A Critical Appraisal of the Protective Activity of Polyphenolic Antioxidants against Iatrogenic Effects of Anticancer Chemotherapeutics #
Abstract
:1. Introduction
2. Methods
3. Sources and Healthy Effects of Polyphenols
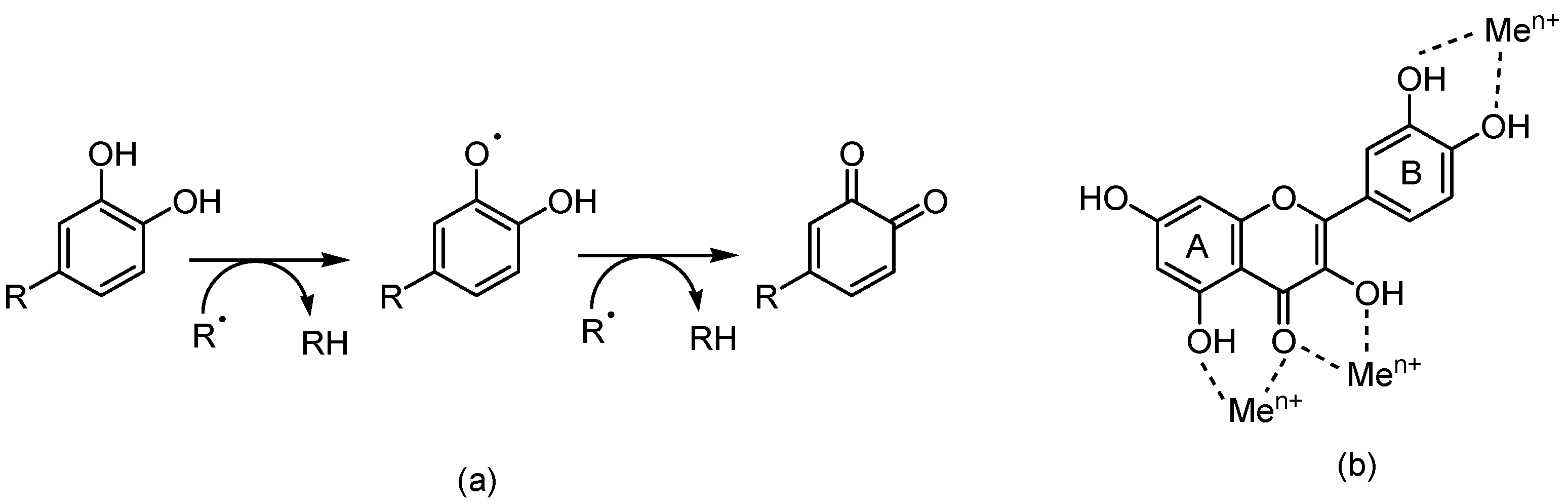
4. Polyphenols and Oxidative Stress
5. Polyphenols and Anticancer Activity
6. Protective Effects of Polyphenols against Adverse Effects of Antitumor Therapies
6.1. Polyphenolic Adjuvants in Anticancer Therapeutic Interventions
6.2. Activity against ROS-Mediated Effects of Chemotherapeutics
6.2.1. Preclinical Findings
6.2.2. Evaluation of Clinical Studies
| Source | Polyphenol/s | Key Findings | Ref. | |
|---|---|---|---|---|
| In vitro models | Wine extracts | Ellagic acid | Pro-apoptotic effect after gamma irradiation on MCF-7 cells | [50] |
| Procyanidins, catechins, flavonols | Antiproliferative effect in PC3 prostatic cancer cells | [51] | ||
| Olive tree | Oleuropein | Apoptosis induction, cell proliferation reduction, and resistance to cisplatin Reduction in ovarian carcinoma cell lines (A2780) | [52] | |
| Salidroside | ROS and superoxide reduction in breast cancer lines | [95] | ||
| Cell cycle arrest of breast cancer lines | [96] | |||
| Animal models | Honey | Coumaric acid, ferulic acid, caffeic acid, eugenol, flavonoids | Prevention of DMBA-induced breast cancer in rats | [55] |
| Olive tree | Hydroxytyrosol | Activation of immune system in mice | [57] | |
| Inhibition of chemokine-like factor 1 (CKLF1), as well as anti-oxidative and anti-apoptosis effects in kidneys of mice during cisplatin treatment | [53] | |||
| Ellagic acid | Attenuation of doxorubicin-related oxidative stress in male Wistar rats | [81] | ||
| Gallic acid | Mortality reduction and heart functional recovery in doxorubicin-treated rats | [84] | ||
| Turmeric alcoholic extract | Curcuminoids | Reduction in doxorubicin-treated male rats mortality as well as increase in heart weight and heart/bodyweight | [90] | |
| MonoHER | Protection from doxorubicin cardiotoxicity in mice | [91] | ||
| Clinical studies | Honey | Coumaric acid, ferulic acid, caffeic acid, eugenol, flavonoids | Oral mucositis reduction in double-blind clinical trial | [58] |
| MonoHER | Doxorubicin activity improvement (partial metastatic soft-tissue sarcoma remission) | [93] | ||
| Salidroside | In co-administration, no significant protection against epirubicin-related cardiotoxicity although well tolerated | [98] | ||
| Aereosol | Polyphenols | Minimization of oral side effects in patients after radiation therapy | [61] | |
| Nutridrink | Recovery of patients undergoing gastrointestinal tumor resection | [62] | ||
| MINDS | Brain protection from toxic side effects of chemotherapy | [64] | ||
| Rich-food | Reduction in radiotherapy side effects in those with breast cancer | [65] |
6.3. Bioavailability Issues
6.4. The Clinical Promise of Nanothecnology-Based Delivery Systems
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nan Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of Dietary Flavonoids: Nomenclature, Occurrence and Intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef]
- Feng, C.; Chen, B.; Fan, R.; Zou, B.; Han, B.; Guo, G. Polyphenol-Based Nanosystems for Next-Generation Cancer Therapy: Multifunctionality, Design, and Challenges. Macromol. Biosci. 2023, 23, 2300167. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary Phytochemicals and Cancer Chemoprevention: A Review of the Clinical Evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef]
- Almeida, S.; Alves, M.G.; Sousa, M.; Oliveira, P.F.; Silva, B.M. Are Polyphenols Strong Dietary Agents Against Neurotoxicity and Neurodegeneration? Neurotox. Res. 2016, 30, 345–366. [Google Scholar] [CrossRef]
- Hurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; De Gaetano, G.; on behalf of the MOLI-SANI Study Investigators. Mediterranean Diet, Dietary Polyphenols and Low Grade Inflammation: Results from the MOLI-SANI Study: Mediterranean Diet, Polyphenols and Low Grade Inflammation. Br. J. Clin. Pharmacol. 2017, 83, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, M.Á.; Estruch, R.; Ros, E. Polyphenol-Rich Foods in the Mediterranean Diet Are Associated with Better Cognitive Function in Elderly Subjects at High Cardiovascular Risk. JAD 2012, 29, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y.; Takimoto, H.; Matsumoto, T.; Kawaguchi, K. Potential Use of Dietary Natural Products, Especially Polyphenols, for Improving Type-1 Allergic Symptoms. CPD 2014, 20, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A Review of the Mechanisms and Effectiveness of Dietary Polyphenols in Reducing Oxidative Stress and Thrombotic Risk. J. Hum. Nutr. Diet. 2014, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P.; Jaquet, V.; Marcucci, F.; Schmidt, H.H.H.W. The Oxidative Stress Theory of Disease: Levels of Evidence and Epistemological Aspects: The Oxidative Stress Theory of Disease. Br. J. Pharmacol. 2017, 174, 1784–1796. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.; Campion, P.; Bishop, K. Reducing Breast Cancer Recurrence: The Role of Dietary Polyphenolics. Nutrients 2016, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov (accessed on 24 November 2023).
- Available online: https://worldwide.espacenet.com (accessed on 24 November 2023).
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of Processing on the Antioxidant Properties of Fruit and Vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.; Cerutti, A.; Beccaro, G. Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus Spp. Buds. Pharm. 2016, 9, 7. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of 62 Fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M.T.; Franceschi, S.; Lerici, C.R. Loss and/or Formation of Antioxidants during Food Processing and Storage. Cancer Lett. 1997, 114, 71–74. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Leongito, M.; Piccirillo, M.; Giudice, A.; Pivonello, C.; De Angelis, C.; Granata, V.; Palaia, R.; Izzo, F. Curcumin AntiCancer Studies in Pancreatic Cancer. Nutrients 2016, 8, 433. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical Trials of Resveratrol: Clinical Trials. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
- Jalili-Baleh, L.; Babaei, E.; Abdpour, S.; Nasir Abbas Bukhari, S.; Foroumadi, A.; Ramazani, A.; Sharifzadeh, M.; Abdollahi, M.; Khoobi, M. A Review on Flavonoid-Based Scaffolds as Multi-Target-Directed Ligands (MTDLs) for Alzheimer’s Disease. Eur. J. Med. Chem. 2018, 152, 570–589. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Zhong, K. Alzheimer’s Disease Drug Development Pipeline: 2018. TRCI 2018, 4, 195–214. [Google Scholar] [CrossRef]
- Study Details | Study of STA-1 as an Add-on Treatment to Donepezil | ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT01255046 (accessed on 22 October 2023).
- Chang, T.-T.; Huang, C.-C.; Hsu, C.-H. Clinical Evaluation of the Chinese Herbal Medicine Formula STA-1 in the Treatment of Allergic Asthma. Phytother. Res. 2006, 20, 342–347. [Google Scholar] [CrossRef]
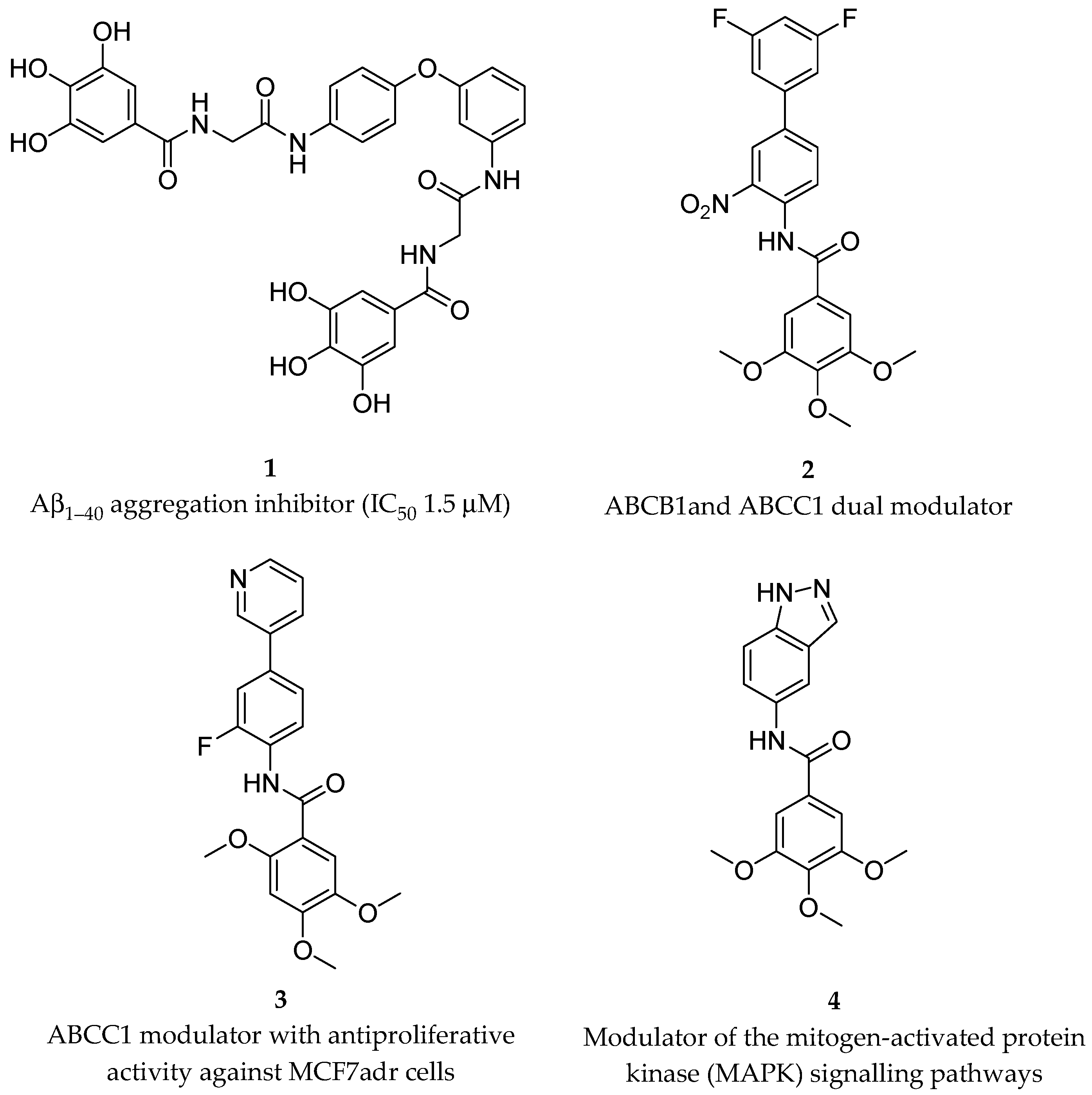
- Cellamare, S.; Stefanachi, A.; Stolfa, D.A.; Basile, T.; Catto, M.; Campagna, F.; Sotelo, E.; Acquafredda, P.; Carotti, A. Design, Synthesis, and Biological Evaluation of Glycine-Based Molecular Tongs as Inhibitors of Aβ1–40 Aggregation in Vitro. Bioorg. Med. Chem. 2008, 16, 4810–4822. [Google Scholar] [CrossRef]
- Pellicani, R.Z.; Stefanachi, A.; Niso, M.; Carotti, A.; Leonetti, F.; Nicolotti, O.; Perrone, R.; Berardi, F.; Cellamare, S.; Colabufo, N.A. Potent Galloyl-Based Selective Modulators Targeting Multidrug Resistance Associated Protein 1 and P-Glycoprotein. J. Med. Chem. 2012, 55, 424–436. [Google Scholar] [CrossRef]
- Tardia, P.; Stefanachi, A.; Niso, M.; Stolfa, D.A.; Mangiatordi, G.F.; Alberga, D.; Nicolotti, O.; Lattanzi, G.; Carotti, A.; Leonetti, F.; et al. Trimethoxybenzanilide-Based P-Glycoprotein Modulators: An Interesting Case of Lipophilicity Tuning by Intramolecular Hydrogen Bonding. J. Med. Chem. 2014, 57, 6403–6418. [Google Scholar] [CrossRef]
- Leo, V.; Stefanachi, A.; Nacci, C.; Leonetti, F.; De Candia, M.; Carotti, A.; Altomare, C.D.; Montagnani, M.; Cellamare, S. Galloyl Benzamide-Based Compounds Modulating Tumour Necrosis Factor α-Stimulated c-Jun N-Terminal Kinase and P38 Mitogen-Activated Protein Kinase Signalling Pathways. J. Pharm. Pharmacol. 2015, 67, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Ross, E.K.; Khatter, S.; Miller, K.; Linseman, D.A. Chemical Basis for the Disparate Neuroprotective Effects of the Anthocyanins, Callistephin and Kuromanin, against Nitrosative Stress. Free Radic. Bio. Med. 2017, 103, 23–34. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative Stress, Mitochondrial Dysfunction and Neurodegenerative Diseases; a Mechanistic Insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Imlay, J.A. Cellular Defenses against Superoxide and Hydrogen Peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals: Prooxidant Activity of Polyphenols and Carotenoids. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Sokolová, R.; Ramešová, Š.; Degano, I.; Hromadová, M.; Gál, M.; Žabka, J. The Oxidation of Natural Flavonoid Quercetin. Chem. Commun. 2012, 48, 3433. [Google Scholar] [CrossRef]
- Watanabe, K.; Tanaka, M.; Yuki, S.; Hirai, M.; Yamamoto, Y. How Is Edaravone Effective Against Acute Ischemic Stroke and Amyotrophic Lateral Sclerosis? J. Clin. Biochem. Nutr. 2018, 62, 20–38. [Google Scholar] [CrossRef]
- Shuaib, A.; Lees, K.R.; Lyden, P.; Grotta, J.; Davalos, A.; Davis, S.M.; Diener, H.-C.; Ashwood, T.; Wasiewski, W.W.; Emeribe, U. NXY-059 for the Treatment of Acute Ischemic Stroke. N. Engl. J. Med. 2007, 357, 562–571. [Google Scholar] [CrossRef]
- Lewandowska, U.; Fichna, J.; Gorlach, S. Enhancement of Anticancer Potential of Polyphenols by Covalent Modifications. Biochem. Pharmacol. 2016, 109, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary Polyphenols in Chemoprevention and Synergistic Effect in Cancer: Clinical Evidences and Molecular Mechanisms of Action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Li, S.; Wang, C.; Cao, N.; Qu, H.; Cheng, C.; Wang, Z.; Wang, L.; Zhou, L. Potential Applications of Polyphenols on Main ncRNAs Regulations as Novel Therapeutic Strategy for Cancer. Biomed. Pharmacother. 2019, 113, 108703. [Google Scholar] [CrossRef] [PubMed]
- Symonds, E.L.; Konczak, I.; Fenech, M. The Australian Fruit Illawarra Plum (Podocarpus Elatus Endl., Podocarpaceae) Inhibits Telomerase, Increases Histone Deacetylase Activity and Decreases Proliferation of Colon Cancer Cells. Br. J. Nutr. 2013, 109, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Pandareesh, M.D.; Mythri, R.B.; Srinivas Bharath, M.M. Bioavailability of Dietary Polyphenols: Factors Contributing to Their Clinical Application in CNS Diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Ashby, C.R.; Samuel, T.; Peraman, R.; Tiwari, A.K. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients 2017, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of Polyphenols and Its Anticancer Properties in Biomedical Research: A Narrative Review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Bishayee, A.; Yousefi, B. Polyphenols: Major Regulators of Key Components of DNA Damage Response in Cancer. DNA Repair. 2019, 82, 102679. [Google Scholar] [CrossRef] [PubMed]
- Ahire, V.; Kumar, A.; Mishra, K.P.; Kulkarni, G. Ellagic Acid Enhances Apoptotic Sensitivity of Breast Cancer Cells to γ-Radiation. Nutr. Cancer 2017, 69, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Tenta, R.; Fragopoulou, E.; Tsoukala, M.; Xanthopoulou, M.; Skyrianou, M.; Pratsinis, H.; Kletsas, D. Antiproliferative Effects of Red and White Wine Extracts in PC-3 Prostate Cancer Cells. Nutr. Cancer 2017, 69, 952–961. [Google Scholar] [CrossRef]
- Hashemi Sheikhshabani, S.; Amini-Farsani, Z.; Rahmati, S.; Jazaeri, A.; Mohammadi-Samani, M.; Asgharzade, S. Oleuropein Reduces Cisplatin Resistance in Ovarian Cancer by Targeting Apoptotic Pathway Regulators. Life Sci. 2021, 278, 119525. [Google Scholar] [CrossRef]
- Chen, C.; Ai, Q.; Wei, Y. Hydroxytyrosol Protects against Cisplatin-Induced Nephrotoxicity via Attenuating CKLF1 Mediated Inflammation, and Inhibiting Oxidative Stress and Apoptosis. Int. Immunopharmacol. 2021, 96, 107805. [Google Scholar] [CrossRef]
- Badolato, M.; Carullo, G.; Cione, E.; Aiello, F.; Caroleo, M.C. From the Hive: Honey, a Novel Weapon against Cancer. Eur. J. Med. Chem. 2017, 142, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Takruri, H.R.; Shomaf, M.S.; Shnaigat, S.F. Multi Floral Honey Has a Protective Effect against Mammary Cancer Induced by 7,12-Dimethylbenz(a)Anthracene in Sprague Dawley Rats. JAS 2017, 9, 196. [Google Scholar] [CrossRef]
- Attia, W.Y.; Gabry, M.S.; El-Shaikh, K.A.; Othman, G.A. The Anti-Tumor Effect of Bee Honey in Ehrlich Ascite Tumor Model of Mice is Coincided with Stimulation of the Immune Cells. EJI 2008, 15, 169–183. [Google Scholar]
- Lalla, R.V.; Brennan, M.M.; Schubert, M.M. Oral complications of cancer therapy. In Pharmacology and Therapeutics for Dentistry, 6th ed.; Yagiela, J.A., Dowd, F.J., Johnson, B., Mariotti, A., Neidle, E.A., Eds.; Mosby Elsevier: Amsterdam, The Netherlands, 2011; pp. 782–798. [Google Scholar]
- Raeessi, M.A.; Raeessi, N.; Panahi, Y.; Gharaie, H.; Davoudi, S.M.; Saadat, A.; Karimi Zarchi, A.A.; Raeessi, F.; Ahmadi, S.M.; Jalalian, H. “Coffee plus Honey” versus “Topical Steroid” in the Treatment of Chemotherapy-Induced Oral Mucositis: A Randomised Controlled Trial. BMC Complement. Altern. Med. 2014, 14, 293. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, A.; Lambrinou, E.; Katodritis, N.; Vomvas, D.; Raftopoulos, V.; Georgiou, M.; Paikousis, L.; Charalambous, M. The Effectiveness of Thyme Honey for the Management of Treatment-Induced Xerostomia in Head and Neck Cancer Patients: A Feasibility Randomized Control Trial. Eur. J. Oncol. Nur 2017, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Neamatallah, T.; El-Shitany, N.A.; Abbas, A.T.; Ali, S.S.; Eid, B.G. Honey Protects against Cisplatin-Induced Hepatic and Renal Toxicity through Inhibition of NF-κB-Mediated COX-2 Expression and the Oxidative Stress Dependent BAX/Bcl-2/Caspase-3 Apoptotic Pathway. Food Funct. 2018, 9, 3743–3754. [Google Scholar] [CrossRef]
- Study Details | Polyphenol Rich Aerosol as a Support for Cancer Patients in Minimizing Side Effects After a Radiation Therapy—NCT05994638| ClinicalTrials.Gov. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05994638 (accessed on 11 November 2023).
- Study Details | Investigation of a Polyphenol-Rich Preparation as Support for Oncology Patients Undergoing Gastrointestinal Tumor Resection—NCT06017661 | ClinicalTrials.Gov. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06017661 (accessed on 4 November 2023).
- Xue, D.; Peng, Y.; Zhang, M.; Zheng, L.; Liang, Q.; Li, H.; Yu, J.S.; Chen, J.T. Compositions and Methods for Preventing and Treating Radiation-Induced Bystander Effects Caused by Radiation or Radio-Therapy—CN111447940. 2020. Available online: https://worldwide.espacenet.com/patent/search/family/065015329/publication/CN111447940A?q=CN111447940 (accessed on 29 October 2023).
- Study Details | Pilot Study of a MIND Diet Intervention in Women Undergoing Active Treatment for Breast Cancer—NCT05984888| ClinicalTrials.Gov. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05984888 (accessed on 10 November 2023).
- Study Details | Supplementation with Dietary Anthocyanins and Side Effects of Radiotherapy for Breast Cancer—NCT02195960| ClinicalTrials.Gov. 2023. Available online: https://clinicaltrials.gov/study/NCT02195960 (accessed on 28 October 2023).
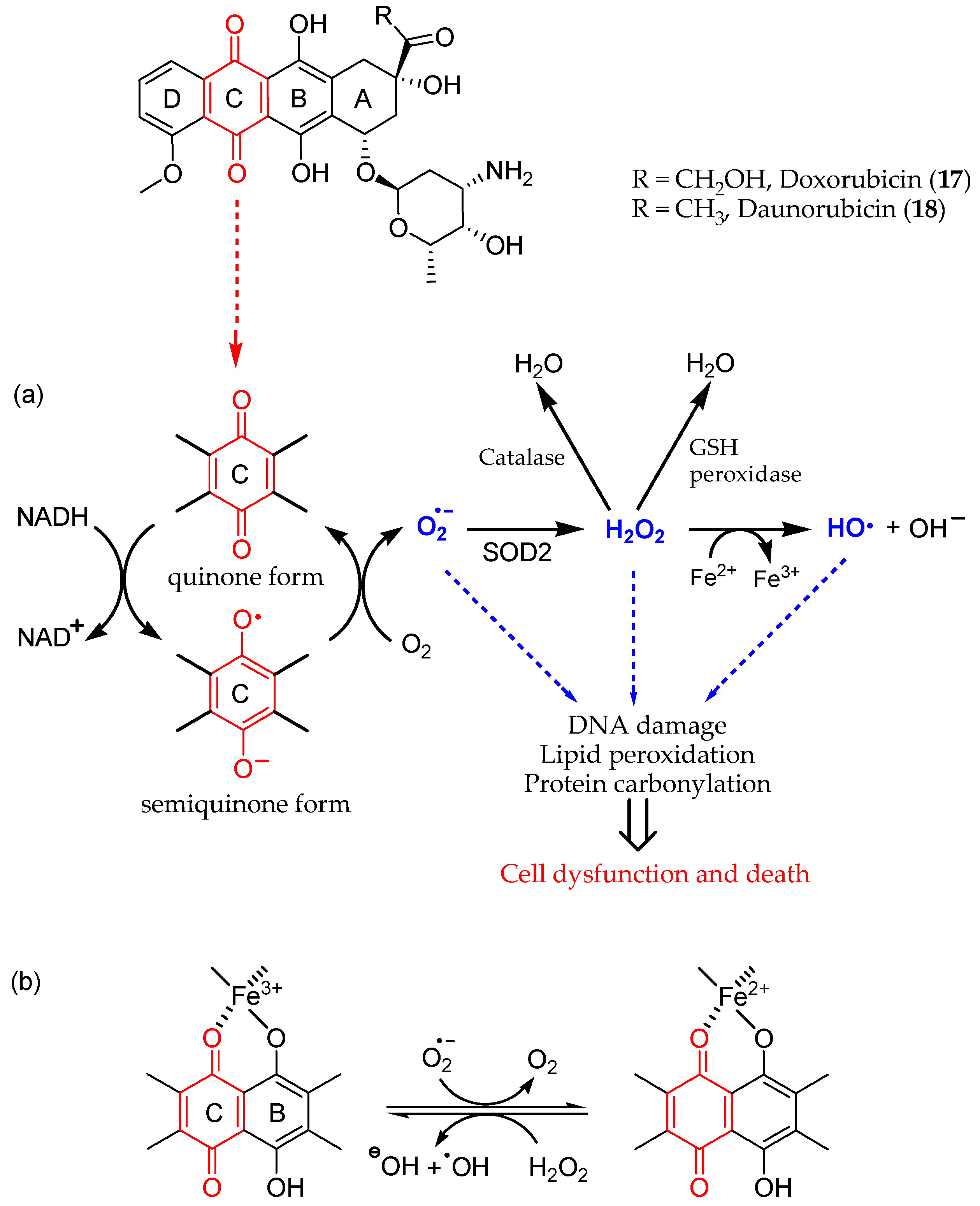
- Fujiwara, A.; Hoshino, T.; Westley, J.W. Anthracycline Antibiotics. Crit. Rev. Biotechnol. 1985, 3, 133–157. [Google Scholar] [CrossRef]
- Brown, S.-A.; Sandhu, N.; Herrmann, J. Systems Biology Approaches to Adverse Drug Effects: The Example of Cardio-Oncology. Nat. Rev. Clin. Oncol. 2015, 12, 718–731. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Volkova, M.; Russell, R. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. CCR 2012, 7, 214–220. [Google Scholar] [CrossRef]
- Vejpongsa, P.; Yeh, E.T.H. Prevention of Anthracycline-Induced Cardiotoxicity. J. Am. Coll. Cardiol. 2014, 64, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Jungsuadee, P. Doxorubicin-Induced Cardiomyopathy: An Update beyond Oxidative Stress and Myocardial Cell Death. Cardiovasc. Regen. Med. 2016, 3, e1127. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of Doxorubicin Is Mediated through Mitochondrial Iron Accumulation. J. Clin. Invest. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Maccarinelli, F.; Buratti, P.; Recalcati, S.; Cairo, G. The Role of Iron in Anthracycline Cardiotoxicity. Front. Pharmacol. 2014, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.K.; Gerdes, S.; West, A.; Richter, S.; Busemann, C.; Hentschel, L.; Lenz, F.; Kopp, H.-G.; Ehninger, G.; Reichardt, P.; et al. Efficacy and Safety of Dexrazoxane (DRZ) in Sarcoma Patients Receiving High Cumulative Doses of Anthracycline Therapy—A Retrospective Study Including 32 Patients. BMC Cancer 2016, 16, 619. [Google Scholar] [CrossRef] [PubMed]
- Lehenbauer Ludke, A.R.; Al-Shudiefat, A.A.-R.S.; Dhingra, S.; Jassal, D.S.; Singal, P.K. A Concise Description of Cardioprotective Strategies in Doxorubicin-Induced cardiotoxicityThis Article Is One of a Selection of Papers Published in a Special Issue Celebrating the 125th Anniversary of the Faculty of Medicine at the University of Manitoba. Can. J. Physiol. Pharmacol. 2009, 87, 756–763. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R. The Effects of Ellagic Acid upon Brain Cells: A Mechanistic View and Future Directions. Neurochem. Res. 2016, 41, 1219–1228. [Google Scholar] [CrossRef]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, Ellagic Acid and Vascular Health. Mol. Aspects Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Khatri, D.; Juvekar, A. Kinetics of Inhibition of Monoamine Oxidase Using Curcumin and Ellagic Acid. Phcog Mag. 2016, 12, 116. [Google Scholar] [CrossRef]
- Farbood, Y.; Sarkaki, A.; Dolatshahi, M.; Mansouri, S.M.T.; Khodadadi, A. Ellagic Acid Protects the Brain Against 6-Hydroxydopamine Induced Neuroinflammation in a Rat Model of Parkinson’s Disease. Basic.Clinic. Neurosci. 2015, 6, 83–89. [Google Scholar]
- Mansouri, M.T.; Farbood, Y.; Naghizadeh, B.; Shabani, S.; Mirshekar, M.A.; Sarkaki, A. Beneficial Effects of Ellagic Acid against Animal Models of Scopolamine- and Diazepam-Induced Cognitive Impairments. Pharm. Biol. 2016, 54, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Warpe, V.S.; Mali, V.R.; S, A.; Bodhankar, S.L.; Mahadik, K.R. Cardioprotective Effect of Ellagic Acid on Doxorubicin Induced Cardiotoxicity in Wistar Rats. JACME 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal Importance of Gallic Acid and Its Ester Derivatives: A Patent Review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Lorenzo, A.; Nabavi, S.; Talas, Z.; Nabavi, S. Polyphenols: Well Beyond The Antioxidant Capacity: Gallic Acid. and Related Compounds as Neuroprotective Agents: You Are What You Eat! CPB 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.; Swamy, A.H.M.V. Cardioprotective Effect of Gallic Acid against Doxorubicin-Induced Myocardial Toxicity in Albino Rats. Indian. J. Health Sci. Biomed. Res. 2015, 8, 28. [Google Scholar] [CrossRef]
- Pothitirat, W.; Gritsanapan, W. Variation of Bioactive Components in Curcuma Longa in Thailand. Curr. Sci. 2006, 91, 1397–1400. [Google Scholar]
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular Mechanisms Underlying Chemopreventive Potential of Curcumin: Current Challenges and Future Perspectives. Life Sci. 2016, 148, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases: Curcumin: From Kitchen to Clinic. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; El-azeem, A.S.A.; Afify, A.A.; Shabana, M.H.; Ahmed, H.H. Cardioprotective Effects of Curcuma Longa, L. Extracts against Doxorubicin-Induced Cardiotoxicity in Rats. J. Med. Plants Res. 2011, 5, 4049–4058. [Google Scholar]
- Van Acker, F.A.A.; Van Acker, S.A.B.E.; Krammer, K.; Haenen, G.R.M.M.; Bast, A.; Van Der Vijgh, W.J.F. 7-Monohydroxyethylrutoside Protects against Chronic Doxorubicin-Induced Cardiotoxicity When Administered Only Once per Week. Clin. Cancer Res. 2000, 6, 1337–1341. [Google Scholar] [PubMed]
- Bruynzeel, A.M.E.; Niessen, H.W.M.; Bronzwaer, J.G.F.; Van Der Hoeven, J.J.M.; Berkhof, J.; Bast, A.; Van Der Vijgh, W.J.F.; Van Groeningen, C.J. The Effect of Monohydroxyethylrutoside on Doxorubicin-Induced Cardiotoxicity in Patients Treated for Metastatic Cancer in a Phase II Study. Br. J. Cancer 2007, 97, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-W.; Hu, J.-J.; Fu, R.-Q.; Liu, X.; Zhang, Y.-H.; Li, J.; Liu, L.; Li, Y.-N.; Deng, Q.; Luo, Q.-S.; et al. Flavonoids Inhibit Cell Proliferation and Induce Apoptosis and Autophagy through Downregulation of PI3Kγ Mediated PI3K/AKT/mTOR/p70S6K/ULK Signaling Pathway in Human Breast Cancer Cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xin, H.; Wu, L.-X.; Zhu, Y.-Z. Salidroside Attenuates Apoptosis in Ischemic Cardiomyocytes: A Mechanism Through a Mitochondria-Dependent Pathway. J. Pharmacol. Sci. 2010, 114, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Schriner, S.E.; Abrahamyan, A.; Avanessian, A.; Bussel, I.; Maler, S.; Gazarian, M.; Holmbeck, M.A.; Jafari, M. Decreased Mitochondrial Superoxide Levels and Enhanced Protection against Paraquat in Drosophila Melanogaster Supplemented with Rhodiola Rosea. Free. Radic. Res. 2009, 43, 836–843. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Qiu, S.; Yu, D.; Lin, S. Salidroside Induces Cell-Cycle Arrest and Apoptosis in Human Breast Cancer Cells. BBRC 2010, 398, 62–67. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, H.; Jin, Z.; Bi, S.; Yang, X.; Yi, D.; Liu, W. Cardioprotection of Salidroside from Ischemia/Reperfusion Injury by Increasing N-Acetylglucosamine Linkage to Cellular Proteins. Eur. J. Pharmacol. 2009, 613, 93–99. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, W.; Gao, C.; Deng, L.; Shen, D. Protective Effects of Salidroside on Epirubicin-Induced Early Left Ventricular Regional Systolic Dysfunction in Patients with Breast Cancer. Drugs R. D. 2012, 12, 101–106. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol Nanoformulation for Cancer Prevention and Therapy: Resveratrol Nanoformulations for Cancer. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31. [Google Scholar] [CrossRef]
- Griesser, M.; Pistis, V.; Suzuki, T.; Tejera, N.; Pratt, D.A.; Schneider, C. Autoxidative and Cyclooxygenase-2 Catalyzed Transformation of the Dietary Chemopreventive Agent Curcumin. J. Biol. Chem. 2011, 286, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.N.; Schneider, C. Vanillin and Ferulic Acid: Not the Major Degradation Products of Curcumin. Trends Mol. Med. 2012, 18, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Lubinu, G.; Madau, P.; Pala, N.; Nurra, S.; Mariani, A.; Sechi, M. Polymeric Nanoparticles Encapsulating White Tea Extract for Nutraceutical Application. J. Agric. Food Chem. 2015, 63, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Nagavarma, B.V.N.; Hemant, K.S.Y.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preparation of polymeric nanoparticles. AJPCR 2012, 5, 16–23. [Google Scholar]
- Bohn, T. Dietary Factors Affecting Polyphenol Bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I Clinical Trial and Pharmacokinetic Evaluation of NK911, a Micelle-Encapsulated Doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Bioavailability and Delivery of Nutraceuticals and Functional Foods Using Nanotechnology. In Bio-Nanotechnology; Bagchi, D., Bagchi, M., Moriyama, H., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 593–604. ISBN 978-0-470-67037-8. [Google Scholar]
- Zaletok, S.; Gulua, L.; Wicker, L.; Shlyakhovenko, V.O.; Gogo, S.; Orlovsky, O.; Karnaushenko, O.; Verbinenko, A.; Milinevska, V.; Samoylenko, O.; et al. Green tea, red wine and lemon extracts reduce experimental tumor growth and cancer drug toxicity. Exp. Onc. 2015, 37, 262–271. [Google Scholar] [CrossRef]
- Cook, M.T. Mechanism of Metastasis Suppression by Luteolin in Breast Cancer. BCTT 2018, 10, 89–100. [Google Scholar] [CrossRef]
- Sabzichi, M.; Hamishehkar, H.; Ramezani, F.; Sharifi, S.; Tabasinezhad, M.; Pirouzpanah, M.; Ghanbari, P.; Samadi, N. Luteolin-Loaded Phytosomes Sensitize Human Breast Carcinoma MDA-MB 231 Cells to Doxorubicin by Suppressing Nrf2 Mediated Signalling. APJCP 2014, 15, 5311–5316. [Google Scholar] [CrossRef]
- Srinivas, P.; Sadanandam; Manthena, S. Phytosomes in Herbal Drug Delivery. J. Nat. Pharm. 2010, 1, 14. [Google Scholar] [CrossRef]
- Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Novel Resveratrol and 5-Fluorouracil Coencapsulated in PEGylated Nanoliposomes Improve Chemotherapeutic Efficacy of Combination against Head and Neck Squamous Cell Carcinoma. BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of Curcumin: An Emerging Paradigm for Improved Remedial Application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Karewicz, A.; Bielska, D.; Loboda, A.; Gzyl-Malcher, B.; Bednar, J.; Jozkowicz, A.; Dulak, J.; Nowakowska, M. Curcumin-Containing Liposomes Stabilized by Thin Layers of Chitosan Derivatives. Colloids Surf. B Biointerfaces 2013, 109, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, L.; Liu, C.; Zhang, D.; Wang, S.; Deng, Z.; Lou, W.; Xu, H.; Bai, Q.; Ma, J. Preparation and Characterization of Cationic Curcumin Nanoparticles for Improvement of Cellular Uptake. Carbohyd. Polym. 2012, 90, 16–22. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Nimmannit, U.; Callery, P.S.; Rojsitthisak, P. Effects of Different Carboxylic Ester Spacers on Chemical Stability, Release Characteristics, and Anticancer Activity of Mono-PEGylated Curcumin Conjugates. J. Pharm. Sci. 2011, 100, 5206–5218. [Google Scholar] [CrossRef]
- Gong, C.; Deng, S.; Wu, Q.; Xiang, M.; Wei, X.; Li, L.; Gao, X.; Wang, B.; Sun, L.; Chen, Y.; et al. Improving Antiangiogenesis and Anti-Tumor Activity of Curcumin by Biodegradable Polymeric Micelles. Biomaterials 2013, 34, 1413–1432. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purgatorio, R.; Boccarelli, A.; Pisani, L.; de Candia, M.; Catto, M.; Altomare, C.D. A Critical Appraisal of the Protective Activity of Polyphenolic Antioxidants against Iatrogenic Effects of Anticancer Chemotherapeutics. Antioxidants 2024, 13, 133. https://doi.org/10.3390/antiox13010133
Purgatorio R, Boccarelli A, Pisani L, de Candia M, Catto M, Altomare CD. A Critical Appraisal of the Protective Activity of Polyphenolic Antioxidants against Iatrogenic Effects of Anticancer Chemotherapeutics. Antioxidants. 2024; 13(1):133. https://doi.org/10.3390/antiox13010133
Chicago/Turabian StylePurgatorio, Rosa, Angelina Boccarelli, Leonardo Pisani, Modesto de Candia, Marco Catto, and Cosimo D. Altomare. 2024. "A Critical Appraisal of the Protective Activity of Polyphenolic Antioxidants against Iatrogenic Effects of Anticancer Chemotherapeutics" Antioxidants 13, no. 1: 133. https://doi.org/10.3390/antiox13010133