Abstract
Herbal teas are used in South Africa as digestives to lower glycaemia and for other indications. However, the chemical composition of the infusions and their effect on enzymes related to metabolic syndrome is poorly known. The composition of infusions and methanol extracts of B. saligna (Scrophulariaceae), Lippia javanica, L. scaberrima, and Phyla dulcis (Verbenaceae) was assessed and the effect of the infusions and extract was determined towards α-glucosidase, α-amylase, and pancreatic lipase. The commercial herbal products were extracted separately with MeOH or hot water to obtain the extract/infusion for comparative studies. Total phenolic, total flavonoid and antioxidant capacity were assessed. The fingerprints of the MeOH extracts and infusions were compared by HPLD-DAD. The extract constituents were tentatively identified by HPLC-MS/MS and NMR analyses. From the extracts/infusions, 57 compounds were identified, including iridoids, phenylpropanoid glycosides, flavonoids, and caffeic acid derivatives, among others. The MeOH extracts and infusions showed strong inhibition towards α-glucosidase with IC50 in the range of 0.13–0.84 µg/mL for the phenolic-enriched infusion extract (PEI) and 0.47–0.50 µg/mL for the MeOH extracts, respectively. The P. dulcis PEI showed higher inhibition towards α-glucosidase, and the MeOH extract of L. scaberrima was better than the PEI. At 100 µg/mL, the PEI from the four herbal teas reduces the activity of α-amylase by 23.03–28.61%, with L. javanica as the most active tea. Three of the species are high in phenylpropanoid glycosides, while P. dulcis contains rosmarinic acid. Some 26 compounds were identified in the infusion from B. saligna, 28 from L. scaberrima, and 21 from P. dulcis. Four of them are common in all the teas, namely decaffeoylverbascoside, verbascoside, isoverbascoside, and tuberonic acid hexoside. Ten compounds occur in two of the teas and seventeen, fifteen, and eleven compounds were detected only in B. saligna, L. scaberrima, and P. dulcis, respectively. Most of the compounds are reported for the first time from the crude drug infusions. The results give some support for the traditional use of herbal teas as digestives and/or indications for diabetes. The chemical fingerprints set the basis for quality control of the crude drugs, based on the main constituents and differential compounds occurring in the samples.
1. Introduction
Herbal teas are used worldwide as digestives and are a way to ingest bioactive compounds following local lore or commercial trends. The best-known South African herbal tea is rooibos (Aspalathus linearis) and the traditional use of the product is related to a glucose lowering effect, including inhibition of the enzyme α-glucosidase [1]. The dihydrochalcone C-glucoside aspalathin has been identified as the main bioactive compound from rooibos tea. Other South African herbal teas can be potential sources of enzyme inhibitors associated with metabolic syndrome.
Metabolic syndrome is the association of obesity, hyperlipidemia, hypertension, and insulin resistance and it precedes an increase in the risk of cardiovascular diseases and the onset of type-2 diabetes. Inhibition of enzymes associated with type-2 diabetes, including α-amylase and α-glucosidase, is an alternative to reducing postprandial hyperglycemia.
The enzymes pancreatic lipase, α-amylase, and α-glucosidase are responsible for the breakdown of lipids and carbohydrates, respectively. The inhibition of these enzymes delays the absorption of fatty acids and monosaccharides, which is an important strategy for treating postprandial hyperglycemia and obesity [2].
Herbal teas are sources of polyphenols that confer organoleptic properties such as color, astringency, bitterness, or flavor, and functional characteristics such as antioxidant activity, and prevention or reduction of metabolic disorders [1,3,4,5], among others [6].
In South Africa, medicinal and aromatic plants are cultivated to develop healthy herbal products with the aim of improving the wellbeing of local communities and the country’s economy. A recent work on commercialized South African herbal teas showed the effect of selected crude drug (herbal tea) infusions on different antioxidant assays, as well as on the inhibition of the enzyme cyclooxygenase and the proliferation of a human cell line [7]. According to the information on the single plants reported, traditional uses are linked mainly to the taste and digestive properties. Buddleja saligna is a medicinal plant used in infusion to control glycaemia and is therefore recommended for treating diabetes [7]. However, evidence is needed to assess the effect of the extract and infusion of the herbal teas on enzymes associated with metabolic syndrome, such as α-glucosidase, α-amylase, and pancreatic lipase. Lippia javanica, L. scaberrima, and P. dulcis are used in infusions as digestive, among other uses [7]. The plant parts used for the herbal teas are the leaves.
In the present work, four plant species used as sources of herbal teas were investigated. The samples comprise the Scrophulariaceae Buddleja saligna Willd., and the Verbenaceae Lippia scaberrima Sond., Lippia javanica (Burm.f.) Spreng., and Phyla dulcis (Trevir.) Moldenke. According to The Plant List (http://www.theplantlist.org, accessed on 2 October 2024), Phyla dulcis (Trevir.) Moldenke is a synonym of Phyla scaberrima (Juss. ex Pers.) Moldenke. In the Flora of Yucatan, Mexico, Phyla dulcis (Trevir.) Moldenke is a basonym of Lippia dulcis Trevir. (https://www.cicy.mx/sitios/flora%20digital/ficha_virtual.php?especie=2258, accessed on 2 October 2024).
Little is known of the polar metabolites occurring in the extracts or infusions of the selected plants. From the aerial parts of L. javanica and L. scaberrima, verbascoside and isoverbascoside were reported [8]. The antimycobacterial and adjuvant effects of L. scaberrima were described [9]. However, the polar constituents occurring in the infusions were not identified due to the detection method selected (G-MS).
The herb Phyla dulcis was rediscovered as the source of the sweet compound hernandulcin studied in ancient Aztec manuscripts [10]. The herb Phyla dulcis (synonyms: Lippia dulcis Trevir. and Lippia dulcis var. mexicana Wehmer) is native to Mexico and Central America. The plants selected for the present study are cultivated and commercialized as herbal teas in the southern part of Africa. Due to the scarce information available, identification of the constituents in the infusions and the potential effect on enzymes related to digestion and metabolic syndrome can be useful for consumers. Our hypothesis is that the infusions from the selected teas will show some effect on enzymes associated with metabolic syndrome and linked to sugar metabolism, such as α-glucosidase or α-amylase.
2. Materials and Methods
2.1. Herbal Teas
The herbal teas were commercial samples from Northwest and Eastern Cape provinces. Traditional uses and botanical identification were described by Matsabisa et al. [7]. Crude drugs included the Scrophulariaceae Buddleja saligna Willd., known under the common name ‘Gancair’, as well as the Verbenaceae Lippia javanica (Burm.f.) Spreng. (‘Zinibar’), Lippia scaberrima Sond. (‘Mosukujane’), and Phyla dulcis (Trevir.) Moldenke (‘Haw Haw’). Plant voucher specimens were deposited at Bolus Herbarium (BLFU) at University of the Free State, Bloemfontein, South Africa under the reference numbers MGM0015, MGM005, MGM012, and MGM0016 for B. saligna, L. javanica, L. scaberrima, and P. dulcis, respectively.
2.2. Extraction of Bioactive Compounds
The teas were produced using plant leaves. The teas were prepared by placing tea bags in boiling water. The powdered plant material contained in the bags ranged from 1 to 2.5 g/bag. The plant material was removed from the bags, weighed, and infused in 400 mL of boiled water. After 10 min at room temperature, the infusion was filtered and activated Amberlite XAD-7 (Supelco, France, commercialized by sigmaaldrich.com, 89555 Steinheim, Germany) was added and stirred for 20 min. Then, the Amberlite was filtered and washed with distilled water, and the retained compounds were desorbed with MeOH. The solution was taken to dryness under reduced pressure and then lyophilized to afford the phenolic-enriched infusions (PEI). On the other hand, methanol extracts were prepared from the leaves by placing the samples in distilled MeOH in a 1:40 plant material–solvent ratio under sonication for 10 min. The process was repeated three times. The combined extracts were filtered, taken to dryness, and lyophilized, yielding the MeOH extracts. The infusion represents the way the herbal teas are consumed. Extraction with MeOH allows us to obtain medium and polar compounds except the most polar constituents, such as sugars and inorganic salts. The MeOH extract is analyzed after being taken to dryness under reduced pressure and lyophilized to a solid powder. The water infusion extracts fewer medium and low polarity compounds, and more sugars and inorganic salts than MeOH. To enrich the infusions in phenolics and remove sugars and salts, infusions were treated with Amberlite to adsorb polar organic compounds for further analysis.
2.3. Total Phenolic, Total Flavonoid and Total Procyanidin Content
The Folin–Ciocalteu reagent was used to determine total phenolic (TP) content. The total flavonoid (TF) content was measured using the aluminum trichloride method [11]. The 4-dimethylaminocinnamaldehyde (DMAC) method was used to determine the total content of proanthocyanidin (PAC) in the MeOH extracts and infusions
2.4. Antioxidant Capacity Assays
For the antioxidant capacity study, complementary assays were used, as described in [11,12]. The positive control was the flavonol quercetin. For FRAP and ORAC, the results are expressed as µmol TE/g extract. The results for TEAC are given as µM TE/g extract and DPPH as SC50 (μg/mL). All determinations were carried out in triplicate.
2.5. Enzyme Inhibition Studies
The MeOH extracts and the PEI of infusions were assessed for inhibition of enzymes α-amylase, α-glucosidase, and pancreatic lipase, as described in previous work [11]. The inhibition of α-amylase was determined at 550 nm wavelength (WL) at the final sample concentration of 100 µg/mL, as described in [11]. For α-glucosidase, the inhibition was measured at 415 nm WL using a concentration of a sample ranging from 0.1 to 100 µg/mL [11]. The inhibition of porcine pancreatic lipase was determined as reported in [11], at 400 nm WL.
2.6. NMR Profiles
To get an insight into the chemical composition of the teas, some 15 mg of the lyophilized infusion were dissolved in DMSO-d6, and 1H-NMR spectra were recorded at 400 MHz. Two-dimensional NMR, 13C-NMR, and COSY experiments were performed to identify the main compounds. The spectral data were processed using the Mestre Nova x64 program (MestreLab Research Laboratories, Santiago de Compostela, Spain).
2.7. HPLC-DAD Analyses
Shimadzu HPLC equipment from Shimadzu Corporation (Kyoto, Japan) was used for the analyses. The equipment included an LC-20AT pump, a CTO-20 AC column oven, and a UV diode array detector (SPD-M20A). The software used was LabSolution software (Version 5.51). The column used was 250 mm × 4.6 mm, with 5 µm of particle size Inertsil ODS-4 RP-18 (GL Sciences Inc., Tokyo, Japan). The column was kept at 30 °C. A linear gradient of solvents was used for the HPLC analyses. The solvents were: (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile (ACN). The gradient was: 0–15 min, 90–85% A; 15–20 min, 85% A; 20–25 min, 85–82% A; 25–75 min, 82–70% A; 75–78 min, 70% A; and 78–82 min, 70–90% A. The compounds eluted were monitored at 360 and 330 nm and UV spectra were measured in the range of 200–600 nm for chromophore characterization. Some 20 μL of a 5 mg/mL solution of the samples was injected and the flow rate was maintained at 0.4 mL/min.
2.8. HPLC-MS/MS Analyses
The mass spectra of the compounds were acquired through a Bruker Daltonik GmbH (Bremen, Germany) UHPLC/HPLC-DAD Bruker Elute LC system. The system was coupled in tandem with a Compact Q-TOF spectrometer. The software used for data analysis was the Compass DataAnalysis 4.4 software from Bruker Daltonik GmbH (Bremen, Germany). The column and precolumn used for chromatographic separations were as follows: Kromasil 100 5 C18, 5 µm particle size, 250 mm × 4.6 mm (Kromasil, Akzo Nobel, Bohus, Sweden), Nova-Pak, Waters Corp., Milford, CT, USA, C-18 Precolumn (22 × 3.9 mm, 4 μm particle size). Samples were dissolved in a mixture of ACN: formic acid 0.1% (1:1, v/v), and 20 µL of this solution were injected at 5 mg/mL for analysis. The same conditions previously described for HPLC-DAD analyses were employed for the chromatographic separation. The ionization was performed by electrospray ionization (ESI), using nitrogen as a nebulizer (9 L/min) and drying gas (4 Bar, 200 °C). The MS conditions were as follows: electrospray needle, −3500 V; compensating endplate, −500 V; separator cone 1, 56.0 V; separator cone 2, 6.0 V; output compensation capillary, 84.6 V; and output capillary, 140.6 V. The collision energy was 10–25 eV in stepping mode, auto MS/MS mode (4 precursor/cycle), 50–1500 m/z (scan 0.2 s centroid mode), using helium as a collision gas. Sodium formate (10% formic acid, 1 M) was employed for internal calibration. The compounds were tentatively identified by comparison of their mass spectra, fragmentation pattern, and UV profile.
2.9. Statistical Analyses
Statistical analyses were performed using GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA, USA). Differences between samples were tested using analysis of variance (ANOVA) one way, followed by Tukey’s multiple comparisons tests, and a p < 0.05 was considered statistically significant. All determinations were conducted in triplicate or quadruplicate, and the results were presented as mean ± standard deviation (SD).
3. Results
3.1. Extraction Yields
The extraction yields of the plant material contained in the tea bags were variable, according to the solvent used (MeOH or hot water) and the plant species (Table 1). The highest content of MeOH soluble was for B. saligna (23.11%), followed by P. dulcis (12.83%) and the two Lippia species (6.82–6.16%). The yield of the PEI of infusions was in the range of 3.95–5.01%, with higher extraction for B. saligna and L. javanica (5.01% and 4.91%, respectively). Infusions dissolve mainly water-soluble polar compounds while MeOH also dissolves less polar constituents, including chlorophyll and lipophilic substances. Therefore, the extraction yields of the crude drugs using MeOH were higher. However, the content of the different phenolics is lower as the extraction is less selective. The rationale in studying the composition of infusions is the fact that they better represent what is contained in the beverages taken by users. For better detection, the infusions were enriched in phenolics and other polar compounds using Amberlite XAD-7 resin, as described in Section 2.2.

Table 1.
Extraction yield from the South African herbal teas using MeOH and PEI of infusion.
3.2. Phenolics and Antioxidant Capacity
Enrichment of components from the infusion by Amberlite XAD to yield the PEI, increased the TP, TF, and TPA (L. scaberrima, P. dulcis) content, with higher activity in the DPPH discoloration assay, FRAP, ORAC, and TEAC values (except for the ORAC value of B. saligna) (Table 2). The best antioxidant capacity was observed for P. dulcis in FRAP and ORAC, while B. saligna was better in the TEAC measurements. When looking at the DPPH results, all extracts presented SC50 values in the range of 5.16–7.00 µg/mL. The TP content of the extracts was higher in the PEIs. The identity of the components plays a relevant role in the antioxidant capacity, measured by the ORAC assay, as can be observed by comparing the B. saligna PEI (29.29 g GAE/100 g PEI) with the best effect towards DPPH and TEAC, but with lower ORAC activity (Table 2).

Table 2.
Total phenolic (TP), total flavonoid (TF), total proanthocyanidin (TPA) content, and antioxidant activity (DPPH, FRAP, TEAC, ORAC) of infusions and methanol extracts from selected South African herbal teas.
3.3. Enzyme Inhibition
All extracts were active as α-glucosidase inhibitors, with IC50 values ranging from 0.13 to 0.84 µg/mL for the PEI and 0.47 to 0.50 µg/mL for the MeOH extracts, respectively. The P. dulcis PEI showed higher inhibition towards α-glucosidase, and the MeOH extract of L. scaberrima was better than the PEI (Table 3). At 100 µg/mL, the PEI from the four herbal teas reduced the activity of α-amylase by 23.14, 28.61, and 23.03% for B. saligna, L. scaberrima, and P. dulcis, respectively, while the most active tea was L. javanica, with an IC50 of 34.27 µg/mL. All MeOH extracts were inactive against pancreatic lipase at 50 µg/mL, but the PEI from B. saligna and L. javanica showed a mild effect, reducing the enzyme activity by 7.25 and 6.87%, respectively. None of the extracts showed an inhibition comparable to that of the reference compound Orlistat® (Table 3).

Table 3.
Inhibitory activity of the PEIs and MeOH extracts from South African herbal teas towards α-glucosidase, α-amylase, and pancreatic lipase.
3.4. NMR Analysis
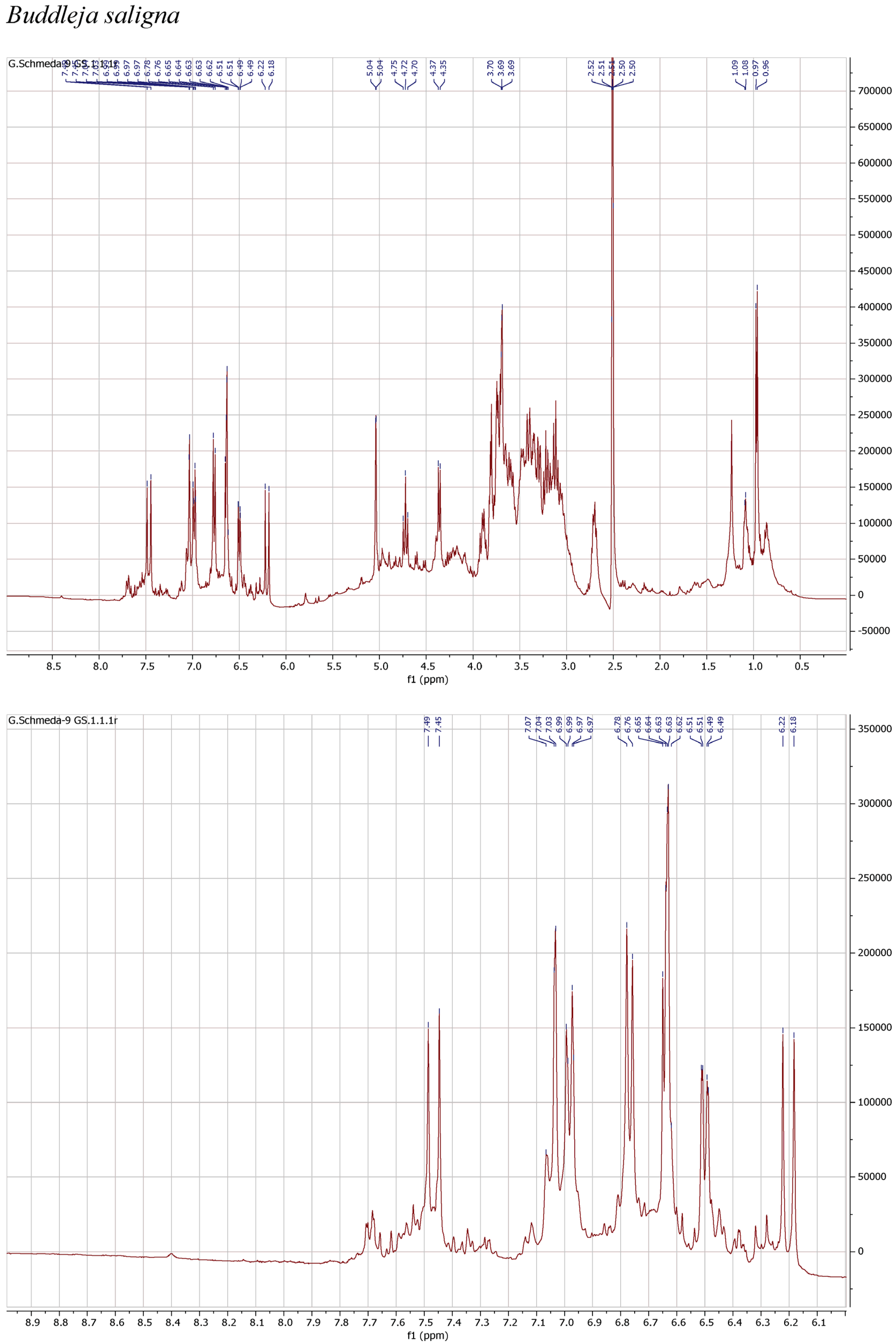
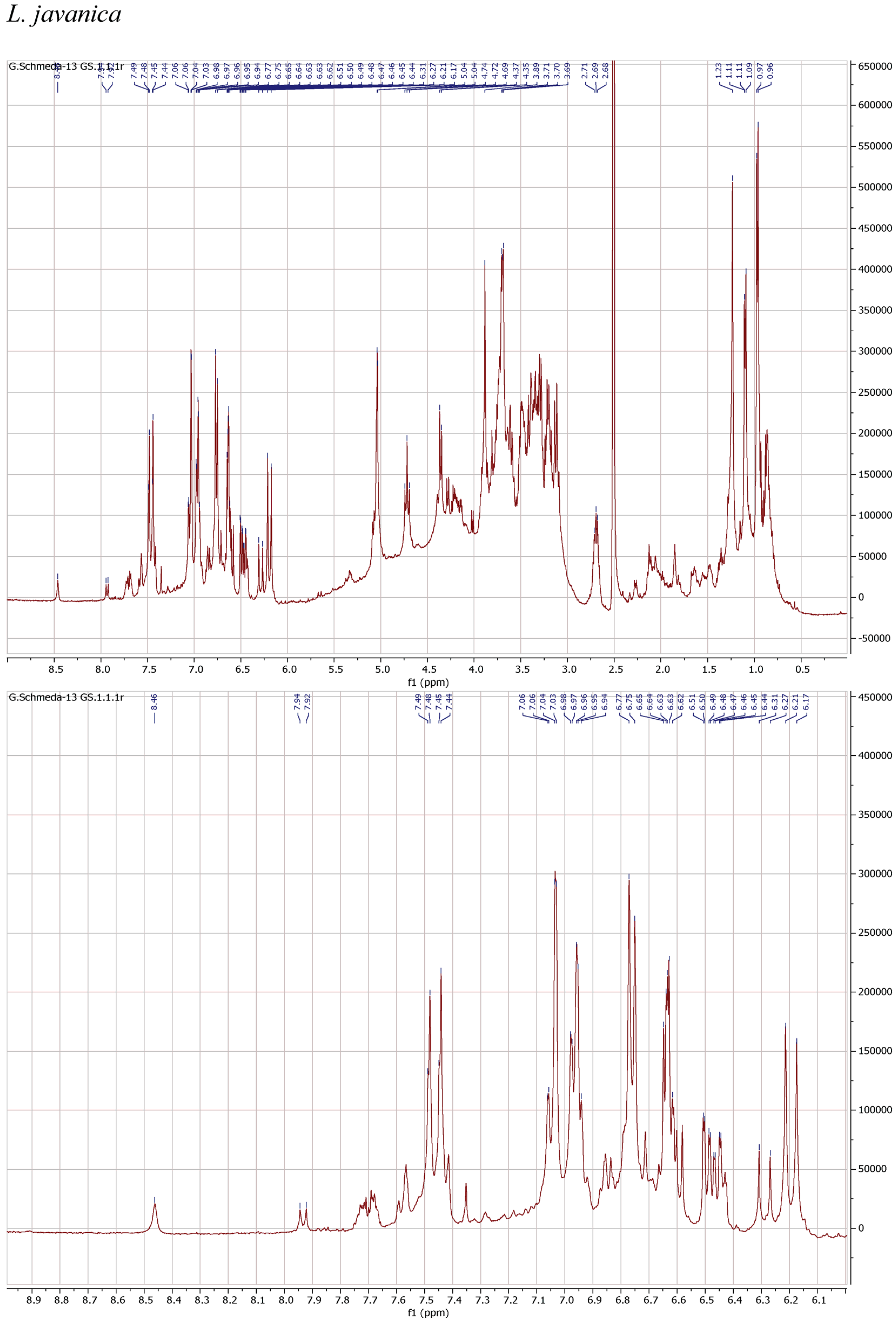
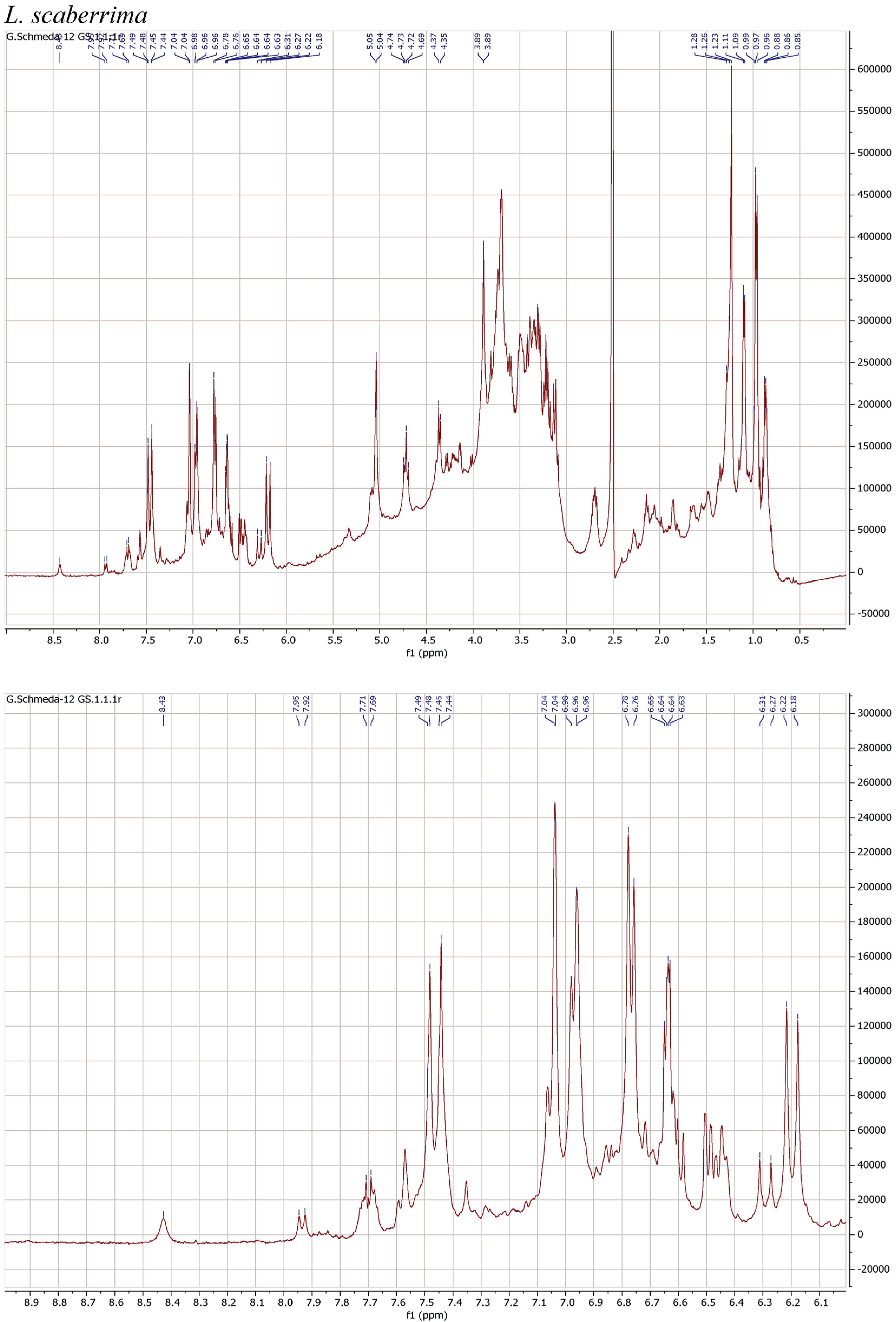
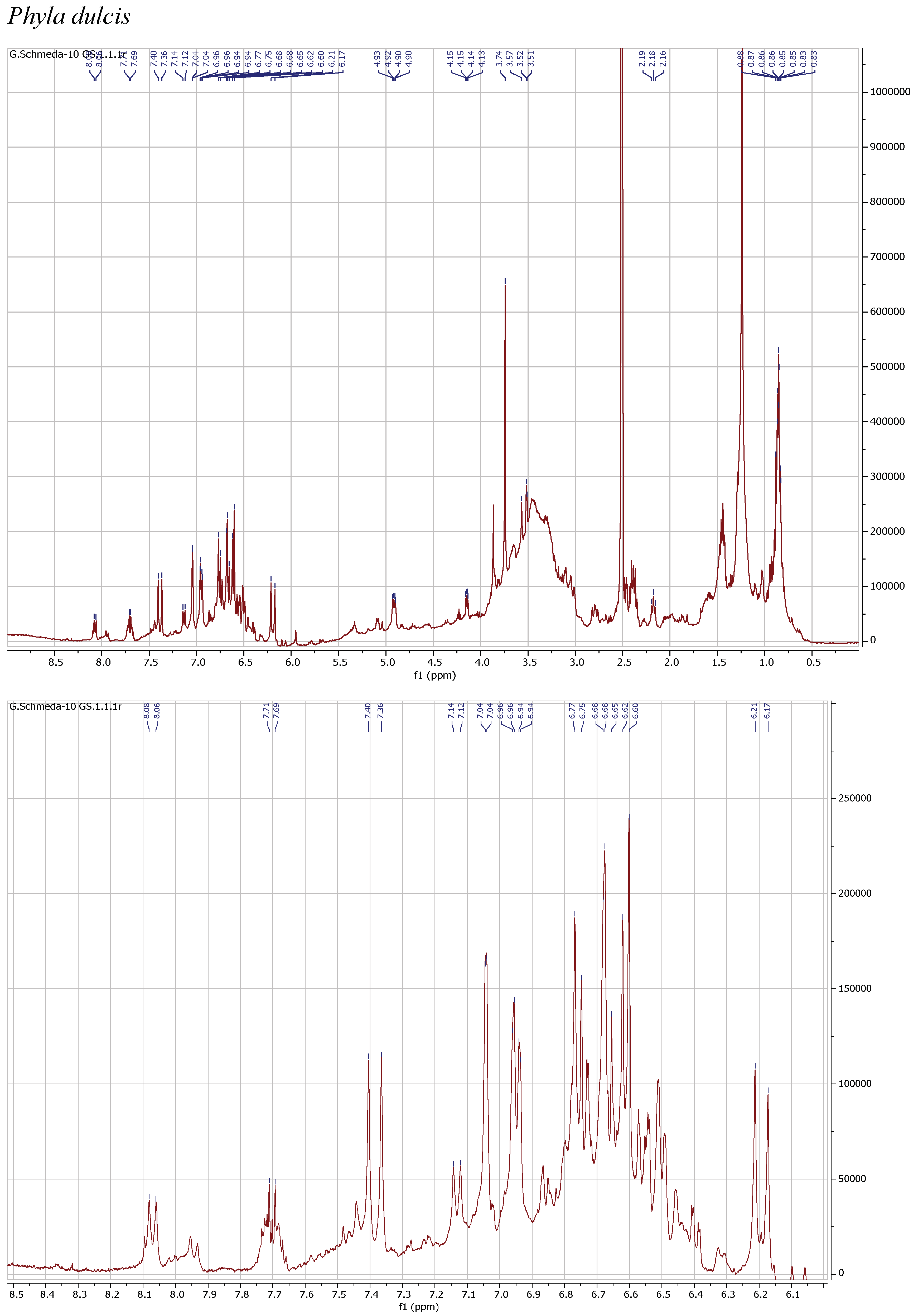
1H NMR spectra of the herbal teas were measured to obtain a first insight into the teas’ compositions. The spectra and enlarged section of the aromatic signals are presented in Figure 1.
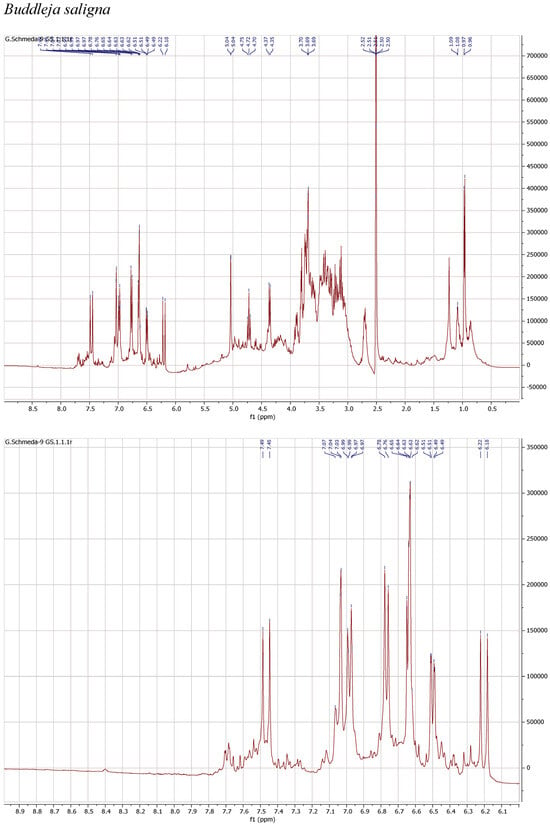
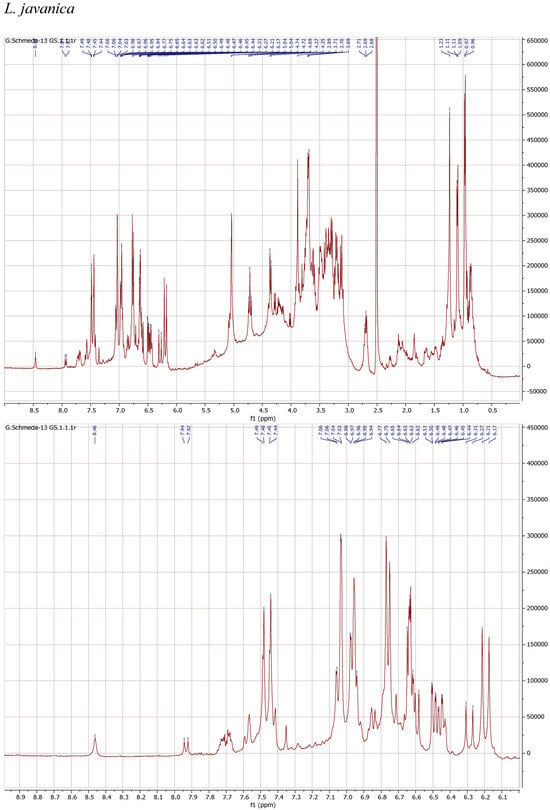
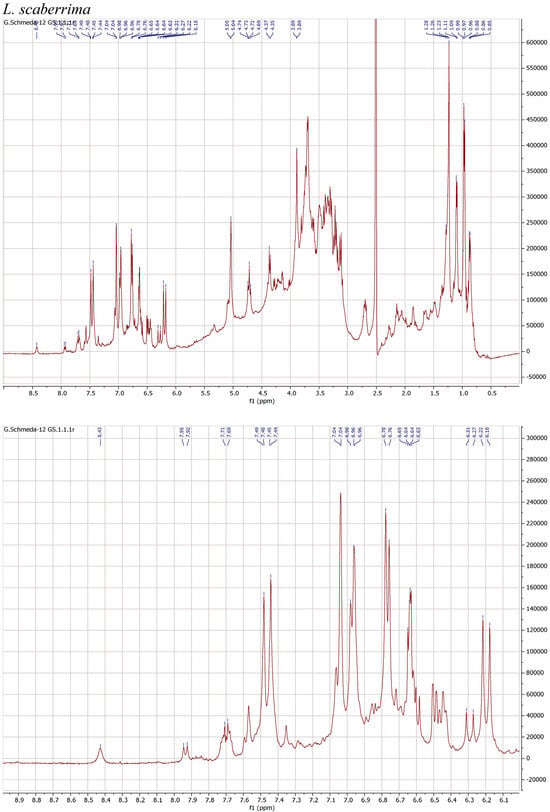
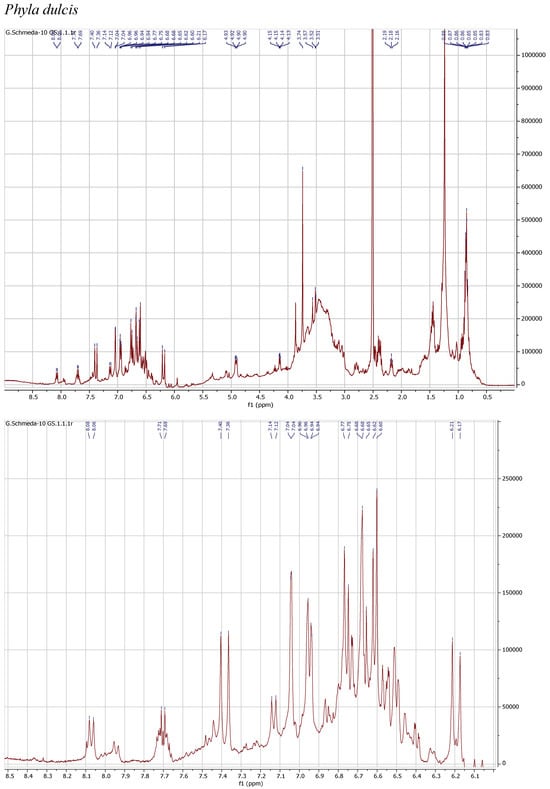
Figure 1.
1H NMR spectra of the infusions from Buddleja saligna, Lippia javanica, L. scaberrima, and Phyla dulcis (400 MHz, DMSO-d6).
The 1H NMR spectrum of the infusion from B. saligna shows a main compound with the typical signals for two 1,3,4-trisubstituted aromatic rings belonging to a caffeoyl and a phenylethyl moieties as well as two anomeric sugar H, overlapping multiplets from the sugar protons in the δ 3.1–4.2 range, and a d at δ 0.97, compatible with a deoxy sugar. The signals at δ 7.47 d (J = 15.9 Hz) (H-β′), 7.03 d (J = 2.2 Hz) (H-2′), 6.98 dd (J = 8.3, 2.2 Hz) (H-6′), 6.77 d (J = 8.3 Hz) (H-5′), and 6.20 d (J = 15.9 Hz) (H-α′) agree with the caffeoyl, while the H at δ 6.64 d (J = 7.8 Hz) (H-5), 6.63 d (J = 2.1 Hz) (H-2), 6.50 dd (J = 7.8, 2.1 Hz) (H-6), 3.90 m (H-α), and 2.70 m (H-β) can be assigned to the phenylethyl aglycone. The signals at δ 5.04 br s and 4.36 d (J = 7.3 Hz) from the anomeric H pointed out a rhamnose and a β-hexose, identified as glucose. The d at δ 0.97 (J = 6.0 Hz) confirms the rhamnose and the t at 4.72 (J = 9.6 Hz) confirms the placement of the caffeic acid ester at the C-4 from the glucose. The NMR data agrees with verbascoside [8].
The teas from L. javanica and L. scaberrima were very similar and showed a mixture of phenylethanoid glycosides as the main compounds. The main difference with B. saligna is the presence of a more complex mixture in Lippia, with two products showing the rhamnose methyl at δ 0.97 (main compound) and δ 1.10, respectively, with slight differences in the α-H of the caffeoyl ester (δ 6.20 and 6.29, respectively). The compounds were assigned as verbascoside and isoverbascoside. Both compounds were reported from L. javanica [8]. Two additional minor constituents can be deduced from additional d at δ 0.87 and 0.86, supporting the presence of isomers.
The 1H NMR spectrum of the PEI from P. dulcis shows a main compound with signals at δ 7.38 d (J =15.9 Hz; H-7′), 7.04 d (J = 2.2 Hz; H-2′), 6.95 dd (J = 8.3, 2.2 Hz; H-6′), 6.76 d (J = 8.3 Hz; H-5′), 6.61 d (J = 7.8 Hz; H-5), 6.68 d (J = 2.1 Hz; H-2), 6.50 dd (J = 7.8, 2.1 Hz; H-6), and 6.19 d (J = 15.9 Hz; H-8′), supporting a caffeoyl acid and an additional phenolic moiety. The dd at δ 4.91 (J = 8.7, 3 Hz; H-8) suggests the occurrence of rosmarinic acid, confirmed with a reference sample. Additional signals in the aromatic region and the sugar H support the presence of flavonoid glycosides.
3.5. HPLC-DAD Profiles
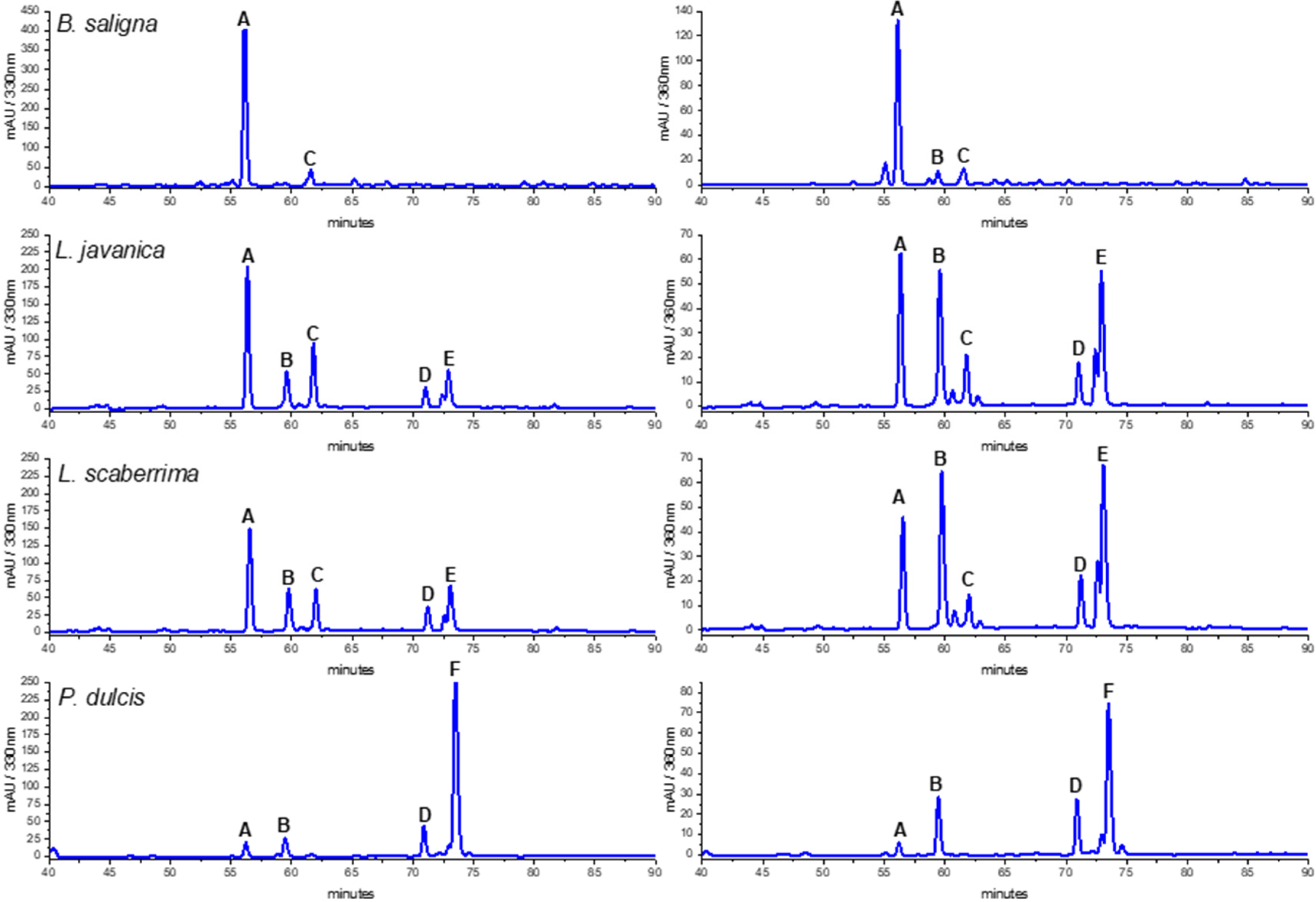
The HPLC traces of the MeOH and water infusions of the crude drugs were compared to select the better conditions for HPLC-MS/MS analysis. The PEI from the teas provided a better insight into the composition of the product consumed than the MeOH extract, as it was obtained after enrichment of the infusions (Figure 2). The reason for the HPLC-DAD analysis is to give the option for identification and quantification of the main compounds using accessible equipment. Furthermore, the HPLC-DAD fingerprints offer the option for quality control of the crude drug and can be used to compare different samples/populations and changes of composition following crude drug processing/storage.
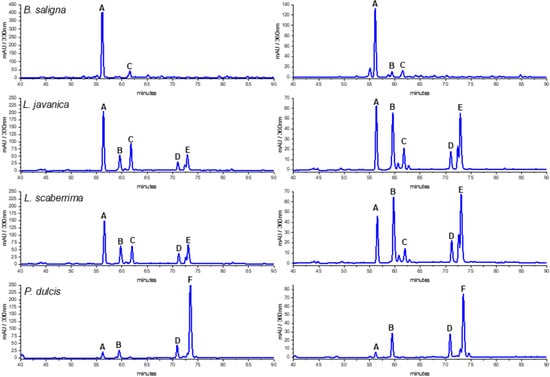
Figure 2.
HPLC-DAD profiles of Buddleja saligna, Lippia javanica, L. scaberrima, and Phyla dulcis infusions at 330 and 360 nm. Compounds: A: verbascoside; B: Quercetin 3-O-glucoside; C: isoverbascoside; D: flavonoid; E: Quercetin derivative; F: Rosmarinic acid.
The HPLC fingerprints of L. javanica and L. scaberrima were similar, with the same main constituents eluting at Rt 56.2 (A), 59.5 (B), 62.0 (C), and 73.1 min (D), respectively. The UV spectra suggest the occurrence of two different groups of compounds, namely phenylethanoid glycosides and flavonoids (Table 4). The main constituent for L. javanica and L. scaberrima was assigned as verbascoside/acteoside (A) according to the literature [8], while rosmarinic acid (F) is the main product in P. dulcis. Verbascoside was the main compound detected in the HPLC trace of B. saligna. According to the literature, the UV maxima of verbascoside are λ 326 and 289 nm, while for isoverbascoside they are 322 and 288, respectively [8]. The compounds B and F were identified as quercetin 3-O-glucoside and rosmarinic acid, respectively, via a comparison with reference standard compounds (PhytoLab, Vestenbergsgreuth, Germany). The identity of verbascoside was confirmed by NMR analyses.

Table 4.
Main compounds detected in the HPLC-DAD traces of Buddleja saligna, Lippia javanica. L. scaberrima, and Phyla dulcis.
3.6. HPLC-MS/MS Analyses
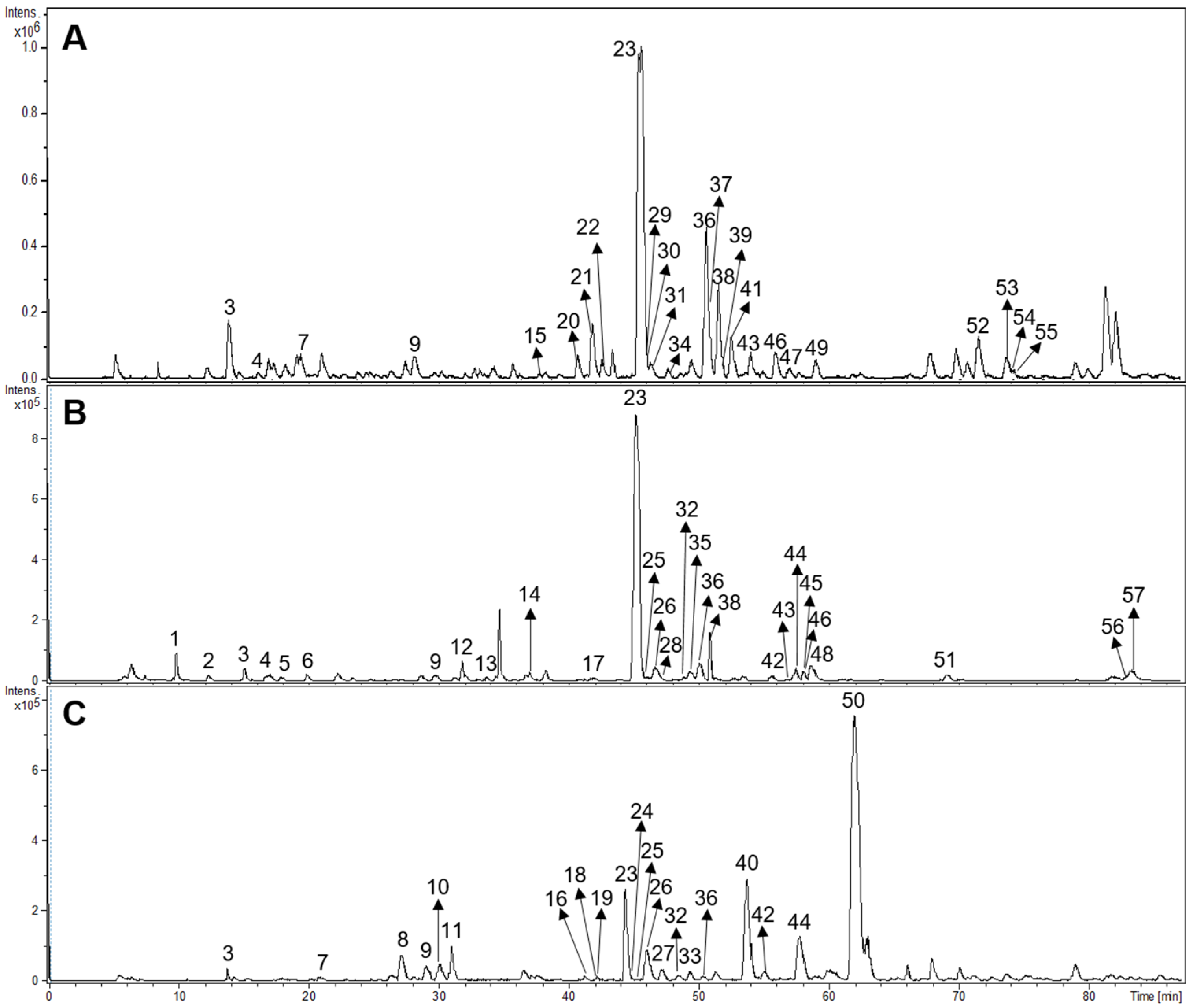
Fifty-seven compounds belonging to different structural groups were tentatively identified from the infusions. They comprised iridoids, flavonoids, phenylpropanoid glycosides, caffeic acid esters, and other compounds. The identification follows from the molecular formula, fragmentation patterns, interpretation of the information, literature, and databases including www.foodb.ca. The HPLC-ESI-MS/MS chromatograms of the samples are shown in Figure 3.
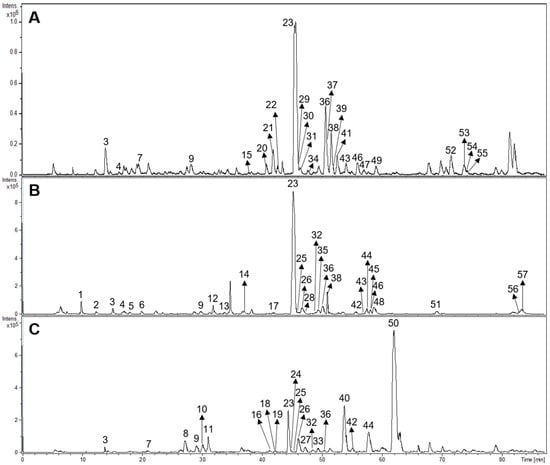
Figure 3.
HPLC-ESI-MS/MS chromatograms of Buddleja saligna (A), Lippia scaberrima (B), and Phyla dulcis (C). For the identity of the compounds please see Table 5.
The tentative identification of the tea constituents by HPLC-MS/MS is summarized in Table 5. Some compounds identified in the samples are shown in Figure 4.

Table 5.
Compounds tentatively identified in the MeOH extracts of Gancair (Buddleja saligna), Mosukujane (Lippia scaberrima), and Haw Haw (Phyla dulcis) through LC-MS in negative ion mode, and occurrence of the compounds in the crude drugs.
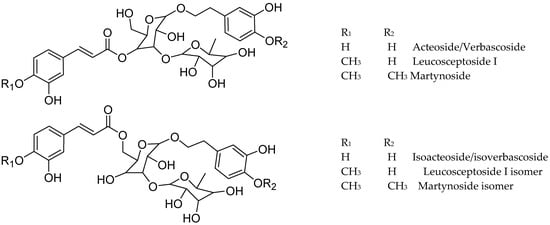
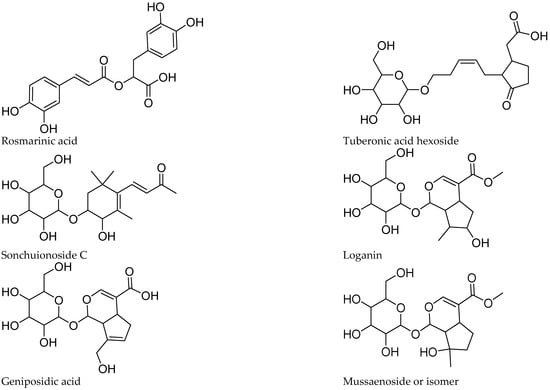
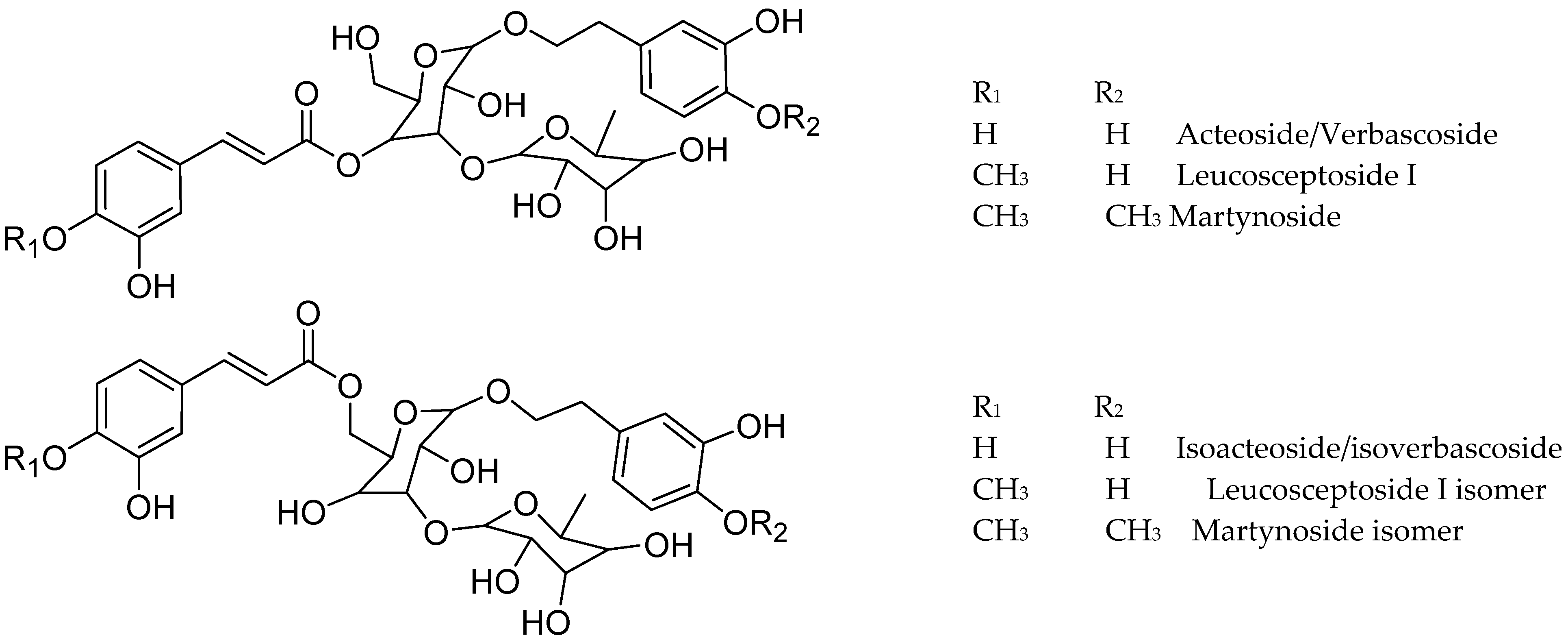
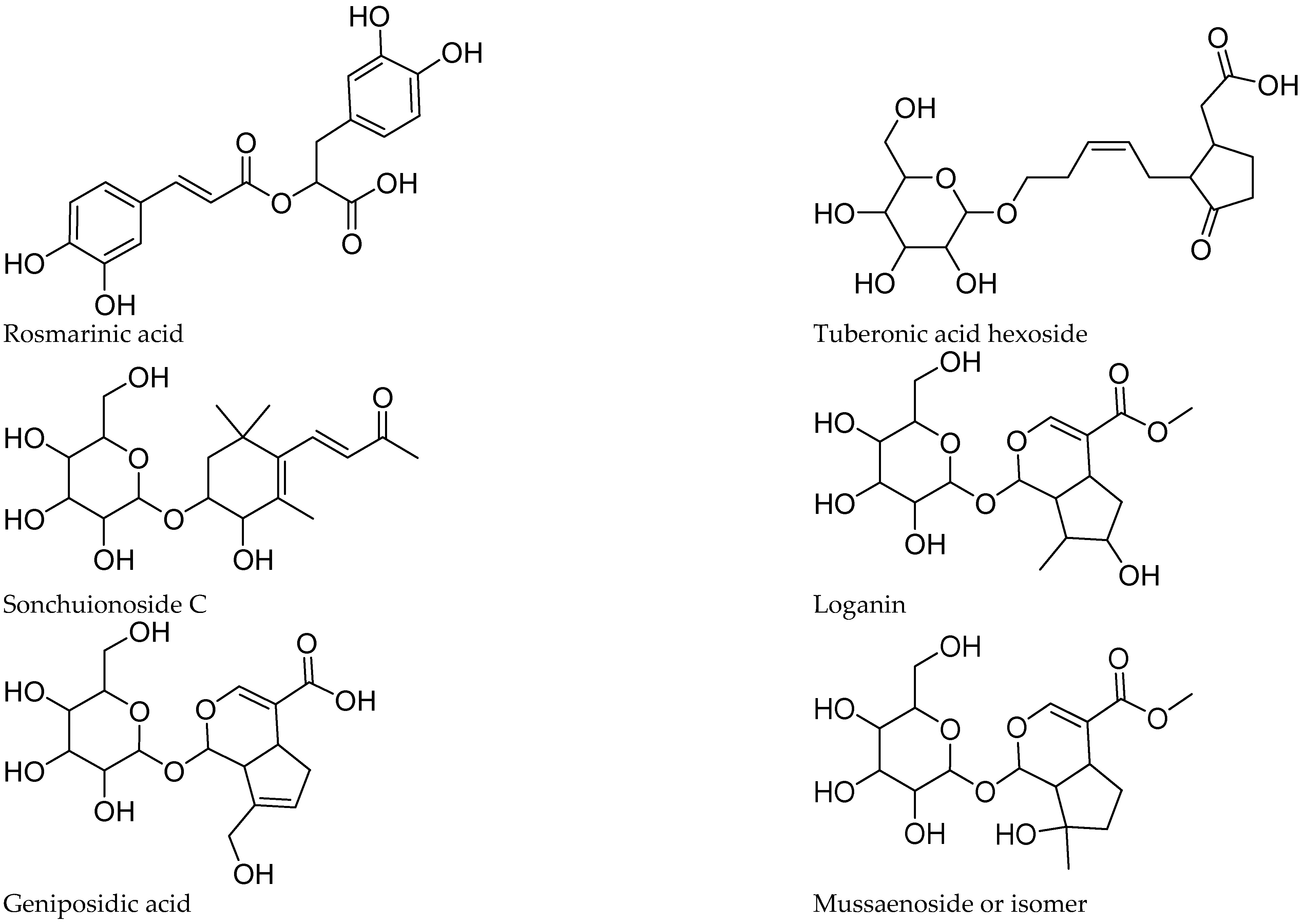
Figure 4.
Structure of some compounds identified/tentatively identified from the selected South African teas.
3.6.1. Iridoids
Five iridoids were tentatively identified in the extracts, including compounds 1, 2, 6, 12, and 14. The proposed identification was supported by the work of [13,14,15] on iridoid glucosides. Compounds 1 and 14 showed a pseudomolecular ion at m/z 389, compatible with the molecular formula C16H22O11. Both lost a hexose (162 amu), yielding a fragment at m/z 227, in agreement with theveside and mussaenoside isomers [16]. Considering their previously reported elution order, compound 1 was tentatively identified as theveside and 14 as mussaenoside. Both iridoids were previously informed in L. alba, L. citriodora, and L. graveolens [16,17]. The [M-H]− ion at m/z 373 of compound 2 and the MS2 fragments at m/z 211, 167, and 123 agree with that reported for geniposidic acid [15]. Compound 12 was detected as [M+HCOOH]− at m/z 435, yielding the fragments at m/z 389 and 227, characteristic of loganin [16]. Compound 6 showed [M+HCOOH]− and MS2 ions at m/z 451, 405, and 243, differing in one oxygen from 12 and was assigned as hydroxyloganin. The exact stereochemistry and placement of the hydroxy group remain to be established. 8-epiloganin and lamiide were reported for L. dulci, and the first for L. alba [16,18], while loganin and its derivatives were informed for L. graveolens [19].
3.6.2. Flavonoids
Seven kaempferol derivatives, including a dihexoside (15), dirhamnoside pentoside (19), hexoside rhamnoside (22), three isomeric hexosides (compounds 25, 31 and 47) and the glucuronide (26) were identified in the teas, showing the characteristic loss of the sugars, leading to the MS2 ion of kaempferol at m/z 285. The position of the sugar residues was theoretically assigned based on the literature [20,21,22]. We proposed that compound 15 was kaempferol 3,7-di-O-hexoside, based on the ions at m/z 447 and 285, and the low formation of the radical ion at m/z 284, indicating 3-O and 7-O substitutions, respectively [21,22]. Compound 19 was tentatively assigned as kaempferol 7-O-rhamnoside 3-O-rhamnoside pentoside because of the intense MS2 ion at m/z 563, consistent with a preferential neutral loss in position 7 and the high formation of the radical ion at m/z 284, suggesting a 3-O substitution [20,22]. Peaks 22, 26, and 47 were tentatively identified as 7-O substituted derivatives based on the low relative abundance of the radical at m/z 284, whereas 25 and 31 were assigned as 3-O hexosides due to the high formation of this ion radical.
The quercetin glycosides 16, 21, 24, and 30 showed the neutral loss of rutinose, hexose, and rhamnose, as well as one hexose unit, with a base peak in agreement with quercetin; they were assigned as quercetin rutinoside (16), hexoside rhamnoside (21), and hexosides (compounds 24 and 30), differing in the identity of the hexose. The position of the sugar residues was inferred to be in 3-O due to the detection of the ion at m/z 271 in all peaks, characteristic of 3-O-substituted quercetin derivatives [22].
The compound 28, with a m/z of 491.0843 shows the neutral loss of glucuronic acid and further fragments to 315.0519, in agreement with rhamnetin/isorhamnetin, and was identified as rhamnetin/isorhamnetin glucuronide. Eriodictyol hexoside (compound 29) was detected in the infusion of B. saligna, but in neither L. javanica, L. scaberrima nor P. dulcis. (2R) and (2S) eriodictyol 7-O-glucopyranoside was reported from several Brazilian Lippia species [23]. The compound 32 showed neutral loss of glucuronic acid and a base peak compatible with hesperetin, being assigned as hesperetin glucuronide. The fragmentation of compound 35, with a m/z of 507.1181, showed the loss of a hexose followed by two methyl groups, suggesting a dimethoxy myricetin hexoside. The compound was tentatively assigned to syringetin hexoside and the placement of the methoxy groups remains to be established [24].
The mass spectrum of compound 8 shows a UV maximum at 332 nm and fragmentation according to a flavone C-glucoside. The spectrometric data agrees with apigenin 6,8-di-C-hexoside [25]. Compound 44 shows loss of glucuronic acid and a base peak at m/z 269, in agreement with apigenin 7-O-hexuronide [21]. The related compounds 45 and 48 show loss of glucuronic acid, leading to the base peak at m/z 329 and 299, respectively, and were assigned as the flavones tricin glucuronide and chrysoeriol glucuronide, respectively.
Compound 54, with a m/z ion at 491 and molecular formula C23H24O12, shows the neutral loss of hexose and a base peak at m/z 329, in agreement with quercetin dimethyl hexoside. The aglycones quercetin methyl ether (compound 56), compatible with rhamnetin/isorhamnetin) and dimethylmyricetin (compound 57), were tentatively identified by the loss of one or two methyl from the [M-H]+ ions.
3.6.3. Phenylpropanoid Glycosides
Phenylpropanoid glycosides were the main compounds in Lippia and Buddleja, with verbascoside/isoverbascoside occurring in B. saligna, L. javanica, and L. scaberrima.
The compounds 23, 33, 36, and 38 show a molecular formula of C29H35O15 for the [M-H]- ion, and similar fragmentation patterns, in agreement with verbascoside (acteoside) and isoverbascoside (isoacteoside) isomers [8,23,26]. The double bond in the caffeic acid moiety can be trans or cis, and the placement of the phenolics in the sugar moiety can explain the differences in the Rt values in the chromatograms. Compound 23 was identified as verbascoside/acteoside based on the 1H NMR analysis of the infusions and 33 was assigned as isoverbascoside/isoacteoside. Compounds 36 and 38 were tentatively assigned as verbascoside/ isoverbascoside isomers 1 and 2, respectively. According to [26], the elution sequence for the isomeric compounds forsythioside A, acteoside, cis-acteoside, and isoacteoside was an Rt of 26.953, 27.497, 28.200, and 30.331 min, respectively. Acteoside, isoacteoside, martynoside, and diacetylmartinoside were reported from L. dulcis cultivated in Japan [18]. Verbascoside (Acteoside) (compound 23) was identified in Lippia and Buddleja as a main compound, in agreement with literature. The related compound 3 shows a molecular formula of [M-H]+ C20H29O12, and differs from verbascoside by a caffeoyl acid moiety, being assigned as a decaffeoyl verbascoside isomer.
Compound 34 shows typical fragmentation for a phenylpropanoid glycoside and differs from verbascoside/isoverbascoside by one deoxysugar unit. The compound agrees with calceolarioside B (desrhamnosyl isoacteoside) [27].
The compounds 37, 42, and 46 showed a molecular formula differing from that of acteoside/isoacteoside by one methoxy group instead a hydroxy in the aromatic moiety. Fragmentation of the [M-H]- ion at m/z 637 led to the base peak at m/z 175, in agreement with Leucosceptoside A [16,28]. Compounds 42 and 46 were assigned as Leucosceptoside A isomer 1 and isomer 2, respectively. The fragmentation of compound 37 shows differences in the placement of the OCH3 in the aromatic moieties. While, in 42 and 46 the methoxy is placed in the phenylpropanoid moiety (ferulic acid), in compound 37 the phenylpropanoid agrees better with coumaric acid (m/z 163 and 145), and the methoxy group should be in the phenylethanoid moiety. A related compound was reported from Forsythia suspensa [29]. Compound 41 showed a molecular formula and fragmentation compatible with a methoxycaffeic acid dihexoside bearing an additional methoxyphenyl ethanoid moiety and was assigned as hydroxy hemiphroside A [30].
Compounds 51 and 53 showed a [M-H]- ion at m/z 651 and a molecular formula of C31H39O15, differing from verbascoside/isoverbascoside by the presence of methoxy instead of hydroxy in the phenolic moiety of the glycoside, and were assigned as martynoside [28] isomer 1 and 2, respectively. Leucosceptoside A and martynoside are the monomethyl ether of acteoside/isoacteoside and the dimethylether of acteoside/isoacteoside, respectively. Compound 55, with a molecular formula of C35H45O20, lost one caffeoyl moiety (162 amu) and yielded MS2 fragments at m/z 623, 461, and 161, in agreement with acteoside/verbascoside [26]. Thus, 55 was tentatively assigned as echinacoside [31].
Compounds 13 and 17 differ from acteoside/isoacteoside by the presence of one additional hydroxy group (compound 13) or methoxy group (compound 17) and were tentatively identified as hydroxy acteoside and methoxy acteoside, respectively. Compound 43, with a [M-H]- ion at m/z 607 and a molecular formula of C29H35O14, differs from verbascoside/isoverbascoside by one oxygen, and the fragmentation pattern agrees with that reported for desoxyacteoside [32].
The mass spectrum of compound 39 shows a molecular formula of C30H37O16, and fragments to the base ion at m/z 161, supporting a caffeic acid derivative. The fragmentation and molecular formula are compatible with campneoside [32]. The related compound 49 shows the loss of 180 amu, leading to m/z 487 and a base peak at m/z 193, in agreement with scroside B. The molecular formula and fragmentation pattern agree with data reported by [30]. Compound 4 was assigned as Cistanoside F by its molecular formula and fragmentation, in agreement with the literature [33].
3.6.4. Caffeic Acid Esters
Caffeic acid (compound 11) was identified in P. dulcis by the UV spectrum and molecular formula and occurs as the hexoside (compound 7) in P. dulcis and B. saligna. Compound 20 shows a [M-H]+ ion at m/z 667 and a molecular formula of C30H35O17. The fragmentation shows the neutral loss of a caffeoyl moiety (162 amu) and the ions at m/z 505, 170, and 161 (base peak), in agreement with Hebitol II [26].
The caffeic acid ester rosmarinic acid (compound 50) occurs in P. dulcis and was identified by its molecular formula, fragmentation pattern, and comparison with a reference standard compound. Compounds 18 and 27 show an m/z of 521 and the [M-H]+ ion with a molecular formula of C24H25O13, with a base peak at m/z 161, and in agreement with rosmarinic acid hexosides. The glycosides were assigned as rosmarinic acid hexoside 1 (18) and hexoside 2 (27), respectively. Compound 40 showed a [M-H]+ ion at m/z 719 and a molecular formula of C36H31O16, suggesting four phenylpropanoid units and fragments with a base peak at m/z 161. The fragmentation and molecular formula suggest the occurrence of a sagerinic acid isomer. The compound was tentatively assigned as sagerinic acid, in agreement with [34,35].
3.6.5. Other Compounds
Tuberonic acid hexoside 9 was identified by its molecular formula and fragmentation, according with previous studies on B. officinalis flowers [26]. In our sample, compound 10 with the pseudomolecular ion [M+HCOOH]+ at m/z 431.1920 and a molecular formula of C19H29O8 shows the neutral loss of hexose, leading to the base peak at m/z 223, which further fragments to m/z 153, in agreement with sonchuionoside C [36].
Compound 5, with a molecular formula of [M-H]+ C15H19O10, shows the neutral loss of hexose, leading to the base peak at m/z 197, in agreement with syringic acid. The compound was identified as syringic acid hexoside. Compound 52 showed the neutral loss of a pentose, leading to a base peak at m/z 289, in agreement with octen-3-yl hexoside and was assigned as the acyl carbohydrate 1-octen-3-yl hexoside pentoside. The occurrence of the different compounds in the infusions of the selected South African teas is summarized in Table 5.
4. Discussion
The infusions and MeOH extracts of selected herbal teas from South Africa were assessed for the inhibition of enzymes associated with metabolic syndrome. All infusions and extracts showed good inhibition of the α-glucosidase. Buddleja saligna is traditionally used for diabetes in South Africa. Different leaf extracts of the plant were previously assayed for α-glucosidase inhibition [37]. According to [37], the hexane extract showed the best α-glucosidase inhibition (IC50 = 260 μg/mL). However, our results using the infusion and a polar methanol extract show a lower IC50 (higher activity) due to a different chemical composition, resembling that of traditional preparations and herbal teas.
The inhibitory effect of the MeOH extracts towards the α-glucosidase was 0.47–0.50 µg/mL while the activity of the PEI was higher for B. saligna and P. dulcis (0.21 and 0.13 µg/mL, respectively). The MeOH extracts of the crude drugs showed a TP content ranging from 9.43 to 11.66 g/100 g extract, while the PEI of B. saligna and P. dulcis contains 29.29 and 17.69 g GAE/100 g extract, respectively. The increase in activity can be explained by higher phenolics and by the identity of the compounds occurring in the samples. The α-glucosidase inhibition by the PEI of L. javanica and L. scaberrima, with a higher TP than the MeOH extract, however, did not show relevant changes, with IC50 values of 0.43 and 0.84 µg/mL, respectively. Rosmarinic acid and verbascoside were the main constituents in the P. dulcis and B. saligna extracts, respectively.
The enzymes α-glucosidase, α-amylase, and pancreatic lipase have different substrates and specificity. The enzyme α-glucosidase is a hydrolase that releases α-glucose from carbohydrates. The enzyme acts by hydrolyzing terminal glycosidic bonds, releasing α-glucose from the nonreducing end of the substrate chain. α-Amylase, a starch hydrolase, is the main digestive enzyme in saliva. It begins the digestion of starch hydrolyzing α-1,4 glycosidic linkages and releases smaller molecules such as disaccharides or trisaccharides. Pancreatic lipase hydrolyzes the ester linkages of triglycerides releasing fatty acids and glycerol. The enzyme is relevant for lipid absorption and digestion of cholesterol and fatty acid esters as well as lipid-soluble vitamins. As the selected enzymes have different structures and biochemical targets, the effect of the plant constituents will be different for the three enzymes. Selectivity is a desired characteristic for a possible application in pharmaceutical food science. A recent review on natural compounds affecting glucose and lipid metabolism shows the potential of this approach [38].
The composition of the teas was determined by HPLC-DAD-MS/MS and 1H NMR. Chemical profiles using HPLC-DAD allow for the identification of the main compounds in the infusions and methanol extracts. The six main phenolics, including the differential marker rosmarinic acid for P. dulcis, the phenylpropanoids verbascoside and isoverbascoside occurring in different ratios in B. saligna, L. javanica, and L. scaberrima, and the flavonoids, can be used for quality controls of the crude drugs.
The HPLC-DAD fingerprints strategy, using reference compounds, is a suitable method to monitor changes in composition related to different populations, select the best harvesting time, and set the basis for further studies on changes related to environmental responses and processing for tea production. Using the more sensible HPLC-MS/MS analyses, 26 compounds were identified in the infusion from B. saligna, 28 from L. scaberrima, and 21 from P. dulcis. A few of them (decaffeoylverbascoside, verbascoside, isoverbascoside, and tuberonic acid hexoside) were found in all the samples. In addition, 10 compounds occur in two of the species. Most of the compounds occurring in low amounts were species-specific, accounting for seventeen, fifteen, and eleven metabolites in B. saligna, L. scaberrima, and P. dulcis, respectively. Most of the compounds reported in this work are described for the first time from the selected South African crude drugs and the few products previously identified in the plants are indicated with an * in Table 5.
The phenylpropanoid glycosides verbascoside and isoverbascoside have been reported as the main phenolics from the aerial parts of L. javanica [8]. Both compounds were isolated from the defatted MeOH extract of the plant and were fully identified by spectroscopic means and comparison with standards. Verbascoside and isoverbascoside were also quantified in MeOH:H2O 80:20 extracts from Lippia species, including L. scaberrima [8]. Verbascoside, also known as acteoside, inhibits the growth of tumor cells [39], reduces sugar absorption, protects pancreatic β-cells against endoplasmic reticulum (ER) stress [40] and, acts as a hepatoprotective, antioxidant, and anti-inflammatory, among other functions [41]. Rosmarinic acid is a well-known antioxidant with anti-inflammatory effects [42], which shows inhibition towards several enzymes and strong therapeutic potential [43,44]. Related studies have been performed with herbal teas from the Anatolian peninsula [45], China [46], and South Africa [47].
Another study on South African crude drugs investigated in this work includes the potential of an ethanol extract from B. saligna in sunscreen formulations [48]. However, the phytochemical information in this study was obtained by GC-MS analyses of an ethanol extract and the polar constituents occurring in the extract were not identified due to the analytical method. Polar Buddleja constituents were described by [26] in B. officinalis flowers and polyphenols from B. globosa leaves were reported by [32]. The effect of B. saligna on the angiotensin converting enzyme and the mechanism involved in lipid metabolism were described [49]. Some triterpenes were isolated and identified from leaves of B. saligna [50].
The in vitro antimycobacterial activity of Lippia scaberrima was reported by [9], along with the phenolic content, antibacterial, and antioxidant in vitro from South African Lippia infusions [51]. A dereplication study on Brazilian Lippia species showed that verbascoside occurs in most of the samples, while isoverbascoside was detected in six out of ten species [23]. A recent work on P. dulcis, using the synonym Lippia dulcis was published [52] with Brazilian material. The authors identified verbascoside by co-chromatography with a standard sample and worked on different extracts obtained with methanol or ethanol. Our extract of P. dulcis shows a different composition, with rosmarinic acid as a main constituent; this supports the importance of verifying the botanical identity and chemical composition of herbal teas in the production place. Neither hernandulcin nor lippidulcine derivatives were detected in the South African grown P. dulcis. In a study on plant material from Japan, the isolation and identification of (+)hernandulcin, (−)epihernandulcin, lippidulcine A, and epilippidulcine was reported [18].
Methanol extracts and infusions of four different South African herbal teas were compared for composition, antioxidant capacity, and inhibition of enzymes related to metabolic syndrome (α-glucosidase, α-amylase, and pancreatic lipase). Three of the species belong to the Verbenaceae family and one of them to the Scrophulariaceae. The four herbal teas inhibited the enzyme α-glucosidase with a better effect for the phenolic-enriched infusions (PEI) of Phyla dulcis and Buddleja saligna (IC50 values of 0.13 and 0.21 µg/mL, respectively). The PEI was mildly active towards α-amylase at 100 µg/mL, suggesting some potential for inhibiting these two enzymes, related to sugar absorption. The metabolites occurring in the teas, including rosmarinic acid, verbascoside, and isoverbascoside among others, might explain, at least in part, the effect of the selected South African herbal teas as inhibitors of the α-glucosidase and α-amylase. Verbascoside was reported as an inhibitor of α-glucosidase in Brazilian Lippia dulcis, a botanical synonym of P. dulcis. The molecular docking and enzyme inhibition of the compound described in [52] confirm its effect on α-glucosidase and help explain the effect of the infusions as strong glucosidase inhibitors in the South African samples. Rosmarinic acid has been reported as an α-glucosidase inhibitor and was isolated from different plant sources, including Rosmarinus officinale, Perilla leaves [53], and Rabdosia serra [54]. Other reported effects of the compounds included antioxidant and antiallergic properties [53], and tyrosinase inhibition [54]. In an in silico reverse docking investigation, rosmarinic acid and flavonol quercetin were identified as inhibitors of the enzymes α-glucosidase and pancreatic α-amylase, and as inhibitors of lipid accumulation in hepatic cells [55]. Taking all this information together, the occurrence of rosmarinic acid as a major compound in P. dulcis supports the notion of herbal tea as a digestive.
The comparison of the samples by HPLC-DAD shows that B. saligna, L. javanica, and L. scaberrima are phenylpropanoid glycosides-containing herbal teas while P. dulcis contains rosmarinic acid as a main bioactive. Our work presents an overview of the chemical constituents of the infusions that can be used for the characterization and quality control of herbal teas. The chemical fingerprints observed in HPLD-DAD allow a clear differentiation among B. saligna, P.dulcis, and the two Lippia species (L. javanica and L. scaberrima). Further work is needed to find additional chemical and/or molecular markers to differentiate L. javanica and L. scaberrima. The fingerprints with the main phenolics from the crude drugs can be used to start new studies on the variability of the constituents and enzyme inhibition according to the production places, agricultural practices, and response to environmental stress.
Author Contributions
G.S.-H. and M.G.M. conceived and designed the study. N.N. and A.B.-E. carried out chromatographic profiling and participated in the HPLC-MS analysis of the samples. C.T. and S.T. performed biological evaluations, data analysis and editing. All authors participated in the data interpretation and writing of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Project EQM170023 for the HPLC-MS/MS measurements. We acknowledge the cooperation and research relationships from communities of Mokgola, Lekubu (Northwest Province), Ntshatshongo and Krwakrwa (Eastern Cape Province), South Africa. Financial assistance from the Department of Science and Innovation (IK-based Technology Innovation (DSI)—DST Contract numbers (DST/CON 0075/2018) and the Department of Agriculture Land Reform and Rural Development (DALRRD) (Contract number 1 047 M4602) is kindly acknowledged. We are also grateful to FONDECYT Project 1210076 (ANID, Chile).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| CE | Catequin equivalent |
| DMAC | dimethylamino cinnamaldehyde |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| FRAP | ferric reducing antioxidant power |
| GAE | Gallic acid equivalent |
| HPLC-DAD-MS/MS | high performance liquid chromatography coupled with diode-array detection and tandem mass spectrometry |
| ORAC | oxygen radical absorbance capacity |
| PAC | total proanthocyanidin |
| PEI | phenolic-enriched infusion |
| TE | Trolox equivalent |
| TEAC | Trolox equivalent antioxidant capacity |
| TF | total flavonoid |
| TP | total phenolic |
References
- Muller, C.J.F.; Joubert, E.; de Beer, D.; Sanderson, M.; Malherbe, C.J.; Fey, S.J.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine 2012, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Söhretoglu, D.; Sari, S. Flavonoids as alpha-glucosidase inhibitors: Mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Lim, J.; Ferruzzi, M.G.; Hamaker, B.R. Structural requirements of flavonoids for the selective inhibition of α-amylase versus α-glucosidase. Food Chem. 2022, 370, 130981. [Google Scholar] [CrossRef]
- Manousi, N.; Sarakatsianos, I.; Samanidou, V. Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants. In Engineering Tools in the Beverage Industry; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 283–314. [Google Scholar] [CrossRef]
- Matsabisa, M.G.; Bala, A.; Tripathy, S.; Digashu, M.M.; Rautenbach, F.; Dassarma, B.; Erhabor, J.O.; Braga, F.C.; Mukherjee, P.K.; Tang, M.; et al. Study on South African indigenous teas—Antioxidant potential, nutritional content, and hypoxia-induced cyclooxygenase inhibition on U87 MG cell line. Molecules 2022, 27, 3505. [Google Scholar] [CrossRef]
- Olivier, D.K.; Shikanga, E.A.; Combrinck, S.; Krause, R.W.M.; Regnier, T.; Dlaminia, T.P. Phenylethanoid glycosides from Lippia javanica. S. Afr. J. Bot. 2010, 76, 58–63. [Google Scholar] [CrossRef]
- Reid, A.; Oosthuizen, C.B.; Lall, N. In vitro antimycobacterial and adjuvant properties of two traditional South African teas, Aspalathus linearis (Burm.f.) R. Dahlgren and Lippia scaberrima Sond. S. Afr. J. Bot. 2020, 128, 257–263. [Google Scholar] [CrossRef]
- Compadre, C.M.; Pezzuto, J.M.; Kinghorn, A.D.; Kamath, S.K. Hernandulcin: An intensely sweet compound discovered by review of ancient literature. Science 1985, 227, 417–419. [Google Scholar] [CrossRef]
- Nina, N.; Theoduloz, C.; Paillán, H.; Jiménez-Aspee, F.; Márquez, K.; Schuster, K.; Becker, L.; Oellig, C.; Frank, J.; Schmeda-Hirschmann, G. Chemical profile and bioactivity of Chilean bean landraces (Phaseolus vulgaris L.). J. Funct. Foods 2023, 104, 105513. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Vieira, M.N.; Rodriguez-Werner, M.A.; Schmalfuss, E.; Winterhalter, P.; Schmeda-Hirschmann, G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in AGS cells. J. Funct. Foods 2016, 26, 11–26. [Google Scholar] [CrossRef]
- Ren, L.; Xue, X.; Zhang, F.; Wang, Y.; Liu, Y.; Li, C.; Liang, X. Studies of iridoid glycosides using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3039–3050. [Google Scholar] [CrossRef] [PubMed]
- Es-Safi, N.-E.; Kerhoas, L.; Ducrot, P.H. Fragmentation study of iridoid glucosides through positive and negative electrospray ionization, collision-induced dissociation and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.T.; Liu, H.; Wen, J.; Guorong Fan, G.; Chai, Y.; Wu, Y. Fragmentation study of iridoid glycosides including epimers by liquid chromatography-diode array detection/electrospray ionization mass spectrometry and its application in metabolic fingerprint analysis of Gardenia jasminoides. Rapid Commun. Mass Spectrom. 2010, 24, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Timóteo, P.; Karioti, A.; Leitão, S.G.; Vincieri, F.F.; Bilia, A.R. A validated HPLC method for the analysis of herbal teas from three chemotypes of Brazilian Lippia alba. Food Chem. 2015, 175, 366–373. [Google Scholar] [CrossRef]
- Cortés-Chitala, M.d.C.; Flores-Martínez, H.; Orozco-Ávila, I.; León-Campos, C.; Suárez-Jacobo, A.; Estarrón-Espinosa, M.; López-Muraira, I. Identification and quantification of phenolic compounds from Mexican oregano (Lippia graveolens HBK) hydroethanolic extracts and evaluation of its antioxidant capacity. Molecules 2021, 26, 702. [Google Scholar] [CrossRef]
- Ono, M.; Morinaga, H.; Masuoka, C.; Ikeda, T.; Okawa, M.; Kinjo, J.; Nohara, T. New bisabolane-type sesquiterpenes from the aerial parts of Lippia dulcis. Chem. Pharm. Bull. 2005, 53, 1175–1177. [Google Scholar] [CrossRef]
- Rastrelli, L.; Caceres, A.; Morales, C.; De Simone, F.; Aquino, R. Iridoids from Lippia graveolens. Phytochemistry 1998, 49, 1829–1832. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef]
- Hvattum, E.; Ekeberg, D. Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry. J. Mass Spectrom. 2003, 38, 43–49. [Google Scholar] [CrossRef]
- Vukics, V.; Guttman, A. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2010, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Funari, C.S.; Eugster, P.J.; Martel, S.; Carrupt, P.-A.; Wolfender, J.-L.; Silva, D.H.S. High resolution ultra high-pressure liquid chromatography–time-of-flight mass spectrometry dereplication strategy for the metabolite profiling of Brazilian Lippia species. J. Chromatogr. A 2012, 1259, 167–178. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, M.; Tonidandel, L.; Larcher, R.; Nicolini, G.; Dalla Vedova, A.; De Marchi, F.; Gardiman, M.; Giust, M.; Flamini, R. Identification of new flavonols in hybrid grapes by combined liquid chromatography-mass spectrometry approaches. Food Chem. 2014, 163, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Gil-Izquierdo, A.; Andrade, P.B.; Valentao, P.; Tomás-Barberán, F.A. Characterization of C-glycosyl flavones O-glycosylated by liquid Chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Xu, Q.; Li, R.; Shi, L.; Han, Y.; Zhu, Y.; Wu, G.; Qin, M. Chemical profiles and quality evaluation of Buddleja officinalis flowers by HPLC-DAD and HPLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 164, 283–295. [Google Scholar] [CrossRef]
- Sun, M.; Luo, Z.; Liu, Y.; Yang, R.; Lu, L.; Yu, G.; Ma, X.; Liu, A.; Guo, Y.; Zhao, H. Identification of the major components of Buddleja officinalis extract and their metabolites in rat urine by UHPLC-LTQ-Orbitrap. J. Food Sci. 2016, 81, H2587–H2596. [Google Scholar] [CrossRef]
- Marchetti, L.; Pellati, F.; Graziosi, R.; Brighenti, V.; Pinetti, D.; Bertelli, D. Identification and determination of bioactive phenylpropanoid glycosides of Aloysia polystachya (Griseb. et Moldenke) by HPLC-MS. J. Pharm. Biomed. Anal. 2019, 166, 364–370. [Google Scholar] [CrossRef]
- Guo, H.; Liu, A.-H.; Ye, M.; Yang, M.; Guo, D.-A. Characterization of phenolic compounds in the fruits of Forsythia suspensa by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 715–729. [Google Scholar] [CrossRef]
- Cao, X.; Qiao, J.; Wang, L.; Ye, X.; Zheng, L.; Jiang, N.; Mo, W. Screening of glycoside isomers in P. scrophulariiflora using ionic liquid-based ultrasonic-assisted extraction and ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 740–748. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Xing, S.; Tu, P.; Li, X. Identification of echinacoside metabolites produced by human intestinal bacteria using ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2015, 63, 6764–6771. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gómez-Alonso, S.; Pérez-Navarro, J.; Alarcón-Enos, J.; Pastene-Navarrete, E. Polyphenolic compounds extracted and purified from Buddleja globosa Hope (Buddlejaceae) leaves using natural deep eutectic solvents and centrifugal partition chromatography. Molecules 2021, 26, 2192. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 2009, 1216, 5391–5397. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Dragićević, M.; Stupar, A.; Uysal, A.; Şenkardes, I.; Sinan, K.I.; Picot-Allain, M.C.N.; Ak, G.; et al. UHPLC-LTQ OrbiTrap MS analysis and biological properties of Origanum vulgare subsp. viridulum obtained by different extraction methods. Ind. Crops Prod. 2020, 154, 112747. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Miyase, T.; Ueno, A.; Usmanghani, K. Sesquiterpene lactone glycosides and ionone derivative glycosides from Sonchus asper. Phytochemistry 1989, 28, 3399–3402. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Amoo, S.O.; de Kock, C.A.; Smith, P.J.; Van Standen, J. Antiplasmodial, acetylcholinesterase and alpha-glucosidase inhibitory and cytotoxicity properties of Buddleja saligna. S. Afr. J. Bot. 2014, 94, 6–8. [Google Scholar] [CrossRef][Green Version]
- Morikawa, T. Pharmaceutical food science: Search for bio-functional molecules obtained from natural resources to prevent and ameliorate lifestyle diseases. Chem. Pharm. Bull. 2023, 71, 756–765. [Google Scholar] [CrossRef]
- Khalaf, H.A.A.; Jasim, R.A.; Ibrahim, I.T. Verbascoside—A review of its antitumor activities. Pharm. Pharmacy 2021, 12, 109–126. [Google Scholar] [CrossRef]
- Galli, A.; Marciani, P.; Marku, A.; Ghislanzoni, S.; Bertuzzi, F.; Rossi, R.; Di Giancamillo, A.; Castagna, M.; Perego, C. Verbascoside protects pancreatic β-cells against ER-stress. Biomedicines 2020, 8, 582. [Google Scholar] [CrossRef]
- Xiao, Y.; Ren, Q.; Wu, L. The pharmacokinetic property and pharmacological activity of acteoside: A review. Biomed. Pharmacother. 2022, 153, 113296. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Amoah, S.K.S.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic acid -Pharmaceutical and clinical aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Dalar, A.; Konczak, I. Phenolic contents, antioxidant capacities and inhibitory activities against key metabolic syndrome relevant enzymes of herbal teas from Eastern Anatolia. Ind. Crops Prod. 2013, 44, 383–390. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, E.; Wei, Z.; Zheng, Y.; Yan, R.; Ma, X. Phytochemical analysis, cellular antioxidant and α-glucosidase inhibitory activities of various herb plant organs. Ind. Crops Prod. 2019, 141, 111771. [Google Scholar] [CrossRef]
- Shai, L.J.; Masoko, P.; Mokgotho, M.P.; Magano, S.R.; Mogale, A.M.; Boaduo, N.; Eloff, J.N. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S. Afr. J. Bot. 2010, 76, 465–470. [Google Scholar] [CrossRef]
- Twilley, D.; Moodley, D.; Rolfes, H.; Moodley, I.; McGaw, L.J.; Madikizela, B.; Summers, B.; Raaff, L.-A.; Lategan, M.; Kgatuke, L.; et al. Ethanolic extracts of South African plants, Buddleja saligna Willd. and Helichrysum odoratissimum (L.) Sweet, as multifunctional ingredients in sunscreen formulations. S. Afr. J. Bot. 2020, 137, 171–182. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Chukwuma, C.I.; Matsabisa, M.G.; Salau, V.F.; Koorbanally, N.A.; Islam, M.S. Buddleja saligna Willd (Loganiaceae) inhibits angiotensin-converting enzyme activity in oxidative cardiopathy with concomitant modulation of nucleotide hydrolyzing enzymatic activities and dysregulated lipid metabolic pathways. J. Ethnopharmacol. 2020, 248, 112358. [Google Scholar] [CrossRef]
- Singh, A.; Venugopala, K.N.; Khedr, M.A.; Pillay, M.; Nwaeze, K.U.; Coovadia, Y.; Shode, F.; Odhav, B. Antimycobacterial, docking and molecular dynamic studies of pentacyclic triterpenes from Buddleja saligna leaves. J. Biomol. Struct. Dyn. 2016, 35, 2654–2664. [Google Scholar] [CrossRef]
- Shikanga, E.A.; Combrinck, S.; Regnier, T. South African Lippia herbal infusions: Total phenolic content, antioxidant and antibacterial activities. S. Afr. J. Bot. 2010, 76, 567–571. [Google Scholar] [CrossRef]
- Ruas, N.R.; Pereira, A.C.; Pereira, L.L.S.; Germano, C.M.; da Cunha, E.F.F.; de Carvalho, A.A.; Lameira, O.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Inhibition of α-glycosidase by Lippia dulcis Trevir. (Verbenaceae) Preparations, quantification of verbascoside, and study of its molecular docking. Chem. Biodiversity 2023, 20, e202200760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. 2014, 62, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dong, Y.; Zhao, H.; Wen, L.; Yang, B.; Zhao, M. Comparative evaluation of rosmarinic acid, methyl rosmarinate and pedalitin isolated from Rabdosia serra (Maxim.) Hara as inhibitors of tyrosinase and α-glucosidase. Food Chem. 2011, 129, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Tshiyoyo, K.S.; Bester, M.J.; Serem, J.C.; Apostolides, Z. In-silico reverse docking and in-vitro studies identified curcumin, 18α-glycyrrhetinic acid, rosmarinic acid, and quercetin as inhibitors of α-glucosidase and pancreatic α-amylase and lipid accumulation in HepG2 cells, important type 2 diabetes targets. J. Mol. Struct. 2022, 1266, 133492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).