Antioxidant Use after Diagnosis of Head and Neck Squamous Cell Carcinoma (HNSCC): A Systematic Review of Application during Radiotherapy and in Second Primary Cancer Prevention
Abstract
:1. Introduction
2. Materials and Methods
3. Results
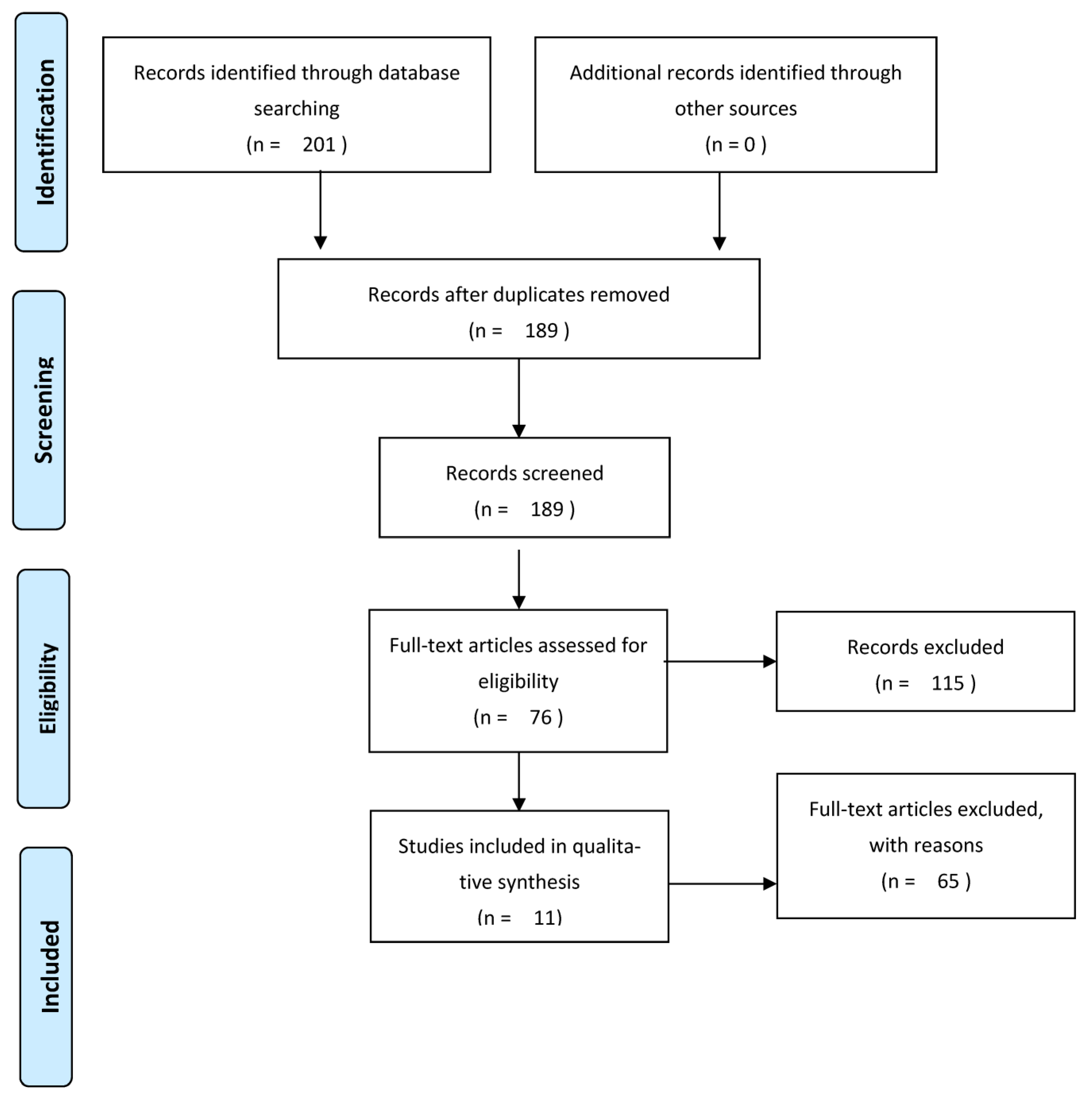
3.1. Systematic Literature Research
3.2. Clinical Trials and Antioxidants
| Authors | Year | PMID | Population | Comparison | Results | Notes | Strength of Evidence OCEBM * | Reference |
|---|---|---|---|---|---|---|---|---|
| Bairati et al. | 2005 | 15812073 | 540 patients treated with RT | α-tocopherol (400 IU/day) and β-carotene (30 mg/day) or placebo on the first day of RT and continued for 3 years after the end of RT | Antioxidant group had higher incidence of second primary cancers. | 2 | [26] | |
| Meyer et al. | 2008 | 18059031 | 540 patients treated with RT | α-tocopherol (400 IU/day) and β-carotene (30 mg/day) or placebo on the first day of RT and continued for 3 years after the end of RT considering smoking | Smoking did not modify the effects of the supplementation. Cigarette smoking increased risk of second primary tumors. | 2 | [28] | |
| Mayne et al. | 2001 | 11245451 | 240 patients | β-carotene (50 mg/day) vs. placebo | No significant effect on second head and neck cancer | Potential increased risk of lung cancer | 2 | [29] |
| Datta et al. | 2018 | 28102098 | 134 patients | JP vs. placebo | No statistically significant effect on second head and neck cancer | 2 | [30] | |
| Seixas-Silva et al. | 2018 | 15837897 | 45 patients | Isotretinoin, interferon α-2a, and vitamin E | The bioadjuvant combination is highly effective in preventing recurrence and second primary tumors | No control group | 3 | [31] |
| Shin et al. | 2001 | v11408495 | 45 patients | Interferon-α, 13-cis-retinoic acid, and α-tocopherol. | The bioadjuvant combination is highly effective in preventing recurrence and second primary tumors | No control group | 3 | [32] |
| van Zandwijk et al. | 2000 | 10861309 | 2592 patients (60% with head and neck cancer and 40% with lung cancer) | (1) retinyl palmitate (300,000 IU/day for 1 year followed by 150,000 IU for successive year), (2) N-acetylcysteine (600 mg/day for 2 years), (3) both compounds, and (4) no intervention. | No difference was seen. Lower incidence of second primary tumors in the no intervention group, not statistically significant. | 2 | [33,34] | |
| Jyothirmayi et al. | 1996 | 9039219 | 49 HNSCC treated with Vitamin A and 42 placebo completed 3-year followup | retinyl palmitate (200,000 IU per week for 1 year) vs. placebo | 2 recurrences in the placebo group, 0 in the experimental one | 2 | [35] | |
| Toma et al. | 2003 | 14534715 | 214 HNSCC stage I-II radically treated (110 experimental vs. 104 placebo) | β-carotene 75 mg/day for 3-month cycles within one month intercycle intervals for a total of 3 years | No statistically significant effect on second head and neck cancer | 2 | [36] | |
| Khuri et al. | 2006 | 16595780 | 1190 stage I or II HNSCC patients | Isotretinoin (13-cis-retinoic acid) (30 mg/day) vs. placebo for 3 years | No statistically significant effect on second head and neck cancer | [7] |
4. Discussion
4.1. Results
4.2. Potential Antioxidant Molecules Not Yet Investigated
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global Cancer Statistics, 2002. CA. Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Rubin, P.; Tupchong, L.; Brady, L.W.; Leibel, S.A.; Laramore, G.E.; Marcial, V.A.; Davis, L.W.; Cox, J.D. Second malignancies in patients who have head and neck cancer: Incidence, effect on survival and implications based on the RTOG experience. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 449–456. [Google Scholar] [CrossRef]
- McDonald, S.; Haie, C.; Rubin, P.; Nelson, D.; Divers, L.D. Second malignant tumors in patients with laryngeal carcinoma: Diagnosis, treatment, and prevention. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Hong, W.K. Second malignant tumors in head and neck squamous cell carcinoma: The overshadowing threat for patients with early-stage disease. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 691–694. [Google Scholar] [CrossRef]
- Vikram, B. Changing Patterns of Failure in Advanced Head and Neck Cancer. Arch. Otolaryngol. 1984, 110, 564–565. [Google Scholar] [CrossRef]
- Khuri, F.R.; Lee, J.J.; Lippman, S.M.; Kim, E.S.; Cooper, J.S.; Benner, S.E.; Winn, R.; Pajak, T.F.; Williams, B.; Shenouda, G.; et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J. Natl. Cancer Inst. 2006, 98, 441–450. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2019, 24, 4771–4778. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control 1991, 2, 325–357. [Google Scholar] [CrossRef]
- Riboli, E.; Norat, T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am. J. Clin. Nutr. 2003, 78, 559S. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Benner, S.E.; Hong, W.K. Cancer chemoprevention. J. Clin. Oncol. 1994, 12, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Eng, C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat. Res. 2003, 114, 15–60. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case–Control Study of Human Papillomavirus and Oropharyngeal Cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Edefonti, V.; Hashibe, M.; Ambrogi, F.; Parpinel, M.; Bravi, F.; Talamini, R.; Levi, F.; Yu, G.; Morgenstern, H.; Kelsey, K.; et al. Nutrient-based dietary patterns and the risk of head and neck cancer: A pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Ann. Oncol. 2012, 23, 1869–1880. [Google Scholar] [CrossRef]
- Chuang, S.C.; Jenab, M.; Heck, J.E.; Bosetti, C.; Talamini, R.; Matsuo, K.; Castellsague, X.; Franceschi, S.; Herrero, R.; Winn, D.M.; et al. Diet and the risk of head and neck cancer: A pooled analysis in the INHANCE consortium. Cancer Causes Control 2012, 23, 69–88. [Google Scholar] [CrossRef]
- McClure, J.B.; Divine, G.; Alexander, G.; Tolsma, D.; Rolnick, S.J.; Stopponi, M.; Richards, J.; Johnson, C.C. A comparison of smokers’ and nonsmokers’ fruit and vegetable intake and relevant psychosocial factors. Behav. Med. 2009, 35, 14–22. [Google Scholar] [CrossRef]
- McPhillips, J.B.; Eaton, C.B.; Gans, K.M.; Derby, C.A.; Lasater, T.M.; McKenney, J.L.; Carleton, R.A. Dietary differences in smokers and nonsmokers from two southeastern New England communities. J. Am. Diet. Assoc. 1994, 94, 287–292. [Google Scholar] [CrossRef]
- Millen, B.E.; Quatromoni, P.A.; Nam, B.H.; O’Horo, C.E.; Polak, J.F.; Wolf, P.A.; D’Agostino, R.B. Dietary patterns, smoking, and subclinical heart disease in women: Opportunities for primary prevention from the Framingham nutrition studies. J. Am. Diet. Assoc. 2004, 104, 208–214. [Google Scholar] [CrossRef]
- Osler, M. The food intake of smokers and nonsmokers: The role of partner’s smoking behavior. Prev. Med. 1998, 27, 438–443. [Google Scholar] [CrossRef]
- Raza, A.; Karimyan, N.; Watters, A.; Emperumal, C.P.; Al-Eryani, K.; Enciso, R. Efficacy of oral and topical antioxidants in the prevention and management of oral mucositis in head and neck cancer patients: A systematic review and meta-analyses. Support. Care Cancer 2022, 30, 8689–8703. [Google Scholar] [CrossRef]
- Seifried, H.E.; McDonald, S.S.; Anderson, D.E.; Greenwald, P.; Milner, J.A. The antioxidant conundrum in cancer. Cancer Res. 2003, 63, 4295–4298. [Google Scholar]
- Ladas, E.J.; Jacobson, J.S.; Kennedy, D.D.; Teel, K.; Fleischauer, A.; Kelly, K.M. Antioxidants and cancer therapy: A systematic review. J. Clin. Oncol. 2004, 22, 517–528. [Google Scholar] [CrossRef]
- Weiger, W.A.; Smith, M.; Boon, H.; Richardson, M.A.; Kaptchuk, T.J.; Eisenberg, D.M. Advising patients who seek complementary and alternative medical therapies for cancer. Ann. Intern. Med. 2002, 137, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Cole, W.C.; Kumar, B.; Che Prasad, K. Pros and cons of antioxidant use during radiation therapy. Cancer Treat. Rev. 2002, 28, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Bairati, I.; Meyer, F.; Gélinas, M.; Fortin, A.; Nabid, A.; Brochet, F.; Mercier, J.-P.; Têtu, B.; Harel, F.; Mâsse, B.; et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J. Natl. Cancer Inst. 2005, 97, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a Combination of Beta Carotene and Vitamin A on Lung Cancer and Cardiovascular Disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Bairati, I.; Fortin, A.; Gélinas, M.; Nabid, A.; Brochet, F.; Têtu, B. Interaction between antioxidant vitamin supplementation and cigarette smoking during radiation therapy in relation to long-term effects on recurrence and mortality: A randomized trial among head and neck cancer patients. Int. J. Cancer 2008, 122, 1679–1683. [Google Scholar] [CrossRef]
- Mayne, S.T.T.; Cartmel, B.; Baum, M.; Shor-Posner, G.; Fallon, B.G.G.; Briskin, K.; Bean, J.; Zheng, T.; Cooper, D.; Friedman, C.; et al. Randomized trial of supplemental beta-carotene to prevent second head and neck cancer. Cancer Res. 2001, 61, 1457–1463. [Google Scholar]
- Datta, M.; Shaw, E.G.E.G.; Lesser, G.J.G.J.; Case, L.D.D.; Vitolins, M.Z.M.Z.; Schneider, C.; Frizzell, B.; Sullivan, C.; Lively, M.; Franzmann, E.; et al. A Randomized Double-Blind Placebo-Controlled Trial of Fruit and Vegetable Concentrates on Intermediate Biomarkers in Head and Neck Cancer. Integr. Cancer Ther. 2018, 17, 115–123. [Google Scholar] [CrossRef]
- Seixas-Silva, J.A.J.; Richards, T.; Khuri, F.R.; Wieand, H.S.; Kim, E.; Murphy, B.; Francisco, M.; Hong, W.K.; Shin, D.M. Phase 2 bioadjuvant study of interferon alfa-2a, isotretinoin, and vitamin E in locally advanced squamous cell carcinoma of the head and neck: Long-term follow-up. Arch. Otolaryngol. Head. Neck Surg. 2005, 131, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.M.; Khuri, F.R.; Murphy, B.; Garden, A.S.; Clayman, G.; Francisco, M.; Liu, D.; Glisson, B.S.; Ginsberg, L.; Papadimitrakopoulou, V.; et al. Combined interferon-alfa, 13-cis-retinoic acid, and alpha-tocopherol in locally advanced head and neck squamous cell carcinoma: Novel bioadjuvant phase II trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 3010–3017. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Dalesio, O.; Pastorino, U.; de Vries, N.; van Tinteren, H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the EUropean Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J. Natl. Cancer Inst. 2000, 92, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Cianfriglia, F.; Iofrida, R.V.; Calpicchio, A.; Manieri, A. The chemoprevention of oral carcinoma with vitamin A and/or N-acetylcysteine. Minerva Stomatol. 1994, 43, 255–261. [Google Scholar] [PubMed]
- Jyothirmayi, R.; Ramadas, K.; Varghese, C.; Jacob, R.; Nair, M.K.; Sankaranarayanan, R. Efficacy of vitamin A in the prevention of loco-regional recurrence and second primaries in head and neck cancer. Eur. J. Cancer B Oral Oncol. 1996, 32, 373–376. [Google Scholar] [CrossRef]
- Toma, S.; Bonelli, L.; Sartoris, A.; Mira, E.; Antonelli, A.; Beatrice, F.; Giordano, C.; Benazzo, M.; Caroggio, A.; Cavalot, A.L.; et al. beta-carotene supplementation in patients radically treated for stage I-II head and neck cancer: Results of a randomized trial. Oncol. Rep. 2003, 10, 1895–1901. [Google Scholar]
- OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence; Oxford Centre for Evidence-Based Medicine: Oxford, UK, 2011. [Google Scholar]
- Lefor, A.T.; Bredenberg, C.E.; Kellman, R.M.; Aust, J.C. Multiple Malignancies of the Lung and Head and Neck: Second Primary Tumor or Metastasis? Arch. Surg. 1986, 121, 265–270. [Google Scholar] [CrossRef]
- Hong, W.K.; Lippman, S.M.; Itri, L.M.; Karp, D.D.; Lee, J.S.; Byers, R.M.; Schantz, S.P.; Kramer, A.M.; Lotan, R.; Peters, L.J.; et al. Prevention of Second Primary Tumors with Isotretinoin in Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 1990, 323, 795–801. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of Effect of Long-Term Supplementation with Beta Carotene on the Incidence of Malignant Neoplasms and Cardiovascular Disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef]
- Virtamo, J. Incidence of Cancer and Mortality Following α-Tocopherol and β-Carotene Supplementation: A Postintervention Follow-up. JAMA 2003, 290, 476–485. [Google Scholar] [CrossRef]
- The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The Effect of Vitamin E and Beta Carotene on the Incidence of Lung Cancer and Other Cancers in Male Smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Stevens, M.H.; Gardner, J.W.; Parkin, J.L.; Johnson, L.P. Head and Neck Cancer Survival and Life-style Change. Arch. Otolaryngol. 1983, 109, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Moore, C. Cigarette Smoking and Cancer of the Mouth, Pharynx, and Larynx: A Continuing Study. JAMA J. Am. Med. Assoc. 1971, 218, 553–558. [Google Scholar] [CrossRef]
- Khuri, F.R.; Kim, E.S.; Lee, J.J.; Winn, R.J.; Benner, S.E.; Lippman, S.M.; Fu, K.K.; Cooper, J.S.; Vokes, E.E.; Vokes, E.E.; et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol. Biomark. Prev. 2001, 10, 823–829. [Google Scholar]
- Meyer, F.; Bairati, I.; Jobin, E.; Gélinas, M.; Fortin, A.; Nabid, A.; Têtu, B. Acute adverse effects of radiation therapy and local recurrence in relation to dietary and plasma beta carotene and alpha tocopherol in head and neck cancer patients. Nutr. Cancer 2007, 59, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Brozyna, A.A.; Kim, T.K.; Elsayed, M.M.; Janjetovic, Z.; Qayyum, S.; Slominski, R.M.; Oak, A.S.W.; Li, C.; Podgorska, E.; et al. CYP11A1-derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas. Int. J. Oncol. 2022, 61, 1–18. [Google Scholar] [CrossRef]
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Li, W.; Shehabi, H.Z.; Janjetovic, Z.; Nguyen, M.N.; Kim, T.K.; Chen, J.; Howell, D.E.; Benson, H.A.E.; Sweatman, T.; et al. Production of 22-hydroxy metabolites of vitamin D 3 by cytochrome P450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab. Dispos. 2011, 39, 1577–1588. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.; Shehabi, H.Z.; Semak, I.; Tang, E.K.Y.; Nguyen, M.N.; Benson, H.A.E.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D 3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Li, W.; Tuckey, R.C. Classical and non-classical metabolic transformation of vitamin D in dermal fibroblasts. Exp. Dermatol. 2016, 25, 231–232. [Google Scholar] [CrossRef]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.K.; Wright, A.C.; Grese, L.N.; Riney, S.J.; Nguyen, M.N.; Tuckey, R.C. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012, 32, 3733–3742. [Google Scholar] [PubMed]
- Wang, J.; Slominski, A.; Tuckey, R.C.; Janjetovic, Z.; Kulkarni, A.; Chen, J.; Postlethwaite, A.E.; Miller, D.; Li, W. 20-Hydroxyvitamin D 3 inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012, 32, 739–746. [Google Scholar] [PubMed]
- Florido, J.; Martinez-Ruiz, L.; Rodriguez-Santana, C.; López-Rodríguez, A.; Hidalgo-Gutiérrez, A.; Cottet-Rousselle, C.; Lamarche, F.; Schlattner, U.; Guerra-Librero, A.; Aranda-Martínez, P.; et al. Melatonin drives apoptosis in head and neck cancer by increasing mitochondrial ROS generated via reverse electron transport. J. Pineal Res. 2022, 73, e12824. [Google Scholar] [CrossRef]
- Yeh, C.M.; Su, S.C.; Lin, C.W.; Yang, W.E.; Chien, M.H.; Reiter, R.J.; Yang, S.F. Melatonin as a potential inhibitory agent in head and neck cancer. Oncotarget 2017, 8, 90545–90556. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; Martinez-Ruiz, L.; Rodríguez-Santana, C.; Shen, Y.Q.; García-Verdugo, J.M.; López-Rodríguez, A.; Rusanova, I.; Quiñones-Hinojosa, A.; et al. Melatonin targets metabolism in head and neck cancer cells by regulating mitochondrial structure and function. Antioxidants 2021, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.Y.; Seo, Y.; Oh, S.J.; Ahn, J.S.; Song, M.H.; Kang, M.J.; Oh, J.M.; Lee, D.; Kim, Y.H.; Sung, E.S.; et al. Melatonin and verteporfin synergistically suppress the growth and stemness of head and neck squamous cell carcinoma through the regulation of mitochondrial dynamics. J. Pineal Res. 2022, 72, e12779. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Y.; Tang, H.; Wang, H.; Jiang, E.; Shao, Z.; Liu, K.; Zhou, X.; Shang, Z. Melatonin inhibits EMT and PD-L1 expression through the ERK1/2/FOSL1 pathway and regulates anti-tumor immunity in HNSCC. Cancer Sci. 2022, 113, 2232–2245. [Google Scholar] [CrossRef]
- Lang, L.; Xiong, Y.; Prieto-Dominguez, N.; Loveless, R.; Jensen, C.; Shay, C.; Teng, Y. FGF19/FGFR4 signaling axis confines and switches the role of melatonin in head and neck cancer metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 1–14. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; García-López, S.; Martinez-Ruiz, L.; Mendivil-Perez, M.; Soto-Mercado, V.; Acuña-Castroviejo, D.; Ortega-Arellano, H.; et al. Combination of melatonin and rapamycin for head and neck cancer therapy: Suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J. Pineal Res. 2018, 64, e12461. [Google Scholar] [CrossRef]
- Guerra, J.; Devesa, J. Usefulness of melatonin and other compounds as antioxidants and epidrugs in the treatment of head and neck cancer. Antioxidants 2022, 11, 35. [Google Scholar] [CrossRef]
- Meliante, P.G.; Barbato, C.; Zoccali, F.; Ralli, M.; Greco, A.; de Vincentiis, M.; Colizza, A.; Petrella, C.; Ferraguti, G.; Minni, A.; et al. Programmed Cell Death-Ligand 1 in Head and Neck Squamous Cell Carcinoma: Molecular Insights, Preclinical and Clinical Data, and Therapies. Int. J. Mol. Sci. 2022, 23, 15384. [Google Scholar] [CrossRef] [PubMed]
- Meliante, P.G.; Zoccali, F.; de Vincentiis, M.; Ralli, M.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Diagnostic Predictors of Immunotherapy Response in Head and Neck Squamous Cell Carcinoma. Diagnostics 2023, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Raman, C.; Chen, J.Y.; Slominski, A.T. How cancer hijacks the body’s homeostasis through the neuroendocrine system. Trends Neurosci. 2023, 46, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meliante, P.G.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Antioxidant Use after Diagnosis of Head and Neck Squamous Cell Carcinoma (HNSCC): A Systematic Review of Application during Radiotherapy and in Second Primary Cancer Prevention. Antioxidants 2023, 12, 1753. https://doi.org/10.3390/antiox12091753
Meliante PG, Petrella C, Fiore M, Minni A, Barbato C. Antioxidant Use after Diagnosis of Head and Neck Squamous Cell Carcinoma (HNSCC): A Systematic Review of Application during Radiotherapy and in Second Primary Cancer Prevention. Antioxidants. 2023; 12(9):1753. https://doi.org/10.3390/antiox12091753
Chicago/Turabian StyleMeliante, Piero Giuseppe, Carla Petrella, Marco Fiore, Antonio Minni, and Christian Barbato. 2023. "Antioxidant Use after Diagnosis of Head and Neck Squamous Cell Carcinoma (HNSCC): A Systematic Review of Application during Radiotherapy and in Second Primary Cancer Prevention" Antioxidants 12, no. 9: 1753. https://doi.org/10.3390/antiox12091753








