenesis | Systemic lupus erythematosus Metabolic syndrome Hepatitis C | [168,169,170,171,172,173] |
| Luteolin | Flavanoid Multiple Targets | ↓Fission ↑Fusion ↑Mitophagy ↑Biogenesis | COVID-1916/08/2023 18:44:00 Autism | [174,175,176,177] |
| Metformin | AMPK | ↓Fission ↑Fusion ↑Mitophagy ↑Biogenesis | Schizophrenia Bipolar Affective Disorder Migraine Stroke | [178,179,180,181,182,183] |
| Methylene Blue | NRF2 Electron Recycling | ↓Fission ↑Fusion ↑Mitophagy ↑Biogenesis | Post-traumatic Stress Disorder Bipolar Affective Disorder Alzheimer’s Disease Post-operative Delirium Frontotemporal Dementia | [184,185,186,187,188,189,190] |
| Nicotinamide | SIRT1 | ↓Fission ↑Fusion ↑Mitophagy ↑Biogenesis | Friedrich’s Ataxia Skin Cancer Diabetes Parkinson’s Disease | [191,192,193,194,195] |
| Oxaloacetate | Antioxidant TCA cycle SIRT1 | ↑Biogenesis | COVID-19 Chronic Fatigue Syndrome Parkinson’s Disease Premenstrual Syndrome | [196,197,198,199] |
| Piracetam | NRF2 | ↓Fission ↑Fusion ↑Biogenesis | Stroke Dementia Sickle Cell Disease Foetal Distress in Labour | [200,201,202,203,204,205] |
| Pterostilbene | PI3K-Akt-mTOR SIRT1 | ↑Mitophagy ↑Biogenesis | Infertility Muscle Injury Amyotrophic Lateral Sclerosis Acute Kidney Injury | [206,207,208,209,210,211] |
| Resveratrol | PPARs | ↓Fission ↓↑Fusion ↑Mitophagy ↑Biogenesis | Diabetes Schizophrenia Alzheimer’s Disease COVID-19 | [212,213,214,215,216] |
| Sulforaphane | NRF2 | ↓Fission ↑Fusion ↑Mitophagy ↑Biogenesis | Autism Depression Schizophrenia | [217,218,219,220] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caddye, E.; Pineau, J.; Reyniers, J.; Ronen, I.; Colasanti, A. Lactate: A Theranostic Biomarker for Metabolic Psychiatry? Antioxidants 2023, 12, 1656. https://doi.org/10.3390/antiox12091656
Caddye E, Pineau J, Reyniers J, Ronen I, Colasanti A. Lactate: A Theranostic Biomarker for Metabolic Psychiatry? Antioxidants. 2023; 12(9):1656. https://doi.org/10.3390/antiox12091656
Chicago/Turabian StyleCaddye, Edward, Julien Pineau, Joshua Reyniers, Itamar Ronen, and Alessandro Colasanti. 2023. "Lactate: A Theranostic Biomarker for Metabolic Psychiatry?" Antioxidants 12, no. 9: 1656. https://doi.org/10.3390/antiox12091656
APA StyleCaddye, E., Pineau, J., Reyniers, J., Ronen, I., & Colasanti, A. (2023). Lactate: A Theranostic Biomarker for Metabolic Psychiatry? Antioxidants, 12(9), 1656. https://doi.org/10.3390/antiox12091656





