Evaluation of Antioxidant Activity and Drug Delivery Potential of Cell-Derived Extracellular Vesicles from Citrus reticulata Blanco cv. ‘Dahongpao’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Extraction of CEVs
2.3. Evaluation of CEVs
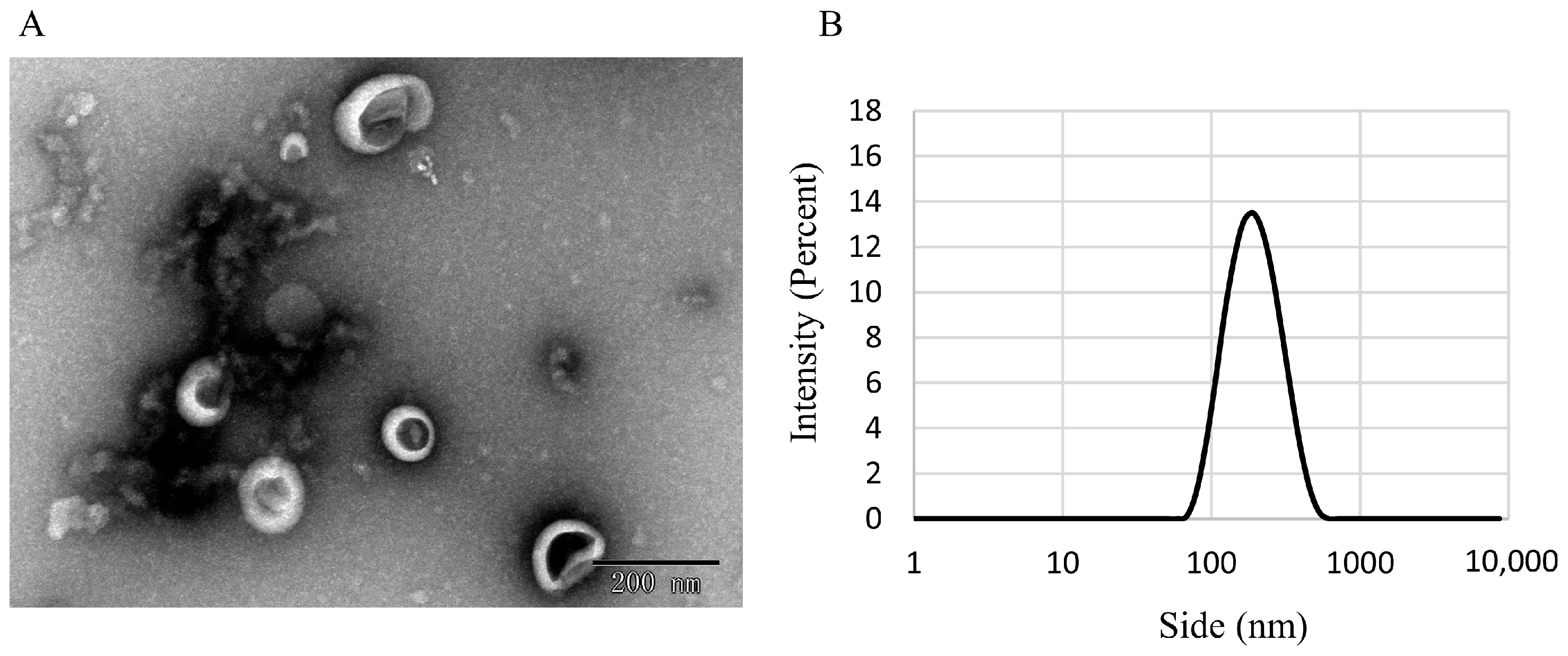
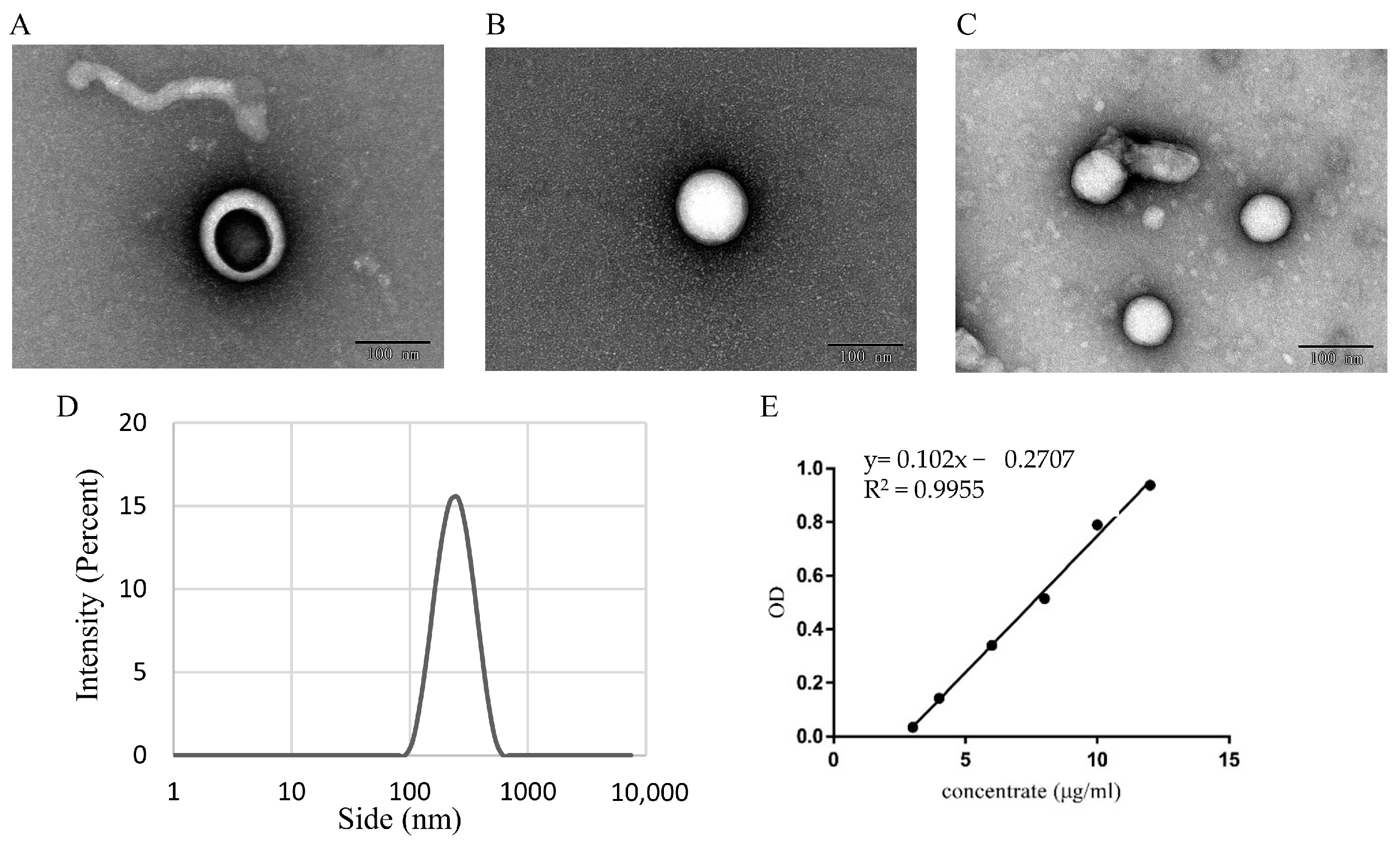
2.3.1. Analysis of CEV Morphology by Using Transmission Electron Microscopy
2.3.2. Malvern Laser Particle Size Analyzer
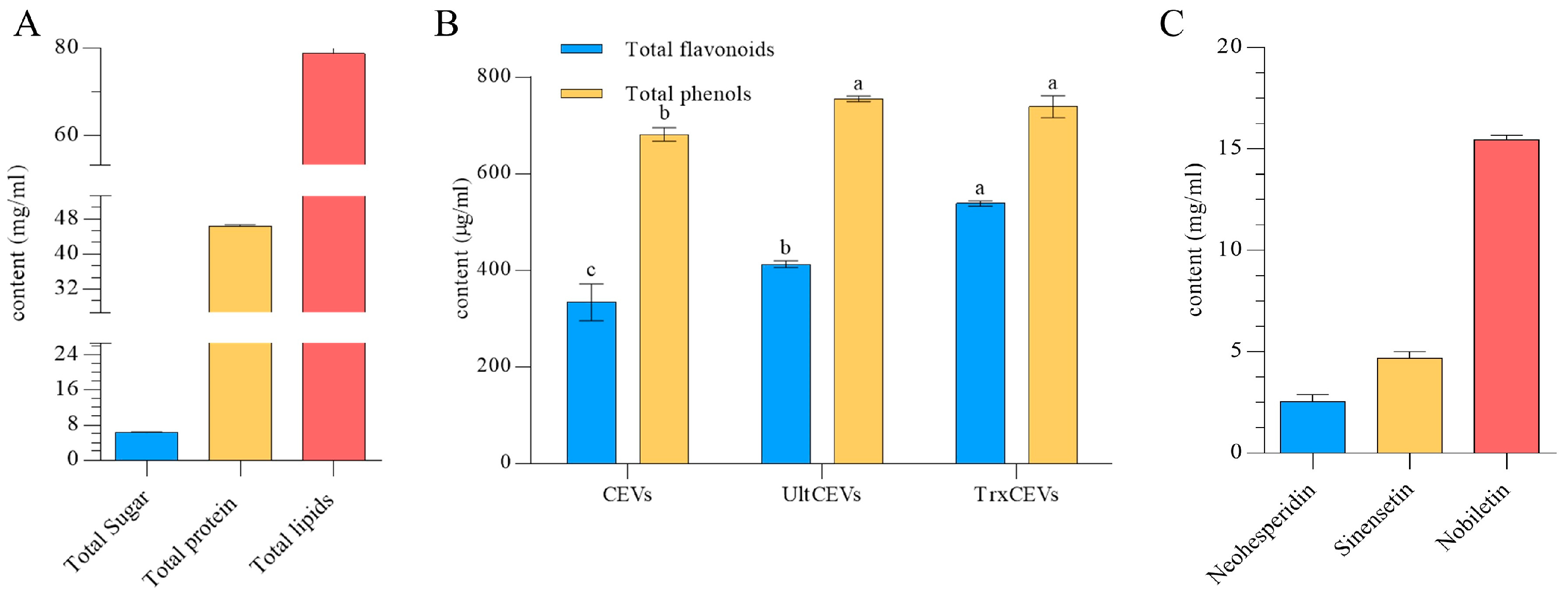
2.3.3. Identification of CEVs Components
Protein Content Determination
Lipid Content Determination
Total Sugar Content Determination
Determination of Total Phenol and Total Flavonoid Content
Determination of Flavonoids
2.3.4. Chemical Evaluation of the Antioxidant Capacity of CEVs
2.3.5. Storage Stability
2.4. Assessment of Anti-Inflammatory and Antioxidant Capacity
2.4.1. Cell Recovery and Culture
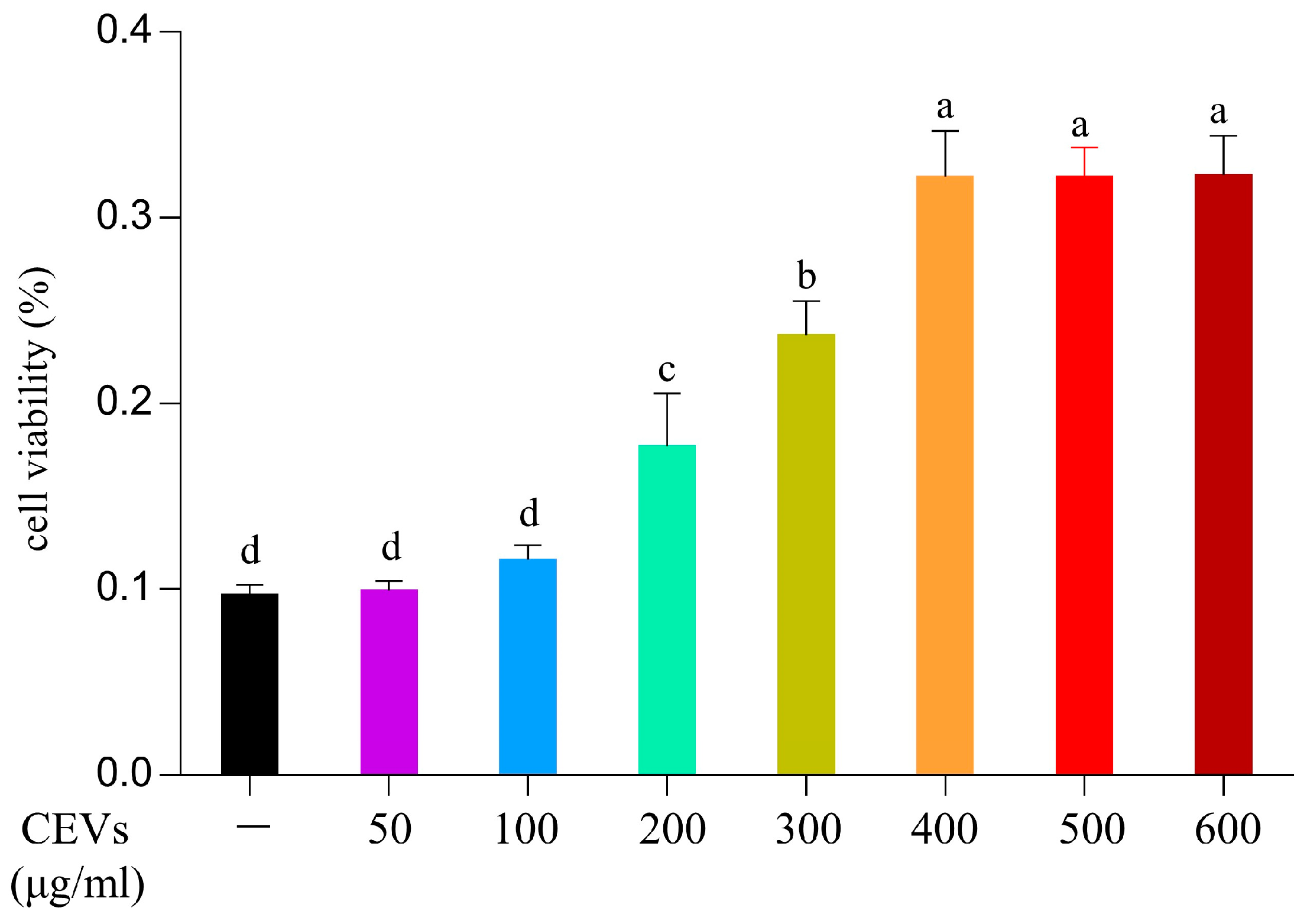
2.4.2. Cell Viability Assay
2.4.3. H2O2-Induced Oxidative Stress
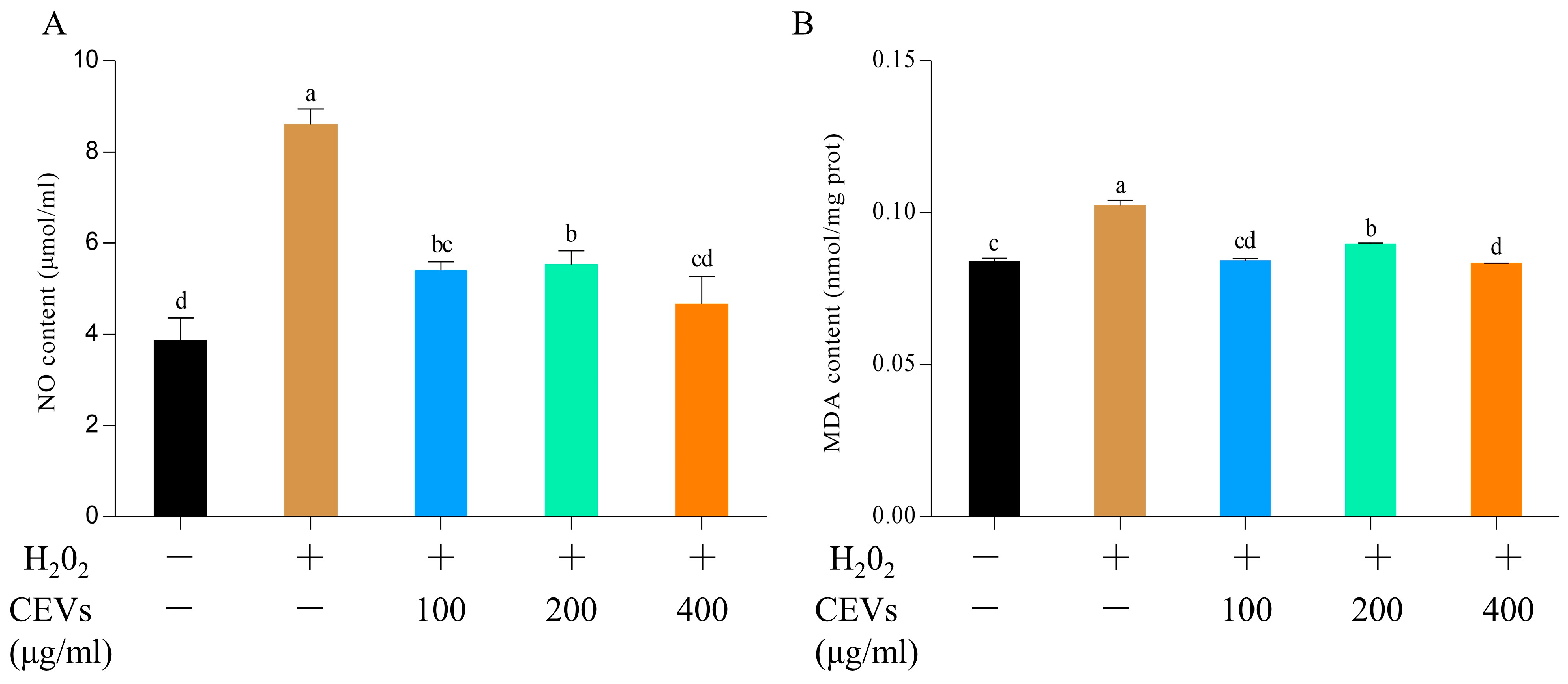
2.4.4. Nitrogen Monoxide (NO) Determination
2.4.5. Malondialdehyde (MDA) Determination
2.4.6. Antioxidant Enzyme Index Determination
2.4.7. LPS Induce Cellular Inflammation
2.4.8. Expression Measurement of Inflammatory Factors
2.5. CEVs Loaded with Tangeretin
2.5.1. Preparation of CEVs-TAN
2.5.2. Drug Encapsulation and Loading Efficiency Evaluation
2.6. Statistical Analysis
3. Results
3.1. Identifification and Characterization of CEVs
3.2. Chemical Composition of CEVs
3.3. Chemical Antioxidant Activity of CEVs
3.4. Storage Stability
3.5. Effect of CEVs on Cell Viability
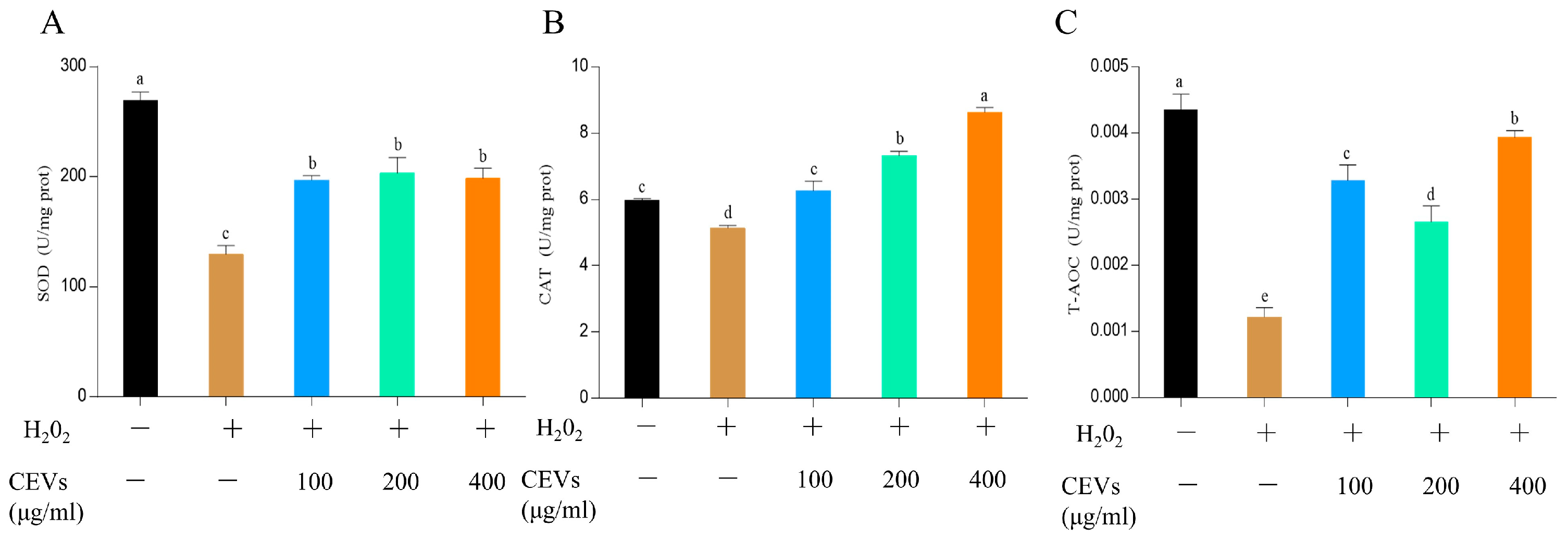
3.6. Cellular Antioxidant Activity of CEVs
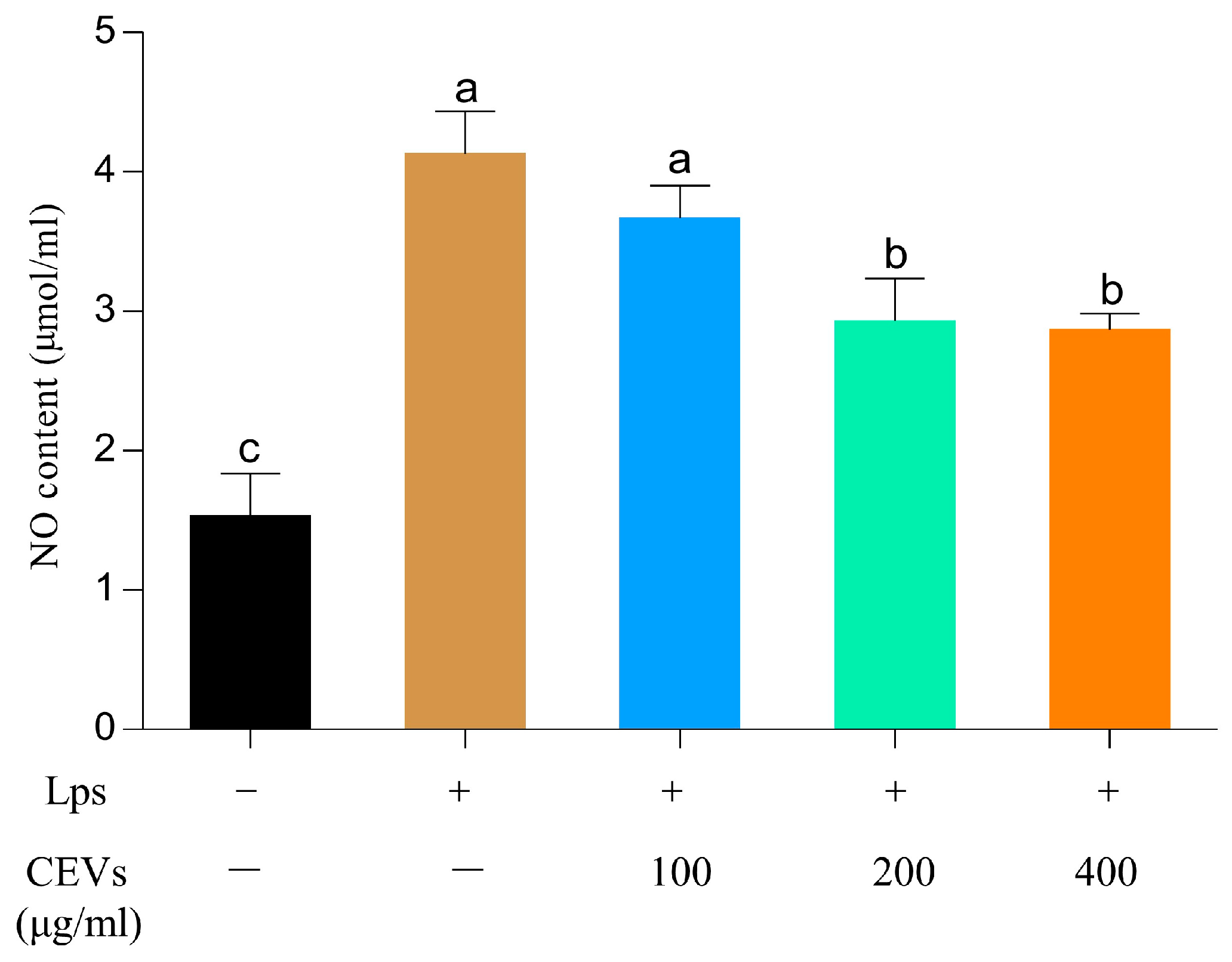
3.6.1. Determination of NO and MDA Indicators
3.6.2. Determination of Enzyme Activity Indicators
3.7. Anti-Inflammatory Activity of CEVs
3.7.1. Determination of NO Indicators
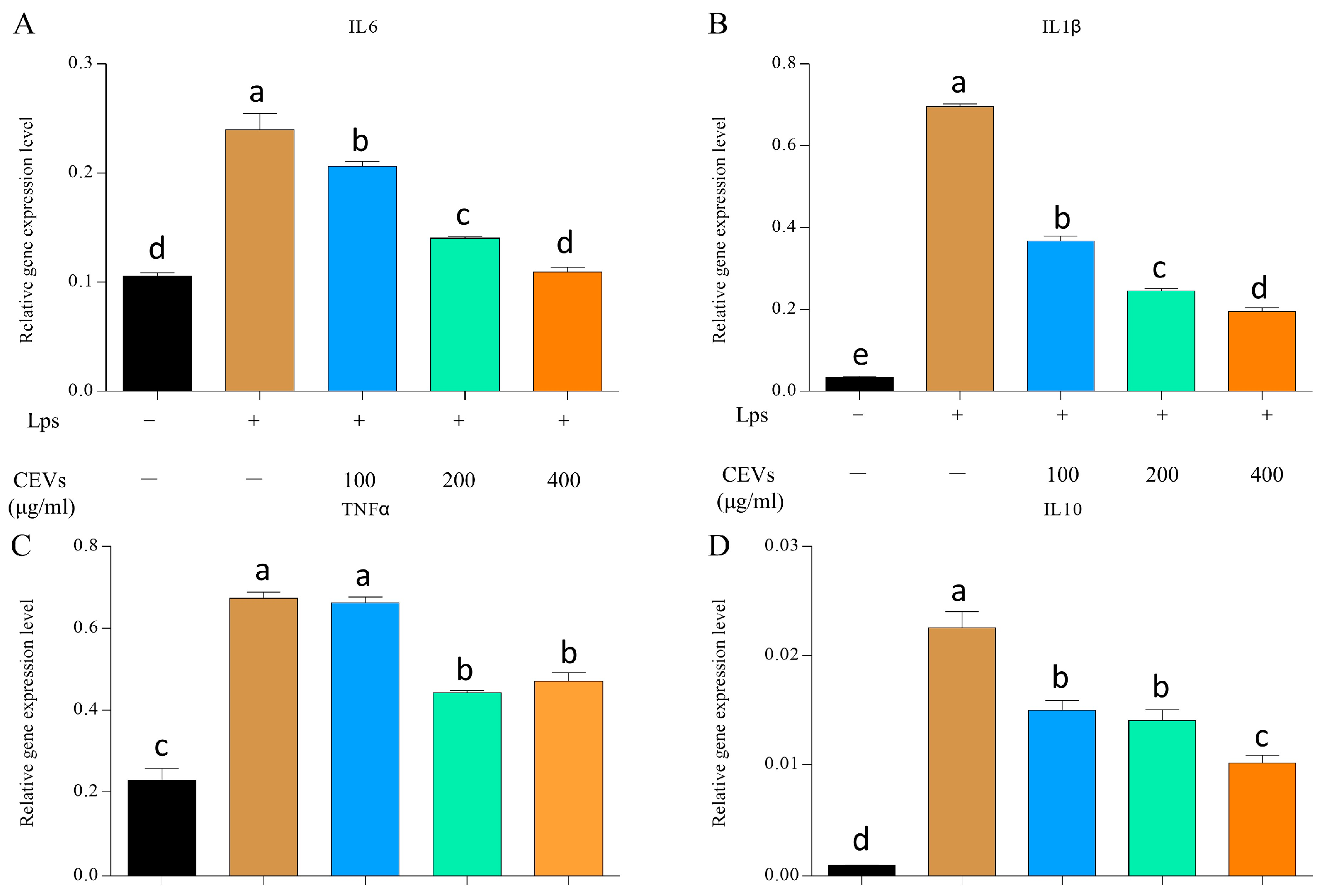
3.7.2. Measurement of Inflammatory Factors
3.8. Preparation of CEVs-TAN and Its Antioxidant and Anti-Inflammatory Evaluation
3.8.1. Loading of Tangeretin by CEVs
3.8.2. Antioxidant and Anti-Inflammatory Evaluation of CEVs-TAN
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Herrmann, I.K.; Stevens, M.M. Cell-Derived Vesicles for Drug Therapy and Diagnostics: Opportunities and Challenges. Nano Today 2015, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Hill, A.F. Therapeutically Harnessing Extracellular Vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.-K.; Giebel, B. Exosomes: Small Vesicles Participating in Intercellular Communication. Int. J. Biochem. Cell Biol. 2012, 44, 11–15. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Court, F.A. Exosomes: Mediators of Communication in Eukaryotes. Biol. Res. 2013, 46, 5–11. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-Derived Exosome-like Nanoparticles and Their Therapeutic Activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.L.; Nielsen, M.E. Plant Exosomes: Using an Unconventional Exit to Prevent Pathogen Entry? J. Exp. Bot. 2017, 69, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Guerriero, G.; Hausman, J.-F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant Extracellular Vesicles and Nanovesicles: Focus on Secondary Metabolites, Proteins and Lipids with Perspectives on Their Potential and Sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-Derived Exosome-like Nanoparticles: A Concise Review on Its Extraction Methods, Content, Bioactivities, and Potential as Functional Food Ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant Derived Edible Nanoparticles as a New Therapeutic Approach against Diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical Review: Vegetables and Fruit in the Prevention of Chronic Diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus Limon-Derived Nanovesicles Inhibit Cancer Cell Proliferation and Suppress CML Xenograft Growth by Inducing TRAIL-Mediated Cell Death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Arai, M.; Komori, H.; Fujita, D.; Tamai, I. Uptake Pathway of Apple-Derived Nanoparticle by Intestinal Cells to Deliver Its Cargo. Pharm. Res. 2021, 38, 523–530. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An Efficient Method to Isolate Lemon Derived Extracellular Vesicles for Gastric Cancer Therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Xu, X.-H.; Yuan, T.-J.; Dad, H.A.; Shi, M.-Y.; Huang, Y.-Y.; Jiang, Z.-H.; Peng, L.-H. Plant Exosomes as Novel Nanoplatforms for MicroRNA Transfer Stimulate Neural Differentiation of Stem Cells In Vitro and In Vivo. Nano Lett. 2021, 21, 8151–8159. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of Cabbage Exosome-like Nanovesicles and Investigation of Their Biological Activities in Human Cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Kim, M.; Jang, H.; Park, J.H. Balloon Flower Root-Derived Extracellular Vesicles: In Vitro Assessment of Anti-Inflammatory, Proliferative, and Antioxidant Effects for Chronic Wound Healing. Antioxidants 2023, 12, 1146. [Google Scholar] [CrossRef]
- Mahdipour, E. Beta Vulgaris Juice Contains Biologically Active Exosome-like Nanoparticles. Tissue Cell 2022, 76, 101800. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; Zhang, H.-G. Delivery of Therapeutic Agents by Nanoparticles Made of Grapefruit-Derived Lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Z.; Wang, F.; Zhou, Z. The Protective Effects of Sour Orange (Citrus Aurantium L.) Polymethoxyflavones on Mice Irradiation-Induced Intestinal Injury. Molecules 2022, 27, 1934. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.-B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies Communication between Plant and Mouse Gut Host Cells through Edible Plant Derived Exosome-like Nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, X.; Han, M.; Smbatyan, G.; Lenz, H.-J.; Zhang, Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 2018, 140, 16413–16417. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and Directions in Studying Cell-Cell Communication by Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Mahaweni, N.M.; Kaijen-Lambers, M.E.H.; Dekkers, J.; Aerts, J.G.J.V.; Hegmans, J.P.J.J. Tumour-Derived Exosomes as Antigen Delivery Carriers in Dendritic Cell-Based Immunotherapy for Malignant Mesothelioma. J. Extracell Vesicles 2013, 2, 22492. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering Exosomes as Refined Biological Nanoplatforms for Drug Delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. A Systematic Analysis of Peptide Linker Length and Liposomal Polyethylene Glycol Coating on Cellular Uptake of Peptide-Targeted Liposomes. ACS Nano 2013, 7, 2935–2947. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and Challenges of Extracellular Vesicle-Based Drug Delivery System: Considering Cell Source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine Milk-Derived Exosomes for Drug Delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Kilasoniya, A.; Garaeva, L.; Shtam, T.; Spitsyna, A.; Putevich, E.; Moreno-Chamba, B.; Salazar-Bermeo, J.; Komarova, E.; Malek, A.; Valero, M.; et al. Potential of Plant Exosome Vesicles from Grapefruit (Citrus × Paradisi) and Tomato (Solanum Lycopersicum) Juices as Functional Ingredients and Targeted Drug Delivery Vehicles. Antioxidants 2023, 12, 943. [Google Scholar] [CrossRef]
- Barzin, M.; Bagheri, A.M.; Ohadi, M.; Abhaji, A.M.; Salarpour, S.; Dehghannoudeh, G. Application of Plant-Derived Exosome-like Nanoparticles in Drug Delivery. Pharm. Dev. Technol. 2023, 28, 383–402. [Google Scholar] [CrossRef]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-Derived Extracellular Vesicles as a Promising Cell-Free Therapeutic Tool for Wound Healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A Cost-Effective Polyethylene Glycol-Based Method for the Isolation of Functional Edible Nanoparticles from Ginger Rhizomes. Sci. Rep. 2020, 10, 4456. [Google Scholar] [CrossRef]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 12, 2722. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Z.; Zhou, Z. Simultaneous Separation and Purification of Five Polymethoxylated Flavones from “Dahongpao” Tangerine (Citrus Tangerina Tanaka) Using Macroporous Adsorptive Resins Combined with Prep-HPLC. Molecules 2018, 23, 2660. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 3, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gu, Y.; Tan, C.; Sundararajan, B.; Li, Z.; Wang, D.; Zhou, Z. Comparative Effects of Five Polymethoxyflavones Purified from Citrus Tangerina on Inflammation and Cancer. Front. Nutr. 2022, 9, 963662. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-Derived Exosomal MicroRNAs Inhibit Lung Inflammation Induced by Exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Zhu, G.; Zeng, L.; Xu, S.; Cheng, H.; Ouyang, Z.; Chen, J.; Pathak, J.L.; Wu, L.; et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front. Immunol. 2022, 13, 896745. [Google Scholar] [CrossRef]
- Yang, W.; Ma, Y.; Xu, H.; Zhu, Z.; Wu, J.; Xu, C.; Sun, W.; Zhao, E.; Wang, M.; Reis, R.L.; et al. Mulberry Biomass-Derived Nanomedicines Mitigate Colitis through Improved Inflamed Mucosa Accumulation and Intestinal Microenvironment Modulation. Research 2023, 6, 0188. [Google Scholar] [CrossRef]
- Li, C.; Song, Q.; Yin, X.; Song, R.; Chen, G. Preparation, Characterization, and In Vitro Anticancer Activity Evaluation of Broccoli-Derived Extracellular Vesicle-Coated Astaxanthin Nanoparticles. Molecules 2022, 27, 3955. [Google Scholar] [CrossRef]
- Teng, Y.; Mu, J.; Hu, X.; Samykutty, A.; Zhuang, X.; Deng, Z.; Zhang, L.; Cao, P.; Yan, J.; Miller, D.; et al. Grapefruit-Derived Nanovectors Deliver MiR-18a for Treatment of Liver Metastasis of Colon Cancer by Induction of M1 Macrophages. Oncotarget 2016, 7, 25683–25697. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yao, X.; Yue, J.; Fang, Y.; Cao, G.; Midgley, A.C.; Nishinari, K.; Yang, Y. Advances in Bioactivity of MicroRNAs of Plant-Derived Exosome-Like Nanoparticles and Milk-Derived Extracellular Vesicles. J. Agric. Food Chem. 2022, 70, 6285–6299. [Google Scholar] [CrossRef] [PubMed]

| NO. | Antioxidant Activities (μmol/g TE) | |||||
|---|---|---|---|---|---|---|
| ABTS | DPPH | FRAP | APC | Rank | ||
| 1 | CEVs | 375.78 ± 1.92 b | 68.56 ± 0.22 c | 185.43 ± 0.58 c | 94.32 | 3 |
| 2 | Ult-CEVs | 369.11 ± 1.92 c | 75.62 ± 0.77 a | 192.60 ± 0.50 a | 98.11 | 1 |
| 3 | Trx-CEVs | 391.33 ± 0.00 a | 73.05 ± 0.22 b | 187.43 ± 0.29 b | 97.97 | 2 |
| Simple Name | Storage Temperature (°C) | Storage Time (Day) | Average Particle Size (nm) | Zero Potential (mV) | PDI |
|---|---|---|---|---|---|
| CEVs | 4 | 1 | 165.63 ± 1.97 d | −5.38 ± 0.03 d | 0.17 ± 0.01 c |
| 5 | 185.93 ± 1.31 c | −6.54 ± 0.35 c | 0.20 ± 0.01 c | ||
| 10 | 247.73 ± 6.00 b | −9.90 ± 0.17 b | 0.52 ± 0.05 b | ||
| 15 | 333.70 ± 11.72 a | −11.00 ± 0.46 a | 0.66 ± 0.10 a | ||
| −80 | 1 | 172.90 ± 1.32 c | −4.46 ± 0.27 a | 0.17 ± 0.02 a | |
| 5 | 202.40 ± 3.86 a | −3.57 ± 0.42 a | 0.18 ± 0.01 a | ||
| 10 | 200.77 ± 1.76 ab | −5.86 ± 0.83 b | 0.20 ± 0.03 a | ||
| 15 | 196.16 ± 1.69 b | −4.13 ± 0.56 a | 0.18 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Ye, Z.; Zhao, L.; Yao, Y.; Zhou, Z. Evaluation of Antioxidant Activity and Drug Delivery Potential of Cell-Derived Extracellular Vesicles from Citrus reticulata Blanco cv. ‘Dahongpao’. Antioxidants 2023, 12, 1706. https://doi.org/10.3390/antiox12091706
Li S, Ye Z, Zhao L, Yao Y, Zhou Z. Evaluation of Antioxidant Activity and Drug Delivery Potential of Cell-Derived Extracellular Vesicles from Citrus reticulata Blanco cv. ‘Dahongpao’. Antioxidants. 2023; 12(9):1706. https://doi.org/10.3390/antiox12091706
Chicago/Turabian StyleLi, Shunjie, Zimao Ye, Lintao Zhao, Yijun Yao, and Zhiqin Zhou. 2023. "Evaluation of Antioxidant Activity and Drug Delivery Potential of Cell-Derived Extracellular Vesicles from Citrus reticulata Blanco cv. ‘Dahongpao’" Antioxidants 12, no. 9: 1706. https://doi.org/10.3390/antiox12091706
APA StyleLi, S., Ye, Z., Zhao, L., Yao, Y., & Zhou, Z. (2023). Evaluation of Antioxidant Activity and Drug Delivery Potential of Cell-Derived Extracellular Vesicles from Citrus reticulata Blanco cv. ‘Dahongpao’. Antioxidants, 12(9), 1706. https://doi.org/10.3390/antiox12091706





