An Overview of the Mechanisms through Which Plants Regulate ROS Homeostasis under Cadmium Stress
Abstract
1. Introduction
2. Antioxidant Systems Involved in Regulating Cd-Induced ROS Bursts
3. Mechanisms by Which Different Mineral Elements Regulate ROS Homeostasis under Cd Stress
3.1. Nitrogen (N)
3.2. Phosphate (P)
3.3. Calcium (Ca)
3.4. Iron (Fe)
3.5. Zinc (Zn)
3.6. Selenium (Se)
3.7. Silicon (Si)
3.8. Sulfur (S)
3.9. Other Minerals
4. The Role of Plant Growth Regulators and Signaling Molecules in Regulating ROS Homeostasis under Cd Stress
4.1. Plant Growth Regulators
4.1.1. Abscisic Acid (ABA)
4.1.2. Auxin
4.1.3. Brassinosteroids (BRs)
4.1.4. Ethylene (ET)
4.1.5. Jasmonic Acid (JA)
4.1.6. Salicylic Acid (SA)
4.1.7. Melatonin (MLT)
4.1.8. Other Phytohormones
4.2. Signaling Molecules
4.2.1. Nitric Oxide (NO)
4.2.2. Hydrogen Peroxide (H2O2)
4.2.3. Hydrogen Sulfide (H2S)
5. Transcriptional and Post-Transcriptional Modification Regulation of ROS Homeostasis under Cd Stress
| TF Name | Plant Species | Functions in Plant | Target Genes | Ref. |
|---|---|---|---|---|
| AtMYB4 | Arabidopsis thaliana | AtMYB4 positively regulates Cd tolerance via promoting PCs and MT accumulation, and enhancing CAT, SOD, and APX activities. | AtPCS1; AtMT1C; AtCAT3; AtSOD; AtAPX1 | [260] |
| AtMYB75 | Arabidopsis thaliana | AtMYB75 positively regulates Cd tolerance via promoting anthocyanin, GSH, and PC biosynthesis and enhancing SOD, and CAT activities. | AtACBP2; AtABCC2 | [33] |
| AtWRKY12 | Arabidopsis thaliana | AtWRKY12 negatively regulates Cd toxicity via decreasing GSH and PC contents. | AtGSH1; AtGSH2; AtPCS1; AtPCS2 | [262] |
| CaPF1 | Capsicum annuum | Overexpression of CaPF1 increased Cd tolerance via enhancing APX, GR and SOD activities in Virginia pine. | None | [267] |
| MsbHLH115 | Medicago sativa | Overexpression of MsbHLH115 increased Cd tolerance via enhancing Fe/Zn accumulation and SOD, POD and CAT acitivities in Arabidopsis. | MsbHLH121; AtSOD1; AtPOD1 | [264] |
| PyWRKY75 | Populus yunnanensis | Overexpression of PyWRKY75 increased Cd tolerance via enhancing POD, SOD, CAT, and APX activities, and increasing AsA, GSH, and PCs accumulation. | None | [263] |
| SaHsfA4c | Sedum alfredii | Overexpression of SaHsfA4c increased Cd tolerance via enhancing CAT, APX, and POD activities in Arabiposis and Sedum alfredii. | SaCAT; SaAPX; SaPOD | [261] |
| ThWRKY7 | Tamarix hispida | ThWRKY7 can specifically bind to and activate ThVHAc1 and improve Cd stress tolerance by enhancing SOD, POD, and GPX acitivities. | ThVHAc1; ThSOD; ThGPX; ThPOD | [268] |
| OsbZIP20 | Oryza sativa | OsbZIP20 can specially bind to and activate the transcription of OsCATA and OsAPX2 and improve Cd stress tolerance by enhancing CAT and APX activities. | OsCATA; OsAPX2 | [51] |
| ZmWRKY4 | Zea mays | Overexpression of ZmWRKY4 positively regulates Cd tolerance via enhancing SOD and APX activities. | ZmSOD4; ZmAPX | [177] |
6. Application of Amendments for Mitigating Cd-Induced Oxidative Stress
6.1. Citric Acid (CA)
6.2. β-Aminobutyric Acid (BABA)
6.3. Nanoenzymes
6.4. Exopolysaccharides (EPSs)
| Amendments | Doses | Cd2+ | Medium | Plant Species | Functions in Plant | Ref. |
|---|---|---|---|---|---|---|
| CA | 0.5 mM/1.0 mM | 0.5 mM /1.0 mM | Solution | Brassica juncea | Increasing the levels of AsA, GSH, and PC, while enhancing the activities of key antioxidant enzymes such as APX, MDHAR, DHAR GR, GPX, SOD, and CAT. | [272] |
| BABA | 200 μM | 100 μM /150 μM | 1/2 MS | Arabidopsis thaliana | Increasing GSH levels. | [273] |
| 20 mM | / | / | Fragaria ananassa | Increasing the endogenous levels of NO, H2S, and AsA. | [277] | |
| 200 μM | 100 μM | Solution | Glycine max | Priming and activating antioxidant defense mechanisms. | [276] | |
| Nanoenzymes | 120 μg/mL | 50 μM | 1/2 MS | Arabidopsis thaliana | Enhancing the activities of SOD, POD and CAT, while mimicking the activities of SOD, CAT, POD, TPX, GPX, and APX. | [278] |
| 150 μM | 1/2 MS | Solanum lycopersicum | ||||
| 300 μM | Solution | |||||
| 27 μg/mg | Soil | |||||
| EPS | 100 mg/L /200 mg/L | 50 μM | Solution | Oryza sativa | Enhancing the activities of CAT and POD, while increasing the levels of GSH. | [281] |
| 250 mg/L | 30 μM | Solution | Oryza sativa | Increasing GSH levels. | [43] | |
| 100 mg/L /400 mg/L | 10 μM /25 μM | Solution | Oryza sativa | Promoting lignin biosynthesis and activating antioxidant activity. | [282] | |
| NAC | 500 μM | 50 μM /200 μM | Solution | Solanum nigrum | Enhancing the biosynthesis of GSH. | [21] |
6.5. N-Acetyl-Cysteine (NAC)
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W.J. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.; Rafiq, M.T.; Li, T.Q.; Liu, D.; He, Z.L.; Stoffella, P.J.; Sun, K.; Xiaoe, Y. Uptake of cadmium by rice grown on contaminated soils and its bioavailability/toxicity in human cell lines (Caco-2/HL-7702). J. Agric. Food Chem. 2015, 63, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Jiang, X.H.; Li, K.; Wu, M.; Zhang, R.F.; Zhang, L.; Chen, G.X. Photosynthetic responses of Oryza sativa L. seedlings to cadmium stress: Physiological, biochemical and ultrastructural analyses. BioMetals 2014, 27, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Aslam, S.; Akbar, M.; Ahmad, A.; Khan, W.U.; Yasin, N.A.; Ali, B.; Rizwan, M.; Ali, S. Combined effect of Bacillus fortis IAGS 223 and zinc oxide nanoparticles to alleviate cadmium phytotoxicity in Cucumis melo. Plant Physiol. Biochem. 2021, 158, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, X.; Islam, E.; Liu, D.; Mahmood, Q. Effects of cadmium on ultrastructure and antioxidative defense system in hyperaccumulator and non-hyperaccumulator ecotypes of Sedum alfredii Hance. J. Hazard. Mater. 2008, 156, 387–397. [Google Scholar] [CrossRef]
- Shamsi, I.H.; Jilani, G.; Zhang, G.P.; Wei, K. Cadmium stress tolerance through potassium nutrition in soybean. Asian J. Chem. 2008, 20, 1099–1108. [Google Scholar]
- Daud, M.K.; Sun, Y.; Dawood, M.; Hayat, Y.; Variath, M.T.; Wu, Y.X.; Raziuddin; Mishkat, U.; Salahuddin; Najeeb, U.; et al. Cadmium-induced functional and ultrastructural alterations in roots of two transgenic cotton cultivars. J. Hazard. Mater. 2009, 161, 463–473. [Google Scholar] [CrossRef]
- Furze, J.M.; Rhodes, M.J.; Parr, A.J.; Robins, R.J.; Withehead, I.M.; Threlfall, D.R. Abiotic factors elicit sesquiterpenoid phytoalexin production but not alkaloid production in transformed root cultures of Datura stramonium. Plant Cell Rep. 1991, 10, 111–114. [Google Scholar] [CrossRef]
- Pinto, A.P.; Mota, A.M.; de Varennes, A.; Pinto, F.C. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci. Total Environ. 2004, 326, 239–247. [Google Scholar] [CrossRef]
- Sanità di Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Kaushik, S.; Ranjan, A.; Sidhu, A.; Singh, A.K.; Sirhindi, G. Cadmium toxicity: Its’ uptake and retaliation by plant defence system and ja signaling. BioMetals 2024, 37, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wen, Q.R.; Ma, T.C.; Zhu, Q.H.; Huang, D.Y.; Zhu, H.H.; Xu, C.; Chen, H.F. Cadmium-induced iron deficiency is a compromise strategy to reduce Cd uptake in rice. Environ. Exp. Bot. 2023, 206, 105155. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Cadmium uptake by wheat (Triticum aestivum L.): An overview. Plants 2020, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Lu, Y.S.; Zhang, X.; Yang, G.Z.; Chao, D.; Wang, Z.G.; Shi, M.X.; Chen, J.G.; Chao, D.Y.; Li, R.B.; et al. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J. Exp. Bot. 2019, 70, 5909–5918. [Google Scholar] [CrossRef]
- Gravot, A.; Lieutaud, A.; Verret, F.; Auroy, P.; Vavasseur, A.; Richaud, P. AtHMA3, a plant P-ATPase, functions as a Cd/Pb transporter in yeast. FEBS Lett. 2004, 561, 22–28. [Google Scholar] [CrossRef]
- Korenkov, V.; Hirschi, K.; Crutchfield, J.D.; Wagner, G.J. Enhancing tonoplast Cd/H antiport activity increases Cd, Zn, and Mn tolerance, and impacts root/shoot Cd partitioning in Nicotiana tabacum L. Planta 2007, 226, 1379–1387. [Google Scholar] [CrossRef]
- Liu, X.S.; Feng, S.J.; Zhang, B.Q.; Wang, M.Q.; Cao, H.W.; Rono, J.K.; Chen, X.; Yang, Z.M. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Banadka, A.; Rashmi, R.; Nagella, P.; Alessa, F.M.; Almaghasla, M.I. Cadmium toxicity in medicinal plants: An overview of the tolerance strategies, biotechnological and omics approaches to alleviate metal stress. Front. Plant Sci. 2022, 13, 1047410. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Long, C.; Cui, Z.X. Effect of biodegradable chelators on induced phytoextraction of uranium- and cadmium-contaminated soil by Zebrina pendula Schnizl. Sci. Rep. 2019, 9, 19187. [Google Scholar] [CrossRef]
- Deng, X.; Xia, Y.; Hu, W.; Zhang, H.; Shen, Z. Cadmium-induced oxidative damage and protective effects of N-acetyl-L-cysteine against cadmium toxicity in Solanum nigrum L. J. Hazard. Mater. 2010, 180, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Eapen, S.; D’Souza, S.F. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 2006, 62, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Shaari, N.E.M.; Tajudin, M.; Khandaker, M.M.; Majrashi, A.; Alenazi, M.M.; Abdullahi, U.A.; Mohd, K.S. Cadmium toxicity symptoms and uptake mechanism in plants: A review. Braz. J. Biol. 2022, 84, e252143. [Google Scholar] [CrossRef] [PubMed]
- Kolahi, M.; Kazemi, E.M.; Yazdi, M.; Goldson-Barnaby, A. Oxidative stress induced by cadmium in lettuce (Lectuca sativa Linn.): Oxidative stress indicators and prediction of their genes. Plant Physiol. Biochem. 2020, 146, 71–89. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Hou, L.; Ji, S.Z.; Zhang, Y.; Wu, X.Z.; Zhang, L.; Liu, P. The mechanism of silicon on alleviating cadmium toxicity in plants: A review. Front. Plant Sci. 2023, 14, 1141138. [Google Scholar] [CrossRef]
- Ketta, M.; Tumová, E.; Stupka, R.; Cítek, J.; Chodová, D. Adverse effects of cadmium on poultry and role of selenium against it: An updated review. Czech J. Anim. Sci. 2021, 66, 339–348. [Google Scholar] [CrossRef]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Mitigating cadmium accumulation and toxicity in plants: The promising role of nanoparticles. Sci. Total Environ. 2024, 912, 168826. [Google Scholar]
- Vitelli, V.; Giamborino, A.; Bertolini, A.; Saba, A.; Andreucci, A. Cadmium stress signaling pathways in plants: Molecular responses and mechanisms. Curr. Issues Mol. Biol. 2024, 46, 6052–6068. [Google Scholar] [CrossRef]
- Zhang, H.W.; Lu, L.L. Transcription factors involved in plant responses to cadmium-induced oxidative stress. Front. Plant Sci. 2024, 15, 1397289. [Google Scholar] [CrossRef]
- Bi, J.W.; Liu, X.C.; Liu, S.R.; Wang, Y.T.; Liu, M. Microstructural and physiological responses to cadmium stress under different nitrogen forms in two contrasting Populus clones. Environ. Exp. Bot. 2020, 169, 103897. [Google Scholar] [CrossRef]
- Han, C.; Wang, L.; Lyu, J.; Shi, W.; Yao, L.; Fan, M.; Bai, M.Y. Brassinosteroid signaling and molecular crosstalk with nutrients in plants. J. Genet. Genom. 2023, 50, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Lu, X.; Yang, F.; Zhang, D. Synergetic modulation of plant cadmium tolerance via MYB75-mediated ROS homeostasis and transcriptional regulation. Plant Cell Rep. 2022, 41, 1515–1530. [Google Scholar] [CrossRef] [PubMed]
- Zaets, I.; Kramarev, S.; Kozyrovska, N. Inoculation with a bacterial consortium alleviates the effect of cadmium overdose in soybean plants. Cent. Eur. J. Biol. 2010, 5, 481–490. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, Y.X.; Guo, Z.P.; Han, M.L.; Yan, X.B. Zinc oxide nanoparticles alleviate cadmium toxicity and promote tolerance by modulating programmed cell death in alfalfa (Medicago sativa L.). J. Hazard. Mater. 2024, 469, 133917. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Scebba, F.; Arduini, I.; Ercoli, L.; Sebastiani, L. Cadmium effects on growth and antioxidant enzymes activities in. Biol. Plant. 2006, 50, 688–692. [Google Scholar] [CrossRef]
- Radotic, K.; Ducic, T.; Mutavdzic, D. Changes in peroxidase activity and isoenzymes in spruce needles after exposure to different concentrations of cadmium. Environ. Exp. Bot. 2000, 44, 105–113. [Google Scholar] [CrossRef]
- Li, F.T.; Qi, J.M.; Zhang, G.Y.; Lin, L.H.; Fang, P.P.; Tao, A.F.; Xu, J.T. Effect of cadmium stress on the growth, antioxidative enzymes and lipid peroxidation in two Kenaf (Hibiscus cannabinus L.) Plant Seedlings. J. Integr. Agric. 2013, 12, 610–620. [Google Scholar] [CrossRef]
- Dixit, V.; Pandey, V.; Shyam, R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J. Exp. Bot. 2001, 52, 1101–1109. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Alharby, H.F.; Razafindrabe, B.H.N.; Fujita, M. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: An intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 2017, 8, 682. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Q.H.; Hou, X.C.; Zhao, C.Q.; Guo, Q. Overexpression of IlHMA2, from Iris lactea, improves the accumulation of and tolerance to cadmium in tobacco. Plants 2023, 12, 3460. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.Y.; Li, Y.; Wei, L.; Peng, S.Y.; Zhang, B.; Xu, D.J.; Cheng, X. Exploring the mechanism of exopolysaccharides in mitigating cadmium toxicity in rice through analyzing the changes of antioxidant system. J. Hazard. Mater. 2024, 461, 132678. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Lee, S.S.; Chung, J.M.; Jung, H.S.; Singh, S.; Mondal, S.; Jang, H.H.; Cho, J.Y.; Bae, H.J.; Chung, B.Y. Site-specific mutagenesis of yeast 2-Cys peroxiredoxin improves heat or oxidative stress tolerance by enhancing its chaperone or peroxidase function. Protoplasma 2017, 254, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Yao, T.T.; Wang, Y.; Wang, J.C.; Song, J.Q.; Cui, C.C.; Ji, G.X.; Cao, J.N.; Muhammad, S.; Ao, H.; et al. CDSP32-overexpressing tobacco plants improves cadmium tolerance by modulating antioxidant mechanism. Plant Physiol. Biochem. 2023, 194, 524–532. [Google Scholar] [CrossRef]
- Xu, J.R.; Wei, Z.; Lu, X.F.; Liu, Y.Z.; Yu, W.J.; Li, C.X. Involvement of nitric oxide and melatonin enhances cadmium resistance of tomato seedlings through regulation of the ascorbate-glutathione cycle and ROS metabolism. Int. J. Mol. Sci. 2023, 24, 9526. [Google Scholar] [CrossRef]
- Wu, Z.C.; Zhang, W.J.; Xu, S.J.; Shi, H.Z.; Wen, D.; Huang, Y.D.; Peng, L.J.; Deng, T.H.B.; Du, R.Y.; Li, F.R.; et al. Increasing ammonium nutrition as a strategy for inhibition of cadmium uptake and xylem transport in rice (Oryza sativa L.) exposed to cadmium stress. Environ. Exp. Bot. 2018, 155, 734–741. [Google Scholar] [CrossRef]
- Di, D.W.; Sun, L.; Zhang, X.N.; Li, G.J.; Kronzucker, H.J.; Shi, W.M. Involvement of auxin in the regulation of ammonium tolerance in rice (Oryza sativa L.). Plant Soil 2018, 432, 373–387. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 1997, 385, 59–61. [Google Scholar] [CrossRef]
- Nasraoui-Hajaji, A.; Chaffei-Haouari, C.; Ghorbel, M.H.; Gouia, H. Growth and nitrate assimilation in tomato (Solanum lycopersicum) grown with different nitrogen source and treated with cadmium. Acta Bot. Gall. 2011, 158, 3–11. [Google Scholar] [CrossRef]
- Di, D.W.; Li, T.T.; Yu, Z.L.; Cheng, J.; Wang, M.; Liu, C.F.; Wang, Y.; Kronzucker, H.J.; Yu, M.; Shi, W. Ammonium mitigates cadmium toxicity by activating the bZIP20-APX2/CATA transcriptional module in rice seedlings in an ABA-dependent manner. J. Hazard. Mater. 2024, 480, 135874. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Kumar, R.G.; Verma, S.; Dubey, R.S. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001, 161, 1135–1144. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, Q.; Yan, T.; Zhang, X.; Xu, S.; Shi, H.; Deng, T.H.; Li, F.; Du, Y.; Du, R.; et al. Ammonium nutrition mitigates cadmium toxicity in rice (Oryza sativa L.) through improving antioxidase system and the glutathione-ascorbate cycle efficiency. Ecotoxicol. Environ. Saf. 2020, 189, 110010. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Recalde, L.; Cabrera, A.; Groppa, M.D.; Benavides, M.P. Does nitrogen source influence cadmium distribution in Arabidopsis plants? Ecotoxicol. Environ. Saf. 2020, 191, 110163. [Google Scholar] [CrossRef]
- Billini, M.; Hoffmann, T.; Kühn, J.; Bremer, E.; Thanbichler, M. The cytoplasmic phosphate level has a central regulatory role in the phosphate starvation response of Caulobacter crescentus. Commun. Biol. 2024, 7, 772. [Google Scholar] [CrossRef]
- Ruangcharus, C.; Kim, S.U.; Hong, C.O. Mechanism of cadmium immobilization in phosphate-amended arable soils. Appl. Biol. Chem. 2020, 63, 36. [Google Scholar] [CrossRef]
- Wang, X.L.; Zou, H.T.; Liu, Q. Effects of phosphate and silicate combined application on cadmium form changes in heavy metal contaminated soil. Sustainability 2023, 15, 4503. [Google Scholar] [CrossRef]
- Arshad, M.; Ali, S.; Noman, A.; Ali, Q.; Rizwan, M.; Farid, M.; Irshad, M.K. Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch. Agron. Soil Sci. 2016, 62, 533–546. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhang, J.L.; Christie, P.; Zhang, F.S. Influence of external zinc and phosphorus supply on Cd uptake by rice (Oryza sativa L.) seedlings with root surface iron plaque. Plant Soil 2007, 300, 105–115. [Google Scholar] [CrossRef]
- Siebers, N.; Siangliw, M.; Tongcumpou, C. Cadmium uptake and subcellular distribution in rice plants as affected by phosphorus: Soil and hydroponic experiments. J. Soil Sci. Plant Nutr. 2013, 13, 833–844. [Google Scholar] [CrossRef]
- Dong, Q.; Wallrad, L.; Almutairi, B.O.; Kudla, J. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2022, 64, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Feng, X.Y.; Qiu, G.Y.; Li, H.; Wang, Y.; Chen, X.D.; Fu, Q.L.; Guo, B. Inhibition roles of calcium in cadmium uptake and translocation in rice: A review. Int. J. Mol. Sci. 2023, 24, 11587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Wang, Z.Q.; Liu, Y.S.; Zhang, T.Q.; Liu, J.M.; You, Z.; Huang, P.P.; Zhang, Z.Q.; Wang, C. Plasma membrane-associated calcium signaling modulates cadmium transport. New Phytol. 2023, 238, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Sinegani, M.S.; Manzoor, M.; Mühling, K.H. Calcium-associated anions play a dual role in modulating cadmium uptake and translocation in wheat. Pollutants 2024, 4, 340–349. [Google Scholar] [CrossRef]
- Cho, S.C.; Chao, Y.Y.; Kao, C.H. Calcium deficiency increases Cd toxicity and Ca is required for heat-shock induced Cd tolerance in rice seedlings. J. Plant Physiol. 2012, 169, 892–898. [Google Scholar] [CrossRef]
- Chen, H.B.; Tang, X.J.; Wang, T.J.; Liao, W.F.; Wu, Z.X.; Wu, M.L.; Song, Z.H.; Li, Y.D.; Luo, P. Calcium polypeptide mitigates Cd toxicity in rice via reducing oxidative stress and regulating pectin modification. Plant Cell Rep. 2024, 43, 163. [Google Scholar] [CrossRef]
- Nazir, M.M.; Noman, M.; Ahmed, T.; Ali, S.; Ulhassan, Z.; Zeng, F.R.; Zhang, G.P. Exogenous calcium oxide nanoparticles alleviate cadmium toxicity by reducing Cd uptake and enhancing antioxidative capacity in barley seedlings. J. Hazard. Mater. 2022, 438, 129498. [Google Scholar] [CrossRef]
- Rahman, A.; Mostofa, M.G.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems calcium-induced cadmium stress tolerance in rice. Braz. J. Bot. 2016, 39, 393–407. [Google Scholar] [CrossRef]
- Okla, M.K.; Saleem, M.H.; Saleh, I.A.; Zomot, N.; Perveen, S.; Parveen, A.; Abasi, F.; Ali, H.; Ali, B.; Alwasel, Y.A.; et al. Foliar application of iron-lysine to boost growth attributes, photosynthetic pigments and biochemical defense system in canola (Brassica napus L.) under cadmium stress. BMC Plant Biol. 2023, 23, 648. [Google Scholar] [CrossRef]
- Tan, Z.; Li, J.; Guan, J.; Wang, C.; Zhang, Z.; Shi, G. Genome-wide identification and expression analysis reveals roles of the NRAMP gene family in iron/cadmium interactions in peanut. Int. J. Mol. Sci. 2023, 24, 1713. [Google Scholar] [CrossRef]
- Wang, R.; Fei, Y.; Pan, Y.; Zhou, P.; Adegoke, J.O.; Shen, R.; Lan, P. IMA peptides function in iron homeostasis and cadmium resistance. Plant Sci. 2023, 336, 111868. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Galisteo, A.P.; Rodriguez-Serrano, M.; Pazmino, D.M.; Gupta, D.K.; Sandalio, L.M.; Romero-Puertas, M.C. S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: Changes under abiotic stress. J. Exp. Bot. 2012, 63, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Noreen, Z.; Rashid, M.; Aslam, M.; Hussain, T.; Younas, A.; Fiaz, S.; Attia, K.A.; Mohammed, A.A. Understanding the role of magnetic (Fe3O4) nanoparticle to mitigate cadmium stress in radish (Raphanus sativus L.). Bot. Stud. 2024, 65, 20. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, S.; Zhou, B.B.; Zia-ur-Rehman, M.; Maqsood, M.A.; Hussain, S.; Bakhsh, G.; Zhang, Z.S.; Yang, Q.; Altaf, A.R. Cadmium stabilization and redox transformation mechanism in maize using nanoscale zerovalent-iron-enriched biochar in cadmium-contaminated soil. Plants 2022, 11, 1074. [Google Scholar] [CrossRef]
- Khavari-Nejad, R.A.; Najafi, F.; Rezaei, M. The influence of cadmium toxicity on some physiological parameters as sffected by iron in rice (Oryza sativa L.) plant. J. Plant Nutr. 2014, 37, 1202–1213. [Google Scholar] [CrossRef]
- Li, M.W.; Liu, C.R.; Zhang, D.Q.; Wang, B.W.; Ding, S. The influence of iron application on the growth and cadmium stress tolerance of poplar. Forests 2022, 13, 2023. [Google Scholar] [CrossRef]
- Shao, G.S.; Chen, M.X.; Wang, W.X.; Mon, R.X.; Zhang, G.P. Iron nutrition affects cadmium accumulation and toxicity in rice plants. Plant Growth Regul. 2007, 53, 33–42. [Google Scholar] [CrossRef]
- Sinclair, S.A.; Kramer, U. The zinc homeostasis network of land plants. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 1553–1567. [Google Scholar] [CrossRef]
- Thompson, M.W. Regulation of zinc-dependent enzymes by metal carrier proteins. BioMetals 2022, 35, 187–213. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Hassan, M.J.; Zhang, G.P.; Wu, F.B.; Wei, K.; Chen, Z.H. Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. J. Plant Nutr. Soil Sci. 2005, 168, 255–261. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, C.; Jiao, Q.J.; Li, G.Z.; Alyemeni, M.N.; Ahmad, P.; Shah, T.R.; Fahad, S.; Zhang, J.J.; Zhao, Y.; et al. Interactive effect of silicon and zinc on cadmium toxicity alleviation in wheat plants. J. Hazard. Mater. 2023, 458, 131933. [Google Scholar] [CrossRef]
- Hassan, M.U.; Huang, G.Q.; Haider, F.U.; Khan, T.A.; Noor, M.A.; Luo, F.; Zhou, Q.; Yang, B.J.; Ul Haq, M.I.; Iqbal, M.M. Application of zinc oxide nanoparticles to mitigate cadmium toxicity: Mechanisms and future prospects. Plants 2024, 13, 1706. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Khan, S.; Jamil, M.; Rehman, S.U.; Rehman, Z.U.; Rha, E.S. Combine effect of ZnO NPs and bacteria on protein and gene’s expression profile of rice (Oryza sativa L.) plant. Toxics 2022, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Bai, Y.S.; Zhang, J.L.; Zada, S.; Khan, N.; Hu, Z.L.; Tang, Y.L. Discovering nature’s shield: Metabolomic insights into green zinc oxide nanoparticles Safeguarding Brassica parachinensis L. from cadmium stress. Plant Physiol. Biochem. 2024, 206, 108126. [Google Scholar] [CrossRef]
- Anwar, T.; Qureshi, H.; Fatimah, H.; Siddiqi, E.H.; Anwaar, S.; Moussa, I.M.; Adil, M.F. Elucidating effect of ZnO-Nanoparticles and melatonin on physiological adjustments and growth of Solanum melongena under salinity stress. Sci. Hortic. 2023, 322, 112455. [Google Scholar] [CrossRef]
- Bashir, A.; Rizwan, M.; Ali, S.; Adrees, M.; Rehman, M.Z.U.; Qayyum, M.F. Effect of composted organic amendments and zinc oxide nanoparticles on growth and cadmium accumulation by wheat; a life cycle study. Environ. Sci. Pollut. Res. 2020, 27, 23926–23936. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Qayyum, M.F.; Nawaz, R.; Ahmad, A.; Asrar, M.; Ahmad, S.R.; Alsahli, A.A.; et al. Combined use of different nanoparticles effectively decreased cadmium (Cd) concentration in grains of wheat grown in a field contaminated with Cd. Ecotoxicol. Environ. Saf. 2021, 215, 112139. [Google Scholar] [CrossRef]
- Karmous, I.; Gammoudi, N.; Chaoui, A. Assessing the potential role of zinc oxide nanoparticles for mitigating cadmium toxicity in Capsicum annuum L. under in vitro conditions. J. Plant Growth Regul. 2023, 42, 719–734. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 2019, 26, 19859–19870. [Google Scholar] [CrossRef]
- Li, Y.Z.; Liang, L.X.; Li, W.; Ashraf, U.; Ma, L.; Tang, X.R.; Pan, S.G.; Tian, H.; Mo, Z.W. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J. Nanobiotechnol. 2021, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Rahman, M.M.; Halim, M.A.; Naidu, R. Growth, metal partitioning and antioxidant enzyme activities of mung beans as influenced by zinc oxide nanoparticles under cadmium stress. Crop Pasture Sci. 2021, 73, 862–876. [Google Scholar] [CrossRef]
- Timilsina, A.; Adhikari, K.; Chen, H. Foliar application of green synthesized ZnO nanoparticles reduced Cd content in shoot of lettuce. Chemosphere 2023, 338, 139589. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Zhao, B.; Niu, T.X. Bamboo biochar and zinc oxide nanoparticles improved the growth of maize (Zea may L.) and decreased cadmium uptake in Cd-contaminated soil. Agriculture 2022, 12, 1507. [Google Scholar] [CrossRef]
- Sharifan, H.; Moore, J.; Ma, X.M. Zinc oxide (ZnO) nanoparticles elevated iron and copper contents and mitigated the bioavailability of lead and cadmium in different leafy greens. Ecotoxicol. Environ. Saf. 2020, 191, 110177. [Google Scholar] [CrossRef]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Alleviation of chromium stress in plants using metal and metal oxide nanoparticles. Environ. Sci. Pollut. Res. 2023, 30, 83180–83197. [Google Scholar] [CrossRef]
- Peng, H.; Shahidi, F. Cannabis and Cannabis Edibles: A Review. J. Agric. Food Chem. 2021, 69, 1751–1774. [Google Scholar] [CrossRef]
- Vankova, R.; Landa, P.; Podlipna, R.; Dobrev, P.I.; Prerostova, S.; Langhansova, L.; Gaudinova, A.; Motkova, K.; Knirsch, V.; Vanek, T. ZnO nanoparticle effects on hormonal pools in. Sci. Total Environ. 2017, 593, 535–542. [Google Scholar] [CrossRef]
- Li, S.B.; Tian, Y.F.; Jiang, P.Y.Z.; Lin, Y.; Liu, X.L.; Yang, H.S. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1448–1469. [Google Scholar] [CrossRef]
- Yu, H.; Park, J.Y.; Kwon, C.W.; Hong, S.C.; Park, K.M.; Chang, P.S. An overview of nanotechnology in food science: Preparative methods, practical applications, and safety. J. Chem. 2018, 2018, 5427978. [Google Scholar] [CrossRef]
- He, X.J.; Hwang, H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.A.H.; Dhankher, O.P.; Tripathi, R.D. Understanding selenium metabolism in plants and its role as a beneficial element. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1937–1958. [Google Scholar] [CrossRef]
- Qureshi, M.T.; Ahmad, M.F.; Iqbal, N.; Waheed, H.; Hussain, S.; Brestic, M.; Anjum, A.; Noorka, I.R. Agronomic bio-fortification of iron, zinc and selenium enhance growth, quality and uptake of different sorghum accessions. Plant Soil Environ. 2021, 67, 10. [Google Scholar] [CrossRef]
- Boorboori, M.R.; Qiu, H.S.; Liu, J.Y.; Zhang, H.Y. Application of silicon and selenium in rice for reducing cadmium stress. Phyton-Int J. Exp. Bot. 2023, 92, 1873–1886. [Google Scholar] [CrossRef]
- Jia, K.Y.; Zhan, Z.P.; Wang, B.Q.; Wang, W.H.; Wei, W.J.; Li, D.W.; Huang, W.; Xu, Z.M. Exogenous selenium enhances cadmium stress tolerance by improving physiological characteristics of cabbage (Brassica oleracea L. var. capitata) seedlings. Horticulturae 2023, 9, 1016. [Google Scholar] [CrossRef]
- Jiang, P.P.; Liu, J.; Chen, M.H.; Yu, G.; You, S.H.; Li, J.Y. Exogenous selenium improves the physiological resistance of cucumber to cadmium stress. Toxicol. Environ. Chem. 2020, 102, 455–472. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, Y.; Sun, S.; Wang, J.; Wang, W.; Han, D.; Shao, H.; Jia, H.; Fu, Y. Selenium modulates the level of auxin to alleviate the toxicity of cadmium in tobacco. Int. J. Mol. Sci. 2019, 20, 3772. [Google Scholar] [CrossRef]
- Manzoor, M.; Abdalla, M.A.; Hussain, M.A.; Mühling, K.H. Silicon-selenium interplay imparts cadmium resistance in wheat through an up-regulating antioxidant system. Int. J. Mol. Sci. 2024, 25, 387. [Google Scholar] [CrossRef]
- Naseem, M.B.B.; Ali, Q.; Ali, S.; Khalid, M.R.; Nawaz, M. Selenium application reduces cadmium uptake in tomato (Lycopersicum esculentum Mill.) by modulating growth, nutrient uptake, gas exchange, root exudates and antioxidant profile. Pak. J. Bot. 2023, 55, 1633–1646. [Google Scholar]
- Sakouhi, L.; Mahjoubi, Y.; Labben, A.; Kharbech, O.; Chaoui, A.; Djebali, W. Effects of cadmium-selenium interaction on glyoxalase and antioxidant systems of germinating Seeds. J. Plant Growth Regul. 2023, 42, 3084–3099. [Google Scholar] [CrossRef]
- Sun, H.Y.; Wang, X.Y.; Yang, N.; Zhou, H.X.; Gao, Y.F.; Yu, J.; Wang, X.X. Effect of exogenous selenium on mineral nutrition and antioxidative capacity in cucumber (Cucumis sativus L.) seedlings under cadmium stress. Plant Soil Environ. 2022, 68, 580–590. [Google Scholar] [CrossRef]
- Wang, B.W.; Zhang, D.Q.; Wang, W.F.; Song, Y.K.; Lu, M.F.; Ding, S. Foliar application of selenium reduces cadmium accumulation in walnut seedlings. Forests 2022, 13, 1493. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.B.; Dang, F.; Cheng, B.X.; Cheng, C.; Ge, C.H.; Zhou, D.M. Common metabolism and transcription responses of low-cadmium-accumulative wheat (Triticum aestivum L.) cultivars sprayed with nano-selenium. Sci. Total Environ. 2024, 948, 174936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Huang, R.Q.; Zhan, N.H.; Qin, L.J. Methyl jasmonate and selenium synergistically mitigative cadmium toxicity in hot pepper (Capsicum annuum L.) plants by improving antioxidase activities and reducing Cd accumulation. Environ. Sci. Pollut. Res. 2023, 30, 82458–82469. [Google Scholar] [CrossRef]
- Ahmad, A.; Javad, S.; Iqbal, S.; Shahzadi, K.; Gatasheh, M.K.; Javed, T. Alleviation potential of green-synthesized selenium nanoparticles for cadmium stress in Solanum lycopersicum L: Modulation of secondary metabolites and physiochemical attributes. Plant Cell Rep. 2024, 43, 1–17. [Google Scholar] [CrossRef]
- Farooq, M.U.; Ishaaq, I.; Barutcular, C.; Skalicky, M.; Maqbool, R.; Rastogi, A.; Hussain, S.; Allakhverdiev, S.I.; Zhu, J. Mitigation effects of selenium on accumulation of cadmium and morpho-physiological properties in rice varieties. Plant Physiol. Biochem. 2022, 170, 1–13. [Google Scholar] [CrossRef]
- Guo, Y.K.; Mao, K.; Cao, H.R.; Ali, W.; Lei, D.; Teng, D.Y.; Chang, C.Y.; Yang, X.F.; Yang, Q.; Niazi, N.K.; et al. Exogenous selenium (cadmium) inhibits the absorption and transportation of cadmium (selenium) in rice. Environ. Pollut. 2021, 268, 115829. [Google Scholar] [CrossRef]
- Nie, L.L.; Zhou, B.Q.; Hong, B.; Wang, X.D.; Chang, T.; Guan, C.Y.; Guan, M. Application of selenium can alleviate the stress of cadmium on rapeseed at different growth stages in soil. Agronomy 2023, 13, 2228. [Google Scholar] [CrossRef]
- Qin, X.M.; Zhao, P.; Liu, O.G.; Nie, Z.J.; Zhu, J.J.; Qin, S.Y.; Li, C. Selenium inhibits cadmium uptake and accumulation in the shoots of winte wheat by altering the transformation of chemical forms of cadmium in soil. Environ. Sci. Pollut. Res. 2022, 29, 8525–8537. [Google Scholar] [CrossRef]
- Kang, Y.Y.; Qin, H.Y.; Wang, G.H.; Lei, B.F.; Yang, X.; Zhong, M. Selenium nanoparticles mitigate cadmium stress in tomato through enhanced accumulation and transport of sulfate/selenite and polyamines. J. Agric. Food Chem. 2024, 72, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Desoky, E.S.M.; Saad, A.M.; Eid, R.S.M.; Selem, E.; Elrys, A.S. Biological silicon nanoparticles improve Phaseolus vulgars L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021, 106, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef]
- Ashraf, H.; Ghouri, F.; Liang, J.; Xia, W.; Zheng, Z.; Shahid, M.Q.; Fu, X. Silicon Dioxide Nanoparticles-based amelioration of Cd toxicity by regulating antioxidant activity and photosynthetic parameters in a line developed from wild rice. Plants 2024, 13, 1715. [Google Scholar] [CrossRef]
- Chen, H.X.; Huang, X.Y.; Chen, H.; Zhang, S.; Fan, C.W.; Fu, T.L.; He, T.B.; Gao, Z.R. Effect of silicon spraying on rice photosynthesis and antioxidant defense system on cadmium accumulation. Sci. Rep. 2024, 14, 15265. [Google Scholar] [CrossRef]
- He, S.; Lian, X.; Zhang, B.; Liu, X.; Yu, J.; Gao, Y.; Zhang, Q.; Sun, H. Nano silicon dioxide reduces cadmium uptake, regulates nutritional homeostasis and antioxidative enzyme system in barley seedlings (Hordeum vulgare L.) under cadmium stress. Environ. Sci. Pollut. Res. 2023, 30, 67552–67564. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, X.Q.; Shi, A.; Yu, Y.S.; Rensing, C.; Zhang, T.X.; Xing, S.H.; Yang, W.H. Exogenous silicon promotes cadmium (Cd) accumulation in Sedum alfredii Hance by enhancing Cd uptake and alleviating Cd toxicity. Front. Plant Sci. 2023, 14, 1134370. [Google Scholar] [CrossRef]
- Huang, X.Y.; Fan, C.W.; Xie, D.Y.; Chen, H.X.; Zhang, S.; Chen, H.; Qin, S.; Fu, T.L.; He, T.B.; Gao, Z.R. Synergistic effects of water management and silicon foliar spraying on the uptake and transport efficiency of cadmium in rice (Oryza sativa L.). Plants 2023, 12, 1414. [Google Scholar] [CrossRef]
- Iwuala, E.; Olajide, O.; Abiodun, I.; Odjegba, V.; Utoblo, O.; Ajewole, T.; Oluwajobi, A.; Uzochukwu, S. Silicon ameliorates cadmium (Cd) toxicity in pearl millet by inducing antioxidant defense system. Heliyon 2024, 10, 3. [Google Scholar] [CrossRef]
- Lai, M.; Ghouri, F.; Sarwar, S.; Alomrani, S.O.; Riaz, M.; Haider, F.U.; Liu, J.; Imran, M.; Ali, S.; Liu, X.; et al. Modulation of metal transporters, oxidative stress and cell abnormalities by synergistic application of silicon and titanium oxide nanoparticles: A strategy for cadmium tolerance in rice. Chemosphere 2023, 345, 140439. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.W.; Dong, Q.Y.; Cai, Y.X.; Cai, K.Z. Silicon-mediated regulation of cadmium transport and activation of antioxidant defense system enhances Pennisetum glaucum resistance to cadmium stress. Plant Physiol. Biochem. 2023, 195, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Sabir, A.; Waraich, E.A.; Ahmad, M.; Hussain, S.; Asghar, H.N.; Haider, A.; Ahmad, Z.; Bibi, S. Silicon-mediated improvement in maize (Zea mays L.) resilience: Unrevealing morpho-physiological, biochemical, and root attributes against cadmium and drought stress. Silicon 2024, 16, 3095–3109. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Vishwakarma, K.; Singh, V.P.; Prakash, V.; Sharma, S.; Muneer, S.; Nikolic, M.; Deshmukh, R.; Vaculík, M.; Corpas, F.J. Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J. Hazard. Mater. 2021, 408, 124820. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, H.Y.; Zhang, S.; Gao, F.W.; Sun, X.; Ren, X.K. Interactive effect of 24-epibrassinolide and silicon on the alleviation of cadmium toxicity in rice (Oryza sativa L.) plants. Environ. Technol. 2023, 13, 4725–4736. [Google Scholar] [CrossRef]
- Xu, L.A.; Xue, X.; Yan, Y.; Zhao, X.T.; Li, L.J.; Sheng, K.; Zhang, Z.Y. Silicon combined with melatonin reduces Cd absorption and translocation in maize. Plants 2023, 12, 3537. [Google Scholar] [CrossRef]
- Yan, G.C.; Jin, H.; Yin, C.; Huang, Q.Y.; Zhou, G.F.; Xu, Y.M.; He, Y.; Liang, Y.C.; Zhu, Z.J. Comparative effects of silicon and silicon nanoparticles on the antioxidant system and cadmium uptake in tomato under cadmium stress. Sci. Total Environ. 2023, 904, 166819. [Google Scholar] [CrossRef]
- Khan, S.R.; Ahmad, Z.; Khan, Z.; Khan, U.; Asad, M.; Shah, T. Synergistic effect of silicon and arbuscular mycorrhizal fungi reduces cadmium accumulation by regulating hormonal transduction and lignin accumulation in maize. Chemosphere 2024, 346, 140507. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Abbas, T.; Hussain, S.; Hussain, J.; Khalil, H.B.; Alharbi, B.M.; El-Yazied, A.A.; et al. Silicon and strigolecton application alleviates the adversities of cadmium toxicity in maize by modulating morpho-physiological and antioxidants defense mechanisms. Agronomy 2023, 13, 2352. [Google Scholar] [CrossRef]
- Shah, T.R.; Khan, Z.; Khan, S.R.; Imran, A.; Asad, M.; Ahmad, A.; Ahmad, P. Silicon inhibits cadmium uptake by regulating the genes associated with the lignin biosynthetic pathway and plant hormone signal transduction in maize plants. Environ. Sci. Pollut. Res. 2023, 30, 123966–123982. [Google Scholar] [CrossRef]
- Liu, S.H.; Ji, X.H.; Chen, Z.L.; Xie, Y.H.; Ji, S.Y.; Wang, X.; Pan, S.F. Silicon facilitated the physical barrier and adsorption of cadmium of iron plaque by changing the biochemical composition to reduce cadmium absorption of rice roots. Ecotoxicol. Environ. Saf. 2023, 256, 114879. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, X.; Li, L.; Muhammad, Z.A.; Li, M.L.; Zheng, T.D.; Guo, Z.; Zhang, Y.; Luo, D.; Ye, X.Y.; et al. Comparative responses of silicon to reduce cadmium and enrich selenium in rice varieties. Foods 2023, 12, 1656. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Wang, F.Y.; Gao, S.C. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 2015, 22, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Li, P.Y. Immobilization of cadmium in paddy soil using a novel active silicon-potassium amendment: A field experimental study. Environ. Monit. Assess. 2023, 195, 1087. [Google Scholar] [CrossRef]
- Alamri, S.; Ali, H.M.; Khan, M.I.R.; Singh, V.P.; Siddiqui, M.H. Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol. Biochem. 2020, 155, 20–34. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Q.F.; Li, J.; Xiong, J.; Zhou, L.N.; He, S.L.; Zhang, J.Q.; Chen, Z.A.; He, S.G.; Liu, H. Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci. Rep. 2019, 9, 7397. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Gautam, H.; Majid, A.; Anjum, N.A.; Masood, A.; Khan, N.A. Appraisal of functional significance of sulfur assimilatory products in plants under elevated metal accumulation. Crop Pasture Sci. 2022, 73, 573–584. [Google Scholar] [CrossRef]
- Alatawi, A.; Wang, X.K.; Maqbool, A.; Saleem, M.H.; Usman, K.; Rizwan, M.; Yasmeen, T.; Arif, M.S.; Noreen, S.; Hussain, A.; et al. S-fertilizer (elemental sulfur) improves the phytoextraction of cadmium through Solanum nigrum L. Int. J. Environ. Res. Public Health 2022, 19, 1655. [Google Scholar] [CrossRef]
- Mir, I.R.; Rather, B.A.; Masood, A.; Khan, N.A. Nitric oxide- and sulfur-mediated reversal of cadmium-inhibited photosynthetic performance involves hydrogen sulfide and regulation of nitrogen, sulfur, and antioxidant metabolism in mustard. Stresses 2022, 2, 550–577. [Google Scholar] [CrossRef]
- Sun, X.X.; Wang, J.N.; Zhang, M.; Liu, Z.Q.; Yang, E.; Meng, J.; He, T.Y. Combined application of biochar and sulfur alleviates cadmium toxicity in rice by affecting root gene expression and iron plaque accumulation. Ecotoxicol. Environ. Saf. 2023, 266, 115596. [Google Scholar] [CrossRef]
- Zang, Y.L.; Zhao, J.; Chen, W.K.; Lu, L.L.; Chen, J.Z.; Lin, Z.; Qiao, Y.B.; Lin, H.Z.; Tian, S.K. Sulfur and water management mediated iron plaque and rhizosphere microorganisms reduced cadmium accumulation in rice. J. Soil Sediments 2023, 23, 3177–3190. [Google Scholar] [CrossRef]
- Hassan, M.J.; Wang, Z.Q.; Zhang, G.P. Sulfur alleviates growth inhibition and oxidative stress caused by cadmium toxicity in rice. J. Plant Nutr. 2005, 28, 1785–1800. [Google Scholar] [CrossRef]
- Kashem, M.A.; Singh, B.R. Metal availability in contaminated soils: II. Uptake of Cd, Ni and Zn in rice plants grown under flooded culture with organic matter addition. Nutr. Cycl. Agroecosyst. 2001, 61, 257–266. [Google Scholar] [CrossRef]
- Shi, G.; Liu, H.; Zhou, D.; Zhou, H.; Fan, G.; Chen, W.; Li, J.; Lou, L.; Gao, Y. Sulfur reduces the root-to-shoot translocation of arsenic and cadmium by regulating their vacuolar sequestration in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 1032681. [Google Scholar] [CrossRef]
- Shi, W.G.; Liu, W.Z.; Ma, C.F.; Zhang, Y.H.; Ding, S.; Yu, W.J.; Deng, S.R.; Zhou, J.; Li, H.; Luo, Z.B. Dissecting MicroRNA-mRNA Regulatory Networks Underlying Sulfur Assimilation and Cadmium Accumulation in Poplar Leaves. Plant Cell Physiol. 2020, 61, 1614–1630. [Google Scholar] [CrossRef]
- Huang, L.J.; Hansen, H.C.B.; Yang, X.S.; Mu, J.; Xie, Z.J.; Li, S.Y.; Wu, G.M.; Hu, Z.Y. Effects of sulfur application on cadmium accumulation in brown rice under wheat-rice rotation. Environ. Pollut. 2021, 287, 117601. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.Q.; Huang, S.Y.; Kong, L.X.; Zhuang, Z.; Wang, Q.; Li, H.F.; Wan, Y.N. The risks of sulfur addition on cadmium accumulation in paddy rice under different water-management conditions. J. Environ. Sci. 2022, 118, 101–111. [Google Scholar] [CrossRef]
- Shen, C.; Fu, H.L.; Liao, Q.; Huang, B.F.; Fan, X.; Liu, X.Y.; Xin, J.L.; Huang, Y.Y. Transcriptome analysis and physiological indicators reveal the role of sulfur in cadmium accumulation and transportation in water spinach (Ipomoea aquatica Forsk.). Ecotoxicol. Environ. Saf. 2021, 225, 112787. [Google Scholar] [CrossRef]
- Wu, J.W.; Zhao, N.; Zhang, P.; Zhu, L.; Lu, Y.; Lei, X.; Bai, Z.Q. Nitrate enhances cadmium accumulation through modulating sulfur metabolism in sweet sorghum. Chemosphere 2023, 313, 137413. [Google Scholar] [CrossRef]
- Wu, M.; Wang, P.Y.; Sun, L.G.; Zhang, J.J.; Yu, J.; Wang, Y.W.; Chen, G.X. Alleviation of cadmium toxicity by cerium in rice seedlings is related to improved photosynthesis, elevated antioxidant enzymes and decreased oxidative stress. Plant Growth Regul. 2014, 74, 251–260. [Google Scholar] [CrossRef]
- Ayub, M.A.; Rehman, M.Z.U.; Ahmad, H.R.; Fox, J.P.; Clubb, P.; Wright, A.L.; Anwar-ul-Haq, M.; Nadeem, M.; Rico, C.M.; Rossi, L. Influence of ionic cerium and cerium oxide nanoparticles on Zea mays seedlings grown with and without cadmium. Environ. Pollut. 2023, 322, 121137. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Chen, D.Q.; Xue, R.R.; Long, J.; Lin, X.H.; Lin, Y.B.; Jia, L.H.; Zeng, R.S.; Song, Y.Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef]
- Sun, Y.H.; Li, Z.J.; Guo, B.; Chu, G.X.; Wei, C.Z.; Liang, Y.C. Arsenic mitigates cadmium toxicity in rice seedlings. Environ. Exp. Bot. 2008, 64, 264–270. [Google Scholar] [CrossRef]
- Shafiq, T.; Yasmin, H.; Shah, Z.A.; Nosheen, A.; Ahmad, P.; Kaushik, P.; Ahmad, A. Titanium Oxide and zinc oxide nanoparticles in combination with cadmium tolerant Bacillus pumilus ameliorates the cadmium toxicity in maize. Antioxidants 2022, 11, 2156. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.B.; Cai, Y.; Liu, L.; Zhang, M.; He, X.Y.; Zhang, G.P.; Wu, F.B. Differences in photosynthesis, yield and grain cadmium accumulation as affected by exogenous cadmium and glutathione in the two rice genotypes. Plant Growth Regul. 2015, 75, 715–723. [Google Scholar] [CrossRef]
- Hu, B.; Deng, F.; Chen, G.; Chen, X.; Gao, W.; Long, L.; Xia, J.; Chen, Z.H. Evolution of abscisic acid signaling for stress responses to toxic metals and metalloids. Front. Plant Sci. 2020, 11, 909. [Google Scholar] [CrossRef]
- Sun, L.; Di, D.W.; Li, G.; Kronzucker, H.J.; Wu, X.; Shi, W. Endogenous ABA alleviates rice ammonium toxicity by reducing ROS and free ammonium via regulation of the SAPK9-bZIP20 pathway. J. Exp. Bot. 2020, 71, 4562–4577. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Dong, Q.; Yang, X.; Liu, Y.; Li, B.; Yuan, S.; Yin, J.; Xu, Q.; Li, T.; et al. Abscisic acid-mediated modifications in water transport continuum are involved in cadmium hyperaccumulation in Sedum alfredii. Chemosphere 2021, 268, 129339. [Google Scholar] [CrossRef]
- Zhang, J.; Hafeez, M.T.; Di, D.W.; Wu, L.; Zhang, L. Precise control of ABA signaling through post-translational protein modification. Plant Growth Regul. 2019, 88, 99–111. [Google Scholar] [CrossRef]
- Shen, C.; Yang, Y.M.; Sun, Y.F.; Zhang, M.; Chen, X.J.; Huang, Y.Y. The regulatory role of abscisic acid on cadmium uptake, accumulation and translocation in plants. Front. Plant Sci. 2022, 13, 953717. [Google Scholar] [CrossRef]
- Pompeu, G.B.; Vilhena, M.B.; Gratao, P.L.; Carvalho, R.F.; Rossi, M.L.; Martinelli, A.P.; Azevedo, R.A. Abscisic acid-deficient sit tomato mutant responses to cadmium-induced stress. Protoplasma 2017, 254, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Dawuda, M.M.; Liao, W.; Hu, L.; Yu, J.; Xie, J.; Calderon-Urrea, A.; Wu, Y.; Tang, Z. Foliar application of abscisic acid mitigates cadmium stress and increases food safety of cadmium-sensitive lettuce (Lactuca sativa L.) genotype. Peerj 2020, 8, e9270. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Wang, S.J.; Zhao, N.; Deng, S.R.; Zhao, C.J.; Li, N.F.; Sun, J.; Zhao, R.; Yi, H.L.; Shen, X.; et al. Exogenous abscisic acid alleviates cadmium toxicity by restricting Cd influx in Populus euphratica cells. J. Plant Growth Regul. 2016, 35, 827–837. [Google Scholar] [CrossRef]
- Leng, Y.; Li, Y.; Ma, Y.H.; He, L.F.; Li, S.W. Abscisic acid modulates differential physiological and biochemical responses of roots, stems, and leaves in mung bean seedlings to cadmium stress. Environ. Sci. Pollut. Res. 2021, 28, 6030–6043. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Chen, S.M.; Li, Y.Y.; Zheng, F.H.; He, B.; Gu, M.H. Exogenous abscisic acid (ABA) promotes cadmium (Cd) accumulation in Sedum alfredii Hance by regulating the expression of Cd stress response genes. Environ. Sci. Pollut. Res. 2020, 27, 8719–8731. [Google Scholar] [CrossRef]
- Shen, G.; Niu, J.; Deng, Z. Abscisic acid treatment alleviates cadmium toxicity in purple flowering stalk (Brassica campestris L. ssp. chinensis var. purpurea Hort.) seedlings. Plant Physiol. Biochem. 2017, 118, 471–478. [Google Scholar] [CrossRef]
- Hong, C.; Cheng, D.; Zhang, G.; Zhu, D.; Chen, Y.; Tan, M. The role of ZmWRKY4 in regulating maize antioxidant defense under cadmium stress. Biochem. Biophys. Res. Commun. 2017, 482, 1504–1510. [Google Scholar] [CrossRef]
- Di, D.W.; Zhang, C.; Luo, P.; An, C.-W. The biosynthesis of auxin: How many paths truly lead to IAA? Plant Growth Regul. 2016, 78, 275–285. [Google Scholar] [CrossRef]
- Luo, P.; Di, D.W. Precise regulation of the TAA1/TAR-YUCCA auxin biosynthesis pathway in plants. Int. J. Mol. Sci. 2023, 24, 8514. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Cai, J.; Chen, Y.; Zhou, C.; Qiao, C.; Wang, Y.; Wang, S. Effect of auxin on cadmium toxicity-induced growth inhibition in Solanum lycopersicum. Toxics 2024, 12, 374. [Google Scholar] [CrossRef]
- Guo, L.; Yang, S.; Tu, Z.; Yu, F.; Qiu, C.; Huang, G.; Fang, S. An indole-3-acetic acid inhibitor mitigated mild cadmium stress by suppressing peroxide formation in rice seedling roots. Plant Physiol. Biochem. 2024, 213, 108823. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, R.; Kim, D.; Modareszadeh, M.; Hwang, S. Cadmium enhances root hair elongation through reactive oxygen species in Arabidopsis. Environ. Exp. Bot. 2022, 196, 104813. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Foresi, N.; Delledonne, M.; Lamattina, L. Auxin induces redox regulation of ascorbate peroxidase 1 activity by S-nitrosylation/denitrosylation balance resulting in changes of root growth pattern in Arabidopsis. J. Exp. Bot. 2013, 64, 3339–3349. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.Y.; Zhang, Y.; Huang, J.; Chen, Z.J.; Shen, R.F.; Zhu, X.F. Auxin is involved in cadmium accumulation in rice through controlling nitric oxide production and the ability of cell walls to bind cadmium. Sci. Total Environ. 2023, 904, 166644. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 2013, 263, 398–403. [Google Scholar] [CrossRef]
- Hu, Y.F.; Zhou, G.; Na, X.F.; Yang, L.; Nan, W.B.; Liu, X.; Zhang, Y.Q.; Li, J.L.; Bi, Y.R. Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J. Plant Physiol. 2013, 170, 965–975. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Geng, Y.; Wu, R.; Wee, C.W.; Xie, F.; Wei, X.; Chan, P.M.; Tham, C.; Duan, L.; Dinneny, J.R. A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell 2013, 25, 2132–2154. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.; Ali, B.; Ahmad, A. 28-homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidants. Environ. Pollut. 2008, 151, 60–66. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef]
- Bajguz, A. Effect of brassinosteroids on nucleic acids and protein content in cultured cells of. Plant Physiol. Biochem. 2000, 38, 209–215. [Google Scholar] [CrossRef]
- Zhu, T.; Wei, C.; Yu, Y.; Zhang, Z.; Zhu, J.; Liang, Z.; Song, X.; Fu, W.; Cui, Y.; Wang, Z.Y.; et al. The BAS chromatin remodeler determines brassinosteroid-induced transcriptional activation and plant growth in Arabidopsis. Dev. Cell 2024, 59, 924–939 e6. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhong, S.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef] [PubMed]
- Arteca, R.N.; Arteca, J.M. Heavy-metal-induced ethylene production in. J. Plant Physiol. 2007, 164, 1480–1488. [Google Scholar] [CrossRef]
- Groppa, M.D.; Benavides, M.P.; Tomaro, M.L. Polyamine metabolism in sunflower and wheat leaf discs under cadmium or copper stress. Plant Sci. 2003, 164, 293–299. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pazmiño, D.M.; Testillano, P.S.; Risueño, M.C.; del Río, L.A.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef]
- Schellingen, K.; Van Der Straeten, D.; Vandenbussche, F.; Prinsen, E.; Remans, T.; Vangronsveld, J.; Cuypers, A. Cadmium-induced ethylene production and responses in rely on ACS2 and ACS6 gene expression. BMC Plant Biol. 2014, 14, 214. [Google Scholar] [CrossRef]
- Keunen, E.; Schellingen, K.; Van Der Straeten, D.; Remans, T.; Colpaert, J.; Vangronsveld, J.; Cuypers, A. ALTERNATIVE OXIDASE1a modulates the oxidative challenge during moderate Cd exposure in Arabidopsis thaliana leaves. J. Exp. Bot. 2015, 66, 2967–2977. [Google Scholar] [CrossRef]
- Millar, A.H.; Whelan, J.; Soole, K.L.; Day, D.A. Organization and regulation of mitochondrial respiration in plants. Annu. Rev. Plant Biol. 2011, 62, 79–104. [Google Scholar] [CrossRef]
- Purvis, A.C.; Shewfelt, R.L. Does the alternative pathway ameliorate chilling injury in sensitive plant-tissues. Physiol. Plant 1993, 88, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Baek, K.H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Raja, V.; Ashraf, M.; Wijaya, L.; Bajguz, A.; Alyemeni, M.N. Jasmonic acid (JA) and gibberellic acid (GA) mitigated Cd-toxicity in chickpea plants through restricted cd uptake and oxidative stress management. Sci. Rep. 2021, 11, 19768. [Google Scholar] [CrossRef] [PubMed]
- Keramat, B.; Kalantari, K.M.; Arvin, M.J. Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant (Glycine max L.). Afr. J. Microbiol. Res. 2009, 3, 240–244. [Google Scholar]
- Singh, I.; Shah, K. Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 2014, 108, 57–66. [Google Scholar] [CrossRef]
- Wang, F.B.; Wan, C.Z.; Wu, W.Y.; Pan, Y.; Cheng, X.M.; Li, C.; Pi, J.L.; Chen, X.H. Exogenous methyl jasmonate (MeJA) enhances the tolerance to cadmium (Cd) stress of okra (Abelmoschus esculentus L.) plants. Plant Cell Tissue Organ Cult. 2023, 155, 923. [Google Scholar] [CrossRef]
- Singh, I.; Shah, K. Evidences for structural basis of altered ascorbate peroxidase activity in cadmium-stressed rice plants exposed to jasmonate. BioMetals 2014, 27, 247–263. [Google Scholar] [CrossRef]
- Kawano, T.; Bouteau, F. Crosstalk between intracellular and extracellular salicylic acid signaling events leading to long-distance spread of signals. Plant Cell Rep. 2013, 32, 1125–1138. [Google Scholar] [CrossRef]
- Liu, Z.P.; Ding, Y.F.; Wang, F.J.; Ye, Y.Y.; Zhu, C. Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Rep. 2016, 35, 719–731. [Google Scholar] [CrossRef]
- Elazab, D.S.; El-Mahdy, M.; Youssef, M.; Eissa, M.A.; Amro, A.; Lambardi, M. Assessment of salicylic acid as a pretreatment on alleviating cadmium toxicity on in vitro banana shoots. J. Plant Growth Regul. 2023, 42, 5700–5712. [Google Scholar] [CrossRef]
- Hayat, U.; Din, K.U.; Haider, A.; Ramzan, T.; Shahzad, B.A.; Ahmad, M.; Zulfiqar, U.; Maqsood, M.F.; Hussain, S.; Alwahibi, M.S.; et al. Salicylic acid-induced antioxidant defense system alleviates cadmium toxicity in wheat. J. Soil Sci. Plant Nutr. 2024, 24, 3068–3086. [Google Scholar] [CrossRef]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, V.P.; Prasad, S.M.; Sharma, S.; Ramawat, N.; Dubey, N.K.; Tripathi, D.K.; Chauhan, D.K. Interactive effect of silicon (Si) and salicylic acid (SA) in maize seedlings and their mechanisms of cadmium (Cd) toxicity alleviation. J. Plant Growth Regul. 2019, 38, 1587–1597. [Google Scholar] [CrossRef]
- Belkhadi, A.; Hediji, H.; Abbes, Z.; Nouairi, I.; Barhoumi, Z.; Zarrouk, M.; Chaibi, W.; Djebali, W. Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linum usitatissimum L. Ecotoxicol. Environ. Saf. 2010, 73, 1004–1011. [Google Scholar] [CrossRef]
- Torun, H.; Cetin, B.; Stojnic, S.; Petrík, P. Salicylic acid alleviates the effects of cadmium and drought stress by regulating water status, ions, and antioxidant defense in Pterocarya fraxinifolia. Front. Plant Sci. 2024, 14, 1339201. [Google Scholar] [CrossRef]
- Durner, J.; Klessig, D.F. Salicylic acid is a modulator of tobacco and mammalian catalases. J. Biol. Chem. 1996, 271, 28492–28501. [Google Scholar] [CrossRef]
- Moussa, H.R.; El-Gamal, S.M. Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol. Plant 2010, 54, 315–320. [Google Scholar]
- He, J.Y.; Ren, Y.F.; Pan, X.B.; Yan, Y.P.; Zhu, C.; Jiang, D. Salicylic acid alleviates the toxicity effect of cadmium on germination, seedling growth, and amylase activity of rice. J. Plant Nutr. Soil Sci. 2010, 173, 300–305. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Hancock, J.T.; Yong, J.W.H. Synergistic interplay between melatonin and hydrogen sulfide enhances cadmium-induced oxidative stress resistance in stock (Matthiola incana L.). Plant Signal. Behav. 2024, 19, 2331357. [Google Scholar] [CrossRef]
- Song, C.; Manzoor, M.A.; Mao, D.; Ren, X.; Zhang, W.W.; Zhang, Y.Y. Photosynthetic machinery and antioxidant enzymes system regulation confers cadmium stress tolerance to tomato seedlings pretreated with melatonin. Sci. Hortic. 2024, 323, 112550. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Alyemeni, M.N.; Bajguz, A.; Ahmad, P. The involvement of hydrogen sulphide in melatonin-induced tolerance to arsenic toxicity in pepper (Capsicum annuum L.) plants by regulating sequestration and subcellular distribution of arsenic, and antioxidant defense system. Chemosphere 2022, 309, 136678. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.B.; Latifi, M.; Aliniaeifard, S.; Bonab, S.S.; Almanghadim, N.N.; Jafari, S.; Mohebbifar, E.; Ahangir, A.; Seifikalhor, M.; Rezadoost, H.; et al. Response to cadmium toxicity: Orchestration of polyamines and microRNAs in maize plant. Plants 2023, 12, 1991. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Toxicity in leaves of rice exposed to cadmium is due to hydrogen peroxide accumulation. Plant Soil 2007, 298, 231–241. [Google Scholar] [CrossRef]
- Shah, T.; Khan, Z.; Asad, M.; Imran, A.; Niazi, M.B.K.; Alsahli, A.A. Alleviation of cadmium toxicity in wheat by strigolactone: Regulating cadmium uptake, nitric oxide signaling, and genes encoding antioxidant defense system. Plant Physiol. Biochem. 2023, 202, 107916. [Google Scholar] [CrossRef]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef]
- Ma, M.; Wendehenne, D.; Philippot, L.; Hansch, R.; Flemetakis, E.; Hu, B.; Rennenberg, H. Physiological significance of pedospheric nitric oxide for root growth, development and organismic interactions. Plant Cell Environ. 2020, 43, 2336–2354. [Google Scholar] [CrossRef]
- Aswani, V.; Rajsheel, P.; Bapatla, R.B.; Sunil, B.; Raghavendra, A.S. Oxidative stress induced in chloroplasts or mitochondria promotes proline accumulation in leaves of pea (Pisum sativum): Another example of chloroplast-mitochondria interactions. Protoplasma 2019, 256, 449–457. [Google Scholar] [CrossRef]
- Khan, E.A.; Ahmed, H.M.I.; Misra, M.; Sharma, P.; Misra, A.N.; Hasanuzzaman, M. Nitric oxide alleviates cadmium-impeded growth by limiting ROS accumulation in pea seedlings. Biocell 2022, 46, 2583–2593. [Google Scholar] [CrossRef]
- Nawaz, M.; Saleem, M.H.; Khalid, M.R.; Ali, B.; Fahad, S. Nitric oxide reduces cadmium uptake in wheat (Triticum aestivum L.) by modulating growth, mineral uptake, yield attributes, and antioxidant profile. Environ. Sci. Pollut. Res. 2024, 31, 9844–9856. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, W.S.; Deng, H.; Chen, B.; Zhang, G.; Wang, P.; Yuan, T.T.; Zhu, Y.S. Improving Endogenous nitric oxide enhances cadmium tolerance in rice through modulation of cadmium accumulation and antioxidant capacity. Agronomy 2023, 13, 1978. [Google Scholar] [CrossRef]
- Liu, F.F.; Qiao, X.H.; Yang, T.; Zhao, P.; Zhu, Z.P.; Zhao, J.H.; Luo, J.M.; Xiong, A.S.; Sun, M. Nitric oxide promoted the seed germination of Cynanchum auriculatum under Cadmium Stress. Agronomy 2024, 14, 86. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, L.J.; Xie, Y.D.; Liu, J.; Sun, G.C.; Li, H.; Liao, M.A.; Wang, Z.H.; Liang, D.; Xia, H.; et al. Melatonin affects the growth and cadmium accumulation of Malachium aquaticum and Galinsoga parviflora. Int. J. Phytoremediat. 2018, 20, 295–300. [Google Scholar] [CrossRef]
- Hasan, M.K.; Liu, C.C.; Wang, F.N.; Ahammed, G.J.; Zhou, J.; Xu, M.X.; Yu, J.Q.; Xia, X.J. Glutathione-mediated regulation of nitric oxide, -nitrosothiol and redox homeostasis confers cadmium tolerance by inducing transcription factors and stress response genes in tomato. Chemosphere 2016, 161, 536–545. [Google Scholar] [CrossRef]
- Innocenti, G.; Pucciariello, C.; Le Gleuher, M.; Hopkins, J.; de Stefano, M.; Delledonne, M.; Puppo, A.; Baudouin, E.; Frendo, P. Glutathione synthesis is regulated by nitric oxide in roots. Planta 2007, 225, 1597–1602. [Google Scholar] [CrossRef]
- Wang, X.; Du, H.X.; Ma, M.; Rennenberg, H. The dual role of nitric oxide (NO) in plant responses to cadmium exposure. Sci. Total Environ. 2023, 892, 164597. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Gravot, A.; Richaud, P.; Auroy, P.; Duc, C.; Gaymard, F.; Taconnat, L.; Renou, J.P.; Pugin, A.; Wendehenne, D. Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol. 2009, 149, 1302–1315. [Google Scholar] [CrossRef]
- De Michele, R.; Vurro, E.; Rigo, C.; Costa, A.; Elviri, L.; Di Valentin, M.; Careri, M.; Zottini, M.; Sanità di Toppi, L.; Lo Schiavo, F. Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol. 2009, 150, 217–228. [Google Scholar] [CrossRef]
- Elviri, L.; Speroni, F.; Careri, M.; Mangia, A.; di Toppi, L.S.; Zottini, M. Identification of in vivo nitrosylated phytochelatins in Arabidopsis thaliana cells by liquid chromatography-direct electrospray-linear ion trap-mass spectrometry. J. Chromatogr. 2010, 1217, 4120–4126. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Tan, X.; Liu, H.; Zeng, G.; Hu, X.; Jian, H.; Gu, Y. Effect of exogenous nitric oxide on antioxidative system and S-nitrosylation in leaves of Boehmeria nivea (L.) Gaud under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Liang, X.; Dong, Y.J.; Xu, L.L.; Zhang, X.W.; Hou, J.; Fan, Z.Y. Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul. 2013, 69, 11–20. [Google Scholar] [CrossRef]
- Xu, L.L.; Dong, Y.J.; Kong, J.; Liu, S. Effects of root and foliar applications of exogenous NO on alleviating cadmium toxicity in lettuce seedlings. Plant Growth Regul. 2014, 72, 39–50. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.X.; Yu, J.Q. OH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J. Exp. Bot. 2014, 65, 595–607. [Google Scholar] [CrossRef]
- Chao, Y.Y.; Hsu, Y.T.; Kao, C.H. Involvement of glutathione in heat shock- and hydrogen peroxide-induced cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Soil 2009, 318, 37–45. [Google Scholar] [CrossRef]
- Chou, T.S.; Chao, Y.Y.; Kao, C.H. Involvement of hydrogen peroxide in heat shock- and cadmium-induced expression of ascorbate peroxidase and glutathione reductase in leaves of rice seedlings. J. Plant Physiol. 2012, 169, 478–486. [Google Scholar] [CrossRef]
- Hu, Y.L.; Ge, Y.; Zhang, C.H.; Ju, T.; Cheng, W.D. Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Regul. 2009, 59, 51–61. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhang, C.H.; Yan, J.L.; Yue, Q.A.; Ge, Y. Effects of sulfur supply and hydrogen peroxide pretreatment on the responses by rice under cadmium stress. Plant Growth Regul. 2015, 77, 299–306. [Google Scholar] [CrossRef]
- Zhang, H.X.; Lv, S.F.; Xu, H.W.; Hou, D.Y.; Li, Y.J.; Wang, F.Y. H2O2 is involved in the metallothionein-mediated rice tolerance to copper and cadmium toxicity. Int. J. Mol. Sci. 2017, 18, 2083. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Djamei, A.; Bitton, F.; Hirt, H. A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol. Plant 2009, 2, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Anand, A. Hydrogen sulfide: An emerging signaling molecule regulating drought stress response in plants. Physiol. Plant 2021, 172, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Dong, J.; Li, R.; Zhao, X.; Zhu, Z.; Zhang, F.; Zhou, K.; Lin, X. Sodium hydrosulfide alleviates aluminum toxicity in Brassica napus through maintaining H2S, ROS homeostasis and enhancing aluminum exclusion. Sci. Total Environ. 2023, 858, 160073. [Google Scholar] [CrossRef]
- Wang, P.; Fang, H.; Gao, R.; Liao, W. Protein persulfidation in plants: Function and mechanism. Antioxidants 2021, 10, 1631. [Google Scholar] [CrossRef]
- Aroca, A.; Serna, A.; Gotor, C.; Romero, L.C. Sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–586. [Google Scholar] [CrossRef]
- Singh, S.K.; Suhel, M.; Husain, T.; Prasad, S.M.; Singh, V.P. Hydrogen sulfide manages hexavalent chromium toxicity in wheat and rice seedlings: The role of sulfur assimilation and ascorbate-glutathione cycle. Environ. Pollut. 2022, 307, 119509. [Google Scholar] [CrossRef]
- Luo, S.L.; Tang, Z.Q.; Yu, J.H.; Liao, W.B.; Xie, J.M.; Lv, J.; Liu, Z.C.; Calderón-Urrea, A. Hydrogen sulfide inhibits cadmium-induced cell death of cucumber seedling root tips by protecting mitochondrial physiological function. J. Plant Growth Regul. 2022, 41, 3421–3432. [Google Scholar] [CrossRef]
- Cao, H.; Song, K.; Hu, Y.; Li, Q.; Ma, T.; Li, R.; Chen, N.; Zhu, S.; Liu, W. The role of exogenous hydrogen sulfide in mitigating cadmium toxicity in plants: A comprehensive meta-analysis. Environ. Sci. Pollut. Res. 2024, 31, 30273–30287. [Google Scholar] [CrossRef]
- Agarwal, P.; Mitra, M.; Banerjee, S.; Roy, S. MYB4 transcription factor, a member of R2R3-subfamily of MYB domain protein, regulates cadmium tolerance via enhanced protection against oxidative damage and increases expression of PCS1 and MT1C in Arabidopsis. Plant Sci. 2020, 297, 110501. [Google Scholar] [CrossRef]
- Chen, S.; Yu, M.; Li, H.; Wang, Y.; Lu, Z.; Zhang, Y.; Liu, M.; Qiao, G.; Wu, L.; Han, X.; et al. SaHsfA4c from Sedum alfredii Hance enhances cadmium tolerance by regulating ROS-scavenger activities and heat shock proteins expression. Front. Plant Sci. 2020, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fan, T.; Zhu, X.; Wu, X.; Ouyang, J.; Jiang, L.; Cao, S. WRKY12 represses GSH1 expression to negatively regulate cadmium tolerance in Arabidopsis. Plant Mol. Biol. 2019, 99, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, Q.; Chen, L.; Tian, F.; Chen, X.; Han, C.; Mi, J.; Lin, X.; Wan, X.; Jiang, B.; et al. A WRKY transcription factor, PyWRKY75, enhanced cadmium accumulation and tolerance in poplar. Ecotoxicol. Environ. Saf. 2022, 239, 113630. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, J.Y.; Dong, S.C.; Chang, M.H.; Zhu, J.X.; Guo, D.L.; Guo, C.H.; Bi, Y.D. Alfalfa MsbHLH115 confers tolerance to cadmium stress through activating the iron deficiency response in Arabidopsis thaliana. Front. Plant Sci. 2024, 15, 1358673. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; López-Jaramillo, J.; Padilla, M.N.; Carreras, A.; Corpas, F.J.; Barroso, J.B. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and -nitrosylation. J. Exp. Bot. 2014, 65, 527–538. [Google Scholar] [CrossRef]
- Zou, J.J.; Li, X.D.; Ratnasekera, D.; Wang, C.; Liu, W.X.; Song, L.F.; Zhang, W.Z.; Wu, W.H. Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 2015, 27, 1445–1460. [Google Scholar] [CrossRef]
- Tang, W.; Charles, T.M.; Newton, R.J. Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine (Pinus Virginiana Mill.) confers multiple stress tolerance and enhances organ growth. Plant Mol. Biol. 2005, 59, 603–617. [Google Scholar] [CrossRef]
- Yang, G.; Wang, C.; Wang, Y.; Guo, Y.; Zhao, Y.; Yang, C.; Gao, C. Overexpression of ThVHAc1 and its potential upstream regulator, ThWRKY7, improved plant tolerance of Cadmium stress. Sci. Rep. 2016, 6, 18752. [Google Scholar] [CrossRef]
- Hu, L.X.; Zhang, Z.F.; Xiang, Z.X.; Yang, Z.J. Exogenous application of citric acid ameliorates the adverse effect of heat stress in Tall Fescue (Lolium arundinaceum). Front. Plant Sci. 2016, 7, 179. [Google Scholar] [CrossRef]
- Freitas, E.V.; Nascimento, C.W.; Souza, A.; Silva, F.B. Citric acid-assisted phytoextraction of lead: A field experiment. Chemosphere 2013, 92, 213–217. [Google Scholar] [CrossRef]
- Gao, Y.; Miao, C.Y.; Mao, L.A.; Zhou, P.; Jin, Z.G.; Shi, W.J. Improvement of phytoextraction and antioxidative defense in Solanum nigrum L. under cadmium stress by application of cadmium-resistant strain and citric acid. J. Hazard. Mater. 2010, 181, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmud, J.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.H.M.B.; Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Q.; Ren, G.; Jiang, L.; Yuan, H.B.; Ma, G.H. The role of β-aminobutyric acid in enhancing cadmium tolerance in Arabidopsis thaliana. Russ. J. Plant Physiol. 2009, 56, 575–579. [Google Scholar] [CrossRef]
- Justyna, P.G.; Ewa, K. Induction of resistance against pathogens by β-aminobutyric acid. Acta Physiol. Plant 2013, 35, 1735–1748. [Google Scholar] [CrossRef]
- Ton, J.; Mauch-Mani, B. β-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J. 2004, 38, 119–130. [Google Scholar] [CrossRef]
- Hossain, Z.; Makino, T.; Komatsu, S. Proteomic study of β-aminobutyric acid-mediated cadmium stress alleviation in soybean. J. Proteom. 2012, 75, 4151–4164. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.R.; Li, M.L.; Liu, L.; Zheng, Y.H.; Zhang, H. β-aminobutyric acid effectively postpones senescence of strawberry fruit by regulating metabolism of NO, H2S, ascorbic acid, and ABA. Horticulturae 2024, 10, 218. [Google Scholar] [CrossRef]
- Shen, X.; Yang, Z.Y.; Dai, X.Y.; Feng, W.; Li, P.; Chen, Y. Calcium hexacyanoferrate nanozyme enhances plant stress resistance by oxidative stress alleviation and heavy metal removal. Adv. Mater. 2024, 36, e2402745. [Google Scholar] [CrossRef]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial exopolysaccharides: Insight into their role in plant abiotic stress tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045–1059. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef]
- Li, K.T.; Peng, S.Y.; Zhang, B.; Peng, W.F.; Yu, S.J.; Cheng, X. Exopolysaccharides from reduces cadmium uptake and mitigates cadmium toxicity in rice seedlings. World J. Microbiol. Biotechnol. 2022, 38, 243. [Google Scholar] [CrossRef]
- Wei, H.Y.; Li, Y.; Yan, J.; Peng, S.Y.; Wei, S.J.; Yin, Y.B.; Li, K.T.; Cheng, X. Root cell wall remodeling: A way for exopolysaccharides to mitigate cadmium toxicity in rice seedling. J. Hazard. Mater. 2023, 443, 130186. [Google Scholar] [CrossRef] [PubMed]
- EL Arroussi, H.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; EL Mernissi, N.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; El Arroussi, H. Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep. 2021, 11, 930. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Singh, A.; Kumar, V. Recent advancements in cadmium-microbe interactive relations and their application for environmental remediation: A mechanistic overview. Environ. Sci. Pollut. Res. 2023, 30, 17009–17038. [Google Scholar] [CrossRef]
- Li, P.; Xiong, Z.Q.; Tian, Y.H.; Zheng, Z.Y.; Liu, Z.X.; Hu, R.W.; Wang, Q.M.; Ao, H.J.; Yi, Z.X.; Li, J. Community-based mechanisms underlying the root cadmium uptake regulated by Cd-tolerant strains in rice (Oryza sativa. L). Front. Plant Sci. 2023, 14, 1196130. [Google Scholar] [CrossRef]
- Zhou, B.B.; Yang, Z.H.; Chen, X.P.; Jia, R.N.; Yao, S.X.; Gan, B.; Fan, D.L.; Yang, X.; Li, W.Q.; Chen, Y.H. Microbiological mechanisms of collaborative remediation of cadmium-contaminated soil with Bacillus cereus and lawn plants. Plants 2024, 13, 1303. [Google Scholar] [CrossRef]
- Zou, D.C.; Du, H.X.; Zhang, F.S.; Gao, L.; Xiong, B.C.; Guo, P.; Ma, M. Responses of co-inoculated with rhizobia and arbuscular mycorrhizal fungi to cadmium under nitrogen excess condition. Plant Soil 2024. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Haider, F.U.; Maqsood, M.F.; Mohy-Ud-Din, W.; Shabaan, M.; Ahmad, M.; Kaleem, M.; Ishfaq, M.; Aslam, Z.; Shahzad, B. Recent advances in microbial-assisted remediation of cadmium-contaminated soil. Plants 2023, 12, 3147. [Google Scholar] [CrossRef]
- Alscher, R.G. Biosynthesis and antioxidant function of glutathione in plants. Physiol. Plant 1989, 77, 457–464. [Google Scholar] [CrossRef]
- Iriti, M.; Castorina, G.; Picchi, V.; Faoro, F.; Gomarasca, S. Acute exposure of the aquatic macrophyte to the herbicide oxadiazon: The protective role of N-acetylcysteine. Chemosphere 2009, 74, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Malanga, G.; Kozak, R.G.; Puntarulo, S. N-acetylcysteine-dependent protection against UV-B damage in two photosynthetic organisms. Plant Sci. 1999, 141, 129–137. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Meng, J.; Dang, S.; Chen, W.F. Effect of biochar on relieving cadmium stress and reducing accumulation in super rice. J. Integr. Agric. 2014, 13, 547–553. [Google Scholar] [CrossRef]
- Sun, Y.B.; Sun, G.H.; Xu, Y.M.; Liu, W.T.; Liang, X.F.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J. Environ. Manag. 2016, 166, 204–210. [Google Scholar] [CrossRef]
- Dong, J.; Wu, F.B.; Zhang, G.P. Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum). Chemosphere 2006, 64, 1659–1666. [Google Scholar] [CrossRef]
- Srivastava, S.; Tripathi, R.D.; Dwivedi, U.N. Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa: An angiospermic parasite. J. Plant Physiol. 2004, 161, 665–674. [Google Scholar] [CrossRef]
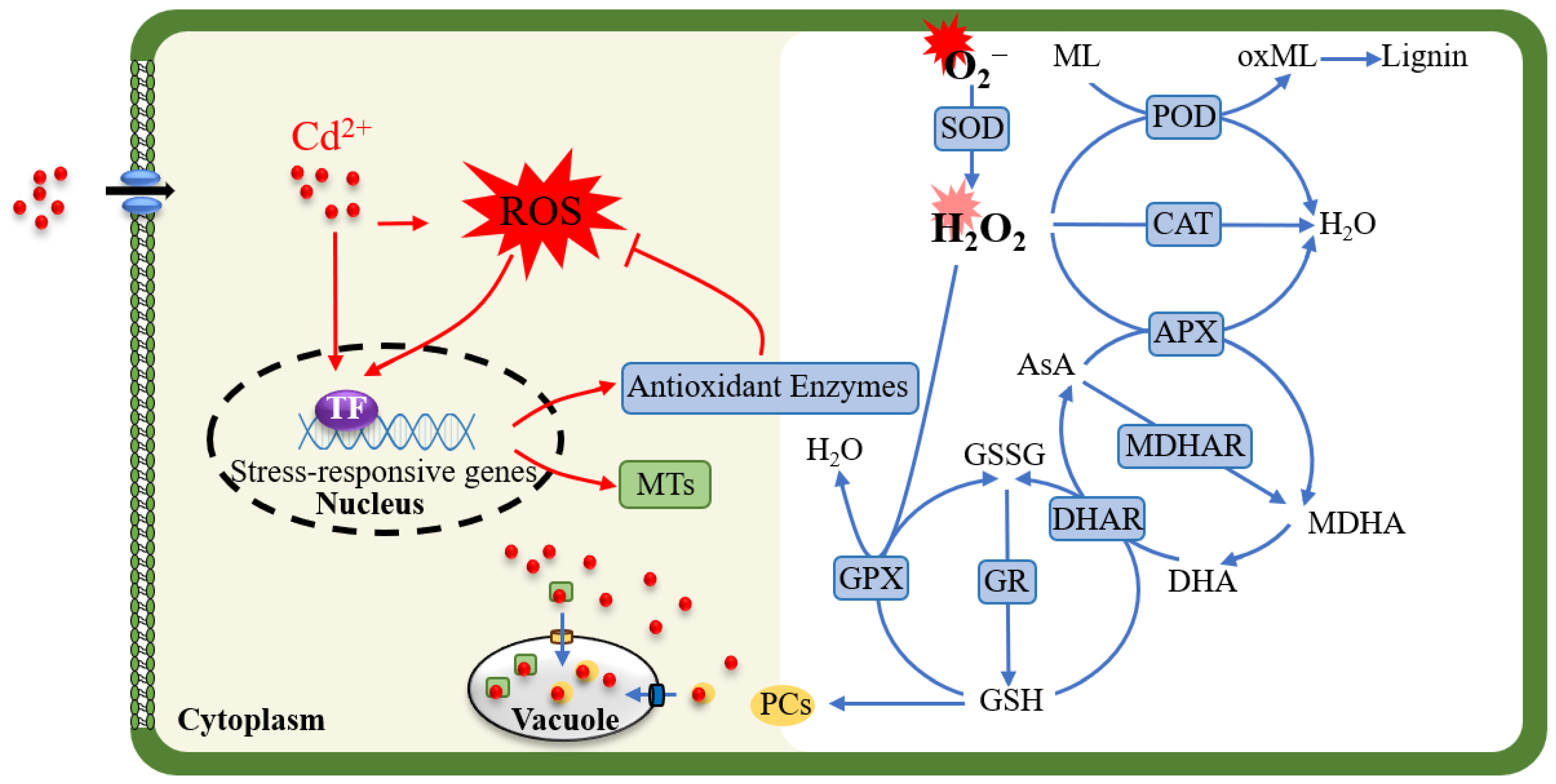
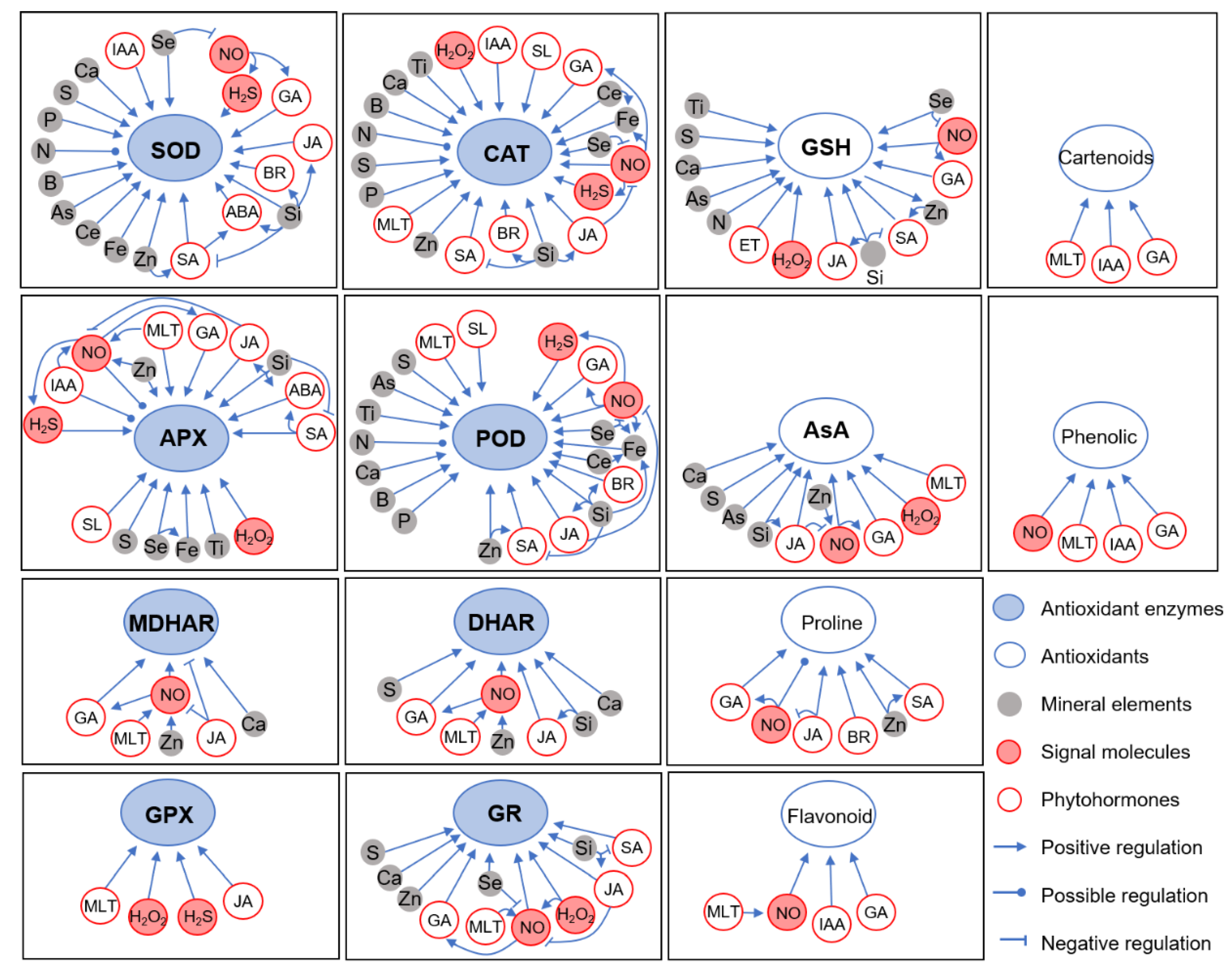
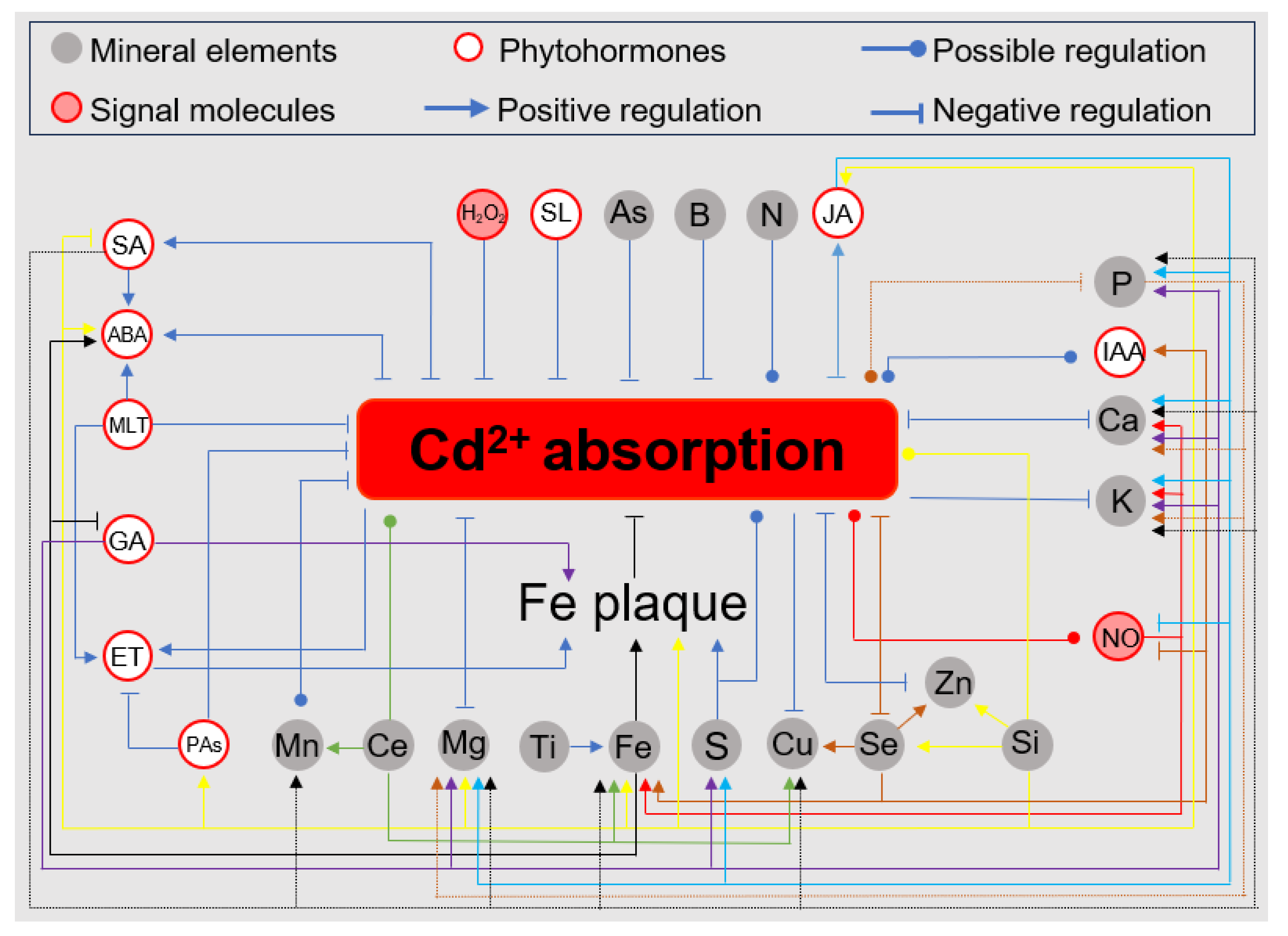
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, P.; Wu, J.; Li, T.-T.; Shi, P.; Ma, Q.; Di, D.-W. An Overview of the Mechanisms through Which Plants Regulate ROS Homeostasis under Cadmium Stress. Antioxidants 2024, 13, 1174. https://doi.org/10.3390/antiox13101174
Luo P, Wu J, Li T-T, Shi P, Ma Q, Di D-W. An Overview of the Mechanisms through Which Plants Regulate ROS Homeostasis under Cadmium Stress. Antioxidants. 2024; 13(10):1174. https://doi.org/10.3390/antiox13101174
Chicago/Turabian StyleLuo, Pan, Jingjing Wu, Ting-Ting Li, Peihua Shi, Qi Ma, and Dong-Wei Di. 2024. "An Overview of the Mechanisms through Which Plants Regulate ROS Homeostasis under Cadmium Stress" Antioxidants 13, no. 10: 1174. https://doi.org/10.3390/antiox13101174
APA StyleLuo, P., Wu, J., Li, T.-T., Shi, P., Ma, Q., & Di, D.-W. (2024). An Overview of the Mechanisms through Which Plants Regulate ROS Homeostasis under Cadmium Stress. Antioxidants, 13(10), 1174. https://doi.org/10.3390/antiox13101174







