Berry Extracts and Their Bioactive Compounds Mitigate LPS and DNFB-Mediated Dendritic Cell Activation and Induction of Antigen Specific T-Cell Effector Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Black Raspberry Extract Preparation for the Study
2.3. Natural Compounds
2.4. Generation of Bone Marrow-Derived DCs by GM-CSF and Flt3 Ligand and Stimulation
2.5. Western Blotting
2.6. Enzyme-Linked Immunosorbent Assay
2.7. T-Cell Activation and Proliferation
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results and Discussion
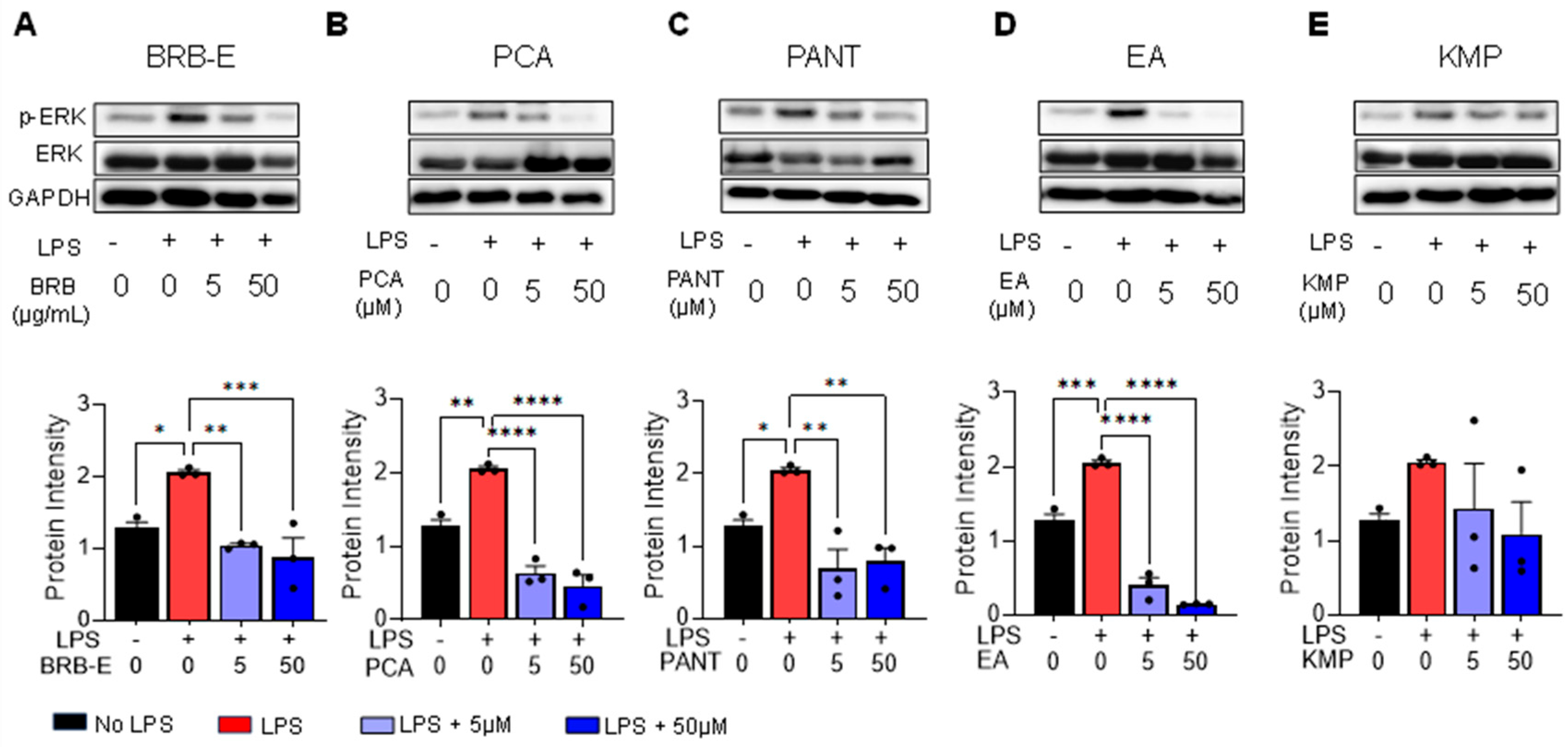
3.1. Effects of BRB-E, Protocatechuic Acid, Proanthocyanidins, Ellagic Acid and Kaempferol on the Activation of ERK in GM-CSF-Derived LPS-Stimulated DCs
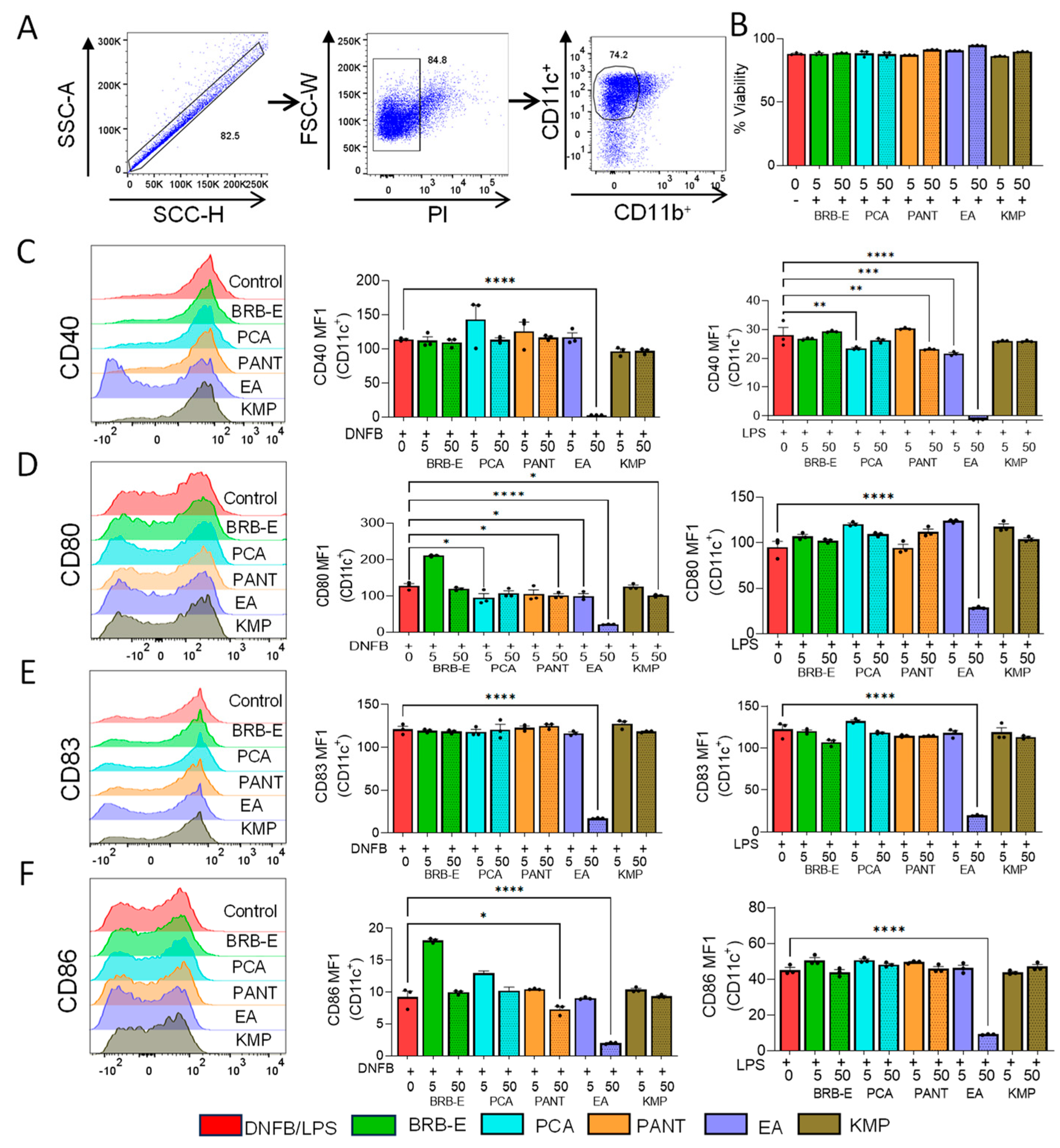
3.2. Effects of BRB-E, Protocatechuic Acid, Proanthocyanidins, Ellagic Acid, and Kaempferol on DNFB/- LPS-Stimulated DCs Activation Markers
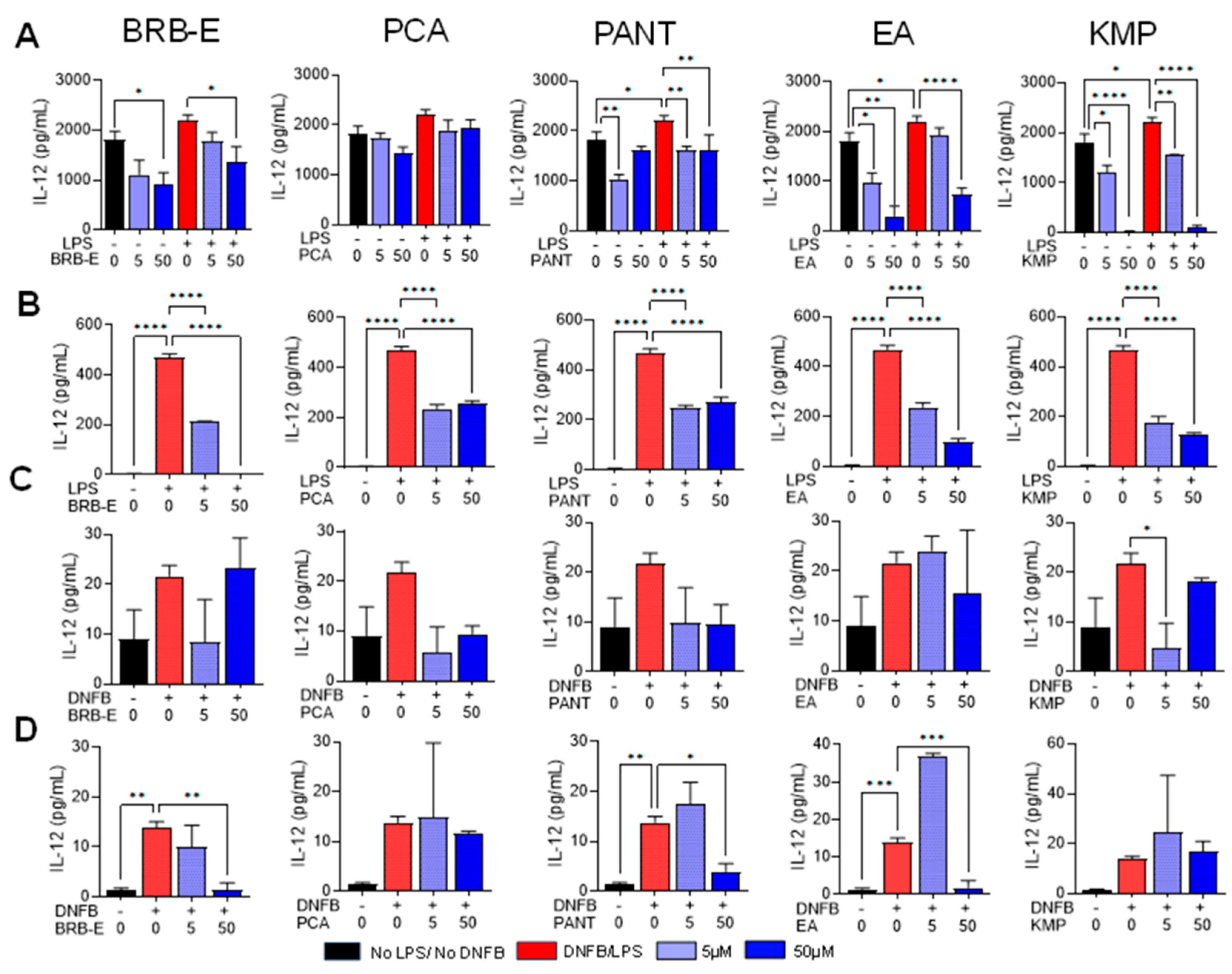
3.3. Effects of BRB-E, Protocatechuic Acid, Proanthocyanidins, Ellagic Acid, and Kaempferol on the Production of IL-12 by Stimulated DCs
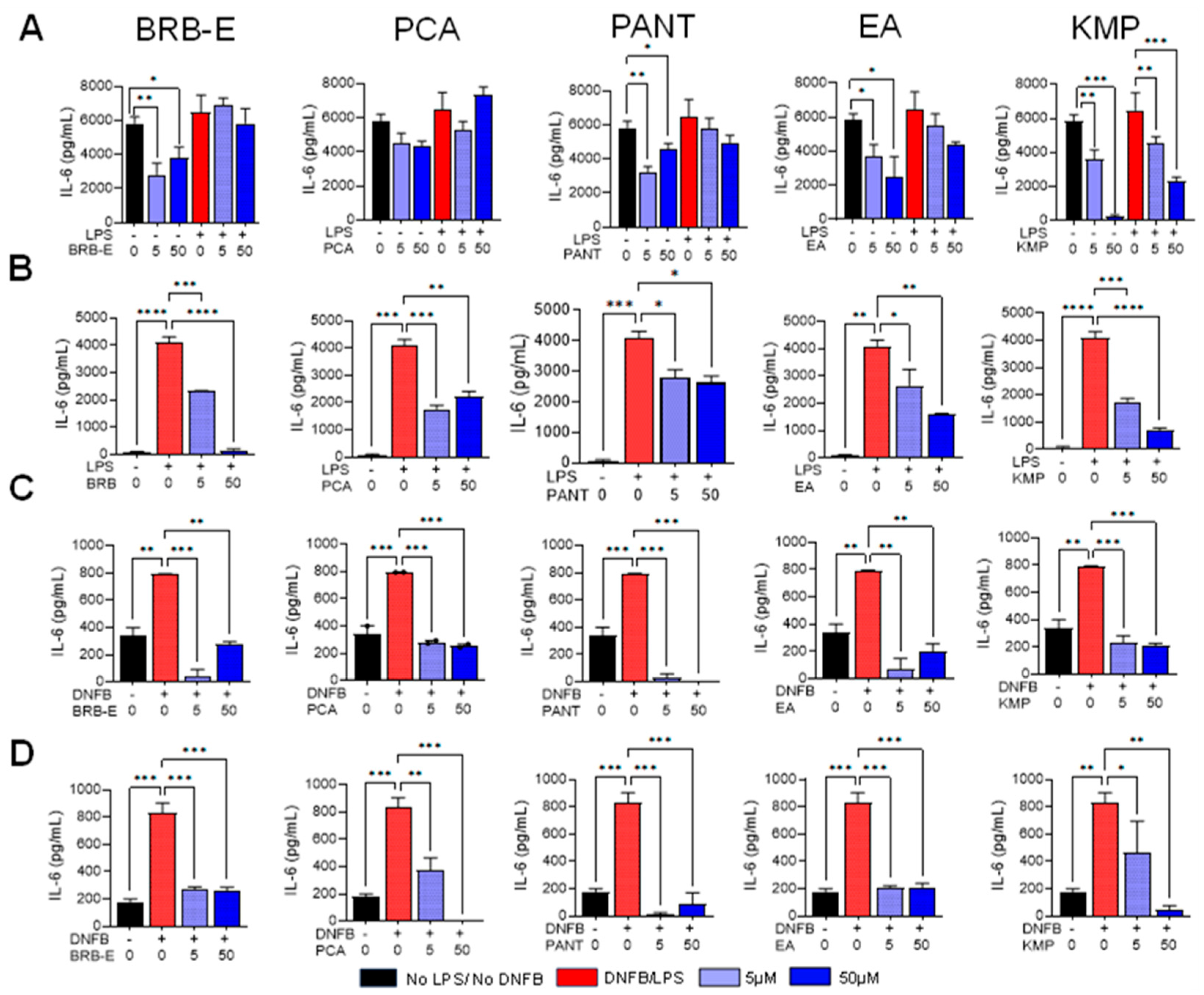
3.4. Effects of BRB-E, Protocatechuic Acid, Proanthocyanidins, Ellagic Acid, and Kaempferol on IL-6 Production by Stimulated DCs
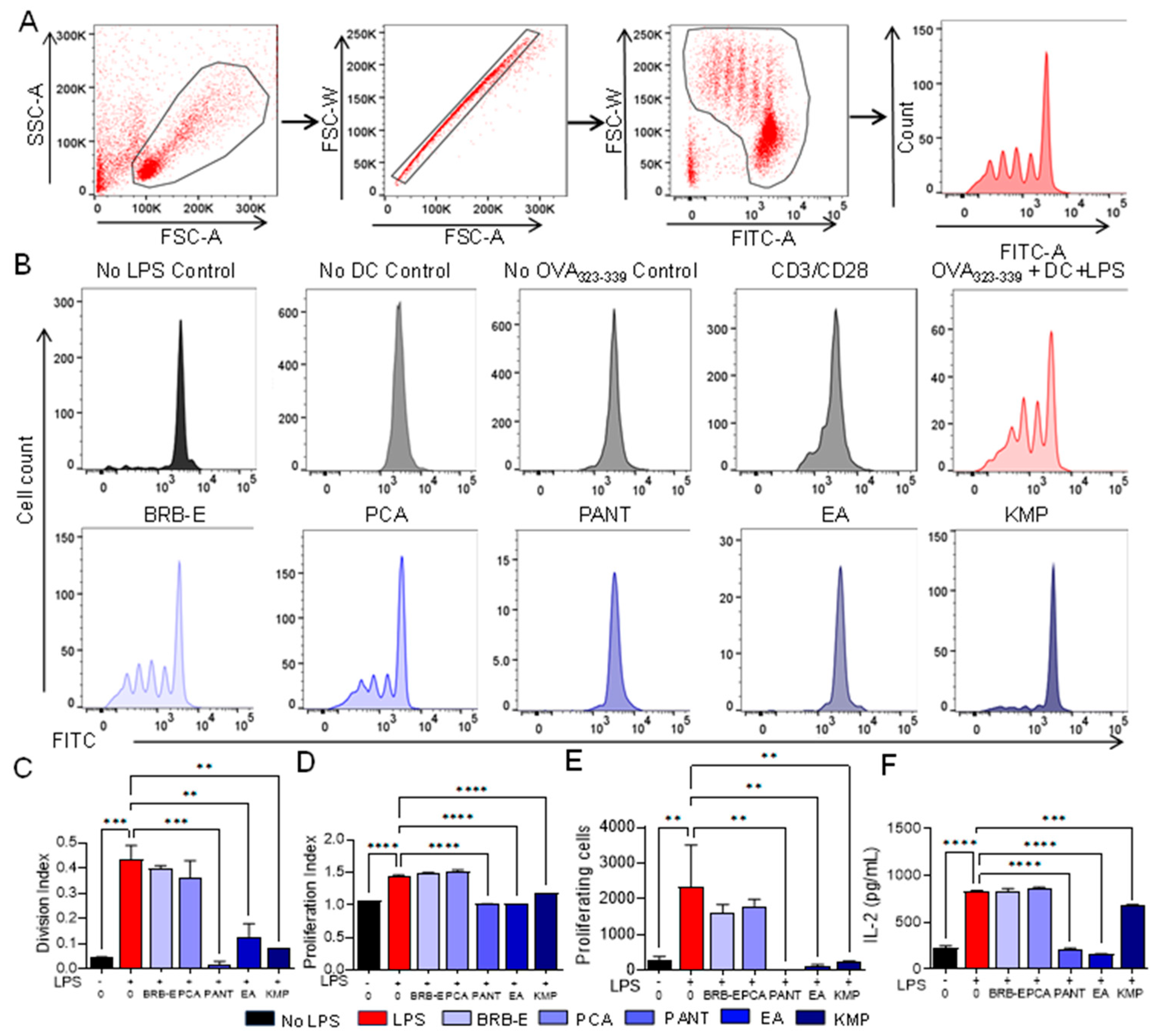
3.5. Proanthocyanidins, Ellagic Acid, and Kaempferol Abrogate Antigen-Specific T-Cell Proliferation and IL-2 Levels Induced by LPS-Stimulated Dendritic Cells
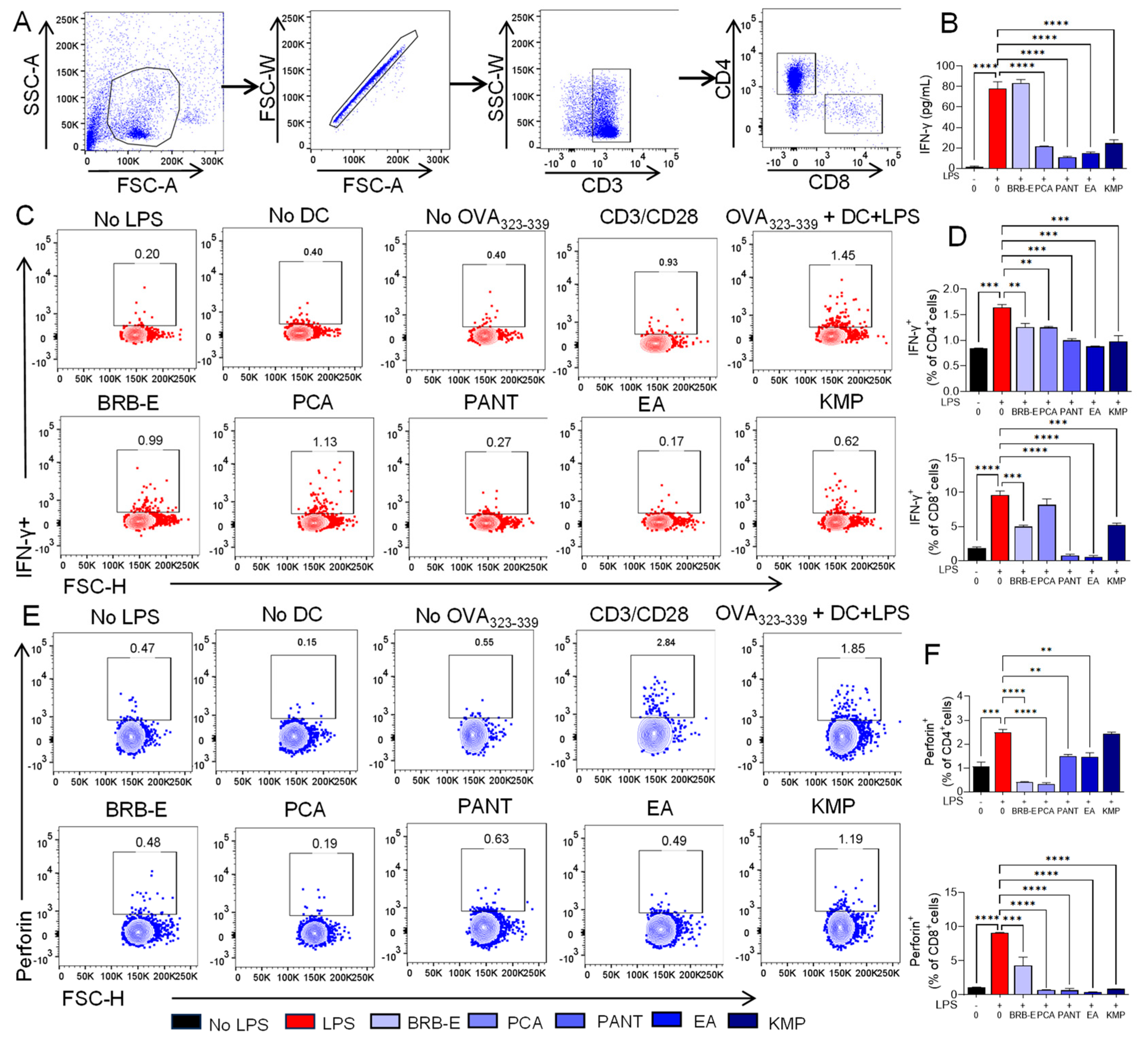
3.6. Effect of BRB-E, Protocatechuic Acid, Proanthocyanidins, Ellagic Acid, and Kaempferol on IFN-γ and Perforin Production during Antigen Specific T-Cell Activation Induced by GM-CSF-Derived LPS-Stimulated DCs
3.7. IL-17 Is Reduced by Protocathechuic Acid, Proanthocyanidins, Ellagic Acid, and Kaempferol during Antigen Specific T-Cell Activation Induced by GM-CSF-Derived LPS-Stimulated DCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, P.B.; Atwater, A.R.; Mueller, M.; Collins, J. Allergic Contact Dermatitis (Nursing). In StatPearls©; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jaulent, C.; Dereure, O.; Raison-Peyron, N. Contact dermatitis caused by polyacrylamide/C13-4 isoparaffin/laureth-7 mix in an emollient cream for atopic skin. Contact Dermat. 2019, 81, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Stone, N. Facial allergic contact dermatitis caused by caprylic/capric triglyceride in a cosmetic cream. Contact Dermat. 2022, 86, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Park, M.E.; Zippin, J.H. Allergic contact dermatitis to cosmetics. Dermatol. Clin. 2014, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.; de Gannes, G.C. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Ski. Ther. Lett. 2011, 16, 1–4. [Google Scholar]
- Roach, K.; Roberts, J. A comprehensive summary of disease variants implicated in metal allergy. J. Toxicol. Environ. Health B Crit. Rev. 2022, 25, 279–341. [Google Scholar] [CrossRef] [PubMed]
- Belsito, D.V.; Fransway, A.F.; Fowler, J.F., Jr.; Sherertz, E.F.; Maibach, H.I.; Mark, J.G., Jr.; Mathias, C.G.; Rietschel, R.L.; Storrs, F.J.; Nethercott, J.R. Allergic contact dermatitis to detergents: A multicenter study to assess prevalence. J. Am. Acad. Dermatol. 2002, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Werfel, T.; White, I.R.; Johansen, J.D. Contact Allergy: A Review of Current Problems from a Clinical Perspective. Int. J. Environ. Res. Public Health 2018, 15, 1108. [Google Scholar] [CrossRef]
- Li, Y.; Li, L. Contact Dermatitis: Classifications and Management. Clin. Rev. Allergy Immunol. 2021, 61, 245–281. [Google Scholar] [CrossRef]
- Dalgard, F.J.; Svensson, Å.; Halvorsen, J.A.; Gieler, U.; Schut, C.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.E.; Misery, L.; et al. Itch and Mental Health in Dermatological Patients across Europe: A Cross-Sectional Study in 13 Countries. J. Investig. Dermatol. 2020, 140, 568–573. [Google Scholar] [CrossRef]
- Vocanson, M.; Hennino, A.; Rozières, A.; Poyet, G.; Nicolas, J.F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 2009, 64, 1699–1714. [Google Scholar] [CrossRef]
- Martin, S.F.; Dudda, J.C.; Bachtanian, E.; Lembo, A.; Liller, S.; Dürr, C.; Heimesaat, M.M.; Bereswill, S.; Fejer, G.; Vassileva, R.; et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J. Exp. Med. 2008, 205, 2151–2162. [Google Scholar] [CrossRef]
- Martin, S.F.; Jakob, T. From innate to adaptive immune responses in contact hypersensitivity. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 289–293. [Google Scholar] [CrossRef]
- Furio, L.; Guezennec, A.; Ducarre, B.; Guesnet, J.; Peguet-Navarro, J. Differential effects of allergens and irritants on early differentiating monocyte-derived dendritic cells. Eur. J. Dermatol. 2008, 18, 141–147. [Google Scholar] [PubMed]
- Weltzien, H.U.; Moulon, C.; Martin, S.; Padovan, E.; Hartmann, U.; Kohler, J. T cell immune responses to haptens. Structural models for allergic and autoimmune reactions. Toxicology 1996, 107, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Dendritic cells and vaccines. In Baylor University Medical Center Proceedings; Taylor & Francis: London, UK, 2008; Volume 21, pp. 3–8. [Google Scholar]
- Everts, B.; Amiel, E.; Huang, S.C.; Smith, A.M.; Chang, C.H.; Lam, W.Y.; Redmann, V.; Freitas, T.C.; Blagih, J.; van der Windt, G.J.; et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014, 15, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Everts, B.; Pearce, E.J. Metabolic control of dendritic cell activation and function: Recent advances and clinical implications. Front. Immunol. 2014, 5, 203. [Google Scholar]
- Dudek, A.M.; Martin, S.; Garg, A.D.; Agostinis, P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front. Immunol. 2013, 4, 438. [Google Scholar] [CrossRef]
- Höper, T.; Siewert, K.; Dumit, V.I.; von Bergen, M.; Schubert, K.; Haase, A. The Contact Allergen NiSO4 Triggers a Distinct Molecular Response in Primary Human Dendritic Cells Compared to Bacterial LPS. Front. Immunol. 2021, 12, 644700. [Google Scholar] [CrossRef]
- Aiba, S.; Manome, H.; Nakagawa, S.; Mollah, Z.U.; Mizuashi, M.; Ohtani, T.; Yoshino, Y.; Tagami, H. p38 Mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and 2,4-dinitrochlorobenzene. J. Investig. Dermatol. 2003, 120, 390–399. [Google Scholar] [CrossRef]
- Arrighi, J.F.; Rebsamen, M.; Rousset, F.; Kindler, V.; Hauser, C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 2001, 166, 3837–3845. [Google Scholar] [CrossRef]
- Boislève, F.; Kerdine-Römer, S.; Rougier-Larzat, N.; Pallardy, M. Nickel and DNCB induce CCR7 expression on human dendritic cells through different signalling pathways: Role of TNF-alpha and MAPK. J. Investig. Dermatol. 2004, 123, 494–502. [Google Scholar] [CrossRef]
- Schäkel, K. Sensory neurons drive adaptive immunity in contact hypersensitivity. J. Allergy Clin. Immunol. 2021, 148, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; DiIulio, N.A.; Fairchild, R.L. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: Interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J. Exp. Med. 1996, 183, 1001–1012. [Google Scholar] [PubMed]
- Akiba, H.; Kehren, J.; Ducluzeau, M.T.; Krasteva, M.; Horand, F.; Kaiserlian, D.; Kaneko, F.; Nicolas, J.F. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ T cytotoxic 1 cells inducing keratinocyte apoptosis. J. Immunol. 2002, 168, 3079–3087. [Google Scholar] [CrossRef]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naive to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Traidl-Hoffmann, C.; Mariani, V.; Hochrein, H.; Karg, K.; Wagner, H.; Ring, J.; Mueller, M.J.; Jakob, T.; Behrendt, H. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J. Exp. Med. 2005, 201, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Liu, Y.J. OX40-OX40L interactions: A promising therapeutic target for allergic diseases? J. Clin. Investig. 2007, 117, 3655–3657. [Google Scholar] [CrossRef]
- Van Och, F.M.; Van Loveren, H.; De Jong, W.H.; Vandebriel, R.J. Cytokine production induced by low-molecular-weight chemicals as a function of the stimulation index in a modified local lymph node assay: An approach to discriminate contact sensitizers from respiratory sensitizers. Toxicol. Appl. Pharmacol. 2002, 184, 46–56. [Google Scholar] [CrossRef]
- Gorbachev, A.V.; DiIulio, N.A.; Fairchild, R.L. IL-12 augments CD8+ T cell development for contact hypersensitivity responses and circumvents anti-CD154 antibody-mediated inhibition. J. Immunol. 2001, 167, 156–162. [Google Scholar] [CrossRef]
- Riemann, H.; Loser, K.; Beissert, S.; Fujita, M.; Schwarz, A.; Schwarz, T.; Grabbe, S. IL-12 breaks dinitrothiocyanobenzene (DNTB)-mediated tolerance and converts the tolerogen DNTB into an immunogen. J. Immunol. 2005, 175, 5866–5874. [Google Scholar] [CrossRef]
- Larsen, J.M.; Bonefeld, C.M.; Poulsen, S.S.; Geisler, C.; Skov, L. IL-23 and T(H)17-mediated inflammation in human allergic contact dermatitis. J. Allergy Clin. Immunol. 2009, 123, 486–492. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wu, L.; Kim, H.K.; Li, H.; Elmets, C.A.; Xu, H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J. Immunol. 2006, 177, 6852–6858. [Google Scholar] [CrossRef] [PubMed]
- Peiser, M. Role of Th17 cells in skin inflammation of allergic contact dermatitis. Clin. Dev. Immunol. 2013, 2013, 261037. [Google Scholar] [CrossRef] [PubMed]
- Diepgen, T.L.; Andersen, K.E.; Chosidow, O.; Coenraads, P.J.; Elsner, P.; English, J.; Fartasch, M.; Gimenez-Arnau, A.; Nixon, R.; Sasseville, D.; et al. Guidelines for diagnosis, prevention and treatment of hand eczema. J. Dtsch. Dermatol. Ges. 2015, 13, e1–e22. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Goyal, A.; Sonthalia, S. Corticosteroid Adverse Effects. In StatPearls©; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Usatine, R.P.; Riojas, M. Diagnosis and management of contact dermatitis. Am. Fam. Physician 2010, 82, 249–255. [Google Scholar] [PubMed]
- Bonnekoh, H.; Vera, C.; Abad-Perez, A.; Radetzki, S.; Neuenschwander, M.; Specker, E.; Mahnke, N.A.; Frischbutter, S.; Latz, E.; Nazaré, M.; et al. Topical inflammasome inhibition with disulfiram prevents irritant contact dermatitis. Clin. Transl. Allergy 2021, 11, e12045. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, K.; Nam, H.; Lee, D. Discovering Health Benefits of Phytochemicals with Integrated Analysis of the Molecular Network, Chemical Properties and Ethnopharmacological Evidence. Nutrients 2018, 10, 1042. [Google Scholar] [CrossRef]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as Anti-Inflammatory Agents in Animal Models of Prevalent Inflammatory Diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Echeveste, C.E.; Oshima, K.; Zhang, J.; Yearsley, M.; Yu, J.; Wang, L.S. Anti-colonic Inflammation by Black Raspberries through Regulating Toll-like Receptor-4 Signaling in Interlukin-10 Knockout Mice. J. Cancer Prev. 2020, 25, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, M.D.; Schwartz, S.J.; Cooperstone, J.L. Profiling the impact of thermal processing on black raspberry phytochemicals using untargeted metabolomics. Food Chem. 2019, 274, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Lamenza, F.F.; Ryan, N.M.; Upadhaya, P.; Siddiqui, A.; Jordanides, P.P.; Springer, A.; Roth, P.; Pracha, H.; Iwenofu, O.H.; Oghumu, S. Inducible TgfbR1 and Pten deletion in a model of tongue carcinogenesis and chemoprevention. Cancer Gene Ther. 2023, 30, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Nedungadi, D.; Ryan, N.; Anderson, K.; Lamenza, F.F.; Jordanides, P.P.; Swingler, M.J.; Rakotondraibe, L.; Riedl, K.M.; Iwenofu, H.; Oghumu, S. Modulation of the oral glucocorticoid system during black raspberry mediated oral cancer chemoprevention. Carcinogenesis 2022, 43, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.M.; Lamenza, F.F.; Upadhaya, P.; Pracha, H.; Springer, A.; Swingler, M.; Siddiqui, A.; Oghumu, S. Black raspberry extract inhibits regulatory T-cell activity in a murine model of head and neck squamous cell carcinoma chemoprevention. Front. Immunol. 2022, 13, 932742. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Ryan, N.; Siddiqui, A.; Pero, T.; Volpedo, G.; Cooperstone, J.L.; Oghumu, S. Black Raspberries and Protocatechuic Acid Mitigate DNFB-Induced Contact Hypersensitivity by Down-Regulating Dendritic Cell Activation and Inhibiting Mediators of Effector Responses. Nutrients 2020, 12, 1701. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, T.J.; Ryan, N.M.; Bruschweiler-Li, L.; Wang, C.; Bernier, M.C.; Somogyi, A.; Yan, P.S.; Cooperstone, J.L.; Mo, X.; Bruschweiler, R.P.; et al. Metabolic Regulation of Glycolysis and AMP Activated Protein Kinase Pathways during Black Raspberry-Mediated Oral Cancer Chemoprevention. Metabolites 2019, 9, 140. [Google Scholar] [CrossRef]
- Oghumu, S.; Casto, B.C.; Ahn-Jarvis, J.; Weghorst, L.C.; Maloney, J.; Geuy, P.; Horvath, K.Z.; Bollinger, C.E.; Warner, B.M.; Summersgill, K.F.; et al. Inhibition of Pro-inflammatory and Anti-apoptotic Biomarkers during Experimental Oral Cancer Chemoprevention by Dietary Black Raspberries. Front. Immunol. 2017, 8, 1325. [Google Scholar] [CrossRef]
- Gu, J.; Thomas-Ahner, J.M.; Riedl, K.M.; Bailey, M.T.; Vodovotz, Y.; Schwartz, S.J.; Clinton, S.K. Dietary Black Raspberries Impact the Colonic Microbiome and Phytochemical Metabolites in Mice. Mol. Nutr. Food Res. 2019, 63, e1800636. [Google Scholar] [CrossRef]
- Sławińska, N.; Prochoń, K.; Olas, B. A Review on Berry Seeds-A Special Emphasis on Their Chemical Content and Health-Promoting Properties. Nutrients 2023, 15, 1422. [Google Scholar] [CrossRef] [PubMed]
- Paudel, L.; Wyzgoski, F.J.; Scheerens, J.C.; Chanon, A.M.; Reese, R.N.; Smiljanic, D.; Wesdemiotis, C.; Blakeslee, J.J.; Riedl, K.M.; Rinaldi, P.L. Nonanthocyanin secondary metabolites of black raspberry (Rubus occidentalis L.) fruits: Identification by HPLC-DAD, NMR, HPLC-ESI-MS, and ESI-MS/MS analyses. J. Agric. Food Chem. 2013, 61, 12032–12043. [Google Scholar] [CrossRef] [PubMed]
- Kresty, L.A.; Fromkes, J.J.; Frankel, W.L.; Hammond, C.D.; Seeram, N.P.; Baird, M.; Stoner, G.D. A phase I pilot study evaluating the beneficial effects of black raspberries in patients with Barrett’s esophagus. Oncotarget 2018, 9, 35356–35372. [Google Scholar] [CrossRef] [PubMed]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Huang, P. Proanthocyanidins may be potential therapeutic agents for the treatment of carotid atherosclerosis: A review. J. Int. Med. Res. 2023, 51, 3000605231167314. [Google Scholar] [CrossRef] [PubMed]
- Eskra, J.N.; Schlicht, M.J.; Bosland, M.C. Effects of Black Raspberries and Their Ellagic Acid and Anthocyanin Constituents on Taxane Chemotherapy of Castration-Resistant Prostate Cancer Cells. Sci. Rep. 2019, 9, 4367. [Google Scholar] [CrossRef] [PubMed]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut-Brain Axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxidative Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Gong, X.; Xiong, H.; Liu, S.; Liu, Y.; Yin, L.; Tu, C.; Wang, H.; Zhao, Z.; Chen, W.; Mei, Z. Qingpeng Ointment Ameliorates Inflammatory Responses and Dysregulation of Itch-Related Molecules for Its Antipruritic Effects in Experimental Allergic Contact Dermatitis. Front. Pharmacol. 2019, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Songkro, S.; Reanmongkol, W. Topical anti-inflammatory potential of standardized pomegranate rind extract and ellagic acid in contact dermatitis. Phytother. Res. 2014, 28, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.B.; Sung, M.L.; Hartman, D.A.; Lock, Y.W.; Bauer, J.; Walter, T.; Carlson, R.P. Cellular and topical in vivo inflammatory murine models in the evaluation of inhibitors of phospholipase A2. Ski. Pharmacol. 1995, 8, 300–308. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hwang, K.A.; Choi, K.C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.K.; Yu, Y.L.; Chen, K.C.; Chang, W.T.; Lee, M.S.; Yang, M.J.; Cheng, H.C.; Liu, C.H.; Chen Dz, C.; Chu, C.L. Kaempferol from Semen cuscutae attenuates the immune function of dendritic cells. Immunobiology 2011, 216, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Kim, M.; Lim, S.J.; Kang, S.W.; Um, B.H.; Nho, C.W. Aceriphyllum rossii extract and its active compounds, quercetin and kaempferol inhibit IgE-mediated mast cell activation and passive cutaneous anaphylaxis. J. Agric. Food Chem. 2014, 62, 3750–3758. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, G.S. Therapeutic effect of kaempferol on atopic dermatitis by attenuation of T cell activity via interaction with multidrug resistance-associated protein 1. Br. J. Pharmacol. 2021, 178, 1772–1788. [Google Scholar] [CrossRef] [PubMed]
- Keppler, K.; Humpf, H.U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorganic Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, J.B.; Chen, K.M.; Sun, Y.W.; Kosinska, W.; Zhou, Y.; Kim, S.A.; Sung, Y.; Gowda, K.; Amin, S.; Stoner, G.D.; et al. Effects of Black Raspberry Extract and Protocatechuic Acid on Carcinogen-DNA Adducts and Mutagenesis, and Oxidative Stress in Rat and Human Oral Cells. Cancer Prev. Res. 2016, 9, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Lin, C.W.; Echeveste, C.E.; Huang, Y.W.; Oshima, K.; Yearsley, M.; Chen, X.; Yu, J.; Wang, L.S. Protocatechuic Acid, a Gut Bacterial Metabolite of Black Raspberries, Inhibits Adenoma Development and Alters Gut Microbiome Profiles in Apc (Min/+) Mice. J. Cancer Prev. 2022, 27, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Widy-Tyszkiewicz, E. Current Evidence for Disease Prevention and Treatment by Protocatechuic Acid (PCA) and Its Precursor Protocatechuic Aldehyde (PCAL) in Animals and Humans. In Plant Antioxidants and Health; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Salas, E.; Dueñas, M.; Schwarz, M.; Winterhalter, P.; Cheynier, V.; Fulcrand, H. Characterization of pigments from different high speed countercurrent chromatography wine fractions. J. Agric. Food Chem. 2005, 53, 4536–4546. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Ali, S.; Audiger, C.; Autenrieth, S.E.; Berod, L.; Bigley, V.; Cyran, L.; Dalod, M.; Dörrie, J.; Dudziak, D.; et al. Guidelines for mouse and human DC generation. Eur. J. Immunol. 2022, 0, 1–52. [Google Scholar] [CrossRef]
- Greter, M.; Helft, J.; Chow, A.; Hashimoto, D.; Mortha, A.; Agudo-Cantero, J.; Bogunovic, M.; Gautier, E.L.; Miller, J.; Leboeuf, M.; et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity 2012, 36, 1031–1046. [Google Scholar] [CrossRef]
- Nakahara, T.; Moroi, Y.; Uchi, H.; Furue, M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J. Dermatol. Sci. 2006, 42, 1–11. [Google Scholar] [CrossRef]
- Birkner, K.; Wasser, B.; Loos, J.; Plotnikov, A.; Seger, R.; Zipp, F.; Witsch, E.; Bittner, S. The Role of ERK Signaling in Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2017, 18, 1990. [Google Scholar] [CrossRef]
- Rescigno, M.; Martino, M.; Sutherland, C.L.; Gold, M.R.; Ricciardi-Castagnoli, P. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 1998, 188, 2175–2180. [Google Scholar] [CrossRef]
- Miyazawa, M.; Ito, Y.; Kosaka, N.; Nukada, Y.; Sakaguchi, H.; Suzuki, H.; Nishiyama, N. Role of MAPK signaling pathway in the activation of dendritic type cell line, THP-1, induced by DNCB and NiSO4. J. Toxicol. Sci. 2008, 33, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zeze, N.; Kido-Nakahara, M.; Tsuji, G.; Maehara, E.; Sato, Y.; Sakai, S.; Fujishima, K.; Hashimoto-Hachiya, A.; Furue, M.; Nakahara, T. Role of ERK Pathway in the Pathogenesis of Atopic Dermatitis and Its Potential as a Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 3467. [Google Scholar] [CrossRef]
- Huang, R.Y.; Yu, Y.L.; Cheng, W.C.; OuYang, C.N.; Fu, E.; Chu, C.L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S.; Eberlein, V.; Schuler, G.; Berking, C.; Heinzerling, L.; Schaft, N.; Dörrie, J. BRAF and MEK Inhibitors Affect Dendritic-Cell Maturation and T-Cell Stimulation. Int. J. Mol. Sci. 2021, 22, 11951. [Google Scholar] [CrossRef] [PubMed]
- Toebak, M.J.; Gibbs, S.; Bruynzeel, D.P.; Scheper, R.J.; Rustemeyer, T. Dendritic cells: Biology of the skin. Contact Dermat. 2009, 60, 2–20. [Google Scholar] [CrossRef]
- Xu, M.; Mizoguchi, I.; Morishima, N.; Chiba, Y.; Mizuguchi, J.; Yoshimoto, T. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin. Dev. Immunol. 2010, 2010, 832454. [Google Scholar] [CrossRef]
- Henry, C.J.; Ornelles, D.A.; Mitchell, L.M.; Brzoza-Lewis, K.L.; Hiltbold, E.M. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J. Immunol. 2008, 181, 8576–8584. [Google Scholar] [CrossRef]
- Dittmar, D.; Schuttelaar, M.L. Immunology and genetics of tumour necrosis factor in allergic contact dermatitis. Contact Dermat. 2017, 76, 257–271. [Google Scholar] [CrossRef]
- Piguet, P.F.; Grau, G.E.; Hauser, C.; Vassalli, P. Tumor necrosis factor is a critical mediator in hapten induced irritant and contact hypersensitivity reactions. J. Exp. Med. 1991, 173, 673–679. [Google Scholar] [CrossRef]
- Chong, S.Z.; Tan, K.W.; Wong, F.H.S.; Chua, Y.L.; Tang, Y.; Ng, L.G.; Angeli, V.; Kemeny, D.M. CD8 T cells regulate allergic contact dermatitis by modulating CCR2-dependent TNF/iNOS-expressing Ly6C+ CD11b+ monocytic cells. J. Investig. Dermatol. 2014, 134, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.B.; Hamid, Q.A.; Norris, D.A.; Kuhn, C.; Giorno, R.C.; Schlievert, P.M.; Farmer, E.R.; Leung, D.Y. Epidermal HLA-DR and the enhancement of cutaneous reactivity to superantigenic toxins in psoriasis. J. Clin. Investig. 1999, 104, 1181–1189. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Guttman-Yassky, E.; Suárez-Fariñas, M.; Nograles, K.E.; Tian, S.; Cardinale, I.; Chimenti, S.; Krueger, J.G. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Investig. Dermatol. 2011, 131, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Llopiz, D.; Ruiz, M.; Infante, S.; Villanueva, L.; Silva, L.; Hervas-Stubbs, S.; Alignani, D.; Guruceaga, E.; Lasarte, J.J.; Sarobe, P. IL-10 expression defines an immunosuppressive dendritic cell population induced by antitumor therapeutic vaccination. Oncotarget 2017, 8, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann. Med. 1995, 27, 537–541. [Google Scholar] [CrossRef]
- Ahrens, B.; Freund, T.; Rha, R.D.; Dittrich, A.M.; Quarcoo, D.; Hutloff, A.; Hamelmann, E. Lipopolysaccharide stimulation of dendritic cells induces interleukin-10 producing allergen-specific T cells in vitro but fails to prevent allergic airway disease. Exp. Lung Res. 2009, 35, 307–323. [Google Scholar] [CrossRef]
- Ghani, S.; Feuerer, M.; Doebis, C.; Lauer, U.; Loddenkemper, C.; Huehn, J.; Hamann, A.; Syrbe, U. T cells as pioneers: Antigen-specific T cells condition inflamed sites for high-rate antigen-non-specific effector cell recruitment. Immunology 2009, 128, e870–e880. [Google Scholar] [CrossRef]
- Kalia, V.; Sarkar, S. Regulation of Effector and Memory CD8 T Cell Differentiation by IL-2-A Balancing Act. Front. Immunol. 2018, 9, 2987. [Google Scholar] [CrossRef]
- Benczik, M.; Gaffen, S.L. The interleukin (IL)-2 family cytokines: Survival and proliferation signaling pathways in T lymphocytes. Immunol. Investig. 2004, 33, 109–142. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Boyman, O.; Kim, H.O.; Hahm, B.; Rubinstein, M.P.; Ramsey, C.; Kim, D.M.; Surh, C.D.; Sprent, J. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J. Exp. Med. 2007, 204, 1787–1801. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Wolint, P.; Walton, S.; Schwarz, K.; Oxenius, A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur. J. Immunol. 2007, 37, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Jung Jang, E.; Kil, Y.S.; Ryeon Park, H.; Oh, S.; Kyeong Kim, H.; Gyeong Jeong, M.; Kyoung Seo, E.; Sook Hwang, E. Suppression of IL-2 production and proliferation of CD4(+) T cells by tuberostemonine O. Chem. Biodivers. 2014, 11, 1954–1962. [Google Scholar] [CrossRef]
- He, D.; Wu, L.; Kim, H.K.; Li, H.; Elmets, C.A.; Xu, H. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J. Immunol. 2009, 183, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Osińska, I.; Popko, K.; Demkow, U. Perforin: An important player in immune response. Cent. Eur. J. Immunol. 2014, 39, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, M.; Huang, S.; Aguet, M.; Ryffel, B. Role of interferon-gamma in contact hypersensitivity assessed in interferon-gamma receptor-deficient mice. Toxicology 1995, 102, 301–312. [Google Scholar] [CrossRef]
- Kehren, J.; Desvignes, C.; Krasteva, M.; Ducluzeau, M.T.; Assossou, O.; Horand, F.; Hahne, M.; Kägi, D.; Kaiserlian, D.; Nicolas, J.F. Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J. Exp. Med. 1999, 189, 779–786. [Google Scholar] [CrossRef]
- Zwicky, P.; Unger, S.; Becher, B. Targeting interleukin-17 in chronic inflammatory disease: A clinical perspective. J. Exp. Med. 2020, 217, e20191123. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Ellagic Acid and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 473–479. [Google Scholar] [CrossRef]
- Stürner, K.H.; Verse, N.; Yousef, S.; Martin, R.; Sospedra, M. Boswellic acids reduce Th17 differentiation via blockade of IL-1β-mediated IRAK1 signaling. Eur. J. Immunol. 2014, 44, 1200–1212. [Google Scholar] [CrossRef]
- Wang, X.Z.; Xue, R.F.; Zhang, S.Y.; Zheng, Y.T.; Zhang, L.Y.; Jiang, Z.Z. Activation of natural killer T cells contributes to triptolide-induced liver injury in mice. Acta Pharmacol. Sin. 2018, 39, 1847–1854. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhaya, P.; Lamenza, F.F.; Shrestha, S.; Roth, P.; Jagadeesha, S.; Pracha, H.; Horn, N.A.; Oghumu, S. Berry Extracts and Their Bioactive Compounds Mitigate LPS and DNFB-Mediated Dendritic Cell Activation and Induction of Antigen Specific T-Cell Effector Responses. Antioxidants 2023, 12, 1667. https://doi.org/10.3390/antiox12091667
Upadhaya P, Lamenza FF, Shrestha S, Roth P, Jagadeesha S, Pracha H, Horn NA, Oghumu S. Berry Extracts and Their Bioactive Compounds Mitigate LPS and DNFB-Mediated Dendritic Cell Activation and Induction of Antigen Specific T-Cell Effector Responses. Antioxidants. 2023; 12(9):1667. https://doi.org/10.3390/antiox12091667
Chicago/Turabian StyleUpadhaya, Puja, Felipe F. Lamenza, Suvekshya Shrestha, Peyton Roth, Sushmitha Jagadeesha, Hasan Pracha, Natalie A. Horn, and Steve Oghumu. 2023. "Berry Extracts and Their Bioactive Compounds Mitigate LPS and DNFB-Mediated Dendritic Cell Activation and Induction of Antigen Specific T-Cell Effector Responses" Antioxidants 12, no. 9: 1667. https://doi.org/10.3390/antiox12091667




