Design, Synthesis, and Anti-Melanogenic Activity of 2-Mercaptomethylbenzo[d]imidazole Derivatives Serving as Tyrosinase Inhibitors: An In Silico, In Vitro, and In Vivo Exploration
Abstract
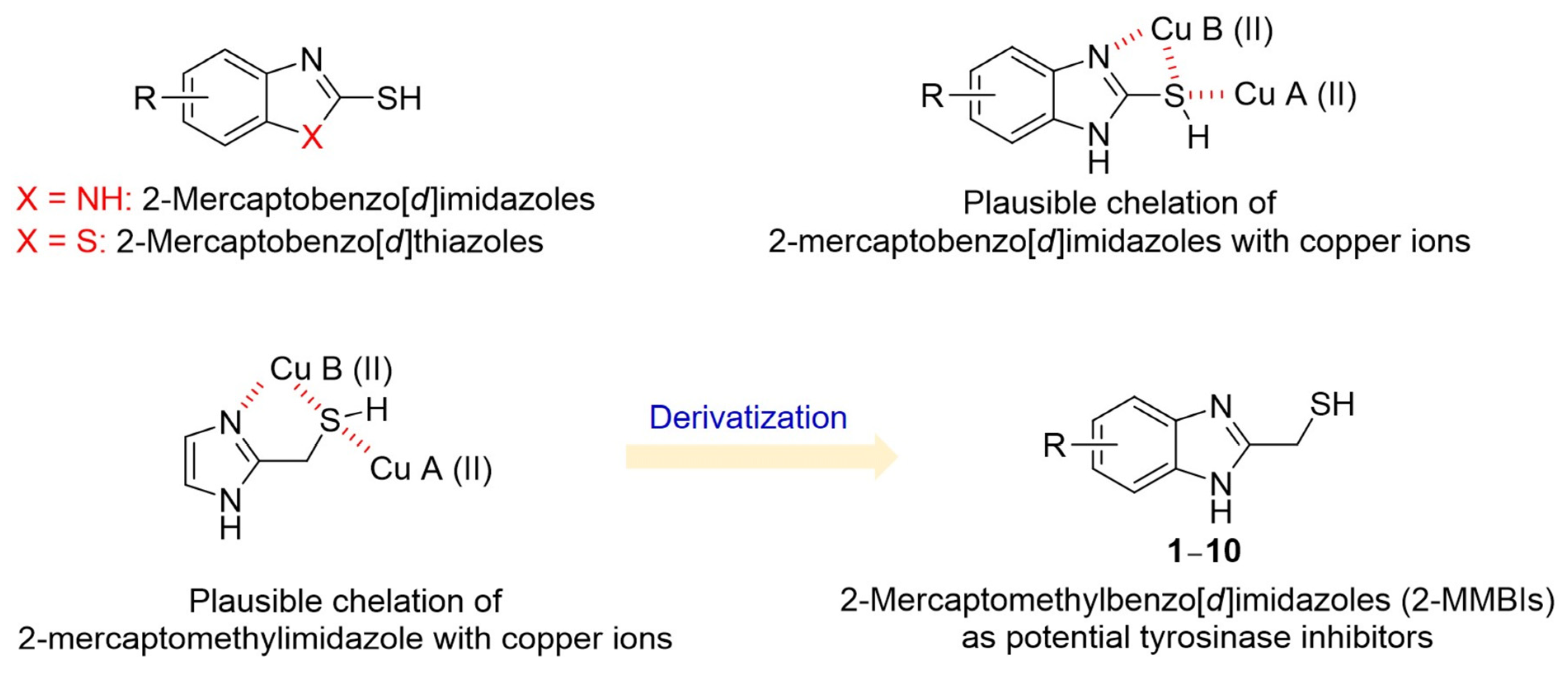
1. Introduction
2. Materials and Methods
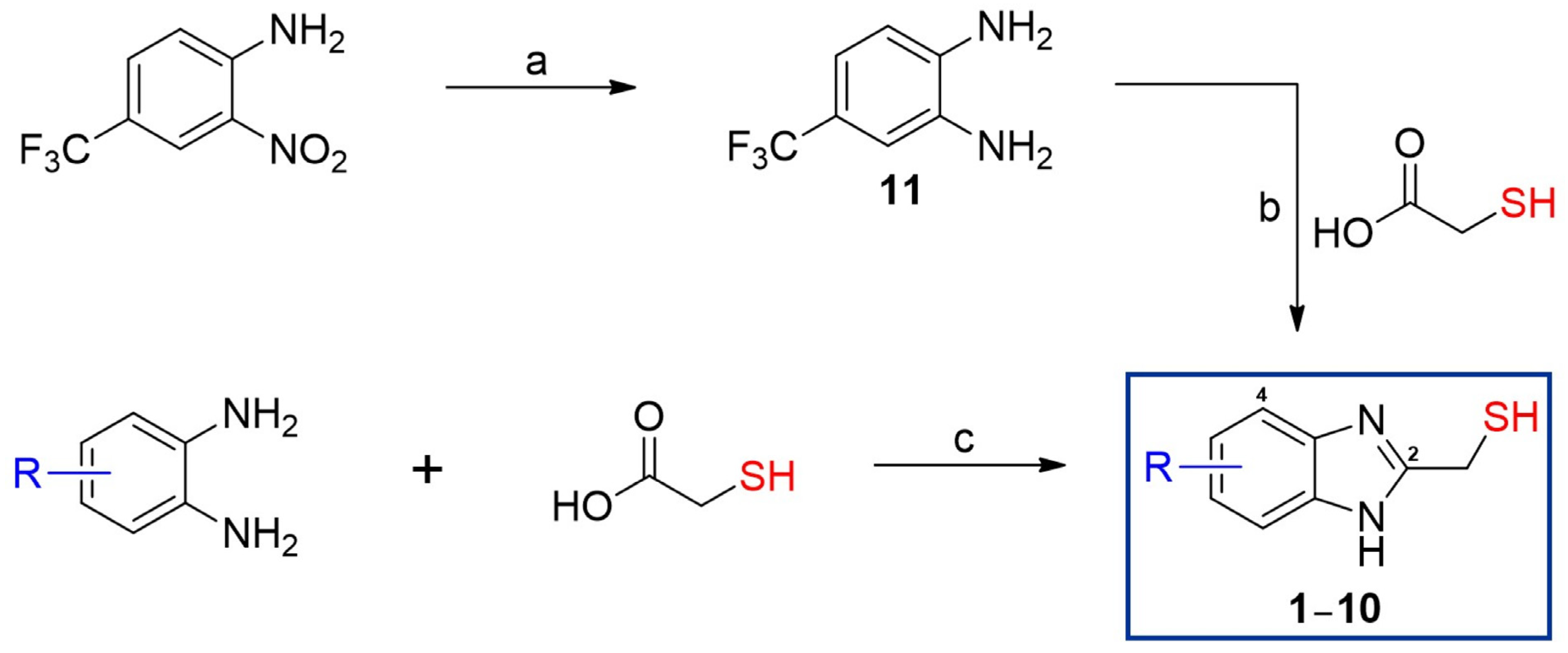
2.1. Synthesis
2.1.1. General Methods
2.1.2. Synthesis of 4-(Trifluoromethyl)benzene-1,2-diamine (11)
2.1.3. General Preparation of 2-MMBI Derivatives 1–10
2.2. Materials for Biological Experiments
2.3. Activity Inhibition Assay Against Mushroom TYR [38,39]
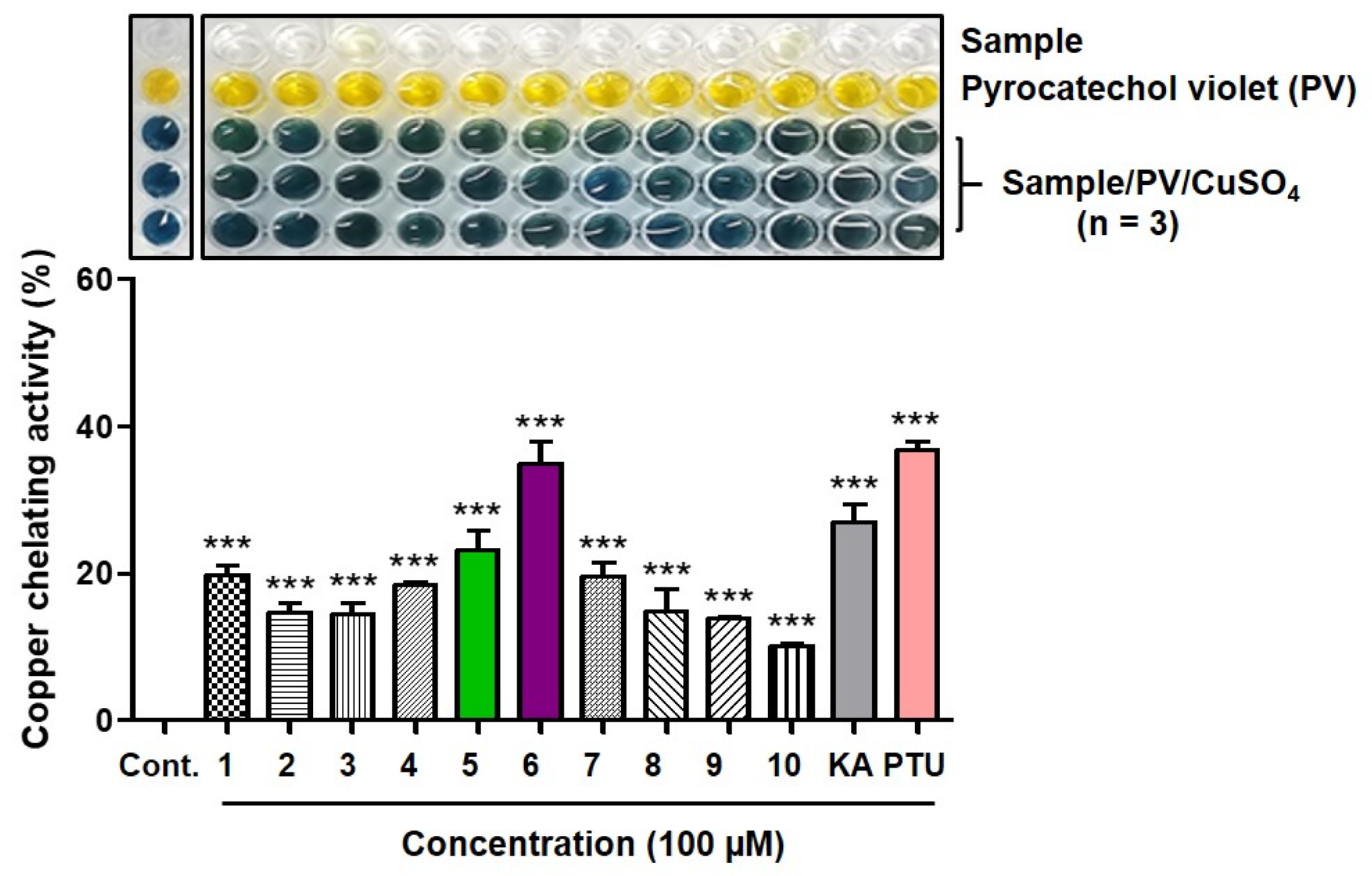
2.4. Copper(II) Ion-Chelating Activity [40]
2.5. Mushroom TYR Activity Assay With and Without CuSO4 [41]
2.6. Kinetic Studies on Mushroom TYR in the Presence of 2-MMBI Derivatives [42,43,44]
2.7. Docking Simulation of 2-MMBI Derivatives and Mushroom TYR [29]
2.8. B16F10 Murine Melanoma Cell and HaCaT Keratinocyte Cell Culture
2.9. B16F10 Cell Cytotoxicity Assay [45,46]
2.10. Melanin Content Measurement Assay [47]
2.11. TYR Activity Assay in B16F10 Cells [45]
2.12. In Situ B16F10 Cellular TYR Activity Assay [48]
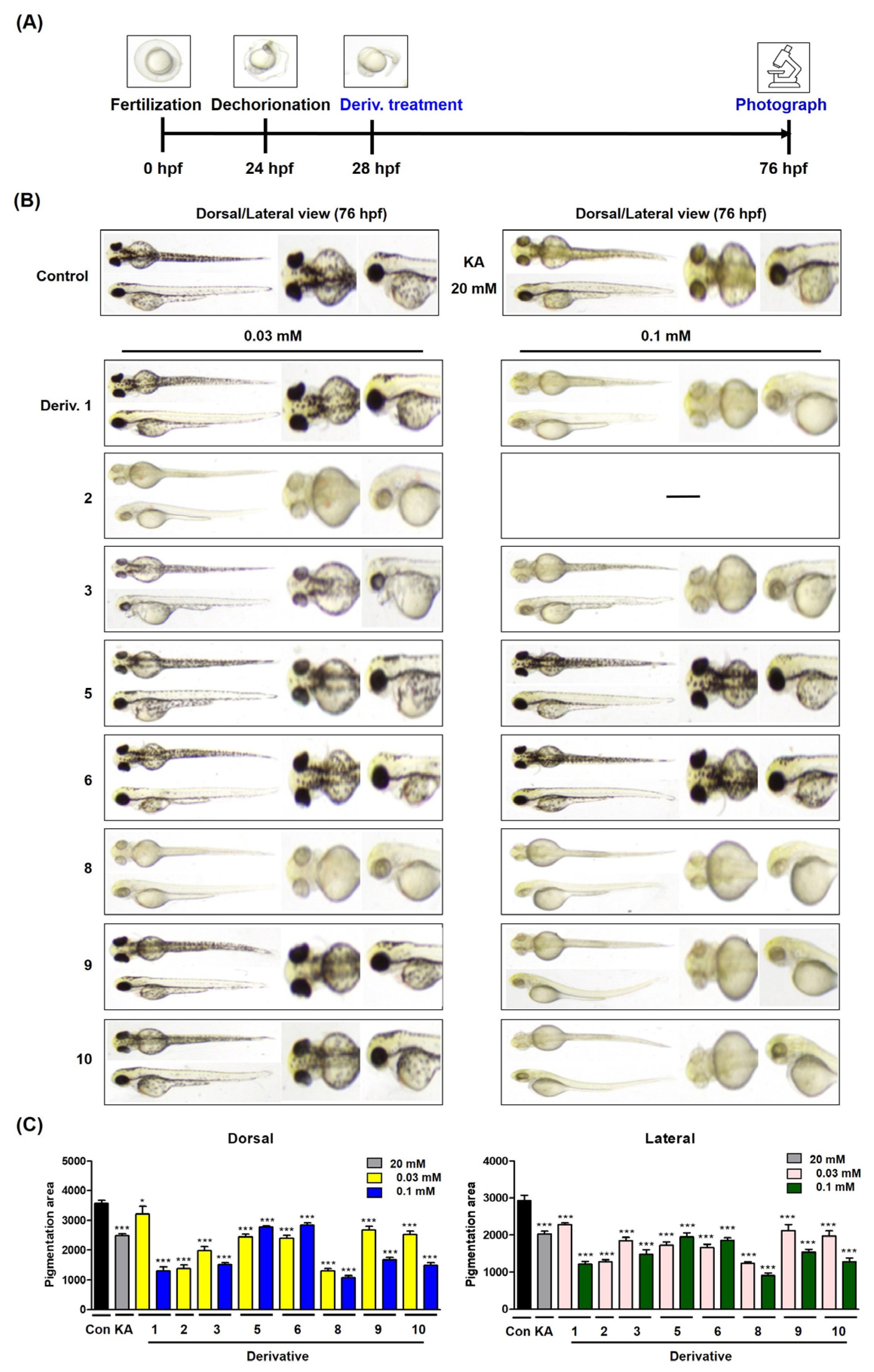
2.13. Melanogenesis Assay Using Zebrafish Embryos [30,49]
2.14. Cell Viability Assay in HaCaT Cells [50]
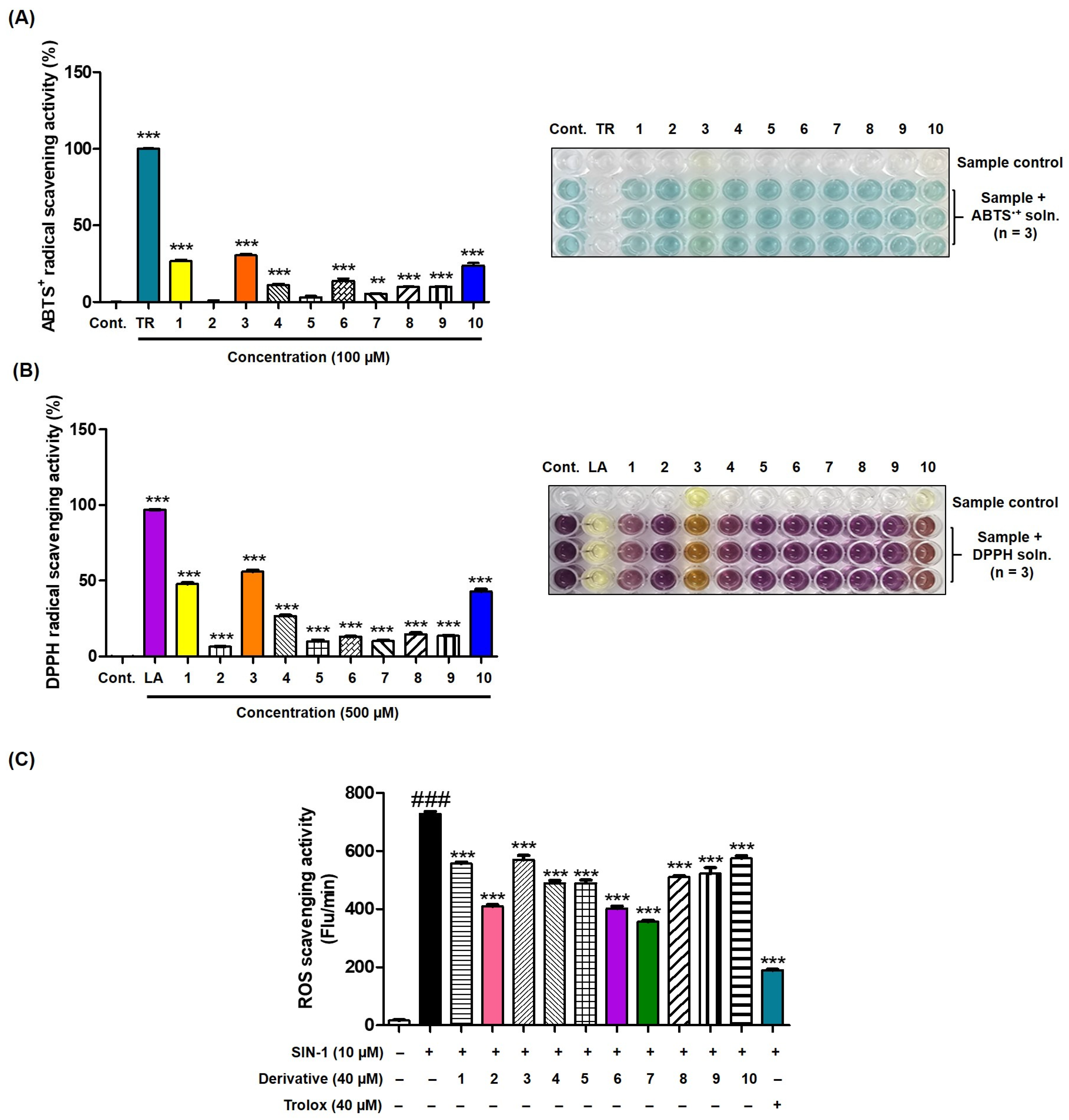
2.15. ABTS●+ Scavenging Assay [51,52]
2.16. DPPH Radical Removal Assay [53]
2.17. Reactive Oxygen Species Removal Activity Assay [54,55]
2.18. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of 2-MMBI Derivatives 1–10
3.2. Inhibitory Effect of 2-MMBI Derivatives on Mushroom TYR Activity
3.3. Copper Ion Chelation Ability of 2-MMBI Derivatives
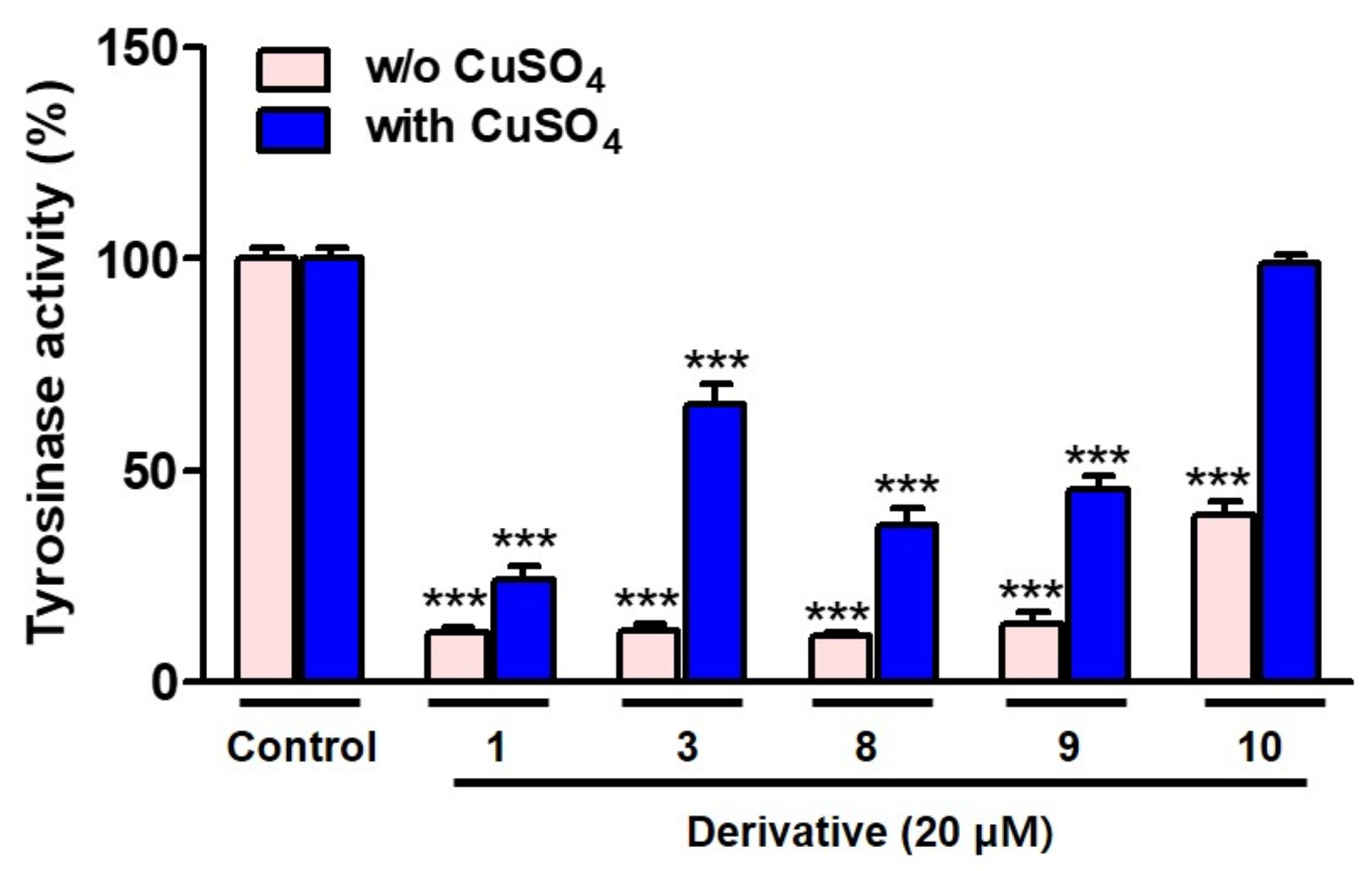
3.4. Decreasing Mushroom TYR Activity in the Presence of CuSO4
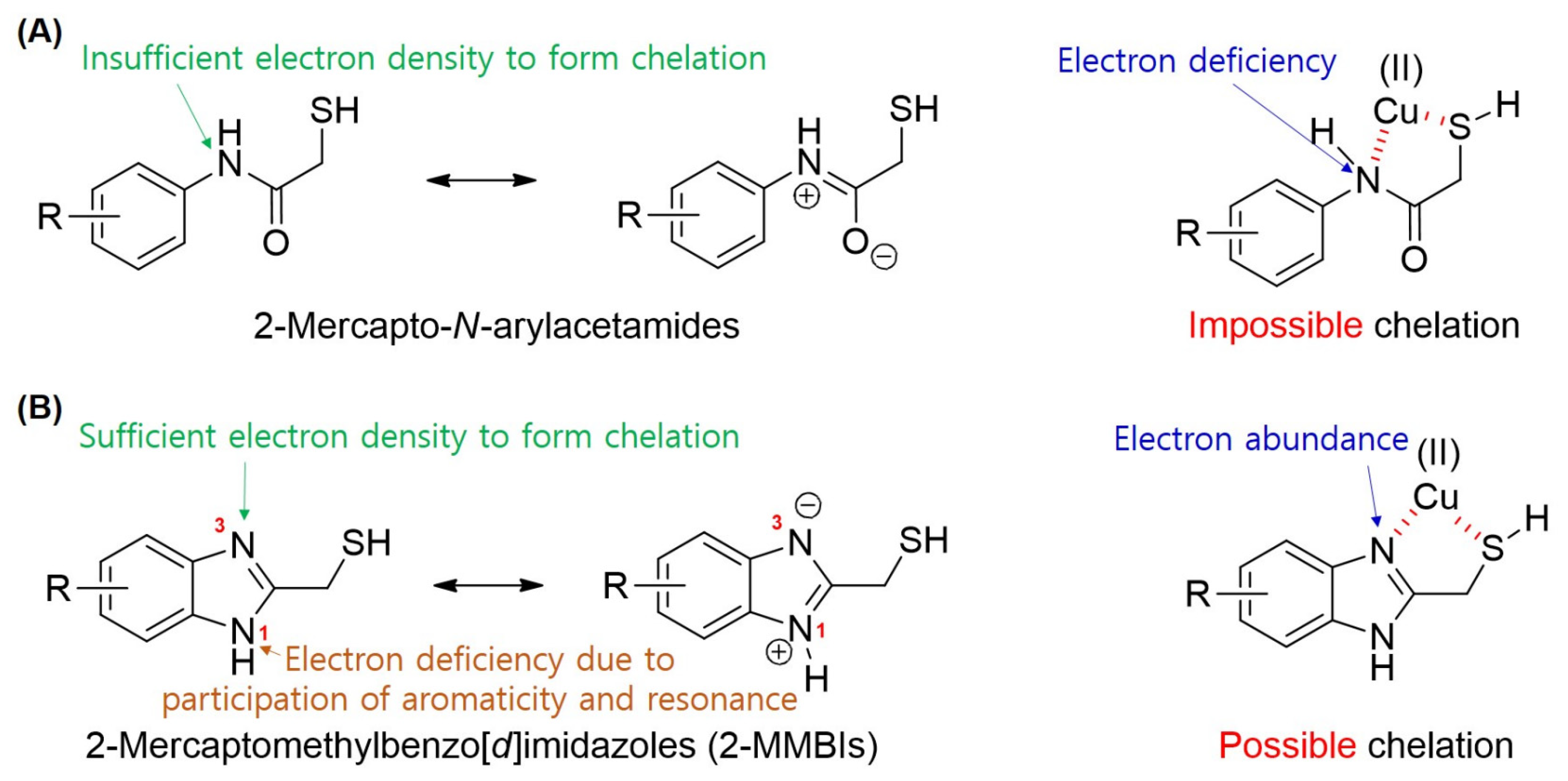
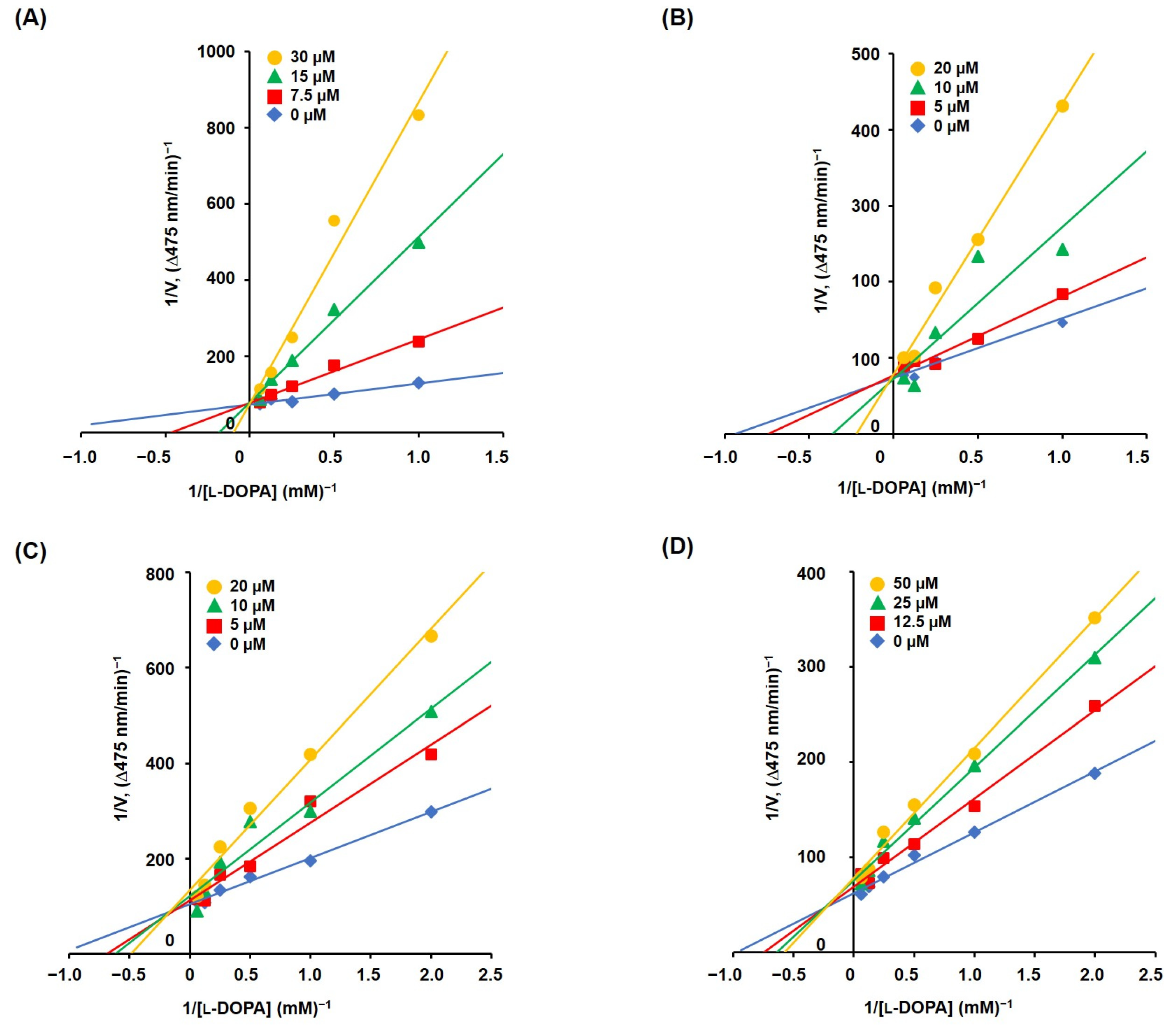
3.5. Mechanism Studies on TYR Inhibition of 2-MMBI Derivatives
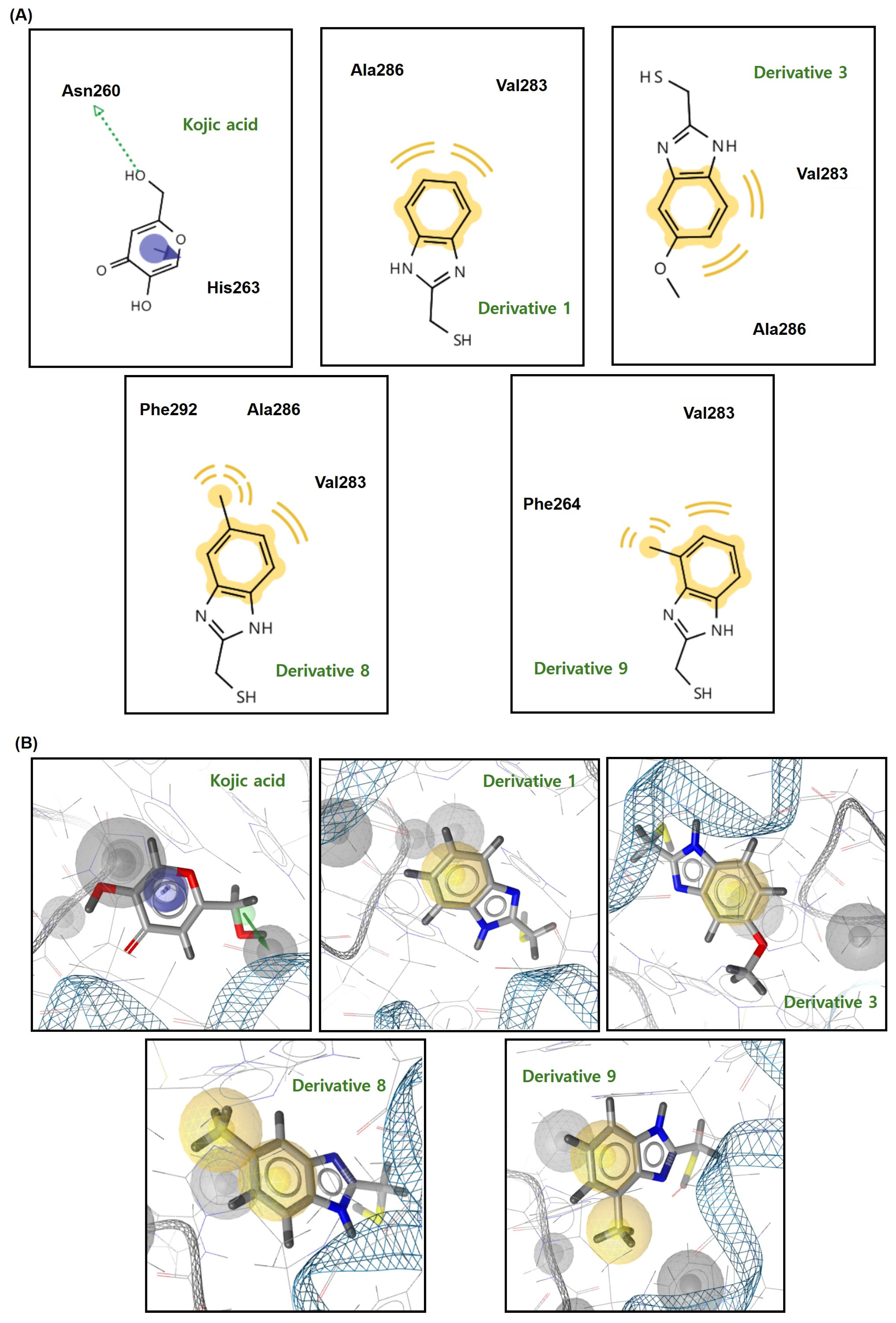
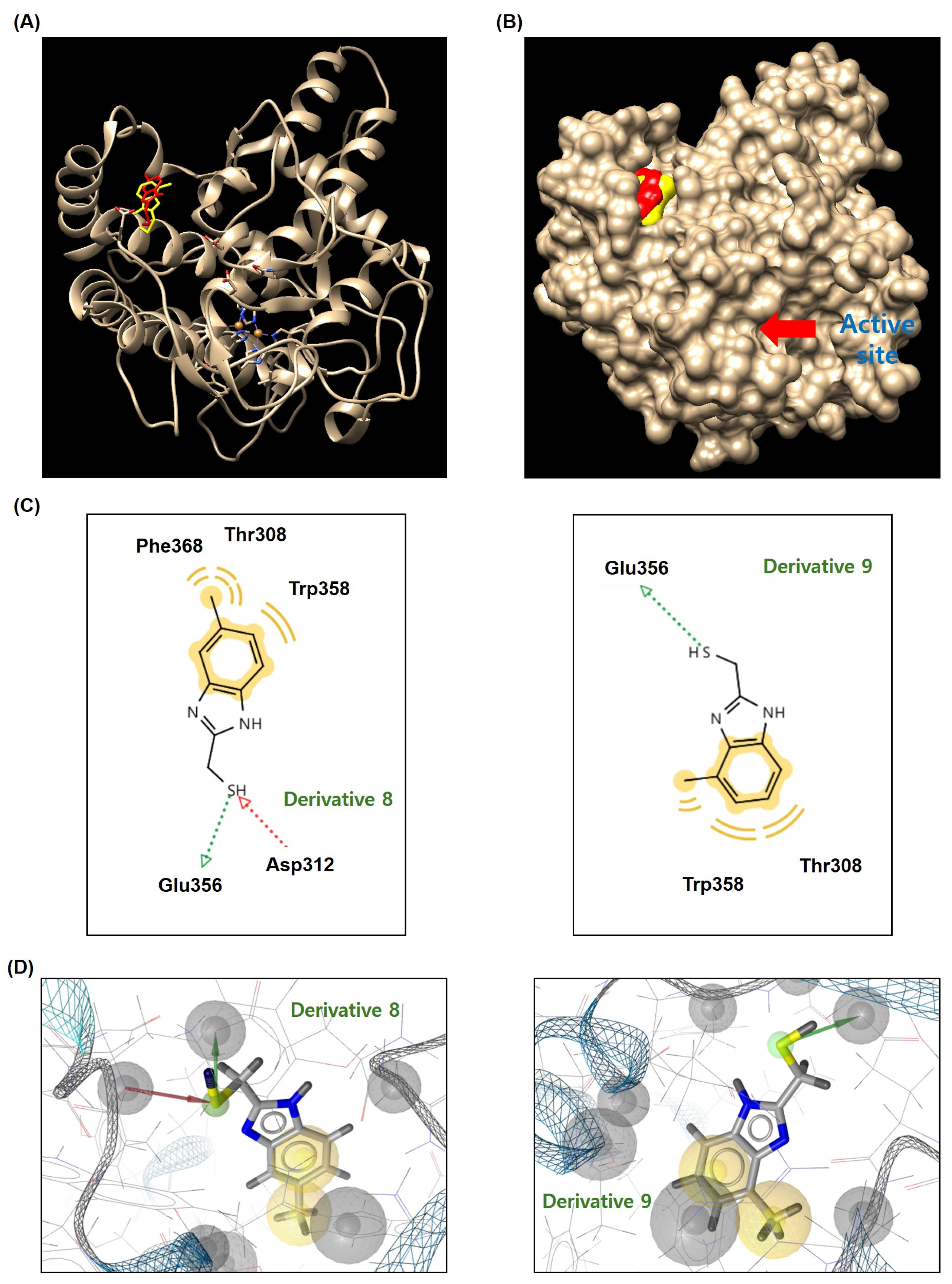
3.6. Docking Simulation of Mushroom TYR and 2-MMBI Derivatives
3.7. B16F10 Cell Viability in the Presence of 2-MMBI Derivatives
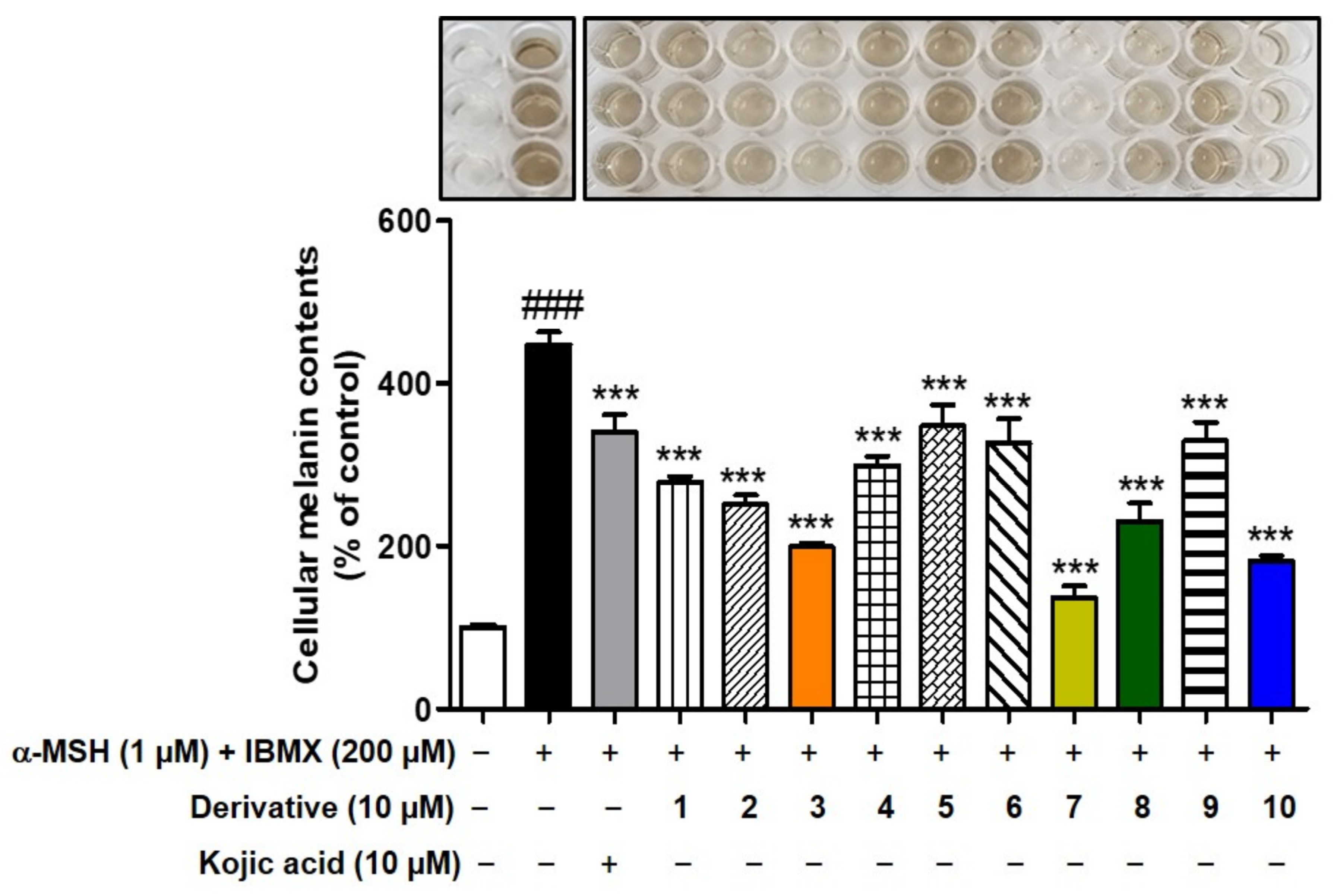
3.8. Effects of 2-MMBI Derivatives on Melanogenesis in B16F10 Cells
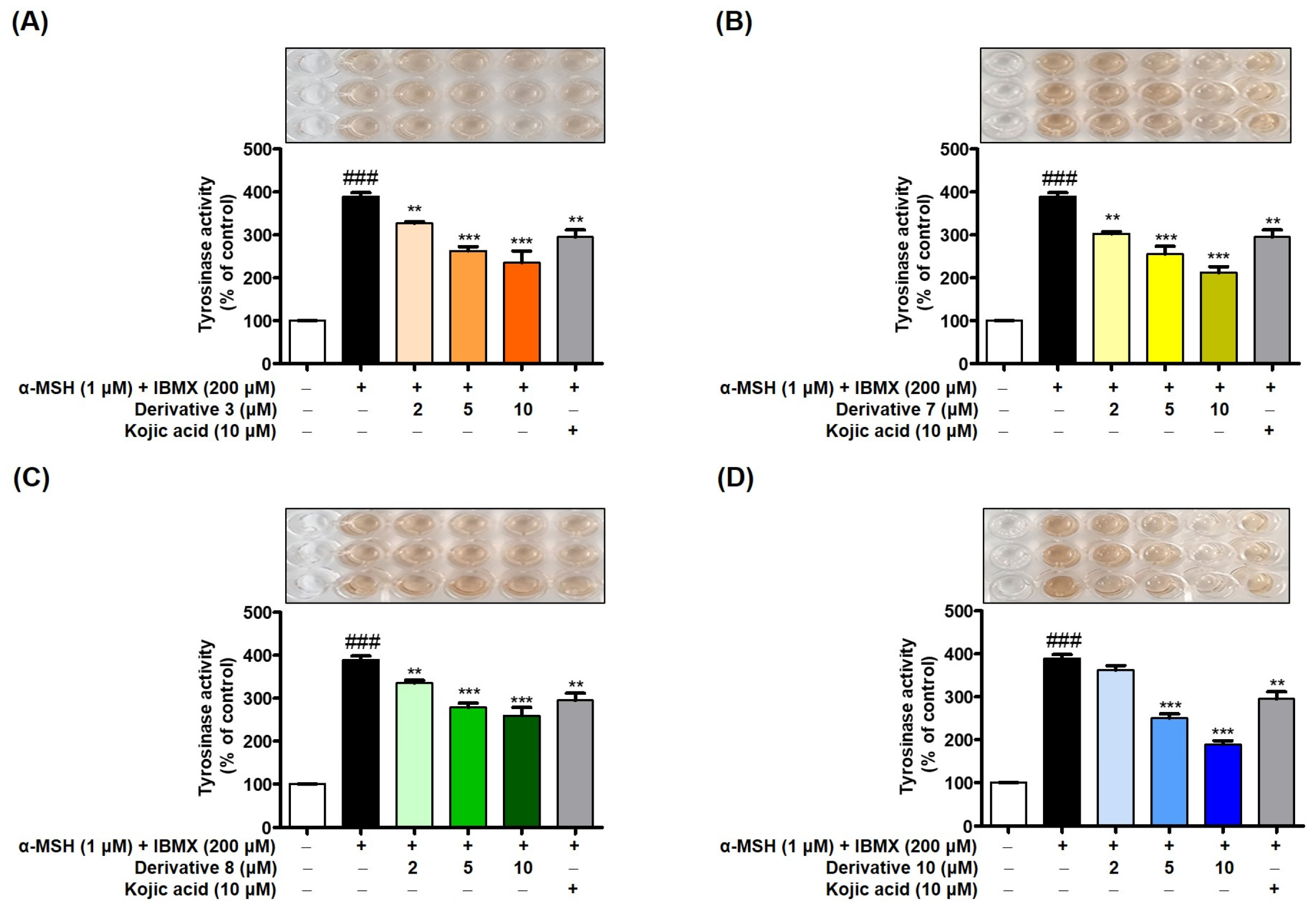
3.9. Effects of 2-MMBI Derivatives on Cellular TYR Activity Inhibition in B16F10 Cells
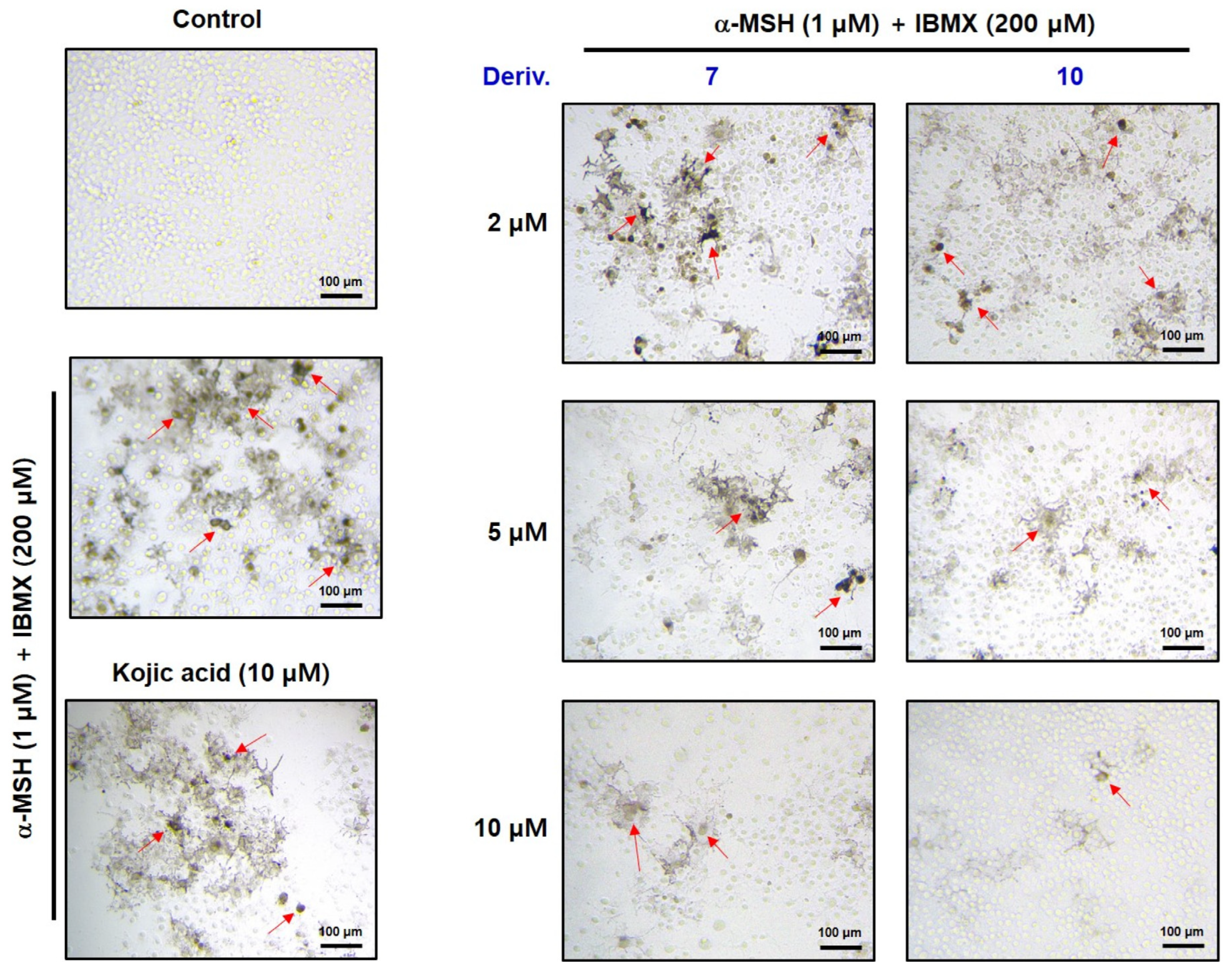
3.10. In Situ B16F10 Cell TYR Activity in the Presence of 2-MMBI Derivatives
3.11. Inhibitory Effect of 2-MMBI Derivatives on Pigmentation in Zebrafish Larvae
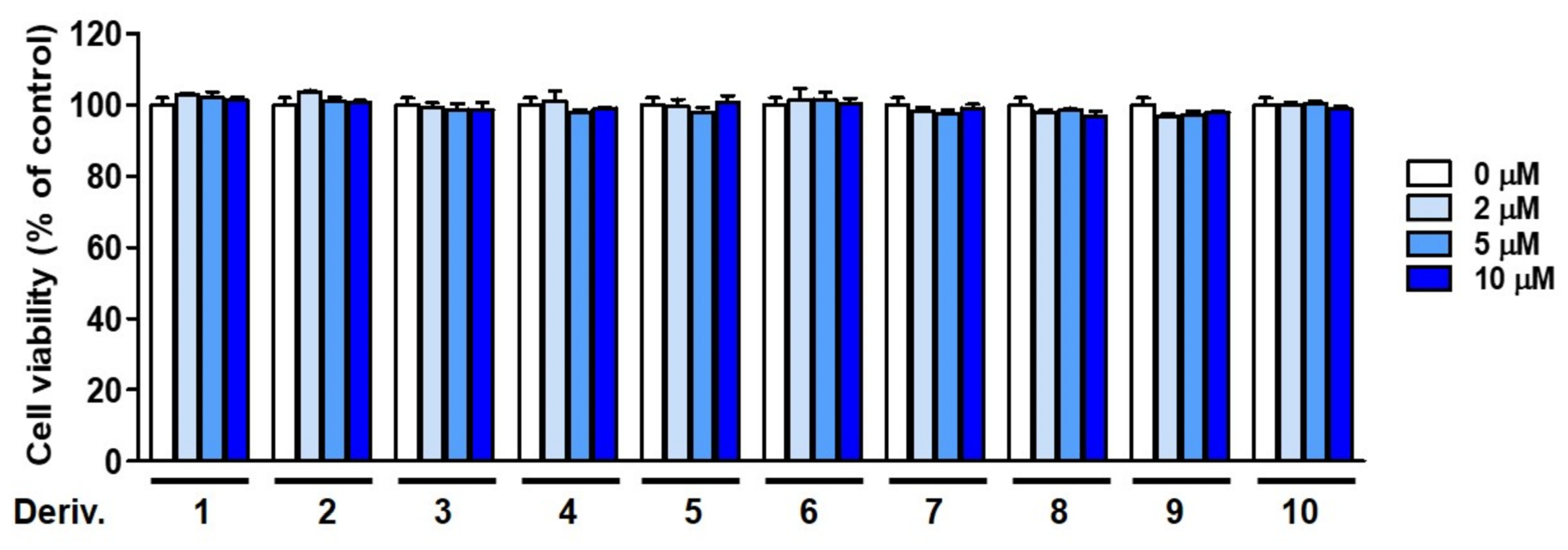
3.12. Cell Viability of 2-MMBI Derivatives in HaCaT Cells
3.13. Antioxidant Activities of 2-MMBI Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thawabteh, A.M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. Skin Pigmentation Types, Causes and Treatment—A Review. Molecules 2023, 28, 4839. [Google Scholar] [CrossRef] [PubMed]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin Pigment in Plants: Current Knowledge and Future Perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Zhang, M.; Wu, Y.; Liu, Y.; Wang, Y.; Zhang, J.; Song, J.; Feng, S.; Sun, Y.; Tan, L. Cloning and characterization of microphthalmia-associated transcription factor-like gene provide insights into Cyclina sinensis clam shell melanin deposition. Aquac. Res. 2022, 53, 1413–1423. [Google Scholar] [CrossRef]
- Wang, R.; Chai, W.-M.; Yang, Q.; Wei, M.-K.; Peng, Y. 2-(4-Fluorophenyl)-quinazolin-4(3H)-one as a novel tyrosinase inhibitor: Synthesis, inhibitory activity, and mechanism. Bioorg. Med. Chem. 2016, 24, 4620–4625. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Maymone, M.B.; Neamah, H.H.; Wirya, S.A.; Patzelt, N.M.; Secemsky, E.A.; Zancanaro, P.Q.; Vashi, N.A. The impact of skin hyperpigmentation and hyperchromia on quality of life: A cross-sectional study. J. Am. Acad. Dermatol. 2017, 77, 775–778. [Google Scholar] [CrossRef]
- Shao, L.L.; Wang, X.L.; Chen, K.; Dong, X.W.; Kong, L.M.; Zhao, D.Y.; Hider, R.C.; Zhou, T. Novel hydroxypyridinone derivatives containing an oxime ether moiety: Synthesis, inhibition on mushroom tyrosinase and application in anti-browning of fresh-cut apples. Food Chem. 2018, 242, 174–181. [Google Scholar] [CrossRef]
- Tessari, I.; Bisaglia, M.; Valle, F.; Samorì, B.; Bergantino, E.; Mammi, S.; Bubacco, L. The reaction of alpha-synuclein with tyrosinase: Possible implications for Parkinson disease. J. Biol. Chem. 2008, 283, 16808–16817. [Google Scholar] [CrossRef]
- Hasegawa, T. Tyrosinase-Expressing Neuronal Cell Line as in Vitro Model of Parkinson’s Disease. Int. J. Mol. Sci. 2010, 11, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Vontzalidou, A.; Zoidis, G.; Chaita, E.; Makropoulou, M.; Aligiannis, N.; Lambrinidis, G.; Mikros, E.; Skaltsounis, A.-L. Design, synthesis and molecular simulation studies of dihydrostilbene derivatives as potent tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5523–5526. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K.; Zucca, F.A.; Zecca, L.; Youdim, M.; Wulf, M.; Riederer, P.; et al. The role of tyrosine hydroxylase as a key player in neuromelanin synthesis and the association of neuromelanin with Parkinson’s disease. J. Neural Transm. 2023, 130, 611–625. [Google Scholar] [CrossRef]
- Mahmoud, M.G.; Awady, M.E.E.; Selim, M.S.; Ibrahim, A.Y.; Ibrahim, F.M.; Mohamed, S.S. Characterization of biologically active exopolysaccharide produced by Streptomyces sp. NRCG4 and its anti-Alzheimer efficacy: In-vitro targets. J. Genet. Eng. Biotechnol. 2023, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765. [Google Scholar] [PubMed]
- Gaskell, M.; McLuckie, K.I.; Farmer, P.B. Genotoxicity of the benzene metabolites para-benzoquinone and hydroquinone. Chem. Biol. Interact. 2005, 153–154, 267–270. [Google Scholar] [CrossRef]
- Chang, T.-S. Natural Melanogenesis Inhibitors Acting Through the Down-Regulation of Tyrosinase Activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Chib, S.; Jamwal, V.L.; Kumar, V.; Gandhi, S.G.; Saran, S. Fungal production of kojic acid and its industrial applications. Appl. Microbiol. Biotechnol. 2023, 107, 2111–2130. [Google Scholar] [CrossRef]
- Ullah, S.; Son, S.; Yun, H.Y.; Kim, D.H.; Chun, P.; Moon, H.R. Tyrosinase inhibitors: A patent review (2011–2015). Expert Opin. Ther. Pat. 2016, 26, 347–362. [Google Scholar] [CrossRef]
- Kanteev, M.; Goldfeder, M.; Fishman, A. Structure-function correlations in tyrosinases. Protein Sci. 2015, 24, 1360–1369. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K. Stilbenes with tyrosinase inhibitory activity. Curr. Sci. 2008, 94, 44–52. [Google Scholar]
- Mayer, A.M. Polyphenol oxidases in plants-recent progress. Phytochemistry 1986, 26, 11–20. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Rodríguez-López, J.N.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-D.; Choi, H.; Abekura, F.; Park, J.-Y.; Yang, W.-S.; Yang, S.-H.; Kim, C.-H. Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation. Int. J. Mol. Sci. 2023, 24, 8226. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.S.; Jung, H.J.; Park, Y.J.; Kang, M.K.; Kim, H.J.; Yoon, D.; Ullah, S.; Kang, D.; Park, Y.; et al. Anti-Browning Effect of 2-Mercaptobenzo[d]imidazole Analogs with Antioxidant Activity on Freshly-Cut Apple Slices and Their Highly Potent Tyrosinase Inhibitory Activity. Antioxidants 2023, 12, 1814. [Google Scholar] [CrossRef]
- Yoon, D.; Jung, H.J.; Lee, J.; Kim, H.J.; Park, H.S.; Park, Y.J.; Kang, M.K.; Kim, G.Y.; Kang, D.; Park, Y.; et al. In vitro and in vivo anti-pigmentation effects of 2-mercaptobenzimidazoles as nanomolar tyrosinase inhibitors on mammalian cells and zebrafish embryos: Preparation of pigment-free zebrafish embryos. Eur. J. Med. Chem. 2024, 266, 116136. [Google Scholar] [CrossRef]
- Jin Jung, H.; Jin Kim, H.; Soo Park, H.; Young Kim, G.; Jung Park, Y.; Lee, J.; Kyung Kang, M.; Yoon, D.; Kang, D.; Park, Y.; et al. Highly potent anti-melanogenic effect of 2-thiobenzothiazole derivatives through nanomolar tyrosinase activity inhibition. Bioorg. Chem. 2024, 150, 107586. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Miao, Y.; Xu, J.; Bi, Q.; Qu, W.; Liu, W.; Feng, F.; Sun, H. A facile and efficient [4 + 2] annulation reaction of sulfur ylides: Access to N-fused benzimidazoles. Org. Chem. Front. 2019, 6, 205–208. [Google Scholar] [CrossRef]
- Karpavičienė, I.; Jonušis, M.; Leduskrasts, K.; Misiūnaitė, I.; Suna, E.; Čikotienė, I. Synthesis and photophysical properties of 3,5-diaryl-2-heteroarylthiophenes. Dye Pigment. 2019, 170, 107646. [Google Scholar] [CrossRef]
- Brembilla, A.; Roizard, D.; Lochon, P. Activité estérolytique de composés associant une fonction thiol et une base hétérocyclique. Exemples de processus bifonctionnel. J. Chim. Phys. 1986, 83, 577–588. [Google Scholar] [CrossRef]
- Alasmary, F.A.; Snelling, A.M.; Zain, M.E.; Alafeefy, A.M.; Awaad, A.S.; Karodia, N. Synthesis and evaluation of selected benzimidazole derivatives as potential antimicrobial agents. Molecules 2015, 20, 15206–15223. [Google Scholar] [CrossRef]
- El-Gohary, N.; Shaaban, M. Synthesis, antimicrobial, antiquorum-sensing and antitumor activities of new benzimidazole analogs. Eur. J. Med. Chem. 2017, 137, 439–449. [Google Scholar] [CrossRef]
- Gümüş, F.; Pamuk, I.; Özden, T.; Yıldız, S.; Diril, N.; Öksüzoǧlu, E.; Gür, S.; Özkul, A. Synthesis, characterization and in vitro cytotoxic, mutagenic and antimicrobial activity of platinum (II) complexes with substituted benzimidazole ligands. J. Inorg. Biochem. 2003, 94, 255–262. [Google Scholar] [CrossRef]
- Hyun, S.K.; Lee, W.-H.; Jeong, D.M.; Kim, Y.; Choi, J.S. Inhibitory Effects of Kurarinol, Kuraridinol, and Trifolirhizin from Sophora flavescens on Tyrosinase and Melanin Synthesis. Biol. Pharm. Bull. 2008, 31, 154–158. [Google Scholar] [CrossRef]
- Suganya, P.; Jeyaprakash, K.; Mallavarapu, G.R.; Murugan, R. Comparison of the chemical composition, tyrosinase inhibitory and anti-inflammatory activities of the essential oils of Pogostemon plectranthoides from India. Ind. Crops Prod. 2015, 69, 300–307. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Ryu, I.Y.; Choi, I.; Jung, H.J.; Ullah, S.; Choi, H.; Al-Amin, M.; Chun, P.; Moon, H.R. In vitro anti-melanogenic effects of chimeric compounds, 2-(substituted benzylidene)-1,3-indanedione derivatives with a β-phenyl-α, β -unsaturated dicarbonyl scaffold. Bioorg. Chem. 2021, 109, 104688. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase Inhibitory Activity of Citrus Essential Oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef]
- Park, S.-A.; Jegal, J.; Chung, K.W.; Jung, H.J.; Noh, S.G.; Chung, H.Y.; Ahn, J.; Kim, J.; Yang, M.H. Isolation of tyrosinase and melanogenesis inhibitory flavonoids from Juniperus chinensis fruits. Biosci. Biotechnol. Biochem. 2018, 82, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Yang, J.H.; Lee, M.K.; Kwon, E.B.; Baek, J.; Hwang, T.; Kim, J.I.; Lee, B. Maclurin Exhibits Antioxidant and Anti-Tyrosinase Activities, Suppressing Melanogenesis. Antioxidants 2022, 11, 1164. [Google Scholar] [CrossRef] [PubMed]
- Khongkarat, P.; Sadangrit, P.; Puthong, S.; Meemongkolkiat, T.; Phuwapraisirisan, P.; Chanchao, C. Anti-tyrosinase and anti-melanogenic effects of piperine isolated from Piper nigrumon B16F10 mouse melanoma cells. Heliyon 2024, 10, e33423. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.O.; Yumnam, S.; Jeong, K.W.; Kim, S.Y. Finasteride inhibits melanogenesis through regulation of the adenylate cyclase in melanocytes and melanoma cells. Arch. Pharmacal Res. 2018, 41, 324–332. [Google Scholar] [CrossRef]
- Matthews, M.; Trevarrow, B.; Matthews, J. A virtual tour of the guide for zebrafish users. Resource 2002, 31, 34–40. [Google Scholar]
- Wang, X.; Zhang, Y.; Wang, D.; Su, N.; Yang, L.; Fu, H.; Zhang, J.; Li, M.; Wang, C. Protective effects of Aureobasidium pullulans lysate on UV-damaged human skin fibroblasts and HaCaT cells. Bioresour. Bioprocess. 2023, 10, 55. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Yamamoto, K. Bovine serum albumin significantly improves the DPPH free radical scavenging potential of dietary polyphenols and gallic acids. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2012, 12, 940–948. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Bondy, S.C. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem. Int. 1990, 17, 435–440. [Google Scholar] [CrossRef]
- Ali, S.F.; LeBel, C.P.; Bondy, S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1992, 13, 637–648. [Google Scholar]
- Reddy, V.M.; Reddy, K.R. Synthesis and biological evaluation of some novel-3-(5-substituted benzimidazol-2-yl)-5-arylisoxazolines. Chin. Chem. Lett. 2010, 21, 1145–1148. [Google Scholar] [CrossRef]
- Elgawish, M.S.; Nafie, M.S.; Yassen, A.S.A.; Yamada, K.; Ghareb, N. The design and synthesis of potent benzimidazole derivatives via scaffold hybridization and evaluating their antiproliferative and proapoptotic activity against breast and lung cancer cell lines. New J. Chem. 2022, 46, 4239–4256. [Google Scholar] [CrossRef]
- Seo, S.-Y.; Sharma, V.K.; Sharma, N. Mushroom Tyrosinase: Recent Prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef]
- Ashooriha, M.; Khoshneviszadeh, M.; Khoshneviszadeh, M.; Moradi, S.E.; Rafiei, A.; Kardan, M.; Emami, S. 1,2,3-Triazole-based kojic acid analogs as potent tyrosinase inhibitors: Design, synthesis and biological evaluation. Bioorg. Chem. 2019, 82, 414–422. [Google Scholar] [CrossRef]
- Li, Q.; Mo, J.; Xiong, B.; Liao, Q.; Chen, Y.; Wang, Y.; Xing, S.; He, S.; Lyu, W.; Zhang, N.; et al. Discovery of Resorcinol-Based Polycyclic Structures as Tyrosinase Inhibitors for Treatment of Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 81–96. [Google Scholar] [CrossRef]



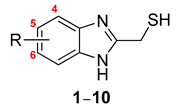 | |||
|---|---|---|---|
| Derivative | R | a IC50 (μM) | |
| l-Tyrosine | l-Dopa | ||
| 1 | H | 4.05 ± 0.48 | 13.78 ± 0.44 |
| 2 | 5-Chloro | 15.36 ± 0.79 | 51.56 ± 4.16 |
| 3 | 5-Methoxy | 6.15 ± 1.05 | 9.77 ± 0.51 |
| 4 | 5-Fluoro | 16.29 ± 0.30 | 35.26 ± 2.98 |
| 5 | 5-Cyano | 21.89 ± 0.46 | 73.56 ± 1.33 |
| 6 | 5-Nitro | 20.91 ± 0.10 | 53.75 ± 5.83 |
| 7 | 5-Trifluoromethyl | 52.37 ± 0.58 | 46.07 ± 4.02 |
| 8 | 5-Methyl | 2.90 ± 0.47 | 10.64 ± 0.42 |
| 9 | 4-Methyl | 11.86 ± 0.21 | 28.97 ± 0.53 |
| 10 | 5,6-Dimethyl | 5.66 ± 0.99 | 54.26 ± 0.72 |
| b Kojic acid | 17.87 ± 1.58 | 23.53 ± 1.52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.J.; Park, H.S.; Kim, H.J.; Park, H.S.; Park, Y.; Chun, P.; Chung, H.Y.; Moon, H.R. Design, Synthesis, and Anti-Melanogenic Activity of 2-Mercaptomethylbenzo[d]imidazole Derivatives Serving as Tyrosinase Inhibitors: An In Silico, In Vitro, and In Vivo Exploration. Antioxidants 2024, 13, 1248. https://doi.org/10.3390/antiox13101248
Jung HJ, Park HS, Kim HJ, Park HS, Park Y, Chun P, Chung HY, Moon HR. Design, Synthesis, and Anti-Melanogenic Activity of 2-Mercaptomethylbenzo[d]imidazole Derivatives Serving as Tyrosinase Inhibitors: An In Silico, In Vitro, and In Vivo Exploration. Antioxidants. 2024; 13(10):1248. https://doi.org/10.3390/antiox13101248
Chicago/Turabian StyleJung, Hee Jin, Hyeon Seo Park, Hye Jin Kim, Hye Soo Park, Yujin Park, Pusoon Chun, Hae Young Chung, and Hyung Ryong Moon. 2024. "Design, Synthesis, and Anti-Melanogenic Activity of 2-Mercaptomethylbenzo[d]imidazole Derivatives Serving as Tyrosinase Inhibitors: An In Silico, In Vitro, and In Vivo Exploration" Antioxidants 13, no. 10: 1248. https://doi.org/10.3390/antiox13101248
APA StyleJung, H. J., Park, H. S., Kim, H. J., Park, H. S., Park, Y., Chun, P., Chung, H. Y., & Moon, H. R. (2024). Design, Synthesis, and Anti-Melanogenic Activity of 2-Mercaptomethylbenzo[d]imidazole Derivatives Serving as Tyrosinase Inhibitors: An In Silico, In Vitro, and In Vivo Exploration. Antioxidants, 13(10), 1248. https://doi.org/10.3390/antiox13101248









