Decreased Klotho Expression Causes Accelerated Decline of Male Fecundity through Oxidative Injury in Murine Testis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fertility Assessment
2.3. Animal and Histological Analysis
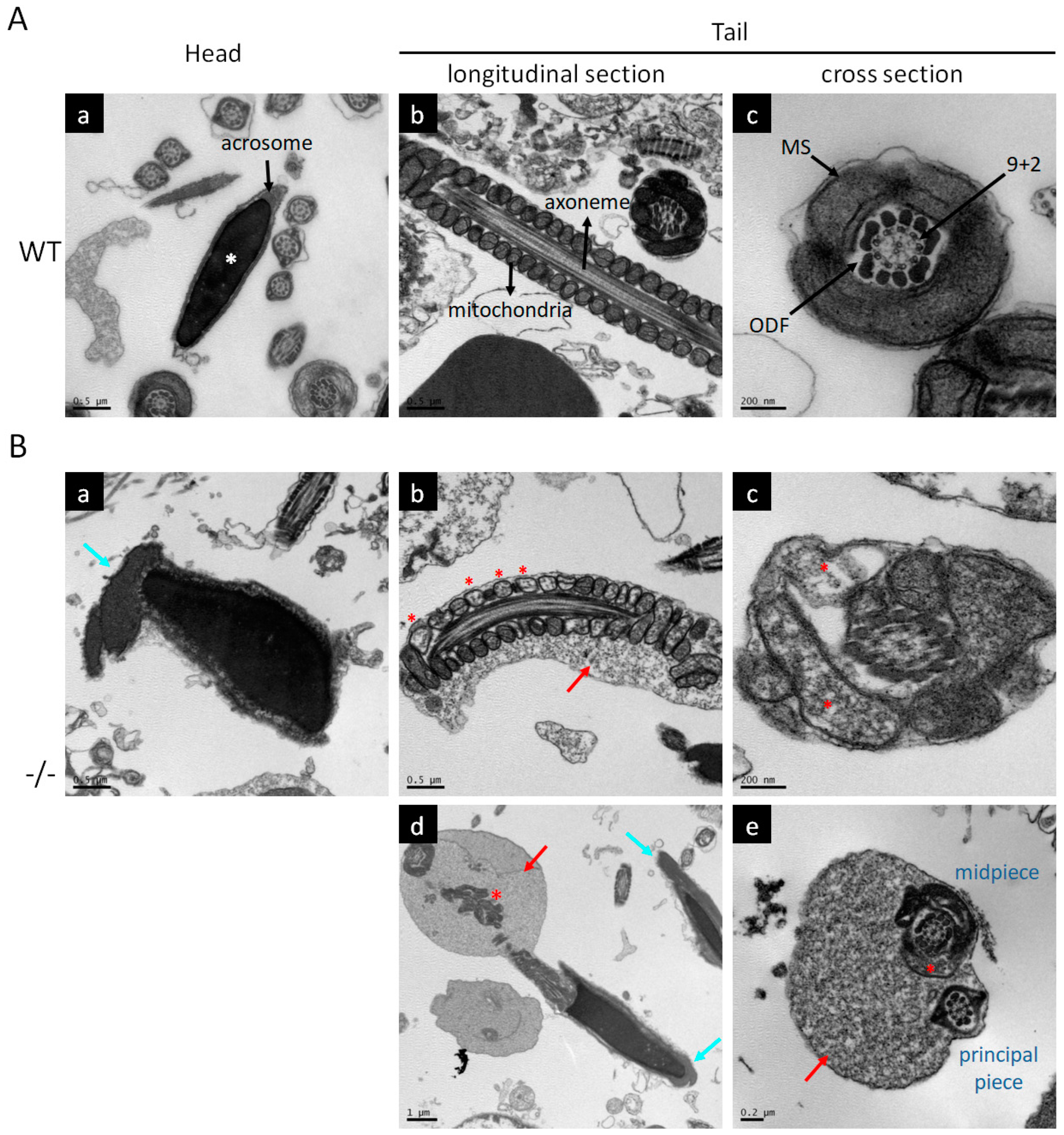
2.4. Electron Microscopy
2.5. Nano Scale Liquid Chromatography Coupled to Tandem Mass Spectrometry (Nano LC-MS/MS) and Western Blotting
2.6. Bioinformatic Analysis of the Testicular Proteome
2.7. Immunofluorescence and Immunocytochemistry Analysis
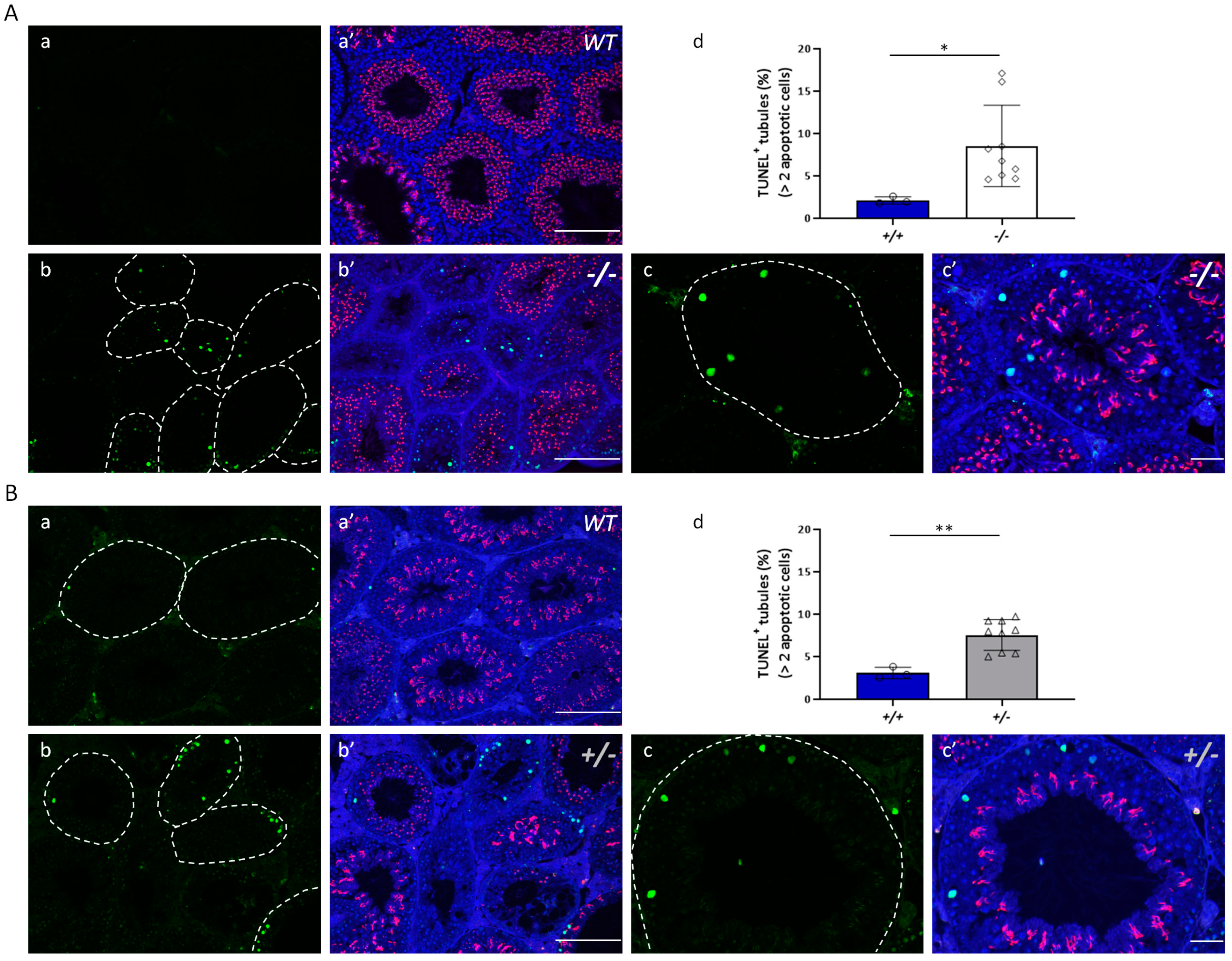
2.8. TUNEL Assay
2.9. Statistical Analysis
3. Results
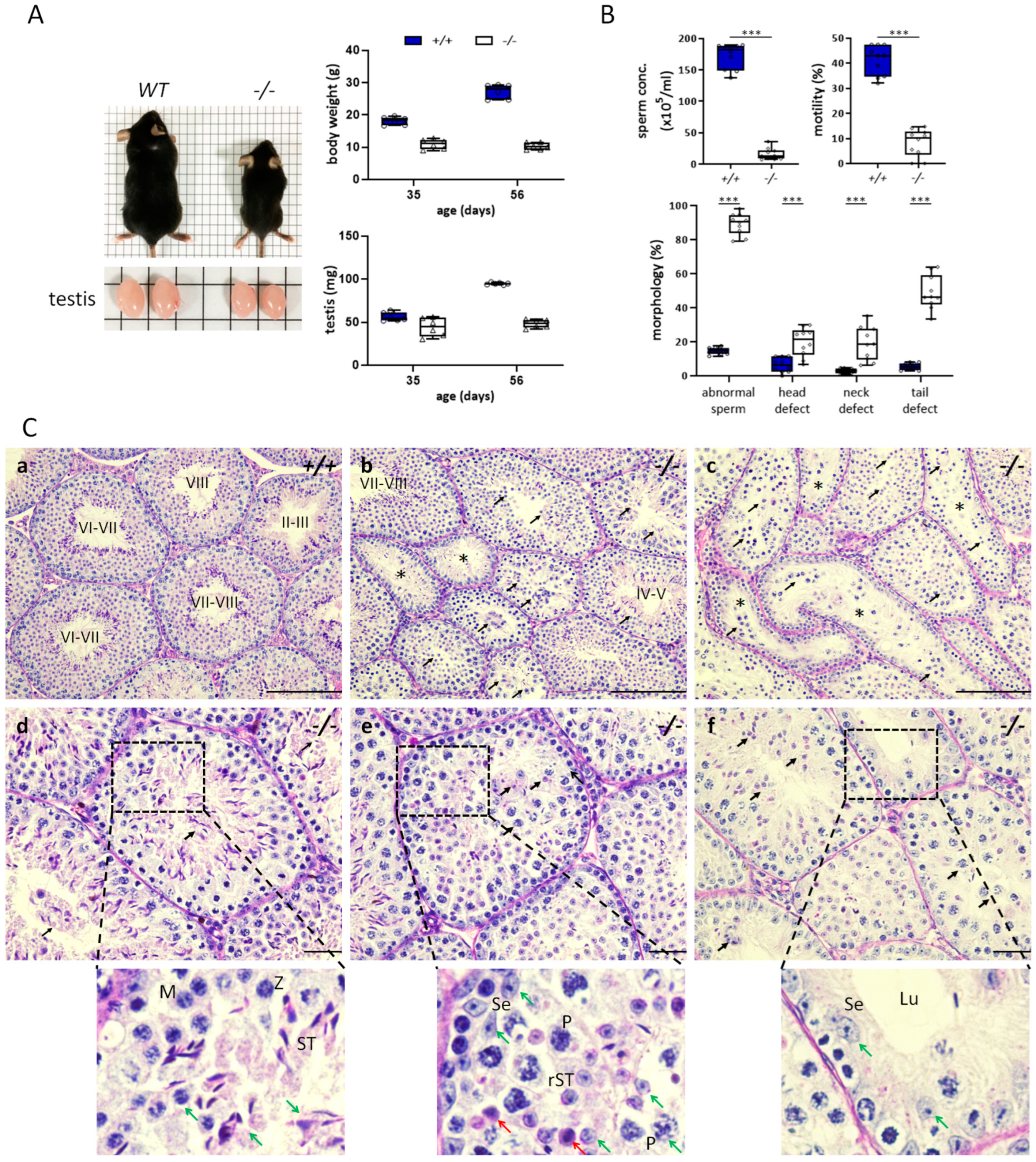
3.1. Decreased Klotho Expression Causes Accelerated Decline in Fecundity
3.2. Characteristics of Impaired Spermatogenesis in Kl KO Mice
3.3. Identification of Factors Related to Klotho Deficiency through Global Proteomic Approach
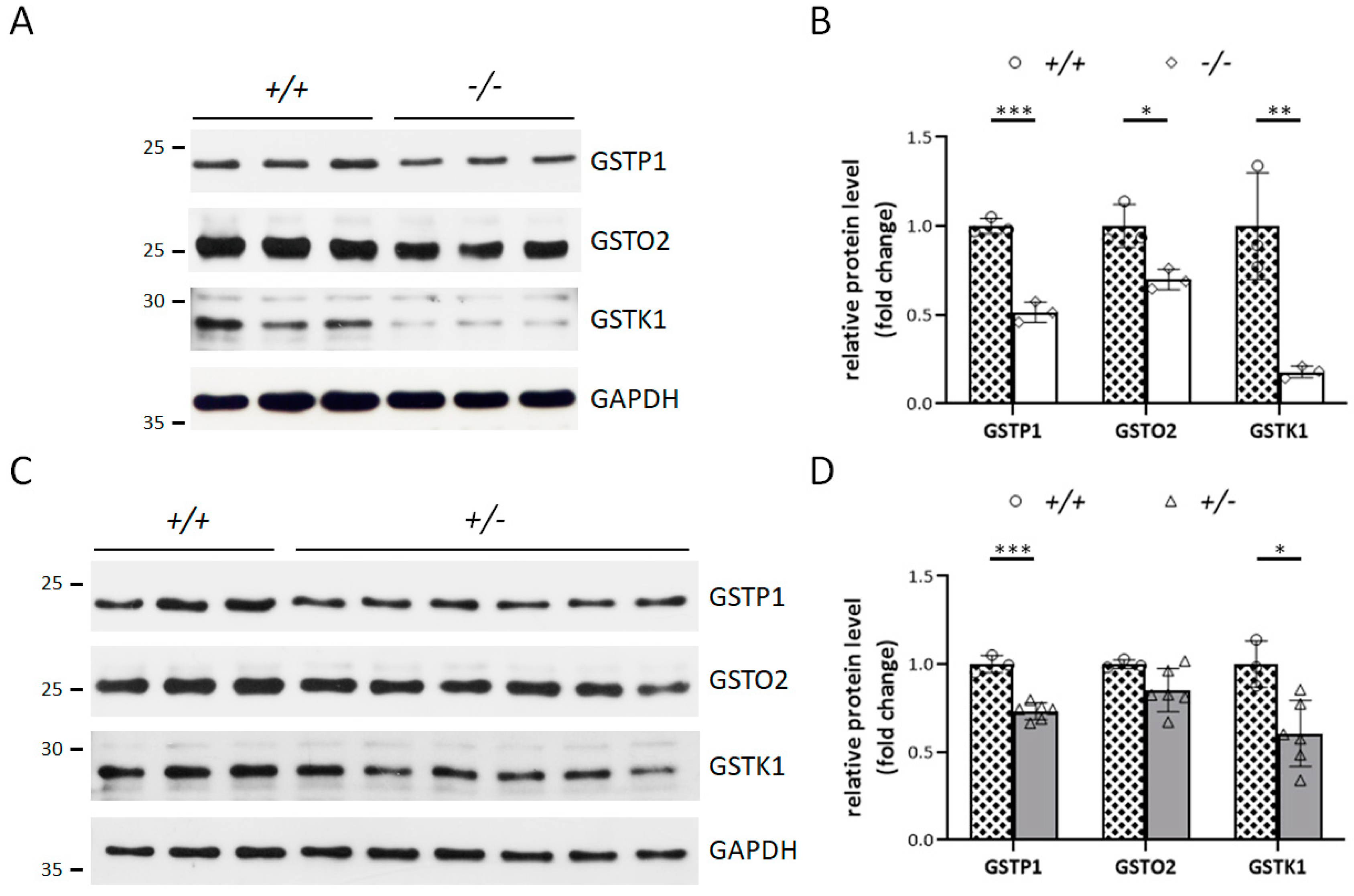
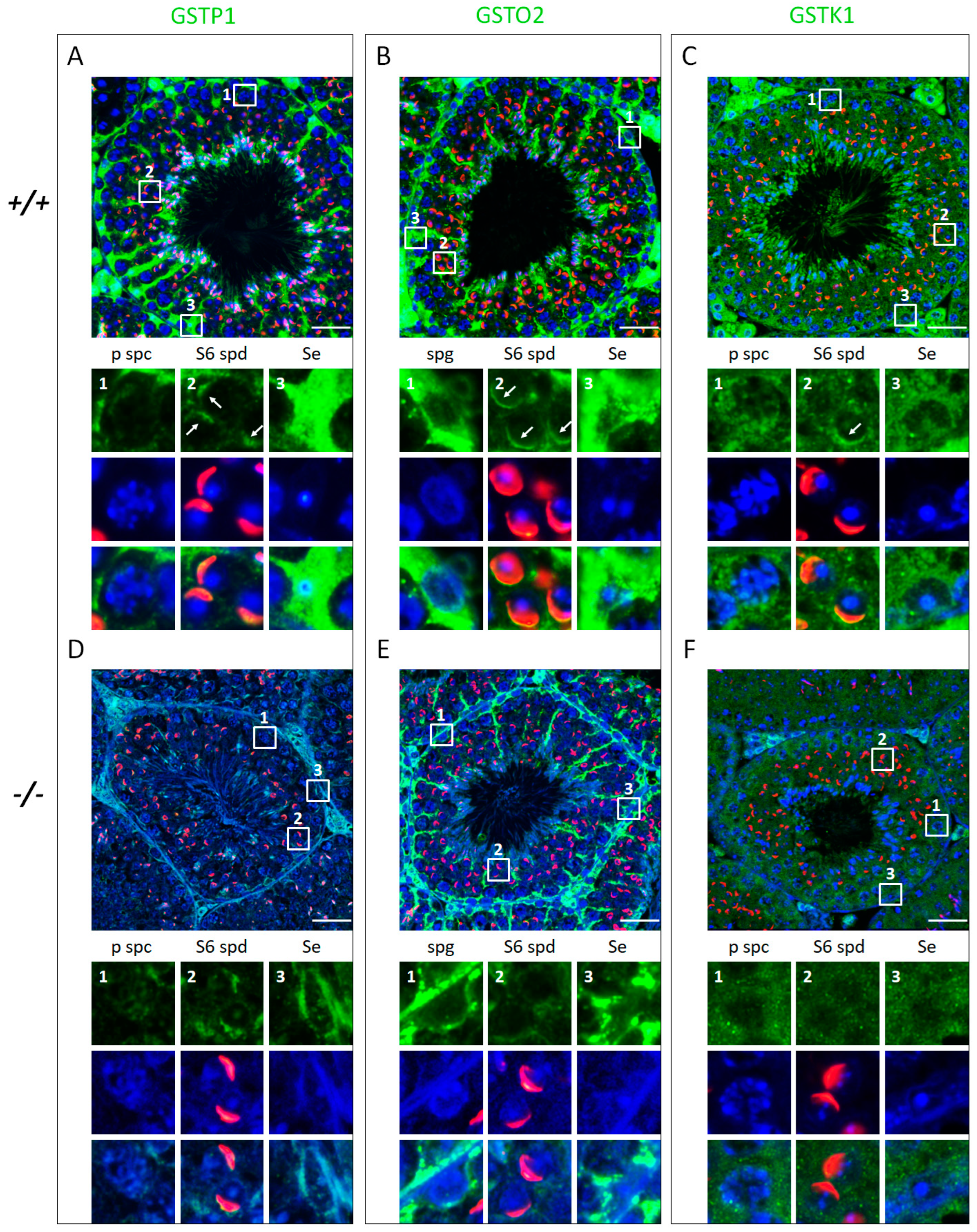
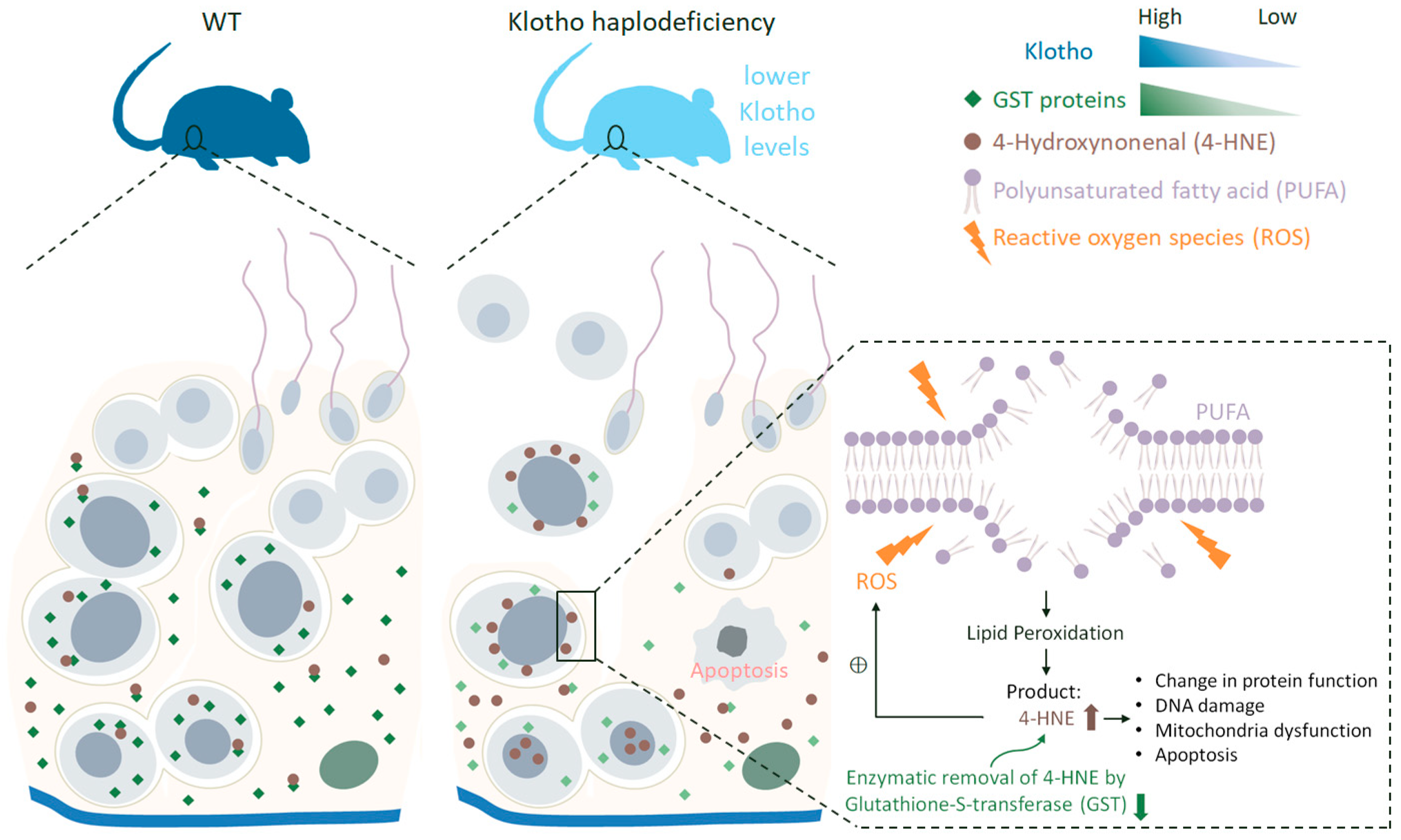
3.4. Klotho Depletion Interferes with Oxidative Balance through Attenuating the Protein Levels of GSTs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef]
- Mazur, D.J.; Lipshultz, L.I. Infertility in the Aging Male. Curr. Urol. Rep. 2018, 19, 54. [Google Scholar] [CrossRef]
- Kefer, J.C.; Agarwal, A.; Sabanegh, E. Role of antioxidants in the treatment of male infertility. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2009, 16, 449–457. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Nallella, K.P.; Thomas, A.J., Jr.; Alvarez, J.G.; Sikka, S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil. Steril. 2006, 86, 878–885. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Li, S.A.; Watanabe, M.; Yamada, H.; Nagai, A.; Kinuta, M.; Takei, K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct. Funct. 2004, 29, 91–99. [Google Scholar] [CrossRef]
- Komaba, H.; Kaludjerovic, J.; Hu, D.Z.; Nagano, K.; Amano, K.; Ide, N.; Sato, T.; Densmore, M.J.; Hanai, J.I.; Olauson, H.; et al. Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int. 2017, 92, 599–611. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Kurt, M.; Wang, Q. Pathobiology of the Klotho Antiaging Protein and Therapeutic Considerations. Front. Aging 2022, 3, 931331. [Google Scholar] [CrossRef]
- Tsujikawa, H.; Kurotaki, Y.; Fujimori, T.; Fukuda, K.; Nabeshima, Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 2003, 17, 2393–2403. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho and aging. Biochim. Biophys. Acta 2009, 1790, 1049–1058. [Google Scholar] [CrossRef]
- Xiao, N.M.; Zhang, Y.M.; Zheng, Q.; Gu, J. Klotho is a serum factor related to human aging. Chin. Med. J. 2004, 117, 742–747. [Google Scholar]
- Espuch-Oliver, A.; Vazquez-Lorente, H.; Jurado-Fasoli, L.; de Haro-Munoz, T.; Diaz-Alberola, I.; Lopez-Velez, M.D.S.; de Haro-Romero, T.; Castillo, M.J.; Amaro-Gahete, F.J. References Values of Soluble alpha-Klotho Serum Levels Using an Enzyme-Linked Immunosorbent Assay in Healthy Adults Aged 18-85 Years. J. Clin. Med. 2022, 11, 2415. [Google Scholar] [CrossRef]
- Koh, N.; Fujimori, T.; Nishiguchi, S.; Tamori, A.; Shiomi, S.; Nakatani, T.; Sugimura, K.; Kishimoto, T.; Kinoshita, S.; Kuroki, T.; et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 2001, 280, 1015–1020. [Google Scholar] [CrossRef]
- Bollehuus Hansen, L.; Kaludjerovic, J.; Nielsen, J.E.; Rehfeld, A.; Poulsen, N.N.; Ide, N.; Skakkebaek, N.E.; Frederiksen, H.; Juul, A.; Lanske, B.; et al. Influence of FGF23 and Klotho on male reproduction: Systemic vs direct effects. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 12436–12449. [Google Scholar] [CrossRef]
- Gao, D.; Wang, S.; Lin, Y.; Sun, Z. In vivo AAV delivery of glutathione reductase gene attenuates anti-aging gene klotho deficiency-induced kidney damage. Redox Biol. 2020, 37, 101692. [Google Scholar] [CrossRef]
- Llavanera, M.; Mateo-Otero, Y.; Bonet, S.; Barranco, I.; Fernandez-Fuertes, B.; Yeste, M. The triple role of glutathione S-transferases in mammalian male fertility. Cell. Mol. Life Sci. 2020, 77, 2331–2342. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ke, C.C.; Chen, Y.L.; Lin, Y.H.; Yu, I.S.; Ku, W.C.; O’Bryan, M.K.; Lin, Y.H. Deficiency of the Tbc1d21 gene causes male infertility with morphological abnormalities of the sperm mitochondria and flagellum in mice. PLoS Genet. 2020, 16, e1009020. [Google Scholar] [CrossRef]
- Lin, Y.H.; Huang, C.Y.; Ke, C.C.; Wang, Y.Y.; Lai, T.H.; Liu, H.C.; Ku, W.C.; Chan, C.C.; Lin, Y.H. ACTN4 Mediates SEPT14 Mutation-Induced Sperm Head Defects. Biomedicines 2020, 8, 518. [Google Scholar] [CrossRef]
- Lin, Y.H.; Ke, C.C.; Wang, Y.Y.; Chen, M.F.; Chen, T.M.; Ku, W.C.; Chiang, H.S.; Yeh, C.H. RAB10 Interacts with the Male Germ Cell-Specific GTPase-Activating Protein during Mammalian Spermiogenesis. Int. J. Mol. Sci. 2017, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.W.; Csordas, A.; Sun, Z.; Jarnuczak, A.; Perez-Riverol, Y.; Ternent, T.; Campbell, D.S.; Bernal-Llinares, M.; Okuda, S.; Kawano, S.; et al. The ProteomeXchange consortium in 2017: Supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017, 45, D1100–D1106. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Platts, A.E.; Dix, D.J.; Chemes, H.E.; Thompson, K.E.; Goodrich, R.; Rockett, J.C.; Rawe, V.Y.; Quintana, S.; Diamond, M.P.; Strader, L.F.; et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum. Mol. Genet. 2007, 16, 763–773. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S. The association of glutathione-S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) with idiopathic male infertility. J. Hum. Genet. 2010, 55, 565–570. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Dadkhah, F.; Ali Asgari, M.; Hosseini, S.Y.; Kolahi, A.A.; Iran-Pour, E. Glutathione S-transferase polymorphisms (GSTM1, GSTT1, GSTP1) and male factor infertility risk: A pooled analysis of studies. Urol. J. 2012, 9, 541–548. [Google Scholar]
- Lakpour, N.; Mirfeizollahi, A.; Farivar, S.; Akhondi, M.M.; Hashemi, S.B.; Amirjannati, N.; Heidari-Vala, H.; Sadeghi, M.R. The association of seminal plasma antioxidant levels and sperm chromatin status with genetic variants of GSTM1 and GSTP1 (Ile105Val and Ala114Val) in infertile men with oligoasthenoteratozoospermia. Dis. Markers 2013, 34, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Li, C.; Huang, J.; Xie, S.; Zhou, L.; Meng, L.; Li, L.; Wei, H.; Zhang, S. Glutathione S-transferase kappa 1 is positively related with sperm quality of porcine sperm. Mol. Reprod. Dev. 2022, 89, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.E.; Suzuki, J.; Aguila, L.; Meinsohn, M.C.; Smith, O.E.; Protopapas, N.; Xu, W.; Sutovsky, P.; Oko, R. Sperm-borne glutathione-S-transferase omega 2 accelerates the nuclear decondensation of spermatozoa during fertilization in micedagger. Biol. Reprod. 2019, 101, 368–376. [Google Scholar] [CrossRef]
- Fisher, H.M.; Aitken, R.J. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J. Exp. Zool. 1997, 277, 390–400. [Google Scholar] [CrossRef]
- Aitken, R.J. The Amoroso Lecture. The human spermatozoon—A cell in crisis? J. Reprod. Fertil. 1999, 115, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bauche, F.; Fouchard, M.H.; Jegou, B. Antioxidant system in rat testicular cells. FEBS Lett. 1994, 349, 392–396. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef]
- Beckman, J.K.; Coniglio, J.G. A comparative study of the lipid composition of isolated rat Sertoli and germinal cells. Lipids 1979, 14, 262–267. [Google Scholar] [CrossRef]
- Aitken, R.J.; Koppers, A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011, 13, 36–42. [Google Scholar] [CrossRef]
- Bromfield, E.G.; Aitken, R.J.; Anderson, A.L.; McLaughlin, E.A.; Nixon, B. The impact of oxidative stress on chaperone-mediated human sperm-egg interaction. Hum. Reprod. 2015, 30, 2597–2613. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.L.H.; De Iuliis, G.N.; Nixon, B.; Bromfield, E.G. Oxidative Stress in the Male Germline: A Review of Novel Strategies to Reduce 4-Hydroxynonenal Production. Antioxidants 2018, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Chandra, A.; Wang, L.F.; Seifert, W.E., Jr.; DaGue, B.B.; Ansari, N.H.; Srivastava, S.K.; Bhatnagar, A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J. Biol. Chem. 1998, 273, 10893–10900. [Google Scholar] [CrossRef]
- Rao, A.V.; Shaha, C. Role of glutathione S-transferases in oxidative stress-induced male germ cell apoptosis. Free. Radic. Biol. Med. 2000, 29, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Olauson, H.; Lindberg, K.; Amin, R.; Jia, T.; Wernerson, A.; Andersson, G.; Larsson, T.E. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J. Am. Soc. Nephrol. 2012, 23, 1641–1651. [Google Scholar] [CrossRef]
- Kuro, O.M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Anour, R.; Andrukhova, O.; Ritter, E.; Zeitz, U.; Erben, R.G. Klotho lacks a vitamin D independent physiological role in glucose homeostasis, bone turnover, and steady-state PTH secretion in vivo. PLoS ONE 2012, 7, e31376. [Google Scholar] [CrossRef]
- Kuro, O.M. Molecular Mechanisms Underlying Accelerated Aging by Defects in the FGF23-Klotho System. Int. J. Nephrol. 2018, 2018, 9679841. [Google Scholar] [CrossRef]
- Morishita, K.; Shirai, A.; Kubota, M.; Katakura, Y.; Nabeshima, Y.; Takeshige, K.; Kamiya, T. The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J. Nutr. 2001, 131, 3182–3188. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef]
- Maltese, G.; Psefteli, P.M.; Rizzo, B.; Srivastava, S.; Gnudi, L.; Mann, G.E.; Siow, R.C. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J. Cell. Mol. Med. 2017, 21, 621–627. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, Y.; Zhu, S.; Cui, Q.; Du, J. Klotho Improves Cardiac Function by Suppressing Reactive Oxygen Species (ROS) Mediated Apoptosis by Modulating Mapks/Nrf2 Signaling in Doxorubicin-Induced Cardiotoxicity. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 5283–5293. [Google Scholar] [CrossRef]
- Xiang, T.; Luo, X.; Ye, L.; Huang, H.; Wu, Y. Klotho alleviates NLRP3 inflammasome-mediated neuroinflammation in a temporal lobe epilepsy rat model by activating the Nrf2 signaling pathway. Epilepsy Behav. 2022, 128, 108509. [Google Scholar] [CrossRef]
- Xing, L.; Guo, H.; Meng, S.; Zhu, B.; Fang, J.; Huang, J.; Chen, J.; Wang, Y.; Wang, L.; Yao, X.; et al. Klotho ameliorates diabetic nephropathy by activating Nrf2 signaling pathway in podocytes. Biochem. Biophys. Res. Commun. 2021, 534, 450–456. [Google Scholar] [CrossRef]
- Nakamura, B.N.; Lawson, G.; Chan, J.Y.; Banuelos, J.; Cortes, M.M.; Hoang, Y.D.; Ortiz, L.; Rau, B.A.; Luderer, U. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free. Radic. Biol. Med. 2010, 49, 1368–1379. [Google Scholar] [CrossRef]
- Bartolini, D.; Commodi, J.; Piroddi, M.; Incipini, L.; Sancineto, L.; Santi, C.; Galli, F. Glutathione S-transferase pi expression regulates the Nrf2-dependent response to hormetic diselenides. Free. Radic. Biol. Med. 2015, 88, 466–480. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Mitchell, L.A.; Lambourne, S.R.; Connaughton, H.S.; De Iuliis, G.N. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol. Reprod. 2012, 87, 110. [Google Scholar] [CrossRef]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.; Schirhagl, R.; Cantineau, A.E.P. Male subfertility and oxidative stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, R.; Ge, Y.; Ma, J.; Guan, J.; Li, S.; Sun, X.; Xue, S.; Han, D. Gene expression profiles in different stages of mouse spermatogenic cells during spermatogenesis. Biol. Reprod. 2003, 69, 37–47. [Google Scholar] [CrossRef]
- Petit, F.M.; Serres, C.; Bourgeon, F.; Pineau, C.; Auer, J. Identification of sperm head proteins involved in zona pellucida binding. Hum. Reprod. 2013, 28, 852–865. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.L.; Pastor, J.; Carranza, D.; Quinones, H.; Griffith, C.; Goetz, R.; Mohammadi, M.; Ye, J.; Zhang, J.; Hu, M.C.; et al. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2015, 30, 223–233. [Google Scholar] [CrossRef]
- Lundy, S.D.; Vij, S.C. Male infertility in renal failure and transplantation. Transl. Androl. Urol. 2019, 8, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lehtihet, M.; Hylander, B. Semen quality in men with chronic kidney disease and its correlation with chronic kidney disease stages. Andrologia 2015, 47, 1103–1108. [Google Scholar] [CrossRef]



| Gene Symbol | Protein Name | Log2 FC | Majority Protein IDs |
|---|---|---|---|
| MCODE_1 | glutathione metabolism | ||
| Ephx1 | Epoxide hydrolase 1 | −2.783 | E9PWK1; Q9D379; Q6PEV0; Q8K2W5 |
| Gsta3 | Glutathione S-transferase A3 | −2.644 | P30115; K9JA27; Q9DCU1; A0A087WQI6 |
| Gstm1 | Glutathione S-transferase Mu 1 | −1.788 | P10649; A2AE89; F6WHQ7 |
| Mgst1 | Microsomal glutathione S-transferase 1 | −1.544 | D3YU60; Q53ZD4; E9QJW0; Q91VS7; A0A0N4SV17; D3YVR3 |
| Gstk1 | Glutathione S-transferase kappa 1 | −1.508 | Q9DCM2 |
| Gstm2 | Glutathione S-transferase Mu 2 | −1.104 | P15626; D3YX76; Q7M0F4 |
| Gsta2 | Glutathione S-transferase A2 | −0.840 | P10648; D3Z6A6; D3YZV3 |
| Gsto2 | Glutathione S-transferase omega-2 | −0.738 | Q9D2J1; A0A494BAY2; Q9D2S1; Q8BW12; Q8K2Q2; A0A494BB82 |
| Gstp1 | Glutathione S-transferase P 1 | −0.707 | A0A494B908; A0A494BAW2; P19157 |
| GPX1 | Glutathione peroxidase 1 | −0.681 | A0A0A6YY34; A0A0A6YVV2; P11352 |
| MCODE_2 | monocarboxylic acid metabolic process | ||
| Acox3 | Peroxisomal acyl-coenzyme A oxidase 3 | −3.109 | Q3TAW3; E9Q296; Q8C178; Q9EPL9 |
| Akr1cl | Aldo-keto reductase family 1, member C-like isoform 1 | −1.861 | Q9D5U2; G3XA14; Q80W25; Q3UXL1 |
| Scp2 | Non-specific lipid-transfer protein | −1.218 | P32020-2; P32020 |
| Aldoc | Fructose-bisphosphate aldolase C | −1.185 | P05063 |
| Acox1 | Peroxisomal acyl-coenzyme A oxidase 1 | −1.008 | Q9R0H0-2; Q9R0H0; A2A848; Q8C168; Q8BW35 |
| Akr1d1 | 3-oxo-5-beta-steroid 4-dehydrogenase | −0.969 | Q8VCX1 |
| Acaa1a | 3-ketoacyl-CoA thiolase A, peroxisomal | −0.957 | Q921H8; Q3UPU8; H3BJZ9; H3BKL5; Q8BLD7 |
| Akr1c12 | Aldo-keto reductase family 1 member C12 | −0.933 | Q9R0M7; Q9JLI0; Q91X42 |
| MCODE_3 | cellular detoxification | ||
| Gpt2 | Alanine aminotransferase 2 | −1.778 | Q8BGT5 |
| Cat | Catalase | −1.258 | Q3UF58; Q91XI2; Q8C6E3; Q542K4; Q3UZE7; Q3TVZ1; P24270 |
| Aldh1a7 | Aldehyde dehydrogenase, cytosolic 1 | −1.254 | B2RTL5; O35945 |
| Ttr | Transthyretin | −1.140 | Q5M9K1; P07309; Q9D6A4 |
| Aldh1a1 | Retinal dehydrogenase 1 | −1.110 | P24549 |
| Aldh1b1 | Aldehyde dehydrogenase X, mitochondrial | −0.844 | Q9CZS1 |
| Tpi1 | Triosephosphate isomerase | −0.679 | P17751; P17751-2; H7BXC3 |
| Aldh2 | Aldehyde dehydrogenase, mitochondrial | −0.610 | Q544B1; Q3UJW1; Q3U9J7; P47738; A0A0G2JEU1; Q3U6I3; Q3TVM2 |
| MCODE_4 | PPAR signaling pathway | ||
| Fabp3 | Fatty acid-binding protein, heart | −2.254 | Q5EBJ0; P11404 |
| Me1 | Malic enzyme; NADP-dependent malic enzyme | −0.703 | Q99LF5; Q921S3; P06801; Q3TQP6 |
| Apoa1 | Apolipoprotein A-I | −0.678 | Q3V2G1; Q00623; Q58EV2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-Y.; Lin, Y.-H.; Wu, V.-C.; Lin, Y.-H.; Huang, C.-Y.; Ku, W.-C.; Sun, C.-Y. Decreased Klotho Expression Causes Accelerated Decline of Male Fecundity through Oxidative Injury in Murine Testis. Antioxidants 2023, 12, 1671. https://doi.org/10.3390/antiox12091671
Wang Y-Y, Lin Y-H, Wu V-C, Lin Y-H, Huang C-Y, Ku W-C, Sun C-Y. Decreased Klotho Expression Causes Accelerated Decline of Male Fecundity through Oxidative Injury in Murine Testis. Antioxidants. 2023; 12(9):1671. https://doi.org/10.3390/antiox12091671
Chicago/Turabian StyleWang, Ya-Yun, Ying-Hung Lin, Vin-Cent Wu, Yu-Hua Lin, Chia-Yen Huang, Wei-Chi Ku, and Chiao-Yin Sun. 2023. "Decreased Klotho Expression Causes Accelerated Decline of Male Fecundity through Oxidative Injury in Murine Testis" Antioxidants 12, no. 9: 1671. https://doi.org/10.3390/antiox12091671
APA StyleWang, Y. -Y., Lin, Y. -H., Wu, V. -C., Lin, Y. -H., Huang, C. -Y., Ku, W. -C., & Sun, C. -Y. (2023). Decreased Klotho Expression Causes Accelerated Decline of Male Fecundity through Oxidative Injury in Murine Testis. Antioxidants, 12(9), 1671. https://doi.org/10.3390/antiox12091671







