NADPH Oxidase 4: Crucial for Endothelial Function under Hypoxia—Complementing Prostacyclin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Models
2.2. Murine Tissue Sampling
2.3. Recruitment of Patients and Preparation of Internal Mammary Artery
2.4. Patients with Peripheral Arterial Disease (PAD)
2.5. Cell Culture of Primary Human Endothelial Cells
2.6. Lentiviral Downregulation of NOX4
2.7. mRNA Stability Assay
2.8. Flow Application on Endothelial Cells
2.9. Griess Assay for Nitrite
2.10. Detection of Extracellular H2O2 Using Amplex Red Assay
2.11. Detection of 6-Keto-PGF1 Alpha
2.12. Real-Time PCR
2.13. Western Blot
2.14. Vascular Function Analysis under Hypoxia
2.15. Statistical Analyses
3. Results
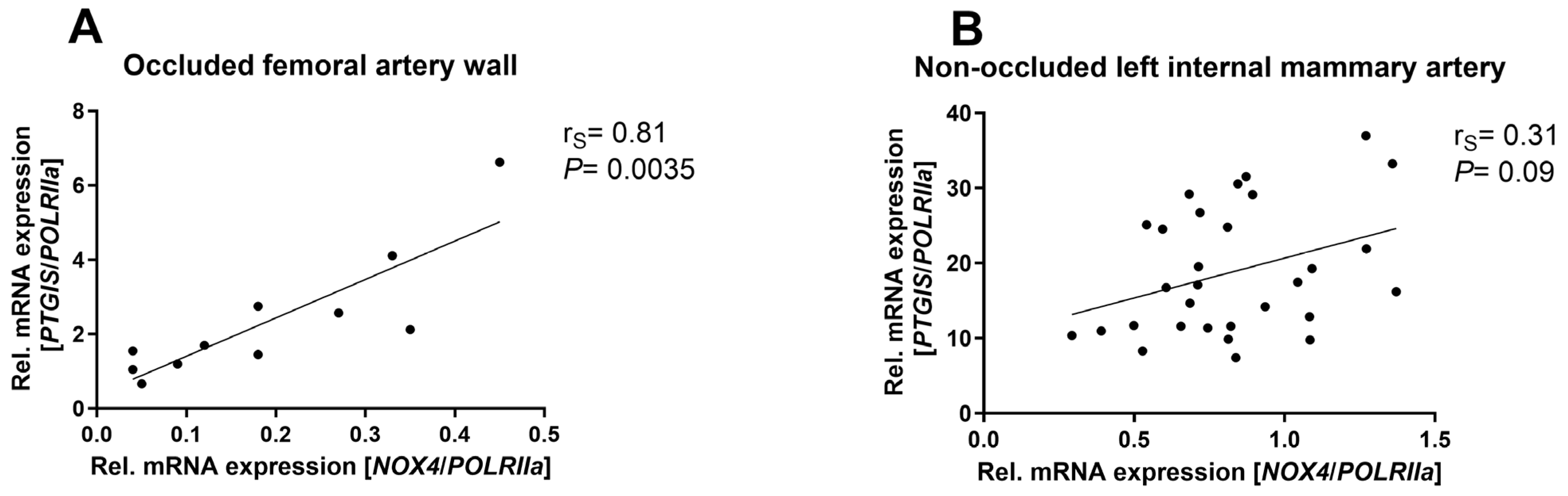
3.1. NOX4 mRNA Expression Highly Correlated with PTGIS Expression in Occluded Arterial Walls of Patients with Peripheral Arterial Disease
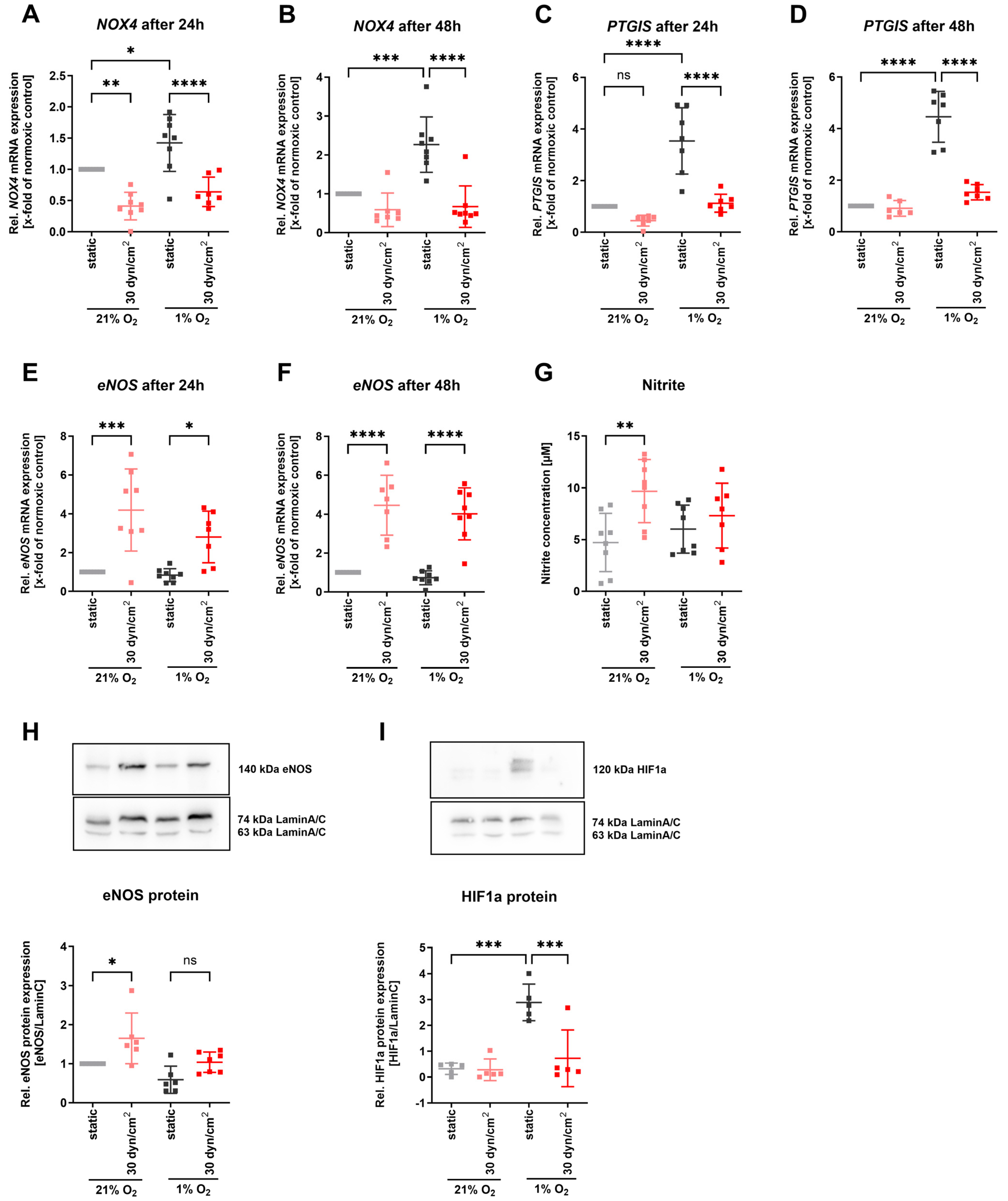
3.2. Hypoxia Elevated NOX4 and PTGIS in Human Endothelial Cells and Murine Vessels
3.3. NOX4 and PTGIS Were Regulated by Different HIFs
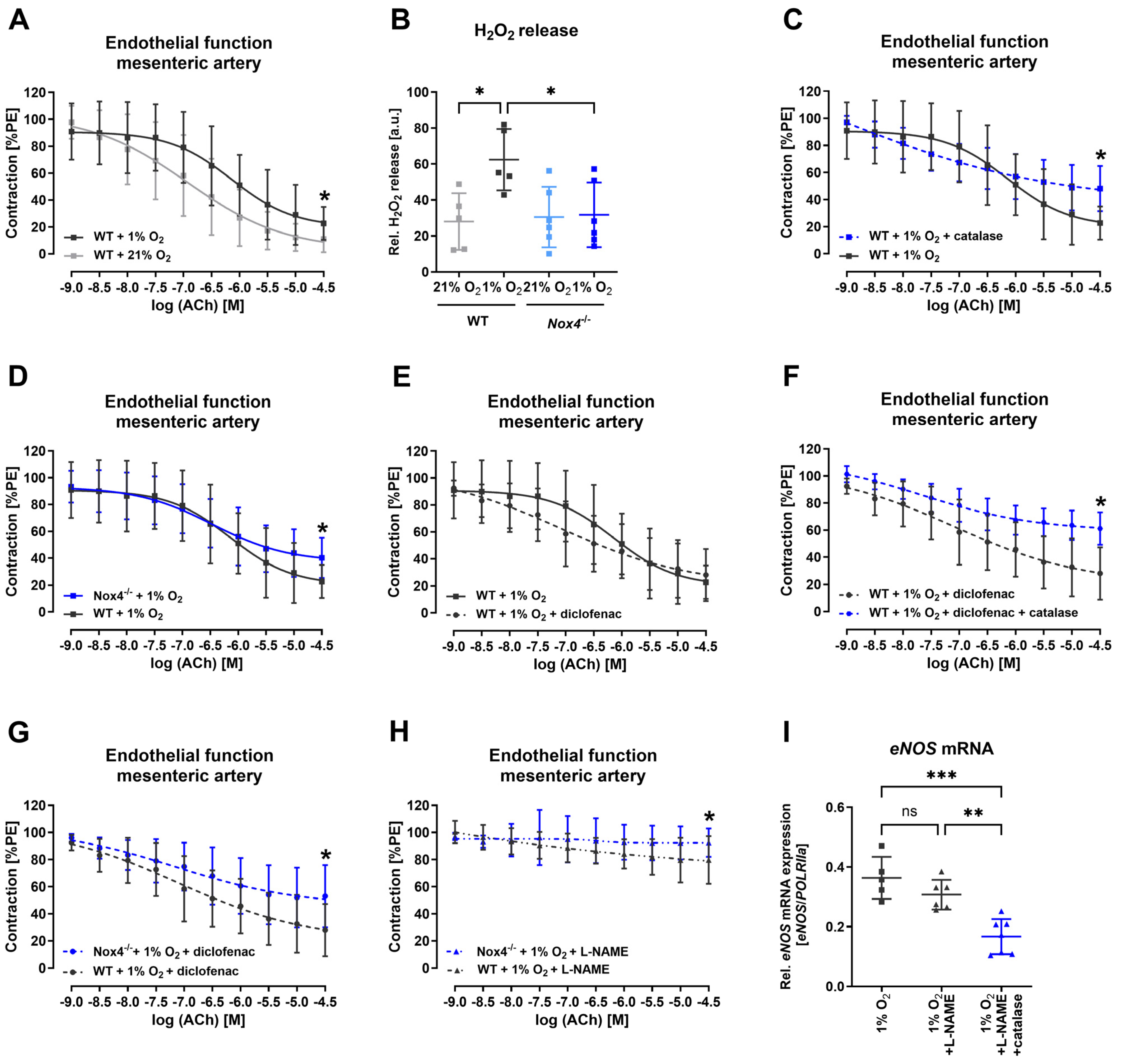
3.4. Nox4 Released Hydrogen Peroxide Maintained Endothelial Function under Hypoxia
3.5. Restored Laminar Flow Prevented Upregulation of NOX4 and PTGIS under Hypoxia
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, K.; Wu, R. Hypoxia-Inducible Factor Regulates Endothelial Metabolism in Cardiovascular Disease. Front. Physiol. 2021, 12, 670653. [Google Scholar] [CrossRef] [PubMed]
- Nallamshetty, S.; Chan, S.Y.; Loscalzo, J. Hypoxia: A master regulator of microRNA biogenesis and activity. Free Radic. Biol. Med. 2013, 64, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Brocato, J.; Chervona, Y.; Costa, M. Molecular responses to hypoxia-inducible factor 1alpha and beyond. Mol. Pharmacol. 2014, 85, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.W.; Kuchnio, A.; Bruning, U.; Carmeliet, P. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem. Sci. 2013, 38, 3–11. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Pouyssegur, J. HIF at a glance. J. Cell Sci. 2009, 122, 1055–1057. [Google Scholar] [CrossRef]
- Suliman, H.B.; Ali, M.; Piantadosi, C.A. Superoxide dismutase-3 promotes full expression of the EPO response to hypoxia. Blood 2004, 104, 43–50. [Google Scholar] [CrossRef]
- Vallet, P.; Charnay, Y.; Steger, K.; Ogier-Denis, E.; Kovari, E.; Herrmann, F.; Michel, J.P.; Szanto, I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience 2005, 132, 233–238. [Google Scholar] [CrossRef]
- Mittal, M.; Roth, M.; König, P.; Hofmann, S.; Dony, E.; Goyal, P.; Selbitz, A.C.; Schermuly, R.T.; Ghofrani, H.A.; Kwapiszewska, G.; et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ. Res. 2007, 101, 258–267. [Google Scholar] [CrossRef]
- Li, S.; Tabar, S.S.; Malec, V.; Eul, B.G.; Klepetko, W.; Weissmann, N.; Grimminger, F.; Seeger, W.; Rose, F.; Hänze, J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid. Redox Signal. 2008, 10, 1687–1698. [Google Scholar] [CrossRef]
- Sylvester, A.L.; Zhang, D.X.; Ran, S.; Zinkevich, N.S. Inhibiting NADPH Oxidases to Target Vascular and Other Pathologies: An Update on Recent Experimental and Clinical Studies. Biomolecules 2022, 12, 823. [Google Scholar] [CrossRef]
- Touyz, R.M.; Briones, A.M.; Sedeek, M.; Burger, D.; Montezano, A.C. NOX isoforms and reactive oxygen species in vascular health. Mol. Interv. 2011, 11, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Noh, S.G.; Park, S.H.; Andtbacka, R.H.I.; Hyngstrom, J.R.; Richardson, R.S. Ageing and endothelium-mediated vascular dysfunction: The role of the NADPH oxidases. J. Physiol. 2023, 601, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, J.; Luo, G.; Zhou, J.; Wang, N.; Wang, S.; Zhao, R.; Cao, X.; Ma, Y.; Liu, G.; et al. Nox4 as a novel therapeutic target for diabetic vascular complications. Redox Biol. 2023, 64, 102781. [Google Scholar] [CrossRef] [PubMed]
- Takac, I.; Schröder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef] [PubMed]
- Peshavariya, H.M.; Liu, G.S.; Chang, C.W.; Jiang, F.; Chan, E.C.; Dusting, G.J. Prostacyclin signaling boosts NADPH oxidase 4 in the endothelium promoting cytoprotection and angiogenesis. Antioxid. Redox Signal. 2014, 20, 2710–2725. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Liu, J.; Zheng, X.; Hu, X.; He, Y. Prostaglandin and prostaglandin receptors: Present and future promising therapeutic targets for pulmonary arterial hypertension. Respir. Res. 2023, 24, 263. [Google Scholar] [CrossRef]
- Vane, J.; Corin, R.E. Prostacyclin: A vascular mediator. Eur. J. Vasc. Endovasc. Surg. 2003, 26, 571–578. [Google Scholar] [CrossRef]
- Busse, R.; Forstermann, U.; Matsuda, H.; Pohl, U. The role of prostaglandins in the endothelium-mediated vasodilatory response to hypoxia. Pflug. Arch. 1984, 401, 77–83. [Google Scholar] [CrossRef]
- Zhang, M.; Brewer, A.C.; Schröder, K.; Santos, C.X.; Grieve, D.J.; Wang, M.; Anilkumar, N.; Yu, B.; Dong, X.; Walker, S.J.; et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18121–18126. [Google Scholar] [CrossRef]
- Cybularz, M.; Langbein, H.; Zatschler, B.; Brunssen, C.; Deussen, A.; Matschke, K.; Morawietz, H. Endothelial function and gene expression in perivascular adipose tissue from internal mammary arteries of obese patients with coronary artery disease. Atheroscler. Suppl. 2017, 30, 149–158. [Google Scholar] [CrossRef]
- Lee, K.; Kang, J.E.; Park, S.K.; Jin, Y.; Chung, K.S.; Kim, H.M.; Lee, K.; Kang, M.R.; Lee, M.K.; Song, K.B.; et al. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem. Pharmacol. 2010, 80, 982–989. [Google Scholar] [CrossRef]
- Zimmer, M.; Ebert, B.L.; Neil, C.; Brenner, K.; Papaioannou, I.; Melas, A.; Tolliday, N.; Lamb, J.; Pantopoulos, K.; Golub, T.; et al. Small-molecule inhibitors of HIF-2a translation link its 5′UTR iron-responsive element to oxygen sensing. Mol. Cell 2008, 32, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Korten, S.; Brunssen, C.; Poitz, D.M.; Großklaus, S.; Brux, M.; Schnittler, H.J.; Strasser, R.H.; Bornstein, S.R.; Morawietz, H.; Goettsch, W. Impact of Hey2 and COUP-TFII on genes involved in arteriovenous differentiation in primary human arterial and venous endothelial cells. Basic Res. Cardiol. 2013, 108, 362. [Google Scholar] [CrossRef] [PubMed]
- Giebe, S.; Cockcroft, N.; Hewitt, K.; Brux, M.; Hofmann, A.; Morawietz, H.; Brunssen, C. Cigarette smoke extract counteracts atheroprotective effects of high laminar flow on endothelial function. Redox Biol. 2017, 12, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Arullampalam, P.; Yang, Z.; Ming, X.F. Hypoxia Enhances Endothelial Intercellular Adhesion Molecule 1 Protein Level Through Upregulation of Arginase Type II and Mitochondrial Oxidative Stress. Front. Physiol. 2019, 10, 1003. [Google Scholar] [CrossRef]
- Craige, S.M.; Chen, K.; Pei, Y.; Li, C.; Huang, X.; Chen, C.; Shibata, R.; Sato, K.; Walsh, K.; Keaney, J.F., Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 2011, 124, 731–740. [Google Scholar] [CrossRef]
- Wang, J.; Hong, Z.; Zeng, C.; Yu, Q.; Wang, H. NADPH oxidase 4 promotes cardiac microvascular angiogenesis after hypoxia/reoxygenation in vitro. Free Radic. Biol. Med. 2014, 69, 278–288. [Google Scholar] [CrossRef]
- Ateghang, B.; Wartenberg, M.; Gassmann, M.; Sauer, H. Regulation of cardiotrophin-1 expression in mouse embryonic stem cells by HIF-1alpha and intracellular reactive oxygen species. J. Cell Sci. 2006, 119, 1043–1052. [Google Scholar] [CrossRef]
- DeNies, M.S.; Johnson, J.; Maliphol, A.B.; Bruno, M.; Kim, A.; Rizvi, A.; Rustici, K.; Medler, S. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol. Rep. 2014, 2, e00204. [Google Scholar] [CrossRef]
- Diebold, I.; Petry, A.; Hess, J.; Gorlach, A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell 2010, 21, 2087–2096. [Google Scholar] [CrossRef]
- Bartoszewski, R.; Moszyńska, A.; Serocki, M.; Cabaj, A.; Polten, A.; Ochocka, R.; Dell’Italia, L.; Bartoszewska, S.; Króliczewski, J.; Dąbrowski, M.; et al. Primary endothelial cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 7929–7941. [Google Scholar] [CrossRef]
- Camacho, M.; Rodríguez, C.; Guadall, A.; Alcolea, S.; Orriols, M.; Escudero, J.R.; Martínez-González, J.; Vila, L. Hypoxia upregulates PGI-synthase and increases PGI2 release in human vascular cells exposed to inflammatory stimuli. J. Lipid Res. 2011, 52, 720–731. [Google Scholar] [CrossRef]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef]
- Cabaj, A.; Moszynska, A.; Charzynska, A.; Bartoszewski, R.; Dabrowski, M. Functional and HRE motifs count analysis of induction of selected hypoxia-responsive genes by HIF-1 and HIF-2 in human umbilical endothelial cells. Cell. Signal. 2022, 90, 110209. [Google Scholar] [CrossRef]
- Mattiussi, S.; Turrini, P.; Testolin, L.; Martelli, F.; Zaccagnini, G.; Mangoni, A.; Barlucchi, L.M.; Antonini, A.; Illi, B.; Cirielli, C.; et al. p21(Waf1/Cip1/Sdi1) mediates shear stress-dependent antiapoptotic function. Cardiovasc. Res. 2004, 61, 693–704. [Google Scholar] [CrossRef]
- Kostyunina, D.S.; Rowan, S.C.; Pakhomov, N.V.; Dillon, E.; Rochfort, K.D.; Cummins, P.M.; O’Rourke, M.J.; McLoughlin, P. Shear Stress Markedly Alters the Proteomic Response to Hypoxia in Human Pulmonary Endothelial Cells. Am. J. Respir. Cell Mol. Biol. 2023, 68, 551–565. [Google Scholar] [CrossRef]
- Veith, C.; Kraut, S.; Wilhelm, J.; Sommer, N.; Quanz, K.; Seeger, W.; Brandes, R.P.; Weissmann, N.; Schröder, K. NADPH oxidase 4 is not involved in hypoxia-induced pulmonary hypertension. Pulm. Circ. 2016, 6, 397–400. [Google Scholar] [CrossRef]
- Nisbet, R.E.; Bland, J.M.; Kleinhenz, D.J.; Mitchell, P.O.; Walp, E.R.; Sutliff, R.L.; Hart, C.M. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am. J. Respir. Cell Mol. Biol. 2010, 42, 482–490. [Google Scholar] [CrossRef]
- Sogawa, K.; Numayama-Tsuruta, K.; Ema, M.; Abe, M.; Abe, H.; Fujii-Kuriyama, Y. Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia. Proc. Natl. Acad. Sci. USA 1998, 95, 7368–7373. [Google Scholar] [CrossRef]
- Wang, F.; Sekine, H.; Kikuchi, Y.; Takasaki, C.; Miura, C.; Heiwa, O.; Shuin, T.; Fujii-Kuriyama, Y.; Sogawa, K. HIF-1alpha-prolyl hydroxylase: Molecular target of nitric oxide in the hypoxic signal transduction pathway. Biochem. Biophys. Res. Commun. 2002, 295, 657–662. [Google Scholar] [CrossRef]
- Huang, L.E.; Willmore, W.G.; Gu, J.; Goldberg, M.A.; Bunn, H.F. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J. Biol. Chem. 1999, 274, 9038–9044. [Google Scholar] [CrossRef]
- Hagen, T.; Taylor, C.T.; Lam, F.; Moncada, S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: Effect on HIF1alpha. Science 2003, 302, 1975–1978. [Google Scholar] [CrossRef]
- Cattaneo, M.G.; Cappellini, E.; Benfante, R.; Ragni, M.; Omodeo-Sale, F.; Nisoli, E.; Borgese, N.; Vicentini, L.M. Chronic deficiency of nitric oxide affects hypoxia inducible factor-1alpha (HIF-1alpha) stability and migration in human endothelial cells. PLoS ONE 2011, 6, e29680. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.R.; Cai, H.; Davis, M.E.; Ramasamy, S.; Harrison, D.G. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ. Res. 2000, 86, 347–354. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Alves-Lopes, R.; Lacchini, S.; Neves, K.B.; Harvey, A.; Montezano, A.C.; Touyz, R.M. Vasoprotective effects of NOX4 are mediated via polymerase and transient receptor potential melastatin 2 cation channels in endothelial cells. J. Hypertens. 2023, 41, 1389–1400. [Google Scholar] [CrossRef]


| Parameter | |
|---|---|
| Age, years, median with range, n | 73.0 (54–81), 11 |
| Sex, male:female, % male | 9:2, 81% |
| BMI, kg/m2, median with range, n | 28.7 (22.9–39.9), 11 |
| Blood glucose, mmol/L, median with range, n | 5.12 (3.91–12.74), 8 |
| LDL cholesterol, mmol/L, median with range, n | 1.6 (1.17–3.26), 6 |
| HDL cholesterol, mmol/L, median with range, n | 1.04 (0.84–1.54), 5 |
| Total cholesterol, mmol/L, median with range, n | 3.24 (2.35–5.53), 6 |
| Triglycerides, mmol/L, median with range, n | 1.77 (1.36–2.98), 6 |
| CRP, mg/L, median with range, n | 7.40 (3.91–12.74), 8 |
| Gene | Primers | Sequence, 5′-3′ | |
|---|---|---|---|
| B2m murine | Forward | TCTCACTGACCGGCCTGTAT | NM_009735.3 |
| Reverse | GATTTCAATGTGAGGCGGGTG | ||
| eNOS human | Forward | GAACCTGTGTGACCCTCACC | NM_000603.5; NM_001160109.2; NM_001160110.1; NM_001160111.1 |
| Reverse | TGGCTAGCTGGTAACTGTGC | ||
| NOX4 human | Forward | TAACCTCAACTGCAGCCTTATC | NM_001143836.3; NM_001143837.2; NM_001291926.2; NM_001291927.1; NM_001291929.2; NM_001300995.1; NM_016931.5 |
| Reverse | CTTTTATCCAACAATCTCCTGGTTCTC | ||
| Nox4 murine | Forward | TGTTGGGCCTAGGATTGTGTT | NM_001285833.1; NM_001285835.1; NM_015760.5 |
| Reverse | AGGGACCTTCTGTGATCCTCG | ||
| POLRIIa human | Forward | ACCTGCGGTCCACGTTGTGT | NM_000937.4 |
| Reverse | CCACCATTTCCCCGGGATGCG | ||
| PTGIS human | Forward | ACTGCCTGGGGAGGAGTTAT | NM_000961.4 |
| Reverse | GGGATCTCCACATCTGCGTT | ||
| Ptgis murine | Forward | GTTGGTGGCGGTGACTTGTT | NM_001420752.1; NM_008968.5; NM_001420756.1; |
| Reverse | CAGCATCTCTCCCAAACTCCA | ||
| TBP human | Forward | CGCCGGCTGTTTAACTTCG | NM_003194.5 |
| Reverse | AGAGCATCTCCAGCACACTC |
| Primary Antibody | Company | Dilution | Molecular Weight |
|---|---|---|---|
| HIF1a | BD Biosciences | 1:1000 | 120 kDa |
| eNOS | BD Biosciences | 1:1000 | 140 kDa |
| Lamin A/C | Cell signaling | 1:2000 | 74 kDa/63 kDa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brendel, H.; Mittag, J.; Hofmann, A.; Hempel, H.; Giebe, S.; Diaba-Nuhoho, P.; Wolk, S.; Reeps, C.; Morawietz, H.; Brunssen, C. NADPH Oxidase 4: Crucial for Endothelial Function under Hypoxia—Complementing Prostacyclin. Antioxidants 2024, 13, 1178. https://doi.org/10.3390/antiox13101178
Brendel H, Mittag J, Hofmann A, Hempel H, Giebe S, Diaba-Nuhoho P, Wolk S, Reeps C, Morawietz H, Brunssen C. NADPH Oxidase 4: Crucial for Endothelial Function under Hypoxia—Complementing Prostacyclin. Antioxidants. 2024; 13(10):1178. https://doi.org/10.3390/antiox13101178
Chicago/Turabian StyleBrendel, Heike, Jennifer Mittag, Anja Hofmann, Helene Hempel, Sindy Giebe, Patrick Diaba-Nuhoho, Steffen Wolk, Christian Reeps, Henning Morawietz, and Coy Brunssen. 2024. "NADPH Oxidase 4: Crucial for Endothelial Function under Hypoxia—Complementing Prostacyclin" Antioxidants 13, no. 10: 1178. https://doi.org/10.3390/antiox13101178
APA StyleBrendel, H., Mittag, J., Hofmann, A., Hempel, H., Giebe, S., Diaba-Nuhoho, P., Wolk, S., Reeps, C., Morawietz, H., & Brunssen, C. (2024). NADPH Oxidase 4: Crucial for Endothelial Function under Hypoxia—Complementing Prostacyclin. Antioxidants, 13(10), 1178. https://doi.org/10.3390/antiox13101178








