Phlorotannins: Novel Orally Administrated Bioactive Compounds That Induce Mitochondrial Dysfunction and Oxidative Stress in Cancer
Abstract
1. Introduction
2. Mitochondrial Dysfunction and Oxidative Stress in Cancer
3. Phlorotannins, a Group of Bioactive Compounds with Cancer-Preventing Potential
| Bioactive Compound | Model Dosis | REDOX Balance | Mechanism | Disease | Reference |
|---|---|---|---|---|---|
| Dieckol, isolated from Ecklonia stolonifera | Hepatocellular carcinoma (Hep3B and Sk-Hep1) cells 100 µM, 24 h | Antioxidant | Release of cytochrome c from mitochondria and induction of apoptosis | Hepatocellular carcinoma | [69] |
| Dieckol | Non-small-cell lung carcinoma A549 cell line 25 and 50 µg/mL (34 and 67 µM), 24 h | Antioxidant | Induction of apoptosis through caspases-3, 8, and 9. Inhibition of proliferation and migration by regulating the PI3K/AKT signaling pathway | Non-small-cell lung cancer | [70] |
| Dieckol | HT29 cells 25 mg/mL (34 mM), 3 and 12 h | Antioxidant | Inhibition of HIF-1α, ROS, migration, and invasion | Colon cancer | [71] |
| Dieckol, isolated from Ecklonia cava | HT1080 cells 25 µg/mL (34 µM), 24 and 48 h | Antioxidant | Reduction of ROS, Rac1, FAK, adhesion, migration, and invasion | Fibrosarcoma | [73] |
| Dieckol, isolated from Ecklonia cava | B16F10 cells 25 µg/mL (34 µM), 24 and 48 h | Antioxidant | Reduction of NADPH oxidase, ROS, Rac1, migration, and invasion | Melanoma | [74] |
| Dieckol, isolated from Ecklonia cava | Rat model of NDEA-induced hepatocarcinogenesis 40 mg/kg, 15 weeks, oral | Antioxidant | Increment of antioxidant enzymes, thereby preventing hepatocarcinogenesis in vivo | Hepatocarcinoma | [75] |
| Dieckol, isolated from Ecklonia cava | Rat model of NDEA-induced hepatocarcinogenesis 40 mg/kg, 15 weeks, oral | Antioxidant | Induction of mitochondria-dependent apoptosis: decreased Bcl-2 and increased Bax, cytochrome c release, and caspase-3 activation. Promotion of inflammation and angiogenesis via NF-κB, COX2, and VEGF. | Hepatocarcinoma | [76] |
| Eckol | Xenograft-bearing mice 1 mg/kg, pretreatment for 7 days + treatment 10 days, oral | Antioxidant | Increased TUNEL-positive apoptotic cells, increased caspase-3 and caspase-9 activation, and reduced expression of Bcl-2, EGFR and EGFR phosphorylation Stimulation of innate and adaptive immune responses | Sarcoma | [77] |
| Phloroglucinol, quinone PMT7 | HL-60, HeLa, K562, and T98G cells 50 µM, 90 min, 24, 48 and 72 h | Antioxidant | Mitochondrial membrane depolarization and inhibition of autophagy | Leukemia, cervical carcinoma, and glioblastoma | [52] |
| Acylphloroglucinols, hyperforin, and myrtucommulone A | HL-60 cells EC50 0.03–0.9 µM | Antioxidant | Mitochondrial membrane depolarization and induction of apoptosis | Leukemia | [78] |
| Eckstolonol and fucofurodiphlorethol, derived from Fucus vesiculosus | Caco-2, HT29, MKN-28, and HFF-1 cells 50–300 µg/mL (0.4–2.4 mM phloroglucinol equivalent), 48 h | Antioxidant | Induction of cell cycle arrest and apoptosis | Colon and gastric cancer | [83] |
| Phlorotannin-rich extract from Ascophyllum nodosum and Fucus vesiculosus | A549 cells 1% extracts, 20 min preincubation | Antioxidant | Reduction of ROS | Lung cancer | [84] |
| Commercial dieckol | MG-63 cells 15/20 µM, 24 h | Pro-oxidant | Induction of ROS generation and apoptosis Inhibition of PI3K/AKT/mTOR pathway Reduction of TNF-α, NF-κB, COX2, IL-6, and matrix metalloproteinase levels | Sarcoma | [85] |
| Extracts from Ecklonia maxima and Ulva rigida | HepG2 cells 200 and 400 µg/mL extracts, 48 h | Pro-oxidant | Reduction of mitochondrial membrane potential. Increment of ROS. Induction of apoptosis. | Liver cancer | [86] |
| Dieckol isolated from Ecklonia cava | SKOV3 cells 60–120 µM, 24 h SKOV3 xenograft mice model 100 mg/kg, 4 weeks, oral | Pro-oxidant | Induction of apoptosis in cancer cells (mitochondrial membrane depolarization, activation of caspases), thereby reducing cancer cell viability and tumor xenograft growth through the increment of ROS | Ovarian cancer | [87] |
| Dieckol-rich extract of Ecklonia cava and cisplatin | SKOV3 cells 100 µg/mL (800 µM), 24 h SKOV3 xenograft mouse model 100 mg/kg, 4 weeks, oral | Pro-oxidant | Induction of ROS and apoptosis. Inhibition of NF-κB and Akt signaling. | Ovarian cancer | [88] |
4. Delivery Strategies Applied to Phlorotannin Encapsulation
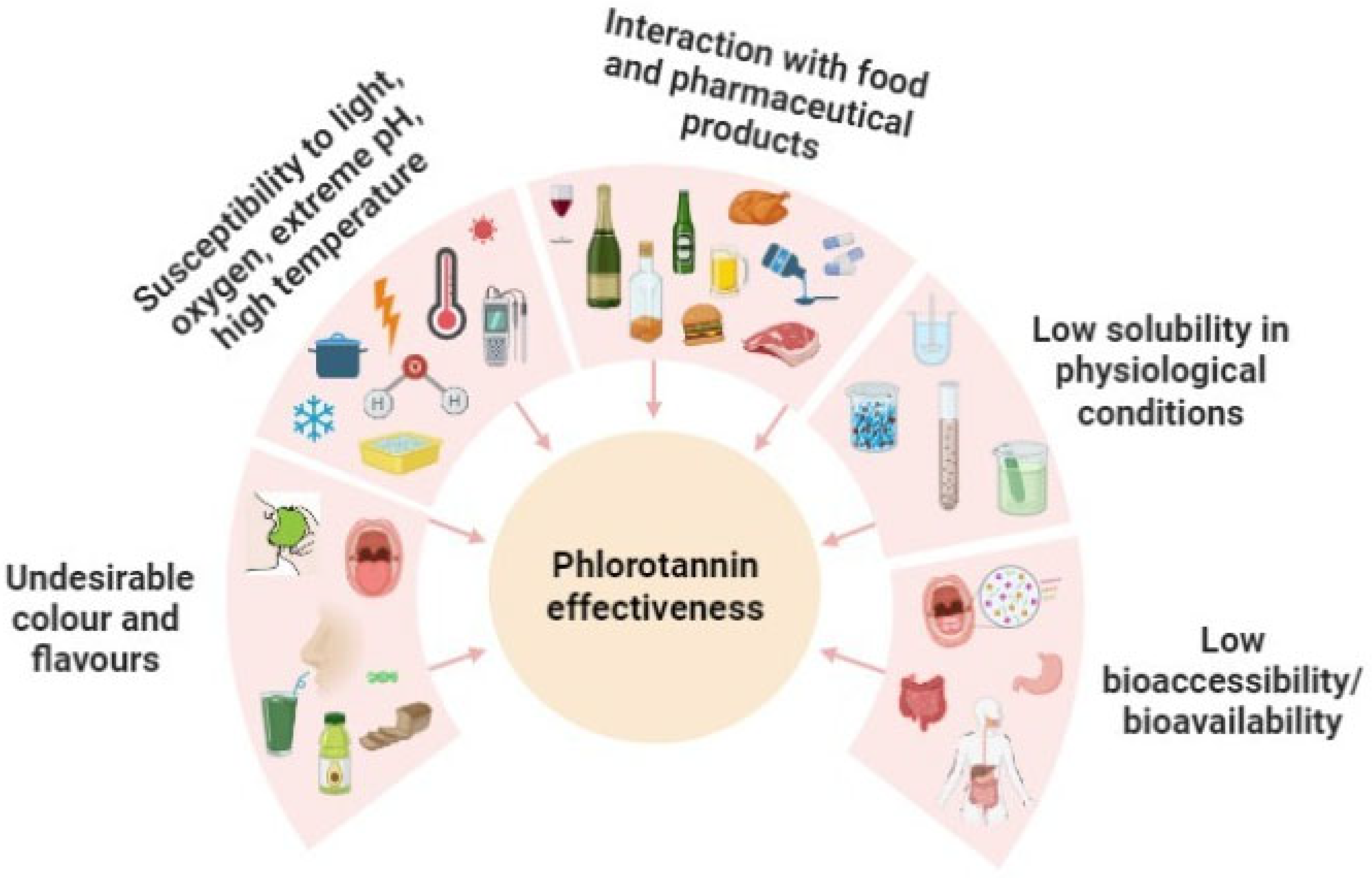
- Protecting the phlorotannins from degradation by environmental factors during their storage (light, oxygen, extreme pH, high temperature, etc.) and gastrointestinal passage [93].
- Avoiding unfavorable interactions between phlorotannins and other components of food, nutraceuticals or pharmaceutical matrices, such as proteins, lipids or complex carbohydrate macromolecules [97].
- Masking the unpleasant organoleptic properties of the phlorotannin extracts [98].
| Method | Technique | Bioactive Compound | Coating Material | Reference |
|---|---|---|---|---|
| Physical encapsulation | Spray-drying | Phlorotannin extract (Sargassum serratum) | Maltodextrin, glucose, and saccharose | [100] |
| Freeze-dried | Phlorotannin extract (Sargassum plagyophyllum) | Maltodextrin | [102] | |
| Chemical encapsulation | Complexation | Phlorotannin extracts (Eisenia bicyclis, Ecklonia cava and Ecklonia kurome) | Soybean protein isolate | [111] |
| Phlorotannin extract (Laminaria digitata) | β-casein (random coil) and bovine serum albumin (globular) | [112] | ||
| Phlorotannins (80% purity) | Polyvinylpyrrolidone nanoparticles | [113] | ||
| Phlorotannin extracts (Undaria pinnatifida) | Myofibrillar protein from Scomberomorus niphonius | [114] | ||
| Emulsion | Phlorotannin extract (Sargassum fusiforme) | Propylene glycol and glycerol | [115] | |
| Liposomes | Phlorotannin extract (Sargassum boveanum) | Soybean lecithin and glycerol | [106] | |
| Phlorotannin extract (Kappaphycus alvarezii) | Folic acid-PEG-DSPE conjugate | [105] | ||
| Physiochemical encapsulation | Spray-drying/ emulsion | Phlorotannin extract (Saccharina japonica) | Polysaccharides (dextrin, maltodextrin, lactose and gum arabic) Proteins (whey protein isolate, gelatin and sodium caseinate) | [101] |
| Freeze-dried/ complexation | Phlorotannin extract (Sargassum incisifolium) | Gold (III) chloride tryhidrate and silver nitrate (AgNO3) | [107] | |
| Phlorotannin extract (Ecklonia cava) | Silver nitrate (AgNO3) | [116] | ||
| Phlorotannin extract (Sargassum ilicifolium) | Chitosan and tripolyphos-phate (TPP) | [95] | ||
| Electrospinning | Phlorotannin (Zhengzhou Bainafo Bioengineering Co., Ltd., Zhengzhou, Henan, China) | Polyethylene oxide and sodium alginate | [117] | |
| Phlorotannin (purchased from Zhengzhou Bainafo Bioengineering Co., Ltd., Zhengzhou, Henan, China) | Momordica charantia polysaccharide | [118] |
5. Essential Variables to Modulate the Bioaccessibility and Bioavailability of Phlorotannin Capsule Systems
5.1. Composition and Chemical Nature of the Encapsulants
5.2. Physicochemical Properties of Capsule Systems
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Díaz-Valdivia, N.; Simón, L.; Díaz, J.; Martinez-Meza, S.; Contreras, P.; Burgos-Ravanal, R.; Pérez, V.I.; Frei, B.; Leyton, L.; Quest, A.F.G. Mitochondrial Dysfunction and the Glycolytic Switch Induced by Caveolin-1 Phosphorylation Promote Cancer Cell Migration, Invasion, and Metastasis. Cancers 2022, 14, 2862. [Google Scholar] [CrossRef] [PubMed]
- McGrail, K.; Granado-Martínez, P.; Esteve-Puig, R.; García-Ortega, S.; Ding, Y.; Sánchez-Redondo, S.; Ferrer, B.; Hernandez-Losa, J.; Canals, F.; Manzano, A.; et al. BRAF activation by metabolic stress promotes glycolysis sensitizing NRASQ61-mutated melanomas to targeted therapy. Nat. Commun. 2022, 13, 7113. [Google Scholar] [CrossRef] [PubMed]
- Amendola, C.R.; Mahaffey, J.P.; Parker, S.J.; Ahearn, I.M.; Chen, W.-C.; Zhou, M.; Court, H.; Shi, J.; Mendoza, S.L.; Morten, M.J.; et al. KRAS4A directly regulates hexokinase 1. Nature 2019, 576, 482–486. [Google Scholar] [CrossRef]
- Matoba, S.; Kang, J.-G.; Patino, W.D.; Wragg, A.; Boehm, M.; Gavrilova, O.; Hurley, P.J.; Bunz, F.; Hwang, P.M. p53 regulates mitochondrial respiration. Science 2006, 312, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Koyama, S.; Itahashi, K.; Tanegashima, T.; Lin, Y.-T.; Togashi, Y.; Kamada, T.; Irie, T.; Okumura, G.; Kono, H.; et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell 2022, 40, 201–218.e9. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Lee, H.; Sim, D.Y.; Im, E.; Park, J.E.; Park, W.Y.; Cho, A.R.; Shim, B.S.; Kim, S. Apoptotic effect of compound K in hepatocellular carcinoma cells via inhibition of glycolysis and Akt/mTOR/c-Myc signaling. Phytother. Res. 2021, 35, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, L.; Chen, W.; Xu, Y.; Wu, M.; Todorova, D.; Tang, Q.; Feng, B.; Jiang, L.; He, J.; et al. Wild-Type p53 Promotes Cancer Metabolic Switch by Inducing PUMA-Dependent Suppression of Oxidative Phosphorylation. Cancer Cell 2019, 35, 191–203.e8. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.E.; Ko, Y.; Domingo-Vidal, M.; Lin, Z.; Whitaker-Menezes, D.; Birbe, R.C.; Tuluc, M.; Győrffy, B.; Caro, J.; Philp, N.J.; et al. TP53 Induced Glycolysis and Apoptosis Regulator and Monocarboxylate Transporter 4 drive metabolic reprogramming with c-MYC and NFκB activation in breast cancer. Int. J. Cancer 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Z.; Zhu, W.; Han, J.; Yang, X.; Zhou, R.; Lu, H.; Yu, H.; Yuan, W.; Li, P.; Tao, J.; et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun. 2021, 41, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 15. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J.-I. ROS-Generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Sharma, L.K.; Li, H.; Xiang, R.; Holstein, D.; Wu, J.; Lechleiter, J.; Naylor, S.L.; Deng, J.J.; Lu, J.; et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum. Mol. Genet. 2009, 18, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.K.; Fang, H.; Liu, J.; Vartak, R.; Deng, J.; Bai, Y. Mitochondrial respiratory complex I dysfunction promotes tumorigenesis through ROS alteration and AKT activation. Hum. Mol. Genet. 2011, 20, 4605–4616. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Xu, R.-H.; Du, M.; Feng, L.; Sasaki, R.; Carew, J.S.; Hu, Y.; Ramdas, L.; Hu, L.; Keating, M.J.; et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J. Cell Biol. 2006, 175, 913–923. [Google Scholar] [CrossRef]
- Guerra, F.; Arbini, A.A.; Moro, L. Mitochondria and cancer chemoresistance. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1858, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Biswas, G.; Anandatheerthavarada, H.K.; Avadhani, N.G. Mechanism of mitochondrial stress-induced resistance to apoptosis in mitochondrial DNA-depleted C2C12 myocytes. Cell Death Differ. 2005, 12, 266–278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muller, F.L.; Liu, Y.; Van Remmen, H. Complex III Releases Superoxide to Both Sides of the Inner Mitochondrial Membrane. J. Biol. Chem. 2004, 279, 49064–49073. [Google Scholar] [CrossRef]
- Lee, S.-R.; Yang, K.-S.; Kwon, J.; Lee, C.; Jeong, W.; Rhee, S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002, 277, 20336–20342. [Google Scholar] [CrossRef]
- Salmeen, A.; Andersen, J.N.; Myers, M.P.; Meng, T.-C.; Hinks, J.A.; Tonks, N.K.; Barford, D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 2003, 423, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Ochoa, E.; Sánchez-Alegría, K.; Gómez-Inclán, C.; Ferrera, P.; Arias, C. Palmitic acid stimulates energy metabolism and inhibits insulin/PI3K/AKT signaling in differentiated human neuroblastoma cells: The role of mTOR activation and mitochondrial ROS production. Neurochem. Int. 2017, 110, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, L.; Irani, K.; Moldovan, N.I.; Finkel, T.; Goldschmidt-Clermont, P.J. The actin cytoskeleton reorganization induced by Rac1 requires the production of superoxide. Antioxid. Redox Signal 2003, 1, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, P.; Pani, G.; Giannoni, E.; Taddei, L.; Colavitti, R.; Raugei, G.; Symons, M.; Borrello, S.; Galeotti, T.; Ramponi, G. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 2003, 161, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Nimnual, A.S.; Taylor, L.J.; Bar-Sagi, D. Redox-dependent downregulation of Rho by Rac. Nat. Cell Biol. 2003, 5, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Del Carlo, M.; Schwartz, D.; Erickson, E.A.; Loeser, R.F. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic. Biol. Med. 2009, 42, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Binker, M.G.; Binker-Cosen, A.A.; Richards, D.; Oliver, B.; Cosen-Binker, L.I. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem. Biophys. Res. Commun. 2009, 379, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-L.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Han, Y.; Wang, Y.; Sun, X.; Yan, S.; Yeh, E.T.H.; Chen, Y.; Cang, H.; Li, H.; Shi, G.; et al. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009, 28, 2748–2762. [Google Scholar] [CrossRef] [PubMed]
- Gerald, D.; Berra, E.; Frapart, Y.M.; Chan, D.A.; Giaccia, A.J.; Mansuy, D.; Pouysségur, J.; Yaniv, M.; Mechta-Grigoriou, F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell 2004, 118, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Xiang, B.; Yang, J.; Sun, X.; Wang, Y.; Cang, H.; Yi, J. Oxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with Apaf-1. Cell Res. 2009, 19, 449–457. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Chanvorachote, P.; Nimmannit, U.; Leonard, S.S.; Stehlik, C.; Wang, L.; Rojanasakul, Y. Mitochondrial superoxide mediates doxorubicin-induced keratinocyte apoptosis through oxidative modification of ERK and Bcl-2 ubiquitination. Biochem. Pharmacol. 2012, 83, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Luanpitpong, S.; Chanvorachote, P.; Stehlik, C.; Tse, W.; Callery, P.S.; Wang, L.; Rojanasakul, Y. Regulation of apoptosis by Bcl-2 cysteine oxidation in human lung epithelial cells. Mol. Biol. Cell 2013, 24, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ueta, E.; Kimura, T.; Yamamoto, T.; Osaki, T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown Algae Phlorotannins: A Marine Alternative to Break the Oxidative Stress, Inflammation and Cancer Network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, S.; Simón, L.; Cifuentes, M.; Quest, A.F.G. The Adipocyte–Macrophage Relationship in Cancer: A Potential Target for Antioxidant Therapy. Antioxidants 2023, 12, 126. [Google Scholar] [CrossRef]
- D’andurain, J.; López, V.; Arazo-Rusindo, M.; Tiscornia, C.; Aicardi, V.; Simón, L.; Mariotti-Celis, M.S. Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review. Nutrients 2023, 15, 2239. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Liu, X.; Yu, C. Extraction and Nano-Sized Delivery Systems for Phlorotannins to Improve Its Bioavailability and Bioactivity. Mar. Drugs 2021, 19, 625. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Balss, J.; Meyer, J.; Mueller, W.; Korshunov, A.; Hartmann, C.; von Deimling, A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008, 116, 597–602. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, W.; Xiang, B.; Li, N.; Huang, S.; Zhou, W.; Sun, Y.; Wang, X.; Ma, J.; Li, G.; et al. Reduced succinate dehydrogenase B expression is associated with growth and de-differentiation of colorectal cancer cells. Tumour Biol. 2013, 34, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.-S.; Chihara, Y.; Yoon, H.-Y.; Kim, Y.-J.; Kim, T.-H.; Woo, S.H.; Yun, S.-J.; Kim, I.Y.; Hirao, Y.; Kim, W.-J. Downregulation of fumarate hydratase is related to tumorigenesis in sporadic renal cell cancer. Urol. Int. 2013, 90, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Locasale, J.W.; Bielas, J.H.; O’Sullivan, J.; Sheahan, K.; Cantley, L.C.; Heiden, M.G.V.; Vitkup, D. Heterogeneity of tumor-induced gene expression changes in the human metabolic network. Nat. Biotechnol. 2013, 31, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.-H.; Wu, C.-C.; Lin, J.-C.; Chi, C.-W.; Wei, Y.-H.; Lee, H.-C. Somatic mutations of mitochondrial genome in hepatocellular carcinoma. Mitochondrion 2010, 10, 174–182. [Google Scholar] [CrossRef]
- Tseng, L.-M.; Yin, P.-H.; Yang, C.-W.; Tsai, Y.-F.; Hsu, C.-Y.; Chi, C.-W.; Lee, H.-C. Somatic mutations of the mitochondrial genome in human breast cancers. Genes Chromosomes Cancer 2011, 50, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-Y.; Wu, C.-W.; Yin, P.-H.; Chang, C.-J.; Li, A.F.-Y.; Chi, C.-W.; Wei, Y.-H.; Lee, H.-C. Somatic mutations in mitochondrial genome and their potential roles in the progression of human gastric cancer. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.F.; Sabelnykova, V.Y.; Weischenfeldt, J.; Simon, R.; Aguiar, J.A.; Alkallas, R.; Heisler, L.E.; Zhang, J.; Watson, J.D.; Chua, M.L.K.; et al. Mitochondrial mutations drive prostate cancer aggression. Nat. Commun. 2017, 8, 656. [Google Scholar] [CrossRef]
- Hsu, C.C.; Tseng, L.M.; Lee, H.C. Role of mitochondrial dysfunction in cancer progression. Exp. Biol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, P.; Hirsilä, M.; Remes, A.M.; Hassinen, I.E.; Kivirikko, K.I.; Myllyharju, J. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: Possible links between cell metabolism and stabilization of HIF. J. Biol. Chem. 2007, 282, 4524–4532. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.M.; Gaude, E.; Turrell, F.K.; Frezza, C.; Martins, C.P. Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature 2016, 531, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lu, W.; Chen, G.; Wang, P.; Chen, Z.; Zhou, Y.; Ogasawara, M.; Trachootham, D.; Feng, L.; Pelicano, H.; et al. K-rasG12V transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res. 2012, 22, 399–412. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Broadley, K.; Larsen, L.; Herst, P.M.; Smith, R.A.J.; Berridge, M.V.; McConnell, M.J. The novel phloroglucinol PMT7 kills glycolytic cancer cells by blocking autophagy and sensitizing to nutrient stress. J. Cell. Biochem. 2011, 112, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapano, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Yalçın, S.; Karakaş, Ö.; Okudan, E.Ş.; Kocaoba, S.; Apak, M.R. Comparative Spectrophotometric and Chromatographic Assessment of Antioxidant Capacity in Different Marine Algae. J. Aquat. Food Prod. Technol. 2023, 32, 81–94. [Google Scholar] [CrossRef]
- de Cedrón, M.G.; Vargas, T.; Madrona, A.; Jiménez, A.; Pérez-Pérez, M.-J.; Quintela, J.-C.; Reglero, G.; San-Felix, A.R.; de Molina, A.R. Novel Polyphenols That Inhibit Colon Cancer Cell Growth Affecting Cancer Cell Metabolism. J. Pharmacol. Exp. Ther. 2018, 366, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Bianchini, F.; Andreucci, E.; Urciuoli, S.; Romani, A.; Tortora, K.; Caderni, G.; Nediani, C.; Calorini, L. Cancer Glycolytic Dependence as a New Target of Olive Leaf Extract. Cancers 2020, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.N.; Cheung, P.C.K.; Ooi, V.E.C.; Ang, P.O. Evaluation of antioxidative activity of extracts from a brown seaweed, Sargassum siliquastrum. J. Agric. Food Chem. 2002, 50, 3862–3866. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Li, Y.; Karadeniz, F.; Kim, M.-M.; Kim, S.-K. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J. Sci. Food Agric. 2009, 89, 1552–1558. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Kang, M.-C.; Jeon, Y.-J. Octaphlorethol A, a novel phenolic compound isolated from Ishige foliacea, protects against streptozotocin-induced pancreatic β cell damage by reducing oxidative stress and apoptosis. Food Chem. Toxicol. 2013, 59, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, M.-H.; Heo, S.-J.; Kang, S.-M.; Ko, S.-C.; Han, J.-S.; Jeon, Y.-J. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2010, 48, 2633–2637. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. The Impact of a Single Dose of a Polyphenol-Rich Seaweed Extract on Postprandial Glycaemic Control in Healthy Adults: A Randomised Cross-Over Trial. Nutrients 2018, 10, 270. [Google Scholar] [CrossRef]
- Kang, M.-C.; Wijesinghe, W.; Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Yang, X.; Kang, N.; Jeon, B.-T.; Kim, J.; Lee, D.-H.; et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type II diabetes in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.; Jeon, Y.; Lee, M.; Lim, Y. Brown Alga Ecklonia cava polyphenol extract ameliorates hepatic lipogenesis, oxidative stress, and inflammation by activation of AMPK and SIRT1 in high-fat diet-induced obese mice. J. Agric. Food Chem. 2015, 63, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Youn, K.; Kim, D.H.; Ahn, M.-R.; Yoon, E.; Kim, O.-Y.; Jun, M. Anti-Neuroinflammatory Property of Phlorotannins from Ecklonia cava on Aβ25-35-Induced Damage in PC12 Cells. Mar. Drugs 2018, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Yadunandam, A.K.; Kim, S.J.; Woo, H.C.; Kim, H.R.; Kim, G.D. Dieckol, isolated from Ecklonia stolonifera, induces apoptosis in human hepatocellular carcinoma Hep3B cells. J. Nat. Med. 2013, 67, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Li, X.F.; Jin, L.F.; Zhao, Y.; Zhu, G.J.; Shen, W.Z. Dieckol inhibits non-small-cell lung cancer cell proliferation and migration by regulating the PI3K/AKT signaling pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22346. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Jeon, Y.J.; Park, S.J. Inhibitory effects of dieckol on hypoxia-induced epithelial-mesenchymal transition of HT29 human colorectal cancer cells. Mol. Med. Rep. 2016, 14, 5148–5154. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Chung, H.Y.; Kim, J.Y.; Son, B.W.; Jung, H.A.; Choi, J.S. Inhibitory phlorotannins from the edible brown algaecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch. Pharm. Res. 2004, 27, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jeon, Y.J. Dieckol from Ecklonia cava suppresses the migration and invasion of HT1080 cells by inhibiting the focal adhesion kinase pathway downstream of Rac1-ROS signaling. Mol. Cells 2012, 33, 141–149. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, Y.T.; Jeon, Y.J. Antioxidant dieckol downregulates the Rac1/ROS signaling pathway and inhibits Wiskott-Aldrich syndrome protein (WASP)-family verprolin-homologous protein 2 (WAVE2)-mediated invasive migration of B16 mouse melanoma cells. Mol. Cells 2012, 33, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Sadeeshkumar, V.; Duraikannu, A.; Ravichandran, S.; Fredrick, W.S.; Sivaperumal, R.; Kodisundaram, P. Protective effects of dieckol on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Biomed. Pharmacother. 2016, 84, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Sadeeshkumar, V.; Duraikannu, A.; Ravichandran, S.; Kodisundaram, P.; Fredrick, W.S.; Gobalakrishnan, R. Modulatory efficacy of dieckol on xenobiotic-metabolizing enzymes, cell proliferation, apoptosis, invasion and angiogenesis during NDEA-induced rat hepatocarcinogenesis. Mol. Cell. Biochem. 2017, 433, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Guo, J.; Hu, X.M.; Zhao, S.Q.; Li, S.L.; Wang, J. An in vivo anti-tumor effect of eckol from marine brown algae by improving the immune response. Food Funct. 2019, 10, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, K.; Müller, H.; Fischer, D.; Jauch, J.; Werz, O. The acylphloroglucinols hyperforin and myrtucommulone A cause mitochondrial dysfunctions in leukemic cells by direct interference with mitochondria. Apoptosis 2015, 20, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Menegazzi, M.; Masiello, P.; Novelli, M. Anti-Tumor Activity of Hypericum perforatum L. and Hyperforin through Modulation of Inflammatory Signaling, ROS Generation and Proton Dynamics. Antioxidants 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Golla, S.; Golla, J.P.; Tanaka, N.; Cai, Y.; Takahashi, S.; Krausz, K.W.; Matsubara, T.; Korboukh, I.; Gonzalez, F.J.S. John’s Wort Attenuates Colorectal Carcinogenesis in Mice through Suppression of Inflammatory Signaling. Cancer Prev. Res. 2015, 8, 786–795. [Google Scholar] [CrossRef]
- Hsu, F.-T.; Chen, W.-T.; Wu, C.-T.; Chung, J.-G. Hyperforin induces apoptosis through extrinsic/intrinsic pathways and inhibits EGFR/ERK/NF-κB-mediated anti-apoptotic potential in glioblastoma. Environ. Toxicol. 2020, 35, 1058–1069. [Google Scholar] [CrossRef]
- Chiang, I.-T.; Chen, W.-T.; Tseng, C.-W.; Chen, Y.-C.; Kuo, Y.-C.; Chen, B.-J.; Weng, M.-C.; Lin, H.-J.; Wang, W.-S. Hyperforin Inhibits Cell Growth by Inducing Intrinsic and Extrinsic Apoptotic Pathways in Hepatocellular Carcinoma Cells. Anticancer Res. 2017, 37, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Fernandes, I.; Oliveira, H.; Carrascal, M.; Ferreira, R.; Silva, A.M.S.; Cruz, M.T.; Mateus, N.; Cardoso, S.M. Antitumor Activity of Fucus vesiculosus-Derived Phlorotannins through Activation of Apoptotic Signals in Gastric and Colorectal Tumor Cell Lines. Int. J. Mol. Sci. 2021, 22, 7604. [Google Scholar] [CrossRef] [PubMed]
- Dutot, M.; Olivier, E.; Fouyet, S.; Magny, R.; Hammad, K.; Roulland, E.; Rat, P.; Fagon, R. In Vitro Chemopreventive Potential of Phlorotannins-Rich Extract from Brown Algae by Inhibition of Benzo[a]pyrene-Induced P2X7 Activation and Toxic Effects. Mar. Drugs 2021, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ren, H.; Sun, H.; Cao, S. Dieckol exerts anticancer activity in human osteosarcoma (MG-63) cells through the inhibition of PI3K/AKT/mTOR signaling pathway. Saudi J. Biol. Sci. 2021, 28, 4908–4915. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O. Antiproliferative and apoptosis—Inducing effects of aqueous extracts from Ecklonia maxima and Ulva rigida on HepG2 cells. J. Food Biochem. 2022, 46, e14498. [Google Scholar] [CrossRef]
- Ahn, J.H.; Yang, Y.I.; Lee, K.T.; Choi, J.H. Dieckol, isolated from the edible brown algae Ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2015, 141, 255–268. [Google Scholar] [CrossRef]
- Yang, Y.I.; Ahn, J.H.; Choi, Y.S.; Choi, J.H. Brown algae phlorotannins enhance the tumoricidal effect of cisplatin and ameliorate cisplatin nephrotoxicity. Gynecol. Oncol. 2015, 136, 355–364. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Ki, J.S. Immunomodulatory, Antioxidant, Anticancer, and Pharmacokinetic Activity of Ulvan, a Seaweed-Derived Sulfated Polysaccharide: An Updated Comprehensive Review. Mar. Drugs 2023, 21, 300. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D’Amen, E. An overview on dietary polyphenols and their biopharmaceutical classification system (Bcs). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Catarino, M.D.; Circuncisão, A.R.; Neves, B.; Marçal, C.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Impact of Gastrointestinal Digestion on the Anti-Inflammatory Properties of Phlorotannins from Himanthalia elongata. Antioxidants 2022, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Ramezanzade, L.; McClements, D.J. Recent advances in nanoencapsulation of hydrophobic marine bioactives: Bioavailability, safety, and sensory attributes of nano-fortified functional foods. Trends Food Sci. Technol. 2021, 109, 322–339. [Google Scholar] [CrossRef]
- Kaushalya, K.G.D.; Gunathilake, K.D.P.P. Encapsulation of phlorotannins from edible brown seaweed in chitosan: Effect of fortification on bioactivity and stability in functional foods. Food Chem. 2022, 377, 132012. [Google Scholar] [CrossRef] [PubMed]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lv, C.; Chen, L.; Bai, G.; Zhao, G.; Xu, C. Encapsulation of anthocyanin molecules within a ferritin nanocage increases their stability and cell uptake efficiency. Food Res. Int. 2014, 62, 183–192. [Google Scholar] [CrossRef]
- Petrotos, K.B.; Karkanta, F.K.; Gkoutsidis, P.E.; Giavasis, I.; Papatheodorou, K.N.; Ntontos, A.C. Production of Novel Bioactive Yogurt Enriched with Olive Fruit Polyphenols. World Acad. Sci. Eng. Technol. 2012, 64, 867–872. [Google Scholar] [CrossRef]
- Franco, W.; Arazo, M.C.R.; Benavides, S. Recent advances in the encapsulation of marine phenolic compounds. In Marine Phenolic Compounds: Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 239–264. [Google Scholar] [CrossRef]
- Cuong, D.X. Antioxidant Nano Phlorotannin Powder from Brown Algae Sargassum serratum: Spray Drying, Antioxidant Activities, Physic-Chemical Characterization. J. Pharm. Res. Int. 2020, 32, 71–85. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Sivagnanam, S.P.; Park, J.S.; Cho, Y.J.; Chun, B.S. Effect of wall materials on the spray drying encapsulation of brown seaweed bioactive compounds obtained by subcritical water extraction. Algal Res. 2021, 58, 102381. [Google Scholar] [CrossRef]
- Anwar, E.; Erianto, H.; Putri, K.S.S. Preparation of powder from brown seaweed (Sargassum plagyophyllum) by freeze-drying with maltodextrin as a stabilizer. Int. J. Appl. Pharm. 2018, 10, 348–353. [Google Scholar] [CrossRef]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of lipid-based encapsulation on the bioaccessibility and bioavailability of phenolic compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Johnson, C.; Hatziantoniou, S.; Demetzos, C. Nanoliposomes and their applications in food nanotechnology. J. Liposome Res. 2008, 18, 309–327. [Google Scholar] [CrossRef]
- Baskararaj, S.; Panneerselvam, T.; Govindaraj, S.; Arunachalam, S.; Parasuraman, P.; Pandian, S.R.K.; Sankaranarayanan, M.; Mohan, U.P.; Palanisamy, P.; Ravishankar, V. Formulation and characterization of folate receptor-targeted PEGylated liposome encapsulating bioactive compounds from Kappaphycus alvarezii for cancer therapy. 3 Biotech 2020, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Savaghebi, D.; Barzegar, M.; Mozafari, M.R. Manufacturing of nanoliposomal extract from Sargassum boveanum algae and investigating its release behavior and antioxidant activity. Food Sci. Nutr. 2020, 8, 299–310. [Google Scholar] [CrossRef]
- Mmola, M.; Le Roes-Hill, M.; Durrell, K.; Bolton, J.J.; Sibuyi, N.; Meyer, M.E.; Beukes, D.R.; Antunes, E. Enhanced antimicrobial and anticancer activity of silver and gold nanoparticles synthesised using Sargassum incisifolium aqueous extracts. Molecules 2016, 21, 1633. [Google Scholar] [CrossRef] [PubMed]
- Trojanowska, A.; Nogalska, A.; Valls, R.G.; Giamberini, M.; Tylkowski, B. Technological solutions for encapsulation. Phys. Sci. Rev. 2017, 2, 20170020. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, H. Trends in Microencapsulation Research. KONA Powder Part. J. 2004, 22, 23–31. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711. [Google Scholar] [CrossRef]
- Vissers, A.M.; Blok, A.E.; Westphal, A.H.; Hendriks, W.H.; Gruppen, H.; Vincken, J.P. Resolubilization of Protein from Water-Insoluble Phlorotannin-Protein Complexes upon Acidification. J. Agric. Food Chem. 2017, 65, 9595–9602. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Y.; Gu, Y.; Zheng, J.; Yu, C.; Qi, H. Preparation, characterization and antioxidant activities of kelp phlorotannin nanoparticles. Molecules 2020, 25, 4550. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Shen, P.; Pu, Y.; Jin, M.; Yu, C.; Qi, H. Enhancement of gel properties of Scomberomorus niphonius myofibrillar protein using phlorotannin extracts under UVA irradiation. J. Food Sci. 2020, 85, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Fu, X.; Duan, D.; Xu, J.; Gao, X.; Zhao, L. Evaluation of bioactivity of phenolic compounds from the brown seaweed of Sargassum fusiforme and development of their stable emulsion. J. Appl. Phycol. 2018, 30, 1955–1970. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K.; Shim, M.S. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials 2016, 6, 235. [Google Scholar] [CrossRef] [PubMed]
- Surendhiran, D.; Cui, H.; Lin, L. Encapsulation of Phlorotannin in Alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag. Shelf Life 2019, 21, 100346. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Abdel-Samie, M.A.; Lin, L. Cold plasma treated phlorotannin/Momordica charantia polysaccharide nanofiber for active food packaging. Carbohydr. Polym. 2020, 239, 116214. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Waldron, D.S.; Smyth, T.J.; O’Brien, N.M.; Kerry, J.P. An examination of the potential of seaweed extracts as functional ingredients in milk. Int. J. Dairy Technol. 2014, 67, 182–193. [Google Scholar] [CrossRef]
- Rønne, M.E.; Madsen, M.; Tandrup, T.; Wilkens, C.; Svensson, B. Gut bacterial alginate degrading enzymes. Essays Biochem. 2023, 67, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef] [PubMed]
- Rewatkar, P.; Kumeria, T.; Popat, A. Size, shape and surface charge considerations of orally delivered nanomedicines. In Nanotechnology for Oral Drug Delivery: From Concept to Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–176. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Lamson, N.G.; Berger, A.; Fein, K.C.; Whitehead, K.A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat. Biomed. Eng. 2020, 4, 84–96. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simón, L.; Arazo-Rusindo, M.; Quest, A.F.G.; Mariotti-Celis, M.S. Phlorotannins: Novel Orally Administrated Bioactive Compounds That Induce Mitochondrial Dysfunction and Oxidative Stress in Cancer. Antioxidants 2023, 12, 1734. https://doi.org/10.3390/antiox12091734
Simón L, Arazo-Rusindo M, Quest AFG, Mariotti-Celis MS. Phlorotannins: Novel Orally Administrated Bioactive Compounds That Induce Mitochondrial Dysfunction and Oxidative Stress in Cancer. Antioxidants. 2023; 12(9):1734. https://doi.org/10.3390/antiox12091734
Chicago/Turabian StyleSimón, Layla, Migdalia Arazo-Rusindo, Andrew F. G. Quest, and María Salomé Mariotti-Celis. 2023. "Phlorotannins: Novel Orally Administrated Bioactive Compounds That Induce Mitochondrial Dysfunction and Oxidative Stress in Cancer" Antioxidants 12, no. 9: 1734. https://doi.org/10.3390/antiox12091734
APA StyleSimón, L., Arazo-Rusindo, M., Quest, A. F. G., & Mariotti-Celis, M. S. (2023). Phlorotannins: Novel Orally Administrated Bioactive Compounds That Induce Mitochondrial Dysfunction and Oxidative Stress in Cancer. Antioxidants, 12(9), 1734. https://doi.org/10.3390/antiox12091734








