Effect of Spicatoside a on Anti-Osteosarcoma MG63 Cells through Reactive Oxygen Species Generation and the Inhibition of the PI3K-AKT-mTOR Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and General Procedures
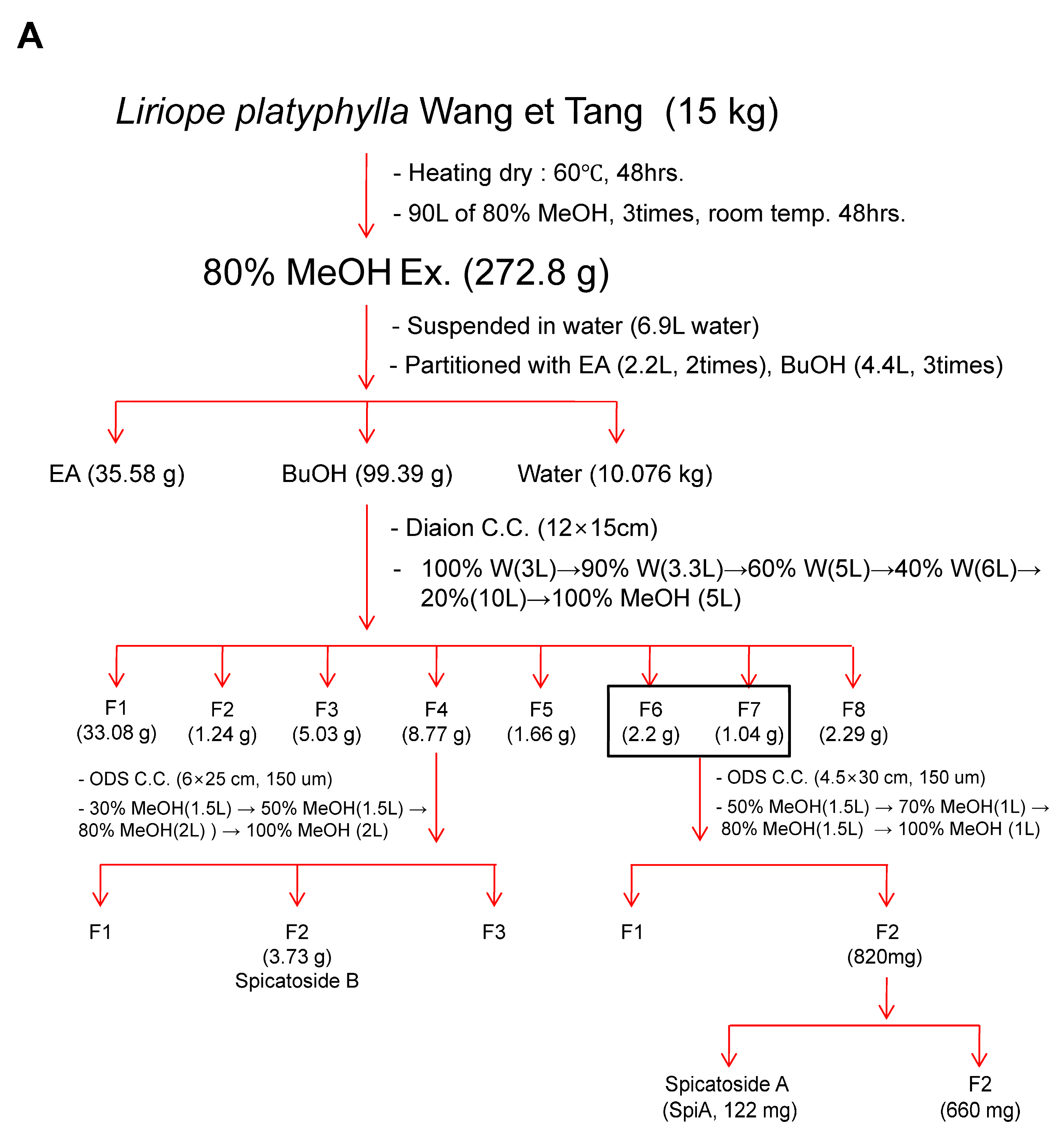
2.2. Extraction and Isolation
2.3. SpiA Stock Solution
2.4. Cell Culture
2.5. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.6. BrdU Cell Proliferation Assay
2.7. Western Blotting
2.8. Proteome Profiler Human Phospho-Kinase Array Kit
2.9. Autophagosome Formation Assay
2.10. ROS and Mitochondrial Membrane Potential
2.11. Terminal Deoxynucleotidyl Transferase-Mediated FITC–dUDP Nick-End Labeling (TUNEL) Assay
2.12. Cell Migration Assay
2.13. Cell Invasion Assay
2.14. Statistical Analysis
3. Results
3.1. Isolation and Characterization of SpiA from L. platyphylla
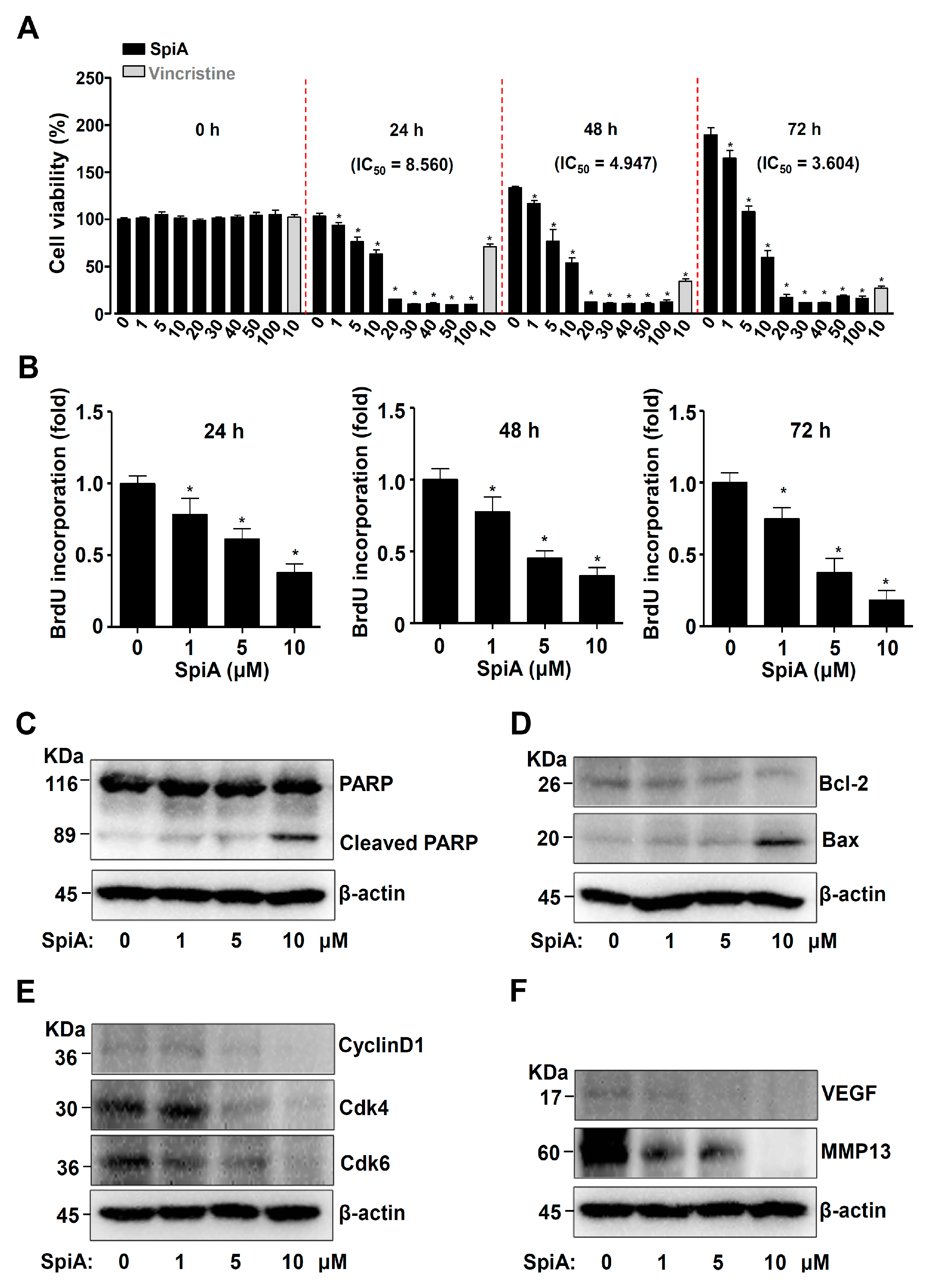
3.2. SpiA Suppresses Proliferation and Induces Apoptotic Cell Death in Human MG63 Cells
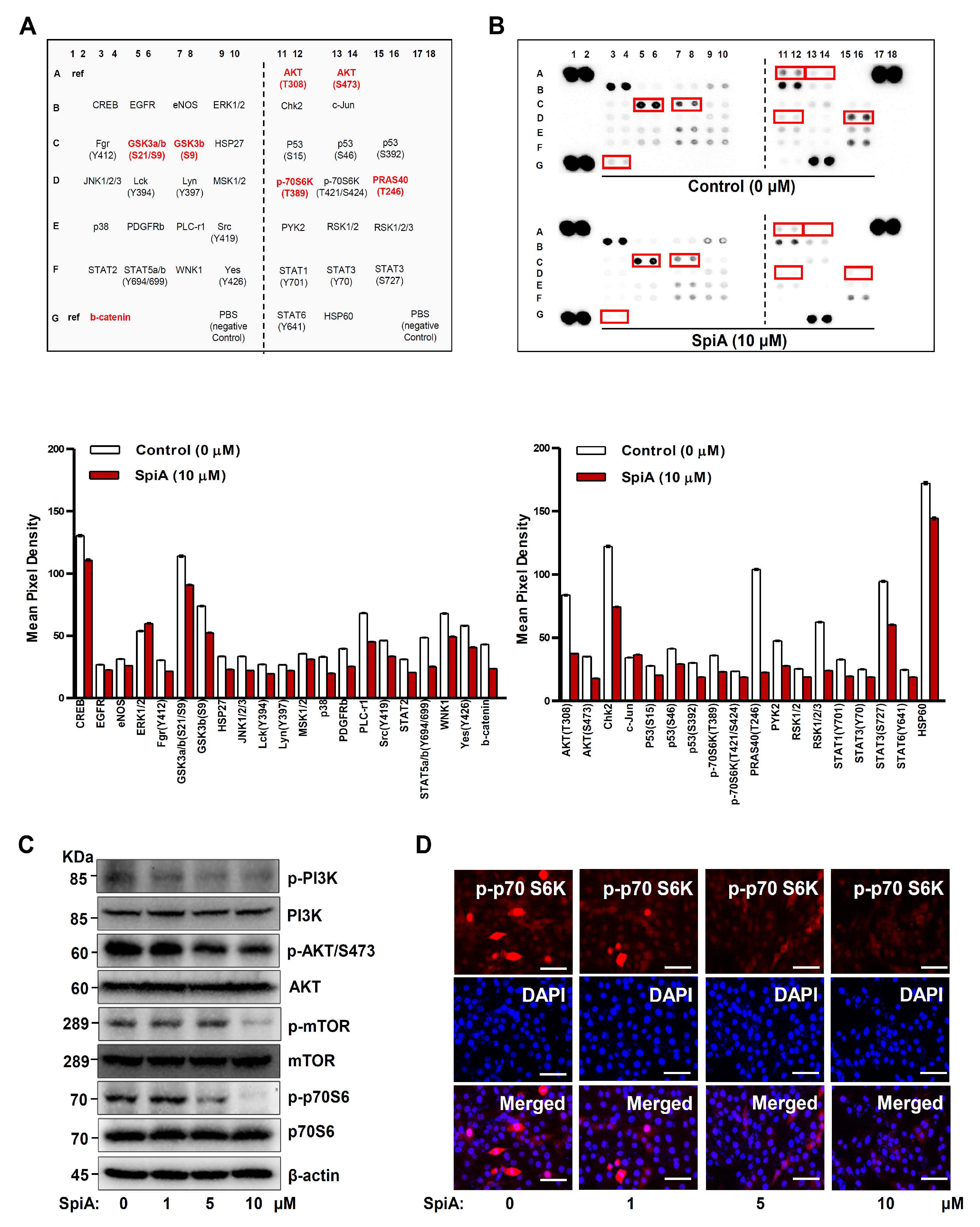
3.3. SpiA Suppresses the PI3K-AKT-Mammalian Target of Rapamycin (mTOR)-p70S6K Pathway in Human MG63 Cells
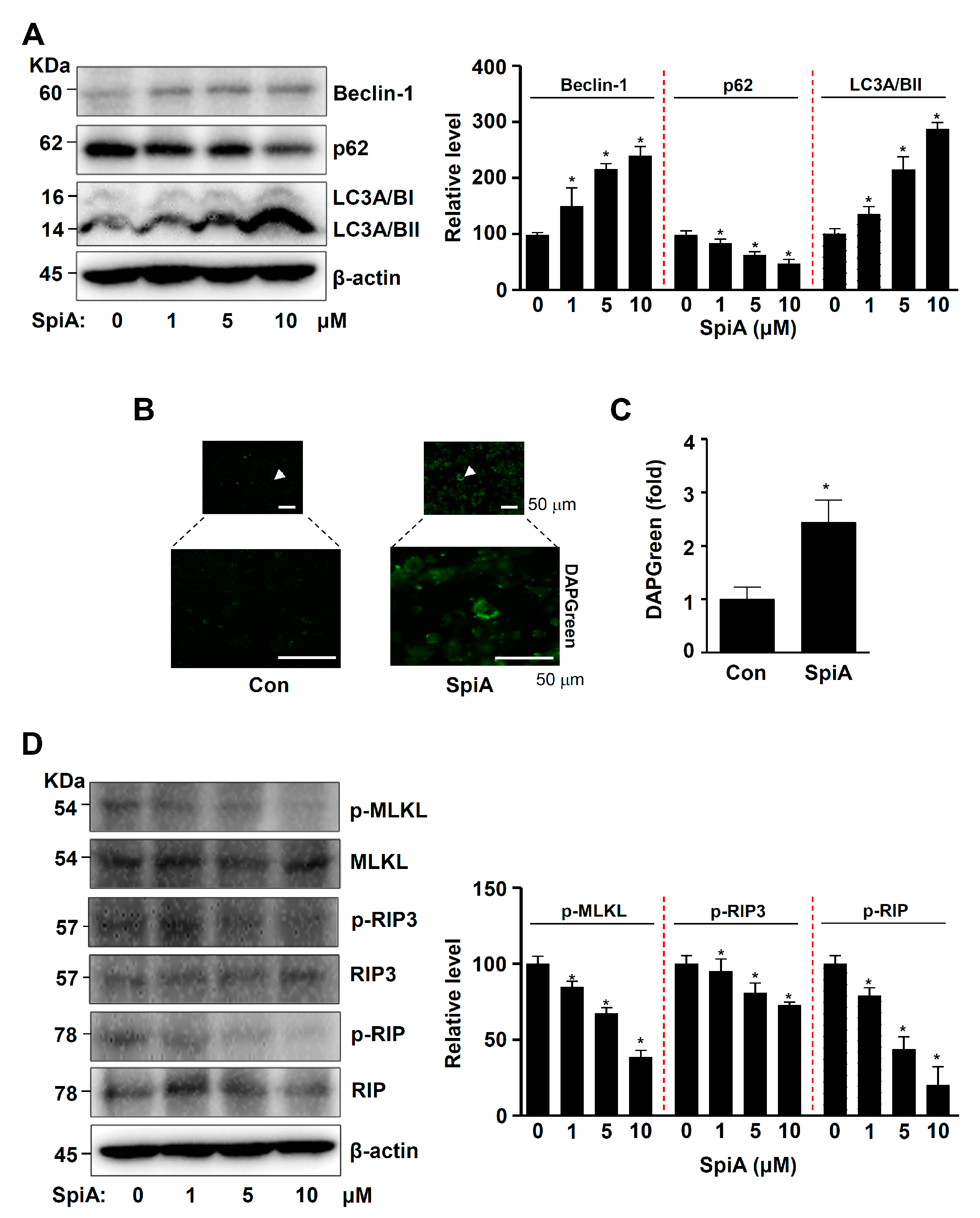
3.4. SpiA Induces Autophagy and Inhibits Necroptosis in Human MG63 Cells
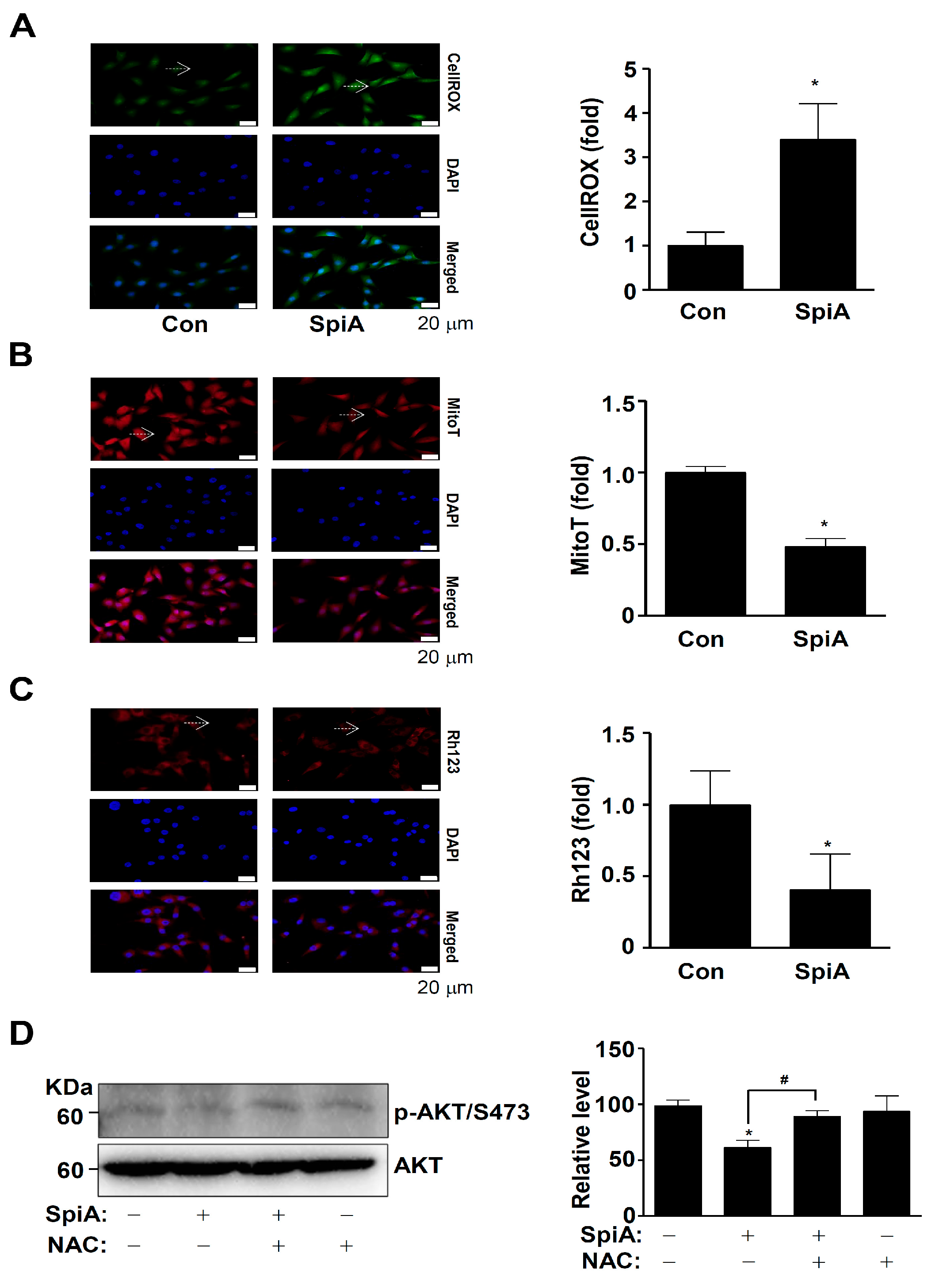
3.5. SpiA Causes ROS Generation and Mitochondria Potential Loss, and Inhibits the Activation of AKT in Human MG63 Cells
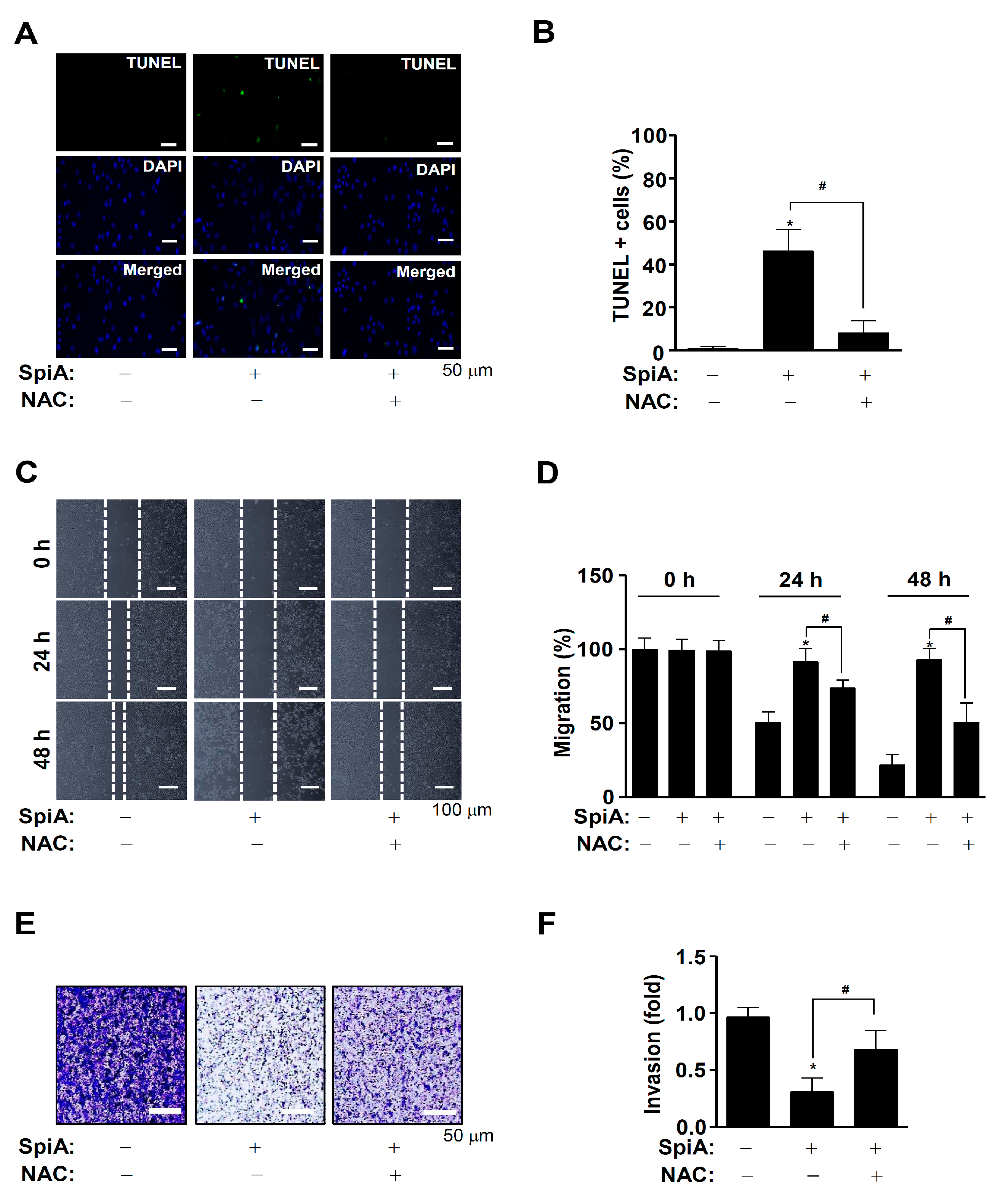
3.6. SpiA Causes Apoptotic Cell Death, Anti-Migration, and Anti-Invasion Effects through ROS Generation in Human MG63 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | AKT serine–threonine kinase |
| L. platyphylla | Liriope platyphylla Wang et Tang |
| NAC | N-acetylcysteine |
| NMR | Nuclear magnetic resonance |
| MLKL | Mixed lineage kinase domain like pseudokinase |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| mTOR | Mammalian target of rapamycin |
| p70S6K | 70-kDa ribosomal protein S6 kinase |
| RIP | Receptor-interacting serine/threonine protein kinase |
| PRAS40 | 40 kDa proline-rich Akt substrate |
| SpiA | Spicatoside A |
| ROS | Reactive oxygen species |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated FITC–dUDP nick-end labeling |
References
- Tian, H.; Cao, J.; Li, B.; Nice, E.C.; Mao, H.; Zhang, Y.; Huang, C. Managing the immune microenvironment of osteosarcoma: The outlook for osteosarcoma treatment. Bone Res. 2023, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of Osteosarcoma and Current Management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Gallyas, F., Jr.; Sumegi, B. Mitochondrial Protection by PARP Inhibition. Int. J. Mol. Sci. 2020, 21, 2767. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Loreth, B.; Battmann, A.; Knoblauch, B.; Stahl, U.; Pollex, U.; Bohle, R.M. Bone matrix production in osteosarcoma. Verh. Dtsch. Ges. Pathol. 1998, 82, 144–153. [Google Scholar] [PubMed]
- Panez-Toro, I.; Munoz-Garcia, J.; Vargas-Franco, J.W.; Renodon-Corniere, A.; Heymann, M.F.; Lezot, F.; Heymann, D. Advances in Osteosarcoma. Curr. Osteoporos. Rep. 2023, 21, 330–343. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, J.; Bai, J.; Shen, H.; Zhang, B.; Deng, L.; Sun, C.; Liu, Y.; Zhang, J.; Zheng, J. Risk and clinicopathological features of osteosarcoma metastasis to the lung: A population-based study. J. Bone Oncol. 2019, 16, 100230. [Google Scholar] [CrossRef]
- Harris, M.A.; Hawkins, C.J. Recent and Ongoing Research into Metastatic Osteosarcoma Treatments. Int. J. Mol. Sci. 2022, 23, 3817. [Google Scholar] [CrossRef]
- Lai, Q.; Ye, C.; Gao, T.; Xiao, J.; Xie, A.; Liu, X.; Yu, X.; Liu, J.; Dai, M.; Liu, H.; et al. Therapeutic effect of neoadjuvant chemotherapy combined with curettage to treat distal femoral osteosarcoma: A case report. Medicine 2017, 96, e8672. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhao, N.; Wang, C.; Kamar, S.; Zhou, Y.; He, Z.; Yang, J.; Sun, B.; Shi, X.; et al. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol. Lett. 2018, 16, 6228–6237. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Tang, J.Y.; Yang, K.H.; Chang, F.R.; Hou, M.F.; Yen, C.Y.; Chang, H.W. The Impact of Oxidative Stress and AKT Pathway on Cancer Cell Functions and Its Application to Natural Products. Antioxidants 2022, 11, 1845. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Mermel, C.H.; Robinson, J.T.; Garraway, L.A.; Golub, T.R.; Meyerson, M.; Gabriel, S.B.; Lander, E.S.; Getz, G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014, 505, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, Y.; Liu, J.; Yang, X. Functional role of MicroRNA/PI3K/AKT axis in osteosarcoma. Front. Oncol. 2023, 13, 1219211. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Xu, C.; Wang, M.; Zandieh Doulabi, B.; Sun, Y.; Liu, Y. Paradox: Curcumin, a Natural Antioxidant, Suppresses Osteosarcoma Cells via Excessive Reactive Oxygen Species. Int. J. Mol. Sci. 2023, 24, 11975. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox. Lancet 2000, 355, 1179–1180. [Google Scholar] [CrossRef]
- Wen, C.; Wang, H.; Wu, X.; He, L.; Zhou, Q.; Wang, F.; Chen, S.; Huang, L.; Chen, J.; Wang, H.; et al. ROS-mediated inactivation of the PI3K/AKT pathway is involved in the antigastric cancer effects of thioredoxin reductase-1 inhibitor chaetocin. Cell Death Dis. 2019, 10, 809. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; He, Z.; Zhu, F.; Wang, M.; He, R.; Zhao, C.; Shi, X.; Zhou, M.; Pan, S.; et al. Inhibition of PI3K/AKT signaling via ROS regulation is involved in Rhein-induced apoptosis and enhancement of oxaliplatin sensitivity in pancreatic cancer cells. Int. J. Biol. Sci. 2021, 17, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhou, J.; Zhou, Z.; Zhu, Q. Abamectin induces apoptosis and autophagy by inhibiting reactive oxygen species-mediated PI3K/AKT signaling in MGC803 cells. J. Biochem. Mol. Toxicol. 2019, 33, e22336. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Pan, S.L.; Xiao, Z.Y.; Hsu, J.L.; Chen, M.C.; Lee, K.H.; Teng, C.M. NPRL-Z-1, as a new topoisomerase II poison, induces cell apoptosis and ROS generation in human renal carcinoma cells. PLoS ONE 2014, 9, e112220. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, D.; Wang, D.; Wu, R.; Zhang, L.; Zhu, H.; He, Q.; Yang, B. ROS-driven Akt dephosphorylation at Ser-473 is involved in 4-HPR-mediated apoptosis in NB4 cells. Free Radic. Biol. Med. 2009, 47, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Youn Hwang, D. Enormous Potential for Development Liriope Platyphylla Wang et Tang as a Therapeutic Drug on the Human Chronic Disease; IntechOpen: London, UK, 2012. [Google Scholar]
- Wang, H.C.; Wu, C.C.; Cheng, T.S.; Kuo, C.Y.; Tsai, Y.C.; Chiang, S.Y.; Wong, T.S.; Wu, Y.C.; Chang, F.R. Active Constituents from Liriope platyphylla Root against Cancer Growth In Vitro. Evid. Based Complement. Alternat Med. 2013, 2013, 857929. [Google Scholar]
- Wang, H.C.; Chang, F.R.; Huang, T.J.; Kuo, C.Y.; Tsai, Y.C.; Wu, C.C. (-)-Liriopein B Suppresses Breast Cancer Progression via Inhibition of Multiple Kinases. Chem. Res. Toxicol. 2015, 28, 897–906. [Google Scholar] [CrossRef]
- Sheng, H.; Lv, W.; Zhu, L.; Wang, L.; Wang, Z.; Han, J.; Hu, J. Liriopesides B induces apoptosis and cell cycle arrest in human non-small cell lung cancer cells. Int. J. Mol. Med. 2020, 46, 1039–1050. [Google Scholar] [CrossRef]
- Kim, W.K.; Pyee, Y.; Chung, H.J.; Park, H.J.; Hong, J.Y.; Son, K.H.; Lee, S.K. Antitumor Activity of Spicatoside A by Modulation of Autophagy and Apoptosis in Human Colorectal Cancer Cells. J. Nat. Prod. 2016, 79, 1097–1104. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S.J. Pharmacological Activities and Applications of Spicatoside A. Biomol. Ther. 2016, 24, 469–474. [Google Scholar] [CrossRef]
- Yun, H.M.; Kwon, H.S.; Lee, J.Y.; Park, K.R. Vitexicarpin Induces Apoptosis and Inhibits Metastatic Properties via the AKT-PRAS40 Pathway in Human Osteosarcoma. Int. J. Mol. Sci. 2024, 25, 3582. [Google Scholar] [CrossRef]
- Park, K.R.; Park, J.I.; Lee, S.; Yoo, K.; Kweon, G.R.; Kwon, I.K.; Yun, H.M.; Hong, J.T. Chi3L1 is a therapeutic target in bone metabolism and a potential clinical marker in patients with osteoporosis. Pharmacol. Res. 2022, 184, 106423. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Yun, H.M.; Hong, J.T. G721-0282 inhibits cell growth and induces apoptosis in human osteosarcoma through down-regulation of the STAT3 pathway. Int. J. Biol. Sci. 2020, 16, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, J.E.; Lee, J.Y.; Park, K.R. Latifolin, a Natural Flavonoid, Isolated from the Heartwood of Dalbergia odorifera Induces Bioactivities through Apoptosis, Autophagy, and Necroptosis in Human Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 13629. [Google Scholar] [CrossRef]
- Yun, H.M.; Kwon, Y.J.; Kim, E.; Chung, H.J.; Park, K.R. Machilin D Promotes Apoptosis and Autophagy, and Inhibits Necroptosis in Human Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2023, 24, 4576. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert. Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Park, K.R.; Yun, H.M.; Quang, T.H.; Oh, H.; Lee, D.S.; Auh, Q.S.; Kim, E.C. 4-Methoxydalbergione suppresses growth and induces apoptosis in human osteosarcoma cells in vitro and in vivo xenograft model through down-regulation of the JAK2/STAT3 pathway. Oncotarget 2016, 7, 6960–6971. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, K.R.; Quang, T.H.; Oh, H.; Hong, J.T.; Kim, Y.C.; Kim, E.C. 4-parvifuran inhibits metastatic and invasive actions through the JAK2/STAT3 pathway in osteosarcoma cells. Arch. Pharm. Res. 2017, 40, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Leem, H.H.; Lee, J.; Kwon, I.K.; Hong, J.T.; Yun, H.M. Anti-cancer effects of Hederoside C, a pentacyclic triterpene saponin, through the intrinsic apoptosis and STAT3 signaling pathways in osteosarcoma. Am. J. Cancer Res. 2021, 11, 4541–4550. [Google Scholar] [PubMed]
- Park, K.R.; Kwon, Y.J.; Cho, M.; Kwon, I.K.; Hong, J.T.; Yun, H.M. 11-O-Galloyl Bergenin from Corylopsis coreanas Leaves Induces Autophagy and Apoptosis in Human Osteosarcoma. Am. J. Chin. Med. 2021, 49, 2017–2031. [Google Scholar] [CrossRef] [PubMed]
- Tewari, M.; Quan, L.T.; O’Rourke, K.; Desnoyers, S.; Zeng, Z.; Beidler, D.R.; Poirier, G.G.; Salvesen, G.S.; Dixit, V.M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 1995, 81, 801–809. [Google Scholar] [CrossRef]
- Bai, X.T.; Moles, R.; Chaib-Mezrag, H.; Nicot, C. Small PARP inhibitor PJ-34 induces cell cycle arrest and apoptosis of adult T-cell leukemia cells. J. Hematol. Oncol. 2015, 8, 117. [Google Scholar] [CrossRef]
- Mashimo, M.; Onishi, M.; Uno, A.; Tanimichi, A.; Nobeyama, A.; Mori, M.; Yamada, S.; Negi, S.; Bu, X.; Kato, J.; et al. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J. Biol. Chem. 2021, 296, 100046. [Google Scholar] [CrossRef]
- Ugarte-Uribe, B.; Garcia-Saez, A.J. Apoptotic foci at mitochondria: In and around Bax pores. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160217. [Google Scholar] [CrossRef]
- Kluck, R.M.; Bossy-Wetzel, E.; Green, D.R.; Newmeyer, D.D. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 1997, 275, 1132–1136. [Google Scholar] [CrossRef]
- Roue, G.; Pichereau, V.; Lincet, H.; Colomer, D.; Sola, B. Cyclin D1 mediates resistance to apoptosis through upregulation of molecular chaperones and consequent redistribution of cell death regulators. Oncogene 2008, 27, 4909–4920. [Google Scholar] [CrossRef]
- Yang, K.; Hitomi, M.; Stacey, D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, X.H.; Yan, Y.G.; Wang, C.; Wang, W.J. PI3K/Akt signaling in osteosarcoma. Clin. Chim. Acta 2015, 444, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Vincent, E.E.; Elder, D.J.; Thomas, E.C.; Phillips, L.; Morgan, C.; Pawade, J.; Sohail, M.; May, M.T.; Hetzel, M.R.; Tavare, J.M. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br. J. Cancer 2011, 104, 1755–1761. [Google Scholar] [CrossRef]
- Gao, Y.; Moten, A.; Lin, H.K. Akt: A new activation mechanism. Cell Res. 2014, 24, 785–786. [Google Scholar] [CrossRef]
- Sadrkhanloo, M.; Paskeh, M.D.A.; Hashemi, M.; Raesi, R.; Bahonar, A.; Nakhaee, Z.; Entezari, M.; Beig Goharrizi, M.A.S.; Salimimoghadam, S.; Ren, J.; et al. New emerging targets in osteosarcoma therapy: PTEN and PI3K/Akt crosstalk in carcinogenesis. Pathol. Res. Pract. 2023, 251, 154902. [Google Scholar] [CrossRef]
- Levine, B. Cell biology: Autophagy and cancer. Nature 2007, 446, 745–747. [Google Scholar] [CrossRef]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, C.; Yang, P.; Li, C.; Li, M. Eldecalcitol induces apoptosis and autophagy in human osteosarcoma MG-63 cells by accumulating ROS to suppress the PI3K/Akt/mTOR signaling pathway. Cell Signal 2021, 78, 109841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.S.; Gao, Z.R.; Zhang, Q.; Tang, X.F.; Lv, Y.F.; Zhang, Z.S.; Zhang, Y.; Tan, Q.L.; Peng, D.B.; Jiang, D.M.; et al. TSSC3 promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR pathway to suppress tumorigenesis and metastasis in osteosarcoma, and predicts a favorable prognosis. J. Exp. Clin. Cancer Res. 2018, 37, 188. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Ahuja, R.; Osafo-Addo, A.D.; Barrows, D.; Kettenbach, A.; Skidan, I.; Teng, X.; Cuny, G.D.; Gerber, S.; Degterev, A. Akt Regulates TNFalpha synthesis downstream of RIP1 kinase activation during necroptosis. PLoS ONE 2013, 8, e56576. [Google Scholar] [CrossRef] [PubMed]
- Molnar, T.; Mazlo, A.; Tslaf, V.; Szollosi, A.G.; Emri, G.; Koncz, G. Current translational potential and underlying molecular mechanisms of necroptosis. Cell Death Dis. 2019, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Nam, Y.W.; Kim, S.; Oh, D.B.; Song, J. Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators. Exp. Mol. Med. 2021, 53, 1007–1017. [Google Scholar] [CrossRef]
- Hu, S.; Chang, X.; Zhu, H.; Wang, D.; Chen, G. PI3K mediates tumor necrosis factor induced-necroptosis through initiating RIP1-RIP3-MLKL signaling pathway activation. Cytokine 2020, 129, 155046. [Google Scholar] [CrossRef]
- Neumann, C.A.; Fang, Q. Are peroxiredoxins tumor suppressors? Curr. Opin. Pharmacol. 2007, 7, 375–380. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Li, K.W.; Liang, Y.Y.; Wang, Q.; Li, Y.; Zhou, S.J.; Wei, H.C.; Zhou, C.Z.; Wan, X.H. Brucea javanica: A review on anticancer of its pharmacological properties and clinical researches. Phytomedicine 2021, 86, 153560. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, C.; Li, J.; Li, J.; Yu, R.; Cai, F.; Qu, G.; Yu, B.; Liu, L.; Zeng, D.; et al. Indirect bilirubin impairs invasion of osteosarcoma cells via inhibiting the PI3K/AKT/MMP-2 signaling pathway by suppressing intracellular ROS. J. Bone Oncol. 2023, 39, 100472. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, H.; Sun, H.; Cao, S. Dieckol exerts anticancer activity in human osteosarcoma (MG-63) cells through the inhibition of PI3K/AKT/mTOR signaling pathway. Saudi J. Biol. Sci. 2021, 28, 4908–4915. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, S.; Bazer, F.W.; Lim, W.; Song, G. Myricetin treatment induces apoptosis in canine osteosarcoma cells by inducing DNA fragmentation, disrupting redox homeostasis, and mediating loss of mitochondrial membrane potential. J. Cell Physiol. 2018, 233, 7457–7466. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Hou, C.H.; Lin, F.L.; Tsao, Y.T.; Hou, S.M. Nimbolide Induces ROS-Regulated Apoptosis and Inhibits Cell Migration in Osteosarcoma. Int. J. Mol. Sci. 2015, 16, 23405–23424. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Gao, Y.; Yang, Y.; Wu, H. Osteosarcoma and Metastasis. Front. Oncol. 2021, 11, 780264. [Google Scholar] [CrossRef] [PubMed]
- Hirahata, M.; Osaki, M.; Kanda, Y.; Sugimoto, Y.; Yoshioka, Y.; Kosaka, N.; Takeshita, F.; Fujiwara, T.; Kawai, A.; Ito, H.; et al. PAI-1, a target gene of miR-143, regulates invasion and metastasis by upregulating MMP-13 expression of human osteosarcoma. Cancer Med. 2016, 5, 892–902. [Google Scholar] [CrossRef]
- Kim, N.H.; Sung, N.J.; Shin, S.; Ryu, D.S.; Youn, H.S.; Park, S.A. Sauchinone inhibits the proliferation, migration and invasion of breast cancer cells by suppressing Akt-CREB-MMP13 signaling pathway. Biosci. Rep. 2021, 41, BSR20211067. [Google Scholar] [CrossRef]

| HPLC Condition | |
|---|---|
| Column | Phenomenex Kinetex C18 (150 mm × 4.6 mm, 2.6 µm, 100A) |
| Mobile phase | A (Water with 0.1% Trifluoroacetic acid) B (ACN with 0.1% Trifluoroacetic acid) 2% B (0min) → 100% B (20min) (gradient for 30 min) |
| Flow rate | 1 mL/min |
| Detector | ELSD |
| Injection volume | 5 µL (1 mg/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-M.; Kim, S.H.; Kwon, Y.-J.; Park, K.-R. Effect of Spicatoside a on Anti-Osteosarcoma MG63 Cells through Reactive Oxygen Species Generation and the Inhibition of the PI3K-AKT-mTOR Pathway. Antioxidants 2024, 13, 1162. https://doi.org/10.3390/antiox13101162
Yun H-M, Kim SH, Kwon Y-J, Park K-R. Effect of Spicatoside a on Anti-Osteosarcoma MG63 Cells through Reactive Oxygen Species Generation and the Inhibition of the PI3K-AKT-mTOR Pathway. Antioxidants. 2024; 13(10):1162. https://doi.org/10.3390/antiox13101162
Chicago/Turabian StyleYun, Hyung-Mun, Soo Hyun Kim, Yoon-Ju Kwon, and Kyung-Ran Park. 2024. "Effect of Spicatoside a on Anti-Osteosarcoma MG63 Cells through Reactive Oxygen Species Generation and the Inhibition of the PI3K-AKT-mTOR Pathway" Antioxidants 13, no. 10: 1162. https://doi.org/10.3390/antiox13101162
APA StyleYun, H.-M., Kim, S. H., Kwon, Y.-J., & Park, K.-R. (2024). Effect of Spicatoside a on Anti-Osteosarcoma MG63 Cells through Reactive Oxygen Species Generation and the Inhibition of the PI3K-AKT-mTOR Pathway. Antioxidants, 13(10), 1162. https://doi.org/10.3390/antiox13101162








