Fruits, Spices and Honey Phenolic Compounds: A Comprehensive Review on Their Origin, Methods of Extraction and Beneficial Health Properties
Abstract
1. Introduction
2. Fruits
2.1. Citrus
2.2. Orange
2.3. Lemon
2.4. Grapefruit
2.5. Prunus
2.5.1. Apricot
2.5.2. Peach
2.5.3. Plum
2.5.4. Sweet Cherry
3. Spices
3.1. Oregano
3.2. Cinnamon
3.3. Clove
3.4. Saffron
3.5. Turmeric
4. Honey
5. Discussion
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haddad, M.A.; El-Qudah, J.; Abu-Romman, S.; Obeidat, M.; Iommi, C.; Jaradat, D.M.M. Phenolics in Mediterranean and Middle East Important Fruits. J. AOAC Int. 2020, 103, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Mizia, S.; Felińczak, A.; Włodarek, D.; Syrkiewicz-Świtała, M. Evaluation of Eating Habits and Their Impact on Health among Adolescents and Young Adults: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 3996. [Google Scholar] [CrossRef] [PubMed]
- Mohammadbeigi, A.; Asgarian, A.; Moshir, E.; Heidari, H.; Afrashteh, S.; Khazaei, S.; Ansari, H. Fast Food Consumption and Overweight/Obesity Prevalence in Students and Its Association with General and Abdominal Obesity. J. Prev. Med. Hyg. 2018, 59, E236–E240. [Google Scholar] [CrossRef]
- Nixon, H.; Doud, L. Do Fast Food Restaurants Cluster around High Schools? A Geospatial Analysis of Proximity of Fast Food Restaurants to High Schools and the Connection to Childhood Obesity Rates. J. Agric. Food Syst. Community Dev. 2011, 2, 181–194. [Google Scholar] [CrossRef][Green Version]
- Al-Otaibi, H.; Basuny, A.M. Fast Food Consumption Associated with Obesity/Overweight Risk among University Female Student in Saudi Arabia. Pak. J. Nutr. 2015, 14, 511–516. [Google Scholar] [CrossRef][Green Version]
- Dumanovsky, T.; Huang, C.Y.; Nonas, C.A.; Matte, T.D.; Bassett, M.T.; Silver, L.D. Changes in Energy Content of Lunchtime Purchases from Fast Food Restaurants after Introduction of Calorie Labelling: Cross Sectional Customer Surveys. BMJ 2011, 343, d4464. [Google Scholar] [CrossRef]
- Fuhrman, J. The Hidden Dangers of Fast and Processed Food. Am. J. Lifestyle Med. 2018, 12, 375–381. [Google Scholar] [CrossRef]
- Cieślik, E.; Gręda, A.; Adamus, W. Contents of Polyphenols in Fruit and Vegetables. Food Chem. 2006, 94, 135–142. [Google Scholar] [CrossRef]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Klimczak, I.; Małecka, M.; Szlachta, M.; Gliszczyńska-Świgło, A. Effect of Storage on the Content of Polyphenols, Vitamin C and the Antioxidant Activity of Orange Juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Gorinstein, S.; Haruenkit, R.; Park, Y.-S.; Jung, S.-T.; Zachwieja, Z.; Jastrzebski, Z.; Katrich, E.; Trakhtenberg, S.; Belloso, O.M. Bioactive Compounds and Antioxidant Potential in Fresh and Dried Jaffa® Sweeties, a New Kind of Citrus Fruit. J. Sci. Food Agric. 2004, 84, 1459–1463. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Beecher, G.R.; Bhagwat, S.A.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in Oranges, Tangerines (Mandarins), Tangors, and Tangelos: A Compilation and Review of the Data from the Analytical Literature. J. Food Compos. Anal. 2006, 19, S66–S73. [Google Scholar] [CrossRef]
- Abad-García, B.; Garmón-Lobato, S.; Sánchez-Ilárduya, M.B.; Berrueta, L.A.; Gallo, B.; Vicente, F.; Alonso-Salces, R.M. Polyphenolic Contents in Citrus Fruit Juices: Authenticity Assessment. Eur. Food Res. Technol. 2014, 238, 803–818. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The Content of Polyphenols and Carotenoids in Three Apricot Cultivars Depending on Stage of Maturity and Geographical Region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Infante, R.; Contador, L.; Rubio, P.; Aros, D.; Peña-Neira, Á. Postharvest Sensory and Phenolic Characterization of Elegant Lady and Carson Peaches. Chil. J. Agric. Res. 2011, 71, 445–451. [Google Scholar] [CrossRef]
- Nandakumar, V.; Singh, T.; Katiyar, S.K. Multi-Targeted Prevention and Therapy of Cancer by Proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC−DAD−ESIMS Analysis of Phenolic Compounds in Nectarines, Peaches, and Plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic Compounds Profile and Antioxidant Properties of Six Sweet Cherry (Prunus avium) Cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef]
- Rizaldy, D.; Insanu, M.; Sabila, N.; Haniffadli, A.; Zahra, A.A.; Pratiwi, S.N.E.; Mudrika, S.N.; Hartati, R.; Fidrianny, I. Lemon (Citrus limon L.): Antioxidative Activity and Its Marker Compound. Biointerface Res. Appl. Chem. 2022, 13, 21. [Google Scholar] [CrossRef]
- Oboh, G.; Bello, F.O.; Ademosun, A.O.; Akinyemi, A.J.; Adewuni, T.M. Antioxidant, Hypolipidemic, and Anti-Angiotensin-1-Converting Enzyme Properties of Lemon (Citrus limon) and Lime (Citrus aurantifolia) Juices. Comp. Clin. Pathol. 2015, 24, 1395–1406. [Google Scholar] [CrossRef]
- Hussain, A.I.; Khosa, M.K.; Chatha, S.A.S.; Zia, K.M.; Riaz, H.; Aslam, K. Spectrophotometric Quantification of Antioxidant Phytochemicals in Juices from Four Different Varieties of Citrus limon, Indigenous to Pakistan. JCSP 2011, 33, 188. [Google Scholar]
- Czech, A.; Malik, A.; Sosnowska, B.; Domaradzki, P. Bioactive Substances, Heavy Metals, and Antioxidant Activity in Whole Fruit, Peel, and Pulp of Citrus Fruits. Int. J. Food Sci. 2021, 2021, 6662259. [Google Scholar] [CrossRef]
- Fejzić, A.; Ćavar, S. Phenolic Compounds and Antioxidant Activity of Some Citruses. Bull. Chem. Technol. Bosnia Herzeg. 2014, 42, 1–4. [Google Scholar]
- Samfira, I.; Rodino, S.; Petrache, P.; Cristina, R.T.; Butu, M.; Butnariu, M. Characterization and identity confirmation of essential oils by mid infrared absorption spectrophotometry. Dig. J. Nanomater. Biostruct. 2015, 10, 557–566. [Google Scholar]
- Singh, N.; Yadav, S.S. A Review on Health Benefits of Phenolics Derived from Dietary Spices. Curr. Res. Food Sci. 2022, 5, 1508–1523. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Strack, D. Phenolic Metabolism. In Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Carmen Seijo, M. Nutritional Value and Antioxidant Activity of Honeys Produced in a European Atlantic Area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Review of the Medicinal Effects of Tualang Honey and a Comparison with Manuka Honey. Malays. J. Med. Sci. 2013, 20, 6–13. [Google Scholar] [PubMed]
- Ntakoulas, D.D.; Tsagkaris, A.S.; Raptis, S.; Pasias, I.N.; Raptopoulou, K.G.; Kharoshka, A.; Schulzova, V.; Proestos, C. Study of Authenticity, Quality Characteristics and Bioactivity in Honey Samples from Different Botanical Origins and Countries. J. Food Compos. Anal. 2024, 136, 106716. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Sulaiman, A.; Alhaddad, H.; Alhadidi, Q. Natural Polyphenols: Influence on Membrane Transporters. J. Intercult. Ethnopharmacol. 2016, 5, 97. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of Dietary Flavonoids and Phenolic Compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. Biomed. Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- An, G.; Wang, X.; Morris, M.E. Flavonoids Are Inhibitors of Human Organic Anion Transporter 1 (OAT1)—Mediated Transport. Drug Metab. Dispos. 2014, 42, 1357–1366. [Google Scholar] [CrossRef]
- Arora, M.; Kaur, P. Antimicrobial & Antioxidant Activity of Orange Pulp and Peel. Int. J. Sci. Res. 2013, 2, 412–415. [Google Scholar]
- Rapisarda, P.; Tomaino, A.; Lo Cascio, R.; Bonina, F.; De Pasquale, A.; Saija, A. Antioxidant Effectiveness as Influenced by Phenolic Content of Fresh Orange Juices. J. Agric. Food Chem. 1999, 47, 4718–4723. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Marin, F.R.; Ortuño, A.; Del Río, J.A. Uses and Properties of Citrus Flavonoids. J. Agric. Food Chem. 1997, 45, 4505–4515. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. The Relative Contributions of Ascorbic Acid and Phenolic Antioxidants to the Total Antioxidant Activity of Orange and Apple Fruit Juices and Blackcurrant Drink. Food Chem. 1997, 60, 331–337. [Google Scholar] [CrossRef]
- Sawalha, S.M.S.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of Main Phenolic Compounds in Sweet and Bitter Orange Peel Using CE–MS/MS. Food Chem. 2009, 116, 567–574. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K. Phenols in Citrus Peel Byproducts. Concentrations of Hydroxycinnamates and Polymethoxylated Flavones in Citrus Peel Molasses. J. Agric. Food Chem. 2001, 49, 3268–3273. [Google Scholar] [CrossRef]
- Lagha-Benamrouche, S.; Madani, K. Phenolic Contents and Antioxidant Activity of Orange Varieties (Citrus sinensis L. and Citrus aurantium L.) Cultivated in Algeria: Peels and Leaves. Ind. Crops Prod. 2013, 50, 723–730. [Google Scholar] [CrossRef]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary Guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Arola-Arnal, A. Optimization of a Polyphenol Extraction Method for Sweet Orange Pulp (Citrus sinensis L.) to Identify Phenolic Compounds Consumed from Sweet Oranges. PLoS ONE 2019, 14, e0211267. [Google Scholar] [CrossRef]
- Ngugi, C.C.; Okoth, E.O.; Muchiri, M. Effects of Dietary Levels of Essential Oil (EO) Extract from Bitter Lemon (Citrus limon) Fruit Peels on Growth, Biochemical, Haemato-Immunological Parameters and Disease Resistance in Juvenile Labeo Victorianus FIngerlings Challenged with Aeromonas Hydrophila. Aquac. Res. 2016, 48, 2253–2265. [Google Scholar] [CrossRef]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds of Citrus Limon for Food and Health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Exploring the Antioxidant Properties of Citrus FFLimon (Lemon) Peel Ultrasound Extract after the Cloud Point Extraction Method. Biomass 2024, 4, 202–216. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of Phenolic Profile and Antioxidant Capacity of Different Fruit Part from Lemon (Citrus limon Burm.) Cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef]
- Singh, V.; Chahal, T.S.; Grewal, S.K.; Gill, P.S. Effect of Fruit Development Stages on Antioxidant Properties and Bioactive Compounds in Peel, Pulp and Juice of Grapefruit Varieties. J. Food Meas. Charact. 2021, 15, 2531–2539. [Google Scholar] [CrossRef]
- Agudelo, C.; Barros, L.; Santos-Buelga, C.; Martínez-Navarrete, N.; Ferreira, I.C.F.R. Phytochemical Content and Antioxidant Activity of Grapefruit (Star Ruby): A Comparison between Fresh Freeze-Dried Fruits and Different Powder Formulations. LWT 2017, 80, 106–112. [Google Scholar] [CrossRef]
- Kelebek, H. Sugars, Organic Acids, Phenolic Compositions and Antioxidant Activity of Grapefruit (Citrus paradisi) Cultivars Grown in Turkey. Ind. Crops Prod. 2010, 32, 269–274. [Google Scholar] [CrossRef]
- Vanamala, J.; Reddivari, L.; Yoo, K.S.; Pike, L.M.; Patil, B.S. Variation in the Content of Bioactive Flavonoids in Different Brands of Orange and Grapefruit Juices. J. Food Compos. Anal. 2006, 19, 157–166. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice Components and Antioxidant Capacity of Citrus Varieties Cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Vincenzo, S.; Pellicanò, T.; Giuffrè, A.; Zappia, C.; Capocasale, M.; Poiana, M. Physical chemical properties and antioxidant capacities of grapefruit juice (Citrus paradisi) extracted from two different varieties. Int. Food Res. J. 2018, 25, 1978–1984. [Google Scholar]
- Potter, D. Prunus. In Wild Crop Relatives: Genomic and Breeding Resources: Temperate Fruits; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 129–145. ISBN 978-3-642-16057-8. [Google Scholar]
- Guerra, M.E.; Rodrigo, J. Japanese Plum Pollination: A Review. Sci. Hortic. 2015, 197, 674–686. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of Phytochemicals, Antioxidant Capacity, and Antidiabetic Activity of Novel Smoothies from Selected Prunus Fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of Phenolic Compounds and Antioxidant Capacity in Fruits of Apricot Genotypes. Molecules 2010, 15, 6285–6305. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64 (Suppl. S3), S112–S120. [Google Scholar] [CrossRef]
- Erdoğan, S.; Erdemoğlu, S. Evaluation of Polyphenol Contents in Differently Processed Apricots Using Accelerated Solvent Extraction Followed by High-Performance Liquid Chromatography-Diode Array Detector. Int. J. Food Sci. Nutr. 2011, 62, 729–739. [Google Scholar] [CrossRef]
- Kan, T.; Bostan, S.Z. Effect of Sulfurization and Process Conditions on Polyphenol Content of Anatolian Apricots (Prunus armeniaca). J. Food Process. Preserv. 2013, 37, 163–170. [Google Scholar] [CrossRef]
- Gundogdu, M.; Ercisli, S.; Berk, S.; Kan, T.; Canan, I.; Gecer, M.K. Diversity on Color and Phenolic Compounds in Apricot Fruits. J. Food Meas. Charact. 2017, 11, 2087–2093. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Cipriano, L.; Nazzaro, F. Apricots: Biochemistry and Functional Properties. Curr. Opin. Food Sci. 2018, 19, 23–29. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, W.; Wang, X.; Cao, J.; Jiang, W. Polyphenol Composition and Antioxidant Capacity in Pulp and Peel of Apricot Fruits of Various Varieties and Maturity Stages at Harvest. Int. J. Food Sci. Technol. 2018, 53, 327–336. [Google Scholar] [CrossRef]
- Ding, T.; Cao, K.; Fang, W.; Zhu, G.; Chen, C.; Wang, X.; Wang, L. Evaluation of Phenolic Components (Anthocyanins, Flavanols, Phenolic Acids, and Flavonols) and Their Antioxidant Properties of Peach Fruits. Sci. Hortic. 2020, 268, 109365. [Google Scholar] [CrossRef]
- Liao, X.; Greenspan, P.; Pegg, R.B. Characterizing the Phenolic Constituents and Antioxidant Capacity of Georgia Peaches. Food Chem. 2019, 271, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Da Costa, G.; Temsamani, H.; Renouf, E.; Mérillon, J.-M.; Madani, K.; Mesnil, M.; Monvoisin, A.; et al. Phenolic Contents and Bioactive Potential of Peach Fruit Extracts. Food Chem. 2016, 202, 212–220. [Google Scholar] [CrossRef]
- Cantín, C.M.; Moreno, M.A.; Gogorcena, Y. Evaluation of the Antioxidant Capacity, Phenolic Compounds, and Vitamin C Content of Different Peach and Nectarine [Prunus persica (L.) Batsch] Breeding Progenies. J. Agric. Food Chem. 2009, 57, 4586–4592. [Google Scholar] [CrossRef]
- Leontowicz, H.; Gorinstein, S.; Lojek, A.; Leontowicz, M.; Číž, M.; Soliva-Fortuny, R.; Park, Y.-S.; Jung, S.-T.; Trakhtenberg, S.; Martin-Belloso, O. Comparative Content of Some Bioactive Compounds in Apples, Peaches and Pears and Their Influence on Lipids and Antioxidant Capacity in Rats. J. Nutr. Biochem. 2002, 13, 603–610. [Google Scholar] [CrossRef]
- Shin, T.-Y.; Park, S.-B.; Yoo, J.-S.; Kim, I.K.; Lee, H.-S.; Kwon, T.K.; Kim, M.K.; Kim, J.C.; Kim, S.-H. Anti-Allergic Inflammatory Activity of the Fruit of Prunus persica: Role of Calcium and NF-ĸB. Food Chem. Toxicol. 2010, 48, 2797–2802. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.-H. Antioxidant Activity of Peach Blossom Extracts. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 46–53. [Google Scholar] [CrossRef]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying Peach and Plum Polyphenols with Chemopreventive Potential against Estrogen-Independent Breast Cancer Cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenolics from Peach (Prunus persica Var. Rich Lady) Inhibit Tumor Growth and Metastasis of MDA-MB-435 Breast Cancer Cells in Vivo. J. Nutr. Biochem. 2014, 25, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Zhang, X.; Mu, Q.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.; Yu, X. Differences in Total Phenolics, Antioxidant Activity and Metabolic Characteristics in Peach Fruits at Different Stages of Ripening. LWT 2023, 178, 114586. [Google Scholar] [CrossRef]
- Satora, P.; Tuszyński, T. Influence of Indigenous Yeasts on the Fermentation and Volatile Profile of Plum Brandies. Food Microbiol. 2010, 27, 418–424. [Google Scholar] [CrossRef]
- Sójka, M.; Kołodziejczyk, K.; Milala, J.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Composition and Properties of the Polyphenolic Extracts Obtained from Industrial Plum Pomaces. J. Funct. Foods 2015, 12, 168–178. [Google Scholar] [CrossRef]
- Hooshmand, S.; Arjmandi, B.H. Viewpoint: Dried Plum, an Emerging Functional Food That May Effectively Improve Bone Health. Ageing Res. Rev. 2009, 8, 122–127. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Byrne, D.; Okie, W.R.; Cisneros-Zevallos, L. Selecting New Peach and Plum Genotypes Rich in Phenolic Compounds and Enhanced Functional Properties. Food Chem. 2006, 96, 273–280. [Google Scholar] [CrossRef]
- Nunes, C.; Guyot, S.; Marnet, N.; Barros, A.S.; Saraiva, J.A.; Renard, C.M.G.C.; Coimbra, M.A. Characterization of Plum Procyanidins by Thiolytic Depolymerization. J. Agric. Food Chem. 2008, 56, 5188–5196. [Google Scholar] [CrossRef] [PubMed]
- Olawuyi, I.F.; Akbarovich, S.A.; Kim, C.K.; Lee, W.Y. Effect of Combined Ultrasound-Enzyme Treatment on Recovery of Phenolic Compounds, Antioxidant Capacity, and Quality of Plum (Prunus salicina L.) Juice. J. Food Process. Preserv. 2021, 45, e15074. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of Phenolic Compounds Profile and Antioxidant Properties of Different Sweet Cherry (Prunus avium L.) Varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Kolniak-Ostek, J.; Oziembłowski, M.; Ticha, A.; Hyšpler, R.; Zadak, Z.; Židová, P.; Paprstein, F. Comparison of Old Cherry Cultivars Grown in Czech Republic by Chemical Composition and Bioactive Compounds. Food Chem. 2017, 228, 136–142. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between Habitual Polyphenol Intake and Incidence of Cardiovascular Events in the PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Wu, T.; Tang, Q.; Yu, Z.; Gao, Z.; Hu, H.; Chen, W.; Zheng, X.; Yu, T. Inhibitory Effects of Sweet Cherry Anthocyanins on the Obesity Development in C57BL/6 Mice. Int. J. Food Sci. Nutr. 2013, 65, 351–359. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.E.; Jenner, A.; Roodenrys, S. Acute Reduction in Blood Pressure Following Consumption of Anthocyanin-Rich Cherry Juice May Be Dose-Interval Dependant: A Pilot Cross-over Study. Int. J. Food Sci. Nutr. 2016, 67, 47–52. [Google Scholar] [CrossRef]
- Faniadis, D.; Drogoudi, P.D.; Vasilakakis, M. Effects of Cultivar, Orchard Elevation, and Storage on Fruit Quality Characters of Sweet Cherry (Prunus avium L.). Sci. Hortic. 2010, 125, 301–304. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhong, F.; Tian, R.; Zhang, K.; Zhang, X.; Li, T. Comparative Study of Phenolic Compounds and Antioxidant Activity in Different Species of Cherries. J. Food Sci. 2011, 76, C633–C638. [Google Scholar] [CrossRef]
- Hu, T.; Subbiah, V.; Wu, H.; BK, A.; Rauf, A.; Alhumaydhi, F.A.; Suleria, H.A.R. Determination and Characterization of Phenolic Compounds from Australia-Grown Sweet Cherries (Prunus avium L.) and Their Potential Antioxidant Properties. ACS Omega 2021, 6, 34687–34699. [Google Scholar] [CrossRef]
- Llupa, J.; Gašić, U.; Brceski, I.; Demertzis, P.; Tešević, V.; Topi, D. LC-MS/MS Characterization of phenolic compounds in the quince (Cydonia oblonga Mill.) and sweet cherry (Prunus avium L.) fruit juices. Agric. For. 2022, 68, 193–205. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S.; Kumar, S.; Narashiman, B. A Review on Traditional Uses, Phytochemistry, Pharmacology, and Clinical Research of Dietary Spice Cuminum cyminum L. Phytother. Res. 2021, 35, 5007–5030. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Singhal, P.; Singla, N.; Sakhare, D.; Sharma, A.K. A Comparative Evaluation of In-Vitro Antioxidant Activity of Some Commonly Used Spices of Northern India. Nat. Prod. J. 2017, 7, 131–136. [Google Scholar] [CrossRef]
- Butnariu, M. Allelopathic Effect of Festuca Rubra on Perennial Grasses. Rom. Biotechnol. Lett. 2013, 18, 8190–8196. [Google Scholar]
- Lazaridis, D.G.; Karabagias, V.K.; Karabagias, I.K.; Andritsos, N.D.; Giannakas, A.E. Physicochemical and Phytochemical Characterization of Green Coffee, Cinnamon Clove, and Nutmeg EEGO, and Aroma Evaluation of the Raw Powders. Eur. Food Res. Technol. 2024, 250, 83–96. [Google Scholar] [CrossRef]
- Rajendran, S.; Rajagopal, P.; Jayaraman, S.; Mahendra, J.; Kasturi, R.; Nagarajan, V.; Karuppan, M. A Review on Dietary Poly Phenols: Herbal Neutraceuticals to Combat Nephrotoxicity. NVEO 2021, 8, 7584–7597. [Google Scholar]
- Mark, R.; Lyu, X.; Lee, J.J.L.; Parra-Saldívar, R.; Chen, W.N. Sustainable Production of Natural Phenolics for Functional Food Applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical Polyphenols: New Analytical Challenges and Opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef]
- Tsao, R.; Deng, Z. Separation Procedures for Naturally Occurring Antioxidant Phytochemicals. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic Acids and Other Cinnamates—Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Vasquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and Spices-Biomarkers of Intake Based on Human Intervention Studies—A Systematic Review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef]
- Saraswathi, K.; Arumugam, P.; Sivaraj, C. Pharmacological Activities of Differential Parts of Selected Essential Indian Spices. J. Pharmacogn. Phytochem. 2020, 9, 2024–2033. [Google Scholar] [CrossRef]
- Muchuweti, M.; Kativu, E.; Mupure, C.H.; Chidewe, C.; Ndhlala, A.R.; Benhura, M.A.N. Phenolic Composition and Antioxidant Properties of Some Spices. Am. J. Food Technol. 2007, 2, 414–420. [Google Scholar] [CrossRef]
- Assefa, A.D.; Keum, Y.-S.; Saini, R.K. A Comprehensive Study of Polyphenols Contents and Antioxidant Potential of 39 Widely Used Spices and Food Condiments. J. Food Meas. Charact. 2018, 12, 1548–1555. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Roussel, A.-M.; Hininger, I.; Benaraba, R.; Ziegenfuss, T.N.; Anderson, R.A. Antioxidant Effects of a Cinnamon Extract in People with Impaired Fasting Glucose That Are Overweight or Obese. J. Am. Coll. Nutr. 2009, 28, 16–21. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Negi, P.S.; Jena, B.S.; Jagan Mohan Rao, L. Antioxidant and Antimutagenic Activities of Cinnamomum zeylanicum Fruit Extracts. J. Food Compos. Anal. 2007, 20, 330–336. [Google Scholar] [CrossRef]
- El-Maati, M.F.A.; Mahgoub, S.A.; Labib, S.M.; Al-Gaby, A.M.A.; Ramadan, M.F. Phenolic Extracts of Clove (Syzygium aromaticum) with Novel Antioxidant and Antibacterial Activities. Eur. J. Integr. Med. 2016, 8, 494–504. [Google Scholar] [CrossRef]
- Nassar, M.; Gaara, A.; El-Ghorab, A.; Farrag, A.R.; Shen, H.; Huq, E.; Mabry, T. Chemical Constituents of Clove (Syzygium aromaticum, Fam. Myrtaceae) and Their Antioxidant Activity. Rev. Latinoam. Química 2007, 35, 47. [Google Scholar]
- Widowati, W.; Ratnawati, H.; Husin, W.; Maesaroh, M. Antioxidant Properties of Spice Extracts. Biomed. Eng. 2015, 1, 24–29. [Google Scholar]
- Rosarior, V.L.; Lim, P.S.; Wong, W.K.; Yue, C.S.; Yam, H.C.; Tan, S.-A. Antioxidant-rich Clove Extract, A Strong Antimicrobial Agent Against Urinary Tract Infections-causing Bacteria in vitro. Trop. Life Sci. Res. 2021, 32, 45–63. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Koutsoumpou, M.; Liakou, V.; Kontakos, S.; Kontominas, M.G. Characterization and geographical discrimination of saffron from Greece, Spain, Iran, and Morocco based on volatile and bioactivity markers, using chemometrics. Eur. Food Res. Technol. 2017, 243, 1577–1591. [Google Scholar] [CrossRef]
- Lachguer, K.; El Merzougui, S.; Boudadi, I.; Laktib, A.; Caid, M.B.E.; Ramdan, B.; Boubaker, H.; Serghini, M.A. Major Phytochemical Compounds, In Vitro Antioxidant, Antibacterial, and Antifungal Activities of Six Aqueous and Organic Extracts of Crocus sativus L. Flower Waste. Waste Biomass Valor. 2023, 14, 1571–1587. [Google Scholar] [CrossRef]
- Singletary, K.W. Oregano: Overview of the Literature on Health Benefits. Nutr. Today 2010, 45, 129–138. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Leontiou, A.A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Shelf Life of Minced Pork in Vacuum-Adsorbed Carvacrol@Natural Zeolite Nanohybrids and Poly-Lactic Acid/Triethyl Citrate/Carvacrol@Natural Zeolite Self-Healable Active Packaging Films. Antioxidants 2024, 13, 776. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, M.; Delgado, A.M.; Caruso, G.; Micali, M.; Barbera, M.; Atrous, H.; Ouslati, A.; Chammem, N. Phenols, Flavors, and the Mediterranean Diet. J. AOAC Int. 2020, 103, 915–924. [Google Scholar] [CrossRef]
- Oregon State University. Flavonoids. Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/flavonoids (accessed on 25 July 2024).
- Vallverdú-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventós, R.M.; Elez-Martínez, P.; Martín-Belloso, O. Changes in the Polyphenol Profile of Tomato Juices Processed by Pulsed Electric Fields. J. Agric. Food Chem. 2012, 60, 9667–9672. [Google Scholar] [CrossRef]
- Exarchou, V.; Godejohann, M.; van Beek, T.A.; Gerothanassis, I.P.; Vervoort, J. LC-UV-Solid-Phase Extraction-NMR-MS Combined with a Cryogenic Flow Probe and Its Application to the Identification of Compounds Present in Greek Oregano. Anal. Chem. 2003, 75, 6288–6294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Antioxidant Properties, Metabolism, Application and Mechanism of Ferulic Acid in Medicine, Food, Cosmetics, Livestock and Poultry. Antioxidants 2024, 13, 853. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.H.; Nhung, N.T.A.; Dao, D.Q. Iron Ions Chelation-Based Antioxidant Potential vs. pro-Oxidant Risk of Ferulic Acid: A DFT Study in Aqueous Phase. Comput. Theor. Chem. 2020, 1185, 112905. [Google Scholar] [CrossRef]
- Das, G.; Gonçalves, S.; Basilio Heredia, J.; Romano, A.; Jiménez-Ortega, L.A.; Gutiérrez-Grijalva, E.P.; Shin, H.S.; Patra, J.K. Cardiovascular Protective Effect of Cinnamon and Its Major Bioactive Constituents: An Update. J. Funct. Foods 2022, 97, 105045. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A Comprehensive Study on the Phenolic Profile of Widely Used Culinary Herbs and Spices: Rosemary, Thyme, Oregano, Cinnamon, Cumin and Bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Hariri, M.; Ghiasvand, R. Cinnamon and Chronic Diseases. In Advances in Experimental Medicine and Biology; Gupta, S., Prasad, S., Aggarwal, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 929, pp. 1–24. [Google Scholar] [CrossRef]
- Gengatharan, A.; Rahim, M.H.A. The Application of Clove Extracts as a Potential Functional Component in Active Food Packaging Materials and Model Food Systems: A Mini-Review. Appl. Food Res. 2023, 3, 100283. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Investigation of the Reactions of Acrylamide during in Vitro Multistep Enzymatic Digestion of Thermally Processed Foods. Food Funct. 2015, 6, 108–113. [Google Scholar] [CrossRef]
- Nazrul, M.; Bhuiyan, M.; Begum, J.; Nandi, N.; Akter, F. Constituents of the Essential Oil from Leaves and Buds of Clove (Syzigium caryophyllatum (L.) Alston). Afr. J. Plant Sci. 2010, 4, 451–454. [Google Scholar]
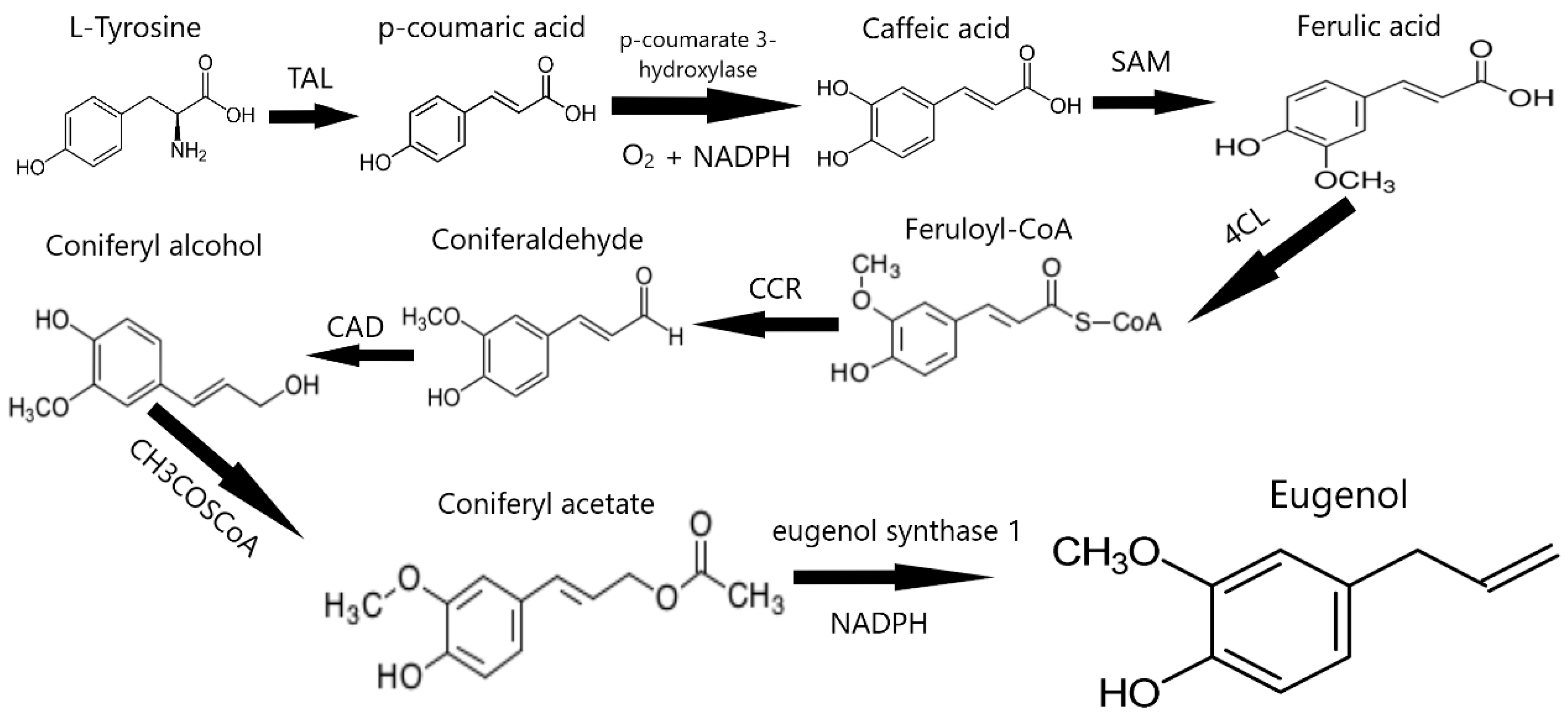
- Harakava, R. Genes Encoding Enzymes of the Lignin Biosynthesis Pathway in Eucalyptus. Genet. Mol. Biol. 2005, 28, 601–607. [Google Scholar] [CrossRef]
- Dewick, P.M. Alkaloids. In Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 311–420. [Google Scholar] [CrossRef]
- Aminzare, M.; Moniri, R.; Azar, H.H.; Mehrasbi, M.R. Evaluation of antioxidant and antibacterial interactions between resveratrol and eugenol in carboxymethyl cellulose biodegradable film. Food Sci. Nutr. 2022, 10, 155–168. [Google Scholar] [CrossRef]
- Lee, K.-G.; Shibamoto, T. Antioxidant Property of Aroma Extract Isolated from Clove Buds [Syzygium aromaticum (L.) Merr. et Perry]. Food Chem. 2001, 74, 443–448. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods. Release 3.1. U.S. Department of Agriculture, Agricultural Research Service. 2014. Available online: http://www.ars.usda.gov/nutrientdata/flav (accessed on 26 July 2024).
- Amanpour, A.; Sonmezdag, A.S.; Kelebek, H.; Selli, S. GC–MS–olfactometric characterization of the most aroma-active components in a representative aromatic extract from Iranian saffron (Crocus sativus L.). Food Chem. 2015, 182, 251–256. [Google Scholar] [CrossRef]
- Dhar, M.K.; Sharma, M.; Bhat, A.; Chrungoo, N.K.; Kaul, S. Functional Genomics of Apocarotenoids in Saffron: Insights from Chemistry, Molecular Biology and Therapeutic Applications. Brief. Funct. Genom. 2017, 16, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Kyriakoudi, A.; Chrysanthou, A.; Mantzouridou, F.; Tsimidou, M.Z. Revisiting extraction of bioactive apocarotenoids from Crocus sativus L. dry stigmas (saffron). Anal. Chim. Acta 2012, 755, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mashmoul, M.; Azlan, A.; Khaza’ai, H.; Yusof, B.N.M.; Noor, S.M. Saffron: A Natural Potent Antioxidant as a Promising Anti-Obesity Drug. Antioxidants 2013, 2, 293–308. [Google Scholar] [CrossRef]
- Kotanidou, E.P.; Tsinopoulou, V.R.; Giza, S.; Ntouma, S.; Angeli, C.; Chatziandreou, M.; Tsopelas, K.; Tseti, I.; Galli-Tsinopoulou, A. The Effect of Saffron Kozanis (Crocus sativus L.) Supplementation on Weight Management, Glycemic Markers and Lipid Profile in Adolescents with Obesity: A Double-Blinded Randomized Placebo-Controlled Trial. Children 2023, 10, 1814. [Google Scholar] [CrossRef]
- KAU Agri-Infotech Portal. Available online: http://www.celkau.in/Crops/Spices/Turmeric/processing.aspx (accessed on 29 July 2024).
- Grynkiewicz, G.; Ślifirsk, P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim. Pol. 2012, 59, 201–212. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Indira Priyadarsini, K. Chemical and Structural Features Influencing the Biological Activity of Curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Tuong, D.T.C.; Moniruzzaman, M.; Smirnova, E.; Chin, S.; Sureshbabu, A.; Karthikeyan, A.; Min, T. Curcumin as a Potential Antioxidant in Stress Regulation of Terrestrial, Avian, and Aquatic Animals: A Review. Antioxidants 2023, 12, 1700. [Google Scholar] [CrossRef] [PubMed]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jaganmohan Rao, L.; Sakariah, K.K. Antioxidant Activities of Curcumin, Demethoxycurcumin and Bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Albright, A. Biological and Social Exposures in Youth Set the Stage for Premature Chronic Diseases. J. Am. Diet. Assoc. 2008, 108, 1843–1845. [Google Scholar] [CrossRef]
- Doll, R. Chronic and Degenerative Disease: Major Causes of Morbidity and Death. Am. J. Clin. Nutr. 1995, 62, 1301S–1305S. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Oddo, L.P.; Piro, R.; Bruneau, É.; Guyot-Declerck, C.; Ivanov, T.; Piskulová, J.; Flamini, C.; Lheritier, J.; Morlot, M.; Russmann, H.; et al. Main European Unifloral Honeys: Descriptive Sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Tan, H.T.; Rahman, R.A.; Gan, S.H.; Halim, A.S.; Hassan, S.A.; Sulaiman, S.A.; Kirnpal-Kaur, B. The Antibacterial Properties of Malaysian Tualang Honey against Wound and Enteric Microorganisms in Comparison to Manuka Honey. BMC Complement. Altern. Med. 2009, 9, 34. [Google Scholar] [CrossRef]
- Koç, A.N.; Silici, S.; Kasap, F.; Hörmet-Oz, H.T.; Mavus-Buldu, H.; Ercal, B.D. Antifungal Activity of the Honeybee Products against Candida spp. and Trichosporon spp. J. Med. Food 2011, 14, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic Acid, Phenolic Acids, and Flavonoids in Malaysian Honey Extracts Demonstrate In Vitro Anti-Inflammatory Activity. Nutr. Res. 2010, 30, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Sulaiman, S.A.; Jaafar, H.; Sirajudeen, K.N.S. Effect of Different Doses of Malaysian Honey on Reproductive Parameters in Adult Male Rats. Andrologia 2012, 44, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Zaid, S.S.M.; Sulaiman, S.A.; Sirajudeen, K.N.M.; Othman, N.H. The Effects of Tualang Honey on Female Reproductive Organs, Tibia Bone and Hormonal Profile in Ovariectomised Rats—Animal Model for Menopause. BMC Complement. Altern. Med. 2010, 10, 82. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Gurtu, S.; Sulaiman, S.A.; Ab Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, M.S.M. Hypoglycemic and Antioxidant Effects of Honey Supplementation in Streptozotocin-Induced Diabetic Rats. Int. J. Vitam. Nutr. Res. 2010, 80, 74–82. [Google Scholar] [CrossRef]
- Al-Waili, N. Intrapulmonary Administration of Natural Honey Solution, Hyperosmolar Dextrose or Hypoosmolar Distill Water to Normal Individuals and to Patients with Type-2 Diabetes Mellitus or Hypertension: Their Effects on Blood Glucose Level, Plasma Insulin and C-Peptide, Blood Pressure and Peaked Expiratory Flow Rate. Eur. J. Med. Res. 2003, 8, 295–303. [Google Scholar]
- Gharzouli, K.; Amira, S.; Gharzouli, A.; Khennouf, S. Gastroprotective Effects of Honey and Glucose-Fructose-Sucrose-Maltose Mixture against Ethanol-, Indomethacin-, and Acidified Aspirin-Induced Lesions in the Rat. Exp. Toxicol. Pathol. 2002, 54, 217–221. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Saloom, K.Y.; Al-Waili, T.N.; Al-Waili, A.N.; Akmal, M.; Al-Waili, F.S.; Al-Waili, H.N. Influence of Various Diet Regimens on Deterioration of Hepatic Function and Hematological Parameters Following Carbon Tetrachloride: A Potential Protective Role of Natural Honey. Nat. Prod. Res. 2006, 20, 1258–1264. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Maruška, A.; Kornyšova, O.; Charczun, N.; Ligor, M.; Buszewski, B. Quantitative and qualitative determination of phenolic compounds in honey. Chem. Technol. 2009, 53, 74–80. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of Honey and Its Mechanisms of Action on the Development and Progression of Cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A Novel Antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.B.; Abdullah, W.Z.; Sulaiman, S.A.; Suen, A.B. A Review of Molecular Mechanisms of the Anti-Leukemic Effects of Phenolic Compounds in Honey. Int. J. Mol. Sci. 2012, 13, 15054–15073. [Google Scholar] [CrossRef]
- Catapano, A.L. Antioxidant Effect of Flavonoids. Angiology 1997, 48, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; Samman, S. Flavonoids-Chemistry, Metabolism, Cardioprotective Effects, and Dietary Sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Nagai, T.; Sakai, M.; Inoue, R.; Inoue, H.; Suzuki, N. Antioxidative Activities of Some Commercially Honeys, Royal Jelly, and Propolis. Food Chem. 2001, 75, 237–240. [Google Scholar] [CrossRef]
- Diplock, A.T.; Rice-Evans, C.A.; Burdon, R.H. Is There a Significant Role for Lipid Peroxidation in the Causation of Malignancy and for Antioxidants in Cancer Prevention? Cancer Res. 1994, 54, 1952s–1956s. [Google Scholar]
- Moore, G.; Brooks, P.; Pappalardo, L.; Boufridi, A. Phenolic Profiles of Australian Monofloral Eucalyptus, Corymbia, Macadamia and Lophostemon Honeys via HPLC-DAD Analysis. Food Chem. 2025, 462, 140900. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Effects of Daily Consumption of Honey Solution on Hematological Indices and Blood Levels of Minerals and Enzymes in Normal Individuals. J. Med. Food 2003, 6, 135–140. [Google Scholar] [CrossRef]
- Santos Filipe, M.; Kowalczyk, T.; Kukula-Koch, W.; Wieczfinska, J.; Bangay, G.; Diaz-Lanza, A.M.; Cardoso, R.V.C.; Mandim, F.; Falcão, S.I.; Vilas-Boas, M.; et al. Evaluating the Quality, Physicochemical Properties, and Biological Activities of Centauri® Honey from Turkey. Food Biosci. 2024, 62, 105028. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, L.; Zhang, J.; Ma, X.; Weng, R. Determination of Endogenous Phenolic Compounds in Honey by HPLC-MS/MS. LWT 2023, 183, 114951. [Google Scholar] [CrossRef]
- Silici, S.; Sarioglu, K.; Dogan, M.; Karaman, K. HPLC-DAD Analysis to Identify the Phenolic Profile of Rhododendron Honeys Collected from Different Regions in Turkey. Int. J. Food Prop. 2014, 17, 1126–1135. [Google Scholar] [CrossRef]
- Mizzi, L.; Chatzitzika, C.; Gatt, R.; Valdramidis, V. HPLC Analysis of Phenolic Compounds and Flavonoids with Overlapping Peaks. Food Technol. Biotechnol. 2020, 58, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, Ι.Κ.; Vavoura, M.V.; Nikolaou, C.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Floral authentication of Greek unifloral honeys based on the combination of phenolic compounds, physicochemical parameters and chemometrics. Food Res. Int. 2014, 62, 753–760. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The Small Intestine Can Both Absorb and Glucuronidate Luminal Flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef]
- Gee, J.M.; DuPont, M.S.; Day, A.J.; Plumb, G.W.; Williamson, G.; Johnson, I.T. Intestinal Transport of Quercetin Glycosides in Rats Involves Both Deglycosylation and Interaction with the Hexose Transport Pathway. J. Nutr. 2000, 130, 2765–2771. [Google Scholar] [CrossRef]


| Cultivar | Fruit Part | Solvent | Extraction | Total Phenolic Content | Total Flavonoid Content | References |
|---|---|---|---|---|---|---|
| Citrus sinensis (L.) Osbeck | Peel | Acetone | Soxhlet | 114 mg GAE/g | - | [40] |
| Citrus sinensis (L.) Osbeck | Peel | Methanol | Soxhlet | 158 mg GAE/g | - | [40] |
| Citrus sinensis (L.) Osbeck | Peel | Water | Soxhlet | 210 mg GAE/g | - | [40] |
| Citrus sinensis (L.) Osbeck | Peel | Hexane | Soxhlet | 79 mg GAE/g | - | [40] |
| Citrus sinensis (L.) Osbeck | Peel | Water | Aqueous | 215 mg GAE/g | - | [40] |
| Citrus sinensis (L.) Osbeck | Pulp | Acetone | Soxhlet | 522 mg GAE/g | - | [40] |
| Citrus sinensis (L.) Osbeck | Pulp | Methanol | Soxhlet | 465 mg GAE/g | - | [40] |
| Citrus sinensis (L.) Osbeck | Pulp | Water | Soxhlet | 330 mg GAE/g | - | [40] |
| Citrus sinensis (L.) Osbeck | Pulp | Hexane | Soxhlet | 201 mg GAE/g | - | [40] |
| Citrus sinensis (L.) Osbeck | Pulp | Water | Aqueous | 326 mg GAE/g | - | [40] |
| Citrus limon (L.) Burm. f. | Peel | n-Hexane | Reflux | 8.9 ± 0.08 mg GAE/g | 27.2 ± 0.03 mg QE/g | [21] |
| Citrus limon (L.) Burm. f. | Peel | Ethyl acetate | Reflux | 13.8 ± 0.07 mg GAE/g | 24.9 ± 0.40 mg QE/g | [21] |
| Citrus limon (L.) Burm. f. | Peel | Ethanol | Reflux | 15.2 ± 0.02 mg GAE/g | 28.9 ± 0.04 mg QE/g | [21] |
| Citrus limon (L.) Burm. f. | Pulp | n-Hexane | Reflux | 1.4 ± 0.02 mg GAE/g | 30.2 ± 0.20 mg QE/g | [21] |
| Citrus limon (L.) Burm. f. | Pulp | Ethyl acetate | Reflux | 9.0 ±0.14 mg GAE/g | 8.5 ± 0.10 mg QE/g | [21] |
| Citrus limon (L.) Burm. f. | Pulp | Ethanol | Reflux | 14.7 ± 0.17 mg GAE/g | 10.4 ± 0.07 mg QE/g | [21] |
| Citrus limon (L.) Burm. f. | Juice | - | - | 64.5 ± 1.4 mg GAE/L | 24.0 ± 0.01 mg GAE/L | [22] |
| Citrus limon (L.) Lisbon | Juice | - | - | 730.46 mg GAE/L | 211.36 mg CE/L | [23] |
| Citrus limon (L.) Eureka | Juice | - | - | 690.62 mg GAE/L | 219.27 mg CE/L | [23] |
| Citrus limon (L.) Mayre | Juice | - | - | 825.37 mg GAE/L | 216.61 mg CE/L | [23] |
| Citrus limon (L.) Bush | Juice | - | - | 998.29 mg GAE/L | 220.34 mg CE/L | [23] |
| Citrus paradisi Red | Whole fruit | 80% aqueous methanol | - | 23.83 ± 34.64 mg GAE/g | - | [24] |
| Citrus paradisi White | Pulp | 80% aqueous methanol | - | 16.96 ± 7.86 mg GAE/g | - | [24] |
| Citrus paradisi Green | Peels | 80% aqueous methanol | - | 20.87 ± 13.81 mg GAE/g | - | [24] |
| Citrus paradisi Red | Juice | - | - | 359 ± 0.029 mg GAE/L | - | [25] |
| Citrus paradisi Red | Peels | Ethanol | UAE | 283 ± 0.018 mg GAE/L | - | [25] |
| Citrus paradisi White | Juice | - | - | 747 ± 0.098 mg GAE/L | - | [25] |
| Citrus paradisi White | Peels | Ethanol | UAE | 192 ± 0.015 mg GAE/L | - | [25] |
| Cultivar | Variety | Fruit Part | Catechol (μg/g DM) | Catechin (μg/g DM) | Chlorogenic Acid (μg/g DM) | Caffeic Acid (μg/g DM) | p-Coumaric Acid (μg/g DM) | Ferulic Acid (μg/g DM) | Gallic Acid (μg/g DM) | TPC (mg GAE/100 g) | TFC (mg RE/100 g) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prunus armeniaca L. | Çataloğlu | Pulp | 13.62 ± 0.02 | 37.23 ± 0.02 | 111.71 ± 0.00 | 5.47 ± 0.00 | 14.55 ± 0.00 | 109.73 ± 0.04 | 15.76 ± 0.05 | - | - | [66] |
| Prunus armeniaca L. | Çöloğlu | Pulp | 6.72 ± 0.01 | 37.50 ± 0.01 | 221.81 ± 0.01 | 41.95 ± 0.00 | 127.92 ± 0.00 | 274.91 ± 0.01 | 31.62 ± 0.01 | - | - | [66] |
| Prunus armeniaca L. | Hasanbey | Pulp | 7.56 ± 0.03 | 32.16 ± 0.01 | 228.71 ± 0.01 | 80.08 ± 0.00 | 198.44 ± 0.01 | 295.09 ± 0.09 | 127.76 ± 0.01 | - | - | [66] |
| Prunus armeniaca L. | Hacıhaliloğlu | Pulp | 44.03 ± 0.02 | 111.62 ± 0.01 | 61.14 ± 0.01 | 21.67 ± 0.00 | 51.21 ± 0.02 | 220.39 ± 0.39 | 18.24 ± 0.08 | - | - | [66] |
| Prunus armeniaca L. | Ordubat | Pulp | 14.91 ± 0.01 | 8.84 ± 0.01 | 42.41 ± 0.01 | 4.96 ± 0.00 | 27.31 ± 0.01 | 93.24 ± 0.06 | 9.68 ± 0.04 | - | - | [66] |
| Prunus armeniaca L. | Wilson delicious | Pulp | 28.17 ± 0.01 | 199.55 ± 0.01 | 147.31 ± 0.00 | 117.10 ± 0.00 | 103.31 ± 0.01 | 110.66 ± 0.08 | 30.78 ± 0.07 | - | - | [66] |
| Prunus armeniaca L. | Stark early orange | Pulp | 7.76 ± 0.00 | 220.12 ± 0.01 | 93.86 ± 0.01 | 62.81 ± 0.00 | 105.54 ± 0.01 | 60.54 ± 0.08 | 14.96 ± 0.01 | - | - | [66] |
| Prunus armeniaca L. | Harcot | Pulp | 119.89 ± 0.01 | 177.32 ± 0.01 | 281.44 ± 0.01 | 167.86 ± 0.00 | 178.51 ± 0.00 | 251.33 ± 0.01 | 47.52 ± 0.02 | - | - | [66] |
| Prunus armeniaca L. | Xiaobai Green mature | Peel | - | - | - | - | - | - | - | 59.3 ± 1.5 | 76.7 ± 3.5 | [68] |
| Prunus armeniaca L. | Xiaobai Green mature | Pulp | - | - | - | - | - | - | - | 76.7 ± 3.5 | 27.9 ± 0.9 | [68] |
| Prunus armeniaca L. | Xiaobai Full mature | Peel | - | - | - | - | - | - | - | 48.5 ± 0.9 | 76.6 ± 1.8 | [68] |
| Prunus armeniaca L. | Xiaobai Full mature | Pulp | - | - | - | - | - | - | - | 28.5 ± 0.4 | 23.0 ± 1.3 | [68] |
| Prunus armeniaca L. | Liguang Green mature | Peel | - | - | - | - | - | - | - | 58.3 ± 2.5 | 81.9 ± 2.4 | [68] |
| Prunus armeniaca L. | Liguang Green mature | Pulp | - | - | - | - | - | - | - | 26.9 ± 1.4 | 23.2 ± 1.0 | [68] |
| Prunus armeniaca L. | Liguang Full mature | Peel | - | - | - | - | - | - | - | 56.9 ± 1.4 | 92.1 ± 2.5 | [68] |
| Prunus armeniaca L. | Liguang Full mature | Pulp | - | - | - | - | - | - | - | 28.4 ± 2.2 | 23.2 ± 0.6 | [68] |
| Prunus armeniaca L. | Katy Green mature | Peel | - | - | - | - | - | - | - | 50.1 ± 1.4 | 150.1 ± 7.5 | [68] |
| Prunus armeniaca L. | Katy Green mature | Pulp | - | - | - | - | - | - | - | 29.8 ± 1.2 | 31.3 ± 1.3 | [68] |
| Prunus armeniaca L. | Katy Full mature | Peel | - | - | - | - | - | - | - | 48.5 ± 0.7 | 143.1 ± 5.4 | [68] |
| Prunus armeniaca L. | Katy Full mature | Pulp | - | - | - | - | - | - | - | 24.2 ± 1.0 | 24.5 ± 0.8 | [68] |
| Prunus armeniaca L. | Chuanzhihong Green mature | Peel | - | - | - | - | - | - | - | 51.0 ± 2.4 | 137.5 ± 3.4 | [68] |
| Prunus armeniaca L. | Chuanzhihong Green mature | Pulp | - | - | - | - | - | - | - | 21.8 ± 0.9 | 19.9 ± 0.7 | [68] |
| Prunus armeniaca L. | Chuanzhihong Full mature | Peel | - | - | - | - | - | - | - | 45.1 ± 1.1 | 83.9 ± 1.7 | [68] |
| Prunus armeniaca L. | Chuanzhihong Full mature | Pulp | - | - | - | - | - | - | - | 16.6 ± 0.7 | 12.3 ± 0.2 | [68] |
| Prunus armeniaca L. | Dajie Green mature | Peel | - | - | - | - | - | - | - | 53.1 ± 1.3 | 110.4 ± 2.2 | [68] |
| Prunus armeniaca L. | Dajie Green mature | Pulp | - | - | - | - | - | - | - | 38.3 ± 0.9 | 63.4 ± 2.9 | [68] |
| Prunus armeniaca L. | Dajie Full mature | Peel | - | - | - | - | - | - | - | 43.8 ± 1.1 | 96.8 ± 3.0 | [68] |
| Prunus armeniaca L. | Dajie Full mature | Pulp | - | - | - | - | - | - | - | 24.7 ± 0.5 | 32.7 ± 1.1 | [68] |
| Prunus armeniaca L. | Shushanggan Green mature | Peel | - | - | - | - | - | - | - | 57.4 ± 3.6 | 73.5 ± 2.4 | [68] |
| Prunus armeniaca L. | Shushanggan Green mature | Pulp | - | - | - | - | - | - | - | 52.2 ± 2.5 | 54.9 ± 1.3 | [68] |
| Prunus armeniaca L. | Shushanggan Full mature | Peel | - | - | - | - | - | - | - | 51.5 ± 1.9 | 59.2 ± 0.7 | [68] |
| Prunus armeniaca L. | Shushanggan Full mature | Pulp | - | - | - | - | - | - | - | 39.7 ± 0.7 | 35.7 ± 1.4 | [68] |
| Cultivar | Variety | Fruit Part | Extraction | Solvent | TPC | TFC | Total Anthocyanins (mg CGE/L) | References |
|---|---|---|---|---|---|---|---|---|
| Prunus domestica L. | Beltsville Elite B70197 | Fresh plum | Ultra-sound | 80% aqueous methanol | 332 ± 3.1 mg GAE/100 g | 237 ± 6.3 mg CE/100 g | - | [16] |
| Prunus domestica L. | Cacak Best | Fresh plum | Ultra-sound | 80% aqueous methanol | 319 ± 1.4 mg GAE/100 g | 200 ± 2.5 mg CE/100 g | - | [16] |
| Prunus domestica L. | French Damson | Fresh plum | Ultra-sound | 80% aqueous methanol | 375 ± 3.8 mg GAE/100 g | 215 ± 9.7 mg CE/100 g | - | [16] |
| Prunus domestica L. | Long John | Fresh plum | Ultra-sound | 80% aqueous methanol | 199 ± 2.5 mg GAE/100 g | 126.3 ± 3.4 mg CE/100 g | - | [16] |
| Prunus domestica L. | Stanley | Fresh plum | Ultra-sound | 80% aqueous methanol | 174 ± 1.5 mg GAE/100 g | 118 ± 2.6 mg CE/100 g | - | [16] |
| Prunus domestica L. | Yugoslavian Elite T101 | Fresh plum | Ultra-sound | 80% aqueous methanol | 217 ± 4.9 mg GAE/100 g | 146 ± 6.0 mg CE/100 g | - | [16] |
| Prunus salicina L. | - | Juice | - | - | 3.77 ± 0.57 mg GAE/L | 1990.8 ± 9.7 mg RE/L | 1.0 ± 0.2 | [84] |
| Prunus salicina L. | - | Juice | Ultra-sound | - | 4.774 ± 0.27 mg GAE/L | 2028.9 ± 13.2 mg RE/L | 1.3 ± 0.1 | [84] |
| Prunus salicina L. | - | Juice | Enzyme | - | 6.381 ± 0.33 mg GAE/L | 2153.9 ± 13.2 mg RE/L | 9.1 ± 0.2 | [84] |
| Prunus salicina L. | - | Juice | Ultra-sound and enzyme 15’ | - | 6.935 ± 0.47 mg GAE/L | 2461.0 ± 3.7 mg RE/L | 6.6 ± 2.3 | [84] |
| Prunus salicina L. | - | Juice | Ultra-sound and enzyme 30’ | - | 6.517 ± 0.40 mg GAE/L | 2240.7 ± 19.1 mg RE/L | 7.6 ± 0.5 | [84] |
| Prunus salicina L. | - | Juice | Ultra-sound and enzyme 60’ | - | 6.546 ± 0.55 mg GAE/L | 2245.0 ± 3.7 mg RE/L | 8.9 ± 0.5 | [84] |
| Cultivar | Variety | Fruit Part | Extraction Solvent | Total Anthocyanins (mg CGE/100 g FW) | TFC | TPC (mg GAE/g) | Total Condensed Tannins (mg CE/g) | References |
|---|---|---|---|---|---|---|---|---|
| Prunus avium L. | Hongyan | Whole fruit pulp | Methanol | 19.93 ± 0.14 | 7.97 ± 4.29 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | Caihong | Whole fruit pulp | Methanol | 20.07 ± 0.11 | 43.55 ± 4.29 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | Rainier | Whole fruit pulp | Methanol | 20.90 ± 0.27 | 77.27 ± 7.07 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | Burlat | Whole fruit pulp | Methanol | 164.76 ± 5.41 | 253.32 ± 19.67 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | Lapins | Whole fruit pulp | Methanol | 104.28 ± 1.84 | 140.01 ± 5.85 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | 5–106 | Whole fruit pulp | Methanol | 147.38 ± 6.06 | 101.61 ± 12.25 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | Tieton | Whole fruit pulp | Methanol | 126.06 ± 1.43 | 70.71 ± 9.73 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | Hongdeng | Whole fruit pulp | Methanol | 142.74 ± 1.17 | 101.61 ± 2.81 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | Zaodaguo | Whole fruit pulp | Methanol | 119.41 ± 0.99 | 120.34 ± 1.62 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | Van | Whole fruit pulp | Methanol | 106.57 ± 1.50 | 68.84 ± 4.29 mg RE/100 g FW | - | - | [91] |
| Prunus avium L. | Bing | Whole fruit pulp | Ethanol | - | 31 ± 5 mg QE/100 g | 1.13 ± 0.04 | 0.03 ± 0.01 | [92] |
| Prunus avium L. | Ron’s | Whole fruit pulp | Ethanol | - | 51 ± 2 mg QE/100 g | 0.87 ± 0.09 | n.d | [92] |
| Prunus avium L. | Merchant | Whole fruit pulp | Ethanol | - | 34 ± 2 mg QE/100 g | 1.23 ± 0.01 | 0.04 ± 0.03 | [92] |
| Prunus avium L. | Lapins | Whole fruit pulp | Ethanol | - | 47 ± 1 mg QE/100 g | 1.73 ± 0.90 | 0.17 ± 0.09 | [92] |
| Prunus avium L. | - | Juice | - | - | 58 g CE/100 g | 0.60 ± 0.04 | - | [93] |
| Spice | Solvent | Extraction Method | Antioxidant Activity (DPPH) | EC50 | TPC | TFC | Caffeic Acid | References |
|---|---|---|---|---|---|---|---|---|
| Origanum vulgare | Aqueous Methanol 50% | Extraction | 58.28 ± 0.33% | - | 15.83 mg GAE/g | - | - | [107] |
| Origanum vulgare | Aqueous Methanol 80% | Shaker extraction 3 h | 53.90 (TE)/g DW | - | 44.08 mg GAE/g | 5.13 mg QE/g | [108] | |
| Origanum vulgare | Enzymes | Enzymatic | 79.6 ± 2.04 μM trolox/100 g dw | - | - | - | 649 ± 0.07 mg/100 g DM | [109] |
| Cinnulin PF | Aqueous | Extraction | - | 0.015 mg/mL | 82.3 mg GAE/g | - | - | [110] |
| Cinnamomum cassia | Aqueous Methanol 80% | Extraction 24 h | - | - | 63.4 ± 0.021 mg GAE/g | - | - | [111] |
| Cinnamomum zeylanicum | Aqueous Methanol 80% | Extraction 24 h | - | - | 119 ± 0.004 mg GAE/g | - | 15.3 mg/100 g of DM | [111] |
| Cinnamomum zeylanicum | Methanol | Soxhlet 4 h | 76% | - | - | - | - | [112] |
| Cinnamomum zeylanicum | Acetone | Soxhlet 4 h | 66% | - | - | - | - | [112] |
| Cinnamomum zeylanicum | Ethyl acetate | Soxhlet 4 h | 56% | - | - | - | - | [112] |
| Cinnamomum zeylanicum | Water | 120 °C/20 min | 94% | - | - | - | - | [112] |
| Cinnamomum burmanni/Syzygium aromaticum (1:1) | Ethanolic extract of grape origin (EEGO) | Extraction 24 h | 55.12 ± 0.01 | 0.08 ± 0.01 mg/L | 1120.24 ± 0.01 mg GAE/L | 634.65 ± 0.01 mg/L | [98] | |
| Syzygium aromaticum | Ethyl acetate | Extraction 60 min | 25.3% | - | 58.8 mg GAE/g extract | 4.70 mg QE/g extract | - | [113] |
| Syzygium aromaticum | Ethanol 80% | Extraction 60 min | Approx. 90% | - | 293 mg GAE/g extract | 12 mg QE /g extract | - | [113] |
| Syzygium aromaticum | Water | Extraction 60 min | 91.4% | - | 230 mg GAE/g extract | 17.5 mg QE/g extract | - | [113] |
| Syzygium aromaticum | Ethanol | Extraction | 93% | - | - | - | - | [114] |
| Syzygium aromaticum | Ethanol 70% | Extraction 24 h | 95% | 0.004 mg/mL | 188.35 mg EE/g | - | - | [115] |
| Syzygium aromaticum | Ethanol 80% | Extraction 24 h | 95% | 0.037 mg/mL | 250.93 ± 1.33 mg GAE/ g extract | - | - | [116] |
| Crocus sativus L. Greece | Methanol | Extraction 24 h | 27.41 ± 1.57% | - | 68.26 ± 31.82 mg GAE/g DM | - | - | [117] |
| Crocus sativus L. Iran | Methanol | Extraction 24 h | 39.49 ± 1.05% | - | 101.93 ± 11.11 mg GAE/g DM | - | - | [117] |
| Crocus sativus L. Morocco | Methanol | Extraction 24 h | 24.56 ± 0.87% | - | 62.93 ± 21.33 mg GAE/g DM | - | - | [117] |
| Crocus sativus L. Spain | Methanol | Extraction 24 h | 26.15 ± 1.70% | - | 43.70 ± 11.58 mg GAE/g DM | - | - | [117] |
| Crocus sativus L. | Water | Extraction 1 h | - | 1.227 ± 0.05286 mg/mL | 104.82 ± 4.36 mg GAE/g DM | 26.88 ± 2.60 mg QE/g DM | - | [118] |
| Crocus sativus L. | Methanol | Extraction 48 h | - | 1.3648 ± 0.09562 mg/mL | 114.66 ± 3.48 mg GAE/g DM | 31.27 ± 0.73 mg QE/g DM | - | [118] |
| Crocus sativus L. | Aqueous ethanol | Soxhlet 6 h | - | 0.88877 ± 0.08736 mg/mL | 130.39 ± 13.79 mg GAE/g DM | 39.06 ± 10.85 mg QE/g DM | - | [118] |
| Crocus sativus L. | Diethyl ether | - | - | 0.30944 ± 0.0333 mg/mL | 214.29 ± 12.68 mg GAE/g DM | 84.46 ± 16.10 mg QE/g DM | - | [118] |
| Crocus sativus L. | Ethyl acetate | - | - | 0.7211 ± 0.03 mg/mL | 154.32 ± 14.03 mg GAE/g DM | 46.88 ± 4.80 mg QE/g DM | - | [118] |
| Crocus sativus L. | n-Butanol | - | - | 0.884 ± 0.1526 mg/mL | 171.8 ± 13.41 mg GAE/g DM | 60.59 ± 11.38 mg QE/g DM | - | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaridis, D.G.; Kitsios, A.-P.; Koutoulis, A.S.; Malisova, O.; Karabagias, I.K. Fruits, Spices and Honey Phenolic Compounds: A Comprehensive Review on Their Origin, Methods of Extraction and Beneficial Health Properties. Antioxidants 2024, 13, 1335. https://doi.org/10.3390/antiox13111335
Lazaridis DG, Kitsios A-P, Koutoulis AS, Malisova O, Karabagias IK. Fruits, Spices and Honey Phenolic Compounds: A Comprehensive Review on Their Origin, Methods of Extraction and Beneficial Health Properties. Antioxidants. 2024; 13(11):1335. https://doi.org/10.3390/antiox13111335
Chicago/Turabian StyleLazaridis, Dimitrios G., Apostolos-Panagiotis Kitsios, Antonios S. Koutoulis, Olga Malisova, and Ioannis K. Karabagias. 2024. "Fruits, Spices and Honey Phenolic Compounds: A Comprehensive Review on Their Origin, Methods of Extraction and Beneficial Health Properties" Antioxidants 13, no. 11: 1335. https://doi.org/10.3390/antiox13111335
APA StyleLazaridis, D. G., Kitsios, A.-P., Koutoulis, A. S., Malisova, O., & Karabagias, I. K. (2024). Fruits, Spices and Honey Phenolic Compounds: A Comprehensive Review on Their Origin, Methods of Extraction and Beneficial Health Properties. Antioxidants, 13(11), 1335. https://doi.org/10.3390/antiox13111335








