Andrographolide Induces ROS-Mediated Cytotoxicity, Lipid Peroxidation, and Compromised Cell Integrity in Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains, Plasmids, Primers, and Growth Conditions
2.2. Fluorescence Microscopy
2.3. HAC1 Splicing
2.4. Intracellular ROS Detection
2.5. Thiobarbituric Acid Reactive Substances (TBARS) Assay
2.6. Sterols Quantification (Ergosterol Biosynthesis Assay)
% Ergosterol + % 24(28) DHE = [(A281.5/290) × F]/total protein weight
2.7. Zymolyase Assay
2.8. Scanning Electron Microscopy
2.9. Preparation of Secreted Biomolecules
2.10. Quantification and Statistical Analysis
3. Results
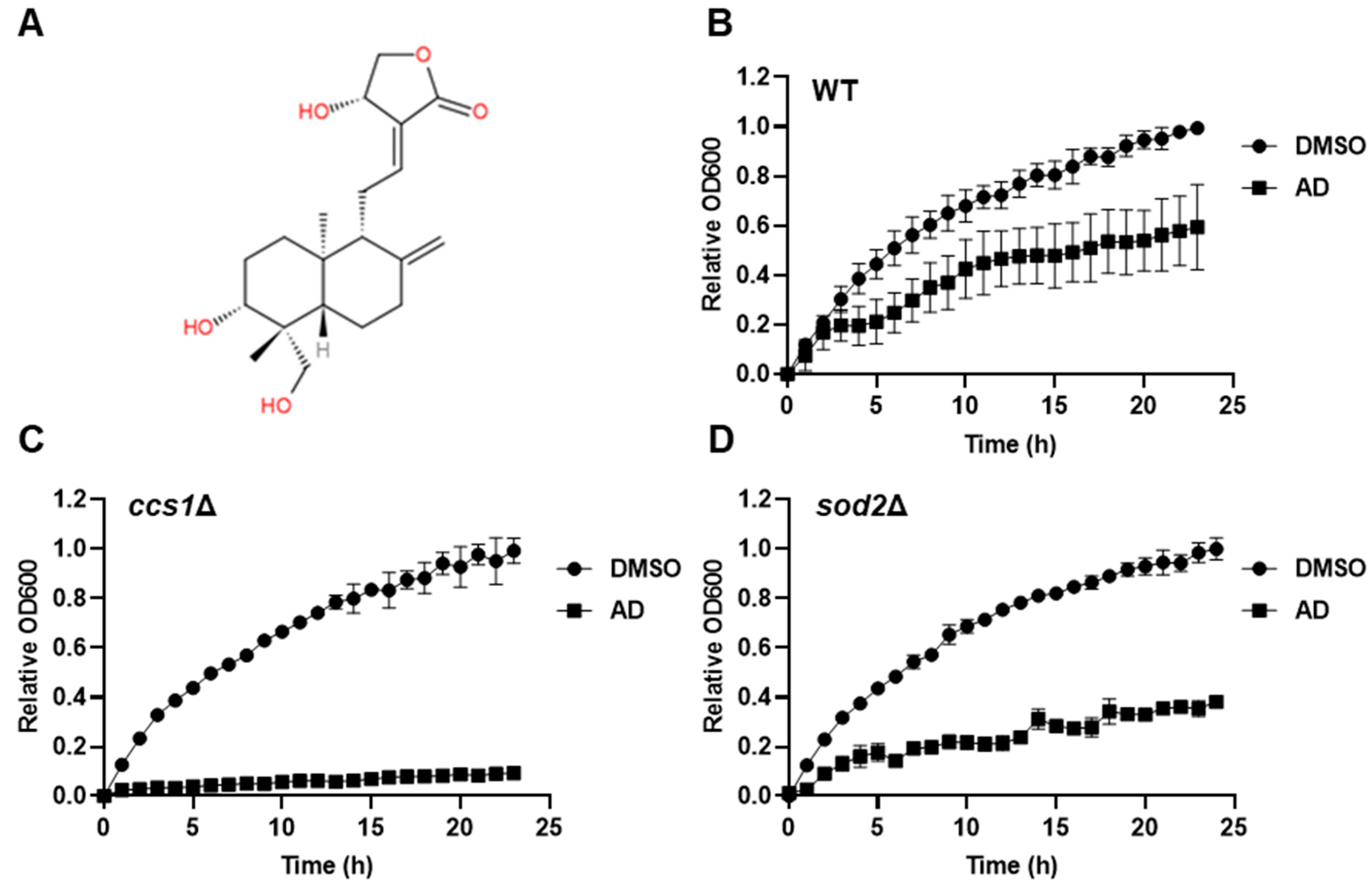
3.1. Andrographolide Exhibits Cytotoxicity in S. cerevisiae with ROS Synergy
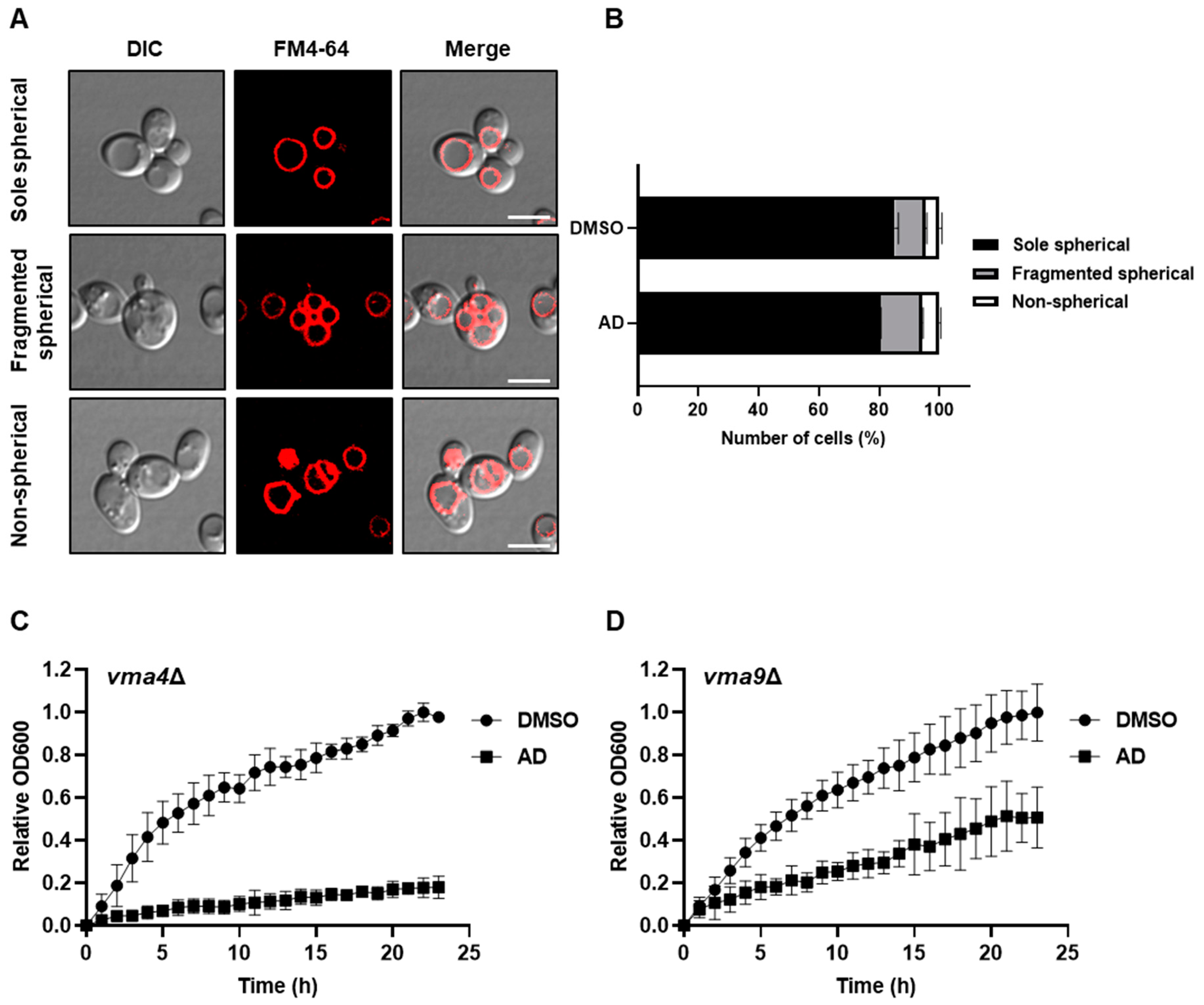
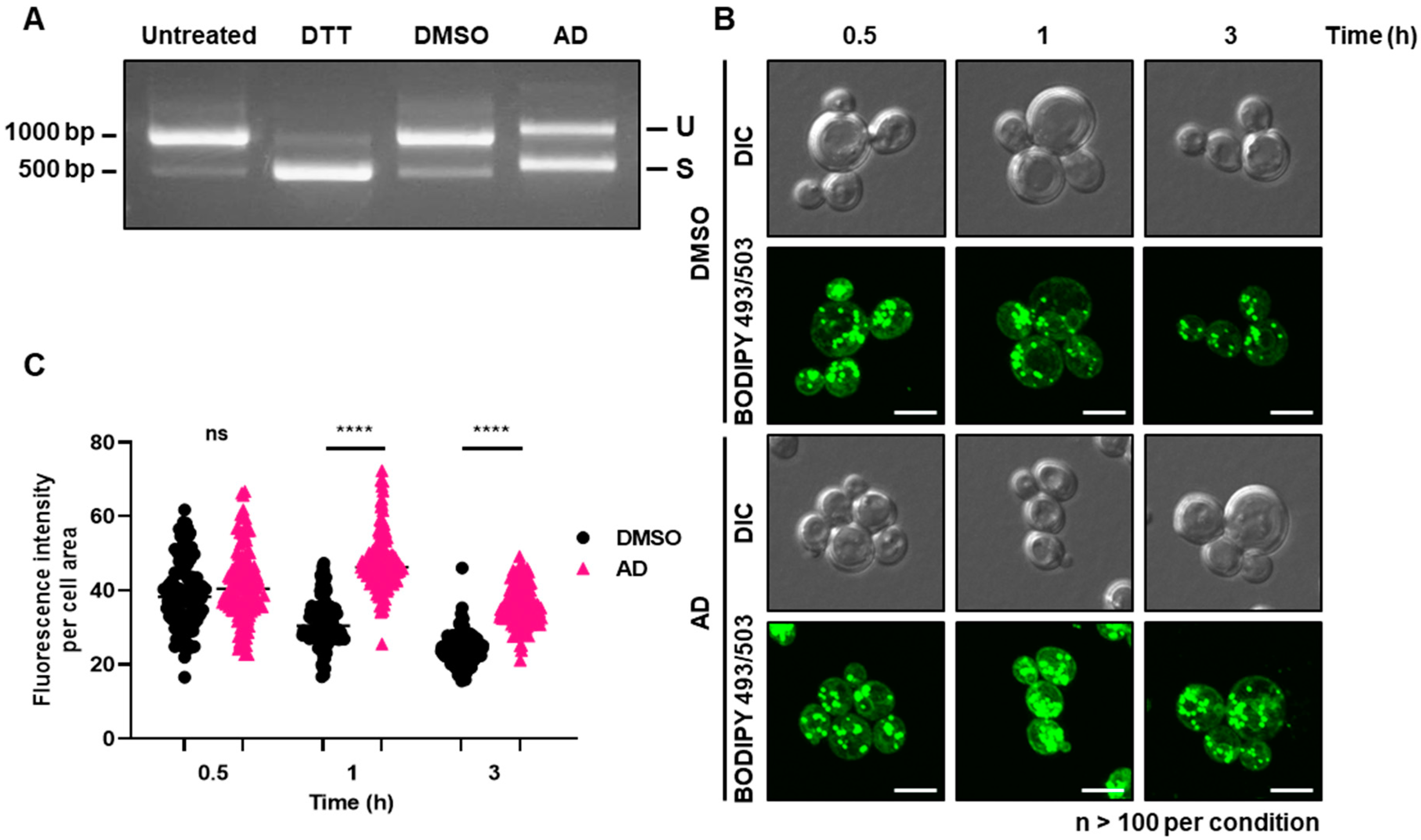
3.2. Andrographolide Induces Vacuole Fragmentation, ER Stress, and Lipid Droplet Accumulation in Yeast
3.3. Andrographolide Induces Lipid Peroxidation and ROS Elevation in Yeast
3.4. Andrographolide Compromises Cell Integrity and Affects the Ergosterol-Dependent Membrane Stability
3.5. Andrographolide Induces Sheet-like Structures Impacting Yeast Cells
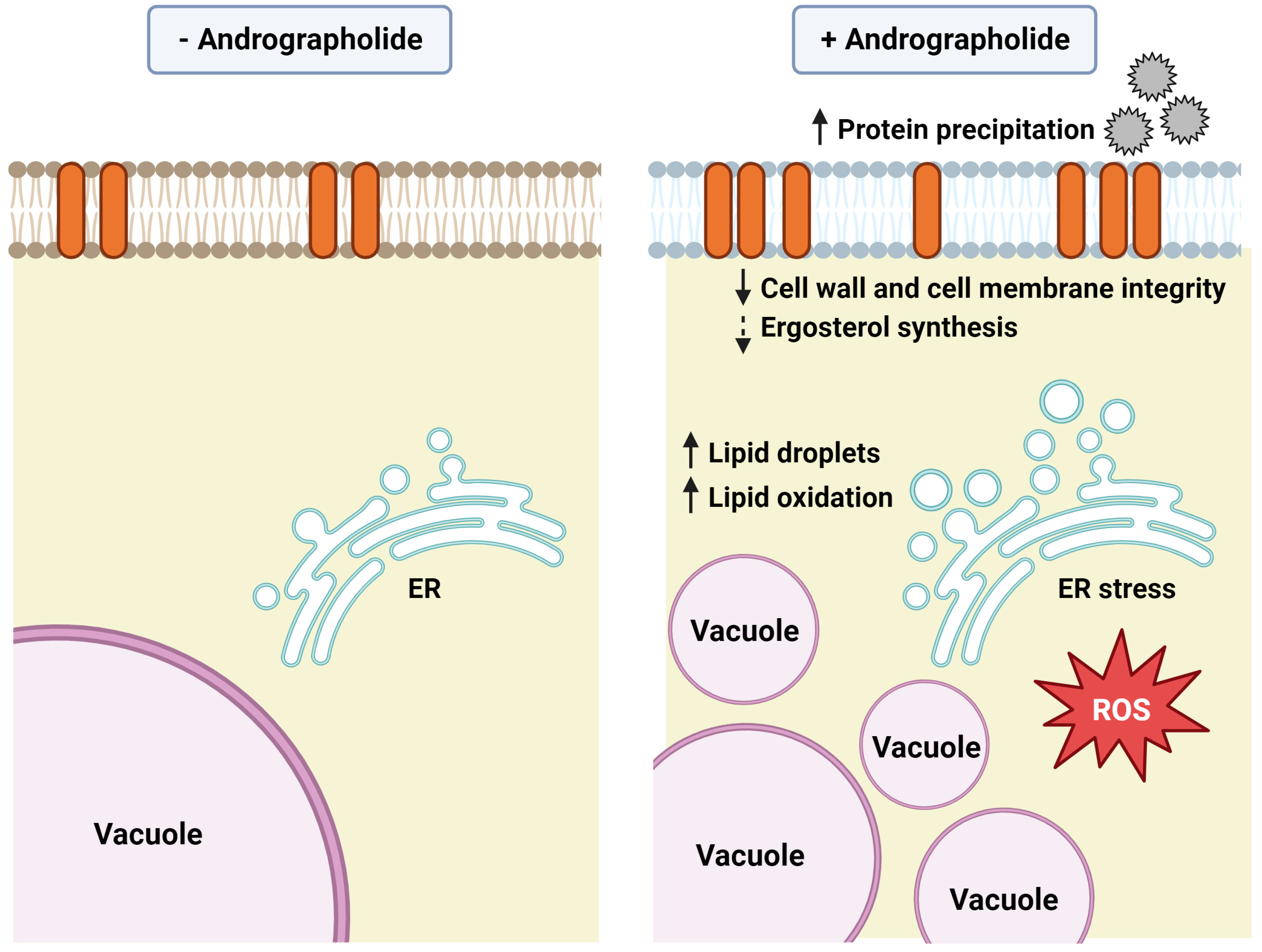
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Li, S.; Si, Y.; Xu, H. Andrographolide and its derivatives: Current achievements and future perspectives. Eur. J. Med. Chem. 2021, 224, 113710. [Google Scholar] [CrossRef]
- Li, X.; Yuan, W.; Wu, J.; Zhen, J.; Sun, Q.; Yu, M. Andrographolide, a natural anti-inflammatory agent: An Update. Front. Pharmacol. 2022, 13, 920435. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mishra, K.P.; Ganju, L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017, 162, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L. Biological properties of andrographolide, an active ingredient of Andrographis paniculata: A narrative review. Ann. Transl. Med. 2021, 9, 1186. [Google Scholar] [CrossRef]
- Shang, Y.X.; Shen, C.; Stub, T.; Zhu, S.J.; Qiao, S.Y.; Li, Y.Q.; Wang, R.T.; Li, J.; Liu, J.P. Adverse Effects of Andrographolide Derivative Medications Compared to the Safe use of Herbal Preparations of Andrographis paniculata: Results of a Systematic Review and Meta-Analysis of Clinical Studies. Front. Pharmacol. 2022, 13, 773282. [Google Scholar] [CrossRef]
- Arifullah, M.; Namsa, N.D.; Mandal, M.; Chiruvella, K.K.; Vikrama, P.; Gopal, G.R. Evaluation of anti-bacterial and anti-oxidant potential of andrographolide and echiodinin isolated from callus culture of Andrographis paniculata Nees. Asian Pac. J. Trop. Biomed. 2013, 3, 604–610. [Google Scholar] [CrossRef]
- Chanarat, S.; Svasti, J. Stress-induced upregulation of the ubiquitin-relative Hub1 modulates pre-mRNA splicing and facilitates cadmium tolerance in Saccharomyces cerevisiae. Biochim. Biophys. Acta 2020, 1867, 118565. [Google Scholar] [CrossRef]
- Janke, C.; Magiera, M.M.; Rathfelder, N.; Taxis, C.; Reber, S.; Maekawa, H.; Moreno-Borchart, A.; Doenges, G.; Schwob, E.; Schiebel, E.; et al. A versatile toolbox for PCR-based tagging of yeast genes: New fluorescent proteins, more markers and promoter substitution cassettes. Yeast 2004, 21, 947–962. [Google Scholar] [CrossRef]
- Bahler, J.; Wu, J.Q.; Longtine, M.S.; Shah, N.G.; McKenzie, A., 3rd; Steever, A.B.; Wach, A.; Philippsen, P.; Pringle, J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 1998, 14, 943–951. [Google Scholar] [CrossRef]
- Rothstein, R. Targeting, disruption, replacement, and allele rescue: Integrative DNA transformation in yeast. Methods Enzymol. 1991, 194, 281–301. [Google Scholar] [CrossRef] [PubMed]
- Karaduman, R.; Chanarat, S.; Pfander, B.; Jentsch, S. Error-Prone Splicing Controlled by the Ubiquitin Relative Hub1. Mol. Cell 2017, 67, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.O.; Sepulveda, L.A.; Xu, H.; Golding, I. Measuring mRNA copy number in individual Escherichia coli cells using single-molecule fluorescent in situ hybridization. Nat. Protoc. 2013, 8, 1100–1113. [Google Scholar] [CrossRef]
- Stauffer, B.; Powers, T. Target of rapamycin signaling mediates vacuolar fragmentation. Curr. Genet. 2017, 63, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Revie, N.M.; Iyer, K.R.; Maxson, M.E.; Zhang, J.; Yan, S.; Fernandes, C.M.; Meyer, K.J.; Chen, X.; Skulska, I.; Fogal, M.; et al. Targeting fungal membrane homeostasis with imidazopyrazoindoles impairs azole resistance and biofilm formation. Nat. Commun. 2022, 13, 3634. [Google Scholar] [CrossRef]
- Liao, P.C.; Yang, E.J.; Pon, L.A. Live-Cell Imaging of Mitochondrial Redox State in Yeast Cells. STAR Protoc. 2020, 1, 100160. [Google Scholar] [CrossRef]
- Davey, H.M.; Hexley, P. Red but not dead? Membranes of stressed Saccharomyces cerevisiae are permeable to propidium iodide. Environ. Microbiol. 2011, 13, 163–171. [Google Scholar] [CrossRef]
- Scrimale, T.; Didone, L.; de Mesy Bentley, K.L.; Krysan, D.J. The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae. Mol. Biol. Cell 2009, 20, 164–175. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakayama, H.; Nagayoshi, Y.; Kakeya, H.; Kohno, S. Dissection of Ire1 functions reveals stress response mechanisms uniquely evolved in Candida glabrata. PLoS Pathog. 2013, 9, e1003160. [Google Scholar] [CrossRef]
- James, J.; Fiji, N.; Roy, D.; Andrew Mg, D.; Shihabudeen, M.S.; Chattopadhyay, D.; Thirumurugan, K. A rapid method to assess reactive oxygen species in yeast using H2DCF-DA. Anal. Methods 2015, 7, 8572–8575. [Google Scholar] [CrossRef]
- Howlett, N.G.; Avery, S.V. Induction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl. Environ. Microbiol. 1997, 63, 2971–2976. [Google Scholar] [CrossRef] [PubMed]
- Sj, S.; Veerabhadrappa, B.; Subramaniyan, S.; Dyavaiah, M. Astaxanthin enhances the longevity of Saccharomyces cerevisiae by decreasing oxidative stress and apoptosis. FEMS Yeast Res. 2019, 19, foy113. [Google Scholar] [CrossRef] [PubMed]
- Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of ergosterol content: Novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Warnock, D.W.; Morrison, C.J. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2081–2085. [Google Scholar] [CrossRef]
- Rather, I.A.; Sabir, J.S.M.; Asseri, A.H.; Wani, M.Y.; Ahmad, A. Triazole Derivatives Target 14alpha-Demethylase (LDM) Enzyme in Candida albicans Causing Ergosterol Biosynthesis Inhibition. J. Fungi 2022, 8, 688. [Google Scholar] [CrossRef] [PubMed]
- Phothichaisri, W.; Chankhamhaengdecha, S.; Janvilisri, T.; Nuadthaisong, J.; Phetruen, T.; Fagan, R.P.; Chanarat, S. Potential Role of the Host-Derived Cell-Wall Binding Domain of Endolysin CD16/50L as a Molecular Anchor in Preservation of Uninfected Clostridioides difficile for New Rounds of Phage Infection. Microbiol. Spectrum 2022, 10, e02361-21. [Google Scholar] [CrossRef]
- Phetruen, T.; Chanarat, S.; Janvilisri, T.; Phanchana, M.; Charoensutthivarakul, S.; Phothichaisri, W.; Chankhamhaengdecha, S. Receptor binding protein of prophage reversibly recognizes the low-molecular weight subunit of the surface-layer protein SlpA in Clostridioides difficile. Front. Microbiol. 2022, 13, 998215. [Google Scholar] [CrossRef]
- de Camara, A.A., Jr.; Dupont, S.; Beney, L.; Gervais, P.; Rosenthal, A.; Correia, R.T.; Pedrini, M.R. Fisetin yeast-based bio-capsules via osmoporation: Effects of process variables on the encapsulation efficiency and internalized fisetin content. Appl. Microbiol. Biotechnol. 2016, 100, 5547–5558. [Google Scholar] [CrossRef]
- Recek, N.; Zhou, R.; Zhou, R.; Te’o, V.S.J.; Speight, R.E.; Mozetic, M.; Vesel, A.; Cvelbar, U.; Bazaka, K.; Ostrikov, K.K. Improved fermentation efficiency of S. cerevisiae by changing glycolytic metabolic pathways with plasma agitation. Sci. Rep. 2018, 8, 8252. [Google Scholar] [CrossRef]
- Turcotte, B.; Liang, X.B.; Robert, F.; Soontorngun, N. Transcriptional regulation of nonfermentable carbon utilization in budding yeast. FEMS Yeast Res. 2010, 10, 2–13. [Google Scholar] [CrossRef]
- Roberts, G.G.; Hudson, A.P. Transcriptome profiling of Saccharomyces cerevisiae during a transition from fermentative to glycerol-based respiratory growth reveals extensive metabolic and structural remodeling. Mol. Genet. Genom. 2006, 276, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, L.M.; Scharfe, C.; Deutschbauer, A.M.; Mokranjac, D.; Herman, Z.S.; Jones, T.; Chu, A.M.; Giaever, G.; Prokisch, H.; Oefner, P.J.; et al. Systematic screen for human disease genes in yeast. Nat. Genet. 2002, 31, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Casler, J.C.; Papanikou, E.; Barrero, J.J.; Glick, B.S. Maturation-driven transport and AP-1-dependent recycling of a secretory cargo in the Golgi. J. Cell Biol. 2019, 218, 1582–1601. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Kane, P.M. The yeast lysosome-like vacuole: Endpoint and crossroads. Biochim. Biophys. Acta 2009, 1793, 650–663. [Google Scholar] [CrossRef]
- Aufschnaiter, A.; Buttner, S. The vacuolar shapes of ageing: From function to morphology. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Zieger, M.; Mayer, A. Yeast vacuoles fragment in an asymmetrical two-phase process with distinct protein requirements. Mol. Biol. Cell 2012, 23, 3438–3449. [Google Scholar] [CrossRef]
- Sambade, M.; Alba, M.; Smardon, A.M.; West, R.W.; Kane, P.M. A genomic screen for yeast vacuolar membrane ATPase mutants. Genetics 2005, 170, 1539–1551. [Google Scholar] [CrossRef]
- Stauffer, B.; Powers, T. Target of rapamycin signaling mediates vacuolar fission caused by endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol. Biol. Cell 2015, 26, 4618–4630. [Google Scholar] [CrossRef]
- Kawahara, T.; Yanagi, H.; Yura, T.; Mori, K. Unconventional splicing of HAC1/ERN4 mRNA required for the unfolded protein response. Sequence-specific and non-sequential cleavage of the splice sites. J. Biol. Chem. 1998, 273, 1802–1807. [Google Scholar] [CrossRef]
- Hooks, K.B.; Griffiths-Jones, S. Conserved RNA structures in the non-canonical Hac1/Xbp1 intron. RNA Biol. 2011, 8, 552–556. [Google Scholar] [CrossRef]
- van Anken, E.; Pincus, D.; Coyle, S.; Aragon, T.; Osman, C.; Lari, F.; Gomez Puerta, S.; Korennykh, A.V.; Walter, P. Specificity in endoplasmic reticulum-stress signaling in yeast entails a step-wise engagement of HAC1 mRNA to clusters of the stress sensor Ire1. eLife 2014, 3, e05031. [Google Scholar] [CrossRef]
- Jarc, E.; Petan, T. Lipid Droplets and the Management of Cellular Stress. Yale J. Biol. Med. 2019, 92, 435–452. [Google Scholar]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Goodman, J.M. The lipid droplet-a well-connected organelle. Front. Cell Dev. Biol. 2015, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Shinohara, Y.; Suzuki, M.; Fujimoto, T. A pitfall in using BODIPY dyes to label lipid droplets for fluorescence microscopy. Histochem. Cell Biol. 2010, 133, 477–480. [Google Scholar] [CrossRef]
- Brookheart, R.T.; Michel, C.I.; Schaffer, J.E. As a matter of fat. Cell Metab. 2009, 10, 9–12. [Google Scholar] [CrossRef]
- Han, J.; Kaufman, R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016, 57, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Li, W.; Fu, H.; Fang, L.; Chai, H.; Ding, B.; Qian, S. Andrographolide induced ferroptosis in multiple myeloma cells by regulating the P38/Nrf2/HO-1 pathway. Arch. Biochem. Biophys. 2023, 742, 109622. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.; Wang, Q.; Zhou, D.; Zhao, B.; Yao, J. Andrographolide promoted ferroptosis to repress the development of non-small cell lung cancer through activation of the mitochondrial dysfunction. Phytomedicine 2023, 109, 154601. [Google Scholar] [CrossRef]
- Ma, R.; Shimura, T.; Yin, C.; Okugawa, Y.; Kitajima, T.; Koike, Y.; Okita, Y.; Ohi, M.; Uchida, K.; Goel, A.; et al. Antitumor effects of Andrographis via ferroptosis-associated genes in gastric cancer. Oncol. Lett. 2021, 22, 523. [Google Scholar] [CrossRef]
- Shimura, T.; Sharma, P.; Sharma, G.G.; Banwait, J.K.; Goel, A. Enhanced anti-cancer activity of andrographis with oligomeric proanthocyanidins through activation of metabolic and ferroptosis pathways in colorectal cancer. Sci. Rep. 2021, 11, 7548. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Bermejo, C.; Grau, C.; Perez, R.; Rodriguez-Pena, J.M.; Francois, J.; Nombela, C.; Arroyo, J. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J. Biol. Chem. 2004, 279, 15183–15195. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Hiraki, T. Mechanistic role of ergosterol in membrane rigidity and cycloheximide resistance in Saccharomyces cerevisiae. Biochim. Biophys. Acta 2009, 1788, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Jorda, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Godinho, C.P.; Vitorino, M.V.; Robalo, T.T.; Fernandes, F.; Rodrigues, M.S.; Sa-Correia, I. Crosstalk between Yeast Cell Plasma Membrane Ergosterol Content and Cell Wall Stiffness under Acetic Acid Stress Involving Pdr18. J. Fungi 2022, 8, 103. [Google Scholar] [CrossRef]
- Faria-Oliveira, F.; Carvalho, J.; Ferreira, C.; Hernaez, M.L.; Gil, C.; Lucas, C. Quantitative differential proteomics of yeast extracellular matrix: There is more to it than meets the eye. BMC Microbiol. 2015, 15, 271. [Google Scholar] [CrossRef]
- Liang, S.T.; Chen, C.; Chen, R.X.; Li, R.; Chen, W.L.; Jiang, G.H.; Du, L.L. Michael acceptor molecules in natural products and their mechanism of action. Front. Pharmacol. 2022, 13, 1033003. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Loh, X.Y.; Wijaya, H.; Wang, J.; Lin, Q.; Lam, Y.; Wong, W.S.; Mok, Y.K. Specificity and inhibitory mechanism of andrographolide and its analogues as antiasthma agents on NF-kappaB p50. J. Nat. Prod. 2015, 78, 208–217. [Google Scholar] [CrossRef]
- Yang, C.H.; Yen, T.L.; Hsu, C.Y.; Thomas, P.A.; Sheu, J.R.; Jayakumar, T. Multi-Targeting Andrographolide, a Novel NF-kappaB Inhibitor, as a Potential Therapeutic Agent for Stroke. Int. J. Mol. Sci. 2017, 18, 1638. [Google Scholar] [CrossRef]
- Wong, D.P.W.; Ng, M.Y.; Leung, J.Y.; Boh, B.K.; Lim, E.C.; Tan, S.H.; Lim, S.; Seah, W.H.; Hu, C.Z.; Ho, B.C.; et al. Regulation of the NRF2 transcription factor by andrographolide and organic extracts from plant endophytes. PLoS ONE 2018, 13, e0204853. [Google Scholar] [CrossRef] [PubMed]
- Casler, J.C.; Glick, B.S. A microscopy-based kinetic analysis of yeast vacuolar protein sorting. eLife 2018, 9, e56844. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phetruen, T.; van Dam, B.; Chanarat, S. Andrographolide Induces ROS-Mediated Cytotoxicity, Lipid Peroxidation, and Compromised Cell Integrity in Saccharomyces cerevisiae. Antioxidants 2023, 12, 1765. https://doi.org/10.3390/antiox12091765
Phetruen T, van Dam B, Chanarat S. Andrographolide Induces ROS-Mediated Cytotoxicity, Lipid Peroxidation, and Compromised Cell Integrity in Saccharomyces cerevisiae. Antioxidants. 2023; 12(9):1765. https://doi.org/10.3390/antiox12091765
Chicago/Turabian StylePhetruen, Tanaporn, Bloem van Dam, and Sittinan Chanarat. 2023. "Andrographolide Induces ROS-Mediated Cytotoxicity, Lipid Peroxidation, and Compromised Cell Integrity in Saccharomyces cerevisiae" Antioxidants 12, no. 9: 1765. https://doi.org/10.3390/antiox12091765
APA StylePhetruen, T., van Dam, B., & Chanarat, S. (2023). Andrographolide Induces ROS-Mediated Cytotoxicity, Lipid Peroxidation, and Compromised Cell Integrity in Saccharomyces cerevisiae. Antioxidants, 12(9), 1765. https://doi.org/10.3390/antiox12091765






