Photoprotective Effects of Two New Morin-Schiff Base Derivatives on UVB-Irradiated HaCaT Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Elucidation Instrumental
2.2. General Procedure for Preparation of Morin-Schiff Derivatives
2.3. Cell Culture and UVB Irradiation of HaCaT Keratinocytes
2.4. Assessment of Cell Viability by Resazurin Assay in HaCaT Keratinocytes
2.5. Analysis of Scavenger Activity by ABTS Test
2.6. Evaluation of Intracellular ROS Production by DCF-DA Assay in UVB-Exposed HaCaT Keratinocytes
2.7. Determination of Lipid Peroxidation by TBARS Assay in UVB-Exposed HaCaT Keratinocytes
2.8. Determination of IL-6 Production by ELISA Kit in UVB-Exposed HaCaT Keratinocytes
2.9. Statistical Analysis
3. Results
3.1. Obtention of Morin Oxime (2) and Morin Semicarbazone (3)
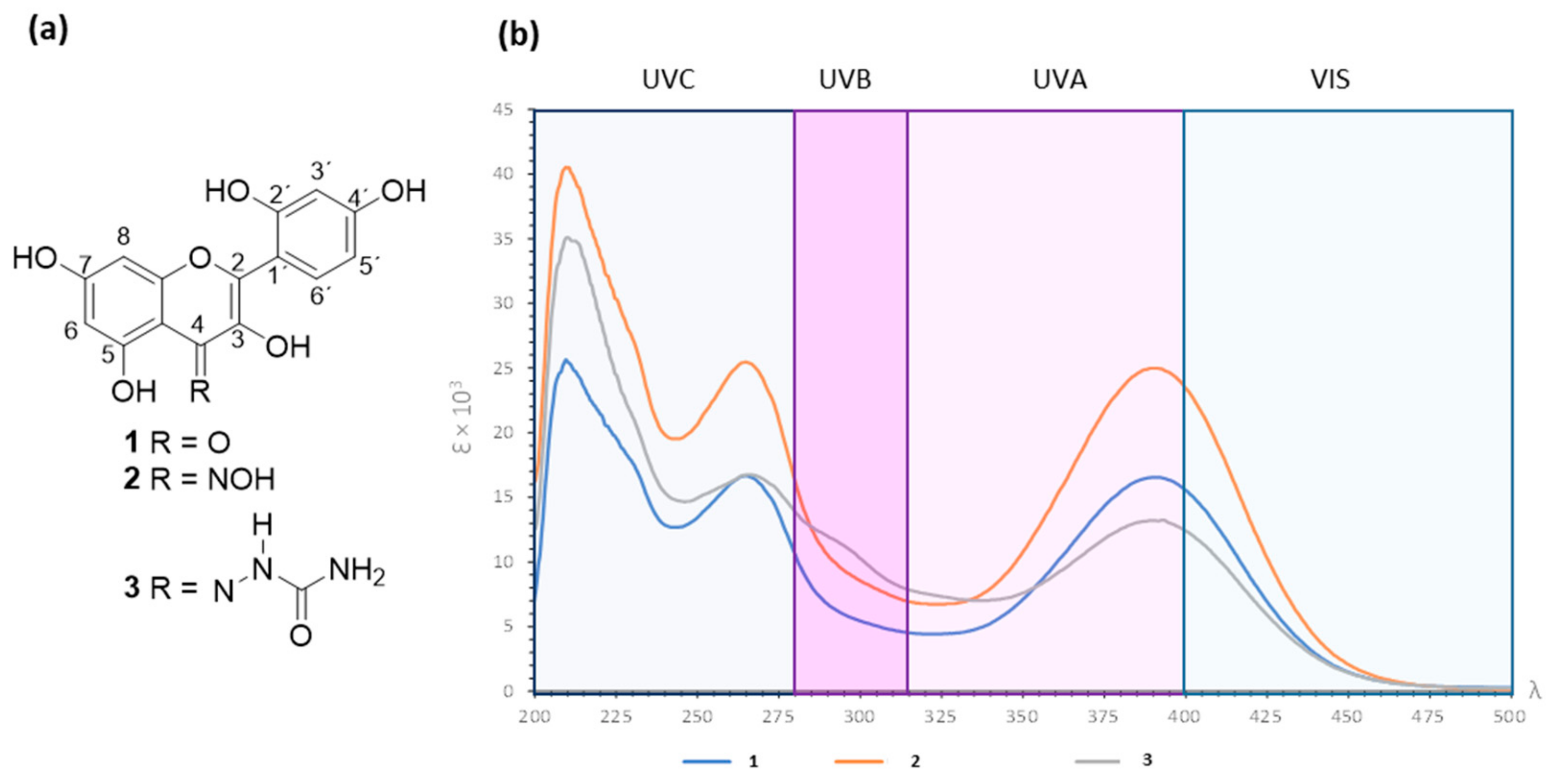
3.2. Absorbance Spectra for Morin (1) and Derivatives 2 and 3 in the UV–Vis Range
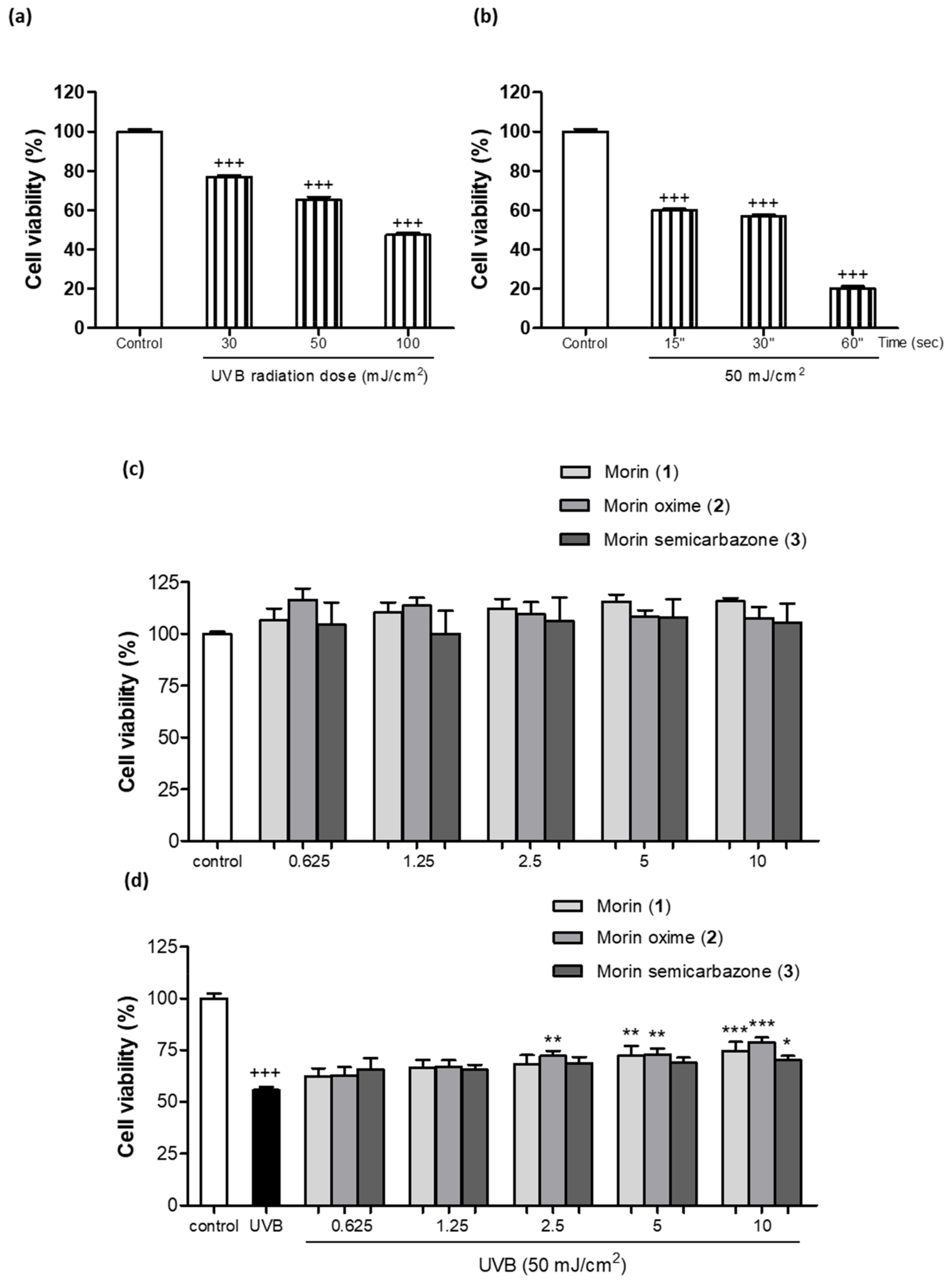
3.3. Photoprotective Effect of Morin (1) and Derivatives 2 and 3 on Cell Viability
3.4. Antioxidant Activity of Morin (1) and Derivatives 2 and 3
3.5. Effect of Morin (1) and Derivatives (2 and 3) Preventing Lipid Peroxidation

3.6. Anti-Inflammatory Activity in UVB-Exposed HaCaT Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pecorelli, A.; Valacchi, G. Oxidative-Stress-Sensitive MicroRNAs in UV-Promoted Development of Melanoma. Cancers 2022, 14, 3224. [Google Scholar] [CrossRef]
- González, S.; Aguilera, J.; Berman, B.; Calzavara-Pinton, P.; Gilaberte, Y.; Goh, C.L.; Lim, H.W.; Schalka, S.; Stengel, F.; Wolf, P.; et al. Expert Recommendations on the Evaluation of Sunscreen Efficacy and the Beneficial Role of Non-Filtering Ingredients. Front. Med. 2022, 9, 790207. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; Park, K.H.; Hahn, J.-H. Alleviation of Ultraviolet-B Radiation-Induced Photoaging by a TNFR Antagonistic Peptide, TNFR2-SKE. Mol. Cells 2019, 42, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Luangpraditkun, K.; Tissot, M.; Joompang, A.; Charoensit, P.; Grandmottet, F.; Viyoch, J.; Viennet, C. Prevention by the Natural Artocarpin of Morphological and Biochemical Alterations on UVB-Induced HaCaT Cells. Oxid. Med. Cell. Longev. 2021, 2021, 5067957. [Google Scholar] [CrossRef] [PubMed]
- González, S.; De Gálvez, M.V.; De Troya, M.; Rodríguez-Luna, A.; Calzavara-Pinton, P. Personalized Medical Photoprotection: Determining Optimal Measures for Susceptible Patient Groups. Open Dermatol. J. 2023, 17, e187437222212300. [Google Scholar] [CrossRef]
- Aguilera, J.; Vicente-Manzanares, M.; de Gálvez, M.V.; Herrera-Ceballos, E.; Rodríguez-Luna, A.; González, S. Booster Effect of a Natural Extract of Polypodium Leucotomos (Fernblock®) That Improves the UV Barrier Function and Immune Protection Capability of Sunscreen Formulations. Front. Med. 2021, 8, 684665. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Eh Suk, V.R.; Gew, L.T. Plant Polyphenols as Green Sunscreen Ingredients: A Systematic Review. J. Cosmet. Dermatol. 2022, 21, 5409–5444. [Google Scholar] [CrossRef]
- Milito, A.; Castellano, I.; Damiani, E. From Sea to Skin: Is There a Future for Natural Photoprotectants? Mar. Drugs 2021, 19, 379. [Google Scholar] [CrossRef]
- Rajput, S.A.; Wang, X.; Yan, H.-C. Morin Hydrate: A Comprehensive Review on Novel Natural Dietary Bioactive Compound with Versatile Biological and Pharmacological Potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef]
- Venu Gopal, J. Morin Hydrate: Botanical Origin, Pharmacological Activity and Its Applications: A Mini-Review. Pharmacogn. J. 2013, 5, 123–126. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A Systematic Review of Natural Products for Skin Applications: Targeting Inflammation, Wound Healing, and Photo-Aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, N.; Soltanian, S.; Raeiszadeh, M.; Moeinzadeh, M.; Ohadi, M.; Sharifi, F.; Pardakhty, A.; Sharififar, F. Characteristics and in Vitro Anti Skin Aging Activity and UV Radiation Protection of Morin Loaded in Niosomes. J. Cosmet. Dermatol. 2022, 21, 6326–6335. [Google Scholar] [CrossRef]
- Solairaja, S.; Andrabi, M.Q.; Dunna, N.R.; Venkatabalasubramanian, S. Overview of Morin and Its Complementary Role as an Adjuvant for Anticancer Agents. Nutr. Cancer 2021, 73, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.J.; Ahn, J.J. Antioxidant and Skin Protection Effect of Morin upon UVA Exposure. Biomed. Dermatol. 2018, 2, 12. [Google Scholar] [CrossRef]
- Lee, M.H.; Cha, H.J.; Choi, E.O.; Han, M.H.; Kim, S.O.; Kim, G.Y.; Hong, S.H.; Park, C.; Moon, S.K.; Jeong, S.J.; et al. Antioxidant and Cytoprotective Effects of Morin against Hydrogen Peroxide-Induced Oxidative Stress Are Associated with the Induction of Nrf-2-Mediated HO-1 Expression in V79-4 Chinese Hamster Lung Fibroblasts. Int. J. Mol. Med. 2017, 39, 672–680. [Google Scholar] [CrossRef]
- Shetty, P.K.; Venuvanka, V.; Jagani, H.V.; Chethan, G.H.; Ligade, V.S.; Musmade, P.B.; Nayak, U.Y.; Reddy, M.S.; Kalthur, G.; Udupa, N.; et al. Development and Evaluation of Sunscreen Creams Containing Morin-Encapsulated Nanoparticles for Enhanced UV Radiation Protection and Antioxidant Activity. Int. J. Nanomed. 2015, 10, 6477–6491. [Google Scholar] [CrossRef]
- Al-Rooqi, M.M.; Mughal, E.U.; Raja, Q.A.; Hussein, E.M.; Naeem, N.; Sadiq, A.; Asghar, B.H.; Moussa, Z.; Ahmed, S.A. Flavonoids and Related Privileged Scaffolds as Potential Urease Inhibitors: A Review. RSC Adv. 2023, 13, 3210–3233. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff Bases: A Short Review of Their Antimicrobial Activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and Rosmarinic Acid Combination Has Anti-Inflammatory Effects through Regulation of NLRP3 Inflammasome in UVB-Exposed HaCaT Keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef]
- Carrasco, C.J.; Montilla, F.; Álvarez, E.; Calderón-Montaño, J.M.; López-Lázaro, M.; Galindo, A. Chirality Influence on the Cytotoxic Properties of Anionic Chiral Bis(N-Heterocyclic Carbene)Silver Complexes. J. Inorg. Biochem. 2022, 235, 111924. [Google Scholar] [CrossRef]
- Muñoz-García, R.; Sánchez-Hidalgo, M.; Montoya, T.; Alcarranza, M.; Ortega-Vidal, J.; Altarejos, J.; Alarcón-de-la-Lastra, C. Effects of Oleacein, a New Epinutraceutical Bioproduct from Extra Virgin Olive Oil, in LPS-Activated Murine Immune Cells. Pharmaceuticals 2022, 15, 1338. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-Containing Cream Prevents Epidermal Hyperplasia and UVB-Induced Skin Erythema in Mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [PubMed]
- Iosageanu, A.; Ilie, D.; Craciunescu, O.; Seciu-Grama, A.M.; Oancea, A.; Zarnescu, O.; Moraru, I.; Oancea, F. Effect of Fish Bone Bioactive Peptides on Oxidative, Inflammatory and Pigmentation Processes Triggered by UVB Irradiation in Skin Cells. Molecules 2021, 26, 2691. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Picardo, M. Antioxidant Activity, Lipid Peroxidation and Skin Diseases. What’s New. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.D.; Sayyad, S.F.; Hatvate, N.T.; Dhumal, V.V.; Pardeshi, S.R.; Chavda, V.P.; Vora, L.K. Drug Delivery Strategies for Avobenzone: A Case Study of Photostabilization. Pharmaceutics 2023, 15, 1008. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.A.; Polonini, H.C.; Suzuki, É.Y.; Raposo, N.R.B.; Da Silva, A.D. Synthesis of Conjugated Bile Acids/Azastilbenes as Potential Antioxidant and Photoprotective Agents. Steroids 2015, 98, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Khanna, P.; Khanna, L. Synthesis, Comparative in Vitro Antibacterial, Antioxidant and UV Fluorescence Studies of Bis Indole Schiff Bases and Molecular Docking with Ct-DNA and SARS-CoV-2 Mpro. Luminescence 2021, 36, 1531–1543. [Google Scholar] [CrossRef]
- Adwin Jose, P.; Sankarganesh, M.; Dhaveethu Raja, J.; Arumugam, S. DNA/BSA Interaction, Anticancer, Antimicrobial and Catalytic Applications of Synthesis of Nitro Substituted Pyrimidine-Based Schiff Base Ligand Capped Nickel Nanoparticles. J. Biomol. Struct. Dyn. 2023, 2, 1–15. [Google Scholar] [CrossRef]
- Adwin Jose, P.; Sankarganesh, M.; Dhaveethu Raja, J.; Senthilkumar, G.S.; Nandini Asha, R.; Raja, S.J.; Sheela, C.D. Bio-Inspired Nickel Nanoparticles of Pyrimidine-Schiff Base: In Vitro Anticancer, BSA and DNA Interactions, Molecular Docking and Antioxidant Studies. J. Biomol. Struct. Dyn. 2022, 40, 10715–10729. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Al-Hamdani, A.A.S.; Kaseem, M. Synthesis and Antioxidant Activities of Schiff Bases and Their Complexes: A Review. Appl. Organomet. Chem. 2016, 30, 810–817. [Google Scholar] [CrossRef]
- Heinert, D.; Martell, A.E. Syntheses and Infrared Spectra of Schiff Bases. J. Am. Chem. Soc. 1962, 84, 3257–3263. [Google Scholar] [CrossRef]
- Baker, P.K.; Flower, K.R. The Preparation of Some Monodentate Coordinated Semicarbazone(O) and Thiosemicarbazone(S) Cationic But-2-Yne Tungsten(II) Complexes. Z. Naturforschung—Sect. B J. Chem. Sci. 1993, 48, 1715–1718. [Google Scholar] [CrossRef]
- Jaisin, Y.; Ratanachamnong, P.; Wongsawatkul, O.; Watthammawut, A.; Malaniyom, K.; Natewong, S. Antioxidant and Anti-Inflammatory Effects of Piperine on UV-B-Irradiated Human HaCaT Keratinocyte Cells. Life Sci. 2020, 263, 118607. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, J.; Shin, Y.K.; Song, J.Y. Protective Mechanism of Morin against Ultraviolet B-Induced Cellular Senescence in Human Keratinocyte Stem Cells. Int. J. Radiat. Biol. 2014, 90, 20–28. [Google Scholar] [CrossRef]
- Shirai, A.; Onitsuka, M.; Maseda, H.; Omasa, T. Effect of Polyphenols on Reactive Oxygen Species Production and Cell Growth of Human Dermal Fibroblasts after Irradiation with Ultraviolet-A Light. Biocontrol Sci. 2015, 20, 27–33. [Google Scholar] [CrossRef]
- Scarim, C.B.; Pavan, F.R. Thiazole, Triazole, Thio- and Semicarbazone Derivatives—Promising Moieties for Drug Development for the Treatment of Tuberculosis. Eur. J. Med. Chem. Rep. 2021, 1, 100002. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gil, S.; Rodríguez-Luna, A.; Ávila-Román, J.; Rodríguez-García, G.; del Río, R.E.; Motilva, V.; Gómez-Hurtado, M.A.; Talero, E. Photoprotective Effects of Two New Morin-Schiff Base Derivatives on UVB-Irradiated HaCaT Cells. Antioxidants 2024, 13, 134. https://doi.org/10.3390/antiox13010134
García-Gil S, Rodríguez-Luna A, Ávila-Román J, Rodríguez-García G, del Río RE, Motilva V, Gómez-Hurtado MA, Talero E. Photoprotective Effects of Two New Morin-Schiff Base Derivatives on UVB-Irradiated HaCaT Cells. Antioxidants. 2024; 13(1):134. https://doi.org/10.3390/antiox13010134
Chicago/Turabian StyleGarcía-Gil, Sara, Azahara Rodríguez-Luna, Javier Ávila-Román, Gabriela Rodríguez-García, Rosa E. del Río, Virginia Motilva, Mario A. Gómez-Hurtado, and Elena Talero. 2024. "Photoprotective Effects of Two New Morin-Schiff Base Derivatives on UVB-Irradiated HaCaT Cells" Antioxidants 13, no. 1: 134. https://doi.org/10.3390/antiox13010134
APA StyleGarcía-Gil, S., Rodríguez-Luna, A., Ávila-Román, J., Rodríguez-García, G., del Río, R. E., Motilva, V., Gómez-Hurtado, M. A., & Talero, E. (2024). Photoprotective Effects of Two New Morin-Schiff Base Derivatives on UVB-Irradiated HaCaT Cells. Antioxidants, 13(1), 134. https://doi.org/10.3390/antiox13010134








