Protective Effects of Anethole in Foeniculum vulgare Mill. Seed Ethanol Extract on Hypoxia/Reoxygenation Injury in H9C2 Heart Myoblast Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Extracts
2.3. H9C2 Cell Culture
2.4. Hypoxia/Reoxygenation (H/R)
2.5. Cell Viability
2.6. Measurement of Intracellular Reactive Oxygen Species (ROS) Levels
2.7. Determination of Mitochondrial Membrane Potential
2.8. Nuclear Condensation
2.9. Immunocytochemistry
2.10. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.11. In Silico Pharmacokinetics Analysis
2.12. Image Acquisition
2.13. Statistical Analysis
3. Results
3.1. Phytochemical Profiling of the FVSE
3.1.1. GC-MS Analysis
3.1.2. The Identities of the Prominent Components
3.2. Druggability and Pharmacokinetic Properties of the FVSE’s Main Compounds
3.2.1. Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) Analysis
3.2.2. Components Boiled-Egg Model and Radar Plot Analysis
3.2.3. Consideration for Potential Nutrients
3.3. Cardiomyocyte-Protective Activities of FVSE and Its Prominent Component Anethole
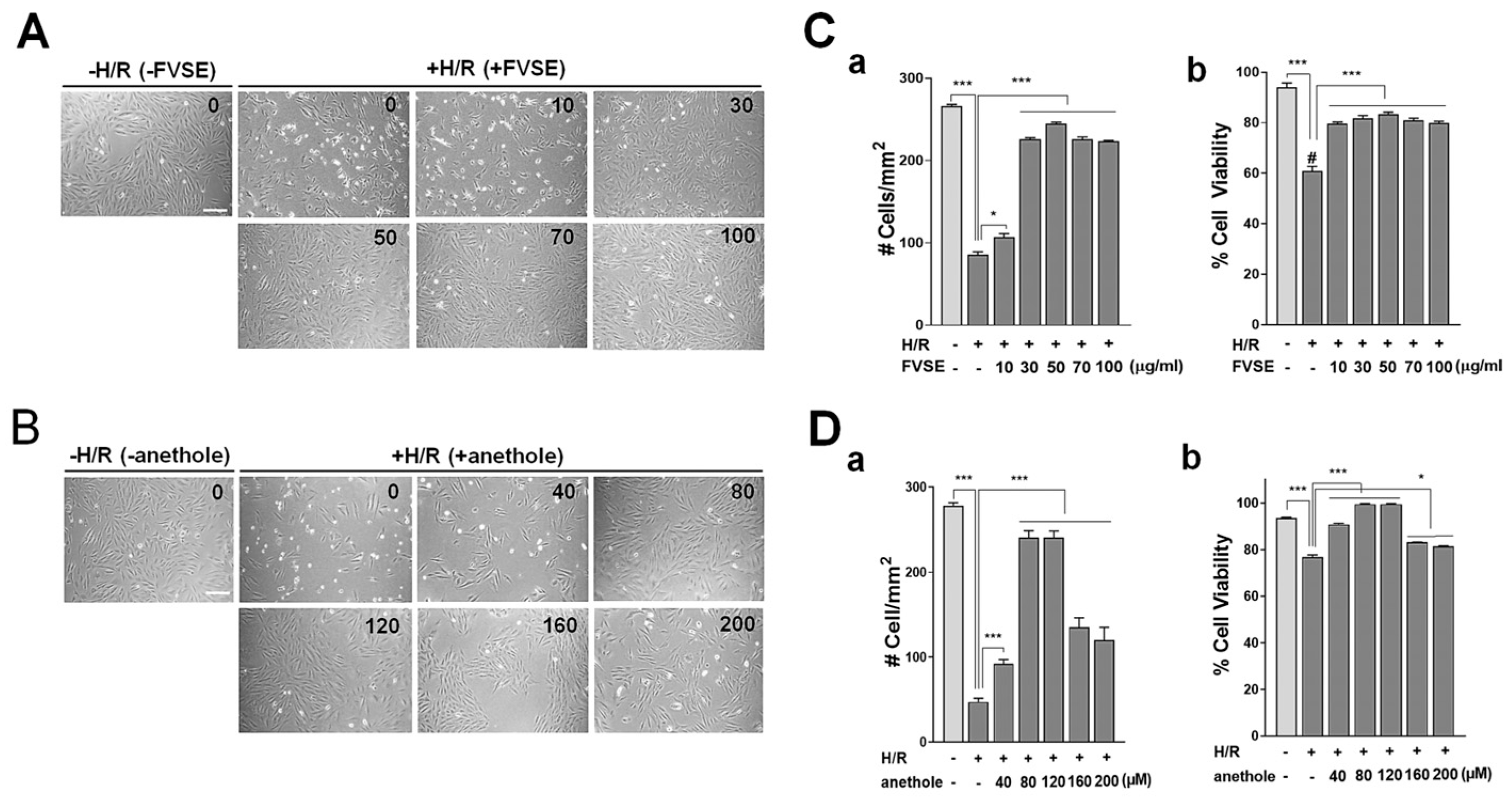
3.3.1. Optimization of FVSE and Anethole Concentration
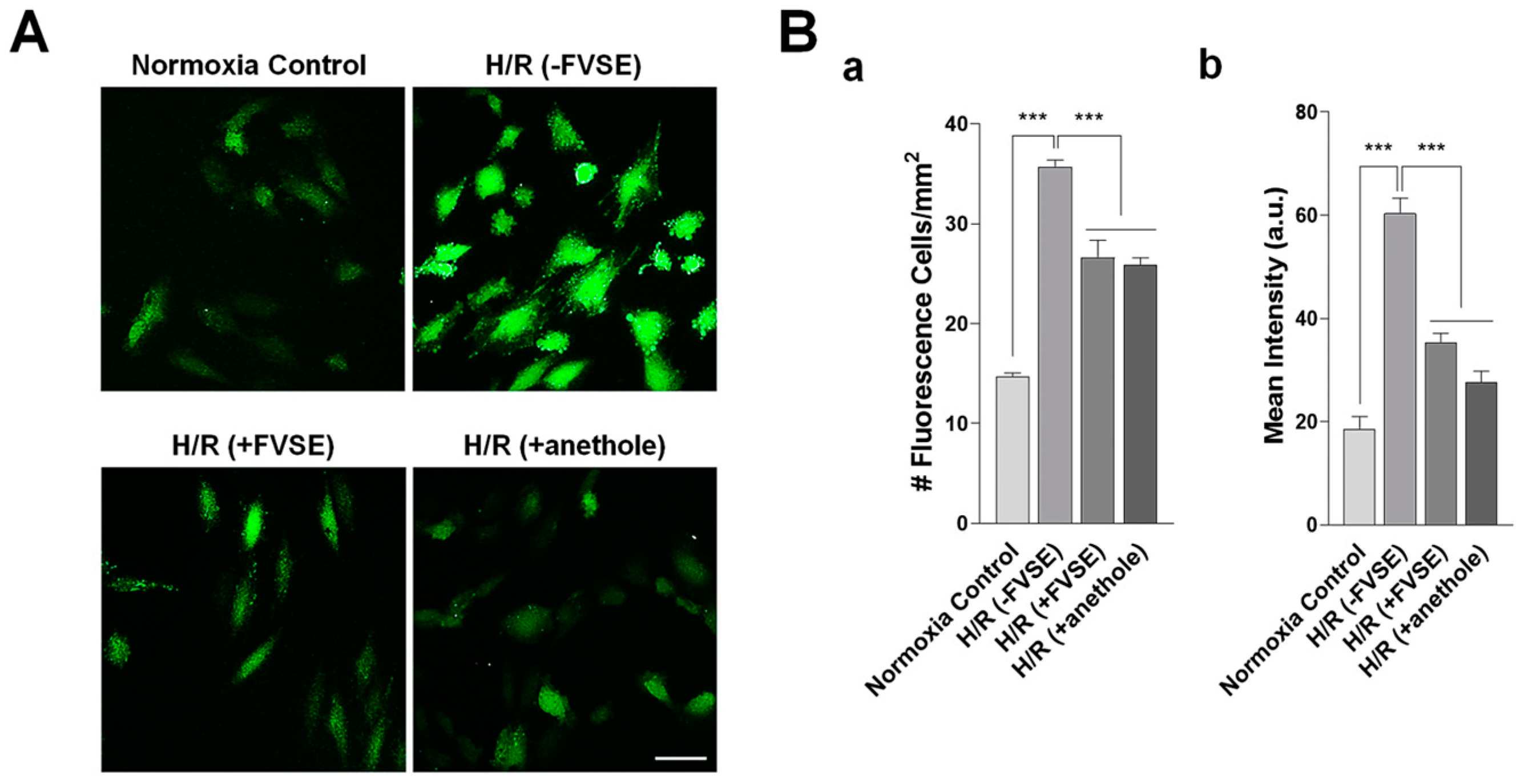
3.3.2. FVSE and Anethole Suppress H/R-Induced ROS Generation
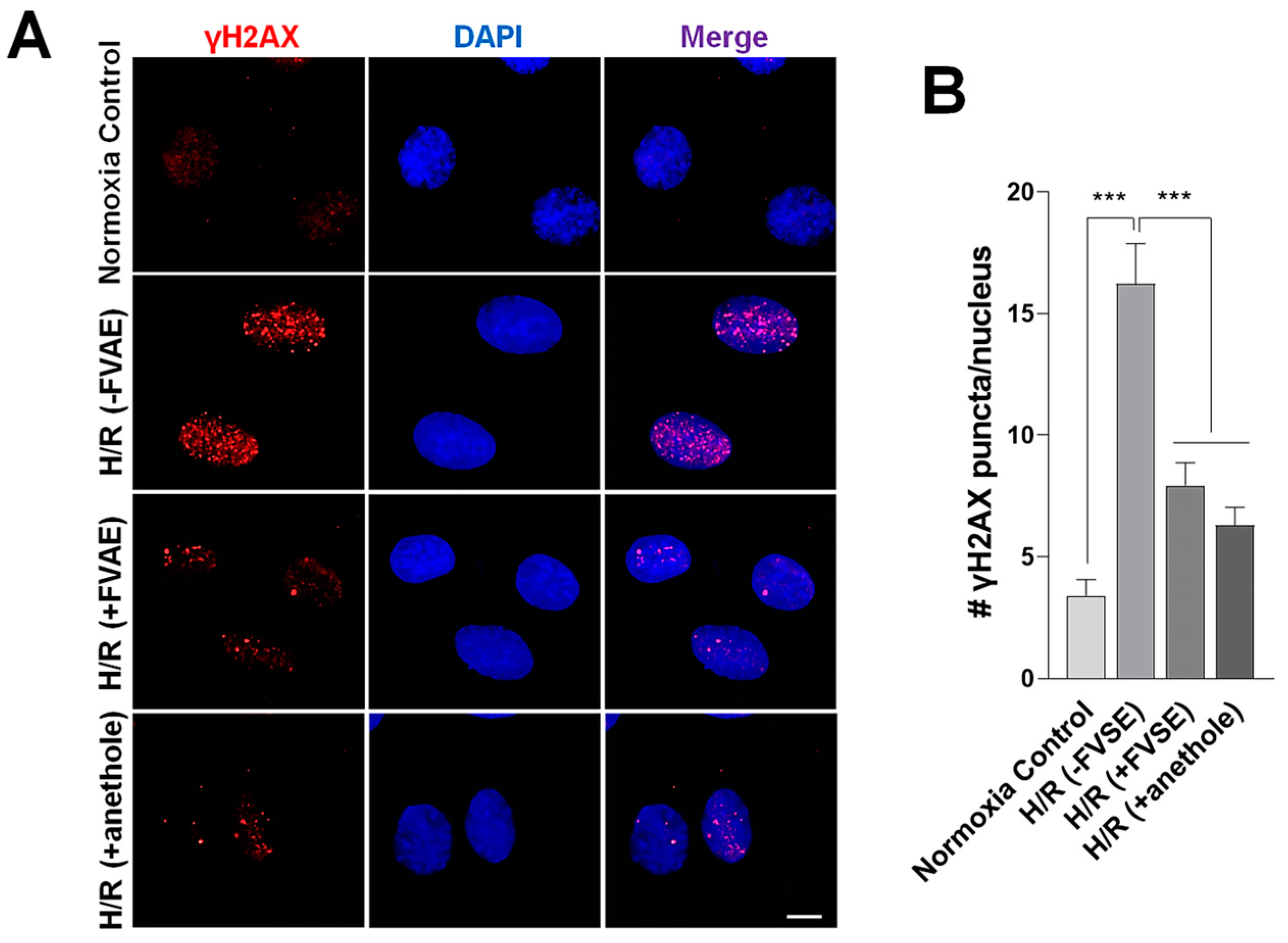
3.3.3. FVSE and Anethole Decrease DNA Damage
3.3.4. FVSE and Anethole Prevent Nuclear Condensation
3.3.5. FVSE and Anethole Preserve ΔΨm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucero García Rojas, E.Y.; Villanueva, C.; Bond, R.A. Hypoxia Inducible Factors as Central Players in the Pathogenesis and Pathophysiology of Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 709509. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Liu, C.; Gao, L.; Xu, X.; Zhu, N.; Cao, L. Hepatoprotective Effect of Aqueous Extract from the Seeds of Orychophragmus violaceus Against Liver Injury in Mice and HepG2 Cells. Int. J. Mol. Sci. 2017, 18, 1197. [Google Scholar] [CrossRef]
- Abe, H.; Semba, H.; Takeda, N. The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J. Atheroscler. Thromb. 2017, 24, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Copple, B.L. Hypoxia Stimulates Hepatocyte Epithelial to Mesenchymal Transition by Hypoxia-inducible factor and transforming growth factor-β-dependent mechanisms. Liver Int. 2010, 30, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Beaudin, A.E.; Waltz, X.; Hanly, P.J.; Poulin, M.J. Impact of Obstructive Sleep Apnoea and Intermittent Hypoxia on Cardiovascular and Cerebrovascular Regulation. Exp. Physiol. 2017, 102, 743–763. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Galli, G.; Wang, Y.; Fan, Q.; Wang, Z.; Wang, X.; Xiao, W. Novel Therapeutic Targets for Hypoxia-Related Cardiovascular Diseases: The Role of HIF-1. Front. Physiol. 2020, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Fornal, E. The Impact of Flavonols on Cardiovascular Risk. Nutrients 2022, 14, 1973. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M. Cardiovascular Risk Prediction: Basic Concepts, Current Status, and Future Directions. Circulation 2010, 121, 1768–1777. [Google Scholar] [CrossRef]
- Yadav, K.; Behera, S.; Singh, M.; Parashar, M.; Goel, S.; Jaswal, N.; Gupta, A. Trend of Risk Factors for Cardiovascular Diseases Among Young and Middle-Aged Indians: Insights From a Nationally Representative Survey. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 19, 200200. [Google Scholar] [CrossRef]
- Singhai, H.; Rathee, S.; Jain, S.K.; Patil, U.K. The Potential of Natural Products in the Management of Cardiovascular Disease. Curr. Pharm. Des. 2024, 30, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Hesari, M.; Mohammadi, P.; Khademi, F.; Shackebaei, D.; Momtaz, S.; Moasefi, N.; Farzaei, M.H.; Abdollahi, M. Current Advances in the Use of Nanophytomedicine Therapies for Human Cardiovascular Diseases. Int. J. Nanomed. 2021, 16, 3293–3315. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. Biomed. Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, S.C.; Wong, H.; Hop, C.E. ADME Properties and Their Dependence on Physicochemical Properties. In Drug Metabolism and Pharmacokinetics Quick Guide; Springer: New York, NY, USA, 2011; pp. 165–181. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and Drug-Like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Kim, H.; Xue, X. Detection of Total Reactive Oxygen Species in Adherent Cells by 2’,7’-Dichlorodihydrofluorescein Diacetate Staining. J. Vis. Exp. 2020, 160. [Google Scholar] [CrossRef]
- Reers, M.; Smith, T.W.; Chen, L.B. J-Aggregate Formation of a Carbocyanine as a Quantitative Fluorescent Indicator of Membrane Potential. Biochemistry 1991, 30, 4480–4486. [Google Scholar] [CrossRef]
- Moon, I.S.; Cho, S.-J.; Jin, I.; Walikonis, R. A Simple Method for Combined Fluorescence in Situ Hybridization and Immunocytochemistry. Mol. Cells 2007, 24, 76–82. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness, and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Russell, A. Mechanisms of Antimicrobial Action of Antiseptics and Disinfectants: An Increasingly Important Area of Investigation. J. Antimicrob. Chemother. 2002, 49, 597–599. [Google Scholar] [CrossRef]
- Sandoval-Powers, M.; Králová, S.; Nguyen, G.-S.; Fawwal, D.V.; Degnes, K.; Lewin, A.S.; Klinkenberg, G.; Wentzel, A.; Liles, M.R. Streptomyces poriferorum sp. nov., a Novel Marine Sponge-Derived Actinobacteria Species Expressing Anti-MRSA Activity. Syst. Appl. Microbiol. 2021, 44, 126244. [Google Scholar] [CrossRef] [PubMed]
- Sianturi, G.L.R.; Trisnawati, E.W.; Koketsu, M.; Suryanti, V. Chemical Constituents and Antioxidant Activity of Britton’s Wild Petunia (Ruellia brittoniana) Flower. Biodiversitas J. Biol. Divers. 2023, 24. [Google Scholar] [CrossRef]
- Rubab, M.; Chelliah, R.; Saravanakumar, K.; Barathikannan, K.; Wei, S.; Kim, J.-R.; Yoo, D.; Wang, M.-H.; Oh, D.-H. Bioactive Potential of 2-Methoxy-4-vinylphenol and Benzofuran from Brassica oleracea L. var. capitate f. rubra (Red Cabbage) on Oxidative and Microbiological Stability of Beef Meat. Foods 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L. Investigating the Biosynthesis of Polyacetylenes: Synthesis of Deuterated Linoleic Acids & Mechanism Studies of DMDS Addition to 1,4-Enynes. Ph.D. Thesis, Miami University, Miami, FL, USA, 2003. [Google Scholar]
- Marinov, V.; Valcheva-Kuzmanova, S. Review on the Pharmacological Activities of Anethole. Scr. Sci. Pharm. 2015, 2, 14–19. [Google Scholar] [CrossRef]
- Moradi Vastegani, S.; Khoshnam, S.E.; Ghafouri, S.; Bakhtiari, N.; Farbood, Y.; Sarkaki, A. Anethole Attenuates Motor Dysfunctions, Striatal Neuronal Activity Deficiency and Blood-Brain Barrier Permeability by Decreasing Striatal α-Synuclein and Oxidative Stress in Rotenone-Induced Parkinson’s Disease of Male Rats. PLoS ONE 2023, 18, e0294612. [Google Scholar] [CrossRef]
- Rostami-Faradonbeh, N.; Amini-Khoei, H.; Zarean, E.; Bijad, E.; Lorigooini, Z. Anethole as a Promising Antidepressant for Maternal Separation Stress in Mice by Modulating Oxidative Stress and Nitrite Imbalance. Sci. Rep. 2024, 14, 7766. [Google Scholar] [CrossRef]
- Yin, W.T.; Maradza, W.; Xu, Y.F.; Ma, X.T.; Shi, R.; Zhao, R.Y.; Wang, X.D. Comparison of Key Aroma-Active Composition and Aroma Perception of Cold-Pressed and Roasted Peanut Oils. Int. J. Food Sci. Technol. 2022, 57, 2968–2979. [Google Scholar] [CrossRef]
- Hamada Saoud, D.; Hadjadj, S.; Bencheikh, S.E.; Goudjil, M.B.; Bouafia, A.; Ladjel, S.; Menaa, F. Phytochemical Screening of Aerial Organs of Wild Fennel Essential Oils from Southeast Algeria: Identification of Chemical Composition, Antioxidant, and Antimicrobial Activities. Biomass Convers. Bioref. 2024, 14, 16257–16271. [Google Scholar] [CrossRef]
- Wang, Y.; Hussain, M.; Jiang, Z.; Wang, Z.; Gao, J.; Ye, F.; Mao, R.; Li, H. Aquilaria Species (Thymelaeaceae) Distribution, Volatile and Non-Volatile Phytochemicals, Pharmacological Uses, Agarwood Grading System, and Induction Methods. Molecules 2021, 26, 7708. [Google Scholar] [CrossRef]
- Paşayeva, L. Foeniculum vulgare Mill. In Novel Drug Targets with Traditional Herbal Medicines: Scientific and Clinical Evidence; Springer: Berlin/Heidelberg, Germany, 2022; pp. 263–288. [Google Scholar]
- Talie, M.; War, J.M.; Nisa, A.U.; Dar, A.H.; Wani, A.H.; Bhat, M.Y. Chemical Composition and Antimicrobial Properties of Morchella crassipes (Ascomycota) From Kashmir Valley (India). Int. J. Med. Mushrooms 2024, 26, 39–51. [Google Scholar] [CrossRef]
- Ayswarya, S.; Radhakrishnan, M.; Manigundan, K.; Gopikrishnan, V.; Soytong, K. Antioxidant Activity of 2,4-Di-Tert-Butylphenol Isolated from Plant Growth Promoting Endophytic Streptomyces KCA-1. J. Biol. Sci. 2022, 18, 2343–2352. [Google Scholar]
- Johney, A.; Yasin, I.M.B.M. Evaluation of Methanolic Etlingera coccinea Crude Extract Against Pathogenic Microorganisms. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2024; Volume 3023. [Google Scholar]
- Maxia, A.; Falconieri, D.; Piras, A.; Porcedda, S.; Marongiu, B.; Frau, M.A.; Gonçalves, M.J.; Cabral, C.; Cavaleiro, C.; Salgueiro, L. Chemical Composition and Antifungal Activity of Essential Oils and Supercritical CO2; Extracts of Apium nodiflorum (L.) Lag. Mycopathologia 2012, 174, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Rayanil, K.; Bunchornmaspan, P.; Tuntiwachwuttikul, P. A New Phenolic Compound with Anticancer Activity from the Wood of Millettia leucantha. Arch. Pharm. Res. 2011, 34, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Rangsinth, P.; Warayanon, W.; Leung, G.P.H.; Chuchawankul, S.; Prasansuklab, A.; Tencomnao, T. Protective Effect of Aquilaria crassna Leaf Extract Against Benzo[a]pyrene-Induced Toxicity in Neuronal Cells and Caenorhabditis elegans: Possible Active Constituent Includes Clionasterol. Nutrients 2023, 15, 3985. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Liu, G.; Tang, Y. In Silico ADMET Prediction: Recent Advances, Current Challenges and Future Trends. Curr. Top. Med. Chem. 2013, 13, 1273–1289. [Google Scholar] [CrossRef]
- Kirchmair, J.; Göller, A.H.; Lang, D.; Kunze, J.; Testa, B.; Wilson, I.D.; Glen, R.C.; Schneider, G. Predicting Drug Metabolism: Experiment and/or Computation? Nat. Rev. Drug Discov. 2015, 14, 387–404. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, J.; Xu, Y.; Zhou, N.; Peng, J.; Xiong, Z.; Liu, X. In Silico ADME/T Modelling for Rational Drug Design. Q. Rev. Biophys. 2015, 48, 488–515. [Google Scholar] [CrossRef]
- Alqahtani, S. In Silico ADME-Tox Modeling: Progress and Prospects. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1147–1158. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. Chem. Med. Chem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Jubb, H.; Blundell, T.L.; Ascher, D.B. Flexibility and Small Pockets at Protein–Protein Interfaces: New Insights into Druggability. Prog. Biophys. Mol. Biol. 2015, 119, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Onda, M.; Higashida, A.; Hirano, T.; Nishio, T.; Hakamata, W. Synthesis of All Stereoisomers of 1-(4-Methoxyphenyl)-2,3,4,9-Tetrahydro-N-Methyl-1H-Pyrido[3,4-b]Indole-3-Carboxamide. Molbank 2018, 2018, M973. [Google Scholar] [CrossRef]
- Ohta, T.; Sasayama, H.; Nakajima, O.; Kurahashi, N.; Fujii, T.; Furukawa, I. Asymmetric Allylic Substitution Catalyzed by Palladium–Yliphos Complex. Tetrahedron Asymmetry 2003, 14, 537–542. [Google Scholar] [CrossRef]
- Zou, Q.; Huang, Y.; Zhang, W.; Lu, C.; Yuan, J. A Comprehensive Review of the Pharmacology, Chemistry, Traditional Uses and Quality Control of Star Anise (Illicium verum Hook. f.): An Aromatic Medicinal Plant. Molecules 2023, 28, 7378. [Google Scholar] [CrossRef] [PubMed]
- Greijer, A.E.; Van der Wall, E. The Role of Hypoxia Inducible Factor 1 (HIF-1) in Hypoxia Induced Apoptosis. J. Clin. Pathol. 2004, 57, 1009–1014. [Google Scholar] [CrossRef]
- Kadam, R.; Roy, N. Recent Trends in Drug-Likeness Prediction: A Comprehensive Review of In Silico Methods. Indian J. Pharm. Sci. 2007, 69, 609. [Google Scholar]
- Pitchai, D.; Manikkam, R.; Sukamaran, S.; Kandhasamy, S.; Periyasamy, V.J. In-Silico Docking Studies on Anticancerous Polyphenolic Phytocompounds Targeting the BH3 Domain of Bcl-XL Receptor in Apoptotic Pathway. J. Pharm. Res. 2011, 4, 1626–1631. [Google Scholar]
- Rafi, S.B.; Hearn, B.R.; Vedantham, P.; Jacobson, M.P.; Renslo, A.R. Predicting and Improving the Membrane Permeability of Peptidic Small Molecules. J. Med. Chem. 2012, 55, 3163–3169. [Google Scholar] [CrossRef]
- Kavitha, K.; Ranjani, P.V. Solubility Enhancement: A Challenge for Hydrophobic Drugs. IOSR J. Pharm. Biol. Sci. 2015, 10, 50–60. [Google Scholar]
- Sambhakar, S.; Saharan, R.; Narwal, S.; Malik, R.; Gahlot, V.; Khalid, A.; Najmi, A. Exploring LIPIDs for Their Potential to Improve Bioavailability of Lipophilic Drug Candidates: A Review. Saudi Pharm. J. 2023, 31, 101870. [Google Scholar]
- Pajouhesh, H.; Lenz, G.R. Medicinal Chemical Properties of Successful Central Nervous System Drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Abu Hassan Shaari, H.; Ramli, M.M.; Mohtar, M.N.; Rahman, N.A.; Ahmad, A. Synthesis and Conductivity Studies of Poly(Methyl Methacrylate) (PMMA) by Co-Polymerization and Blending with Polyaniline (PANi). Polymers 2021, 13, 1939. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, D.; Zhao, Y. Development of Synthesis and Application of High Molecular Weight Poly(Methyl Methacrylate). Polymers 2022, 14, 2632. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Saxena, S.N.; Sharma, L.K.; Lal, G. Prevalence of Essential and Fatty Oil Constituents in Fennel (Foeniculum vulgare Mill) Genotypes Grown in Semi Arid Regions of India. J. Essent. Oil Bear. Plants 2018, 21, 40–51. [Google Scholar] [CrossRef]
- Ponte, E.L.; Sousa, P.L.; Rocha, M.V.A.P.; Soares, P.M.G.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H.; Assreuy, A. Comparative Study of the Anti-Edematogenic Effects of Anethole and Estragole. Pharmacol. Rep. 2012, 64, 984–990. [Google Scholar] [CrossRef]
- Smith, R.L.; Adams, T.B.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S. Safety Assessment of Allylalkoxybenzene Derivatives Used as Flavouring Substances—Methyl Eugenol and Estragole. Food Chem. Toxicol. 2002, 40, 851–870. [Google Scholar] [CrossRef]
- Yea, S.S.; Jeong, H.S.; Choi, C.Y.; Park, K.R.; Oh, S.; Shin, J.G.; Yun, C.H. Inhibitory Effect of Anethole on T-Lymphocyte Proliferation and Interleukin-2 Production Through Down-Regulation of the NF-AT and AP-1. Int. Immunopharmacol. 2006, 20, 1098–1105. [Google Scholar] [CrossRef]
- Freire, R.S.; Morais, S.M.; Catunda-Junior, F.E.A.; Pinheiro, D.C.S.N. Synthesis and Antioxidant, Anti-Inflammatory, and Gastroprotector Activities of Anethole and Related Compounds. Bioorg. Med. Chem. 2005, 13, 4353–4358. [Google Scholar] [CrossRef]
- Bone, K.; Mills, S. Principles and Practice of Phytotherapy: Modern Herbal Medicine; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]


| No. | RT | Compound Name | CAS No. | % Area | Match Factor | Pharmacological Activities (Ref.) |
|---|---|---|---|---|---|---|
| 1 | 12.9749 | Phenol | 108-95-2 | 3.00 | 90.6 | Antiseptic [21] |
| 2 | 13.1057 | 2-Hydroxy-gamma-butyrolactone | 19444-84-9 | 1.43 | 81.3 | Antimicrobial [22] |
| 3 | 13.516 | Furan, 2-butyltetrahydro- | 1004-29-1 | 1.36 | 65.4 | No activities |
| 4 | 13.6349 | Cyclopropanemethanol, alpha-butyl- | 4379-16-2 | 0.69 | 64.7 | No activities |
| 5 | 13.9441 | Phenol, 2-methoxy- | 1990-05-01 | 0.44 | 76.1 | Antioxidant [23] |
| 6 | 14.9906 | 5-Methyl-1R,3-trans-cyclohexanediol | 549277 | 0.25 | 61.3 | No activities |
| 7 | 15.2522 | 4-Vinylphenol | 2628-17-3 | 0.69 | 67.6 | Antimicrobial [24] |
| 8 | 15.3355 | 2-Oxabicyclo[2.2.2]octan-6-ol, 1,3,3-trimethyl- | 5418-86-0 | 0.59 | 77.1 | No activities |
| 9 | 15.389 | Methane, tris(methylthio)- | 18679-48-6 | 1.07 | 83.4 | No activities |
| 10 | 15.8528 | 2-Hydroxymethyl-2-methylbrendane | 584818 | 0.69 | 70.7 | No activities |
| 11 | 15.9479 | 9-Decyn-1-ol | 17643-36-6 | 1.00 | 75.4 | Antifungal [25] |
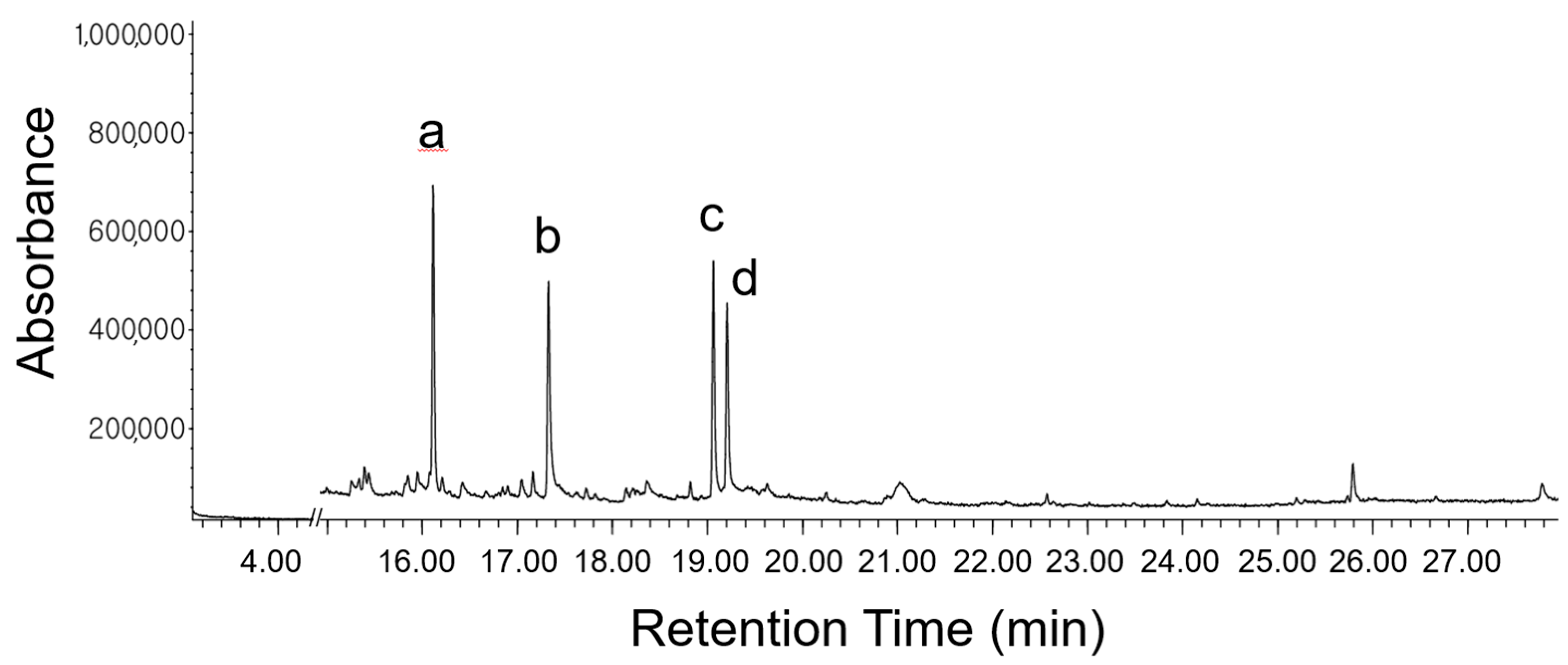
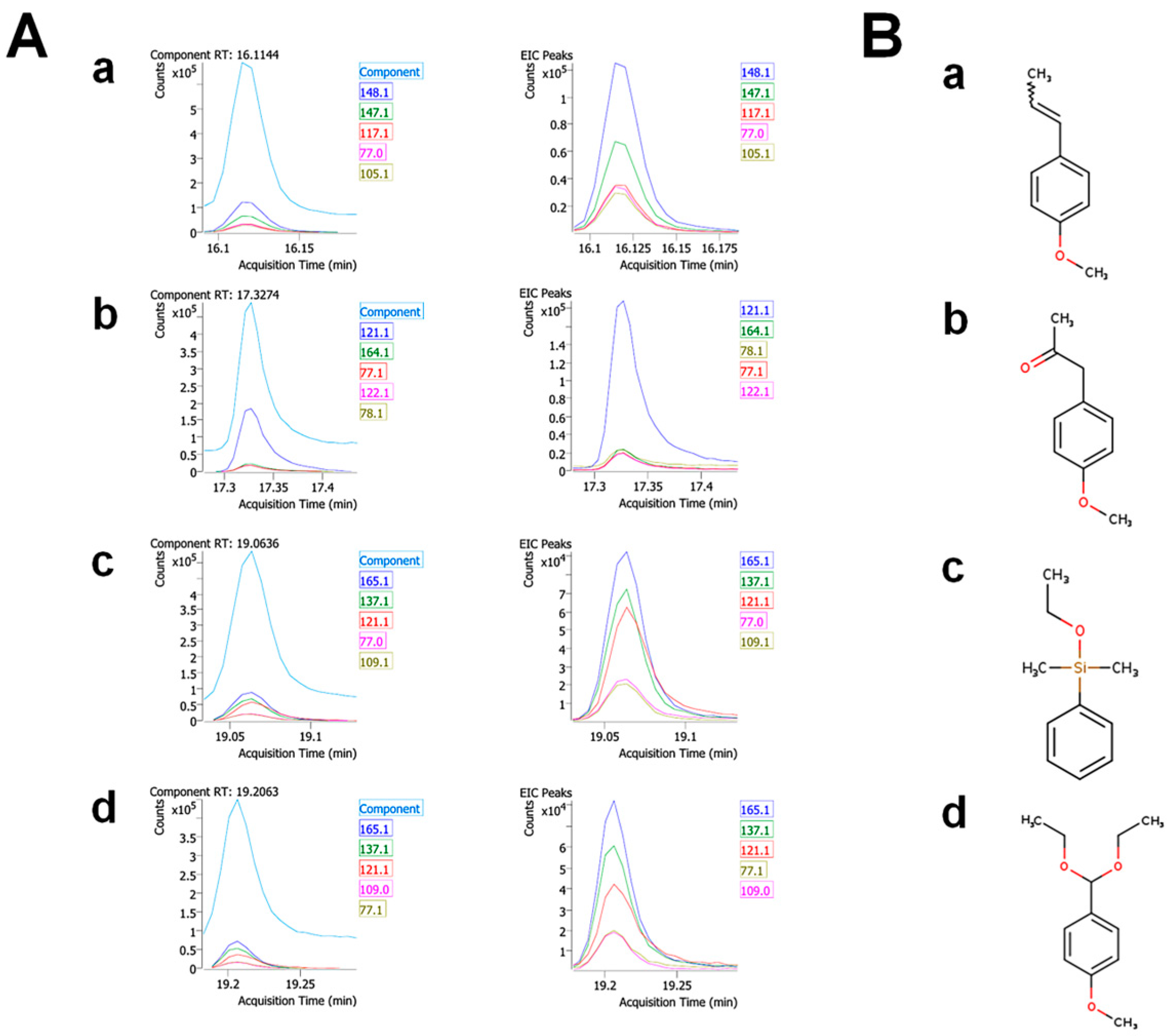
| 12 | 16.1144 | Anethole | 104-46-1 | 17.84 | 99 | Anti-inflammatory, Antioxidant, anticancer, neuroprotective, cardio-protective, gastro-protective, antifungal, and antispasmodic [26,27,28] |
| 13 | 16.2095 | Cyclohexane, 2-ethenyl-1,1-dimethyl-3-methylene- | 95452-08-7 | 0.95 | 71.9 | No activities |
| 14 | 16.4236 | Phenol, 5-ethenyl-2-methoxy- | 621-58-9 | 1.45 | 83.9 | Antioxidant [29] |
| 15 | 16.6674 | 2,5-Dimethylcyclohexanol | 3809-32-3 | 0.46 | 62.6 | No activities |
| 16 | 16.8458 | 1,2-Cyclohexanediol, 1-methyl-4-(1-methylethenyl)- | 1946-00-5 | 0.38 | 78.9 | No activities |
| 17 | 16.8993 | Bicyclo(3.1.1)heptane-2,3-diol, 2,6,6-trimethyl- | 53404-49-2 | 0.37 | 63.8 | Antioxidant [30] |
| 18 | 17.042 | Benzene, 1-methoxy-4-(1-methylpropyl)- | 4917-90-2 | 1.15 | 80.7 | No activities |
| 19 | 17.1609 | 3(2H)-Furanone, 4-methoxy-2,5-dimethyl- | 4077-47-8 | 1.37 | 69.4 | No activities |
| 20 | 17.3274 | 2-Propanone, 1-(4-methoxyphenyl)- | 122-84-9 | 17.32 | 89 | Neuroprotective and cardioprotective [31,32] |
| 21 | 17.7198 | 1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octane-6,7-endo,endo-diol | 56084-15-2 | 0.66 | 76.6 | No activities |
| 22 | 17.8209 | 4-Hydroxybutyl acrylate, TMS | 53954667 | 0.24 | 59.3 | No activities |
| 23 | 18.1479 | Epoxy-linalooloxide | 537453 | 0.76 | 67.4 | Antimicrobial [33] |
| 24 | 18.2133 | 2,4,6-Heptanetrione | 12285 | 0.64 | 47.9 | No activities |
| 25 | 18.2609 | 1-Propanone, 1-(3-methoxyphenyl)- | 37951-49-8 | 0.09 | 81.2 | No activities |
| 26 | 18.362 | S-(p-Methoxybenzoyl)thiohydroxylamine | 35124-66-4 | 1.23 | 81.2 | No activities |
| 27 | 18.8198 | 2,4-Di-tert-butylphenol | 96-76-4 | 1.01 | 86.3 | Antioxidant [34] |
| 28 | 19.0636 | Silane, ethoxydimethylphenyl- | 1825-58-7 | 16.60 | 83.7 | No activities |
| 29 | 19.2063 | para-Anisaldehyde diethyl acetal | 75468 | 12.69 | 83.2 | No activities |
| 30 | 19.6285 | 2-Dodecenal, (E)- | 20407-84-5 | 0.58 | 69.1 | Antimicrobial [35] |
| 31 | 20.2528 | 1,3-Benzodioxole, 4,5-dimethoxy-6-(2-propenyl)- | 484-31-1 | 0.23 | 77.8 | Antifungal [36] |
| 32 | 21.0258 | 1-Hydroxy-1-(4-methoxyphenyl) propane-2-one | 15482-29-8 | 4.78 | 78.3 | Anticencer [37] |
| 33 | 22.5717 | Neophytadiene | 504-96-1 | 0.50 | 74.3 | Anti-inflammatory [38] |
| 34 | 23.8382 | Dibutyl phthalate | 84-74-2 | 0.21 | 67.8 | No activities |
| 35 | 24.1534 | Hexadecanoic acid, ethyl ester | 628-97-7 | 0.49 | 61.9 | No activities |
| 36 | 25.1939 | 9-Octadecenoic acid (Z)-, methyl ester | 112-62-9 | 0.43 | 65.2 | No activities |
| 37 | 25.7409 | 2-Chloroethyl linoleate | 25525-76-2 | 0.41 | 70.3 | No activities |
| 38 | 25.7944 | Ethyl Oleate | 111-62-6 | 3.30 | 57.3 | No activities |
| 39 | 26.6685 | (2R,3S,5S,6R)-2,5-bis(4-Methoxyphenyl)-3,6-dimethyl-1,4-dioxane-rel- | 212516-42-2 | 0.36 | 57.3 | No activities |
| 40 | 27.7804 | 9-Octadecenamide, (Z)- | 301-02-0 | 1.68 | 80.4 | Neuroprotective [39] |
| 41 | 28.6426 | Silane, ethyldimethylphenyl- | 17873-23-3 | 0.45 | 57 | No activities |
| RT (min) | Compound Name | CAS No. | % of Total | Match Factor |
|---|---|---|---|---|
| 16.1 | Anethole | 104-46-1 | 17.8 | 99 |
| 17.3 | 1-(4-methoxyphenyl)-2-propanone | 122-84-9 | 17.3 | 89 |
| 19.1 | Ethoxydimethylphenyl-silane | 1825-58-7 | 16.6 | 83.7 |
| 19.2 | Para-Anisaldehyde diethyl acetal | 75468 | 12.7 | 83.2 |
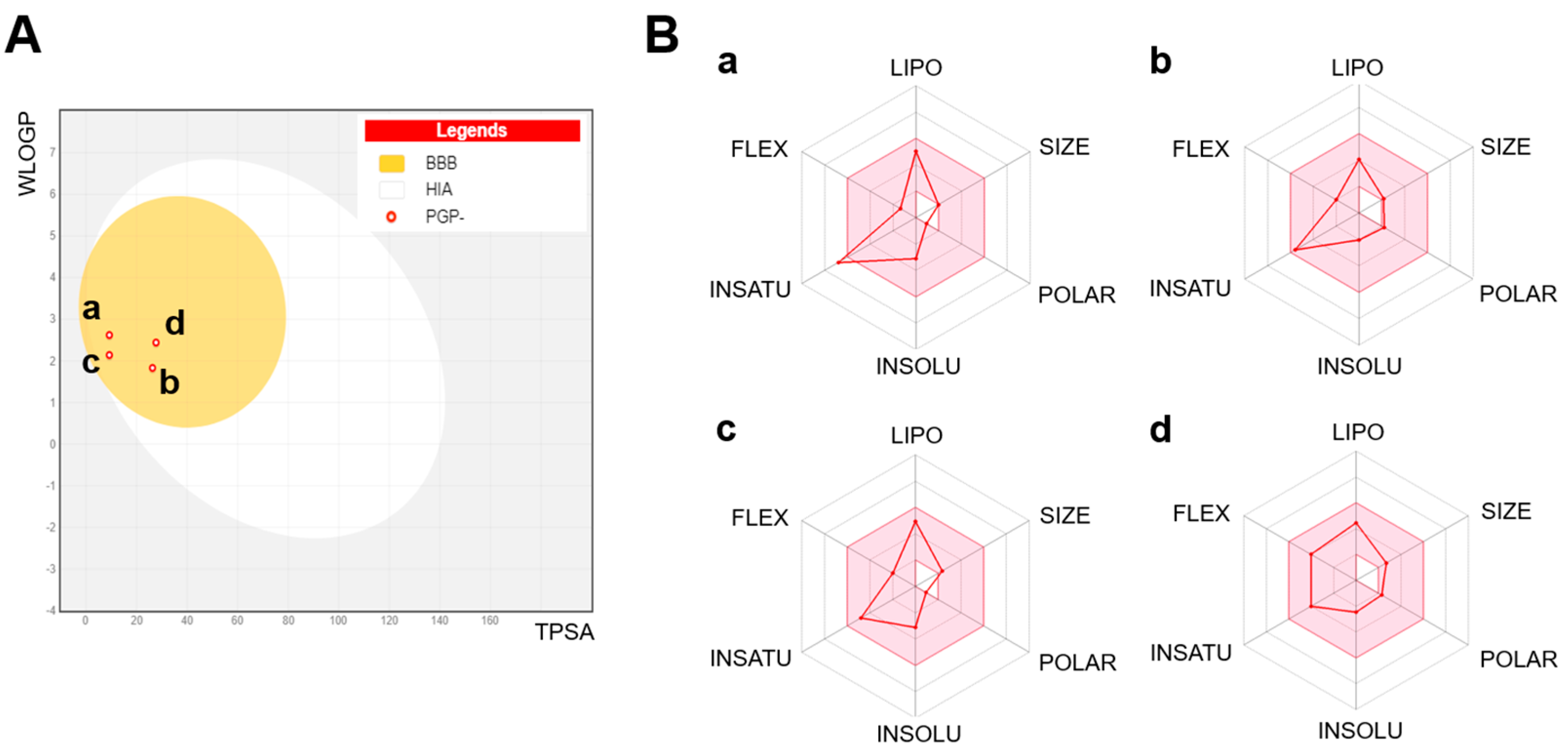
| Compound Name | Physicochemical Properties | Lipophilicity (MLogP) | Pharmacokinetics | Drug-Likeness | Medicinal Score |
|---|---|---|---|---|---|
| Anethole | MW: 148.2 Rotatable bonds: 2 H-bond donors: 0 H-bond acceptors: 1 TPSA: 9.23 Å2 | 2.67 | BBB permeation: Yes GI absorption: High CYP inhibition: Yes on CYP1A2 | Lipinski’s rule: Compliant | Predicted bioavailability: 0.55 P-gp substrate: No |
| 1-(4-Methoxyphenyl)-2-propanone | MW: 180.32, Rotatable bonds: 3 H-bond donors: 0 H-bond acceptors: 1 TPSA: 9.23 Å2 | 1.74 | BBB permeation: Yes GI absorption: High CYP inhibition: Yes on CYP1A2 and CYP3A4 | Lipinski’s rule: Compliant | Predicted bioavailability: 0.55 P-gp substrate: No |
| Ethoxydimethylphenylsilane | MW: 210.27 Rotatable bonds: 6 H-bond donors: 0 H-bond acceptors: 3 TPSA: 27.67 Å2 | 2.49 | BBB permeation: Yes GI absorption: High CYP inhibition: Yes on CYP1A2 and CYP3A4 | Lipinski’s rule: Compliant | Predicted bioavailability: 0.55 P-gp substrate: No |
| Para-Anisaldehyde Diethyl Acetal | MW: 164.2 Rotatable bonds: 3 H-bond donors: 0 H-bond acceptors: 2 TPSA: 26.3 Å2 | 2.20 | BBB permeation: Yes GI absorption: High CYP inhibition: Yes on CYP1A2 | Lipinski’s rule: Compliant | Predicted bioavailability: 0.55 P-gp substrate: No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.W.; Habiba, S.U.; Munni, Y.A.; Choi, H.J.; Aktar, A.; Mazumder, K.; Nah, D.-Y.; Yang, I.-J.; Moon, I.S. Protective Effects of Anethole in Foeniculum vulgare Mill. Seed Ethanol Extract on Hypoxia/Reoxygenation Injury in H9C2 Heart Myoblast Cells. Antioxidants 2024, 13, 1161. https://doi.org/10.3390/antiox13101161
Seo JW, Habiba SU, Munni YA, Choi HJ, Aktar A, Mazumder K, Nah D-Y, Yang I-J, Moon IS. Protective Effects of Anethole in Foeniculum vulgare Mill. Seed Ethanol Extract on Hypoxia/Reoxygenation Injury in H9C2 Heart Myoblast Cells. Antioxidants. 2024; 13(10):1161. https://doi.org/10.3390/antiox13101161
Chicago/Turabian StyleSeo, Jeong Won, Sarmin Ummey Habiba, Yeasmin Akter Munni, Ho Jin Choi, Asma Aktar, Kishor Mazumder, Deuk-Young Nah, In-Jun Yang, and Il Soo Moon. 2024. "Protective Effects of Anethole in Foeniculum vulgare Mill. Seed Ethanol Extract on Hypoxia/Reoxygenation Injury in H9C2 Heart Myoblast Cells" Antioxidants 13, no. 10: 1161. https://doi.org/10.3390/antiox13101161
APA StyleSeo, J. W., Habiba, S. U., Munni, Y. A., Choi, H. J., Aktar, A., Mazumder, K., Nah, D.-Y., Yang, I.-J., & Moon, I. S. (2024). Protective Effects of Anethole in Foeniculum vulgare Mill. Seed Ethanol Extract on Hypoxia/Reoxygenation Injury in H9C2 Heart Myoblast Cells. Antioxidants, 13(10), 1161. https://doi.org/10.3390/antiox13101161








