Diabetic Keratopathy: Redox Signaling Pathways and Therapeutic Prospects
Abstract
1. Introduction
2. Pathophysiology of Diabetic Keratopathy
2.1. Corneal Cell Damage in Diabetic Keratopathy
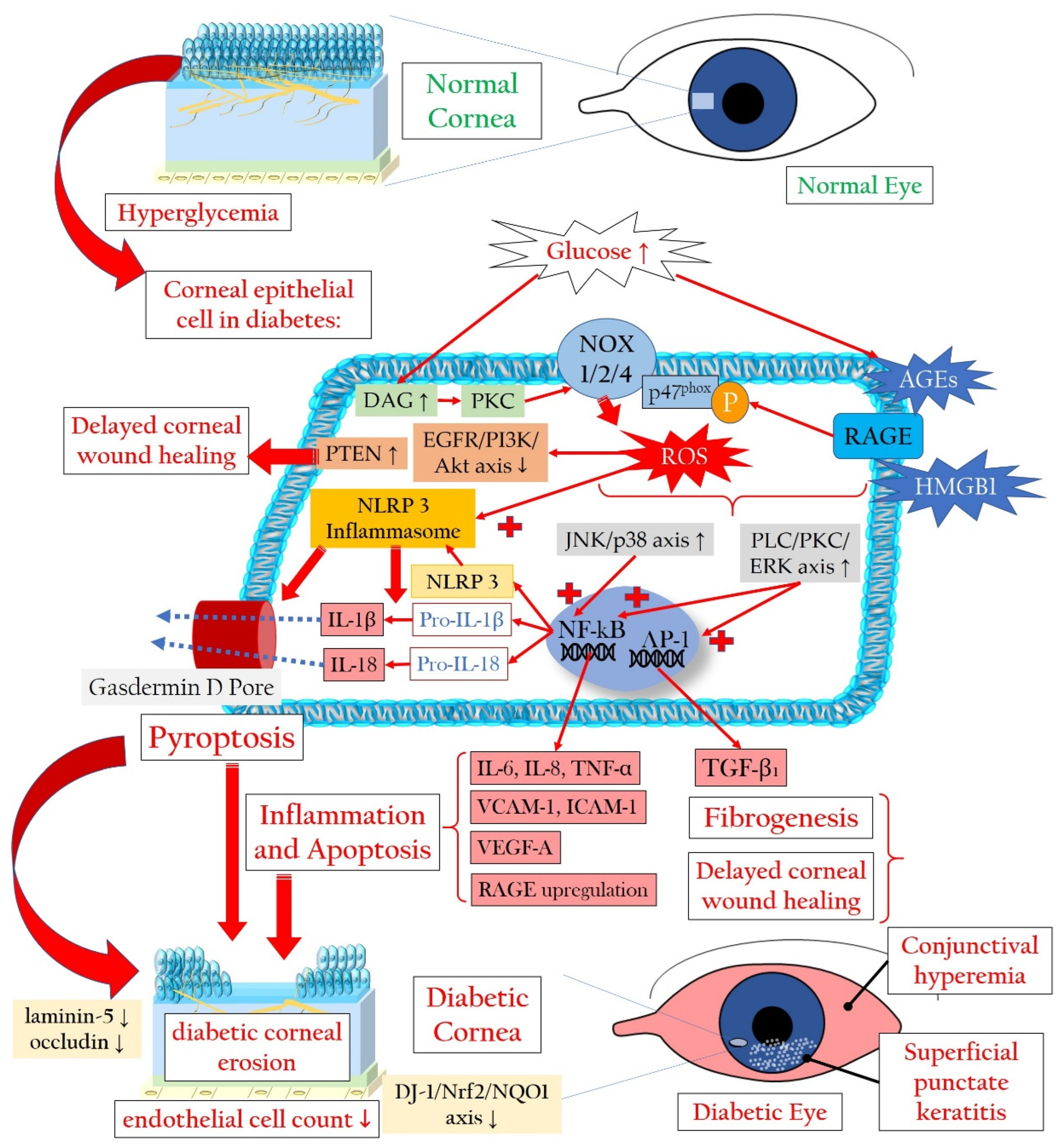
2.1.1. Redox Signaling in the Diabetic Cornea
2.1.2. Pyroptosis in the Corneal Epithelium Triggered by Hyperglycemia
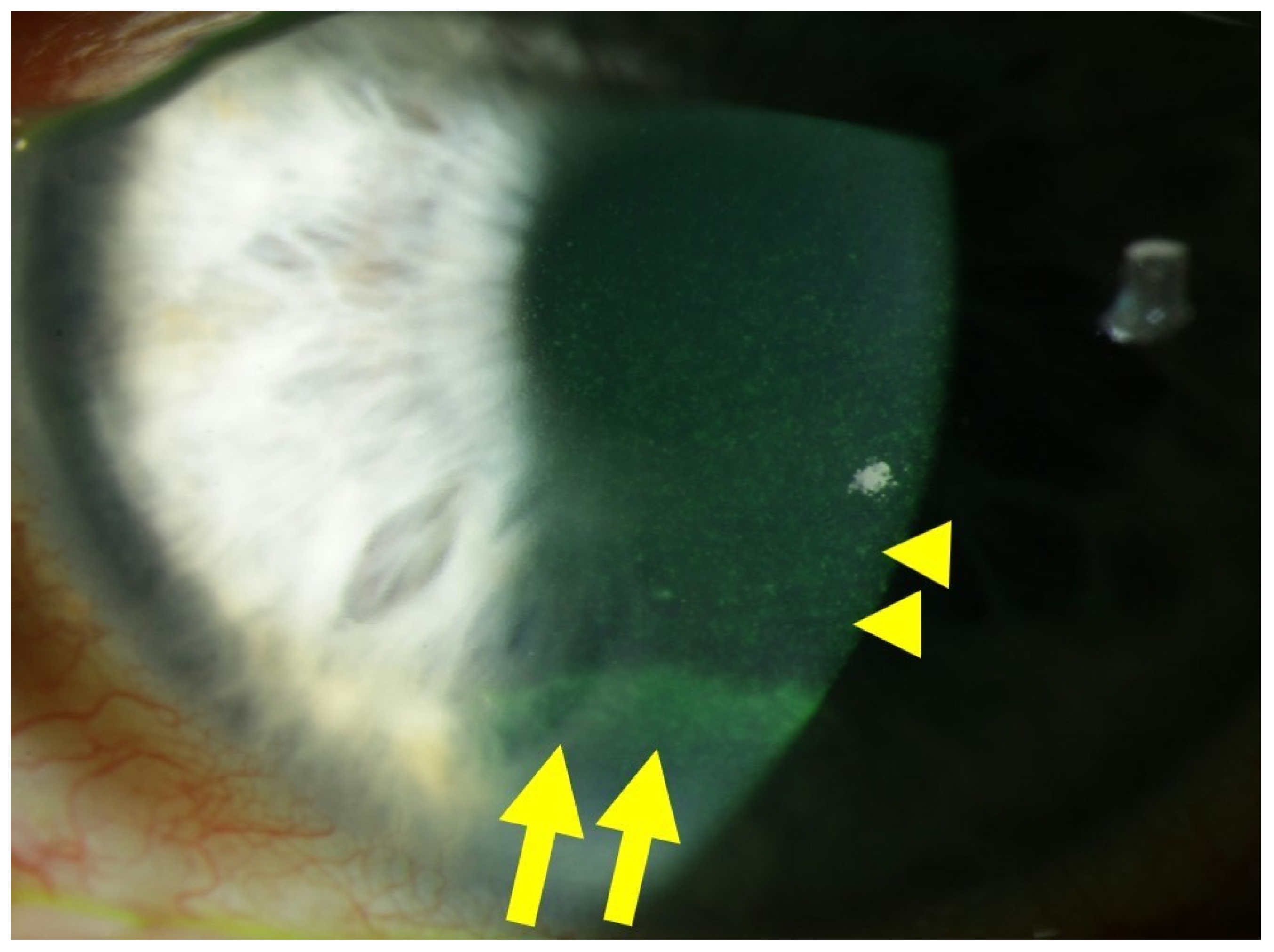
2.1.3. Involvement of Stroma, Endothelium, and Tight Junctions in Diabetic Damage
2.2. Diabetic Corneal Neuropathy
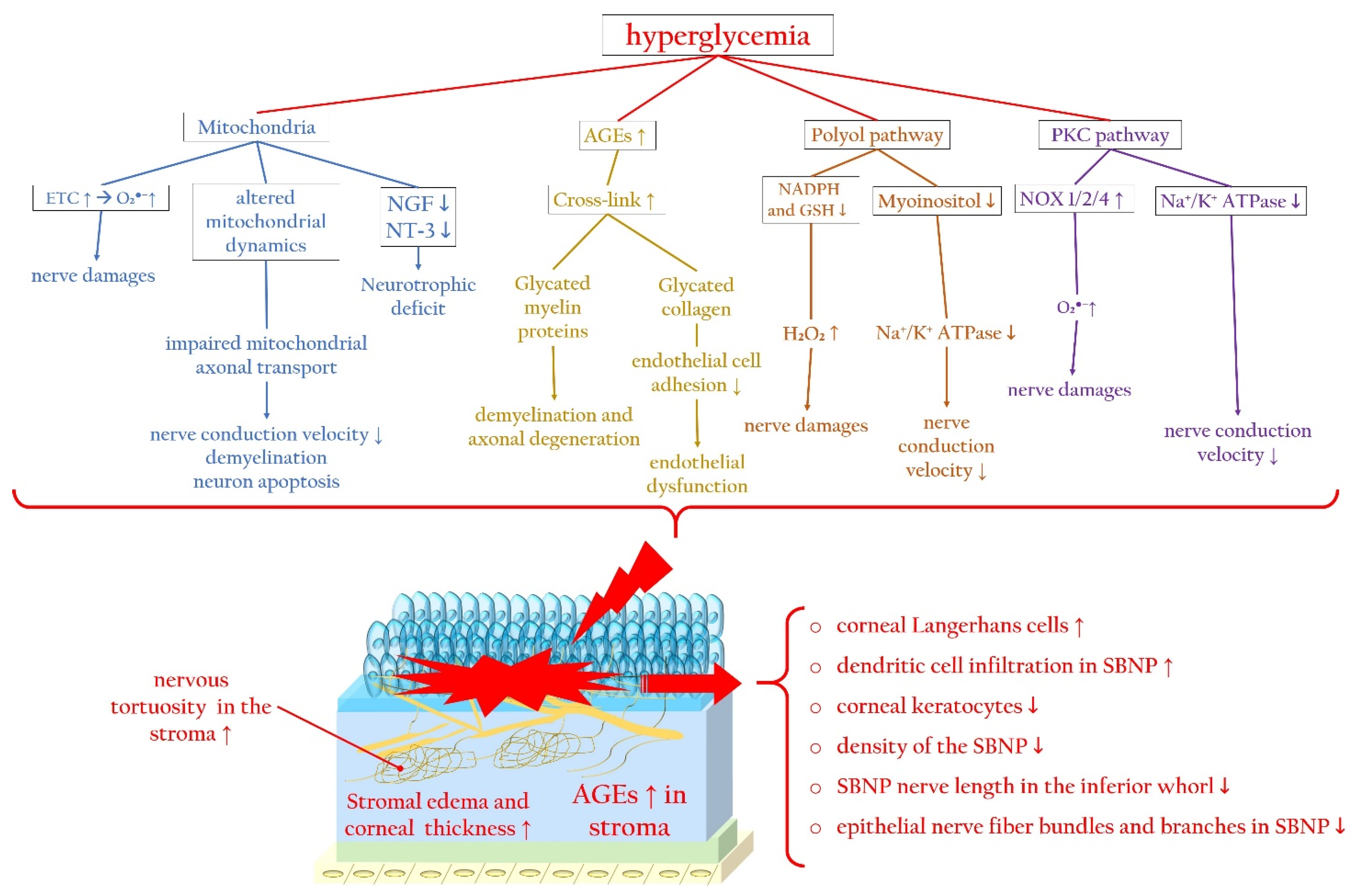
2.2.1. Evidence of Corneal Nerve Damage in Diabetes
2.2.2. Molecular Pathways Leading to Corneal Nerve Damage
Hyperglycemia-Related Mitochondrial Dysfunction
AGEs and Axonal Degeneration
Effects of the Polyol Pathway and PKC Cascade
2.3. Diabetes-Associated Dry Eye Disease Promotes Oxidatives Stress and Inflammation
3. Innovative Antioxidative Approaches for Diabetic Keratopathy
3.1. Targeting Pyroptosis in Diabetic Keratopathy
3.2. Targeting Nrf2 and SIRT1 in Diabetic Keratopathy
3.3. Counteracting Oxidative Stress and Inflammation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, L.; Deng, S.; Sun, X.; Wang, N. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J. Ophthalmol. 2016, 2016, 8201053. [Google Scholar] [CrossRef] [PubMed]
- Manaviat, M.R.; Rashidi, M.; Afkhami-Ardekani, M.; Shoja, M.R. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008, 8, 10. [Google Scholar] [CrossRef]
- Seifart, U.; Strempel, I. The dry eye and diabetes mellitus. Ophthalmologe 1994, 91, 235–239. [Google Scholar] [PubMed]
- Pan, L.Y.; Kuo, Y.K.; Chen, T.H.; Sun, C.C. Dry eye disease in patients with type II diabetes mellitus: A retrospective, population-based cohort study in Taiwan. Front. Med. 2022, 9, 980714. [Google Scholar] [CrossRef]
- Naik, K.; Magdum, R.; Ahuja, A.; Kaul, S.; Johnson, S.; Mishra, A.; Patil, M.; Dhore, D.N.; Alapati, A. Ocular Surface Diseases in Patients With Diabetes. Cureus 2022, 14, e23401. [Google Scholar] [CrossRef]
- Nepp, J.; Abela, C.; Polzer, I.; Derbolav, A.; Wedrich, A. Is there a correlation between the severity of diabetic retinopathy and keratoconjunctivitis sicca? Cornea 2000, 19, 487–491. [Google Scholar] [CrossRef]
- Weng, J.; Ross, C.; Baker, J.; Alfuraih, S.; Shamloo, K.; Sharma, A. Diabetes-Associated Hyperglycemia Causes Rapid-Onset Ocular Surface Damage. Invest. Ophthalmol. Vis. Sci. 2023, 64, 11. [Google Scholar] [CrossRef]
- Yetkin, E.; Aksoy Aydemir, G.; Bilen, A.; Pehlivanoglu, B.; Asik, A.; Kocaay, F.; Ozkoyuncu, D.; Aydemir, E. Evaluation of Dry Eye Disease Characteristics of Children With Type 1 and Type 2 Diabetes Mellitus and MODY. Eye Contact Lens 2023. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, L.; Zhu, C.; Nan, W.; Ding, X.; Dong, Y.; Zhao, M. The effect of diabetes on corneal endothelium: A meta-analysis. BMC Ophthalmol. 2021, 21, 78. [Google Scholar] [CrossRef]
- Mansoor, H.; Tan, H.C.; Lin, M.T.Y.; Mehta, J.S.; Liu, Y.C. Diabetic Corneal Neuropathy. J. Clin. Med. 2020, 9, 3956. [Google Scholar] [CrossRef] [PubMed]
- Kaji, Y. Prevention of diabetic keratopathy. Br. J. Ophthalmol. 2005, 89, 254–255. [Google Scholar] [PubMed]
- Bikbova, G.; Oshitari, T.; Tawada, A.; Yamamoto, S. Corneal changes in diabetes mellitus. Curr. Diabetes Rev. 2012, 8, 294–302. [Google Scholar]
- Lutty, G.A. Effects of diabetes on the eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF81–ORSF87. [Google Scholar]
- Gao, F.; Lin, T.; Pan, Y. Effects of diabetic keratopathy on corneal optical density, central corneal thickness, and corneal endothelial cell counts. Exp. Ther. Med. 2016, 12, 1705–1710. [Google Scholar] [CrossRef]
- Schultz, R.O.; Van Horn, D.L.; Peters, M.A.; Klewin, K.M.; Schutten, W.H. Diabetic keratopathy. Trans. Am. Ophthalmol. Soc. 1981, 79, 180–199. [Google Scholar]
- Priyadarsini, S.; Whelchel, A.; Nicholas, S.; Sharif, R.; Riaz, K.; Karamichos, D. Diabetic keratopathy: Insights and challenges. Surv. Ophthalmol. 2020, 65, 513–529. [Google Scholar] [CrossRef]
- Yeung, A.; Dwarakanathan, S. Diabetic keratopathy. Dis. Mon. 2021, 67, 101135. [Google Scholar] [CrossRef]
- Ljubimov, A.V. Diabetic complications in the cornea. Vis. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef]
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul. Surf. 2018, 16, 45–57. [Google Scholar] [PubMed]
- Murphy, P.J.; Patel, S.; Kong, N.; Ryder, R.E.; Marshall, J. Noninvasive assessment of corneal sensitivity in young and elderly diabetic and nondiabetic subjects. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1737–1742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mukhija, R.; Gupta, N.; Vashist, P.; Tandon, R.; Gupta, S.K. Population-based assessment of visual impairment and pattern of corneal disease: Results from the CORE (Corneal Opacity Rural Epidemiological) study. Br. J. Ophthalmol. 2020, 104, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Tai, M.-C.; Ho, C.-H.; Chu, C.-C.; Wang, J.-J.; Tseng, S.-H.; Jan, R.-L. Risk of Corneal Ulcer in Patients with Diabetes Mellitus: A Retrospective Large-Scale Cohort Study. Sci. Rep. 2020, 10, 7388. [Google Scholar] [CrossRef]
- Friend, J.; Snip, R.C.; Kiorpes, T.C.; Thoft, R.A. Insulin sensitivity and sorbitol production of the normal rabbit corneal epithelium in vitro. Investig. Ophthalmol. Vis. Sci. 1980, 19, 913–919. [Google Scholar]
- Cunha, D.A.; Carneiro, E.M.; Alves, M.d.C.; Jorge, A.G.; Sousa, S.M.d.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A.; Rocha, E.M. Insulin secretion by rat lachrymal glands: Effects of systemic and local variables. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, E768–E775. [Google Scholar] [CrossRef]
- Mueckler, M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes 1990, 39, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front. Endocrinol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Zhu, L.; Titone, R.; Robertson, D.M. The impact of hyperglycemia on the corneal epithelium: Molecular mechanisms and insight. Ocul. Surf. 2019, 17, 644–654. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Strokov, I.A.; Nosikov, V.V.; Savel’yeva, E.L.; Sitnikov, V.F.; Yegorov, Y.E.; Lankin, V.Z. The Role of Oxidative Stress in Diabetic Neuropathy: Generation of Free Radical Species in the Glycation Reaction and Gene Polymorphisms Encoding Antioxidant Enzymes to Genetic Susceptibility to Diabetic Neuropathy in Population of Type I Diabetic Patients. Cell Biochem. Biophys. 2015, 71, 1425–1443. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [PubMed]
- Ramya, R.; Coral, K.; Bharathidevi, S.R. RAGE silencing deters CML-AGE induced inflammation and TLR4 expression in endothelial cells. Exp. Eye Res. 2021, 206, 108519. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglio, F.; Böhm, E.W.; Pfeiffer, N.; Gericke, A. Oxidative Stress: A Suitable Therapeutic Target for Optic Nerve Diseases? Antioxidants 2023, 12, 1465. [Google Scholar]
- Böhm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.-S.; Sohn, E.; Jeong, I.-H.; Kim, H.; Kim, J.S. Involvement of advanced glycation end products, oxidative stress and nuclear factor-kappaB in the development of diabetic keratopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yu, X.; Yang, H.; Wu, X. Advanced Glycation End Products Induce Human Corneal Epithelial Cells Apoptosis through Generation of Reactive Oxygen Species and Activation of JNK and p38 MAPK Pathways. PLoS ONE 2013, 8, e66781. [Google Scholar] [CrossRef]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar]
- Chen, Y.-H.; Chen, Z.-W.; Li, H.-M.; Yan, X.-F.; Feng, B. AGE/RAGE-induced EMP release via the NOX-derived ROS pathway. J. Diabetes Res. 2018, 2018, 6823058. [Google Scholar] [CrossRef]
- Dobi, A.; Bravo, S.B.; Veeren, B.; Paradela-Dobarro, B.; Álvarez, E.; Meilhac, O.; Viranaicken, W.; Baret, P.; Devin, A.; Rondeau, P. Advanced glycation end-products disrupt human endothelial cells redox homeostasis: New insights into reactive oxygen species production. Free Radic. Res. 2019, 53, 150–169. [Google Scholar] [CrossRef]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.K.; Warnholtz, A.; et al. Mechanisms Underlying Endothelial Dysfunction in Diabetes Mellitus. Circ. Res. 2001, 88, e14–e22. [Google Scholar] [CrossRef]
- Giordo, R.; Nasrallah, G.K.; Posadino, A.M.; Galimi, F.; Capobianco, G.; Eid, A.H.; Pintus, G. Resveratrol-Elicited PKC Inhibition Counteracts NOX-Mediated Endothelial to Mesenchymal Transition in Human Retinal Endothelial Cells Exposed to High Glucose. Antioxidants 2021, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: A review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tao, M.; Zhao, Y.; Hu, X.; Wang, M. 4′-Methoxyresveratrol Alleviated AGE-Induced Inflammation via RAGE-Mediated NF-κB and NLRP3 Inflammasome Pathway. Molecules 2018, 23, 1447. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.P.; Li, Y.; Ljubimov, A.V.; Yu, F.S.X. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes 2009, 58, 1077–1085. [Google Scholar] [CrossRef]
- Taguchi, K.; Fukami, K. RAGE signaling regulates the progression of diabetic complications. Front. Pharmacol. 2023, 14, 1128872. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.M.; Albon, J.; Anderson, I.; Kamma, C.; Boulton, M. Differential Regulation of Key Stages in Early Corneal Wound Healing by TGF-β Isoforms and Their Inhibitors. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1886–1894. [Google Scholar] [CrossRef]
- Hou, Y.; Lan, J.; Zhang, F.; Wu, X. Expression profiles and potential corneal epithelial wound healing regulation targets of high-mobility group box 1 in diabetic mice. Exp. Eye Res. 2021, 202, 108364. [Google Scholar] [CrossRef]
- Wan, L.; Bai, X.; Zhou, Q.; Chen, C.; Wang, H.; Liu, T.; Xue, J.; Wei, C.; Xie, L. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int. J. Biol. Sci. 2022, 18, 809–825. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Wooff, Y.; Fernando, N.; Wong, J.H.C.; Dietrich, C.; Aggio-Bruce, R.; Chu-Tan, J.A.; Robertson, A.A.B.; Doyle, S.L.; Man, S.M.; Natoli, R. Caspase-1-dependent inflammasomes mediate photoreceptor cell death in photo-oxidative damage-induced retinal degeneration. Sci. Rep. 2020, 10, 2263. [Google Scholar] [CrossRef]
- Evavold, C.L.; Hafner-Bratkovič, I.; Devant, P.; D’Andrea, J.M.; Ngwa, E.M.; Boršić, E.; Doench, J.G.; LaFleur, M.W.; Sharpe, A.H.; Thiagarajah, J.R. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell 2021, 184, 4495–4511.e4419. [Google Scholar]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.G.; Hunter, K.; Salloum, F.N. Chapter Five—Cardiac complications of cancer therapies. In Advances in Cancer Research; Gewirtz, D.A., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 155, pp. 167–214. [Google Scholar]
- Bitirgen, G.; Ozkagnici, A.; Malik, R.; Kerimoglu, H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet. Med. 2014, 31, 431–438. [Google Scholar] [PubMed]
- Zhang, X.; Qiu, J.; Huang, F.; Shan, K.; Zhang, C. Type 2 Diabetes Mellitus Makes Corneal Endothelial Cells Vulnerable to Ultraviolet A-Induced Oxidative Damage Via Decreased DJ-1/Nrf2/NQO1 Pathway. Invest. Ophthalmol. Vis. Sci. 2022, 63, 25. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Cui, J.; Lin, Q.; Chen, X.Y.; Zhang, J.; Gao, E.H.; Wei, B.; Zhao, W. Protection of the enhanced Nrf2 deacetylation and its downstream transcriptional activity by SIRT1 in myocardial ischemia/reperfusion injury. Int. J. Cardiol. 2021, 342, 82–93. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Wang, M.X.; Zhao, J.; Zhang, H.; Li, K.; Niu, L.Z.; Wang, Y.P.; Zheng, Y.J. Potential Protective and Therapeutic Roles of the Nrf2 Pathway in Ocular Diseases: An Update. Oxid. Med. Cell Longev. 2020, 2020, 9410952. [Google Scholar] [CrossRef]
- Lu, W.; Ebihara, N.; Miyazaki, K.; Murakami, A. Reduced Expression of Laminin-5 in Corneal Epithelial Cells Under High Glucose Condition. Cornea 2006, 25, 61–67. [Google Scholar] [CrossRef]
- Huang, C.; Liao, R.; Wang, F.; Tang, S. Characteristics of Reconstituted Tight Junctions After Corneal Epithelial Wounds and Ultrastructure Alterations of Corneas in Type 2 Diabetic Rats. Curr. Eye Res. 2016, 41, 783–790. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Shi, D.; Chen, P.; Yu, Y.; Yang, L.; Xie, L. Overexpression of SIRT1 Promotes High Glucose–Attenuated Corneal Epithelial Wound Healing via p53 Regulation of the IGFBP3/IGF-1R/AKT Pathway. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3806–3814. [Google Scholar]
- Jiang, Q.-W.; Kaili, D.; Freeman, J.; Lei, C.-Y.; Geng, B.-C.; Tan, T.; He, J.-F.; Shi, Z.; Ma, J.-J.; Luo, Y.-H. Diabetes inhibits corneal epithelial cell migration and tight junction formation in mice and human via increasing ROS and impairing Akt signaling. Acta Pharmacol. Sin. 2019, 40, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Graue-Hernandez, E.O.; Tran, V.; Reid, B.; Pu, J.; Mannis, M.J.; Zhao, M. Downregulation of PTEN at Corneal Wound Sites Accelerates Wound Healing through Increased Cell Migration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qi, X.; Wang, X.; Li, W.; Li, Y.; Zhou, Q. PTEN Inhibition Facilitates Diabetic Corneal Epithelial Regeneration by Reactivating Akt Signaling Pathway. Transl. Vis. Sci. Technol. 2020, 9, 5. [Google Scholar] [CrossRef]
- Gekka, M.; Miyata, K.; Nagai, Y.; Nemoto, S.; Sameshima, T.; Tanabe, T.; Maruoka, S.; Nakahara, M.; Kato, S.; Amano, S. Corneal epithelial barrier function in diabetic patients. Cornea 2004, 23, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef]
- He, J.; Bazan, N.G.; Bazan, H.E.P. Mapping the entire human corneal nerve architecture. Exp. Eye Res. 2010, 91, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar]
- Müller, L.J.; Vrensen, G.F.; Pels, L.; Cardozo, B.N.; Willekens, B. Architecture of human corneal nerves. Invest. Ophthalmol. Vis. Sci. 1997, 38, 985–994. [Google Scholar]
- Müller, L.J.; Pels, L.; Vrensen, G. Ultrastructural organization of human corneal nerves. Investig. Ophthalmol. Vis. Sci. 1996, 37, 476–488. [Google Scholar]
- Dartt, D.A.; Bex, P.; D’Amore, P.; Dana, R.; Mcloon, L.; Niederkorn, J. Ocular Periphery and Disorders; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Belmonte, C.; Garcia-Hirschfeld, J.; Gallar, J. Neurobiology of ocular pain. Prog. Retin. Eye Res. 1997, 16, 117–156. [Google Scholar]
- Maugeri, G.; D’Amico, A.G.; Amenta, A.; Saccone, S.; Federico, C.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; Longo, A.; et al. Protective effect of PACAP against ultraviolet B radiation-induced human corneal endothelial cell injury. Neuropeptides 2020, 79, 101978. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hill, L.J.; Downie, L.E.; Chinnery, H.R. Neuroimmune crosstalk in the cornea: The role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Prog. Retin. Eye Res. 2022, 91, 101105. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S. Neuronal changes in the diabetic cornea: Perspectives for neuroprotection. BioMed Res. Int. 2016, 2016, 5140823. [Google Scholar]
- Tomlinson, D.; Fernyhough, P.; Diemel, L. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes 1997, 46, S43–S49. [Google Scholar]
- Puri, S.; Kenyon, B.M.; Hamrah, P. Immunomodulatory Role of Neuropeptides in the Cornea. Biomedicines 2022, 10, 1985. [Google Scholar] [CrossRef]
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 2014, 63, 4262–4274. [Google Scholar] [CrossRef]
- Chikamoto, N.; Chikama, T.-i.; Yamada, N.; Nishida, T.; Ishimitsu, T.; Kamiya, A. Efficacy of substance P and insulin-like growth factor-1 peptides for preventing postsurgical superficial punctate keratopathy in diabetic patients. Jpn. J. Ophthalmol. 2009, 53, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Nakamura, M.; Nakata, K.; Yamaguchi, T.; Takase, K.; Okahara, A.; Ikuse, T.; Nishida, T. Effects of Substance P and IGF-1 in Corneal Epithelial Barrier Function and Wound Healing in a Rat Model of Neurotrophic Keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3810–3815. [Google Scholar] [CrossRef]
- Yang, L.W.Y.; Mehta, J.S.; Liu, Y.-C. Corneal neuromediator profiles following laser refractive surgery. Neural Regen. Res. 2021, 16, 2177. [Google Scholar]
- Zhang, Y.; Gao, N.; Wu, L.; Lee, P.S.; Me, R.; Dai, C.; Xie, L.; Yu, F.-S.X. Role of VIP and sonic hedgehog signaling pathways in mediating epithelial wound healing, sensory nerve regeneration, and their defects in diabetic corneas. Diabetes 2020, 69, 1549–1561. [Google Scholar]
- Dogru, M.; Katakami, C.; Inoue, M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology 2001, 108, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Cousen, P.; Cackett, P.; Bennett, H.; Swa, K.; Dhillon, B. Tear production and corneal sensitivity in diabetes. J. Diabetes Its Complicat. 2007, 21, 371–373. [Google Scholar]
- Kabosova, A.; Kramerov, A.A.; Aoki, A.M.; Murphy, G.; Zieske, J.D.; Ljubimov, A.V. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp. Eye Res. 2003, 77, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Said, D.G.; Messmer, E.M.; Rolando, M.; Benitez-del-Castillo, J.M.; Hossain, P.N.; Shortt, A.J.; Geerling, G.; Nubile, M.; Figueiredo, F.C. Neurotrophic keratopathy. Prog. Retin. Eye Res. 2018, 66, 107–131. [Google Scholar] [PubMed]
- Barsegian, A.; Lee, J.; Salifu, M.O.; McFarlane, S.I. Corneal neuropathy: An underrated manifestation of diabetes mellitus. J. Clin. Endocrinol. Diabetes 2018, 2. [Google Scholar]
- De Clerck, E.E.B.; Schouten, J.; Berendschot, T.; Koolschijn, R.S.; Nuijts, R.; Schram, M.T.; Schaper, N.C.; Henry, R.M.A.; Dagnelie, P.C.; Ruggeri, A.; et al. Reduced corneal nerve fibre length in prediabetes and type 2 diabetes: The Maastricht Study. Acta Ophthalmol. 2020, 98, 485–491. [Google Scholar] [CrossRef]
- Zhou, T.; Lee, A.; Lo, A.C.Y.; Kwok, J. Diabetic Corneal Neuropathy: Pathogenic Mechanisms and Therapeutic Strategies. Front. Pharmacol. 2022, 13, 816062. [Google Scholar] [CrossRef]
- Chen, X.; Graham, J.; Petropoulos, I.N.; Ponirakis, G.; Asghar, O.; Alam, U.; Marshall, A.; Ferdousi, M.; Azmi, S.; Efron, N. Corneal nerve fractal dimension: A novel corneal nerve metric for the diagnosis of diabetic sensorimotor polyneuropathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1113–1118. [Google Scholar]
- Li, Q.; Zhong, Y.; Zhang, T.; Zhang, R.; Zhang, Q.; Zheng, H.; Ji, L.; Sun, W.; Zhu, X.; Zhang, S. Quantitative analysis of corneal nerve fibers in type 2 diabetics with and without diabetic peripheral neuropathy: Comparison of manual and automated assessments. Diabetes Res. Clin. Pract. 2019, 151, 33–38. [Google Scholar] [CrossRef]
- Lewis, E.J.; Lovblom, L.E.; Ferdousi, M.; Halpern, E.M.; Jeziorska, M.; Pacaud, D.; Pritchard, N.; Dehghani, C.; Edwards, K.; Srinivasan, S. Rapid corneal nerve fiber loss: A marker of diabetic neuropathy onset and progression. Diabetes Care 2020, 43, 1829–1835. [Google Scholar] [CrossRef]
- Sady, C.; Khosrof, S.; Nagaraj, R. Advanced Maillard reaction and crosslinking of corneal collagen in diabetes. Biochem. Biophys. Res. Commun. 1995, 214, 793–797. [Google Scholar] [PubMed]
- Mocan, M.C.; Durukan, I.; Irkec, M.; Orhan, M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea 2006, 25, 769–773. [Google Scholar]
- Saghizadeh, M.; Brown, D.J.; Castellon, R.; Chwa, M.; Huang, G.H.; Ljubimova, J.Y.; Rosenberg, S.; Spirin, K.S.; Stolitenko, R.B.; Adachi, W. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: A possible mechanism of basement membrane and integrin alterations. Am. J. Pathol. 2001, 158, 723–734. [Google Scholar] [CrossRef]
- Gül, M.; Emre, S.; Esrefoglu, M.; Vard, N. Protective effects of melatonin and aminoguanidine on the cornea in streptozotocin-induced diabetic rats. Cornea 2008, 27, 795–801. [Google Scholar] [PubMed]
- Leppin, K.; Behrendt, A.K.; Reichard, M.; Stachs, O.; Guthoff, R.F.; Baltrusch, S.; Eule, J.C.; Vollmar, B. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest. Ophthalmol. Vis. Sci. 2014, 55, 3603–3615. [Google Scholar] [CrossRef]
- Sierra-Silvestre, E.; Andrade, R.J.; Holguín-Colorado, L.; Edwards, K.; Coppieters, M.W. Occurrence of corneal sub-epithelial microneuromas and axonal swelling in people with diabetes with and without (painful) diabetic neuropathy. Diabetologia 2023, 66, 1719–1734. [Google Scholar] [CrossRef]
- De Cilla, S.; Ranno, S.; Carini, E.; Fogagnolo, P.; Ceresara, G.; Orzalesi, N.; Rossetti, L.M. Corneal subbasal nerves changes in patients with diabetic retinopathy: An in vivo confocal study. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5155–5158. [Google Scholar] [CrossRef]
- He, J.; Bazan, H.E. Mapping the nerve architecture of diabetic human corneas. Ophthalmology 2012, 119, 956–964. [Google Scholar] [CrossRef]
- Davidson, E.P.; Coppey, L.J.; Kardon, R.H.; Yorek, M.A. Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1222–1230. [Google Scholar]
- Wang, F.; Gao, N.; Yin, J.; Fu-Shin, X.Y. Reduced innervation and delayed re-innervation after epithelial wounding in type 2 diabetic Goto-Kakizaki rats. Am. J. Pathol. 2012, 181, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Stem, M.S.; Hussain, M.; Lentz, S.I.; Raval, N.; Gardner, T.W.; Pop-Busui, R.; Shtein, R.M. Differential reduction in corneal nerve fiber length in patients with type 1 or type 2 diabetes mellitus. J. Diabetes Its Complicat. 2014, 28, 658–661. [Google Scholar]
- Zhivov, A.; Winter, K.; Hovakimyan, M.; Peschel, S.; Harder, V.; Schober, H.-C.; Kundt, G.; Baltrusch, S.; Guthoff, R.F.; Stachs, O. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS ONE 2013, 8, e52157. [Google Scholar]
- Kalteniece, A.; Ferdousi, M.; Azmi, S.; Marshall, A.; Soran, H.; Malik, R.A. Keratocyte density is reduced and related to corneal nerve damage in diabetic neuropathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3584–3590. [Google Scholar] [CrossRef]
- Ferdousi, M.; Romanchuk, K.; Mah, J.K.; Virtanen, H.; Millar, C.; Malik, R.A.; Pacaud, D. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci. Rep. 2019, 9, 8758. [Google Scholar] [PubMed]
- Qu, J.-h.; Li, L.; Tian, L.; Zhang, X.-y.; Thomas, R.; Sun, X.-G. Epithelial changes with corneal punctate epitheliopathy in type 2 diabetes mellitus and their correlation with time to healing. BMC Ophthalmol. 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Lagali, N.S.; Allgeier, S.; Guimaraes, P.; Badian, R.A.; Ruggeri, A.; Köhler, B.; Utheim, T.P.; Peebo, B.; Peterson, M.; Dahlin, L.B. Reduced corneal nerve fiber density in type 2 diabetes by wide-area mosaic analysis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6318–6327. [Google Scholar] [CrossRef]
- Issar, T.; Tummanapalli, S.S.; Kwai, N.C.G.; Chiang, J.C.B.; Arnold, R.; Poynten, A.M.; Markoulli, M.; Krishnan, A.V. Associations between acute glucose control and peripheral nerve structure and function in type 1 diabetes. Diabet. Med. 2020, 37, 1553–1560. [Google Scholar] [CrossRef]
- Yang, A.Y.; Chow, J.; Liu, J. Focus: Sensory biology and pain: Corneal innervation and sensation: The eye and beyond. Yale J. Biol. Med. 2018, 91, 13. [Google Scholar]
- Tavakoli, M.; Kallinikos, P.A.; Efron, N.; Boulton, A.J.; Malik, R.A. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care 2007, 30, 1895–1897. [Google Scholar] [CrossRef]
- Rosenberg, M.E.; Tervo, T.M.; Immonen, I.J.; Müller, L.J.; Grönhagen-Riska, C.; Vesaluoma, M.H. Corneal structure and sensitivity in type 1 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2915–2921. [Google Scholar]
- Didenko, T.N.; Smoliakova, G.P.; Sorokin, E.L.; Egorov, V.V. Clinical and pathogenetic features of neurotrophic corneal disorders in diabetes. Vestn. Oftalmol. 1999, 115, 7–11. [Google Scholar] [PubMed]
- Vincent, A.M.; Edwards, J.L.; McLean, L.L.; Hong, Y.; Cerri, F.; Lopez, I.; Quattrini, A.; Feldman, E.L. Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol. 2010, 120, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.S.; Mervak, C.M.; Münch, A.E.; Robell, N.J.; Hayes, J.M.; Porzio, M.T.; Singleton, J.R.; Smith, A.G.; Feldman, E.L.; Lentz, S.I. Hyperglycemia-and neuropathy-induced changes in mitochondria within sensory nerves. Ann. Clin. Transl. Neurol. 2014, 1, 799–812. [Google Scholar] [CrossRef]
- Vincent, A.M.; Mclean, L.L.; Backus, C.; Feldman, E.L. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005, 19, 1–24. [Google Scholar] [CrossRef]
- Ishibashi, F.; Kojima, R.; Taniguchi, M.; Kosaka, A.; Uetake, H.; Tavakoli, M. The expanded bead size of corneal C-nerve fibers visualized by corneal confocal microscopy is associated with slow conduction velocity of the peripheral nerves in patients with type 2 diabetes mellitus. J. Diabetes Res. 2016, 2016, 3653459. [Google Scholar] [CrossRef]
- Zherebitskaya, E.; Akude, E.; Smith, D.R.; Fernyhough, P. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: Role of glucose-induced oxidative stress. Diabetes 2009, 58, 1356–1364. [Google Scholar] [CrossRef]
- Ryle, C.; Donaghy, M. Non-enzymatic glycation of peripheral nerve proteins in human diabetics. J. Neurol. Sci. 1995, 129, 62–68. [Google Scholar]
- Sugimoto, K.; Yasujima, M.; Yagihashi, S. Role of advanced glycation end products in diabetic neuropathy. Curr. Pharm. Des. 2008, 14, 953–961. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nishizawa, Y.; Horiuchi, S.; Yagihashi, S. Localization in human diabetic peripheral nerve of Nɛ-carboxymethyllysine-protein adducts, an advanced glycation endproduct. Diabetologia 1997, 40, 1380–1387. [Google Scholar] [CrossRef]
- Kaji, Y.; Amano, S.; Usui, T.; Suzuki, K.; Tanaka, S.; Oshika, T.; Nagai, R.; Horiuchi, S. Advanced glycation end products in Descemet’s membrane and their effect on corneal endothelial cell. Curr. Eye Res. 2001, 23, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Zeng, X.; Li, F.; Zhao, S. The effect of the duration of diabetes on dry eye and corneal nerves. Contact Lens Anterior Eye 2019, 42, 380–385. [Google Scholar] [CrossRef]
- Greene, D.A.; Sima, A.A.; Stevens, M.J.; Feldman, E.L.; Lattimer, S.A. Complications: Neuropathy, pathogenetic considerations. Diabetes Care 1992, 15, 1902–1925. [Google Scholar] [PubMed]
- Lehning, E.J.; LoPachin, R.M.; Mathew, J.; Eichberg, J. Changes in Na-K ATPase and protein kinase C activities in peripheral nerve of acrylamide-treated rats. J. Toxicol. Environ. Health Part. A Curr. Issues 1994, 42, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef]
- Rieger, G. The importance of the precorneal tear film for the quality of optical imaging. Br. J. Ophthalmol. 1992, 76, 157–158. [Google Scholar] [CrossRef]
- Yang, C.-J.; Anand, A.; Huang, C.-C.; Lai, J.-Y. Unveiling the Power of Gabapentin-Loaded Nanoceria with Multiple Therapeutic Capabilities for the Treatment of Dry Eye Disease. ACS Nano 2023, 17, 25118–25135. [Google Scholar] [CrossRef]
- Imam, S.; Elagin, R.B.; Jaume, J.C. Diabetes-associated dry eye syndrome in a new humanized transgenic model of type 1 diabetes. Mol. Vis. 2013, 19, 1259–1267. [Google Scholar]
- Research in Dry Eye: Report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 179–193. [CrossRef]
- Kesarwani, D.; Rizvi, S.W.A.; Khan, A.A.; Amitava, A.K.; Vasenwala, S.M.; Siddiqui, Z. Tear film and ocular surface dysfunction in diabetes mellitus in an Indian population. Indian. J. Ophthalmol. 2017, 65, 301–304. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Buckner, B.R.; Zhu, M.; Cavanagh, H.D.; Robertson, D.M. Elevated IGFBP3 Levels in Diabetic Tears: A Negative Regulator of IGF-1 Signaling in the Corneal Epithelium. Ocul. Surf. 2012, 10, 100–107. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.; Liu, J.; Shi, B.; He, S.; Yao, X.; Willcox, M.D. Advanced glycation end product (AGE) modified proteins in tears of diabetic patients. Mol. Vis. 2010, 16, 1576–1584. [Google Scholar] [PubMed]
- Liu, H.; Sheng, M.; Liu, Y.; Wang, P.; Chen, Y.; Chen, L.; Wang, W.; Li, B. Expression of SIRT1 and oxidative stress in diabetic dry eye. Int. J. Clin. Exp. Pathol. 2015, 8, 7644–7653. [Google Scholar] [PubMed]
- Wang, B.; Zeng, H.; Zuo, X.; Yang, X.; Wang, X.; He, D.; Yuan, J. TLR4-Dependent DUOX2 Activation Triggered Oxidative Stress and Promoted HMGB1 Release in Dry Eye. Front. Med. 2021, 8, 781616. [Google Scholar] [CrossRef]
- Qu, M.; Wan, L.; Dong, M.; Wang, Y.; Xie, L.; Zhou, Q. Hyperglycemia-induced severe mitochondrial bioenergetic deficit of lacrimal gland contributes to the early onset of dry eye in diabetic mice. Free Radic. Biol. Med. 2021, 166, 313–323. [Google Scholar] [CrossRef]
- Richdale, K.; Chao, C.; Hamilton, M. Eye care providers’ emerging roles in early detection of diabetes and management of diabetic changes to the ocular surface: A review. BMJ Open Diabetes Res. Care 2020, 8, e001094. [Google Scholar] [CrossRef]
- Abdul-Hamid, M.; Moustafa, N. Amelioration of alloxan-induced diabetic keratopathy by beta-carotene. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2014, 66, 49–59. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki Hagiagha, A.; Heshmati, J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 139, 141–152. [Google Scholar] [CrossRef]
- Wiseman, H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993, 326, 285–288. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, J.; Xiang, J.; Li, Y.; Wu, D.; Xu, J. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019, 21, 101093. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, L.; Zhang, Z.; Li, J.; Qu, M.; Zhou, Q. Topical calcitriol application promotes diabetic corneal wound healing and reinnervation through inhibiting NLRP3 inflammasome activation. Exp. Eye Res. 2021, 209, 108668. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Z.; Lu, J.; Watsky, M. Effects of Topical 1,25 and 24,25 Vitamin D on Diabetic, Vitamin D Deficient and Vitamin D Receptor Knockout Mouse Corneal Wound Healing. Biomolecules 2023, 13, 1065. [Google Scholar]
- Liu, X.; Liu, H.; Lu, X.; Zhao, S. N-acetylcysteine alleviates ocular surface damage in STZ-induced diabetic mice by inhibiting the ROS/NLRP3/Caspase-1/IL-1β signaling pathway. Exp. Eye Res. 2021, 209, 108654. [Google Scholar] [CrossRef]
- Li, Z.; Han, Y.; Ji, Y.; Sun, K.; Chen, Y.; Hu, K. The effect of a-Lipoic acid (ALA) on oxidative stress, inflammation, and apoptosis in high glucose-induced human corneal epithelial cells. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 735–748. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Spinella, R.; Oliverio, G.W.; Postorino, E.I.; Signorino, G.A.; Rusciano, D.; Aragona, P. Effects of the Topical Use of the Natural Antioxidant Alpha-Lipoic Acid on the Ocular Surface of Diabetic Patients with Dry Eye Symptoms. Front. Biosci. (Landmark Ed.) 2022, 27, 202. [Google Scholar] [CrossRef]
- Bierbrauer, K.L.; Comini, L.R.; Leonhard, V.; Escobar Manzanelli, M.A.; Castelli, G.; Farfán, S.; Alasino, R.V.; Beltramo, D.M. Eudragit Films as Carriers of Lipoic Acid for Transcorneal Permeability. Polymers 2023, 15, 1793. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Shi, C.; Jiao, F.; Gong, Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol. Med. Rep. 2019, 20, 4081–4090. [Google Scholar] [CrossRef]
- Somayajulu, M.; McClellan, S.A.; Pitchaikannu, A.; Bessert, D.; Liu, L.; Steinle, J.; Hazlett, L.D. Effects of Glycyrrhizin Treatment on Diabetic Cornea. J. Ocul. Pharmacol. Ther. 2021, 37, 12–23. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Li, Z.; Li, H.; Chen, Y.; Chen, X.; Su, W.; Liang, D. Subconjunctival injections of dimethyl fumarate inhibit lymphangiogenesis and allograft rejection in the rat cornea. Int. Immunopharmacol. 2021, 96, 107580. [Google Scholar] [CrossRef]
- Giurdanella, G.; Longo, A.; Salerno, L.; Romeo, G.; Intagliata, S.; Lupo, G.; Distefano, A.; Platania, C.B.M.; Bucolo, C.; Li Volti, G.; et al. Glucose-impaired Corneal Re-epithelialization Is Promoted by a Novel Derivate of Dimethyl Fumarate. Antioxidants 2021, 10, 831. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Han, F.; Wang, K.; Bai, X.; Jia, Y.; Li, Z.; Cai, W.; Zhang, W.; Su, L.; et al. SIRT1 activation promotes angiogenesis in diabetic wounds by protecting endothelial cells against oxidative stress. Arch. Biochem. Biophys. 2019, 661, 117–124. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Wu, X.; Dai, Y.; Chen, P.; Xie, L. microRNA-182 Mediates Sirt1-Induced Diabetic Corneal Nerve Regeneration. Diabetes 2016, 65, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Zhao, X.; Chen, P.; Xie, L. MicroRNA-204-5p-Mediated Regulation of SIRT1 Contributes to the Delay of Epithelial Cell Cycle Traversal in Diabetic Corneas. Invest. Ophthalmol. Vis. Sci. 2015, 56, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Guo, R.; Tsao, J.-R.; He, Y.; Wang, C.; Jiang, J.; Zhang, D.; Chen, T.; Yue, T.; Hu, K. Salidroside alleviates oxidative stress in dry eye disease by activating autophagy through AMPK-Sirt1 pathway. Int. Immunopharmacol. 2023, 121, 110397. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Zhao, X.; Guo, Q.; Yang, R.; Zhang, C.; Huang, Y.; Ma, L.; Zhao, S. Effect of PPARγ on oxidative stress in diabetes-related dry eye. Exp. Eye Res. 2023, 231, 109498. [Google Scholar] [CrossRef]
- Inaba, T.; Ohnishi-Kameyama, M.; Liu, Y.; Tanaka, Y.; Kobori, M.; Tamaki, S.; Ito, T.; Higa, K.; Shimazaki, J.; Tsubota, K. Quercetin improves lacrimal gland function through its anti-oxidant actions: Evidence from animal studies, and a pilot study in healthy human volunteers. Front. Nutr. 2022, 9, 974530. [Google Scholar] [CrossRef]
- Ji, Y.; Leng, Y.; Lei, S.; Qiu, Z.; Ming, H.; Zhang, Y.; Zhang, A.; Wu, Y.; Xia, Z. The mitochondria-targeted antioxidant MitoQ ameliorates myocardial ischemia-reperfusion injury by enhancing PINK1/Parkin-mediated mitophagy in type 2 diabetic rats. Cell Stress. Chaperones 2022, 27, 353–367. [Google Scholar] [CrossRef]
- Yang, M.Y.; Fan, Z.; Zhang, Z.; Fan, J. MitoQ protects against high glucose-induced brain microvascular endothelial cells injury via the Nrf2/HO-1 pathway. J. Pharmacol. Sci. 2021, 145, 105–114. [Google Scholar] [CrossRef]
- Fink, B.; Coppey, L.; Davidson, E.; Shevalye, H.; Obrosov, A.; Chheda, P.R.; Kerns, R.; Sivitz, W.; Yorek, M. Effect of mitoquinone (Mito-Q) on neuropathic endpoints in an obese and type 2 diabetic rat model. Free Radic. Res. 2020, 54, 311–318. [Google Scholar] [CrossRef]
- Pu, Q.; Guo, X.X.; Hu, J.J.; Li, A.L.; Li, G.G.; Li, X.Y. Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomed. Pharmacother. 2022, 147, 112659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, Y.; Wei, C.; Zhao, X.; Zhou, Q.; Xie, L. DNase I improves corneal epithelial and nerve regeneration in diabetic mice. J. Cell Mol. Med. 2020, 24, 4547–4556. [Google Scholar] [CrossRef]
- Berryman, A.M.; Maritim, A.C.; Sanders, R.A.; Watkins, J.B., III. Influence of treatment of diabetic rats with combinations of pycnogenol, beta-carotene, and alpha-lipoic acid on parameters of oxidative stress. J. Biochem. Mol. Toxicol. 2004, 18, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.A.; Farag, A.; Zahran, I.S.; Hafez, A.; Rizk, M.A.; Abass, M. Pycnogenol a promising remedy for diabetic keratopathy in experimentally induced corneal alkali burns in diabetic rats. BMC Vet. Res. 2022, 18, 209. [Google Scholar] [CrossRef]
- Ho, J.H.; Tseng, K.C.; Ma, W.H.; Chen, K.H.; Lee, O.K.; Su, Y. Thymosin beta-4 upregulates anti-oxidative enzymes and protects human cornea epithelial cells against oxidative damage. Br. J. Ophthalmol. 2008, 92, 992–997. [Google Scholar] [CrossRef]
- Sosne, G.; Rimmer, D.; Kleinman, H.K.; Ousler, G. Thymosin Beta 4: A Potential Novel Therapy for Neurotrophic Keratopathy, Dry Eye, and Ocular Surface Diseases. Vitam. Horm. 2016, 102, 277–306. [Google Scholar] [CrossRef] [PubMed]
- Reihanifar, T.; Şahin, M.; Stefek, M.; Ceylan, A.F.; Karasu, Ç. Cemtirestat, an aldose reductase inhibitor and antioxidant compound, induces ocular defense against oxidative and inflammatory stress in rat models for glycotoxicity. Cell Biochem. Funct. 2023, 41, 622–632. [Google Scholar] [CrossRef]
- Jaworski, M.; Lorenc, A.; Leszczyński, R.; Mrukwa-Kominek, E. Topical Insulin in Neurotrophic Keratopathy: A Review of Current Understanding of the Mechanism of Action and Therapeutic Approach. Pharmaceutics 2024, 16, 15. [Google Scholar] [CrossRef]
- Peterson, C.; Chandler, H.L. Insulin facilitates corneal wound healing in the diabetic environment through the RTK-PI3K/Akt/mTOR axis in vitro. Mol. Cell Endocrinol. 2022, 548, 111611. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Zhang, Z.; Dan, J.; Zhou, Q.; Wang, X.; Li, W.; Zhou, L.; Yang, L.; Xie, L. Insulin Promotes Corneal Nerve Repair and Wound Healing in Type 1 Diabetic Mice by Enhancing Wnt/β-Catenin Signaling. Am. J. Pathol. 2020, 190, 2237–2250. [Google Scholar] [CrossRef]
- Leong, C.Y.; Naffi, A.A.; Wan Abdul Halim, W.H.; Bastion, M.C. Usage of topical insulin for the treatment of diabetic keratopathy, including corneal epithelial defects. World J. Diabetes 2023, 14, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Fai, S.; Ahem, A.; Mustapha, M.; Mohd Noh, U.K.; Bastion, M.C. Randomized Controlled Trial of Topical Insulin for Healing Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Asia Pac. J. Ophthalmol. 2017, 6, 418–424. [Google Scholar] [CrossRef]
- Aniah Azmi, N.; Bastion, M.C. Short-Term Results of Trial of Topical Insulin for Treatment of Dry Eyes in Diabetics. Eye Contact Lens 2020, 46 (Suppl. 1), S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Valle, D.; Burgos-Blasco, B.; Rego-Lorca, D.; Puebla-Garcia, V.; Perez-Garcia, P.; Benitez-del-Castillo, J.M.; Herrero-Vanrell, R.; Vicario-de-la-Torre, M.; Gegundez-Fernandez, J.A. Comparison of the efficacy of topical insulin with autologous serum eye drops in persistent epithelial defects of the cornea. Acta Ophthalmol. 2022, 100, e912–e919. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Lu, X.; Tombran-Tink, J.; Zhao, S. PEDF Attenuates Ocular Surface Damage in Diabetic Mice Model Through Its Antioxidant Properties. Curr. Eye Res. 2021, 46, 302–308. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Pang, S.; Wang, L.; Fan, Z.; Ma, C.; Yang, S.; Banda, J.; Hui, Q.; Lv, F.; et al. rhFGF-21 accelerates corneal epithelial wound healing through the attenuation of oxidative stress and inflammatory mediators in diabetic mice. J. Biol. Chem. 2023, 299, 105127. [Google Scholar] [CrossRef]


| Molecule | Chemistry/Biologic Effect | Study Design, Route, Model | Molecular Target and Study Outcome | Ref. |
|---|---|---|---|---|
| β-carotene | vitamin A derivative | in vivo, oral application, diabetic rat model | antioxidant and hypoglycemic effect, ameliorating corneal changes | [139] |
| calcitriol | vitamin D derivative | in vitro, high glucose-treated human corneal epithelial cells | inhibition of ROS–NLRP3–IL-1β signaling via activation of Nrf2 antioxidant signaling | [143] |
| in vivo, topical administration, diabetic mouse model | promotion of diabetic corneal wound healing and reinnervation via NLRP3 suppression | [144] | ||
| in vivo, topical route with 1,25 Vit. D or 24,25 Vit. D, diabetic mouse model | improvement of corneal wound healing | [145] | ||
| NAC | N-acetylated derivative of the natural amino acid L-cysteine | in vivo, topical administration, diabetic mouse model | mitigation of ocular surface damage via suppression of the ROS/NLRP3/caspase-1/IL-1β signaling pathway | [146] |
| ALA | (R)-enantiomer of lipoic acid: vitamin-like fatty acid | in vitro, high glucose-exposed human corneal epithelial cells | suppression of AGE–RAGE–TLR4-NLRP3 pathway-induced inflammation and amelioration of oxidative stress, apoptosis, and inflammation | [147] |
| Eye drops based on a combination of ALA and HPMC in diabetic patients with DED | effectiveness in the treatment of diabetic DED and self-regeneration, improving corneal defects | [148] | ||
| GLY | naturally occurring saponin | in vitro, in vivo, ex vivo, oral application, diabetic mouse model | downregulation, among others, of HMGB1, IL-1β, TLR2, TLR4, and NLRP3, leading to attenuation of corneal inflammation and oxidative stress | [151] |
| in vivo, subconjunctival injection, diabetic mouse model | attenuated activation of RAGE and TLR4 molecular pathways, promoting corneal epithelial wound healing | [47] | ||
| VP13/126 | DMF derivative | in vitro, glucose-impaired rabbit corneal epithelial cells | activation of the Nrf2/HO-1 pathway, inducing corneal re-epithelialization | [153] |
| SIRT1 modulators | miRNA | in vivo, subconjunctival injection, diabetic mouse model | miRNA-182 upregulates SIRT1 and downregulates NOX4, promoting diabetic corneal nerve regeneration | [155] |
| in vivo, subconjunctival injection, diabetic murine model | blockade of microRNA-204-5p favors corneal epithelial wound healing via SIRT1 | [156] | ||
| salidroside | glycoside, extract from Rhodiola crenulata, natural antioxidant | in vitro and in vivo, eye drops, DED murine model | mitigation of oxidative stress in DED through Nrf2 via AMPK–SIRT1 signaling on the ocular surface | [157] |
| rosiglitazone | thiazolidinedione, insulin-sensitizing drug | in vivo, oral gavage, diabetes-related DED in a mouse model | decrease in oxidative stress in the lacrimal gland in part by activating PPARγ, inducing overexpression of antioxidants such as GPx3 | [158] |
| quercetin | flavonol, naturally occurring antioxidant | in vivo, diet route, diabetic mouse model | improvement of tear function in diabetic mice via upregulation of SOD1 and SOD2 in the lacrimal gland, reduction of ROS formation, and promotion of cell survival | [159] |
| mito-Q | synthetic drug, mitochondria-specific antioxidant | in vivo, diet route, diet-induced obese or type 2 diabetic rat models | amelioration of nerve conduction velocity, corneal and intraepidermal nerve fiber density, corneal sensitivity, and thermal nociception | [162] |
| NMN | nucleotide | in vitro, high glucose-treated human corneal epithelial cells | enhancement of cell viability by reducing apoptosis, increasing cell migration, and restoring tight junctions via activation of the SIRT1/Nrf2/HO-1 axis | [163] |
| DNAse I | enzyme responsible for DNA degradation | in vivo, topical administration, diabetic mouse model | improvement of corneal epithelial wound healing and nerve regeneration by activating Akt, IGFR-1, SIRT1, while inhibiting NOX2 and NOX4 upregulation, reducing ROS | [164] |
| pycnogenol | mixture of flavonoids and procyanidins | in vivo, eye drops, diabetic rat model | acceleration of wound re-epithelialization | [166] |
| thymosin β-4 | naturally occurring polypeptide | in vitro, human corneal epithelial cells exposed to oxidative stress | upregulation of antioxidants such as SOD | [167] |
| SkQ1 | mitochondria-targeted antioxidant | in vivo, topical administration, diabetic mouse model | amelioration of DED severity and diabetic keratopathy via improvement of mitochondrial function | [137] |
| cemtirestat | aldose reductase inhibitor and antioxidant | in vivo, oral administration, rodent model for glycotoxicity | reduction of inflammation and oxidative stress via TNF-α, IL-1β, NF-kB, and caspase-3 downregulation | [169] |
| insulin | growth factor with regenerative and antiapoptotic effects | in vitro, human and canine corneal epithelial cells | activation of the PI3K/Akt axis, leading to antiapoptotic effects, favoring cell proliferation and migration, accelerating corneal wound healing | [171] |
| in vivo, eye drops, diabetic murine model | enhancement of the corneal nerve repair via activation of the Wnt/β-catenin pathway | [172] | ||
| insulin eye drops in diabetic patients with diverse dose regimens (0.5 or 1.0 unit/drop, 2–4 times daily) | enhancement of corneal epithelial wound healing, mitigation of diabetic DED, improvement of re-epithelialization compared with autologous serum in persistent corneal defects | [174,175,176] | ||
| PEDF | growth factor with antioxidant effects | in vivo, topical administration, diabetic mouse model | reduction of corneal epithelial defects via mitigation of ROS generation, decreased RAGE expression, and upregulation of SOD-1 | [177] |
| rhFGF-21 | growth factor with anti-inflammatory and antioxidant properties | in vitro on human corneal epithelial cells and in vivo on a diabetic mouse model | promotes corneal epithelial wound healing by reducing pro-inflammatory markers like TNF-α, IL-6, IL-1β and promoting antioxidant enzyme expression such as that of SOD-1 | [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonfiglio, F.; Wasielica-Poslednik, J.; Pfeiffer, N.; Gericke, A. Diabetic Keratopathy: Redox Signaling Pathways and Therapeutic Prospects. Antioxidants 2024, 13, 120. https://doi.org/10.3390/antiox13010120
Buonfiglio F, Wasielica-Poslednik J, Pfeiffer N, Gericke A. Diabetic Keratopathy: Redox Signaling Pathways and Therapeutic Prospects. Antioxidants. 2024; 13(1):120. https://doi.org/10.3390/antiox13010120
Chicago/Turabian StyleBuonfiglio, Francesco, Joanna Wasielica-Poslednik, Norbert Pfeiffer, and Adrian Gericke. 2024. "Diabetic Keratopathy: Redox Signaling Pathways and Therapeutic Prospects" Antioxidants 13, no. 1: 120. https://doi.org/10.3390/antiox13010120
APA StyleBuonfiglio, F., Wasielica-Poslednik, J., Pfeiffer, N., & Gericke, A. (2024). Diabetic Keratopathy: Redox Signaling Pathways and Therapeutic Prospects. Antioxidants, 13(1), 120. https://doi.org/10.3390/antiox13010120








