Chemical Profiling, Enzyme Inhibitory Activity and Antioxidant Capacity of South African Herbal Teas: Buddleja saligna, Lippia javanica, L. scaberrima and Phyla dulcis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herbal Teas
2.2. Extraction of Bioactive Compounds
2.3. Total Phenolic, Total Flavonoid and Total Procyanidin Content
2.4. Antioxidant Capacity Assays
2.5. Enzyme Inhibition Studies
2.6. NMR Profiles
2.7. HPLC-DAD Analyses
2.8. HPLC-MS/MS Analyses
2.9. Statistical Analyses
3. Results
3.1. Extraction Yields
3.2. Phenolics and Antioxidant Capacity
3.3. Enzyme Inhibition
3.4. NMR Analysis
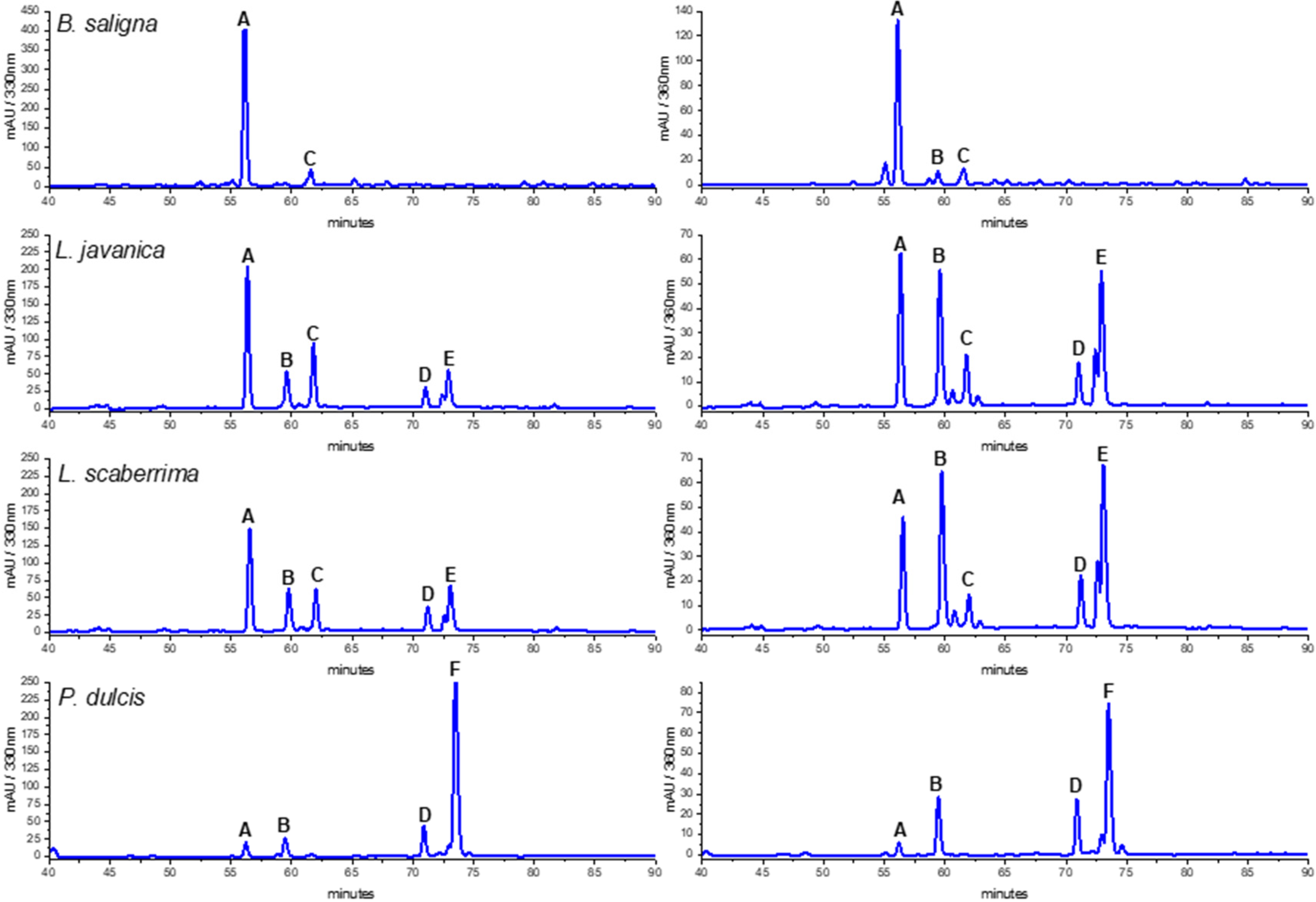
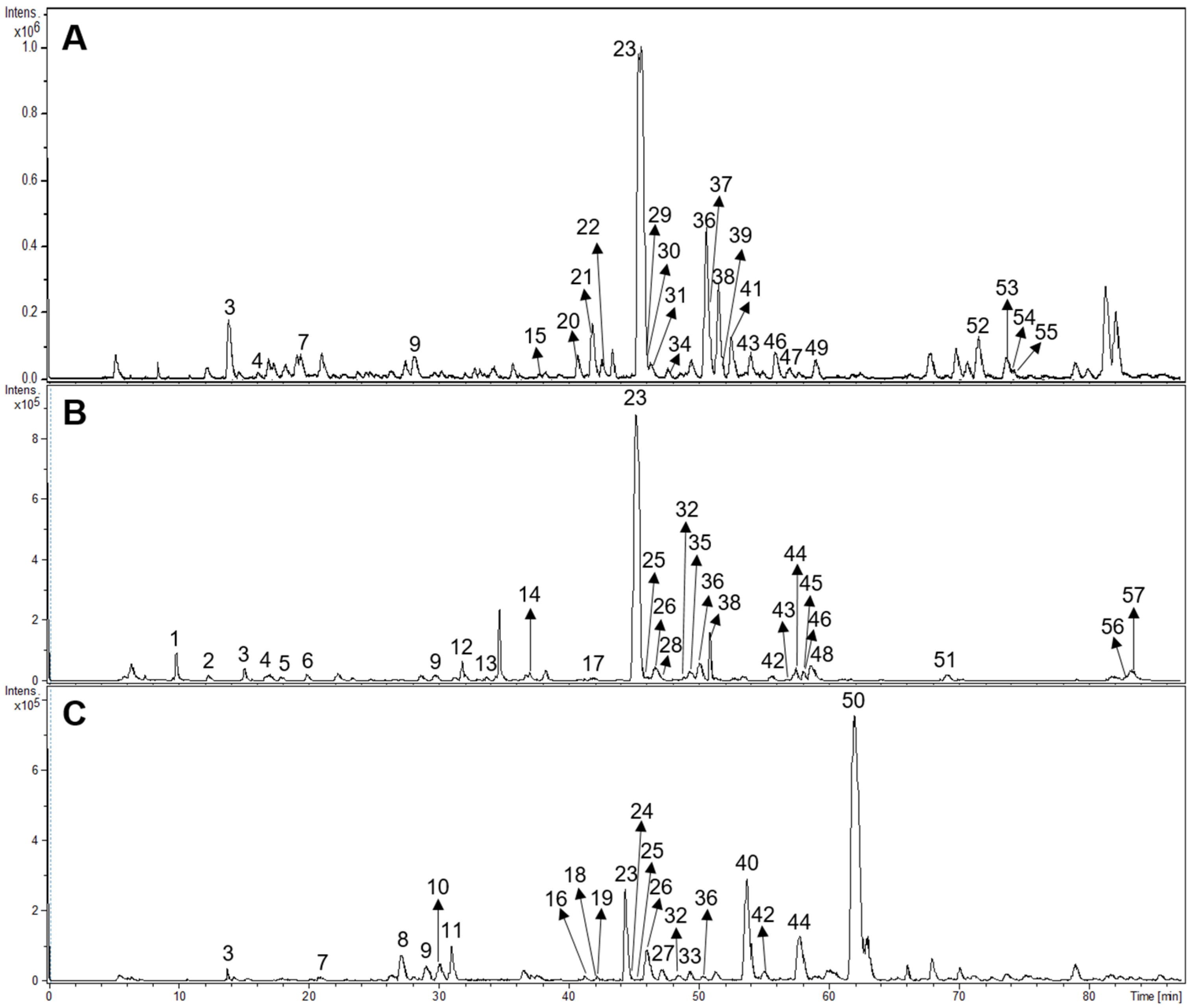
3.5. HPLC-DAD Profiles
3.6. HPLC-MS/MS Analyses
3.6.1. Iridoids
3.6.2. Flavonoids
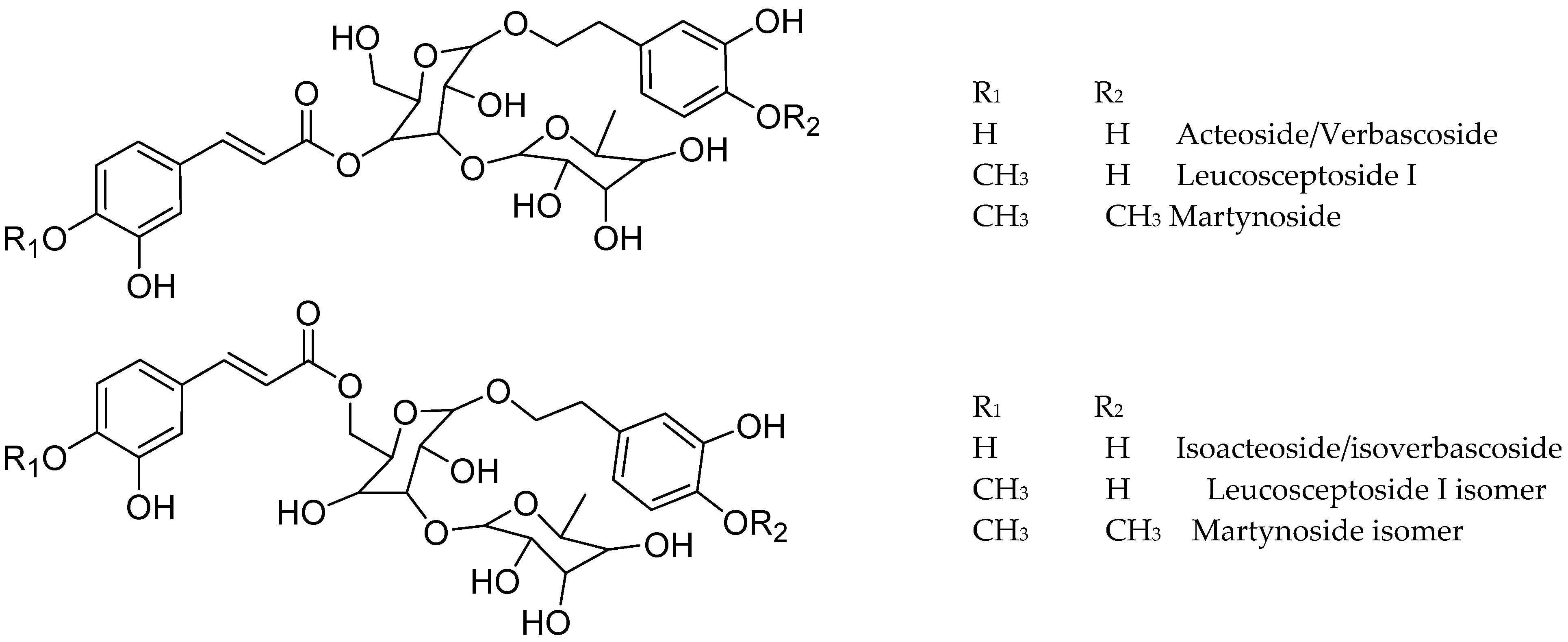
3.6.3. Phenylpropanoid Glycosides
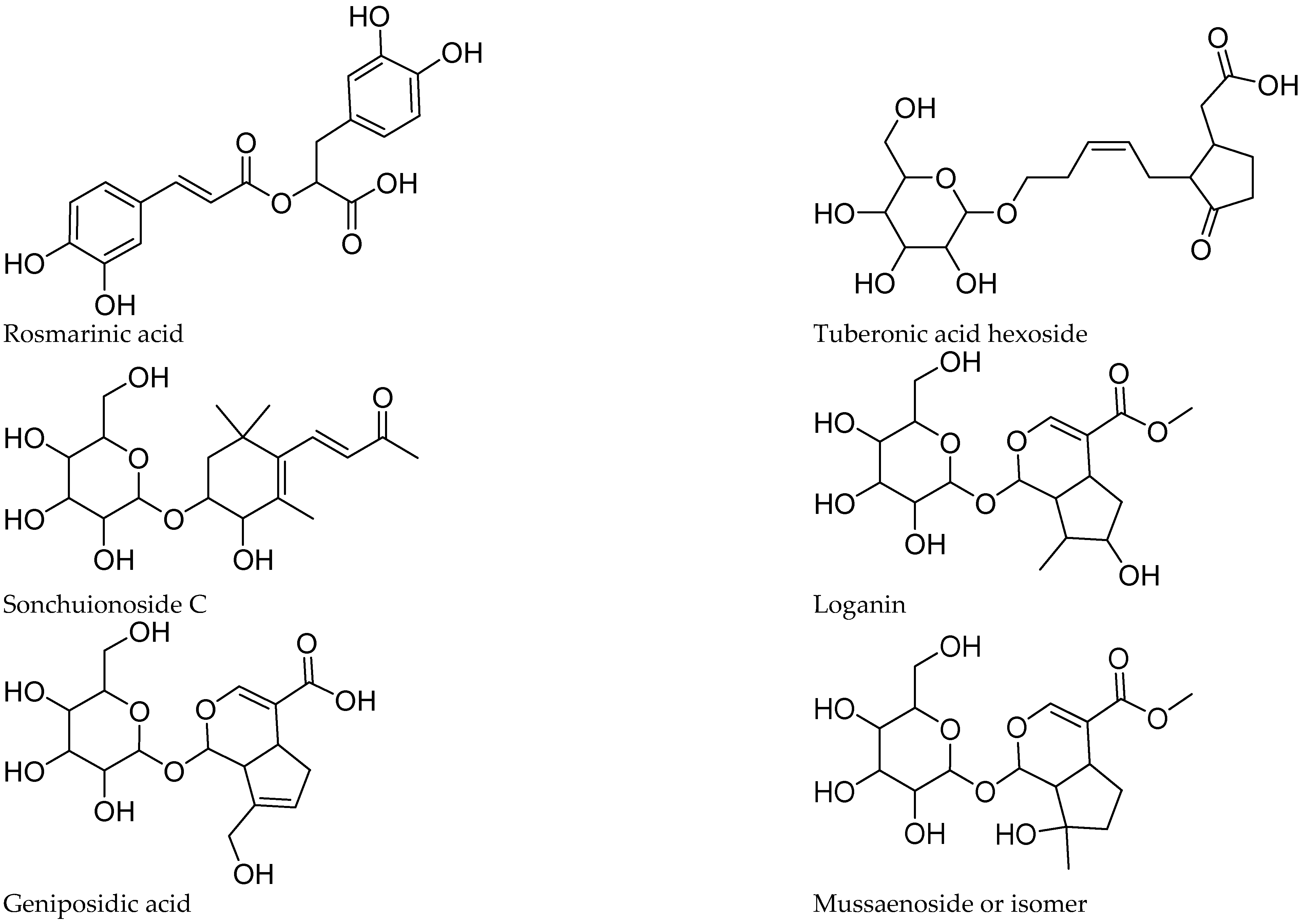
3.6.4. Caffeic Acid Esters
3.6.5. Other Compounds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| CE | Catequin equivalent |
| DMAC | dimethylamino cinnamaldehyde |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| FRAP | ferric reducing antioxidant power |
| GAE | Gallic acid equivalent |
| HPLC-DAD-MS/MS | high performance liquid chromatography coupled with diode-array detection and tandem mass spectrometry |
| ORAC | oxygen radical absorbance capacity |
| PAC | total proanthocyanidin |
| PEI | phenolic-enriched infusion |
| TE | Trolox equivalent |
| TEAC | Trolox equivalent antioxidant capacity |
| TF | total flavonoid |
| TP | total phenolic |
References
- Muller, C.J.F.; Joubert, E.; de Beer, D.; Sanderson, M.; Malherbe, C.J.; Fey, S.J.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine 2012, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Söhretoglu, D.; Sari, S. Flavonoids as alpha-glucosidase inhibitors: Mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Lim, J.; Ferruzzi, M.G.; Hamaker, B.R. Structural requirements of flavonoids for the selective inhibition of α-amylase versus α-glucosidase. Food Chem. 2022, 370, 130981. [Google Scholar] [CrossRef]
- Manousi, N.; Sarakatsianos, I.; Samanidou, V. Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants. In Engineering Tools in the Beverage Industry; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 283–314. [Google Scholar] [CrossRef]
- Matsabisa, M.G.; Bala, A.; Tripathy, S.; Digashu, M.M.; Rautenbach, F.; Dassarma, B.; Erhabor, J.O.; Braga, F.C.; Mukherjee, P.K.; Tang, M.; et al. Study on South African indigenous teas—Antioxidant potential, nutritional content, and hypoxia-induced cyclooxygenase inhibition on U87 MG cell line. Molecules 2022, 27, 3505. [Google Scholar] [CrossRef]
- Olivier, D.K.; Shikanga, E.A.; Combrinck, S.; Krause, R.W.M.; Regnier, T.; Dlaminia, T.P. Phenylethanoid glycosides from Lippia javanica. S. Afr. J. Bot. 2010, 76, 58–63. [Google Scholar] [CrossRef]
- Reid, A.; Oosthuizen, C.B.; Lall, N. In vitro antimycobacterial and adjuvant properties of two traditional South African teas, Aspalathus linearis (Burm.f.) R. Dahlgren and Lippia scaberrima Sond. S. Afr. J. Bot. 2020, 128, 257–263. [Google Scholar] [CrossRef]
- Compadre, C.M.; Pezzuto, J.M.; Kinghorn, A.D.; Kamath, S.K. Hernandulcin: An intensely sweet compound discovered by review of ancient literature. Science 1985, 227, 417–419. [Google Scholar] [CrossRef]
- Nina, N.; Theoduloz, C.; Paillán, H.; Jiménez-Aspee, F.; Márquez, K.; Schuster, K.; Becker, L.; Oellig, C.; Frank, J.; Schmeda-Hirschmann, G. Chemical profile and bioactivity of Chilean bean landraces (Phaseolus vulgaris L.). J. Funct. Foods 2023, 104, 105513. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Vieira, M.N.; Rodriguez-Werner, M.A.; Schmalfuss, E.; Winterhalter, P.; Schmeda-Hirschmann, G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in AGS cells. J. Funct. Foods 2016, 26, 11–26. [Google Scholar] [CrossRef]
- Ren, L.; Xue, X.; Zhang, F.; Wang, Y.; Liu, Y.; Li, C.; Liang, X. Studies of iridoid glycosides using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3039–3050. [Google Scholar] [CrossRef] [PubMed]
- Es-Safi, N.-E.; Kerhoas, L.; Ducrot, P.H. Fragmentation study of iridoid glucosides through positive and negative electrospray ionization, collision-induced dissociation and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.T.; Liu, H.; Wen, J.; Guorong Fan, G.; Chai, Y.; Wu, Y. Fragmentation study of iridoid glycosides including epimers by liquid chromatography-diode array detection/electrospray ionization mass spectrometry and its application in metabolic fingerprint analysis of Gardenia jasminoides. Rapid Commun. Mass Spectrom. 2010, 24, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Timóteo, P.; Karioti, A.; Leitão, S.G.; Vincieri, F.F.; Bilia, A.R. A validated HPLC method for the analysis of herbal teas from three chemotypes of Brazilian Lippia alba. Food Chem. 2015, 175, 366–373. [Google Scholar] [CrossRef]
- Cortés-Chitala, M.d.C.; Flores-Martínez, H.; Orozco-Ávila, I.; León-Campos, C.; Suárez-Jacobo, A.; Estarrón-Espinosa, M.; López-Muraira, I. Identification and quantification of phenolic compounds from Mexican oregano (Lippia graveolens HBK) hydroethanolic extracts and evaluation of its antioxidant capacity. Molecules 2021, 26, 702. [Google Scholar] [CrossRef]
- Ono, M.; Morinaga, H.; Masuoka, C.; Ikeda, T.; Okawa, M.; Kinjo, J.; Nohara, T. New bisabolane-type sesquiterpenes from the aerial parts of Lippia dulcis. Chem. Pharm. Bull. 2005, 53, 1175–1177. [Google Scholar] [CrossRef]
- Rastrelli, L.; Caceres, A.; Morales, C.; De Simone, F.; Aquino, R. Iridoids from Lippia graveolens. Phytochemistry 1998, 49, 1829–1832. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef]
- Hvattum, E.; Ekeberg, D. Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry. J. Mass Spectrom. 2003, 38, 43–49. [Google Scholar] [CrossRef]
- Vukics, V.; Guttman, A. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2010, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Funari, C.S.; Eugster, P.J.; Martel, S.; Carrupt, P.-A.; Wolfender, J.-L.; Silva, D.H.S. High resolution ultra high-pressure liquid chromatography–time-of-flight mass spectrometry dereplication strategy for the metabolite profiling of Brazilian Lippia species. J. Chromatogr. A 2012, 1259, 167–178. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, M.; Tonidandel, L.; Larcher, R.; Nicolini, G.; Dalla Vedova, A.; De Marchi, F.; Gardiman, M.; Giust, M.; Flamini, R. Identification of new flavonols in hybrid grapes by combined liquid chromatography-mass spectrometry approaches. Food Chem. 2014, 163, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Gil-Izquierdo, A.; Andrade, P.B.; Valentao, P.; Tomás-Barberán, F.A. Characterization of C-glycosyl flavones O-glycosylated by liquid Chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Xu, Q.; Li, R.; Shi, L.; Han, Y.; Zhu, Y.; Wu, G.; Qin, M. Chemical profiles and quality evaluation of Buddleja officinalis flowers by HPLC-DAD and HPLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 164, 283–295. [Google Scholar] [CrossRef]
- Sun, M.; Luo, Z.; Liu, Y.; Yang, R.; Lu, L.; Yu, G.; Ma, X.; Liu, A.; Guo, Y.; Zhao, H. Identification of the major components of Buddleja officinalis extract and their metabolites in rat urine by UHPLC-LTQ-Orbitrap. J. Food Sci. 2016, 81, H2587–H2596. [Google Scholar] [CrossRef]
- Marchetti, L.; Pellati, F.; Graziosi, R.; Brighenti, V.; Pinetti, D.; Bertelli, D. Identification and determination of bioactive phenylpropanoid glycosides of Aloysia polystachya (Griseb. et Moldenke) by HPLC-MS. J. Pharm. Biomed. Anal. 2019, 166, 364–370. [Google Scholar] [CrossRef]
- Guo, H.; Liu, A.-H.; Ye, M.; Yang, M.; Guo, D.-A. Characterization of phenolic compounds in the fruits of Forsythia suspensa by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 715–729. [Google Scholar] [CrossRef]
- Cao, X.; Qiao, J.; Wang, L.; Ye, X.; Zheng, L.; Jiang, N.; Mo, W. Screening of glycoside isomers in P. scrophulariiflora using ionic liquid-based ultrasonic-assisted extraction and ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 740–748. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Xing, S.; Tu, P.; Li, X. Identification of echinacoside metabolites produced by human intestinal bacteria using ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2015, 63, 6764–6771. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gómez-Alonso, S.; Pérez-Navarro, J.; Alarcón-Enos, J.; Pastene-Navarrete, E. Polyphenolic compounds extracted and purified from Buddleja globosa Hope (Buddlejaceae) leaves using natural deep eutectic solvents and centrifugal partition chromatography. Molecules 2021, 26, 2192. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 2009, 1216, 5391–5397. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Dragićević, M.; Stupar, A.; Uysal, A.; Şenkardes, I.; Sinan, K.I.; Picot-Allain, M.C.N.; Ak, G.; et al. UHPLC-LTQ OrbiTrap MS analysis and biological properties of Origanum vulgare subsp. viridulum obtained by different extraction methods. Ind. Crops Prod. 2020, 154, 112747. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Miyase, T.; Ueno, A.; Usmanghani, K. Sesquiterpene lactone glycosides and ionone derivative glycosides from Sonchus asper. Phytochemistry 1989, 28, 3399–3402. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Amoo, S.O.; de Kock, C.A.; Smith, P.J.; Van Standen, J. Antiplasmodial, acetylcholinesterase and alpha-glucosidase inhibitory and cytotoxicity properties of Buddleja saligna. S. Afr. J. Bot. 2014, 94, 6–8. [Google Scholar] [CrossRef]
- Morikawa, T. Pharmaceutical food science: Search for bio-functional molecules obtained from natural resources to prevent and ameliorate lifestyle diseases. Chem. Pharm. Bull. 2023, 71, 756–765. [Google Scholar] [CrossRef]
- Khalaf, H.A.A.; Jasim, R.A.; Ibrahim, I.T. Verbascoside—A review of its antitumor activities. Pharm. Pharmacy 2021, 12, 109–126. [Google Scholar] [CrossRef]
- Galli, A.; Marciani, P.; Marku, A.; Ghislanzoni, S.; Bertuzzi, F.; Rossi, R.; Di Giancamillo, A.; Castagna, M.; Perego, C. Verbascoside protects pancreatic β-cells against ER-stress. Biomedicines 2020, 8, 582. [Google Scholar] [CrossRef]
- Xiao, Y.; Ren, Q.; Wu, L. The pharmacokinetic property and pharmacological activity of acteoside: A review. Biomed. Pharmacother. 2022, 153, 113296. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Amoah, S.K.S.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic acid -Pharmaceutical and clinical aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Dalar, A.; Konczak, I. Phenolic contents, antioxidant capacities and inhibitory activities against key metabolic syndrome relevant enzymes of herbal teas from Eastern Anatolia. Ind. Crops Prod. 2013, 44, 383–390. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, E.; Wei, Z.; Zheng, Y.; Yan, R.; Ma, X. Phytochemical analysis, cellular antioxidant and α-glucosidase inhibitory activities of various herb plant organs. Ind. Crops Prod. 2019, 141, 111771. [Google Scholar] [CrossRef]
- Shai, L.J.; Masoko, P.; Mokgotho, M.P.; Magano, S.R.; Mogale, A.M.; Boaduo, N.; Eloff, J.N. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S. Afr. J. Bot. 2010, 76, 465–470. [Google Scholar] [CrossRef]
- Twilley, D.; Moodley, D.; Rolfes, H.; Moodley, I.; McGaw, L.J.; Madikizela, B.; Summers, B.; Raaff, L.-A.; Lategan, M.; Kgatuke, L.; et al. Ethanolic extracts of South African plants, Buddleja saligna Willd. and Helichrysum odoratissimum (L.) Sweet, as multifunctional ingredients in sunscreen formulations. S. Afr. J. Bot. 2020, 137, 171–182. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Chukwuma, C.I.; Matsabisa, M.G.; Salau, V.F.; Koorbanally, N.A.; Islam, M.S. Buddleja saligna Willd (Loganiaceae) inhibits angiotensin-converting enzyme activity in oxidative cardiopathy with concomitant modulation of nucleotide hydrolyzing enzymatic activities and dysregulated lipid metabolic pathways. J. Ethnopharmacol. 2020, 248, 112358. [Google Scholar] [CrossRef]
- Singh, A.; Venugopala, K.N.; Khedr, M.A.; Pillay, M.; Nwaeze, K.U.; Coovadia, Y.; Shode, F.; Odhav, B. Antimycobacterial, docking and molecular dynamic studies of pentacyclic triterpenes from Buddleja saligna leaves. J. Biomol. Struct. Dyn. 2016, 35, 2654–2664. [Google Scholar] [CrossRef]
- Shikanga, E.A.; Combrinck, S.; Regnier, T. South African Lippia herbal infusions: Total phenolic content, antioxidant and antibacterial activities. S. Afr. J. Bot. 2010, 76, 567–571. [Google Scholar] [CrossRef]
- Ruas, N.R.; Pereira, A.C.; Pereira, L.L.S.; Germano, C.M.; da Cunha, E.F.F.; de Carvalho, A.A.; Lameira, O.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Inhibition of α-glycosidase by Lippia dulcis Trevir. (Verbenaceae) Preparations, quantification of verbascoside, and study of its molecular docking. Chem. Biodiversity 2023, 20, e202200760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. 2014, 62, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dong, Y.; Zhao, H.; Wen, L.; Yang, B.; Zhao, M. Comparative evaluation of rosmarinic acid, methyl rosmarinate and pedalitin isolated from Rabdosia serra (Maxim.) Hara as inhibitors of tyrosinase and α-glucosidase. Food Chem. 2011, 129, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Tshiyoyo, K.S.; Bester, M.J.; Serem, J.C.; Apostolides, Z. In-silico reverse docking and in-vitro studies identified curcumin, 18α-glycyrrhetinic acid, rosmarinic acid, and quercetin as inhibitors of α-glucosidase and pancreatic α-amylase and lipid accumulation in HepG2 cells, important type 2 diabetes targets. J. Mol. Struct. 2022, 1266, 133492. [Google Scholar] [CrossRef]

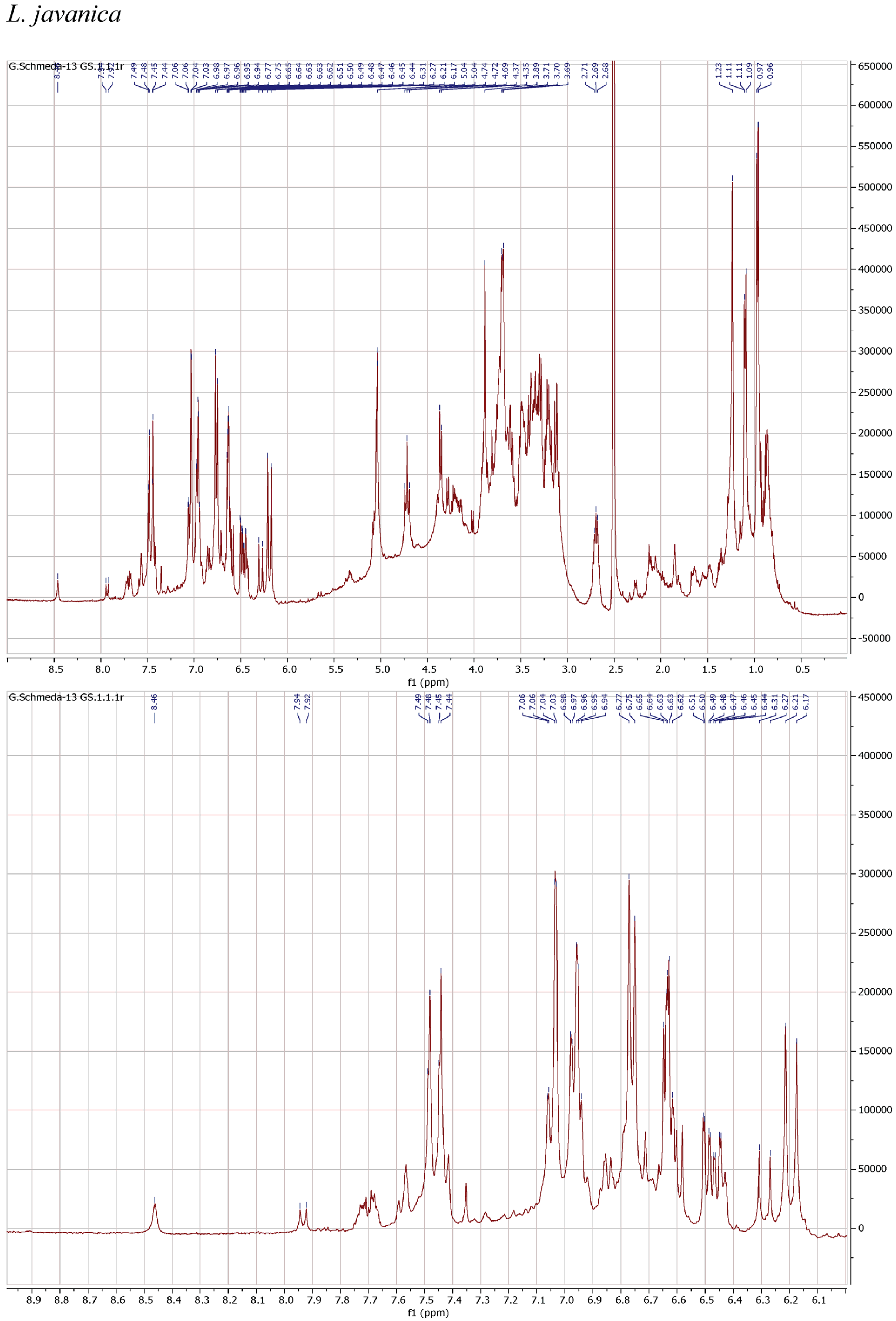
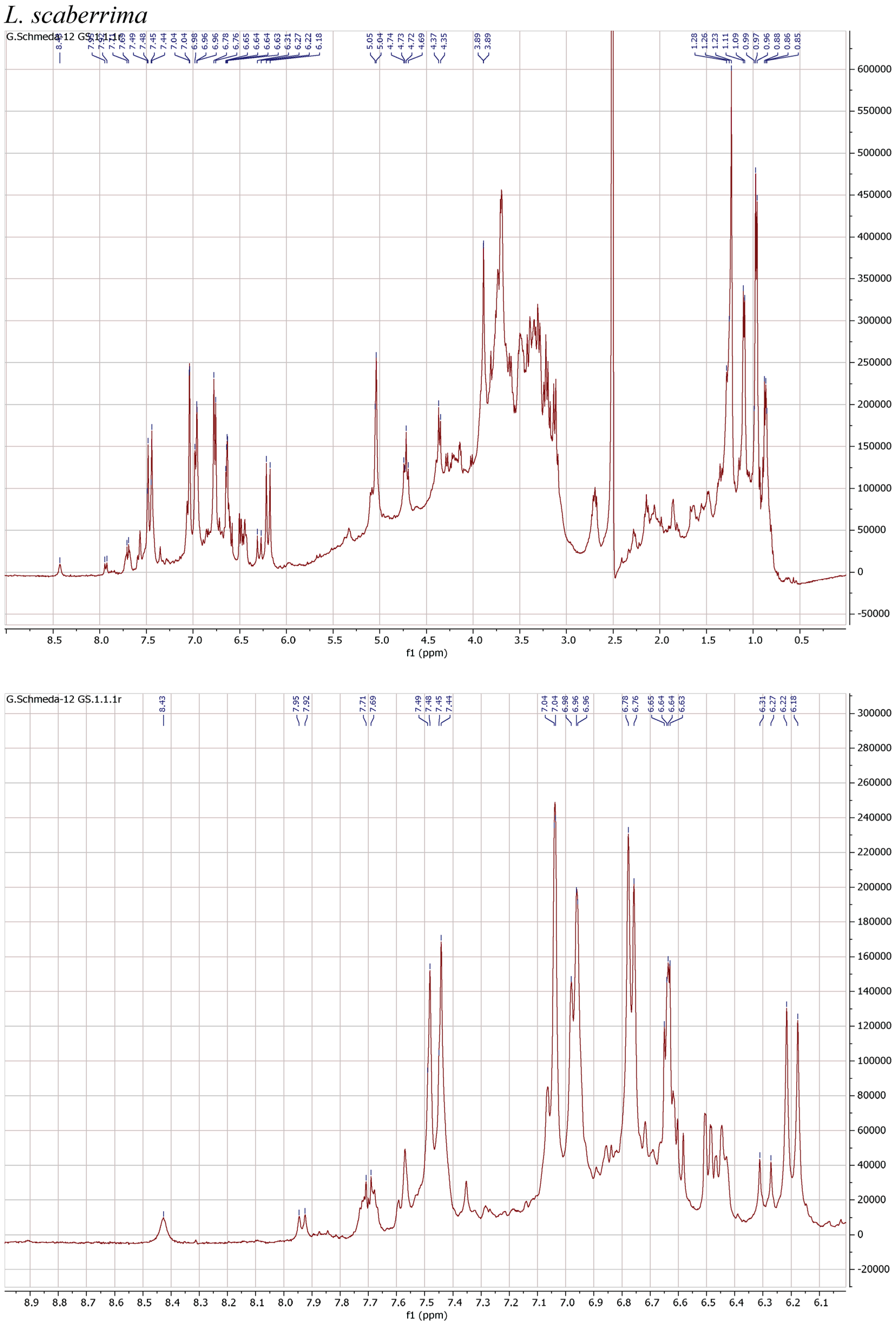
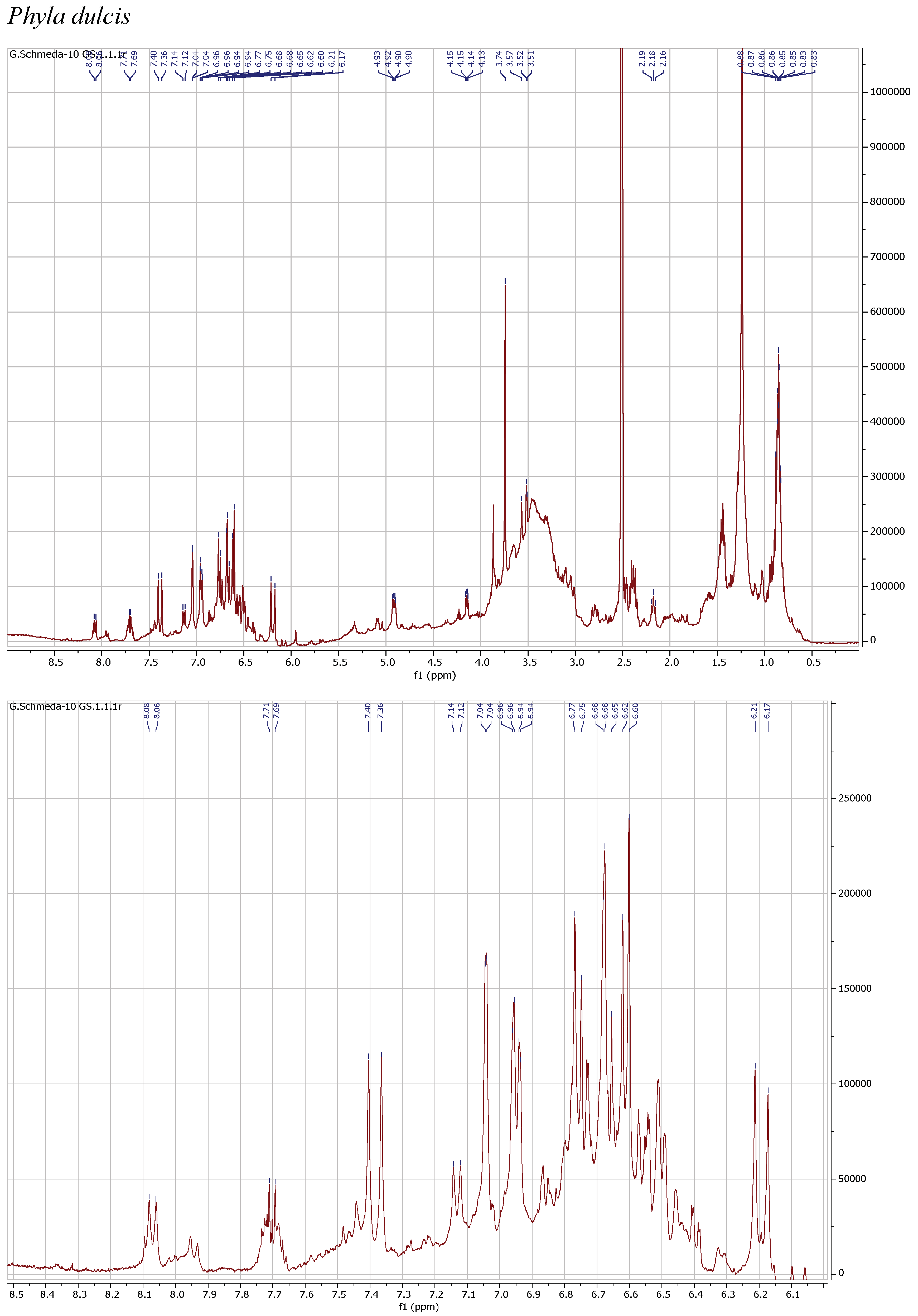
| Scientific Name | Plant Family and Plant Part | Trade Name | % Yield MeOH Extract | % Yield PEI of Infusion |
|---|---|---|---|---|
| Buddleja saligna Willd. | Scrophulariaceae, L | Gancair | 23.11 | 5.01 |
| Lippia javanica (Burm.f.) Spreng | Verbenaceae, L | Zinibar | 6.82 | 4.91 |
| Lippia scaberrima Sond. | Verbenaceae, L | Mosukujane | 6.16 | 4.07 |
| Phyla dulcis (Trevir.) Moldenke | Verbenaceae, L | Haw Haw | 12.83 | 3.95 |
| Plant Species and Extract Type | TP (g GAE/100 g Extract) | TF (g CE/100 g Extract) | TPA (g CE/100 g Extract | DPPH (SC50, µg/mL) | FRAP (µmol TE/g Extract) | TEAC (µM TE/g Extract) | ORAC (µmol TE/g Extract) |
|---|---|---|---|---|---|---|---|
| MeOH extract | |||||||
| Buddleja saligna | 9.71 ± 0.11 a | 0.79 ± 0.03 a | not detected | 23.62 ± 0.39 a | 221.31 ± 6.37 a | 766.92 ± 30.71 a | 197.14 ± 16.76 a |
| Lippia javanica | 11.66 ± 0.07 b | 0.97 ± 0.05 a,b | not detected | 18.28 ± 0.48 b | 267.28 ± 9.57 b | 1091.43 ± 45.50 b | 182.55 ± 6.20 a |
| Lippia scaberrima | 10.79 ± 0.12 c | 0.99 ± 0.04 b | not detected | 22.42 ± 0.80 a | 260.92 ± 16.21 b | 806.97 ± 32.50 a | 271.17 ± 10.15 b |
| Phyla dulcis | 9.43 ± 0.10 d | 2.86± 0.18 c | 0.48 ± 0.03 | 26.70 ± 1.01 c | 337.31 ± 14.44 c | 1019.69 ± 40.03 b | 192.33 ± 13.58 a |
| PEI | |||||||
| Buddleja saligna | 29.29 ± 0.61 a | 13.82 ± 0.39 a | not detected | 5.16 ± 0.10 a | 1277.18 ± 60.17 a | 2214.17 ± 114.90 a | 909.49 ± 78.49 a |
| Lippia javanica | 17.95 ± 0.03 b | 12.13 ± 0.58 b,d | not detected | 5.97 ± 0.05 b | 1028.90 ± 24.10 b | 1609.18 ± 83.01 b | 518.51 ± 17.58 b |
| Lippia scaberrima | 23.84 ± 0.41 c | 22.94 ± 0.04 c | 0.31 ± 0.01 | 7.00 ± 0.26 c | 977.88 ± 55.62 b | 1528.75 ± 76.69 b | 423.92 ± 28.62 b |
| Phyla dulcis | 17.69 ± 0.17 b | 13.01 ± 0.15 a,d | 2.81 ± 0.16 | 5.77 ± 0.20 b | 1805.56 ± 38.90 c | 2025.03 ± 104.81 a | 1037.81 ± 12.48 c |
| Quercetin # | - | - | - | 8.05 ± 0.41 | 1090.23 ± 17.45 | 8180.66 ± 20.88 | 23374.06 ± 897.39 |
| Sample | α-Glucosidase (IC50, µg/mL) | α-Amylase (% at 100 µg/mL or IC50, µg/mL) | Lipase (% at 50 µg/mL or IC50, µg/mL) |
|---|---|---|---|
| MeOH | |||
| Buddleja saligna | 0.47 ± 0.03 a | inactive | inactive |
| Lippia javanica | 0.47 ± 0.02 a | inactive | inactive |
| Lippia scaberrima | 0.49 ± 0.02 a | 0.47 ± 0.00% | inactive |
| Phyla dulcis | 0.50 ± 0.03 a | inactive | inactive |
| PEI | |||
| Buddleja saligna | 0.21 ± 0.02 a | 23.14 ± 1.96% | 7.25 ± 0.38% |
| Lippia javanica | 0.43 ± 0.01 b | 34.27 ± 2.04 | 6.87 ± 0.65% |
| Lippia scaberrima | 0.84 ± 0.13 c | 28.61 ± 1.63% | inactive |
| Phyla dulcis | 0.13 ± 0.06 a | 23.03 ± 2.01% | inactive |
| Acarbose # | 137.73 ± 1.31 | 28.48 ± 0.29 | - |
| Orlistat # | - | - | 0.04 ± 0.00 |
| Peak | Rt (min) | UV Maxima (nm) | Identification | Sample |
|---|---|---|---|---|
| A | 56.2 | 330, 288sh, 245, 219sh | Verbascoside | B. saligna, L. javanica, L. scaberrima, P. dulcis |
| B | 59.5 | 348, 268sh, 254 | Quercetin-3-O-glucoside | L. javanica, L. scaberrima, P. dulcis |
| C | 62.0 | 326, 288sh, 245sh | Isoverbascoside | B. saligna, L. javanica, L. scaberrima |
| D | 71.0 | 337, 267 | Flavonoid | L. javanica, L. scaberrima, P. dulcis |
| E | 73.1 | 346, 268sh, 252 | Quercetin derivative | L. javanica, L. scaberrima |
| F | 73.5 | 328, 288sh, 244sh | Rosmarinic acid | P. dulcis |
| Peak | Rt (Min) | UV Max | [M-H]− Measured | Molecular Formula | [M-H]− Theoretical | Error (ppm) | MS/MS | Tentative Identification | B. saligna (Gancair) | L. scaberrima (Mosukujane) | P. dulcis (Haw Haw) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9.9 | 389.1086 | C16H21O11 | 389.1089 | 0.77 | 389.1072(100), 227.0588(16), 209.0441(40), 191.0352 (55) | Theveside | X | |||
| 2 | 12.5 | 373.1132 | C16H21O10 | 373.1140 | 2.14 | 211.0643 (84), 167.0732 (54), 123.0457 (100) | Geniposidic acid | X | |||
| 3 | 15.3 | 461.1659 | C20H29O12 | 461.1664 | 1.08 | 461.1678 (100), 315.1027 (4), 113.0244 (21) | Decaffeoylverbascoside | X | X | X * | |
| 4 | 17.1 | 487.1475 | C21H27O13 | 487.1457 | −3.69 | 179.0366 (100), 161.0253 (27), 135.0451 (27) | Cistanoside F | X | X | ||
| 5 | 18.1 | 359.0987 | C15H19O10 | 359.0984 | −0.83 | 197.0471 (100) | Syringic acid hexoside | X | |||
| 6 | 20.1 | 451.1473 | C18H27O13 | 451.1457 | −3.54 | 405.1410 (100), 243.0877 (80) | Hydroxyloganin isomer [M+HCOOH]− | X | |||
| 7 | 21.1 | 341.0872 | C15H17O9 | 341.0878 | 1.75 | 179.0355(100) | Caffeoyl hexoside | X | X | ||
| 8 | 27.2 | 332 | 593.1503 | C27H29O15 | 593.1512 | 1.51 | 593.1473 (100), 383.0749 (61), 353.0655 (98) | Apigenin 6,8-di-C-hexoside | X | ||
| 9 | 29.0 | 387.1652 | C18H27O9 | 387.1660 | 2.06 | 387.1657 (100), 207.1038 (13) | Tuberonic acid hexoside | X | X | X | |
| 10 | 30.1 | 431.1920 | C20H31O10C19H29O8 | 431.1922 | 0.46 | 385.1851 (100) [M-H]+ (C19H29O8), 223.1339 (50), 153.0922 (42) | Sonchuionoside C [M+HCOOH]− | X | |||
| 11 | 31.1 | 323 | 179.0359 | C9H7O4 | 179.0350 | −5.02 | 135.0456 (100) | Caffeic acid | X | ||
| 12 | 31.9 | 435.1523 | C18H27O12 | 435.1508 | −3.44 | 389.1452 (16) [M-H]+ (C17H25O10), 227.0931 (100), 101.0268 (37) | Loganin [M+HCOOH]- | X | |||
| 13 | 33.7 | 639.1953 | C29H35O16 | 639.1930 | −3.59 | 161.0250 (100) | Hydroxy acteoside | X | |||
| 14 | 37.0 | 389.1089 | C16H21O11 | 389.1089 | 0.20 | 227.0905 (24), 161.0101(100) | Mussaenoside | X | |||
| 15 | 39.1 | 609.1417 | C27H29O16 | 609.1461 | 7.22 | 447.0837 (56), 285.0387 (100), 284.0291(14) | Kaempferol 3,7-di-O-hexoside | X | |||
| 16 | 41.3 | 609.1440 | C27H29O16 | 609.1461 | 3.45 | 300.0289 (100), 301.0331(35), 271.0235 (38), 179.0012 (16) | Rutin (Quercetin 3-O-rhamnoside glucoside) (rutinoside) | X | |||
| 17 | 41.7–42.5 | 653.2035 | C30H37O16 | 653.2087 | 7.96 | 416.8155 (11), 161.0248 (100) | Methoxy acteoside | X | |||
| 18 | 42.2 | 521.1292 | C24H25O13 | 521.1301 | 1.72 | 359.0749 (35), 161.0252 (100) | Rosmarinic acid hexoside 1 | X | |||
| 19 | 42.3 | 709.1979 | C32H37O18 | 709.1985 | 0.85 | 709.1967(100), 563.1320 (64), 430.0908 (28), 285.0407(30), 284.0311 (75), 255.0283(10) | Kaempferol 7-O-rhamnoside 3-O-rhamnoside pentoside | X | |||
| 20 | 42.2 | 326 | 667.1847 | C30H35O17 | 667.1879 | 4.49 | 505.1532(44), 179.0353(37), 161.0246(100) | Caffeoyl hebitol II | X | ||
| 21 | 43.2 | 349 | 609.1437 | C27H29O16 | 609.1461 | 3.93 | 300.0273 (100), 301.0335(40), 271.0237(11) | Quercetin 3-O-hexoside rhamnoside | X | ||
| 22 | 44.0 | 347 | 593.1480 | C27H29O15 | 593.1511 | 5.22 | 285.0394 (100), 284.0316(10) | Kaempferol 7-O-hexoside rhamnoside | X | ||
| 23 | 44.2–45.9 | 329 | 623.1998 | C29H35O15 | 623.1981 | −2.72 | 315.1091 (11), 161.0260 (100) | Verbascoside/Acteoside | X | X * | X * |
| 24 | 44.8 | 463.0875 | C21H19O12 | 463.0882 | 1.51 | 301.0397(73), 300.0259 (100), 271.0252 (99) | Quercetin 3-O-hexoside 1 | X | |||
| 25 | 45.2–46.0 | 447.0943 | C21H19O11 | 447.0933 | −2.23 | 285.0408 (100), 284.0305(68) | Kaempferol 3-O-hexoside 1 | X | X | ||
| 26 | 45.9–46.8 | 347 | 461.0724 | C21H17O12 | 461.0726 | 0.43 | 285.0414 (100), 284.0360(2) | Kaempferol 7-O-glucuronide | X | X | |
| 27 | 46.9–48.0 | 521.1311 | C24H25O13 | 521.1301 | −1.91 | 521.1288 (29), 359.0795 (53), 323.0780 (27), 179.0360 (34), 161.0251 (100) | Rosmarinic acid hexoside 2 | X | |||
| 28 | 47.2 | 491.0843 | C22H19O13 | 491.0831 | −2.44 | 491.0851 (51), 315.0519 (84), 300.0292 (100), 204.0475 (9), 147.0666 (11) | Rhamnetin/isorhamnetin glucuronide | X | |||
| 29 | 47.3 | 449.1102 | C21H21O11 | 449.1089 | −2.89 | 287.0596 (100), 135.0465 (46) | Eridictyol hexoside | X | |||
| 30 | 47.3 | 463.0889 | C21H19O12 | 463.0882 | −1.51 | 301.0350 (50), 300.0273 (99), 271.0259 (100) | Quercetin 3-O-hexoside 2 | X | |||
| 31 | 47.7 | 447.0928 | C21H19O11 | 447.0933 | 1.11 | 284.0350 (100), 285.0397(73) | Kaempferol 3-O-hexoside 2 | X | |||
| 32 | 48.5 | 477.1053 | C22H21O12 | 477.1039 | −2.93 | 300.0926 (100) | Hesperetin glucuronide | X | X | ||
| 33 | 49.3 | 623.1999 | C29H35O15 | 623.1981 | −2.89 | 315.1048 (18), 161.0261 (100) | Isoverbascoside/isoacteoside | X * | |||
| 34 | 49.1 | 477.1387 | C23H25O11 | 477.1402 | 3.14 | 179.0356 (7), 161.0258 (100) | Calceolarioside B | X | |||
| 35 | 49.3 | 507.1172 | C23H23O13 | 507.1144 | −5.52 | 345.0642 (22), 329.0340 (100), 314.01112 (42) | Syringetin hexoside | X | |||
| 36 | 49.9–51.8 | 623.1979 | C29H35O15 | 623.1981 | 0.32 | 461.1670 (1), 315.1099 (6), 161.0253 (100) | Verbascoside/Isoverbascoside isomer 1 | X | X * | X * | |
| 37 | 52.6 | 637.2135 | C30H37O15 | 637.2137 | −0.31 | 637.2138(100), 163.0428 (79), 145.0309 (37) | Leucosceptoside A related derivative | X | |||
| 38 | 52.8 | 623.1962 | C29H35O15 | 623.1981 | 3.04 | 461.1658 (5), 269.0461 (8), 161.0256 (100) | Verbascoside/Isoverbascoside isomer 2 | X | X | ||
| 39 | 52.8 | 653.2068 | C30H37O16 | 653.2087 | 2.90 | 429.1461 (14), 161.0281 (100) | Campneoside I | X | |||
| 40 | 53.7–54.2 | 282 | 719.1635 | C36H31O16 | 719.1618 | −2.36 | 719.1631 (40), 359.0773 (14), 197.0462 (41), 161.0247 (100) | Sagerinic acid isomer | X | ||
| 41 | 53.9 | 326 | 683.2199 | C31H39O17 | 683.2192 | −1.02 | 193.0518 (100) | Hydroxy hemiphroside A | X | ||
| 42 | 55.8 | 637.2182 | C30H37O15 | 637.2137 | −7.06 | 461.1648 (7), 315.1113 (8), 175.0418 (100) | Leucosceptoside A isomer 1 | X | X | ||
| 43 | 56.7–56.9 | 314 | 607.2047 | C29H35O14 | 607.2032 | −2.47 | 315.1052 (5), 163.0420 (15), 145.0311 (100) | Desoxyacteoside | X | X | |
| 44 | 57.5 | 336 | 445.0775 | C21H17O11 | 445.0776 | 0.22 | 445.0748 (7), 270.0502 (16), 269.0453 (100) | Apigenin 7-O-hexuronide | X | X | |
| 45 | 57.6–58.1 | 505.1015 | C23H21O13 | 505.0988 | −5.34 | 329.0663 (100), 314.0455 (88), 299.0208 (66), 148.0556 (23) | Tricin glucuronide | X | |||
| 46 | 57.8–58.3 | 637.2147 | C30H37O15 | 637.2137 | −1.56 | 315.0494 (19), 175.0419 (100) | Leucosceptoside A isomer 2 | X | X | ||
| 47 | 58.5 | 447.0933 | C21H19O11 | 447.0933 | 0.02 | 285.0420 (100), 284.0318 (1) | Kaempferol 7-O-hexoside | X | |||
| 48 | 58.6 | 349 | 475.0914 | C22H19O12 | 475.0882 | −6.73 | 299.0583 (62), 284.0349 (100), 113.0245 (9) | Chrysoeriol glucuronide | X | ||
| 49 | 60.6 | 667.2247 | C31H39O16 | 667.2243 | −0.59 | 487.1257 (17), 193.0510 (100) | Scroside B | X | |||
| 50 | 61.8–63.0 | 330 | 359.0781 | C18H15O8 | 359.0772 | −2.50 | 197.0466 (54), 161.0255 (100), 133.0301 (57) | Rosmarinic acid | X | ||
| 51 | 69.1 | 651.2319 | C31H39O15 | 651.2294 | −3.83 | 502.9688 (10), 329.1591 (14), 175.0413 (100) | Martynoside | X | |||
| 52 | 72.6 | 421.2054 | C19H33O10 | 421.2079 | 5.93 | 289.1652 (100), 130.9705 (93) | 1-Octen-3-yl hexoside pentoside | X | |||
| 53 | 74.8 | 651.2298 | C31H39O15 | 651.2294 | −0.61 | 476.8643 (19), 442.8783 (22), 175.0407 (100) | Martynoside isomer 1 | X | |||
| 54 | 75.2 | 491.1174 | C23H23O12 | 491.1195 | 4.27 | 329.0658 (100), 314.0437 (33), 299.0207 (77) | Quercetin dimethyl hexoside | X | |||
| 55 | 75.3 | 785.2282 | C38H41O18 | 785.2298 | 2.03 | 623.2197 (12), 461.1676 (22), 161.0236 (100) | Echinacoside | X | |||
| 56 | 82.7 | 315.0506 | C16H11O7 | 315.0510 | 1.26 | 300.0284 (100) | Quercetin methyl ether (Rhamnetin/isorhamnetin) | X | |||
| 57 | 82.9 | 345.0625 | C17H13O8 | 345.0616 | −2.80 | 330.0381 (100), 315.0162 (19) | Dimethylmyricetin | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nina, N.; Burgos-Edwards, A.; Theoduloz, C.; Tripathy, S.; Matsabisa, M.G.; Schmeda-Hirschmann, G. Chemical Profiling, Enzyme Inhibitory Activity and Antioxidant Capacity of South African Herbal Teas: Buddleja saligna, Lippia javanica, L. scaberrima and Phyla dulcis. Antioxidants 2024, 13, 1219. https://doi.org/10.3390/antiox13101219
Nina N, Burgos-Edwards A, Theoduloz C, Tripathy S, Matsabisa MG, Schmeda-Hirschmann G. Chemical Profiling, Enzyme Inhibitory Activity and Antioxidant Capacity of South African Herbal Teas: Buddleja saligna, Lippia javanica, L. scaberrima and Phyla dulcis. Antioxidants. 2024; 13(10):1219. https://doi.org/10.3390/antiox13101219
Chicago/Turabian StyleNina, Nélida, Alberto Burgos-Edwards, Cristina Theoduloz, Satyajit Tripathy, Motlalepula Gilbert Matsabisa, and Guillermo Schmeda-Hirschmann. 2024. "Chemical Profiling, Enzyme Inhibitory Activity and Antioxidant Capacity of South African Herbal Teas: Buddleja saligna, Lippia javanica, L. scaberrima and Phyla dulcis" Antioxidants 13, no. 10: 1219. https://doi.org/10.3390/antiox13101219
APA StyleNina, N., Burgos-Edwards, A., Theoduloz, C., Tripathy, S., Matsabisa, M. G., & Schmeda-Hirschmann, G. (2024). Chemical Profiling, Enzyme Inhibitory Activity and Antioxidant Capacity of South African Herbal Teas: Buddleja saligna, Lippia javanica, L. scaberrima and Phyla dulcis. Antioxidants, 13(10), 1219. https://doi.org/10.3390/antiox13101219






