Golden Tomato Juice Enhances Hepatic PPAR-α Expression, Mitigates Metabolic Dysfunctions and Influences Redox Balance in a High-Fat-Diet Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Tomato Seedling Cultivation and Morphological Characteristics
2.2. Process of Turning Golden Tomatoes into Juice
2.2.1. Chemical and Nutritional Properties of Golden Tomato Juice
- Energy calculation
2.2.2. Analysis of Total Polyphenols by Folin Ciocalteu Assay in GTJ
2.2.3. Identification and Quantification of 9-Oxo-10(E),12(E)-Octadecadienoic Acid in GTJ by HPLC System
- Sample preparation
- Identification and quantification of 9-oxo-10(E),12(E)-ODA by HPLC
2.3. Animals
2.3.1. Experimental Groups
2.3.2. Preparation of the Orally Administered Tomato Solutions
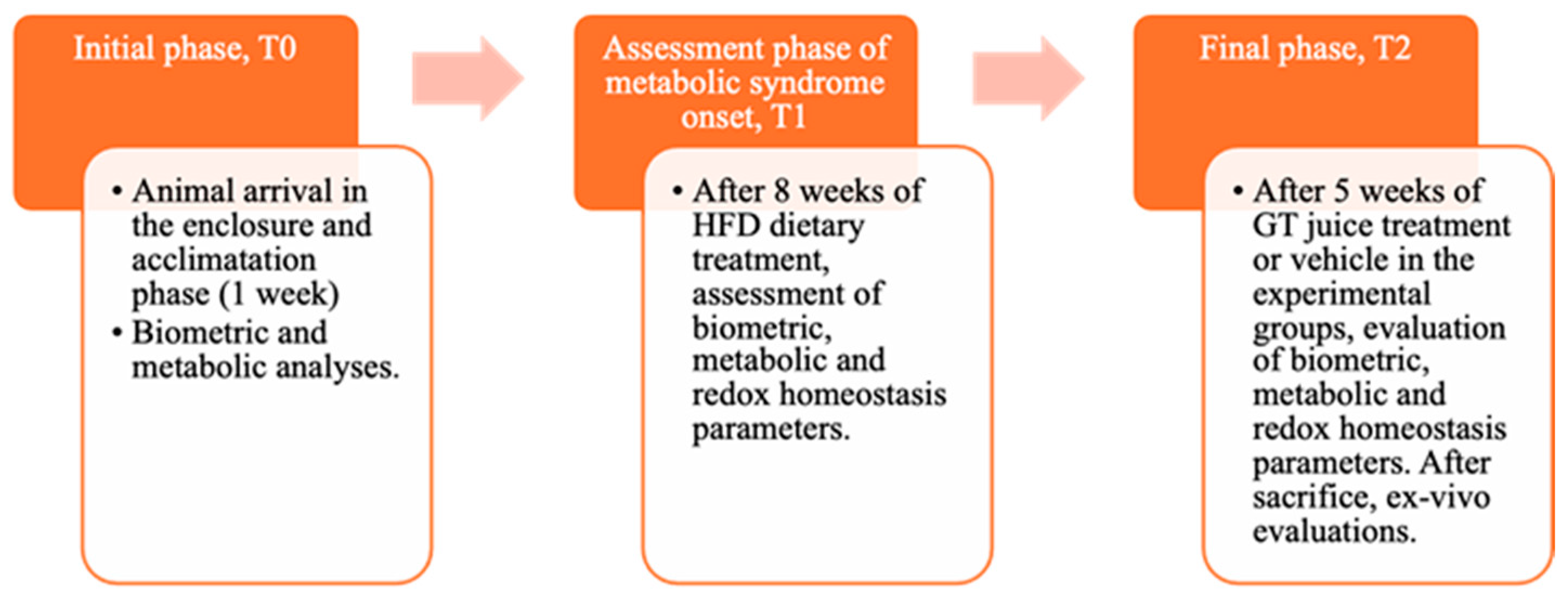
2.4. Experimental Design
2.4.1. Biometric Parameters and Leptin Levels
2.4.2. Histological Analysis and PPAR-α Expression by Real-Time PCR of Hepatic Tissue
2.4.3. Metabolic Assays: Glucose Tolerance and Lipid Homeostasis
2.4.4. Systemic and Hepatic Redox Homeostasis Parameters
MDA Evaluation in Hepatic Tissue
RONS Evaluation in Hepatic Tissue
2.5. Statistical Analyses
3. Results
3.1. Morphological Characteristics of Tomato Plants
3.2. Chemical and Nutritional Properties of Golden Tomatoes After Juice Processing
3.3. Fatty Acid Content “9-Oxo-10(E),12(E)-ODA” in GTJ Compared with Red and Golden Tomatoes
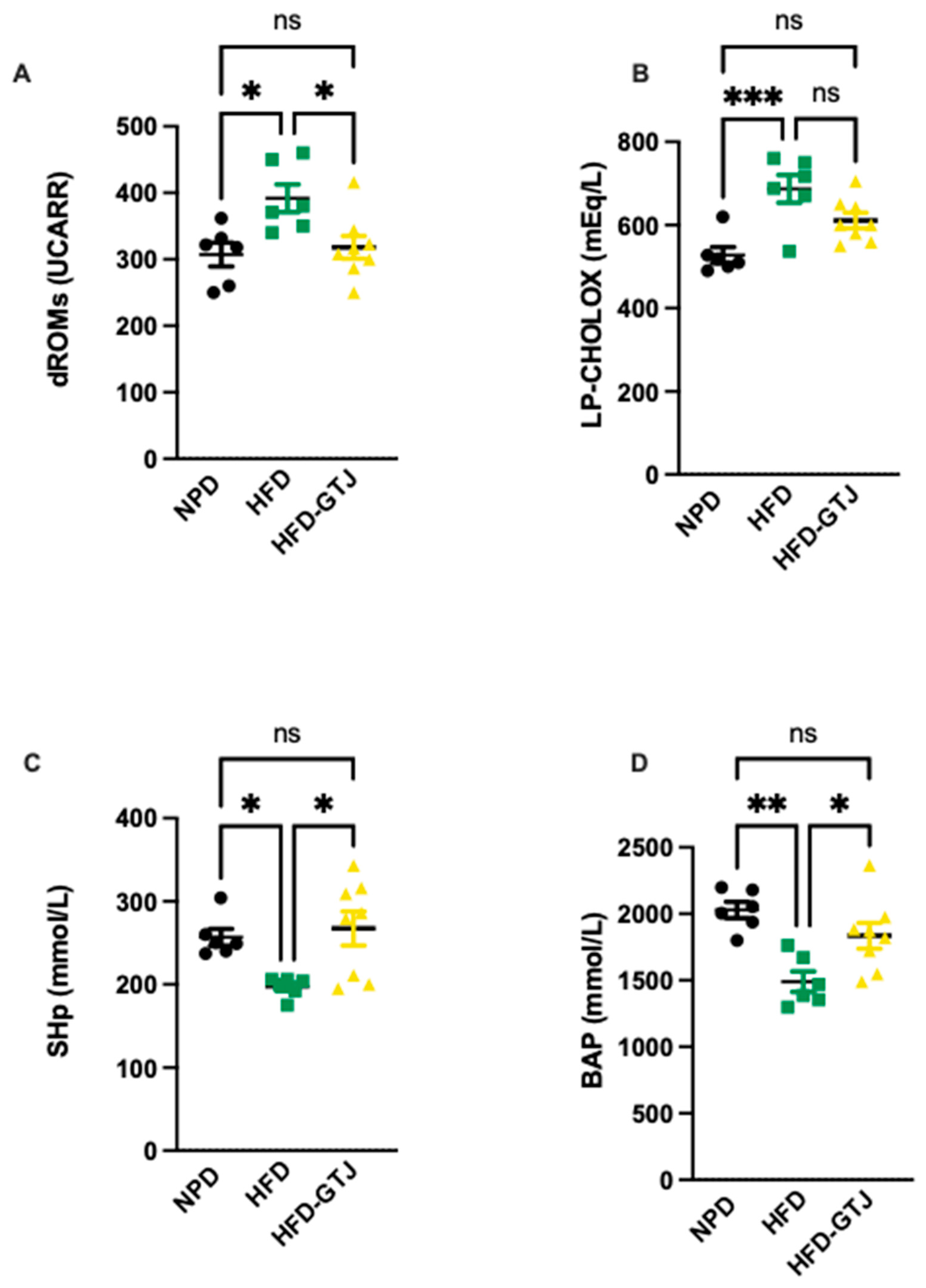
3.4. Parameters Assessed at T1 Time to Verify the Induction of Metabolic Syndrome
3.5. Effects of GTJ Treatment on Body Weight, Food Intake, and Leptin Levels in MetS
3.6. Effects of GT Juice in the Liver on Steatosis and PPAR-α Levels in MetS
3.7. Metabolic Effects of GT Juice in MetS: Glucose Tolerance and Lipid Profile
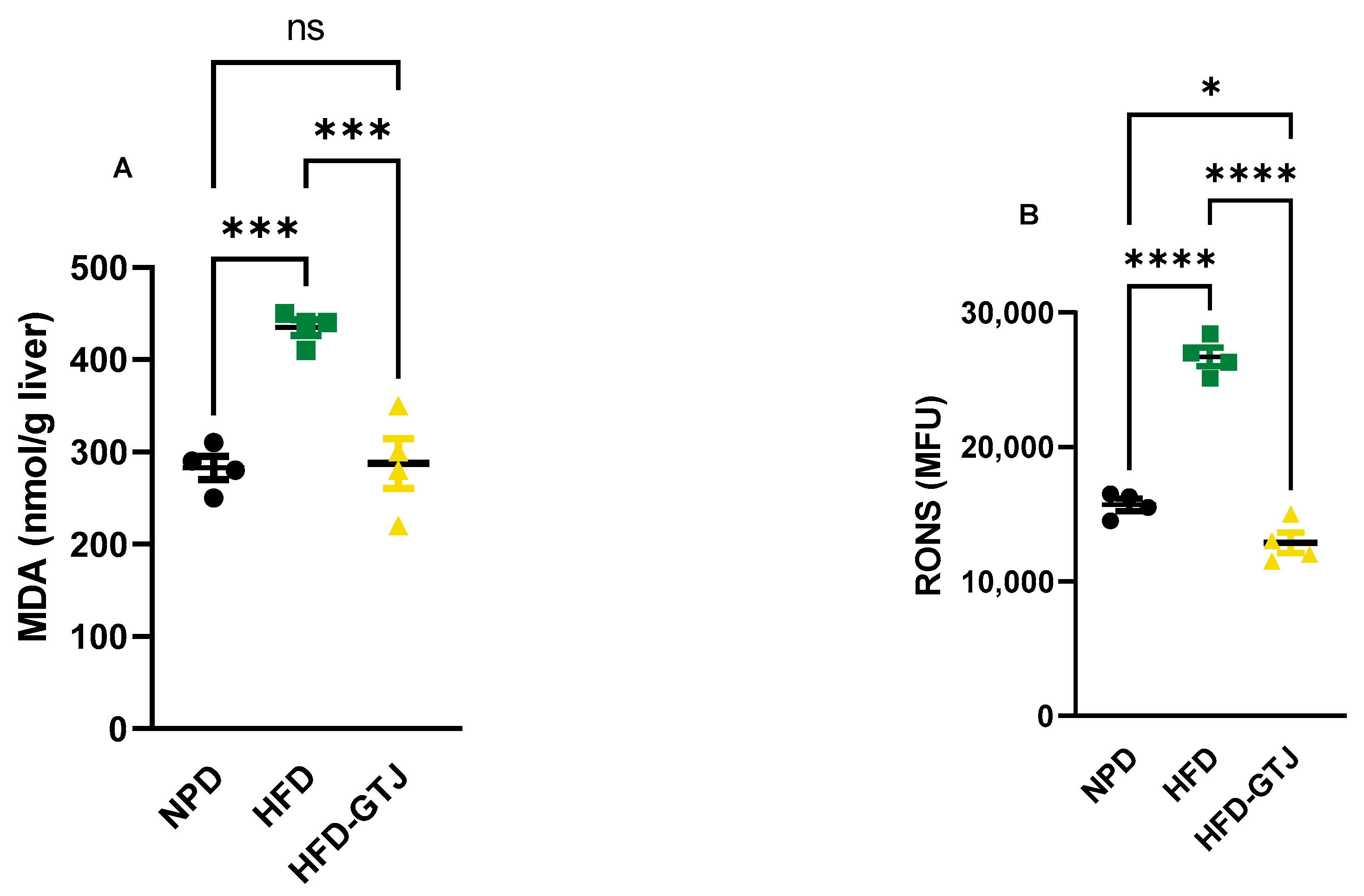
3.8. Effects of GT Juice on Systemic and Hepatic Redox Homeostasis in MetS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gambino, G.; Giglia, G.; Schiera, G.; Di Majo, D.; Epifanio, M.S.; La Grutta, S.; Lo Baido, R.; Ferraro, G.; Sardo, P. Haptic Perception in Extreme Obesity: qEEG Study Focused on Predictive Coding and Body Schema. Brain Sci. 2020, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Di Majo, D.; Cacciabaudo, F.; Accardi, G.; Gambino, G.; Giglia, G.; Ferraro, G.; Candore, G.; Sardo, P. Ketogenic and Modified Mediterranean Diet as a Tool to Counteract Neuroinflammation in Multiple Sclerosis: Nutritional Suggestions. Nutrients 2022, 14, 2384. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, M.; Lantieri, L.; Leto, G.; Pescia, F.; Di Majo, D. Nutrition, Obesity and Hormones. J. Biol. Res. 2018, 91. [Google Scholar] [CrossRef]
- Spahis, S.; Borys, J.-M.; Levy, E. Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid. Redox Signal. 2017, 26, 445–461. [Google Scholar] [CrossRef]
- Kim, B.; Feldman, E.L. Insulin Resistance as a Key Link for the Increased Risk of Cognitive Impairment in the Metabolic Syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic Syndrome--a New World-Wide Definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome Proliferator-Activated Receptor Targets for the Treatment of Metabolic Diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef]
- Vatashchuk, M.V.; Bayliak, M.M.; Hurza, V.V.; Storey, K.B.; Lushchak, V.I. Metabolic Syndrome: Lessons from Rodent and Models. Biomed. Res. Int. 2022, 2022, 5850507. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and Nutritional Overview of Diet-Induced Metabolic Syndrome Models in Rats: What Is the Best Choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Di Majo, D.; Sardo, P.; Giglia, G.; Di Liberto, V.; Zummo, F.P.; Zizzo, M.G.; Caldara, G.F.; Rappa, F.; Intili, G.; van Dijk, R.M.; et al. Correlation of Metabolic Syndrome with Redox Homeostasis Biomarkers: Evidence from High-Fat Diet Model in Wistar Rats. Antioxidants 2022, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, B.; Gong, J.; Li, L.; Chen, G.; Zhang, J.; Chen, Y.; Tian, X.; Han, B.; Guo, Y.; et al. Chickpea Extract Ameliorates Metabolic Syndrome Symptoms via Restoring Intestinal Ecology and Metabolic Profile in Type 2 Diabetic Rats. Mol. Nutr. Food Res. 2021, 65, e2100007. [Google Scholar] [CrossRef]
- Zhao, M.; Cai, H.; Jiang, Z.; Li, Y.; Zhong, H.; Zhang, H.; Feng, F. Glycerol-Monolaurate-Mediated Attenuation of Metabolic Syndrome Is Associated with the Modulation of Gut Microbiota in High-Fat-Diet-Fed Mice. Mol. Nutr. Food Res. 2019, 63, e1801417. [Google Scholar] [CrossRef]
- Gambino, G.; Giglia, G.; Allegra, M.; Di Liberto, V.; Zummo, F.P.; Rappa, F.; Restivo, I.; Vetrano, F.; Saiano, F.; Palazzolo, E.; et al. “Golden” Tomato Consumption Ameliorates Metabolic Syndrome: A Focus on the Redox Balance in the High-Fat-Diet-Fed Rat. Antioxidants 2023, 12, 1121. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Fernández-Ruiz, V.; Sánchez-Mata, M.-C.; Cámara, R.M.; Domínguez, L.; Sesso, H.D. Scientific Evidence of the Beneficial Effects of Tomato Products on Cardiovascular Disease and Platelet Aggregation. Front. Nutr. 2022, 9, 849841. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Zito, R.; Gambino, G.; Di Maria, G.; Javed, A.; Lupo, G.; Giglia, G.; Sardo, P.; Ferraro, G.; Rappa, F.; et al. Red and Golden Tomato Administration Improves Fat Diet-Induced Hepatic Steatosis in Rats by Modulating HNF4α, Lepr, and GK Expression. Front. Nutr. 2023, 10, 1221013. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Frinchi, M.; Giglia, G.; Scordino, M.; Urone, G.; Ferraro, G.; Mudò, G.; Sardo, P.; Di Majo, D.; Di Liberto, V. Impact of “Golden” Tomato Juice on Cognitive Alterations in Metabolic Syndrome: Insights into Behavioural and Biochemical Changes in a High-Fat Diet Rat Model. J. Funct. Foods 2024, 112, 105964. [Google Scholar] [CrossRef]
- Motojima, K.; Passilly, P.; Peters, J.M.; Gonzalez, F.J.; Latruffe, N. Expression of Putative Fatty Acid Transporter Genes Are Regulated by Peroxisome Proliferator-Activated Receptor Alpha and Gamma Activators in a Tissue- and Inducer-Specific Manner. J. Biol. Chem. 1998, 273, 16710–16714. [Google Scholar] [CrossRef]
- Yu, S.; Rao, S.; Reddy, J.K. Peroxisome Proliferator-Activated Receptors, Fatty Acid Oxidation, Steatohepatitis and Hepatocarcinogenesis. Curr. Mol. Med. 2003, 3, 561–572. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Takahashi, H.; Kim, Y.-I.; Hirai, S.; Goto, T.; Ohyane, C.; Tsugane, T.; Konishi, C.; Fujii, T.; Inai, S.; Iijima, Y.; et al. Comparative and Stability Analyses of 9- and 13-Oxo-Octadecadienoic Acids in Various Species of Tomato. Biosci. Biotechnol. Biochem. 2011, 75, 1621–1624. [Google Scholar] [CrossRef]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally Diverse Metabolites from Fatty Acid Oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Al Dhaheri, A.S.; Alkhatib, D.H.; Jaleel, A.; Tariq, M.N.M.; Feehan, J.; Apostolopoulos, V.; Osaili, T.M.; Mohamad, M.N.; Cheikh Ismail, L.; Saleh, S.T.; et al. Proximate Composition and Mineral Content of Spices Increasingly Employed in the Mediterranean Diet. J. Nutr. Sci. 2023, 12, e79. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of Organic Acids in Fruits and Vegetables by Liquid Chromatography with Tandem-Mass Spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Merrill, A.L.; Watt, B.K. Energy Value of Foods: Basis and Derivation; US Department of Agriculture: Washington, DC, USA, 1973. [Google Scholar]
- Scordino, M.; Urone, G.; Frinchi, M.; Valenza, C.; Bonura, A.; Cipollina, C.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M.; Mudò, G.; et al. Anti-Apoptotic and Anti-Inflammatory Properties of Grapefruit IntegroPectin on Human Microglial HMC3 Cell Line. Cells 2024, 13, 355. [Google Scholar] [CrossRef]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. Metabolic Phenotyping Guidelines: Assessing Glucose Homeostasis in Rodent Models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef]
- Lee, S.; Hashimoto, J.; Suzuki, T.; Satoh, A. The Effects of Exercise Load during Development on Oxidative Stress Levels and Antioxidant Potential in Adulthood. Free Radic. Res. 2017, 51, 179–186. [Google Scholar] [CrossRef]
- Macri, A.; Scanarotti, C.; Bassi, A.M.; Giuffrida, S.; Sangalli, G.; Traverso, C.E.; Iester, M. Evaluation of Oxidative Stress Levels in the Conjunctival Epithelium of Patients with or without Dry Eye, and Dry Eye Patients Treated with Preservative-Free Hyaluronic Acid 0.15% and Vitamin B12 Eye Drops. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 425–430. [Google Scholar] [CrossRef]
- Mancini, S.; Mariani, F.; Sena, P.; Benincasa, M.; Roncucci, L. Myeloperoxidase Expression in Human Colonic Mucosa Is Related to Systemic Oxidative Balance in Healthy Subjects. Redox Rep. 2017, 22, 399–407. [Google Scholar] [CrossRef]
- Jansen, E.; Ruskovska, T. Serum Biomarkers of (Anti)Oxidant Status for Epidemiological Studies. Int. J. Mol. Sci. 2015, 16, 27378–27390. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Attanzio, A.; Calvi, P.; Mulè, F.; Tesoriere, L.; Allegra, M.; Amato, A. Indicaxanthin from Fruit Ameliorates Glucose Dysmetabolism and Counteracts Insulin Resistance in High-Fat-Diet-Fed Mice. Antioxidants 2021, 11, 80. [Google Scholar] [CrossRef]
- Cruz-Carrión, Á.; Ruiz de Azua, M.J.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Suárez, M.; Arola-Arnal, A. Tomatoes Consumed in-Season Prevent Oxidative Stress in Fischer 344 Rats: Impact of Geographical Origin. Food Funct. 2021, 12, 8340–8350. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Chávez-Manzanera, E.; Meza-Arana, C.E.; Brito-Córdova, G.; Mehta, R.; Pérez-Méndez, O.; Gómez-Pérez, F.J. Effect of Tomato Consumption on High-Density Lipoprotein Cholesterol Level: A Randomized, Single-Blinded, Controlled Clinical Trial. Diabetes Metab. Syndr. Obes. 2013, 6, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.d.O.B.; Dos Santos, C.A.; Leite, J.I.A.; Caldas, A.P.S.; Bressan, J. Impact of Nutrients and Food Components on Dyslipidemias: What Is the Evidence? Adv. Nutr. 2015, 6, 703–711. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Foods of the Mediterranean Diet: Tomato, Olives, Chili Pepper, Wheat Flour and Wheat Germ. J. Prev. Med. Hyg. 2022, 63, E4–E11. [Google Scholar]
- Willcox, J.K.; Catignani, G.L.; Lazarus, S. Tomatoes and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2003, 43, 1–18. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and Lycopene Supplementation and Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef]
- Islam, N.; Akhter, N. Comparative Study of Protective Effect of Tomato Juice and N-Hexane Extract of Tomato on Blood Lipids and Oxidative Stress in Cholesterol-Fed Rats. Anwer Khan Mod. Med. Coll. J. 2017, 8, 30–37. [Google Scholar] [CrossRef]
- Khayat Nouri, M.H.; Namvaran Abbas Abad, A. Comparative Study of Tomato and Tomato Paste Supplementation on the Level of Serum Lipids and Lipoproteins Levels in Rats Fed with High Cholesterol. Iran. Red Crescent Med. J. 2013, 15, 287–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Shan, Y.; Wang, J.; Sun, B.; Xu, Y.; Zhang, W.; Zhang, Y. Recent Trends in Aroma Release and Perception during Food Oral Processing: A Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 3441–3457. [Google Scholar] [CrossRef] [PubMed]
- Oruna-Concha, M.-J.; Methven, L.; Blumenthal, H.; Young, C.; Mottram, D.S. Differences in Glutamic Acid and 5′-Ribonucleotide Contents between Flesh and Pulp of Tomatoes and the Relationship with Umami Taste. J. Agric. Food Chem. 2007, 55, 5776–5780. [Google Scholar] [CrossRef]
- Wu, J.L.; Wu, Q.P.; Huang, J.M.; Chen, R.; Cai, M.; Tan, J.B. Effects of L-Malate on Physical Stamina and Activities of Enzymes Related to the Malate-Aspartate Shuttle in Liver of Mice. Physiol. Res. 2007, 56, 213–220. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Hirai, S.; Takahashi, H.; Goto, T.; Ohyane, C.; Tsugane, T.; Konishi, C.; Fujii, T.; Inai, S.; Iijima, Y.; et al. 9-Oxo-10(E),12(E)-Octadecadienoic Acid Derived from Tomato Is a Potent PPAR α Agonist to Decrease Triglyceride Accumulation in Mouse Primary Hepatocytes. Mol. Nutr. Food Res. 2011, 55, 585–593. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Khaire, A.A. Leptin as a Predictive Marker for Metabolic Syndrome. Cytokine 2019, 121, 154735. [Google Scholar] [CrossRef]
- Zhao, S.; Li, N.; Zhu, Y.; Straub, L.; Zhang, Z.; Wang, M.-Y.; Zhu, Q.; Kusminski, C.M.; Elmquist, J.K.; Scherer, P.E. Partial Leptin Deficiency Confers Resistance to Diet-Induced Obesity in Mice. Mol. Metab. 2020, 37, 100995. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Chen, L.; Lin, X.; Xu, Y.; Ren, J.; Fu, J.; Qiu, Y. Effect of Oleoylethanolamide on Diet-Induced Nonalcoholic Fatty Liver in Rats. J. Pharmacol. Sci. 2015, 127, 244–250. [Google Scholar] [CrossRef]
- Contreras, A.V.; Torres, N.; Tovar, A.R. PPAR-α as a Key Nutritional and Environmental Sensor for Metabolic Adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef]
- Haluzík, M.M.; Haluzík, M. PPAR-Alpha and Insulin Sensitivity. Physiol. Res. 2006, 55, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome Proliferator-Activated Receptors and Their Ligands: Nutritional and Clinical Implications--a Review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Bahne, E.; Sun, E.W.L.; Young, R.L.; Hansen, M.; Sonne, D.P.; Hansen, J.S.; Rohde, U.; Liou, A.P.; Jackson, M.L.; de Fontgalland, D.; et al. Metformin-Induced Glucagon-like Peptide-1 Secretion Contributes to the Actions of Metformin in Type 2 Diabetes. JCI Insight 2018, 3, e93936. [Google Scholar] [CrossRef]
- Song, S.; Attia, R.R.; Connaughton, S.; Niesen, M.I.; Ness, G.C.; Elam, M.B.; Hori, R.T.; Cook, G.A.; Park, E.A. Peroxisome Proliferator Activated Receptor Alpha (PPARalpha) and PPAR Gamma Coactivator (PGC-1alpha) Induce Carnitine Palmitoyltransferase IA (CPT-1A) via Independent Gene Elements. Mol. Cell. Endocrinol. 2010, 325, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Fuior, E.V.; Zvintzou, E.; Filippatos, T.; Giannatou, K.; Mparnia, V.; Simionescu, M.; Gafencu, A.V.; Kypreos, K.E. Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease. Biomedicines 2023, 11, 2696. [Google Scholar] [CrossRef]
- Vu-Dac, N.; Schoonjans, K.; Laine, B.; Fruchart, J.C.; Auwerx, J.; Staels, B. Negative Regulation of the Human Apolipoprotein A-I Promoter by Fibrates Can Be Attenuated by the Interaction of the Peroxisome Proliferator-Activated Receptor with Its Response Element. J. Biol. Chem. 1994, 269, 31012–31018. [Google Scholar] [CrossRef]
- Reidy, S.P.; Weber, J. Leptin: An Essential Regulator of Lipid Metabolism. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 285–298. [Google Scholar] [CrossRef]
- Lin, Q.; Weis, S.; Yang, G.; Weng, Y.-H.; Helston, R.; Rish, K.; Smith, A.; Bordner, J.; Polte, T.; Gaunitz, F.; et al. Heme Oxygenase-1 Protein Localizes to the Nucleus and Activates Transcription Factors Important in Oxidative Stress. J. Biol. Chem. 2007, 282, 20621–20633. [Google Scholar] [CrossRef]
- Ding, G.; Fu, M.; Qin, Q.; Lewis, W.; Kim, H.W.; Fukai, T.; Bacanamwo, M.; Chen, Y.E.; Schneider, M.D.; Mangelsdorf, D.J.; et al. Cardiac Peroxisome Proliferator-Activated Receptor Gamma Is Essential in Protecting Cardiomyocytes from Oxidative Damage. Cardiovasc. Res. 2007, 76, 269–279. [Google Scholar] [CrossRef]
- Hawkes, H.-J.K.; Karlenius, T.C.; Tonissen, K.F. Regulation of the Human Thioredoxin Gene Promoter and Its Key Substrates: A Study of Functional and Putative Regulatory Elements. Biochim. Biophys. Acta 2014, 1840, 303–314. [Google Scholar] [CrossRef]
- Muzio, G.; Barrera, G.; Pizzimenti, S. Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer. Antioxidants 2021, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Teruel, T.; Smith, S.A.; Peterson, J.; Clapham, J.C. Synergistic Activation of UCP-3 Expression in Cultured Fetal Rat Brown Adipocytes by PPARalpha and PPARgamma Ligands. Biochem. Biophys. Res. Commun. 2000, 273, 560–564. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Equation Applied |
|---|---|
| % Total dry matter | Weight [(dry sample + dish) − dish] initial weight of sample × 100 |
| % Total moisture | 100 − % total dry matter |
| % Protein | % nitrogen × protein factor (6,25) |
| % Crude Fat | weight [(cup + fat residue) − cup empty] initial sample weight × 100 |
| % Carbohydrate | 100 − [(% total moisture + % ash + % protein + % crude fat + % total dietary fiber)] |
| (A) | |||||||
| Components | GT | GTJ | Statistical Values | ||||
| Water (g/100 g) | 91.5 ± 0.3 | 94 ± 0.6 ** | p = 0.003 t = 6.455, df = 4 | ||||
| Proteins (g/100 g) | 5.44 ± 0.4 | 1.31 **** ± 0.06 | p < 0.0001 t = 17.6856; df = 4 | ||||
| Lipids (g/100 g) | 0.2 ± 0.02 | 0.19 ± 0.05 | n.s. | ||||
| Carbohydrates (g/100 g) | 4.80 ± 0.49 | 4.08 ±0.56 | n.s. | ||||
| Energy (Kcal) | 43 ± 2.0 | 23 ± 3.5 ** | p = 0.001 t = 8.593, df = 4 | ||||
| (B) | |||||||
| Parameters | GT | GTJ | Statistical Values | ||||
| Dry matter (g/Kg−1) | 85 ± 1.4 | 158 ± 20.5 ** | p = 0.0035 t = 6.153; df = 4 | ||||
| pH | 4.4 ± 0.1 | 4.2 ± 0.1 | n.s. | ||||
| Brix, °Bx | 5.3 ± 0.2 | 6.6 ± 0.05 *** | p = 0.0003 t = 10.92; df = 4 | ||||
| Titratable acidity (mg%) | 0.61 ± 0.01 | 0.49 ± 0.03 ** | p = 0.0028 t = 6.573; df = 4 | ||||
| Total Polyphenols (mg/100 g) | 87.6 ± 13.5 | 82.5 ± 12.7 | n.s. | ||||
| (C) | |||||||
| Micronutrients | GT | GTJ | RDA (mg/die) | UL (mg/die) | Statistical Values | ||
| Na (mg/100 g) | 90.9 ± 8.1 | 14.4 ± 4.7 *** | n.a. | 2000 * | p = 0.0001 t = 14.15, df = 4 | ||
| K (mg/100 g) | 930.1 ± 7.5 | 113 ± 6.7 **** | n.a. | n.a. | p < 0.0001 t = 140.7, df = 4 | ||
| Mg (mg/100 g) | 164.5 ± 10.5 | 10.8 ± 2.4 **** | 170 | 250 | p < 0.0001 t = 24.72, df = 4 | ||
| Ca (mg/100 g) | 277.4 ± 9.3 | 8.8 ± 2.3 **** | 1000 | 2500 | p < 0.0001 t = 48.56, df = 4 | ||
| Zn (mg/100 g) | 6.7 ± 1.5 | 0.2 ± 0.03 ** | p = 0.0017 t = 7.504, df = 4 | ||||
| Fe (mg/100 g) | 37.9 ± 5.7 | 0.84 ± 0.02 *** | Woman | Man | n.a. | p = 0.0004 t = 11.26, df = 4 | |
| 18–10 | 10 | ||||||
| Cu | 4 ± 0.5 | 0.04 ± 0.02 *** | 0.9 | 5 | p = 0.0002 t = 13.71, df = 4 | ||
| Ni | 0.24 ± 0.06 | 0.007 ± 0.001 ** | p = 0.0025 t = 6.725, df = 4 | ||||
| Mn | 1.7 ± 0.3 | 0.07 ± 0.04 *** | Woman 2.3 | Man 2.7 | n.a. | p = 0.0007 t = 9.328, df = 4 | |
| Al | 95.2 ± 4.87 | 0.65 ± 0.03 **** | p < 0.0001 t = 33.63, df = 4 | ||||
| Vit. A (β-carotene, mg/100 g) | 391 ± 1.3 | 398 ± 3.6 * | 7.5 (μg 1250 RE) | 3000 μg | p = 0.0339 t = 3.168, df = 4 | ||
| Ascorbic acid, Vit. C (mg/100 g) | 17.04 ± 3.5 | 4.4 ± 2.4 ** | 1000 | n.a. | p = 0.0067 t = 5.159, df = 4 | ||
| Samples | R.T. (Minutes) | 9-oxo-10(E),12(E)-ODA (μg/mL) | R.T. (Minutes) | 9-oxo-10(E),12(Z)-ODA (μg/mL) |
|---|---|---|---|---|
| RT | 8.1 | 0.76 ± 0.02 | 7.4 | 0.610 ± 0.007 |
| GT | 8.1 | 1.04 ± 0.05 * | 7.4 | 1.43 ± 0.03 * |
| GTJ | 8.2 | 0.22 ± 0.01 | 7.5 | 0.23 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Majo, D.; Ricciardi, N.; Moncada, A.; Allegra, M.; Frinchi, M.; Di Liberto, V.; Pitonzo, R.; Rappa, F.; Saiano, F.; Vetrano, F.; et al. Golden Tomato Juice Enhances Hepatic PPAR-α Expression, Mitigates Metabolic Dysfunctions and Influences Redox Balance in a High-Fat-Diet Rat Model. Antioxidants 2024, 13, 1324. https://doi.org/10.3390/antiox13111324
Di Majo D, Ricciardi N, Moncada A, Allegra M, Frinchi M, Di Liberto V, Pitonzo R, Rappa F, Saiano F, Vetrano F, et al. Golden Tomato Juice Enhances Hepatic PPAR-α Expression, Mitigates Metabolic Dysfunctions and Influences Redox Balance in a High-Fat-Diet Rat Model. Antioxidants. 2024; 13(11):1324. https://doi.org/10.3390/antiox13111324
Chicago/Turabian StyleDi Majo, Danila, Nicolò Ricciardi, Alessandra Moncada, Mario Allegra, Monica Frinchi, Valentina Di Liberto, Rosa Pitonzo, Francesca Rappa, Filippo Saiano, Filippo Vetrano, and et al. 2024. "Golden Tomato Juice Enhances Hepatic PPAR-α Expression, Mitigates Metabolic Dysfunctions and Influences Redox Balance in a High-Fat-Diet Rat Model" Antioxidants 13, no. 11: 1324. https://doi.org/10.3390/antiox13111324
APA StyleDi Majo, D., Ricciardi, N., Moncada, A., Allegra, M., Frinchi, M., Di Liberto, V., Pitonzo, R., Rappa, F., Saiano, F., Vetrano, F., Miceli, A., Giglia, G., Ferraro, G., Sardo, P., & Gambino, G. (2024). Golden Tomato Juice Enhances Hepatic PPAR-α Expression, Mitigates Metabolic Dysfunctions and Influences Redox Balance in a High-Fat-Diet Rat Model. Antioxidants, 13(11), 1324. https://doi.org/10.3390/antiox13111324










