Chemical Profiling, Antioxidant, and Anti-Inflammatory Activities of Hyoseris radiata L., a Plant Used in the Phytoalimurgic Tradition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
2.2. Plant Material and Extract Preparation
2.3. Extract Fractionation and Isolation of Pure Components
2.4. Chemical Characterization of the Extract by LC-HR-Orbitrap/ESI-MS
2.5. NMR Quali-Quantitative Profiling of H. radiata Extract
2.6. Radical-Scavenging Activity Assays
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Cellular Antioxidant Activity Assay
2.10. Measurement of COX-2 Expression
2.11. Data Analysis
3. Results
3.1. Isolation and Identification of Pure Components
3.2. Chemical Fingerprimt and Amount of Components via LC-MS/MS Analysis
3.2.1. Qualitative Analysis
3.2.2. Quantitative Analysis
3.3. NMR-Based Metabolomic Profiling
3.3.1. Qualitative Analysis
3.3.2. Quantitative Analysis
3.4. Antioxidant Activity
3.4.1. Cell-Free Assays
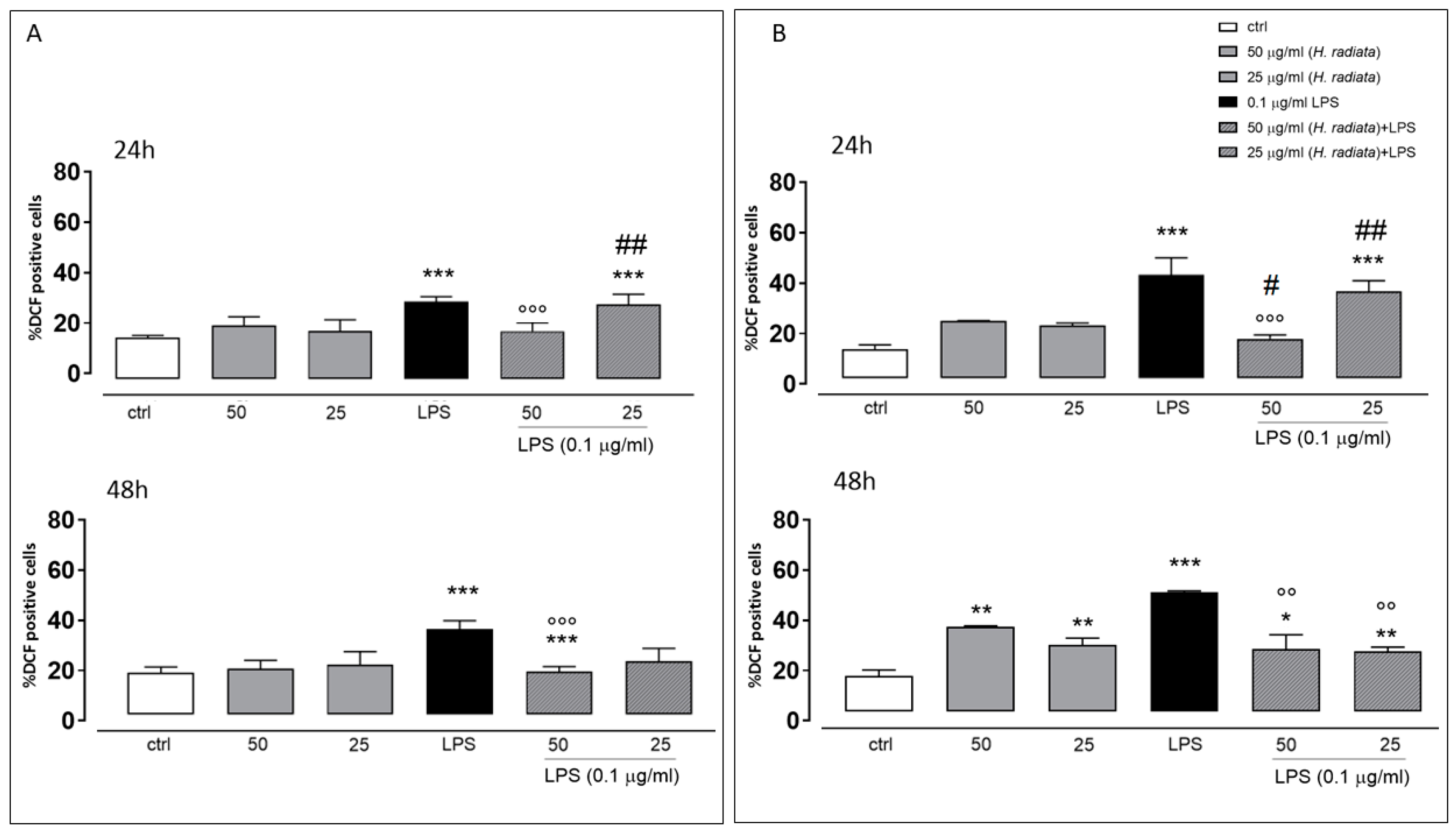
3.4.2. In-Cell Assay
3.5. Anti-Inflammatory Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bacchetta, L.; Visioli, F.; Cappelli, G.; Caruso, E.; Martin, G.; Nemeth, E.; Bacchetta, G.; Bedini, G.; Wezel, A.; On Behalf of the Eatwild Consortium; et al. A manifesto for the valorization of wild edible plants. J. Ethnopharmacol. 2016, 191, 180–187. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Pieroni, A.; Tardío, J.; Pardo-de-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R. Wild food plant use in 21st century Europe: The disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Plants of the World Online (POW). Royal Botanic Gardens, Kew. 2019. Available online: https://www.plantsoftheworldonline.org (accessed on 20 October 2023).
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea: Plantaginaceae to Compositae (and Rubiaceae); Cambridge University Press: Cambridge, UK, 1976; Volume 4, p. 307. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 2018; Volume 4, pp. 1043–1044. [Google Scholar]
- Guarrera, P.M.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef] [PubMed]
- Biscotti, N.; Bonsanto, D.; Del Viscio, G. The traditional food use of wild vegetables in Apulia (Italy) in the light of Italian ethnobotanical literature. Ital. Bot. 2018, 5, 1–24. [Google Scholar] [CrossRef]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef] [PubMed]
- Ranfa, A.; Maurizi, A.; Romano, B.; Bodesmo, M. The importance of traditional uses and nutraceutical aspects of some edible wild plants in human nutrition: The case of Umbria (central Italy). Plant Biosyst. 2014, 148, 297–306. [Google Scholar] [CrossRef]
- Atzei, A.D. Le Piante Nella Tradizione Popolare Della Sardegna; C. Delfino: Sassari, Italy, 2003; p. 85. [Google Scholar]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnobiol. Ethnomed. 2018, 14, 14. [Google Scholar] [CrossRef]
- Pasta, S.; La Rosa, A.; Garfì, G.; Marcenò, C.; Silvestre Gristina, A.; Carimi, F.; Guarino, R. An updated checklist of the Sicilian native edible plants: Preserving the traditional ecological knowledge of century-old agro-pastoral landscapes. Front. Plant Sci. 2020, 11, 388. [Google Scholar] [CrossRef]
- Picchi, G.; Pieroni, A. Atlante dei Prodotti Tipici: Le Erbe; AGRA, RAI-ERI: Roma, Italy, 2005. [Google Scholar]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional uses of the plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- Bisio, A.; Minuto, L. The prebuggiun. In Erbi Boni, Erbi degli Streghi; Pieroni, A., Ed.; Pieroni: Viareggio, Italy, 1999. [Google Scholar]
- Tomei, P.E.; Camangi, F. Tradizioni Fitoalimurgiche in Toscana: Piante Spontanee e Coltivate nella Preparazione delle Zuppe; Pacini Fazzi Editore: Lucca, Italy, 2014; pp. 11–12. [Google Scholar]
- Biscotti, N.; Pieroni, A. The hidden Mediterranean diet: Wild vegetables traditionally gathered and consumed in the Gargano area, Apulia, SE Italy. Acta Soc. Bot. Pol. 2015, 84, 327–338. [Google Scholar] [CrossRef]
- Sicari, V.; Loizzo, M.R.; Sanches Silva, A.; Romeo, R.; Spampinato, G.; Tundis, R.; Leporini, M.; Musarella, C.M. The effect of blanching on phytochemical content and bioactivity of Hypochaeris and Hyoseris species (Asteraceae), vegetables traditionally used in southern Italy. Foods 2021, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M. Usi e Tradizioni della Flora Italiana; Aracne editrice, s.r.l: Roma, Italy, 2006. [Google Scholar]
- Lancioni, M.C.; Ballero, M.; Mura, L.; Maxia, A. Usi alimentari e terapeutici nella tradizione popolare del Goceano (Sardegna Centrale). Atti Soc. Tosc. Sci. Nat., Mem., Serie B 2007, 114, 45–56. [Google Scholar]
- Licata, M.; Tuttolomondo, T.; Leto, C.; Virga, G.; Bonsangue, G.; Cammaleri, I.; Gennaro, M.C.; La Bella, S. A survey of wild plant species for food use in Sicily (Italy)—Results of a 3-year study in four Regional Parks. J. Ethnobiol. Ethnomed. 2016, 12, 12. [Google Scholar] [CrossRef]
- Vanzani, P.; Rossetto, M.; De Marco, V.; Sacchetti, L.E.; Paoletti, M.G.; Rigo, A. Wild Mediterranean plants as traditional food: A valuable source of antioxidants. J. Food Sci. 2011, 76, C46–C51. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, S.; Ghazy, N.M.; Zdero, C.; Bohlmann, F. Polyacetylenic compounds. Part 264. An acetylenic triol from Hyoseris lucida. Phytochemistry 1983, 22, 592–593. [Google Scholar] [CrossRef]
- Cioni, E.; Migone, C.; Ascrizzi, R.; Muscatello, B.; De Leo, M.; Piras, A.M.; Zambito, Y.; Flamini, G.; Pistelli, L. Comparing metabolomic and essential oil fingerprints of Citrus australasica F. Muell (finger lime) varieties and their in vitro antioxidant activity. Antioxidants 2022, 11, 2047. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 2017, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Donadio, G.; Bellone, M.L.; Mensitieri, F.; Parisi, V.; Santoro, V.; Vitiello, M.; Dal Piaz, F.; De Tommasi, N. Characterization of health beneficial components in discarded leaves of three escarole (Cichorium endivia L.) cultivar and study of their antioxidant and anti-inflammatory activities. Antioxidants 2023, 12, 1402. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Sansone, F.; Auriemma, G.; Franceschelli, S.; Pecoraro, M.; Picerno, P.; Aquino, R.P.; Mencherini, T. Study on Ajuga reptans extract: A natural antioxidant in microencapsulated powder form as an active ingredient for nutraceutical or pharmaceutical purposes. Pharmaceutics 2020, 12, 671. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Franceschelli, S.; Pascale, M. Lumacaftor and matrine: Possible therapeutic combination to counteract the inflammatory process in cystic fibrosis. Biomolecules 2021, 11, 422. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Hu, F.; Miyakawa, T.; Tanokura, M. Two-dimensional 1H-13C nuclear magnetic resonance (NMR)-based comprehensive analysis of roasted coffee bean extract. J. Agric. Food. Chem. 2011, 59, 9065–9073. [Google Scholar] [CrossRef]
- Veit, M.; Strack, D.; Czygan, F.-C.; Wray, V.; Witte, L. Di-E-caffeoyl-meso-tartaric acid in the barren sprouts of Equisetum arvense. Phytochemistry 1991, 30, 527–529. [Google Scholar] [CrossRef]
- Xu, X.-H.; Tan, C.-H.; Jiang, S.-H.; Zhu, D.-Y. Debilosides A–C: Three new megastigmane glucosides from Equisetum debile. Helv. Chim. Acta. 2006, 89, 1422–1426. [Google Scholar] [CrossRef]
- Otsuka, H.; Kamada, K.; Yao, M.; Yuasa, K.; Kida, I.; Takeda, Y. Alangionosides C–F, megastigmane glycosides from Alangium premnifolium. Phytochemistry 1995, 38, 1431–1435. [Google Scholar] [CrossRef]
- Inoshiri, S.; Sasaki, M.; Kohda, H.; Otsuka, H.; Yakasaki, K. Aromatic glycosides from Berchemia racemosa. Phytochemistry 1987, 26, 2811–2814. [Google Scholar] [CrossRef]
- Kuwajima, H.; Morita, M.; Takaishi, K.; Inoue, K.; Fujita, T.; He, Z.-D.; Yang, C.-R. Secoiridoid, coumarin, and secoiridoid-coumarin glucosides from Fraxinus chinensis. Phytochemistry 1992, 31, 1277–1280. [Google Scholar] [CrossRef]
- Agrawal, P.K. Carbon-13 NMR of Flavonoids, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 134–135. [Google Scholar]
- Abou-hussein, D.R.; Badr, J.M.; Youssef, D.T.A. Nucleoside constituents of the Egyptian tunicate Eudistoma laysani. Nat. Prod. Sci. 2007, 13, 229–233. [Google Scholar]
- Bourafai-Aziez, A.; Jacob, D.; Charpentier, G.; Cassin, E.; Rousselot, G.; Moing, A.; Deborde, C. Development, validation, and use of 1H-NMR spectroscopy for evaluating the quality of acerola-based food supplements and quantifying ascorbic acid. Molecules 2022, 27, 5614. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Birsa, M.L.; Sarbu, L.G. Health benefits of key constituents in Cichorium intybus L. Nutrients 2023, 15, 1322. [Google Scholar] [CrossRef]
- Perovića, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Schlernitzauer, A.; Oiry, C.; Hamad, R.; Galas, S.; Cortade, F.; Chabi, B.; Casas, F.; Pessemesse, L.; Fouret, G.; Feillet-Coudray, C.; et al. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PLoS ONE, 2013, 8, e78788. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Sun, Q.; Park, Y. The bioactive effects of chicoric acid as a functional food ingredient. J. Med. Food 2019, 22, 645–652. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Shigi, K.; Fukuda, K. Reactive oxygen species induce Cox-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio 2015, 5, 492–501. [Google Scholar] [CrossRef]
- De Leo, M.; Iannuzzi, A.M.; Germanò, M.P.; D’Angelo, V.; Camangi, F.; Sevi, F.; Diretto, G.; De Tommasi, N.; Braca, A. Comparative chemical analysis of six ancient Italian sweet cherry (Prunus avium L.) varieties showing antiangiogenic activity. Food Chem. 2021, 360, 129999. [Google Scholar] [CrossRef]
- Cioni, E.; De Leo, M.; Cacciola, A.; D’Angelo, V.; Germanò, M.P.; Camangi, F.; Ricci, D.; Fabene, D.; Diretto, G.; De Tommasi, N.; et al. Re-discovering Prunus fruit varieties as antiangiogenic agents by metabolomic and bioinformatic approach. Food Chem. 2024, 435, 137574. [Google Scholar] [CrossRef] [PubMed]





| N. a | Compound | tR (min) | [M − H]− | Product Ions b | Formula | Error |
|---|---|---|---|---|---|---|
| Hydroxycinnamic acids | ||||||
| 3 | Caftaric acid | 2.6 | 311.0403 | 292.89, 274.88, 179.03, 149.01 | C13H12O9 | −1.46 |
| 5 | Caffeic acid hexoside | 3.6 | 341.0872 | 179.03, 135.05 | C15H18O9 | −1.79 |
| 6 | Chlorogenic acid (isomer I) c | 4.7 | 353.0879 | 191.05, 173.04 | C16H18O9 | +0.25 |
| 9 | Chlorogenic acid (isomer II) | 6.3 | 353.0879 | 191.05, 173.04 | C16H18O9 | +0.25 |
| 11 | Feruloylquinic acid | 7.9 | 367.1027 | 191.06, 173.04 | C17H20O9 | −2.04 |
| 12 | Chicoric acid (isomer I) c | 8.2 | 473.0717 | 311.04, 293.03, 179.03, 149.01 | C22H18O12 | −1.80 |
| 14 | Chicoric acid (isomer II) | 8.9 | 473.0717 | 311.04, 293.03, 179.03, 149.01 | C22H18O12 | −1.80 |
| 19 | Dicaffeoylquinic acid (isomer I) | 9.9 | 515.1186 | 353.09, 191.06, 173.04 | C25H24O12 | −1.73 |
| 26 | Caffeoylferuloyltartaric acid (isomer I) | 10.8 | 487.0873 | 325.05, 293.03, 193.05, 179.03, 112.99 | C23H20O12 | −1.83 |
| 27 | Dicaffeoylquinic acid (isomer II) | 11.1 | 515.1186 | 353.09, 191.06, 173.04 | C25H24O12 | −1.73 |
| 32 | Caffeoylferuloyltartaric acid (isomer II) | 12.7 | 487.0873 | 325.05, 293.03, 193.05, 179.03, 163.02, 112.99 | C23H20O12 | −1.83 |
| Flavonoids | ||||||
| 13 | Kaempferol/Luteolin O-dihexoside | 8.4 | 609.1454 | 447.09, 285.04 | C27H30O16 | −1.15 |
| 17 | Rutin | 9.2 | 609.1456 | 300.02 | C27H30O16 | −0.84 |
| 18 | Quercetin hexoside | 9.4 | 463.0877 | 300.02 | C21H20O12 | −1.08 |
| 20 | Luteolin 7-O-rutinoside c | 9.9 | 593.1505 | 447.09, 285.04 | C27H30O15 | −1.16 |
| 21 | Luteolin/Kaempferol hexoside (isomer I) | 9.9 | 447.0926 | 285.04 | C21H20O11 | −1.52 |
| 22 | Kaempferol/Luteolin O-uronide | 10.2 | 461.0720 | 285.04 | C21H18O12 | −1.17 |
| 25 | Kaempferol 3-O-glucoside c | 10.5 | 447.0926 | 285.04 | C21H20O11 | −1.52 |
| 28 | Apigenin hexoside | 11.4 | 431.0977 | 269.04 | C21H20O10 | −1.55 |
| 29 | Apigenin uronide | 11.7 | 445.0770 | 269.04 | C21H18O11 | −1.41 |
| 30 | Luteolin/Kaempferol hexoside (isomer II) | 12.1 | 447.0926 | 285.04 | C21H20O11 | −1.52 |
| 31 | Luteolin c | 12.5 | 285.0406 | C15H10O6 | +1.96 | |
| Megastigmane glucosides | ||||||
| 8 | Alangionoside E c | 6.2 | 433.2069 [M + HCOO]− | 387.20, 165.04 | C19H32O8 | −2.25 |
| 10 | Plucheoside B c | 6.8 | 433.2069 [M + HCOO]− | 387.20, 161.04, 113.02, 101.02 | C19H32O8 | −2.25 |
| 23 | Debiloside C c | 10.2 | 405.1762 | 243.12, 225.12, 181.23, 163.12 | C18H30O10 | −1.04 |
| Cumarins | ||||||
| 7 | Cichoriin c | 5.3 | 339.0715 | 177.02 | C15H16O9 | −1.77 |
| Lignans | ||||||
| 15 | Secoisolariciresinol glucoside c | 8.9 | 523.2173 | 361.17 | C26H36O11 | −2.25 |
| 24 | Secoisolariciresinol glucoside isomer | 10.2 | 569.2231 [M + HCOO]− | 361.17 | C26H36O11 | −1.51 |
| Monoterpenes | ||||||
| 16 | Loliolide d | 9.0 | 197.1167 [M + H]+ | 179.11, 161.10, 135.12 | C11H16O3 | −2.64 |
| Primary metabolites | ||||||
| 1 | Hexosylglutamic acid | 0.8 | 290.0879 | 290.09, 272.07, 254.07, 230.07, 200.06, 128.03 | C11H17O8N | −0.83 |
| 2 | Adenosine c,d | 1.1 | 268.1032 [M + H]+ | 136.06 | C10H13N5O4 | −3.10 |
| 4 | Tryptophan | 2.8 | 203.0819 | 186.05, 159.09, 142.06, 116.05 | C11H12O2N2 | −3.45 |
| Fatty acids | ||||||
| 33 | Trihydroxyoctadecadienoic acid | 14.5 | 327.2170 | 327.21, 309.21, 291.20, 229.14, 211.13 | C18H32O5 | −2.14 |
| 34 | Trihydroxyoctadecenoic acid | 15.1 | 329.2329 | 329.23, 311.22, 293.21 | C18H34O5 | −1.37 |
| 35 | Dodecenoic acid | 15.5 | 227.1287 | 209.12, 183.34 | C12H20O4 | −0.79 |
| 36 | Dihydroxyhexadecanoic acid | 15.8 | 287.2223 | 269.21 | C16H32O4 | −1.67 |
| 37 | Dioxooctadecadienoic acid isomer I | 17.1 | 307.1910 | 289.18, 260.96, 235.14 | C18H28O4 | −1.56 |
| 38 | Dioxooctadecadienoic acid isomer II | 17.6 | 307.1910 | 289.18, 260.96, 235.14 | C18H28O4 | −1.56 |
| 39 | Dihydroxyoctadecatrienoic acid | 17.8 | 309.2066 | 309.21, 291.20, 273.18 | C18H30O4 | −1.71 |
| 40 | Dihydroxyoctadecenoic acid | 18.1 | 313.2380 | 313.24, 295.22, 277.22 | C18H34O4 | −1.37 |
| 41 | Dioxooctadecatrienoic acid | 18.2 | 305.1752 | 287.16, 249.15, 135.08 | C18H26O4 | −2.06 |
| 42 | Dihydroxyoctadecadienoic acid | 18.5 | 311.2223 | 311.22, 293.21, 274.88 | C18H32O4 | −1.54 |
| 43 | Hydroxyoctadecatrienoic acid isomer I | 19.1 | 293.2116 | 293.21, 275.20, 249.16, 183.14 | C18H30O3 | −2.11 |
| 44 | Hydroxyoctadecatrienoic acid isomer II | 19.3 | 293.2116 | 293.21, 275.20, 249.16, 183.14 | C18H30O3 | −2.11 |
| 45 | Hydroxyoctadecadienoic acid | 19.7 | 295.2272 | 295.23, 277.22, 259.21, 195.14 | C18H32O3 | −2.23 |
| 46 | Oxooctadecatrienoic acid | 20.1 | 291.1989 | 273.18 | C18H28O3 | −0.69 |
| 47 | Octadecatrienoic acid | 21.5 | 277.2168 | 259.21 | C18H30O2 | −1.80 |
| 48 | Octadecadienoic acid | 21.8 | 279.2323 | 261.22 | C18H32O2 | −1.97 |
| Peak | Compound | mg/g DW ± SD |
|---|---|---|
| Flavonoids | ||
| 18 | Quercetin hexoside | 0.695 ± 0.1 |
| 20 | Luteolin 7-O-rutinoside | 0.576 ± 0.06 |
| 21 | Luteolin/Kaempferol hexoside (isomer I) | 2.31 ± 0.06 |
| 22 | Luteolin/Kaempferol uronide | 2.60 ± 0.02 |
| 30 | Luteolin/Kaempferol hexoside (isomer II) | 0.120 ± 0.01 |
| 31 | Luteolin | 2.15 ± 0.03 |
| Hydroxycinnamic acids | ||
| 3 | Caftaric acid | 0.126 ± 0.004 |
| 5 | Caffeic acid hexoside | 0.102 ± 0.007 |
| 6 + 9 | Chlorogenic acid | 0.936 ± 0.04 |
| 12 | Chicoric acid | 2.62 ± 0.04 |
| 19 + 27 | Dicaffeoylquinic acid | 0.628 ± 0.04 |
| 26 | Caffeoylferuloyltartaric acid | 0.0482 ± 0.01 |
| Total flavonoids | 8.45 ± 0.3 | |
| Total hydroxycinnamic acids | 4.46 ± 0.1 | |
| Total | 12.9 ± 0.4 | |
| Compound | Chemical Shift (ppm) Multiplicity (J in Hz) | Identification Confirmation | MSI Status a |
|---|---|---|---|
| Valine | 0.99 d (J = 7.4), 1.04 d (J = 7.4) | Spike, HSQC | 1 |
| Threonine | 1.33 (J = 6.8) | Spike, HSQC | 1 |
| Alanine | 1.48 d (J = 7.2) | Spike, HSQC | 1 |
| Arginine | 1.54 m | Spike, HSQC | 1 |
| Proline | 2.03 m, 2.35 m | Spike, HSQC | 1 |
| Asparagine | 2.85 dd (J = 16.7; 7.3), 2.96 dd (J = 16.2; 4.0) | Spike, HSQC | 1 |
| Choline | 3.20 s | HSQC | 2 |
| α-Glucose | 5.16 d (J = 4.40) | HSQC | 2 |
| β-Glucose | 4.54 d (J = 7.95) | HSQC | 2 |
| Sucrose | 5.40 d (J = 3.80) | Spike, HSQC | 1 |
| Fructose | 4.01 m | Spike, HSQC | 1 |
| α-Galactose | 5.20 d (J = 3.90) | HSQC | 2 |
| Chlorogenic acid | 7.64 d (J = 16.0), 6.38 d (J = 16.0) | HSQC | 2 |
| Phenylalanine | 7.37 m | HSQC | 2 |
| Chicoric acid | 7.71 d (J = 17.0), 6.47 d (J = 17.0), 7.23 s, 6.90 d (J = 8.0) | HSQC | 2 |
| Trigonelline | 9.13 s, 8.07 t | HSQC | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitiello, M.; Pecoraro, M.; De Leo, M.; Camangi, F.; Parisi, V.; Donadio, G.; Braca, A.; Franceschelli, S.; De Tommasi, N. Chemical Profiling, Antioxidant, and Anti-Inflammatory Activities of Hyoseris radiata L., a Plant Used in the Phytoalimurgic Tradition. Antioxidants 2024, 13, 111. https://doi.org/10.3390/antiox13010111
Vitiello M, Pecoraro M, De Leo M, Camangi F, Parisi V, Donadio G, Braca A, Franceschelli S, De Tommasi N. Chemical Profiling, Antioxidant, and Anti-Inflammatory Activities of Hyoseris radiata L., a Plant Used in the Phytoalimurgic Tradition. Antioxidants. 2024; 13(1):111. https://doi.org/10.3390/antiox13010111
Chicago/Turabian StyleVitiello, Maria, Michela Pecoraro, Marinella De Leo, Fabiano Camangi, Valentina Parisi, Giuliana Donadio, Alessandra Braca, Silvia Franceschelli, and Nunziatina De Tommasi. 2024. "Chemical Profiling, Antioxidant, and Anti-Inflammatory Activities of Hyoseris radiata L., a Plant Used in the Phytoalimurgic Tradition" Antioxidants 13, no. 1: 111. https://doi.org/10.3390/antiox13010111
APA StyleVitiello, M., Pecoraro, M., De Leo, M., Camangi, F., Parisi, V., Donadio, G., Braca, A., Franceschelli, S., & De Tommasi, N. (2024). Chemical Profiling, Antioxidant, and Anti-Inflammatory Activities of Hyoseris radiata L., a Plant Used in the Phytoalimurgic Tradition. Antioxidants, 13(1), 111. https://doi.org/10.3390/antiox13010111










