ERK2 Is a Promoter of Cancer Cell Growth and Migration in Colon Adenocarcinoma
Abstract
1. Introduction
2. Methods
2.1. Tissue Staining
2.2. Mice
2.3. Cell Lines
2.4. Gene Expression Analysis
2.5. Western Blot Analysis
2.6. Pull-Down Assay for MEF SOD3 Clones
2.7. Growth Analysis
2.8. Matrigel Migration
2.9. Wound Healing
2.10. Limitations of the Study
2.11. Statistical Analysis
3. Results
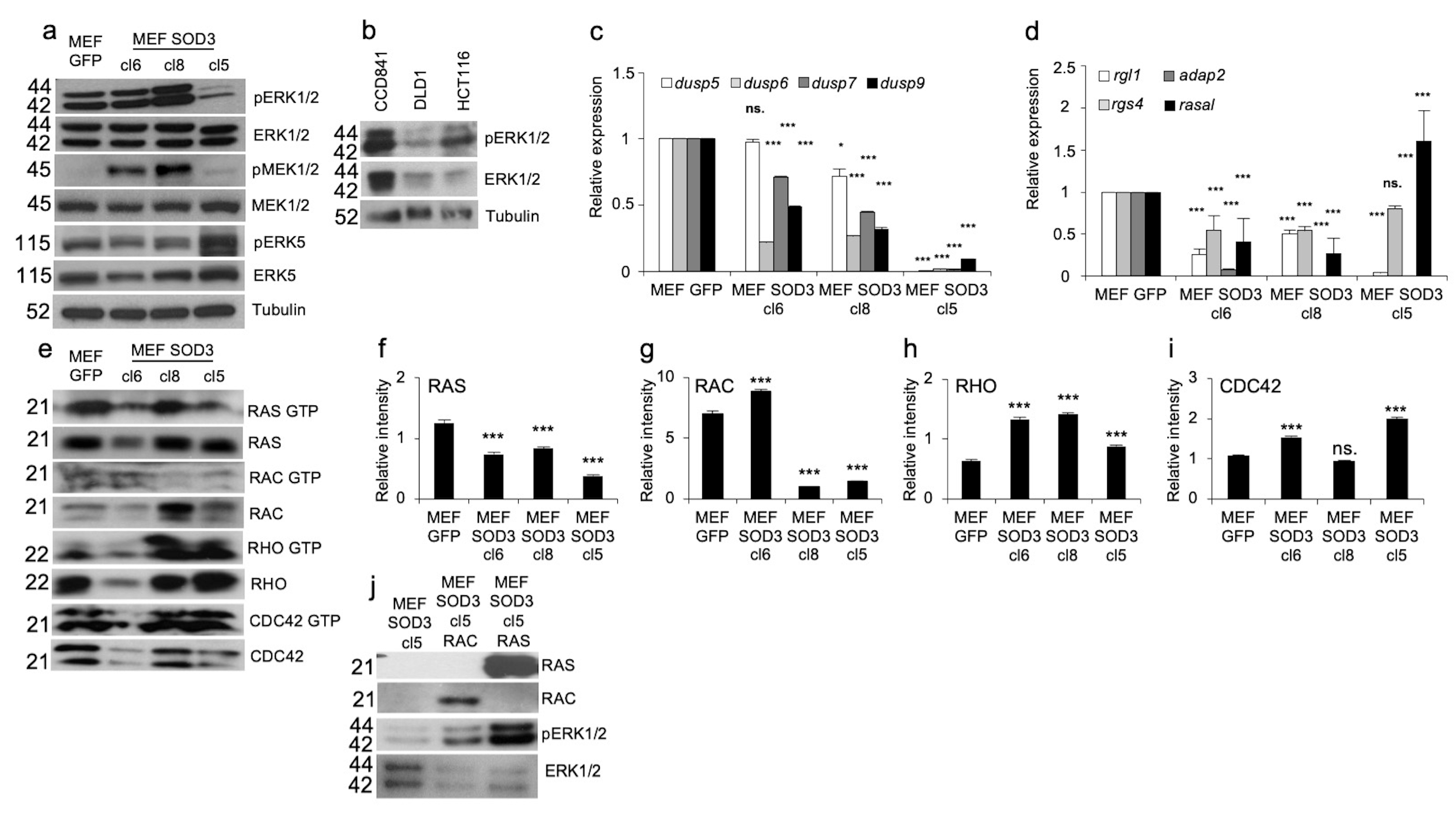
3.1. Inverse Correlation of ERK/2 Phosphorylation and Progression of Colon Tumorigenesis
3.2. ERK1 and ERK2 Expression In Vitro Models
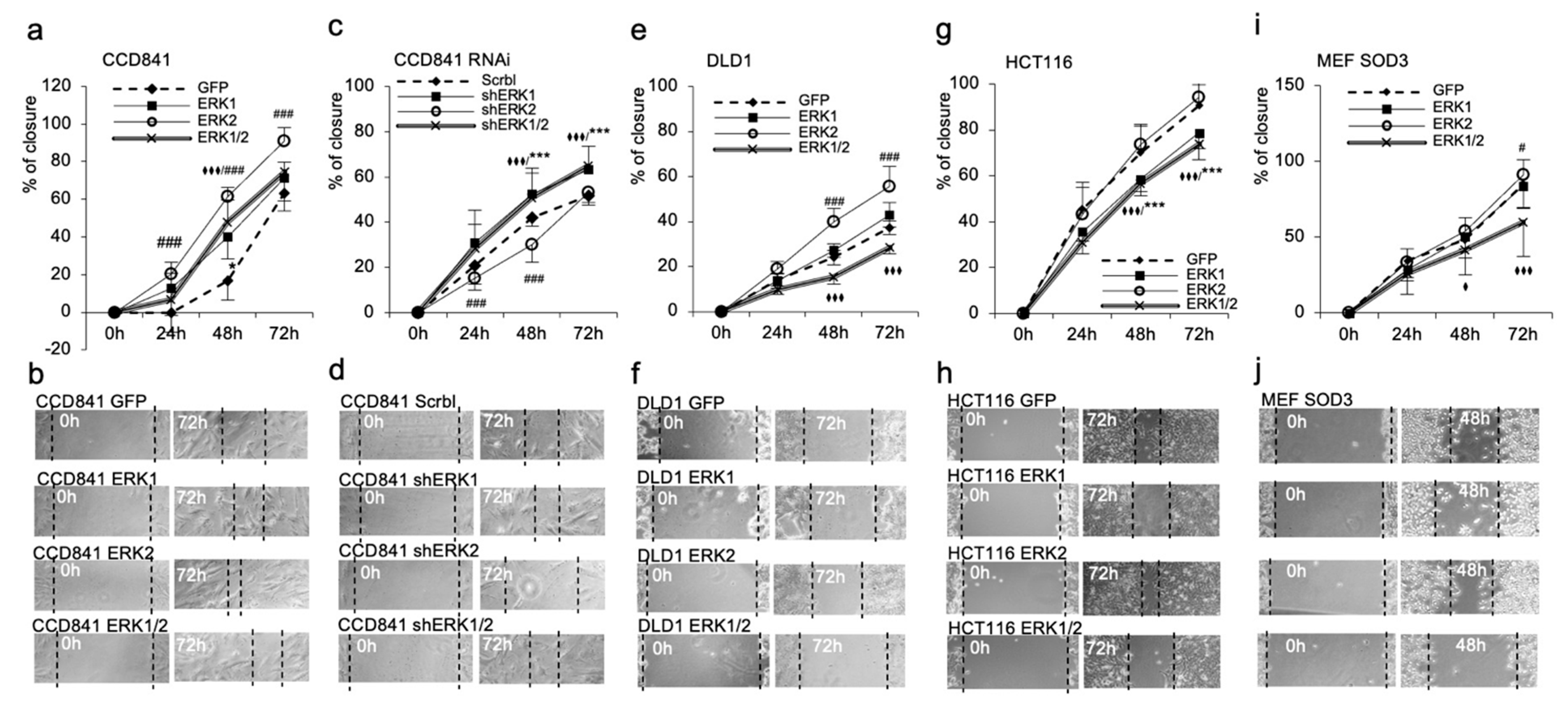
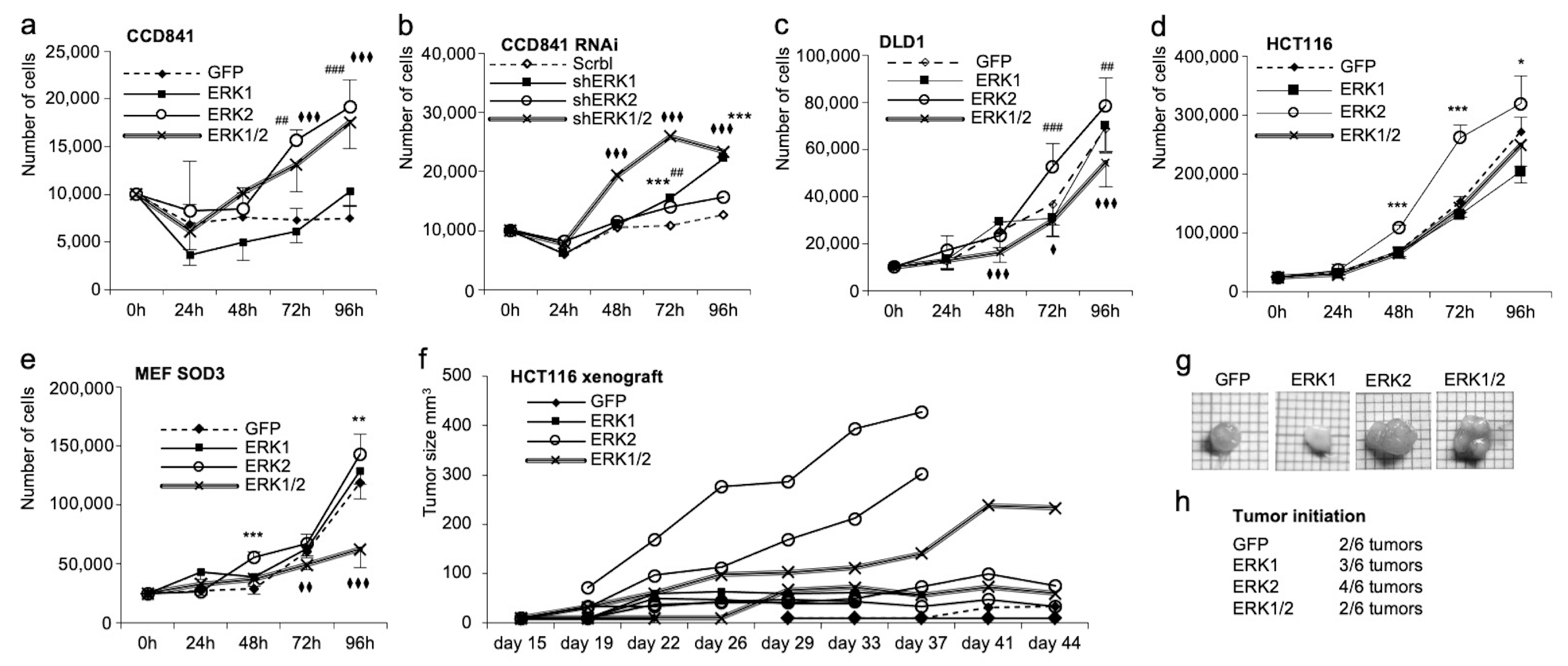
3.3. ERK1 and ERK2 Have Different Functions in Cell Migration and Proliferation In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koul, H.K.; Pal, M.; Koul, S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer 2013, 4, 342–359. [Google Scholar] [CrossRef]
- Del Reino, P.; Alsina-Beauchamp, D.; Escos, A.; Cerezo-Guisado, M.I.; Risco, A.; Aparicio, N.; Zur, R.; Fernandez-Estevez, M.; Collantes, E.; Montans, J.; et al. Pro-oncogenic role of alternative p38 mitogen-activated protein kinases p38gamma and p38delta, linking inflammation and cancer in colitis-associated colon cancer. Cancer Res. 2014, 74, 6150–6160. [Google Scholar] [CrossRef]
- Pattingre, S.; Bauvy, C.; Codogno, P. Amino acids interfere with the ERK1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J. Biol. Chem. 2003, 278, 16667–16674. [Google Scholar] [CrossRef] [PubMed]
- Balmanno, K.; Cook, S.J. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009, 16, 368–377. [Google Scholar] [CrossRef]
- Andreyev, H.J.; Norman, A.R.; Cunningham, D.; Oates, J.; Dix, B.R.; Iacopetta, B.J.; Young, J.; Walsh, T.; Ward, R.; Hawkins, N.; et al. Kirsten ras mutations in patients with colorectal cancer: The ‘RASCAL II’ study. Br. J. Cancer 2001, 85, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Wortzel, I.; Seger, R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer 2011, 2, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.K.; Becker, A.; Park, J.I. Growth Inhibitory Signaling of the Raf/MEK/ERK Pathway. Int. J. Mol. Sci. 2020, 21, 5436. [Google Scholar] [CrossRef]
- Hacohen Lev-Ran, A.; Seger, R. Retention of ERK in the cytoplasm mediates the pluripotency of embryonic stem cells. Stem Cell Rep. 2023, 18, 305–318. [Google Scholar] [CrossRef]
- Lemieux, E.; Boucher, M.J.; Mongrain, S.; Boudreau, F.; Asselin, C.; Rivard, N. Constitutive activation of the MEK/ERK pathway inhibits intestinal epithelial cell differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G719–G730. [Google Scholar] [CrossRef]
- Guo, D.; Zhou, H.; Wu, Y.; Zhou, F.; Xu, G.; Wen, H.; Zhang, X. Involvement of ERK1/2/NF-kappaB signal transduction pathway in TF/FVIIa/PAR2-induced proliferation and migration of colon cancer cell SW620. Tumor Biol. 2011, 32, 921–930. [Google Scholar] [CrossRef]
- Lake, D.; Correa, S.A.; Muller, J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell. Mol. Life Sci. 2016, 73, 4397–4413. [Google Scholar] [CrossRef] [PubMed]
- Leikam, C.; Hufnagel, A.; Schartl, M.; Meierjohann, S. Oncogene activation in melanocytes links reactive oxygen to multinucleated phenotype and senescence. Oncogene 2008, 27, 7070–7082. [Google Scholar] [CrossRef] [PubMed]
- Mitsushita, J.; Lambeth, J.D.; Kamata, T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004, 64, 3580–3585. [Google Scholar] [CrossRef] [PubMed]
- Parascandolo, A.; Laukkanen, M.O. Carcinogenesis and Reactive Oxygen Species Signaling: Interaction of the NADPH Oxidase NOX1-5 and Superoxide Dismutase 1-3 Signal Transduction Pathways. Antioxid. Redox Signal. 2019, 30, 443–486. [Google Scholar] [CrossRef] [PubMed]
- Laurila, J.P.; Castellone, M.D.; Curcio, A.; Laatikainen, L.E.; Haaparanta-Solin, M.; Gronroos, T.J.; Marjamaki, P.; Martikainen, S.; Santoro, M.; Laukkanen, M.O. Extracellular superoxide dismutase is a growth regulatory mediator of tissue injury recovery. Mol. Ther. 2009, 17, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, M.O.; Cammarota, F.; Esposito, T.; Salvatore, M.; Castellone, M.D. Extracellular superoxide dismutase regulates the expression of small gtpase regulatory proteins GEFs, GAPs, and GDI. PLoS ONE 2015, 10, e0121441. [Google Scholar] [CrossRef]
- Cammarota, F.; Fiscardi, F.; Esposito, T.; de Vita, G.; Salvatore, M.; Laukkanen, M.O. Clinical relevance of thyroid cell models in redox research. Cancer Cell Int. 2015, 15, 113. [Google Scholar] [CrossRef]
- Van Emburgh, B.O.; Sartore-Bianchi, A.; Di Nicolantonio, F.; Siena, S.; Bardelli, A. Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol. Oncol. 2014, 8, 1084–1094. [Google Scholar] [CrossRef]
- Eggstein, S.; Franke, M.; Kutschka, I.; Manthey, G.; von Specht, B.U.; Ruf, G.; Farthmann, E.H. Expression and activity of mitogen activated protein kinases in human colorectal carcinoma. Gut 1999, 44, 834–838. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, N.G.; Kim, J.J.; Kim, H.; Ahn, Y.H.; Choi, K.Y. Differential regulation of MAP kinase cascade in human colorectal tumorigenesis. Br. J. Cancer 1999, 81, 1116–1121. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Ding, Q.; Dong, Z.; Ehlers, R.A.; Evers, B.M. Downregulation of mitogen-activated protein kinases in human colon cancers. Anticancer Res. 2000, 20, 75–83. [Google Scholar] [PubMed]
- de Jong, P.R.; Taniguchi, K.; Harris, A.R.; Bertin, S.; Takahashi, N.; Duong, J.; Campos, A.D.; Powis, G.; Corr, M.; Karin, M.; et al. ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation. Nat. Commun. 2016, 7, 11551. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Gomes, S.E.; Borralho, P.M.; Rodrigues, C.M.P. MEK5/ERK5 activation regulates colon cancer stem-like cell properties. Cell Death Discov. 2019, 5, 68. [Google Scholar] [CrossRef]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Castellone, M.D.; Langella, A.; Cantara, S.; Laurila, J.P.; Laatikainen, L.E.; Bellelli, R.; Pacini, F.; Salvatore, M.; Laukkanen, M.O. Extracellular superoxide dismutase induces mouse embryonic fibroblast proliferative burst, growth arrest, immortalization, and consequent in vivo tumorigenesis. Antioxid. Redox Signal. 2014, 21, 1460–1474. [Google Scholar] [CrossRef]
- Tavaluc, R.T.; Hart, L.S.; Dicker, D.T.; El-Deiry, W.S. Effects of low confluency, serum starvation and hypoxia on the side population of cancer cell lines. Cell Cycle 2007, 6, 2554–2562. [Google Scholar] [CrossRef]
- Kai, K.; Nagano, O.; Sugihara, E.; Arima, Y.; Sampetrean, O.; Ishimoto, T.; Nakanishi, M.; Ueno, N.T.; Iwase, H.; Saya, H. Maintenance of HCT116 colon cancer cell line conforms to a stochastic model but not a cancer stem cell model. Cancer Sci. 2009, 100, 2275–2282. [Google Scholar] [CrossRef]
- Rajput, A.; Dominguez San Martin, I.; Rose, R.; Beko, A.; Levea, C.; Sharratt, E.; Mazurchuk, R.; Hoffman, R.M.; Brattain, M.G.; Wang, J. Characterization of HCT116 human colon cancer cells in an orthotopic model. J. Surg. Res. 2008, 147, 276–281. [Google Scholar] [CrossRef]
- Parascandolo, A.; Laukkanen, M.O. SOD3 Is a Non-Mutagenic Growth Regulator Affecting Cell Migration and Proliferation Signal Transduction. Antioxidants 2021, 10, 635. [Google Scholar] [CrossRef]
- Boulton, T.G.; Nye, S.H.; Robbins, D.J.; Ip, N.Y.; Radziejewska, E.; Morgenbesser, S.D.; DePinho, R.A.; Panayotatos, N.; Cobb, M.H.; Yancopoulos, G.D. ERKs: A family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 1991, 65, 663–675. [Google Scholar] [CrossRef]
- Oshikawa, J.; Urao, N.; Kim, H.W.; Kaplan, N.; Razvi, M.; McKinney, R.; Poole, L.B.; Fukai, T.; Ushio-Fukai, M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS ONE 2010, 5, e10189. [Google Scholar] [CrossRef]
- Friedl, P.; Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009, 10, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Krantz, S.B.; You, M.; Zhao, Z. Synergistic activation of MAP kinase (ERK1/2) by erythropoietin and stem cell factor is essential for expanded erythropoiesis. Blood 1998, 92, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.R.; Kwan, Y.W.; Cho, C.K.; Bae, S.; Lee, S.J.; Soh, J.W.; Chung, H.Y.; Lee, Y.S. PKCalpha induces differentiation through ERK1/2 phosphorylation in mouse keratinocytes. Exp. Mol. Med. 2004, 36, 292–299. [Google Scholar] [CrossRef]
- Cammarota, F.; de Vita, G.; Salvatore, M.; Laukkanen, M.O. Ras oncogene-mediated progressive silencing of extracellular superoxide dismutase in tumorigenesis. BioMed Res. Int. 2015, 2015, 780409. [Google Scholar] [CrossRef] [PubMed]
- Irani, K.; Xia, Y.; Zweier, J.L.; Sollott, S.J.; Der, C.J.; Fearon, E.R.; Sundaresan, M.; Finkel, T.; Goldschmidt-Clermont, P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 1997, 275, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Sarkisian, C.J.; Keister, B.A.; Stairs, D.B.; Boxer, R.B.; Moody, S.E.; Chodosh, L.A. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 2007, 9, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Courtney, M.; Laukkanen, M.O. Redox-Activated Signal Transduction Pathways Mediating Cellular Functions in Inflammation, Differentiation, Degeneration, Transformation, and Death. Oxid. Med. Cell Longev. 2016, 2016, 8479718. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Zeidler, J.D.; Kashyap, S.; Warner, G.; Chini, E.N. Evolving concepts in NAD(+) metabolism. Cell Metab. 2021, 33, 1076–1087. [Google Scholar] [CrossRef]
- Lee, B.W.L.; Ghode, P.; Ong, D.S.T. Redox regulation of cell state and fate. Redox Biol. 2019, 25, 101056. [Google Scholar] [CrossRef]
- Laukkanen, M.O.; Leppanen, P.; Turunen, P.; Porkkala-Sarataho, E.; Salonen, J.T.; Yla-Herttuala, S. Gene transfer of extracellular superoxide dismutase to atherosclerotic mice. Antioxid. Redox. Signal. 2001, 3, 397–402. [Google Scholar] [CrossRef]
- Hatano, N.; Mori, Y.; Oh-hora, M.; Kosugi, A.; Fujikawa, T.; Nakai, N.; Niwa, H.; Miyazaki, J.; Hamaoka, T.; Ogata, M. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells 2003, 8, 847–856. [Google Scholar] [CrossRef]
- Di Benedetto, B.; Hitz, C.; Holter, S.M.; Kuhn, R.; Vogt Weisenhorn, D.M.; Wurst, W. Differential mRNA distribution of components of the ERK/MAPK signalling cascade in the adult mouse brain. J. Comp. Neurol. 2007, 500, 542–556. [Google Scholar] [CrossRef]
- Kondoh, K.; Torii, S.; Nishida, E. Control of MAP kinase signaling to the nucleus. Chromosoma 2005, 114, 86–91. [Google Scholar] [CrossRef]
- Casar, B.; Pinto, A.; Crespo, P. Essential role of ERK dimers in the activation of cytoplasmic but not nuclear substrates by ERK-scaffold complexes. Mol. Cell 2008, 31, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, L.; Chng, W.J.; Ding, J.L. Comprehensive Analysis of ERK1/2 Substrates for Potential Combination Immunotherapies. Trends Pharmacol. Sci. 2019, 40, 897–910. [Google Scholar] [CrossRef]
- Whitehurst, A.W.; Robinson, F.L.; Moore, M.S.; Cobb, M.H. The death effector domain protein PEA-15 prevents nuclear entry of ERK2 by inhibiting required interactions. J. Biol. Chem. 2004, 279, 12840–12847. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Roux, D.; Lenormand, P.; Dowd, S.; Keyse, S.; Pouyssegur, J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999, 18, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Khokhlatchev, A.V.; Canagarajah, B.; Wilsbacher, J.; Robinson, M.; Atkinson, M.; Goldsmith, E.; Cobb, M.H. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 1998, 93, 605–615. [Google Scholar] [CrossRef]
- Tie, J.; Lipton, L.; Desai, J.; Gibbs, P.; Jorissen, R.N.; Christie, M.; Drummond, K.J.; Thomson, B.N.; Usatoff, V.; Evans, P.M.; et al. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin. Cancer Res. 2011, 17, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Urosevic, J.; Blasco, M.T.; Llorente, A.; Bellmunt, A.; Berenguer-Llergo, A.; Guiu, M.; Canellas, A.; Fernandez, E.; Burkov, I.; Clapes, M.; et al. ERK1/2 Signaling Induces Upregulation of ANGPT2 and CXCR4 to Mediate Liver Metastasis in Colon Cancer. Cancer Res. 2020, 80, 4668–4680. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parascandolo, A.; Benincasa, G.; Corcione, F.; Laukkanen, M.O. ERK2 Is a Promoter of Cancer Cell Growth and Migration in Colon Adenocarcinoma. Antioxidants 2024, 13, 119. https://doi.org/10.3390/antiox13010119
Parascandolo A, Benincasa G, Corcione F, Laukkanen MO. ERK2 Is a Promoter of Cancer Cell Growth and Migration in Colon Adenocarcinoma. Antioxidants. 2024; 13(1):119. https://doi.org/10.3390/antiox13010119
Chicago/Turabian StyleParascandolo, Alessia, Giulio Benincasa, Francesco Corcione, and Mikko O. Laukkanen. 2024. "ERK2 Is a Promoter of Cancer Cell Growth and Migration in Colon Adenocarcinoma" Antioxidants 13, no. 1: 119. https://doi.org/10.3390/antiox13010119
APA StyleParascandolo, A., Benincasa, G., Corcione, F., & Laukkanen, M. O. (2024). ERK2 Is a Promoter of Cancer Cell Growth and Migration in Colon Adenocarcinoma. Antioxidants, 13(1), 119. https://doi.org/10.3390/antiox13010119






