Effects of Taurine and Vitamin C on the Improvement of Antioxidant Capacity, Immunity and Hypoxia Tolerance in Gibel Carp (Carrassius auratus gibeilo)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Experimental Procedure
2.3. Sample Collection
2.4. Chemical Analysis
2.5. RNA Extraction and Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Whole-Body Composition
3.3. Swimming Ability
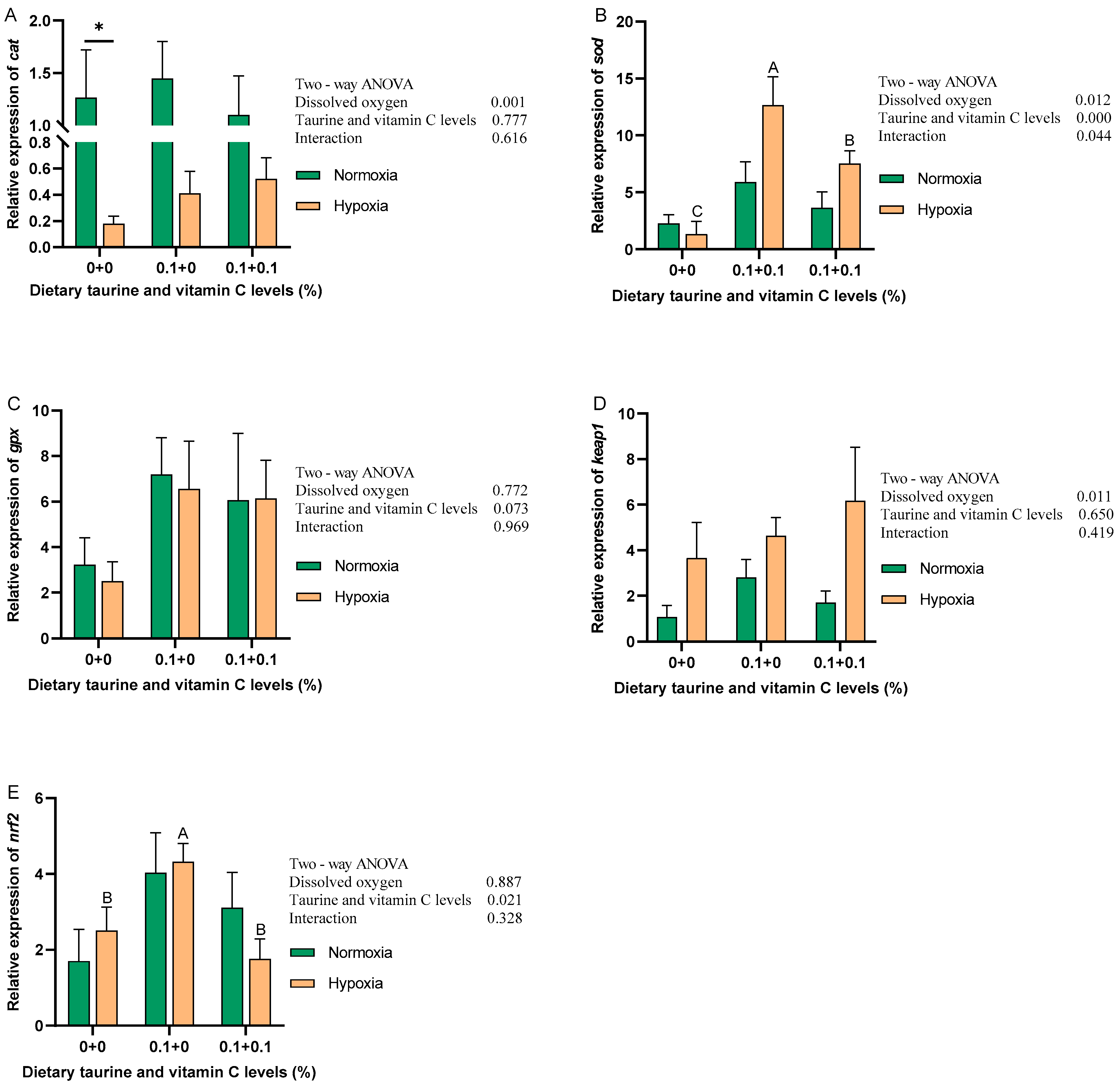
3.4. Antioxidant Capacity of the Liver (Nrf2 Signaling Pathway)
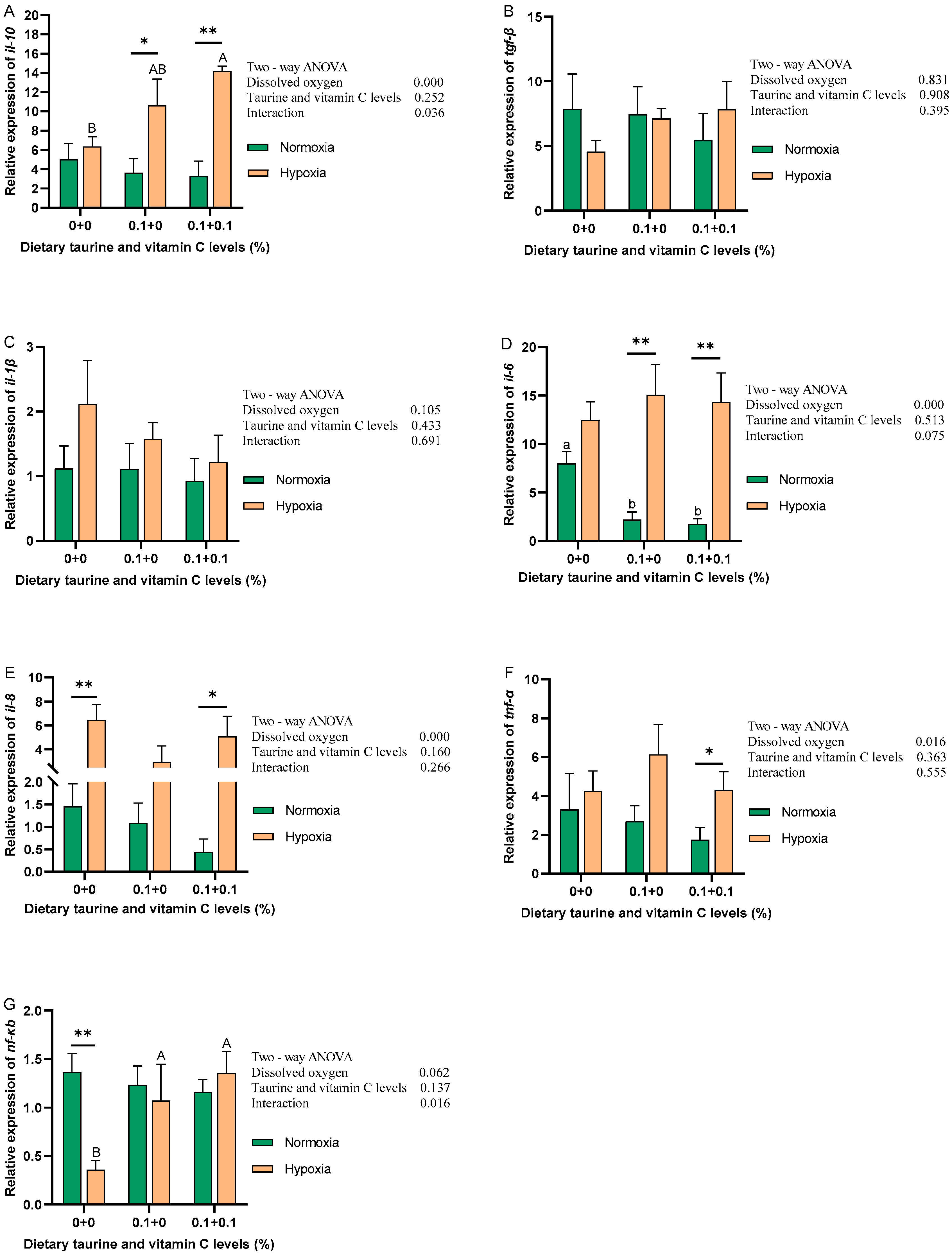
3.5. Immune Response of the Liver (NF-κB Signaling Pathway)
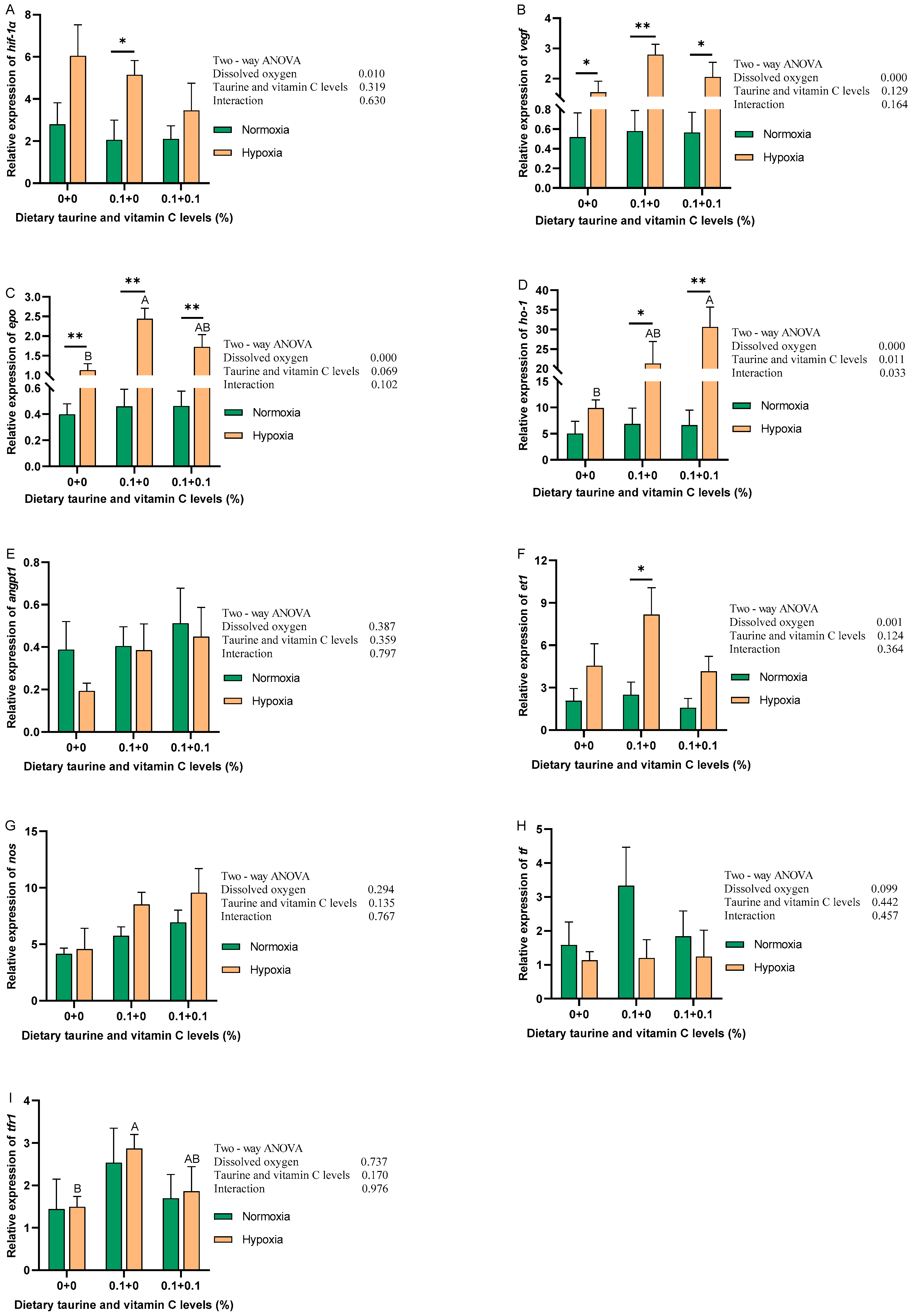
3.6. Hypoxia Signaling Pathway (HIF-1 Signaling Pathway)
3.7. Survival Rate of Fish and Mitochondrial Number in Gill Cells in Hypoxia
4. Discussion
4.1. Growth Performance and Whole-Body Composition
4.2. Antioxidant Capacity of the Liver (Nrf2 Signaling Pathway)
4.3. Immune Response of the Liver (NF-κB Signaling Pathway)
4.4. Hypoxia Signaling Pathway (HIF-1 Signaling Pathway)
4.5. Survival Rate and Mitochondrial Number in Gill Cells of Gibel Carp in Hypoxia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, F.; Shi, M.; Yuan, H.; Yuan, L.; Lu, W.; Zhang, J.; Tong, J.; Song, X. Dietary nano-selenium relieves hypoxia stress and, improves immunity and disease resistance in the Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2016, 54, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sun, Z.; Qi, H.; Zhou, X.; Xu, C.; Wu, D.; Fang, F.; Feng, J.; Zhang, N. Effects of Rice-Fish Co-culture on Oxygen Consumption in Intensive Aquaculture Pond. Rice Sci. 2019, 26, 50–59. [Google Scholar] [CrossRef]
- Tanner, C.A.; Burnett, L.E.; Burnett, K.G. The effects of hypoxia and pH on phenoloxidase activity in the Atlantic blue crab, Callinectes sapidus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, R. Hypoxia: From molecular responses to ecosystem responses. Mar. Pollut. Bull. 2002, 45, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.S.S.; Zhou, B.S.; Randall, D.J.; Woo, N.Y.S.; Lam, P.K.S. Aquatic Hypoxia Is an Endocrine Disruptor and Impairs Fish Reproduction. Environ. Sci. Technol. 2003, 37, 1137–1141. [Google Scholar] [CrossRef]
- Varghese, T.; Rejish Kumar, V.; Gopan, A.; Valappil, R.K.; Sajina, K.A.; Mishal, P.; Pal, A.K. Dietary arginine modulates nonspecific immune responses in Indian Major Carp, Cirrhinus mrigala exposed to hypoxia. Aquaculture 2020, 529, 735613. [Google Scholar] [CrossRef]
- Varghese, T.; Rejish Kumar, V.J.; Anand, G.; Dasgupta, S.; Pal, A. Dietary GABA enhances hypoxia tolerance of a bottom-dwelling carp, Cirrhinus mrigala by modulating HIF-1α, thyroid hormones and metabolic responses. Fish Physiol. Biochem. 2020, 46, 199–212. [Google Scholar] [CrossRef]
- Li, H.; Lu, L.; Wu, M.; Xiong, X.; Luo, L.; Ma, Y.; Liu, Y. The effects of dietary extract of mulberry leaf on growth performance, hypoxia-reoxygenation stress and biochemical parameters in various organs of fish. Aquac. Rep. 2020, 18, 100494. [Google Scholar] [CrossRef]
- Tiedemann, F.; Gmelin, L. Einige neue Bestandtheile der Galle des Ochsen. Ann. Der Phys. 2006, 85, 326–337. [Google Scholar] [CrossRef]
- Kuzmina, V.; Gavrovskaya, L.; Ryzhova, O. Taurine. Effect on Exotrophia and Metabolism in Mammals and Fish. J. Evol. Biochem. Physiol. 2010, 46, 19–27. [Google Scholar] [CrossRef]
- Jacobsen, J.G.; Smith, L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968, 48, 424–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, K.G.; Kim, K.D.; Kim, K.W.; Son, M.H.; Rust, M.; Johnson, R. Effect of dietary taurine levels on the conjugated bile acid composition and growth of juvenile Korean rockfish Sebastes schlegeli (Hilgendorf). Aquac. Res. 2015, 46, 2768–2775. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Ai, Q.; Mao, P.; Tian, Q.; Zhong, L.; Xiao, T.; Chu, W. Effect of dietary taurine supplementation on growth performance, digestive enzyme activities and antioxidant status of juvenile black carp (Mylopharyngodon piceus) fed with low fish meal diet. Aquac. Res. 2018, 49, 3187–3195. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Z.; Liu, G.; Deng, K.; Yang, M.; Pan, M.; Gu, Z.; Liu, D.; Zhang, W.; Mai, K. Synergistic effects of dietary carbohydrate and taurine on growth performance, digestive enzyme activities and glucose metabolism in juvenile turbot Scophthalmus maximus L. Aquaculture 2019, 499, 32–41. [Google Scholar] [CrossRef]
- Salze, G.; McLean, E.; Craig, S.R. Dietary taurine enhances growth and digestive enzyme activities in larval cobia. Aquaculture 2012, 362–363, 44–49. [Google Scholar] [CrossRef]
- Nakashima, T.; Seto, Y.; Nakajima, T.; Shima, T.; Sakamoto, Y.; Cho, N.; Sano, A.; Iwai, M.; Kagawa, K.; Okanoue, T.; et al. Calcium-associated cytoprotective effect of taurine on the calcium and oxygen paradoxes in isolated rat hepatocytes. Liver 1990, 10, 167–172. [Google Scholar] [CrossRef]
- Yang, H.; Tian, L.; Huang, J.; Liang, G.; Liu, Y. Dietary taurine can improve the hypoxia-tolerance but not the growth performance in juvenile grass carp Ctenopharyngodon idellus. Fish Physiol. Biochem. 2013, 39, 1071–1078. [Google Scholar] [CrossRef]
- Lim, C.; Lovell, R.T. Pathology of the vitamin C deficiency syndrome in channel catfish (Ictalurus punctatus). J. Nutr. 1978, 108, 1137. [Google Scholar] [CrossRef]
- Eo, J.; Lee, K. Effect of dietary ascorbic acid on growth and non-specific immune responses of tiger puffer, Takifugu rubripes. Fish Shellfish Immunol. 2008, 25, 611–616. [Google Scholar] [CrossRef]
- Xu, C.; Yu, H.; Li, L.; Li, M.; Qiu, X.; Fan, X.; Fan, Y.; Shan, L. Effects of Dietary Vitamin C on the Growth Performance, Biochemical Parameters, and Antioxidant Activity of Coho Salmon Oncorhynchus kisutch (Walbaum, 1792) Postsmolts. Aquac. Nutr. 2022, 2022, 6866578. [Google Scholar] [CrossRef]
- Khan, M.; Fatima, M.; Shah, S.Z.H.; Khan, N.; Khizar, A.; Nadeem, H.; Khan, F. Evaluation of dietary Vitamin C requirement of Hypophthalmichthys molitrix fingerlings and its effects on growth, haematology and serum enzyme activities. Aquac. Res. 2022, 53, 5582–5593. [Google Scholar] [CrossRef]
- Leal, E.; Zarza, C.; Tafalla, C. Effect of vitamin C on innate immune responses of rainbow trout (Oncorhynchus mykiss) leukocytes. Fish Shellfish Immunol. 2017, 67, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Huang, X.; Chen, N.; Apraku, A.; Wang, W.; Cornel, A.; Rahman, M.M. Impact of dietary vitamin c on plasma metabolites, antioxidant capacity and innate immunocompetence in juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 2020, 17, 100383. [Google Scholar] [CrossRef]
- Zou, W.; Lin, Z.; Huang, Y.; Limbu, S.M.; Rong, H.; Yu, C.; Lin, F.; Wen, X. Effect of dietary vitamin C on growth performance, body composition and biochemical parameters of juvenile Chu’s croaker (Nibea coibor). Aquac. Nutr. 2020, 26, 60–73. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Hou, Y.; Qiu, H.; Zhou, Q. Effect of dietary vitamin C on the growth performance, antioxidant ability and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco Richardson). Aquac. Res. 2017, 48, 149–160. [Google Scholar] [CrossRef]
- Imanpoor, M.; Imanpoor, M.R.; Roohi, Z. Effects of dietary vitamin C on skeleton abnormalities, blood biochemical factors, haematocrit, growth, survival and stress response of Cyprinus carpio fry. Aquac. Int. 2017, 25, 793–803. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Brijesh Kumar, T. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Wu, L.; Xu, W.; Li, H.; Dong, B.; Geng, H.; Jin, J.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; et al. Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio). Antioxidants 2022, 11, 935. [Google Scholar] [CrossRef]
- Huang, D.; Liang, H.; Ge, X.; Zhu, J.; Li, S.; Wang, Y.; Ren, M.; Chen, X. Effects of Dietary Lysine Levels on Growth Performance and Glycolipid Metabolism via the AKT/FoxO1 Pathway in Juvenile Largemouth Bass, Micropterus salmoides. Aquac. Nutr. 2022, 2022, 1372819. [Google Scholar] [CrossRef]
- Ren, M.; Liao, Y.; Xie, J.; Liu, B.; Zhou, Q.; Ge, X.; Cui, H.; Pan, L.; Chen, R. Dietary arginine requirement of juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2013, 414–415, 229–234. [Google Scholar] [CrossRef]
- Tang, B.; Chen, X.; Hu, C.; Dai, J.; Wang, X.; Zhang, J. Flow velocity effects on swimming behavior and exercise physiology of juvenile Acanthopagrus Schlegel II. Acta Hydrobiol. Sin. 2023, 47, 1993–2002. (In Chinese) [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 2003. [Google Scholar]
- Zhao, F.; Xu, P.; Xu, G.; Huang, D.; Zhang, L.; Ren, M.; Liang, H. Dietary valine affects growth performance, intestinal immune and antioxidant capacity in juvenile largemouth bass (Micropterus salmoides). Anim. Feed Sci. Technol. 2023, 295, 115541. [Google Scholar] [CrossRef]
- Sun, X.; Yu, H.; Xing, K.; Tian, Y.; Chen, C.; Guo, Y.; Shi, H.; Yang, S.; Chen, S.; Wang, Q. Effects of taurine levels in feed on blood biochemical parameters and antioxidant indexes of Cynoglossus semilaevis and their responses to fishing stress. Isr. J. Aquac. Bamidgeh 2021, 73, 1–16. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Z.; Niu, J. Growth performance, intestinal histomorphology, body composition, hematological and antioxidant parameters of Oncorhynchus mykiss were not detrimentally affected by replacement of fish meal with concentrated dephenolization cottonseed protein. Aquac. Rep. 2021, 19, 100557. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, W.; Li, X.; Duan, Z.; Dang, J.; Cao, K.; Leng, X. Dietary emulsifier and antioxidant improved astaxanthin utilization and antioxidant capacity of rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2021, 27, 2416–2426. [Google Scholar] [CrossRef]
- Khieokhajonkhet, A.; Suwannalers, P.; Aeksiri, N.; Ratanasut, K.; Chitmanat, C.; Inyawilert, W.; Phromkunthong, W.; Kaneko, G. Effects of dietary red pepper extracts on growth, hematology, pigmentation, disease resistance, and growth- and immune-related gene expressions of goldfish (Carassius auratus). Anim. Feed Sci. Technol. 2023, 301, 115658. [Google Scholar] [CrossRef]
- Yang, K.; Qi, X.; He, M.; Song, K.; Luo, F.; Qu, X.; Wang, G.; Ling, F. Dietary supplementation of salidroside increases immune response and disease resistance of crucian carp (Carassius auratus) against Aeromonas hydrophila. Fish Shellfish Immunol. 2020, 106, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, K.; Xi, B.; Xie, J.; Bing, X. Protective effects of paeonol against lipopolysaccharide-induced liver oxidative stress and inflammation in gibel carp (Carassius auratus gibelio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109339. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Q.; Wang, R.; Sun, K.; Li, S.; Lin, G.; Lei, P.; Xu, H. Effect of dietary poly-γ-glutamic acid on growth, digestive enzyme activity, antioxidant capacity, and TOR pathway gene expression of gibel carp (Carassius auratus gibelio). Aquac. Rep. 2022, 27, 101412. [Google Scholar] [CrossRef]
- Matsunari, H.; Takeuchi, T.; Takahashi, M.; Mushiake, K. Effect of dietary taurine supplementation on growth performance of yellowtail juveniles Seriola quinqueradiata. Fish. Sci. 2005, 71, 1131–1135. [Google Scholar] [CrossRef]
- Kim, S.; Matsunari, H.; Takeuchi, T.; Yokoyama, M.; Murata, Y.; Ishihara, K. Effect of different dietary taurine levels on the conjugated bile acid composition and growth performance of juvenile and fingerling Japanese flounder Paralichthys olivaceus. Aquaculture 2007, 273, 595–601. [Google Scholar] [CrossRef]
- Qi, G.; Ai, Q.; Mai, K.; Xu, W.; Liufu, Z.; Yun, B.; Zhou, H. Effects of dietary taurine supplementation to a casein-based diet on growth performance and taurine distribution in two sizes of juvenile turbot (Scophthalmus maximus L.). Aquaculture 2012, 358–359, 122–128. [Google Scholar] [CrossRef]
- Mellisa, S.; Fitria Hasri, I.; Nurfadillah, N.; Arisa, I.I. The effectiveness of feeding artemia enriched with vitamin c on the growth performance and survival of Lemeduk fish larvae (Barbonymus schwanenfeldii). Iop Conf. Ser. Earth Environ. Sci. 2021, 674, 12061. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Supplementation of Vitamins, Minerals, Enzymes and Antioxidants in Fish Feeds. In Feeds for the Aquaculture Sector; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 63–103. [Google Scholar] [CrossRef]
- Kandeel, M.M.A.; Magouz, F.I.; Omar, A.A.; Amer, A.A.; Zaineldin, A.I.; Ashry, A.M.; Dawood, M.A.O. Combined effects of butyl hydroxytoluene and vitamin C on the growth performance, blood biochemistry, and antioxidative status of common carp (Cyprinus carpio). Ann. Anim. Sci. 2024, 24, 881–888. [Google Scholar] [CrossRef]
- Carr, W.E.S.; Netherton, I.J.C.; Gleeson, R.A.; Derby, C.D. Stimulants of Feeding Behavior in Fish: Analyses of Tissues of Diverse Marine Organisms. Biol. Bull. 1996, 190, 149–160. [Google Scholar] [CrossRef]
- Martinez, J.; Chatzifotis, S.; Divanach, P.; Takeuchi, T. Effect of dietary taurine supplementation on growth performance and feed selection of sea bass Dicentrarchus labrax fry fed with demand-feeders. Fish. Sci. 2004, 70, 74–79. [Google Scholar] [CrossRef]
- Espe, M.; Ruohonen, K.; El-Mowafi, A. Effect of taurine supplementation on metabolism and body lipid to protein ratio in juvenile Atlantic salmon (Salmo salar). Aquac. Res. 2011, 43, 349–360. [Google Scholar] [CrossRef]
- Nasar, M.F.; Shah, S.Z.H.; Aftab, K.; Fatima, M.; Bilal, M.; Hussain, M. Dietary vitamin C requirement of juvenile grass carp (Ctenopharyngodon idella) and its effects on growth attributes, organ indices, whole-body composition and biochemical parameters. Aquac. Nutr. 2021, 27, 1903–1911. [Google Scholar] [CrossRef]
- Al-Amoudi, M.M.; El-Nakkadi, A.M.N.; El-Nouman, B.M. Evaluation of optimum dietary requirement of vitamin C for the growth of Oreochromis spilurus fingerlings in water from the Red Sea. Aquaculture 1992, 105, 165–173. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2008, 37, 43–53. [Google Scholar] [CrossRef]
- Yun, B.; Ai, Q.; Mai, K.; Xu, W.; Qi, G.; Luo, Y. Synergistic effects of dietary cholesterol and taurine on growth performance and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.) fed high plant protein diets. Aquaculture 2012, 324–325, 85–91. [Google Scholar] [CrossRef]
- Dehghani, R.; Oujifard, A.; Torfi Mozanzadeh, M.; Morshedi, V.; Bagheri, D. Effects of dietary taurine on growth performance, antioxidant status, digestive enzymes activities and skin mucosal immune responses in yellowfin seabream, Acanthopagrus latus. Aquaculture 2019, 517, 734795. [Google Scholar] [CrossRef]
- Salze, G.P.; Davis, D.A. Taurine: A critical nutrient for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Ming, J.; Xie, J.; Xu, P.; Ge, X.; Liu, W.; Ye, J. Effects of emodin and vitamin C on growth performance, biochemical parameters and two HSP70s mRNA expression of Wuchang bream (Megalobrama amblycephala Yih) under high temperature stress. Fish Shellfish Immunol. 2012, 32, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Borković, S.S.; Pavlović, S.Z.; Kovačević, T.B.; Štajn, A.Š.; Petrović, V.M.; Saičić, Z.S. Antioxidant defence enzyme activities in hepatopancreas, gills and muscle of Spiny cheek crayfish (Orconectes limosus) from the River Danube. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 122–128. [Google Scholar] [CrossRef]
- Yu, B. Cellular Defenses Against Damage From Reactive Oxygen Species. Physiol. Rev. 1995, 75, 236. [Google Scholar] [CrossRef]
- Xie, X.; Xu, Z.; Xu, K.; Xiao, Y. DUSP19 mediates spinal cord injury-induced apoptosis and inflammation in mouse primary microglia cells via the NF-kB signaling pathway. Neurol. Res. 2019, 42, 31–38. [Google Scholar] [CrossRef]
- Qiu, W.; Hu, J.; Magnuson, J.; Greer, J.; Yang, M.; Chen, Q.; Fang, M.; Zheng, C.; Schlenk, D. Evidence linking exposure of fish primary macrophages to antibiotics activates the NF-kB pathway. Environ. Int. 2020, 138, 105624. [Google Scholar] [CrossRef]
- Zhang, F.; Mao, Y.; Qiao, H.; Jiang, H.; Zhao, H.; Chen, X.; Tong, L.; Sun, X. Protective effects of taurine against endotoxin-induced acute liver injury after hepatic ischemia reperfusion. Amino Acids 2009, 38, 237–245. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Gao, J. Effects of dietary highly unsaturated fatty acid levels on growth, fatty acid profiles, antioxidant activities, mucus immune responses and hepatic lipid metabolism related gene expressions in loach (Misgurnus anguillicaudatus) juveniles. Aquac. Res. 2019, 50, 2486–2495. [Google Scholar] [CrossRef]
- Liu, J.; Pan, M.; Liu, Y.; Huang, D.; Luo, K.; Wu, Z.; Zhang, W.; Mai, K. Taurine alleviates endoplasmic reticulum stress, inflammatory cytokine expression and mitochondrial oxidative stress induced by high glucose in the muscle cells of olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2022, 123, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Haque, M.N.; Shin, Y.K.; Park, H.S.; Rhee, J. Constant and intermittent hypoxia modulates immunity, oxidative status, and blood components of red seabream and increases its susceptibility to the acute toxicity of red tide dinoflagellate. Fish Shellfish Immunol. 2020, 105, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Effects of dissolved oxygen and fish size on Nile tilapia, Oreochromis niloticus (L.): Growth performance, whole-body composition, and innate immunity. Aquac. Int. 2015, 23, 1261–1274. [Google Scholar] [CrossRef]
- Gu, J.; Liang, H.; Ge, X.; Xia, D.; Pan, L.; Mi, H.; Ren, M. A study of the potential effect of yellow mealworm (Tenebrio molitor) substitution for fish meal on growth, immune and antioxidant capacity in juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2022, 120, 214–221. [Google Scholar] [CrossRef]
- Xiao, W. The hypoxia signaling pathway and hypoxic adaptation in fishes. Science China. Life Sci. 2015, 58, 148–155. [Google Scholar] [CrossRef]
- Wanner, R.M.; Spielmann, P.; Stroka, D.M.; Camenisch, G.; Wenger, R.H. Epolones induce erythropoietin expression via hypoxia-inducible factor-1α activation. Blood 2000, 96, 1558–1565. [Google Scholar] [CrossRef]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef]
- Lee, P.; Jiang, B.; Chin, B.Y.; Iyer, N.; Alam, J.; Semenza, G.; Choi, A. Hypoxia-inducible Factor-1 Mediates Transcriptional Activation of the Heme Oxygenase-1 Gene in Response to Hypoxia. J. Biol. Chem. 1997, 272, 5375–5381. [Google Scholar] [CrossRef]
- Tacchini, L.; Bianchi, L.; Zazzera-A, B.; Cairo, G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J. Biol. Chem. 1999, 274, 24142–24146. [Google Scholar] [CrossRef]
- Amano, H.; Maruyama, K.; Naka, M.; Tanaka, T. Target validation in hypoxia-induced vascular remodeling using transcriptome/metabolome analysis. Pharmacogenom. J. 2003, 3, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Mansfield, K.; Bertozzi, C.; Rudenko, V.; Chan, D.; Giaccia, A.; Simon, M. Multiple Factors Affecting Cellular Redox Status and Energy Metabolism Modulate Hypoxia-Inducible Factor Prolyl Hydroxylase Activity In Vivo and In Vitro. Mol. Cell. Biol. 2007, 27, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Malcangio, M.; Bartolini, A.; Ghelardini, C.; Bennardini, F.; Malmberg-Aiello, P.; Franconi, F.; Giotti, A. Effect of ICV taurine on the impairment of learning, convulsions and death caused by hypoxia. Psychopharmacology 1989, 98, 316–320. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, X.; Wang, M.; Guo, H.; Liu, W.; Guang-zhen, J. Metabolism, antioxidant and immunity in acute and chronic hypoxic stress and the improving effect of vitamin C in the channel catfish (Ictalurus punctatus). Fish Physiol. Biochem. 2023, 50, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Roche, D.G.; Binning, S.A.; Bosiger, Y.; Johansen, J.L.; Rummer, J.L. Finding the best estimates of metabolic rates in a coral reef fish. J. Exp. Biol. 2013, 216, 2103–2110. [Google Scholar] [CrossRef]
- John, J.S.; Thometz, N.M.; Boerner, K.; Denum, L.; Kendall, T.L.; Richter, B.P.; Gaspard, J.C.; Williams, T.M. Metabolic trade-offs in tropical and subtropical marine mammals: Unique maintenance and locomotion costs in West Indian manatees and Hawaiian monk seals. J. Exp. Biol. 2021, 224, jeb237628. [Google Scholar] [CrossRef]
- Killen, S.S.; Glazier, D.S.; Rezende, E.L.; Clark, T.D.; Atkinson, D.; Willener, A.S.T.; Halsey, L.G. Ecological Influences and Morphological Correlates of Resting and Maximal Metabolic Rates across Teleost Fish Species. Am. Nat. 2016, 187, 592–606. [Google Scholar] [CrossRef]
- Zhu, X.; Xiao, Z.; Xu, Y.; Zhao, X.; Cheng, P.; Cui, N.; Cui, M.; Li, J.; Zhu, X. Differential Impacts of Soybean and Fish Oils on Hepatocyte Lipid Droplet Accumulation and Endoplasmic Reticulum Stress in Primary Rabbit Hepatocytes. Gastroenterol. Res. Pract. 2016, 2016, 9717014. [Google Scholar] [CrossRef]
- Kriváková, P.; Cervinkova, Z.; Lotkova, H.; Kucera, O.; Rousar, T. Mitochondria and their role in cell metabolism. Acta Med. 2005, 48, 57–67. [Google Scholar]
- Nilsson, G.E.; Dymowska, A.; Stecyk, J.A.W. New insights into the plasticity of gill structure. Respir. Physiol. Neurobiol. 2012, 184, 214–222. [Google Scholar] [CrossRef]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef] [PubMed]
- Nishinaka, Y.; Sugiyama, S.; Yokota, M.; Saito, H.; Ozawa, T. The effects of a high dose of ascorbate on ischemia-reperfusion-induced mitochondrial dysfunction in canine hearts. Heart Vessel. 1992, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | |
|---|---|
| Fish meal 1 | 14.00 |
| Chicken meal | 4.00 |
| Soybean meal 1 | 22.00 |
| Cottonseed meal | 5.00 |
| Rapeseed meal 1 | 22.00 |
| Wheat flour 1 | 14.15 |
| Rice bran | 10.00 |
| Soybean oil | 4.00 |
| Monocalcium phosphate | 2.00 |
| Vitamin premix 2 | 0.20 |
| Mineral premix 3 | 2.00 |
| Lysine | 0.30 |
| Methionine | 0.10 |
| Vc phosphate | 0.05 |
| Choline chloride | 0.20 |
| Analyzed proximate composition | |
| Crude protein (%) | 39.43 ± 0.43 |
| Crude lipid (%) | 7.08 ± 0.33 |
| Crude ash (%) | 9.98 ± 0.25 |
| Index | Measurement Methods | Note |
|---|---|---|
| CAT | Ammonium molybdenum acid method | Assay kits purchased from Jian Cheng Bioengineering Institute (Nanjing, China); Spectrophotometer (Thermo Fisher Multiskan GO, Shanghai, China). |
| T-AOC | ABTS method | |
| SOD | WST-1 method | |
| MDA | TBA method | |
| GSH | Microplate method | |
| GSH-Px | Colorimetric method |
| Genes | Forward (5′–3′) | Reverse (5′–3′) | Primer Source |
|---|---|---|---|
| il-10 | AGTGAGACTGAAGGAGCTCCG | TGGCAGAATGGTGTCCAAGTA | [37] |
| tgf-β | GTTGGCGTAATAACCAGAAGG | AACAGAACAAGTTTGTACCGATAAG | [38] |
| il-1β | GCGCTGCTCAACTTCATCTTG | GTGACACATTAAGCGGCTTCA C | [38] |
| il-6 | CGGAGGGGCTTAACAGGATG | GCTGGCTCAGGAATGGGTAT | DQ861993.1 |
| il-8 | ATTGGTGAAGGAATGAGTCT | CCACAGATGACCTTGACAT | KC184490.1 |
| tnf-α | CATTCCTACGGATGGCATTTACTT | CCTCAGGAATGTCAGTCTTGCAT | [38] |
| nf-κb | GCTCTGACTGCGGTCTTATAC | GCGCTTCATCGAGGATAGTT | [39] |
| cat | TGAAGTTCTACACCGATGAG | CTGAGAGTGGACGAAGGA | XM_026238665.1 |
| sod | TCGGAGACCTTGGTAATGT | CGCCTTCTCATGGATCAC | JQ776518.1 |
| gpx | GAAGTGAACGGTGTGAACGC | GATCCCCCATCAAGGACACG | DQ983598.1 |
| keap1 | CTCCGCTGAATGCTACAA | GGTCATAACACTCCACACT | XM_026245355.1 |
| nrf2 | TACCAAAGACAAGCAGAAGAAACG | GCCTCGTTGAGCTGGTGTTTGG | [40] |
| hif-1α | CTGCCGATCAGTCTGTCTCC | TTTGTGGAGTCTGGACCACG | DQ306727.1 |
| vegf | ATCGAGCACACGTACATCCC | CCTTTGGCCTGCATTCACAC | NM_131408.3 |
| epo | CGAAGTGTCAGCATACCGGA | GCAGATGACGCACTTTTCCC | KC460317.1 |
| ho-1 | GCAAACCAAGAGAAGCCACC | GGAAGTAGACGGGCTGAACC | KC758864 |
| angpt1 | CCAAACCTCACCAAGCAAGC | GGATTACAGTCCAGCCTCCG | XM_059556208.1 |
| et1 | TAAAGCAGCGTCAGACAGGG | CTGCCAGCTTGTGTTTGCAT | NM_131519.1 |
| nos | GGGGACCCTCCTGAAAATGG | TTCTGTCCTCAACGCTGGTG | AY644726.1 |
| tf | CCGAGAAGATGCACGCAAAG | TGTGCATGCCTTGACCAGAT | AF518747.1 |
| tfr1 | CTTTGTCAACGAAGTGGCTGAAT | TACCAAAGAAAATGTGGCGGAAC | XM_052542523.1 |
| β-actin | TCCATTGTTGGACGACCCAG | TGGGCCTCATCTCCCACATA | LC382464.1 |
| Parameters | Dietary Taurine and Vitamin C Levels (%) | ||
|---|---|---|---|
| 0 + 0 | 0.1 + 0 | 0.1 + 0.1 | |
| Growth performance | |||
| IBW (g) 1 | 41.92 ± 0.08 | 41.82 ± 0.04 | 41.78 ± 0.11 |
| FBW (g) 2 | 101.57 ± 0.58 | 102.97 ± 0.84 | 104.20 ± 0.77 |
| FCR 3 | 1.37 ± 0.02 | 1.39 ± 0.02 | 1.43 ± 0.01 |
| SGR (% day-1) 4 | 0.95 ± 0.01 b | 0.97 ± 0.01 ab | 0.98 ± 0.01 a |
| WGR (%) 5 | 142 ± 0.02 b | 146 ± 0.02 ab | 149 ± 0.02 a |
| SR (%) 6 | 100.0 ± 0.00 | 100.0 ± 0.00 | 100.0 ± 0.00 |
| Whole-body composition (%) | |||
| Moisture | 75.21 ± 1.02 | 74.88 ± 0.95 | 74.99 ± 0.62 |
| Crude protein | 15.92 ± 0.38 | 15.80 ± 0.42 | 16.42 ± 0.36 |
| Crude lipid | 2.62 ± 0.93 | 2.37 ± 0.75 | 1.82 ± 0.26 |
| Ash | 4.56 ± 0.16 | 4.75 ± 0.06 | 4.76 ± 0.14 |
| Swimming ability | |||
| SST (sec) 7 | 37.17 ± 8.63 b | 43.00 ± 8.79 b | 142.20 ± 8.63 a |
| Experimental Groups | Parameters | |||||
|---|---|---|---|---|---|---|
| Dissolved Oxygen (mg/L)/Taurine and Vitamin C Levels (%) | CAT (U/mgprot) 1 | T-AOC (mmol/gprot) 2 | SOD (U/mgprot) 3 | MDA (nmol/mgprot) 4 | GSH (μmol/gprot) 5 | GSH-Px (U/mgprot) 6 |
| 6.5/(0 + 0) | 235.26 ± 6.40 c | 0.20 ± 0.03 b | 9.72 ± 0.41 b | 8.12 ± 0.43 | 341.36 ± 39.41 b | 389.08 ± 24.74 a |
| 6.5/(0.1 + 0) | 256.69 ± 4.69 b | 0.47 ± 0.06 a | 11.29 ± 0.28 a | 6.95 ± 0.24 | 492.17 ± 16.72 a | 344.99 ± 29.15 ab |
| 6.5/(0.1 + 0.1) | 287.47 ± 2.43 a | 0.34 ± 0.05 ab | 11.07 ± 0.30 a | 7.13 ± 0.67 | 416.84 ± 39.82 ab | 299.65 ± 8.92 b |
| 0.8/(0 + 0) | 307.08 ± 10.93 A | 0.39 ± 0.04 A | 7.90 ± 0.53 | 3.17 ± 0.41 | 355.38 ± 26.57 B | 448.10 ± 31.79 |
| 0.8/(0.1 + 0) | 258.22 ± 14.23 B | 0.46 ± 0.06 A | 7.42 ± 0.53 | 2.70 ± 0.43 | 476.97 ± 41.14 A | 529.97 ± 28.06 |
| 0.8/(0.1 + 0.1) | 255.88 ± 13.15 B | 0.19 ± 0.02 B | 6.76 ± 0.19 | 3.48 ± 0.26 | 377.81 ± 21.70 B | 483.13 ± 15.06 |
| Dissolved oxygen (mg/L) | ||||||
| 6.5 | 261.54 ± 6.14 | 0.34 ± 0.04 | 10.75 ± 0.24 y | 7.34 ± 0.27 y | 416.79 ± 25.22 | 344.54 ± 15.55 x |
| 0.8 | 272.53 ± 9.63 | 0.35 ± 0.04 | 7.33 ± 0.26 x | 3.10 ± 0.22 x | 401.79 ± 20.87 | 487.34 ± 17.47 y |
| Taurine and vitamin C levels (%) | ||||||
| 0 + 0 | 267.18 ± 13.80 | 0.32 ± 0.04 b | 8.82 ± 0.44 | 5.15 ± 0.86 | 349.15 ± 21.47 b | 418.59 ± 21.39 |
| 0.1 + 0 | 257.45 ± 7.07 | 0.46 ± 0.04 a | 9.35 ± 0.65 | 4.83 ± 0.68 | 483.72 ± 22.89 a | 447.75 ± 37.64 |
| 0.1 + 0.1 | 274.83 ± 7.19 | 0.26 ± 0.03 b | 8.92 ± 0.67 | 5.11 ± 0.71 | 395.55 ± 21.32 b | 381.20 ± 33.15 |
| Two-way ANOVA | ||||||
| Dissolved oxygen | 0.073 | 0.725 | 0.000 | 0.000 | 0.619 | 0.009 |
| Taurine and vitamin C levels | 0.219 | 0.000 | 0.341 | 0.138 | 0.002 | 0.210 |
| Interaction | 0.000 | 0.006 | 0.009 | 0.331 | 0.714 | 0.025 |
| Parameters | Dietary Taurine and Vitamin C levels (%) | ||
|---|---|---|---|
| 0 + 0 | 0.1 + 0 | 0.1 + 0.1 | |
| SR (%) 1 | 12.50 ± 12.50 a | 8.33 ± 4.17 a | 33.33 ± 4.17 a |
| NCM (pcs) 2 | 2.00 ± 1.00 a | 6.00 ± 1.53 a | 5.67 ± 2.91 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, L.; Liang, H.; Huang, D.; Ren, M. Effects of Taurine and Vitamin C on the Improvement of Antioxidant Capacity, Immunity and Hypoxia Tolerance in Gibel Carp (Carrassius auratus gibeilo). Antioxidants 2024, 13, 1169. https://doi.org/10.3390/antiox13101169
Zhang L, Zhang L, Liang H, Huang D, Ren M. Effects of Taurine and Vitamin C on the Improvement of Antioxidant Capacity, Immunity and Hypoxia Tolerance in Gibel Carp (Carrassius auratus gibeilo). Antioxidants. 2024; 13(10):1169. https://doi.org/10.3390/antiox13101169
Chicago/Turabian StyleZhang, Leimin, Lu Zhang, Hualiang Liang, Dongyu Huang, and Mingchun Ren. 2024. "Effects of Taurine and Vitamin C on the Improvement of Antioxidant Capacity, Immunity and Hypoxia Tolerance in Gibel Carp (Carrassius auratus gibeilo)" Antioxidants 13, no. 10: 1169. https://doi.org/10.3390/antiox13101169







