Therapeutic Potential of Green-Engineered ZnO Nanoparticles on Rotenone-Exposed D. melanogaster (Oregon R+): Unveiling Ameliorated Biochemical, Cellular, and Behavioral Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Harvesting and Identification of Plant Materials
2.2.1. Extraction of Plants
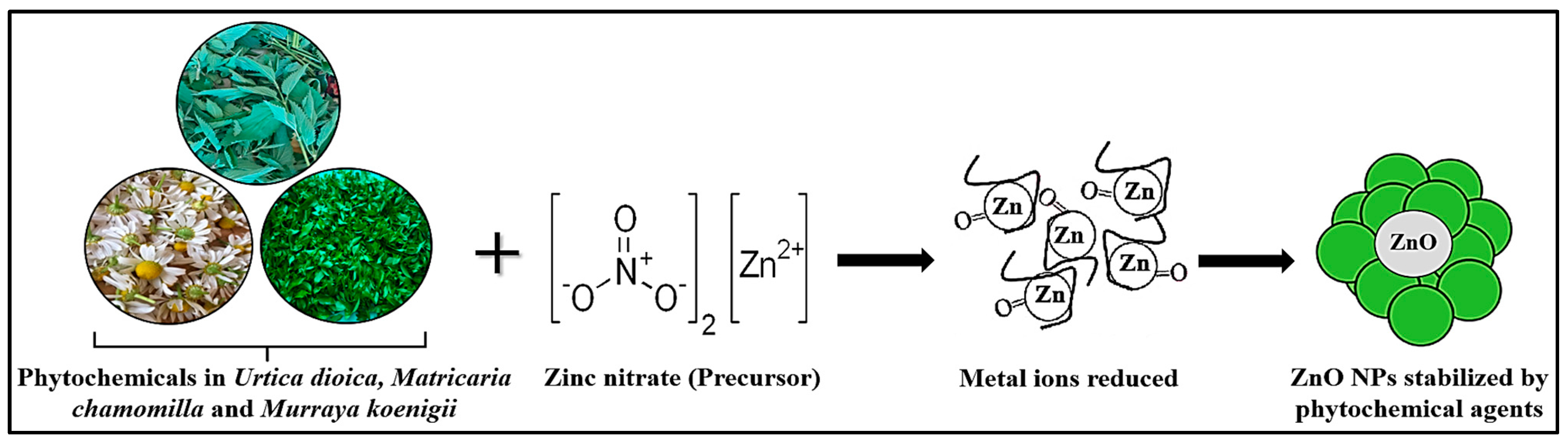
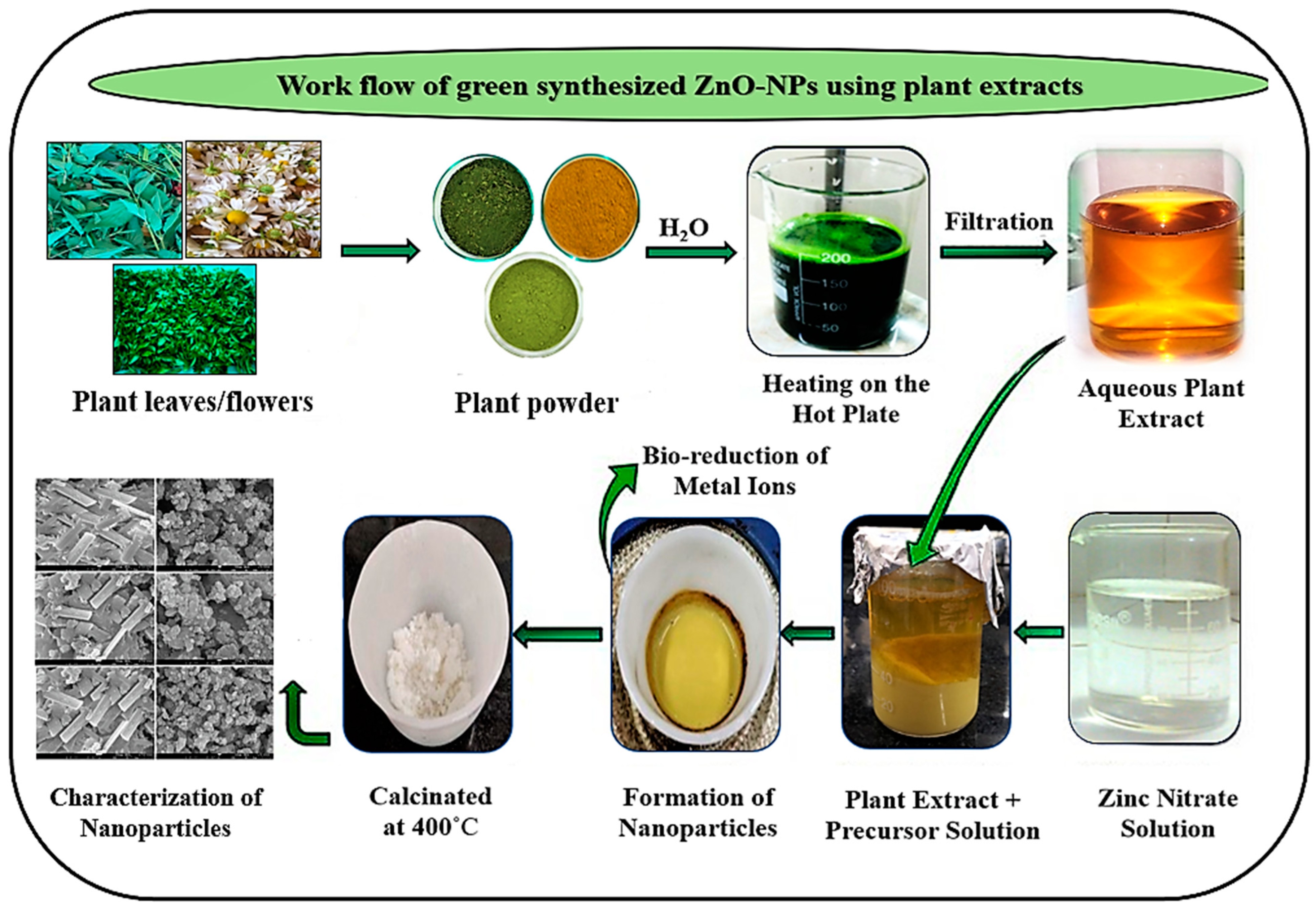
2.2.2. Biosynthesis of ZnO Nanoparticles
2.2.3. Characterization of Synthesized ZnO-NPs Using Microscopy Techniques
2.2.4. UV–Visible Analysis
2.2.5. Powder X-ray Diffraction (XRD)
2.2.6. Fourier Transform Infrared Spectroscopy
2.2.7. Zeta Potentiometer
2.2.8. Dynamic Light Scattering (DLS) Analysis
2.2.9. Scanning Electron Microscopy Analysis
2.2.10. Energy-Dispersive X-ray Spectroscopy
2.2.11. DPPH Free Radical Scavenging Activity
2.2.12. ABTS Free Radical Scavenging Activity
2.2.13. EC50 (Dose–Response Relationship)
2.3. Microorganisms and Fly Strain
2.3.1. Antimicrobial Activity
2.3.2. Biosynthesized ZnO-NP Concentration and Rotenone Exposure
2.3.3. Experimental Design
2.3.4. Dye Exclusion (Trypan Blue) Assay
2.3.5. Homogenate Preparation
2.3.6. Acetylcholinesterase (AChE) Enzymatic Assay
2.3.7. Protein Estimation Assay
2.3.8. Jumping Assay
2.3.9. Negative Geotaxis (Climbing Assay)
2.3.10. Memory Assay
2.3.11. Larval Crawling Assay
2.4. Statistical Analysis
3. Results
3.1. Analytical Assays
3.1.1. Plant-Based Extraction Mechanism of ZnO Nanoparticle Synthesis
3.1.2. Characterization of Biosynthesized Zinc Oxide Nanoparticles (ZnO-NPs)
3.1.3. Optical Properties of ZnO-NPs Using UV–Vis Spectroscopy
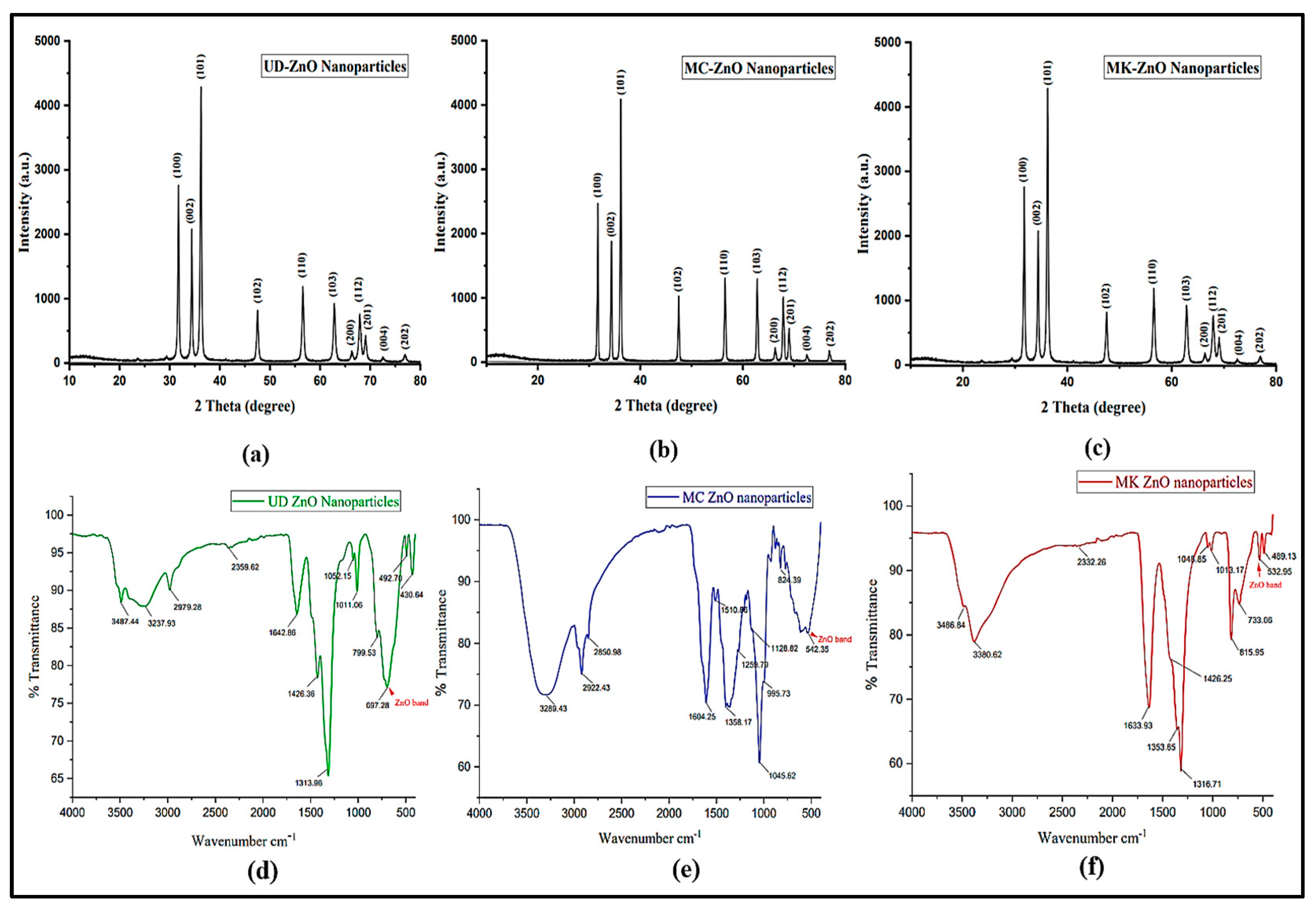
3.1.4. Crystallographic Analysis of ZnO-NPs Using X-ray Diffraction
3.1.5. Fourier Transform Infrared (FT-IR) Analysis
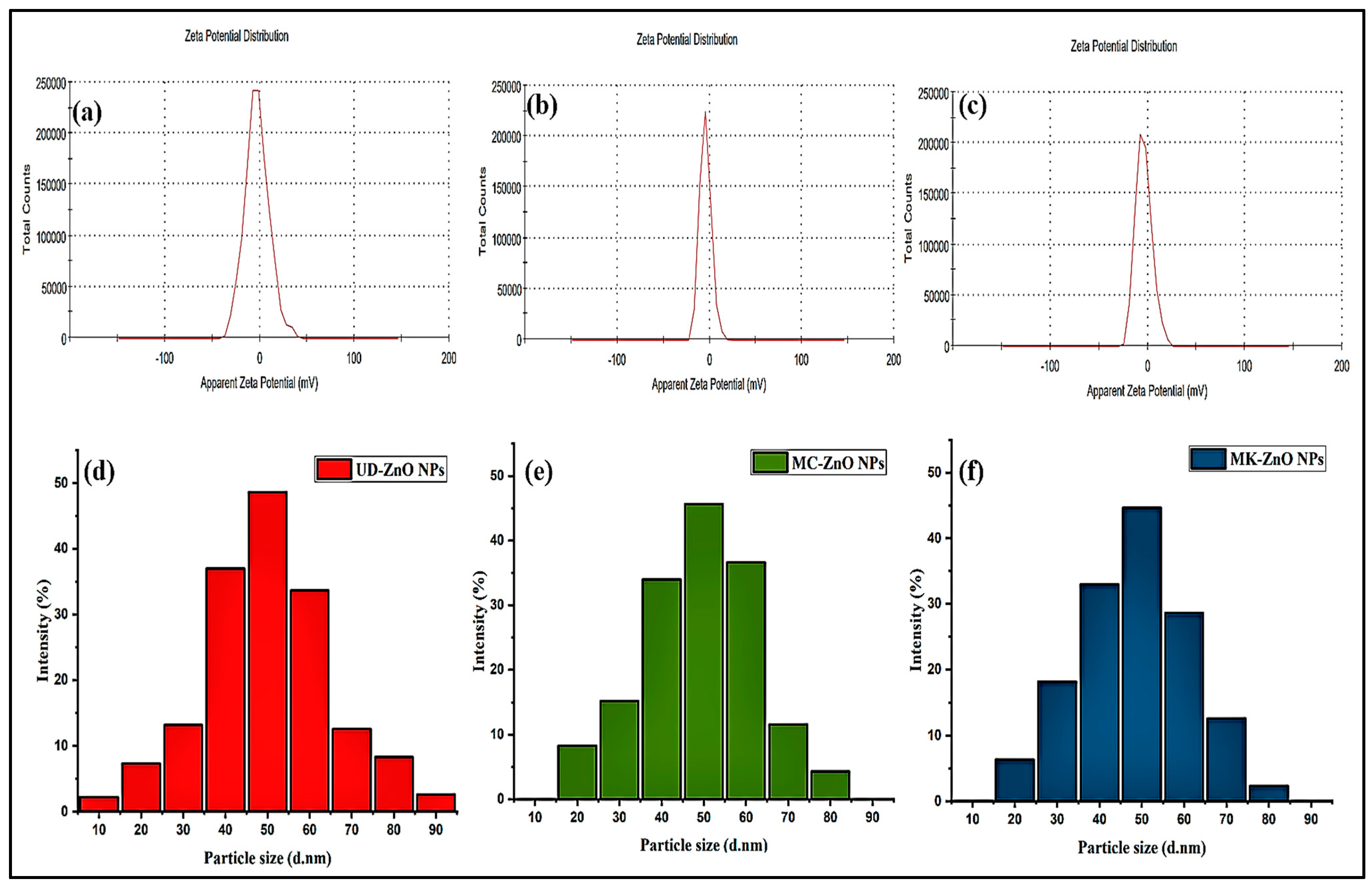
3.1.6. Analysis of Particle Size Using a Zeta Potentiometer and Dynamic Light Scattering
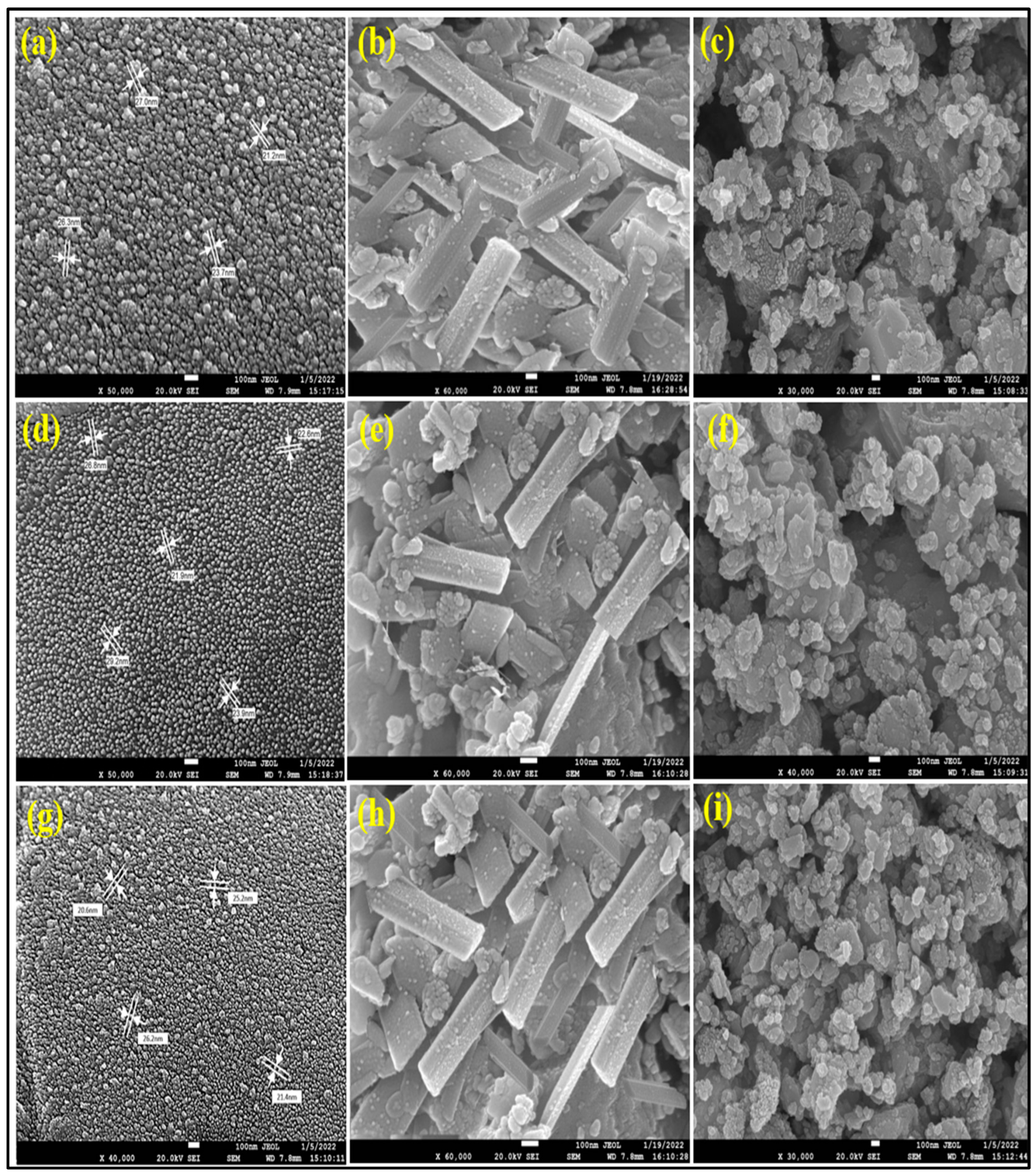
3.1.7. Field Emission Scanning Electron Microscopy
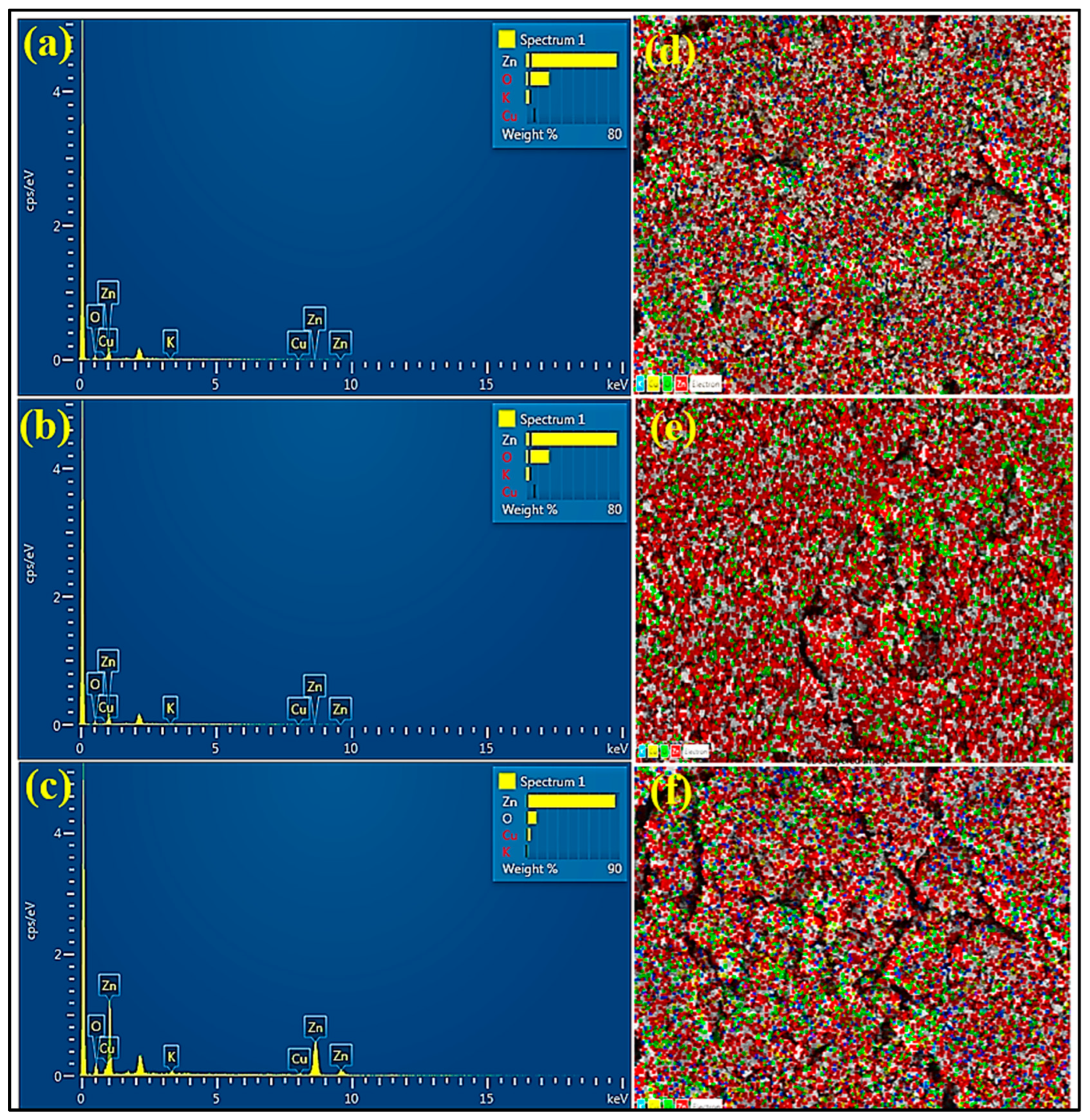
3.1.8. Energy Dispersive X-ray Spectroscopy
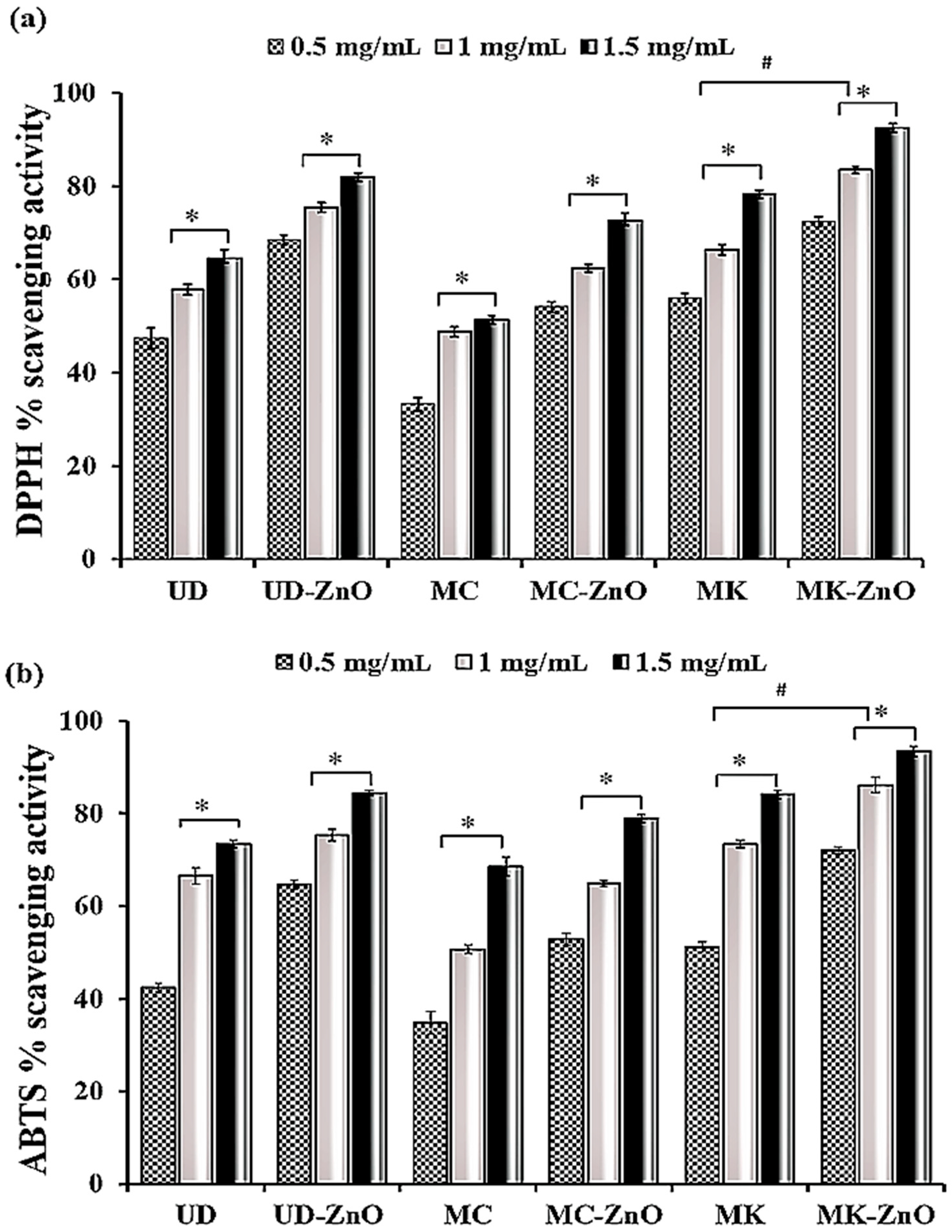
3.1.9. Dose-Dependent Antioxidant Potential of Biosynthesized ZnO-NPs Using DPPH and ABTS
3.1.10. EC50 Calculation Using Statistical Models
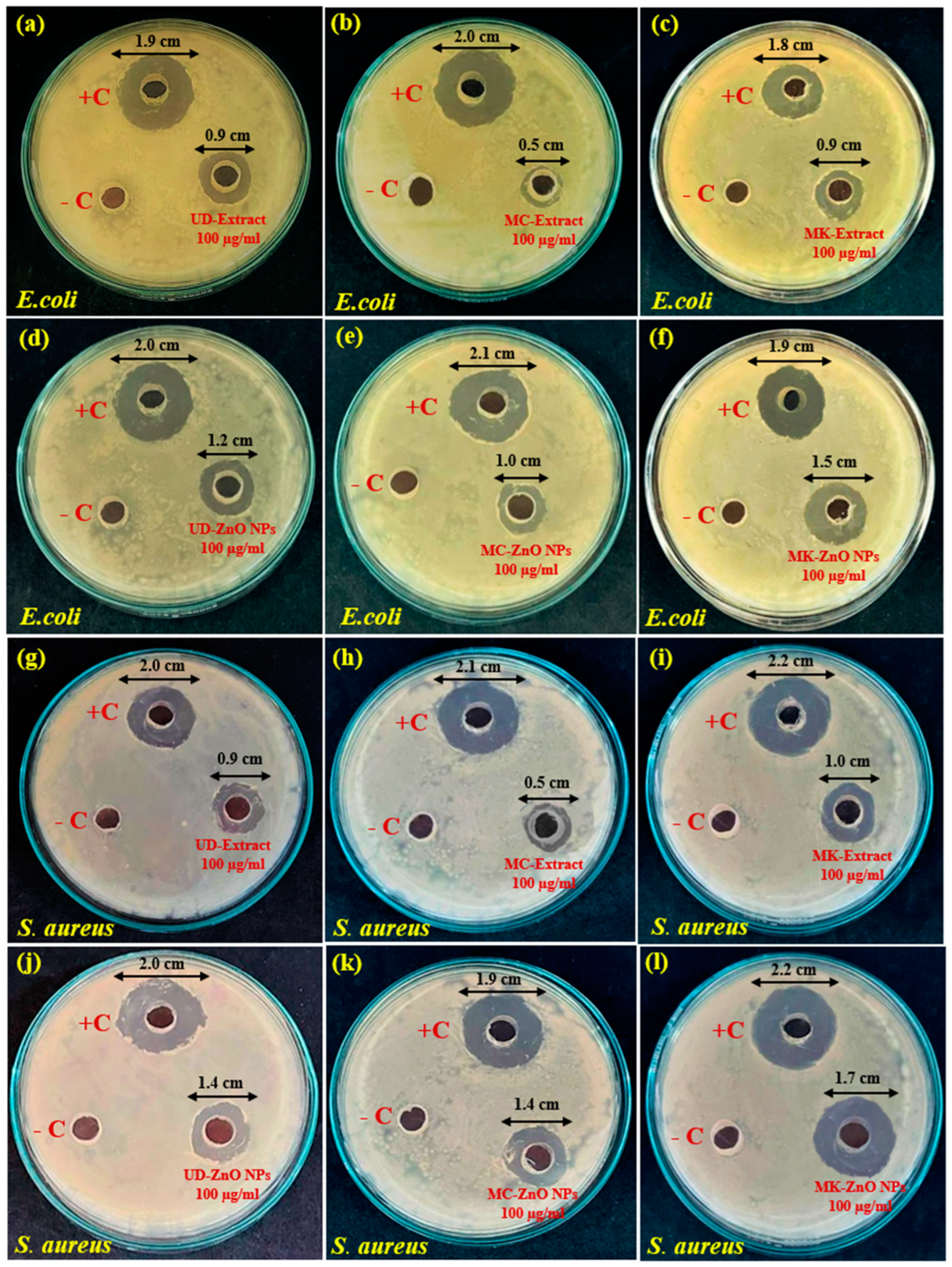
3.1.11. The Antibacterial Potential of Green-Engineered ZnO-NPs against S. aureus and E. coli
3.2. Cellular Assay
Trypan Blue Staining in Tissues of Green-Engineered ZnO-NP-Exposed D. melanogaster
3.3. Biochemical Assays
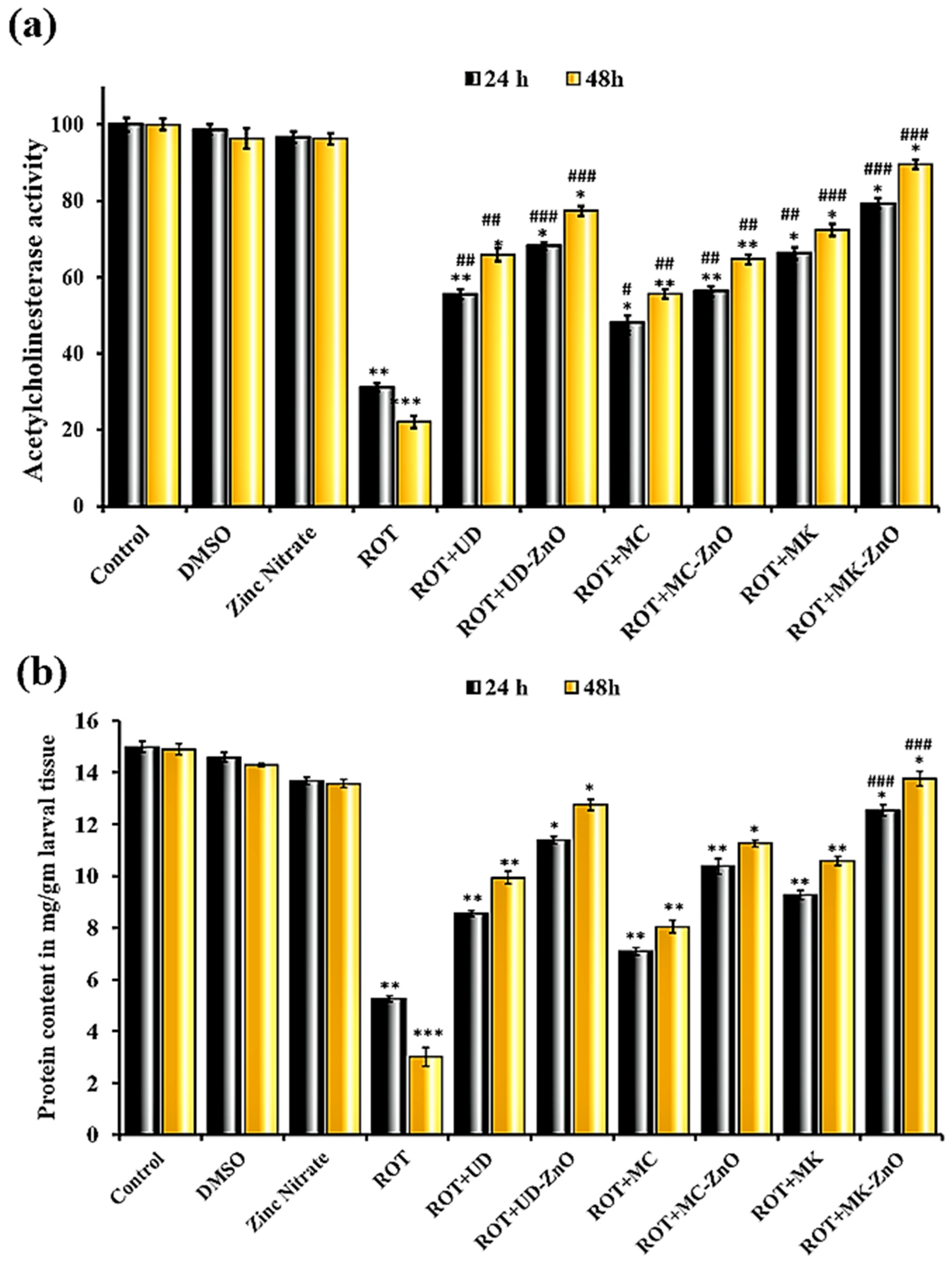
3.3.1. Biosynthesized ZnO-NPs Enhanced AchE Activity in Rotenone-Exposed D. melanogaster
3.3.2. Decreased Protein Concentration in Drosophila Exposed to ROT after 24 and 48 h
3.4. Behavioral Assays
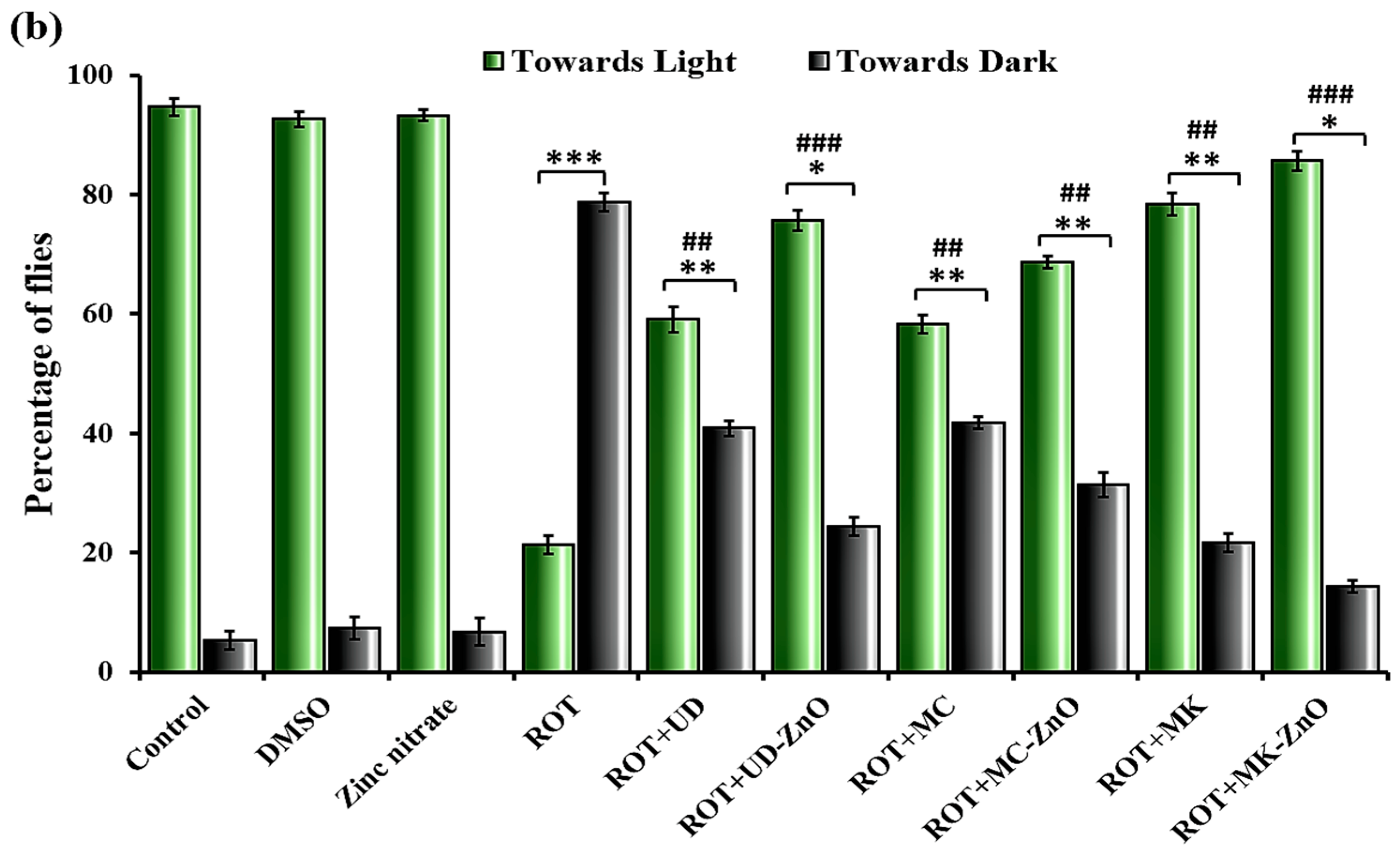
3.4.1. Significant Variation in Locomotor Activity of ROT-Treated Drosophila
3.4.2. Phyto-Synthesized ZnO-NPs Enhanced Memory in Rotenone-Exposed D. melanogaster
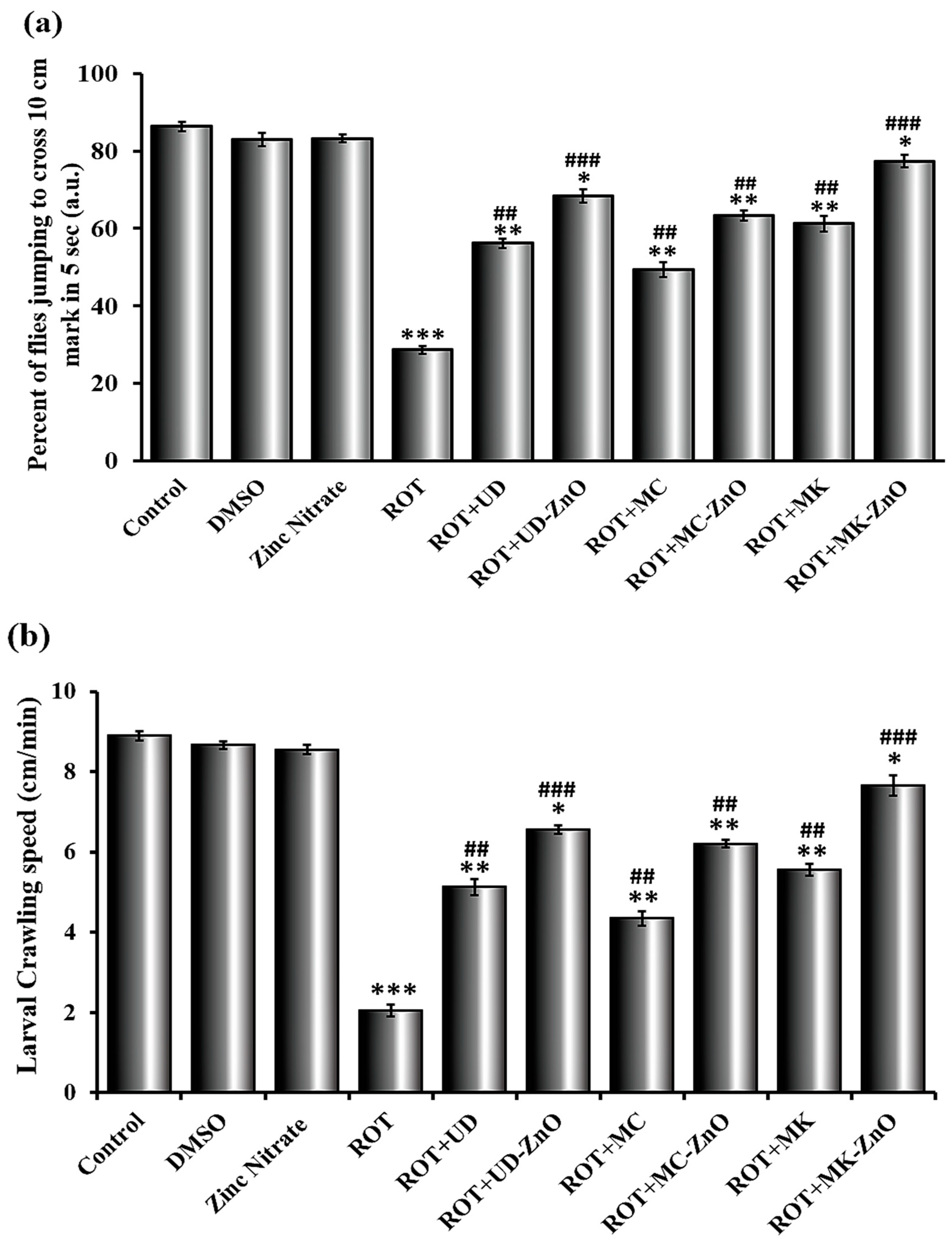
3.4.3. Rotenone Affects Jumping Activity in Drosophila
3.4.4. Green ZnO-NPs Elevated Crawling Activity in ROT-Treated Drosophila Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuznetsov, A.V.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. The Complex Interplay between Mitochondria, ROS, and Entire Cellular Metabolism. Antioxidants 2022, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhen, Y.; Wang, G.; Liu, B. Deconvoluting the Complexity of Reactive Oxygen Species (ROS) in Neurodegenerative Diseases. Front. Neuroanat. 2022, 16, 910427. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Singh, N.; Verma, M.; Kamal, R.; Tiwari, R.; Sanjay Chivate, M.; Rai, S.N.; Kumar, A.; Singh, A.; Singh, M.P.; et al. Hexavalent-Chromium-Induced Oxidative Stress, and the Protective Role of Antioxidants against Cellular Toxicity. Antioxidants 2022, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.M.; Li, C.; Pryma, D.A.; Brem, S.; Coukos, G.; Muzykantov, V. Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: Crossing the blood–brain barrier divide. Expert Opin. Drug Deliv. 2013, 10, 907–926. [Google Scholar] [CrossRef]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef]
- Camberos-Luna, L.; Massieu, L. Therapeutic strategies for ketosis induction and their potential efficacy for the treatment of acute brain injury and neurodegenerative diseases. Neurochem. Int. 2020, 133, 104614. [Google Scholar] [CrossRef]
- Shabir, S.; Singh, M.P. The aging: Introduction, theories, principles, and future prospective. In Anti-Aging Drug Discovery on the Basis of Hallmarks of Aging; Academic Press: New York, NY, USA, 2022; pp. 1–17. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.S.; Lee, K.J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, J.; Zhang, W.; Yu, J.; Zhuang, C. Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radic. Biol. Med. 2020, 159, 87–102. [Google Scholar] [CrossRef]
- Nath, M.; Debnath, P. Therapeutic role of traditionally used Indian medicinal plants and spices in combating COVID-19 pandemic situation. J. Biomol. Struct. Dyn. 2022, 41, 5894–5913. [Google Scholar] [CrossRef]
- Anand, U.; Tudu, C.K.; Nandy, S.; Sunita, K.; Tripathi, V.; Loake, G.J.; Dey, A.; Proćków, J. Ethnodermatological use of medicinal plants in India: From ayurvedic formulations to clinical perspectives—A review. J. Ethnopharmacol. 2022, 284, 114744. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, S.; Shabir, S.; Singh, M.P. Protection Against Drug-Induced Liver Injuries through Nutraceuticals via Amelioration of Nrf-2 Signaling. J. Am. Nutr. Assoc. 2022, 42, 495–515. [Google Scholar] [CrossRef]
- Shabir, S.; Yousuf, S.; Singh, S.K.; Vamanu, E.; Singh, M.P. Ethnopharmacological Effects of Urtica dioica, Matricaria chamomilla, and Murraya koenigii on Rotenone-Exposed D. melanogaster: An Attenuation of Cellular, Biochemical, and Organismal Markers. Antioxidants 2022, 11, 1623. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Shimoni, O.; Banks, W.A.; Bush, A.I.; Gamble, J.R.; Shi, B. Development of Novel Therapeutics Targeting the Blood–Brain Barrier: From Barrier to Carrier. Adv. Sci. 2021, 8, e2101090. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood–brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Soares GA, B.E.; Chopra, H.; Rahman, M.M.; Hasan, Z.; Swain, S.S.; Cavalu, S. Applications of Phyto-Nanotechnology for the Treatment of Neurodegenerative Disorders. Materials 2022, 15, 804. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Prasad, S.; Lall, R. Zinc-curcumin based complexes in health and diseases: An approach in chemopreventive and therapeutic improvement. JTEMIN 2022, 73, 127023. [Google Scholar] [CrossRef]
- Singh, M.P.; Shabir, S.; Deopa, A.S.; Raina, S.R.; Bantun, F.; Jalal, N.A.; Abdel-Razik, N.E.; Jamous, Y.F.; Alhumaidi, M.S.; Altammar, K.A.; et al. Synthesis of Green Engineered Silver Nanoparticles through Urtica dioica: An Inhibition of Microbes and Alleviation of Cellular and Organismal Toxicity in Drosophila melanogaster. Antibiotics 2022, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Zhang, F.; Abdallah, Y.; Zhang, M.; Wang, Y.; Sun, G.; Qiu, W.; Li, B. Biosynthesis and characterization of magnesium oxide and manganese dioxide nanoparticles using Matricaria chamomilla L. extract and its inhibitory effect on Acidovorax oryzae strain RS-2. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2230–2239. [Google Scholar] [CrossRef]
- Philip, D.; Unni, C.; Aromal, S.A.; Vidhu, V.K. Murraya Koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2011, 78, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, S.; Shabir, S.; Kauts, S.; Minocha, T.; Obaid, A.A.; Khan, A.A.; Mujalli, A.; Jamous, Y.F.; Almaghrabi, S.; Baothman, B.K.; et al. Appraisal of the Antioxidant Activity, Polyphenolic Content, and Characterization of Selected Himalayan Herbs: Anti-Proliferative Potential in HepG2 Cells. Molecules 2022, 27, 8629. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Ashokkumar, S. Bio fabrication of zinc oxide nanoparticles using leaf extract of curry leaf (Murraya koenigii) and its antimicrobial activities. Mater. Sci. Semicond. Process. 2015, 34, 365–372. [Google Scholar] [CrossRef]
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.S.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 212–220. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed AM, H.A. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization, and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef]
- Ismail, M.M.; Morsy, G.M.; Mohamed, H.M.; El-Mansy, M.A.; Abd-Alrazk, M.M. FT-IR spectroscopic analyses of 4-hydroxy-1-methyl-3-[2-nitro-2-oxoacetyl-2(1H)quinolinone (HMNOQ). Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2013, 113, 191–195. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah Akhtar, N.; Khattak, A.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Hamad, S.M.; Abdulrahman, A.F.; Biro, S.J.; Ghafor, A.A. Biosynthesis, Characterization and Mechanism of Formation of ZnO Nanoparticles Using Petroselinum Crispum Leaf Extract. Curr. Org. Synth. 2020, 17, 558–566. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2021, 27, 50. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH· assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Reddy, M.M.; Mathur, N.; Saxena, D.K.; Chowdhuri, D.K. Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene, and xylene exposed Drosophila melanogaster: Role of ROS generation. Toxicol. Appl. Pharmacol. 2009, 235, 226–243. [Google Scholar] [CrossRef]
- Ifeanyichukwu, U.L.; Fayemi, O.E.; Ateba, C.N. Green Synthesis of Zinc Oxide Nanoparticles from Pomegranate (Punica granatum) Extracts and Characterization of Their Antibacterial Activity. Molecules 2020, 25, 4521. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.U.; Anwar, M.R.; Zeb, M.A.; Liaqat, W. Green synthesis, characterization, antibacterial activity of metal nanoparticles and composite oxides using leaves extract of Ocimum basilicum L. Microsc. Res. Tech. 2022, 85, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Hosamani, R.; Muralidhara. Neuroprotective efficacy of Bacopa monnieri against rotenone induced oxidative stress and neurotoxicity in Drosophila melanogaster. Neurotoxicology 2009, 30, 977–985. [Google Scholar] [CrossRef]
- Kumar, P.P.; Bawani, S.S.; Anandhi, D.U.; Prashanth KV, H. Rotenone mediated developmental toxicity in Drosophila melanogaster. Environ. Toxicol. Pharmacol. 2022, 93, 103892. [Google Scholar] [CrossRef]
- Ayajuddin, M.; Phom, L.; Koza, Z.; Modi, P.; Das, A.; Chaurasia, R.; Thepa, A.; Yenisetti, S.C. Adult health and transition stage-specific rotenone-mediated Drosophila model of Parkinson’s disease: Impact on late-onset neurodegenerative disease models. Front. Mol. Neurosci. 2022, 15, 896183. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Ram, K.R.; Mishra, M.; Shrivastava, M.; Saxena, D.K.; Chowdhuri, D.K. Effects of coexposure of benzene, toluene, and xylene to Drosophila melanogaster: Alteration in hsp70, hsp60, hsp83, hsp26, ROS generation and oxidative stress markers. Chemosphere 2010, 79, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Mishra, M.; Sharma, A.; Shukla, A.K.; Mudiam, M.K.; Patel, D.K.; Ram, K.R.; Chowdhuri, D.K. Genotoxicity, and apoptosis in Drosophila melanogaster exposed to benzene, toluene, and xylene: Attenuation by quercetin and curcumin. Toxicol. Appl. Pharmacol. 2011, 253, 14–30. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Akinade, T.C.; Babatunde, O.O.; Adedara, A.O.; Adeyemi, O.E.; Otenaike, T.A.; Ashaolu, O.P.; Johnson, T.O.; Terriente-Felix, A.; Whitworth, A.J.; Abolaji, A.O. Protective capacity of carotenoid trans-astaxanthin in rotenone-induced toxicity in Drosophila melanogaster. Sci. Rep. 2022, 12, 4594. [Google Scholar] [CrossRef]
- D’Souza, L.C.; Dwivedi, S.; Raihan, F.; Yathisha, U.G.; Raghu, S.V.; Mamatha, B.S.; Sharma, A. Hsp70 overexpression in Drosophila hemocytes attenuates benzene-induced immune and developmental toxicity by regulating ROS/JNK signaling pathway. Environ. Toxicol. 2022, 37, 1723–1739. [Google Scholar] [CrossRef]
- Sharma, A.; Mishra, M.; Shukla, A.K.; Kumar, R.; Abdin, M.Z.; Chowdhuri, D.K. Organochlorine pesticide, endosulfan induced cellular and organismal response in Drosophila melanogaster. J. Hazard. Mater. 2012, 221–222, 275–287. [Google Scholar] [CrossRef]
- Singh, M.P.; Himalian, R.; Shabir, S.; Obaid, A.A.; Alamri, A.S.; Galanakis, C.M.; Singh, S.K.; Vamanu, E. Protection of Phytoextracts against Rotenone-Induced Organismal Toxicities in Drosophila melanogaster via the Attenuation of ROS Generation. Appl. Sci. 2022, 12, 9822. [Google Scholar] [CrossRef]
- Ali, Y.O.; Escala, W.; Ruan, K.; Zhai, R.G. Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. J. Vis. Exp. JoVE 2011, 49, 2504. [Google Scholar] [CrossRef]
- Sood, K.; Kaur, J.; Singh, H.; Kumar Arya, S.; Khatri, M. Comparative toxicity evaluation of graphene oxide (GO) and zinc oxide (ZnO) nanoparticles on Drosophila melanogaster. Toxicol. Rep. 2019, 6, 768–781. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Basnet, P.; Inakhunbi Chanu, T.; Samanta, D.; Chatterjee, S. A review on biosynthesized zinc oxide nanoparticles using plant extracts as reductants and stabilizing agents. J. Photochem. Photobiol. B Biol. 2018, 183, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; Alterary, S.; Ali Abdullrahman Almoghim, R.; Awad, M.A.; Aldosari, N.S.; Fahad Alghannam, S.; Nasser Alabdan, A.; Alharbi, S.; Ali Mohammed Alateeq, B.; Abdulrahman Al Mohsen, A.; et al. Greener Synthesis of Zinc Oxide Nanoparticles: Characterization and Multifaceted Applications. Molecules 2020, 25, 4198. [Google Scholar] [CrossRef] [PubMed]
- Alhujaily, M.; Albukhaty, S.; Yusuf, M.; Mohammed MK, A.; Sulaiman, G.M.; Al-Karagoly, H.; Alyamani, A.A.; Albaqami, J.; AlMalki, F.A. Recent Advances in Plant-Mediated Zinc Oxide Nanoparticles with Their Significant Biomedical Properties. Bioengineering 2022, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorganic Chem. 2020, 94, 103423. [Google Scholar] [CrossRef] [PubMed]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; HM Abd El-Azim, M. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and their Cytotoxic Activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2021, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Valan Arasu, M. Environmentally Friendly Green Approach for the Production of Zinc Oxide Nanoparticles and Their Anti-Fungal, Ovicidal, and Larvicidal Properties. Nanomaterials 2018, 8, 500. [Google Scholar] [CrossRef]
- Kiani, B.H.; Haq, I.U.; Alhodaib, A.; Basheer, S.; Fatima, H.; Naz, I.; Ur-Rehman, T. Comparative Evaluation of Biomedical Applications of Zinc Nanoparticles Synthesized by Using Withania somnifera Plant Extracts. Plants 2022, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, G.; Mohammad, K.S.; Zarei, M.; Shokoohi, M.; Oskoueian, E.; Poorbagher, M.R.M.; Karimi, E. Zinc oxide nanoparticles synthesized using Hyssopus officinalis L. Extract Induced oxidative stress and changes the expression of key genes involved in inflammatory and antioxidant Systems. Biol. Res. 2022, 55, 24. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.S.; Kumbhakar, D.V.; Pasha, A.; Pawar, S.C.; Grace, A.N.; Singh, R.P.; Nguyen, V.H.; Le, Q.V.; Peng, W. Crotalaria verrucosa Leaf Extract Mediated Synthesis of Zinc Oxide Nanoparticles: Assessment of Antimicrobial and Anticancer Activity. Molecules 2020, 25, 4896. [Google Scholar] [CrossRef] [PubMed]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Cheema, A.I.; Ahmed, T.; Abbas, A.; Noman, M.; Zubair, M.; Shahid, M. Antimicrobial activity of the biologically synthesized zinc oxide nanoparticles against important rice pathogens. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2022, 28, 1955–1967. [Google Scholar] [CrossRef]
- Hashemi, S.; Asrar, Z.; Pourseyedi, S.; Nadernejad, N. Green synthesis of ZnO nanoparticles by Olive (Olea europaea). IET Nanobiotechnol. 2016, 10, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Barzinjy, A.A.; Azeez, H.H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Alyamani, A.A.; Albukhaty, S.; Aloufi, S.; AlMalki, F.A.; Al-Karagoly, H.; Sulaiman, G.M. Green Fabrication of Zinc Oxide Nanoparticles Using Phlomis Leaf Extract: Characterization and In Vitro Evaluation of Cytotoxicity and Antibacterial Properties. Molecules 2021, 26, 6140. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G. Scanning electron microscopy: Preparation and imaging for SEM. Methods Mol. Biol. 2012, 915, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gur, T.; Meydan, I.; Seckin, H.; Bekmezci, M.; Sen, F. Green synthesis, characterization, and bioactivity of biogenic zinc oxide nanoparticles. Environ. Res. 2022, 204 Pt A, 111897. [Google Scholar] [CrossRef]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed. 2018, 14, 87–100. [Google Scholar] [CrossRef]
- Khaleghi, S.; Khayatzadeh, J.; Neamati, A. Biosynthesis of Zinc Oxide nanoparticles using Origanum majorana L. leaf extract, its antioxidant, and cytotoxic activities. Mater. Technol. 2022, 37, 2522–2531. [Google Scholar] [CrossRef]
- Tettey, C.O.; Shin, H.M. Evaluation of the antioxidant and cytotoxic activities of zinc oxide nanoparticles synthesized using Scutellaria baicalensis root. Sci. Afr. 2019, 6, e00157. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, V.S.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem.-Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir ACS J. Surf. Colloids 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Aldeen, T.S.; Mohamed HE, A.; Maaza, M. ZnO nanoparticles prepared via a green synthesis approach: Physical properties, photocatalytic and antibacterial activity. J. Phys. Chem. Solids 2022, 160, 110313. [Google Scholar] [CrossRef]
- Azimpanah, R.; Solati, Z.; Hashemi, M. Synthesis of ZnO nanoparticles with Antibacterial properties using Terminalia catappa leaf extract. Chem. Eng. Technol. 2022, 45, 658–666. [Google Scholar] [CrossRef]
- Siima, A.A.; Stephano, F.; Munissi, J.J.E.; Nyandoro, S.S. Ameliorative effects of flavonoids and polyketides on the rotenone induced Drosophila model of Parkinson’s disease. Neurotoxicology 2020, 81, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Kumar, M.; Jain, M.; Dhawan, D.K. Combined effects of curcumin and piperine in ameliorating benzo(a)pyrene induced DNA damage. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 3002–3006. [Google Scholar] [CrossRef]
- Pandareesh, M.D.; Shrivash, M.K.; Naveen Kumar, H.N.; Misra, K.; Srinivas Bharath, M.M. Curcumin Monoglucoside Shows Improved Bioavailability and Mitigates Rotenone Induced Neurotoxicity in Cell and Drosophila Models of Parkinson’s Disease. Neurochem. Res. 2016, 41, 3113–3128. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Kumar, M.; Jain, M.; Dhawan, D.K. Synergistic effects of piperine and curcumin in modulating benzo(a)pyrene induced redox imbalance in mice lungs. Toxicol. Mech. Methods 2012, 22, 74–80. [Google Scholar] [CrossRef]
- Chopra, G.; Shabir, S.; Yousuf, S.; Kauts, S.; Bhat, S.A.; Mir, A.H.; Singh, M.P. Proteinopathies: Deciphering Physiology and Mechanisms to Develop Effective Therapies for Neurodegenerative Diseases. Mol. Neurobiol. 2022, 59, 7513–7540. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Chakrabarty, R.; Shabir, S.; Yousuf, S.; Obaid, A.A.; Moustafa, M.; Al-Shehri, M.; Al-Emam, A.; Alamri, A.S.; Alsanie, W.F.; et al. Influence of the Gut Microbiota on the Development of Neurodegenerative Diseases. Mediat. Inflamm. 2022, 2022, 3300903. [Google Scholar] [CrossRef]
- Akintunde, J.K.; Farai, T.I.; Arogundade, M.R.; Adeleke, J.T. Biogenic zinc-oxide nanoparticles of Moringa oleifera leaves abrogates rotenone induced neuroendocrine toxicity by regulation of oxidative stress and acetylcholinesterase activity. Biochem. Biophys. Rep. 2021, 26, 100999. [Google Scholar] [CrossRef]
- Riemensperger, T.; Issa, A.R.; Pech, U.; Coulom, H.; Nguyễn, M.V.; Cassar, M.; Jacquet, M.; Fiala, A.; Birman, S. A single dopamine pathway underlies progressive locomotor deficits in a Drosophila model of Parkinson disease. Cell Rep. 2013, 5, 952–960. [Google Scholar] [CrossRef]
- Teixeira, A.; Sárria, M.P.; Pinto, I.; Espiña, B.; Gomes, A.C.; Dias AC, P. Protection against Paraquat-Induced Oxidative Stress by Curcuma longa Extract-Loaded Polymeric Nanoparticles in Zebrafish Embryos. Polymers 2022, 14, 3773. [Google Scholar] [CrossRef]
- Sehgal, A.; Kumar, M.; Jain, M.; Dhawan, D.K. Modulatory effects of curcumin in conjunction with piperine on benzo(a)pyrene-mediated DNA adducts and biotransformation enzymes. Nutr. Cancer 2013, 65, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Slama, I.B.; Omri, K.; El Ghoul, J.; El Mir, L.; Rhouma, K.B.; Abdelmelek, H.; Sakly, M. Effects of nanoparticle zinc oxide on emotional behavior and trace elements homeostasis in rat brain. Toxicol. Ind. Health 2015, 31, 1202–1209. [Google Scholar] [CrossRef]
- Adedara, A.O.; Otenaike, T.A.; Olabiyi, A.A.; Adedara, I.A.; Abolaji, A.O. Neurotoxic and behavioral deficit in Drosophila melanogaster coexposed to rotenone and iron. Metab. Brain Dis. 2022, 38, 349–360. [Google Scholar] [CrossRef]
- Nitta, Y.; Sugie, A. Studies of neurodegenerative diseases using Drosophila and the development of novel approaches for their analysis. Fly 2022, 16, 275–298. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Rani, R.; Singh, S. Biogenic Zinc Oxide Nanoparticles and Their Biomedical Applications: A Review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1437–1452. [Google Scholar] [CrossRef]
- Demir, E.; Demir, F.T.; Marcos, R. Drosophila as a Suitable In Vivo Model in the Safety Assessment of Nanomaterials. Adv. Exp. Med. Biol. 2022, 1357, 275–301. [Google Scholar] [CrossRef]
- Carmona, E.R.; Inostroza-Blancheteau, C.; Rubio, L.; Marcos, R. Genotoxic and oxidative stress potential of nanosized and bulk zinc oxide particles in Drosophila melanogaster. Toxicol. Ind. Health 2016, 32, 1987–2001. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Yong, L.Q.; Hande, M.P.; Ong, C.N.; Yu, L.E.; Bay, B.H.; Baeg, G.H. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int. J. Nanomed. 2017, 12, 1621–1637. [Google Scholar] [CrossRef]
- Ardelean, A.V.; Morosanu, A.M.; Ardelean, I.; Moisescu, C.; Cornea, C.P. Review on knowledge gap in Brachiaria grass research and utilization: Ethiopian perspective. AgroLife Sci. J. 2022, 11, 9–17. [Google Scholar] [CrossRef]

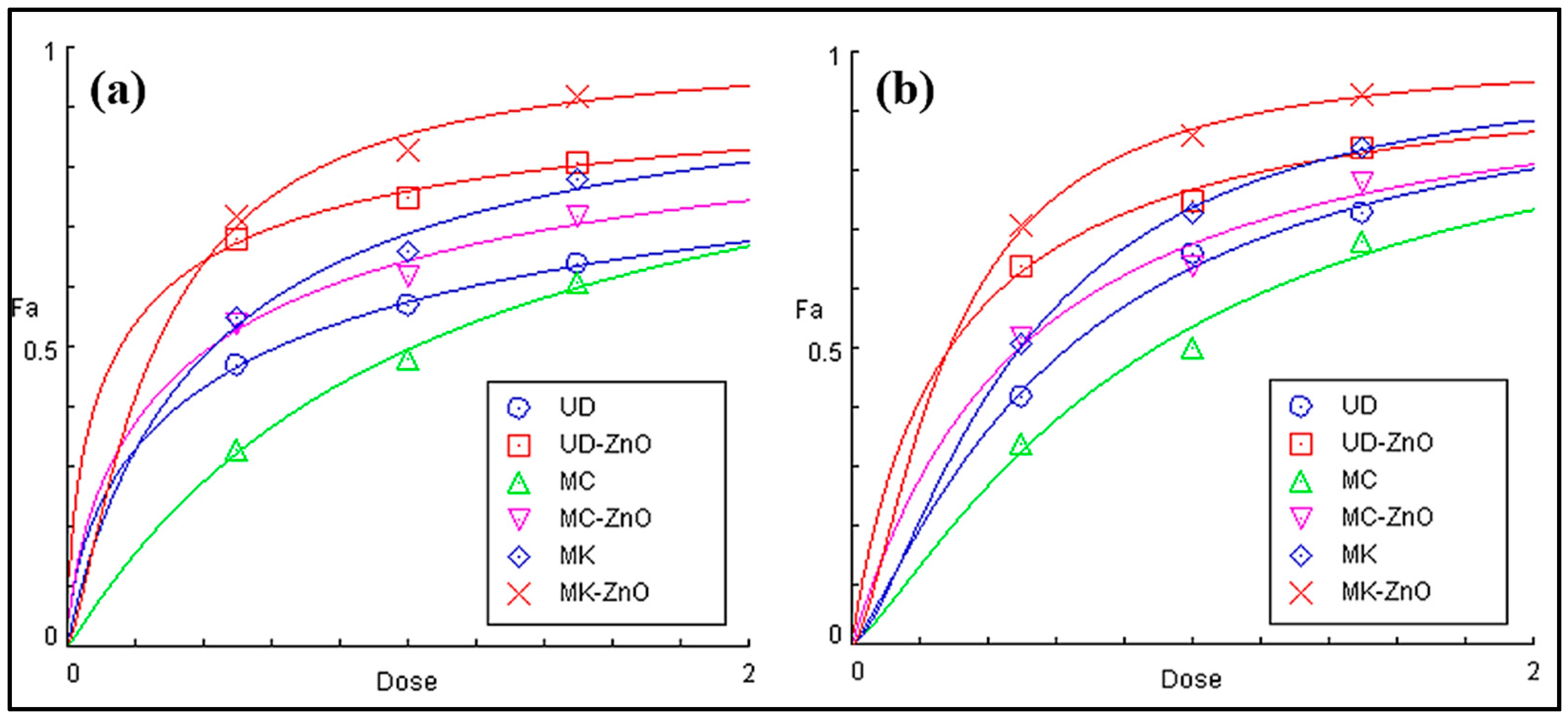


| S. No. | Drug | EC50 (mg/mL) of DPPH | EC50 (mg/mL) of ABTS |
|---|---|---|---|
| 1. | Ascorbic Acid | 0.4 ± 0.02 | 0.11 ± 0.05 |
| 2. | UD | 0.61 ± 0.12 | 0.63 ± 0.11 |
| 3. | UD-ZnO | 0.25 ± 0.18 | 0.28 ± 0.08 |
| 4. | MC | 1.01 ± 0.47 | 0.88 ± 0.10 |
| 5. | MC-ZnO | 0.41 ± 0.21 | 0.47 ± 0.05 |
| 6. | MK | 0.42 ± 0.14 | 0.48 ± 0.17 |
| 7. | MK-ZnO | 0.15 ± 0.03 | 0.21 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabir, S.; Sehgal, A.; Dutta, J.; Devgon, I.; Singh, S.K.; Alsanie, W.F.; Alamri, A.S.; Alhomrani, M.; Alsharif, A.; Basalamah, M.A.M.; et al. Therapeutic Potential of Green-Engineered ZnO Nanoparticles on Rotenone-Exposed D. melanogaster (Oregon R+): Unveiling Ameliorated Biochemical, Cellular, and Behavioral Parameters. Antioxidants 2023, 12, 1679. https://doi.org/10.3390/antiox12091679
Shabir S, Sehgal A, Dutta J, Devgon I, Singh SK, Alsanie WF, Alamri AS, Alhomrani M, Alsharif A, Basalamah MAM, et al. Therapeutic Potential of Green-Engineered ZnO Nanoparticles on Rotenone-Exposed D. melanogaster (Oregon R+): Unveiling Ameliorated Biochemical, Cellular, and Behavioral Parameters. Antioxidants. 2023; 12(9):1679. https://doi.org/10.3390/antiox12091679
Chicago/Turabian StyleShabir, Shabnam, Amit Sehgal, Joydeep Dutta, Inderpal Devgon, Sandeep K. Singh, Walaa F. Alsanie, Abdulhakeem S. Alamri, Majid Alhomrani, Abdulaziz Alsharif, Mohammed Abubaker Mohammed Basalamah, and et al. 2023. "Therapeutic Potential of Green-Engineered ZnO Nanoparticles on Rotenone-Exposed D. melanogaster (Oregon R+): Unveiling Ameliorated Biochemical, Cellular, and Behavioral Parameters" Antioxidants 12, no. 9: 1679. https://doi.org/10.3390/antiox12091679











