Abstract
Oxidative stress (OS), defined as a disruption in redox balance favoring oxidants, has emerged as a major contributor to numerous diseases in human and veterinary medicine. While several reviews have explored the implication of OS in human pathology, an exhaustive review of the canine species is lacking. This comprehensive review aims to summarize the existing literature on the role of OS in canine diseases, highlighting its potentially detrimental effect on various organs and systems. Some inconsistencies among studies exist, likely due to varying biomarkers and sample types. However, there is substantial evidence supporting the involvement of OS in the development or progression of numerous canine disorders, such as cardiovascular, oncologic, endocrine, gastrointestinal, hematologic, renal, neurologic, infectious, and parasitic diseases, among others. Additionally, this review discusses the efficacy of antioxidant and pro-oxidant therapeutic agents for these conditions. Dietary interventions to counteract OS in dogs have gained significant attention in recent years, although further research on the topic is needed. This review aims to serve as a foundational resource for future investigations in this promising field.
1. Introduction
Reduction-oxidation (redox) reactions are central mechanisms of life in biological systems [1]. The concept of “oxidative stress” (OS) arose in 1985, describing a potentially harmful imbalance between the production of oxidants and the organism’s antioxidant defenses, favoring the oxidants [2]. Since then, the knowledge of redox biology has undergone significant advancements and the concept has been redefined to account for the broad implications of redox homeostasis [1,3,4,5]. Pro-oxidant agents include a wide variety of molecules, some of which are free radicals and others are non-radicals, collectively referred to as ‘reactive species’, such as reactive oxygen species (ROS), reactive nitrogen species (RNS), and others [6]. These species originate from endogenous sources, such as normal cellular metabolism, inflammation, or immune cell activation, as well as from exogenous sources, including exposure to pollutants, chemicals, or radiation [4,7,8]. Several metals and metalloproteins, such as iron, copper, chromium, and cobalt, also contribute to the generation of reactive species, primarily through Fenton-like reactions [9,10].
When maintained at controlled concentrations, reactive species play a crucial role in various physiological processes, including cellular signaling, phagocytosis, and regulation of vascular tone [8,11,12]. Nonetheless, elevated levels of reactive species and their by-products can cause severe damage to biomolecules and contribute to the pathogenesis of numerous diseases [1,3,5].
Given the variety of compounds and pathways implicated in redox regulation, numerous methods have been developed to evaluate OS, leading to the identification of multiple measurable biomarkers. The direct measurement of reactive species (ROS, RNS) and reactive oxygen metabolites (d-ROMs) can be challenging due to their very short half-life and the requirement for expensive equipment. Therefore, a more practical approach involves measuring quantifiable products of oxidative damage to biomolecules, such as lipids, proteins, and DNA [13,14,15]. The most frequently measured biomarkers of lipid peroxidation, such as the oxidation products of polyunsaturated fatty acids (PUFAs), are malondialdehyde (MDA), 4-hydroxy-2nonenal (4-HNE), Isoprostanes (IsoP), and acrolein [15,16]. Reactive species can also lead to DNA modifications in several ways, resulting in DNA-oxidation biomarkers such as 8-hydroxy-2′-deoxyguanosine (8-OHdG), one of the most extensively studied [7,17,18]. The oxidation of proteins can be measured as advanced oxidation protein products (AOPPs) and protein carbonyls (PCs) [19]. Another approach to evaluating OS is assessing antioxidant defenses, which include non-enzymatic agents (e.g., vitamins, metals (selenium, zinc), thiol groups, and reduced glutathione (GSH)) and numerous antioxidant enzymes, such as glutathione peroxidase (GPX), superoxide dismutase (SOD), or catalase (CAT) [7,15,19,20]. Additionally, several widely used indexes reflect the overall antioxidant capacity of a sample, such as the 2,2′-azinobis(3-ethylbenzthiazolin-6-sulfonic acid) (ABTS) test, also known as the Total Antioxidant Status (TAS) assay; the Cupric-Reducing Antioxidant Power (CUPRAC) test; and the Ferric-Reducing Antioxidant Power (FRAP) assay [19,21,22,23]. Table 1 summarizes the most common OS biomarkers used in the literature. Further information on this topic can be found in the reviews by Tejchman et al. [15], Frijhoff et al. [18], Sánchez-Rodríguez et al. [19], Dalle-Donne et al. [13], and Sies et al. [1].

Table 1.
Oxidative stress biomarkers—classification and some examples.
OS has been implicated in the development and progression of numerous diseases in humans. While the exact mechanisms in some cases remain unclear, a triad of OS, inflammation, and functional impairment appears to be involved in the pathogenesis of many clinical conditions [1,3,8,15,24]. In recent years, this field has gained growing interest in veterinary medicine, with mounting evidence suggesting a relevant role of OS in the pathogenesis of many canine diseases (Figure 1). Redox homeostasis in dogs also appears to be influenced by factors such as psychogenic stress, housing, and exercise, though this aspect remains unclear [23,25,26,27,28]. While numerous reviews exist in human medicine, to the best of our knowledge, no comprehensive review has been conducted for the canine species. To fill this gap, we performed an extensive literature search in the PubMed database, focusing on original research articles, experimental studies, clinical trials, and reviews related to the association between OS and canine diseases. Search terms included ‘oxidative stress’, ‘oxidation’, ‘antioxidant’, ‘redox’, ‘dog’, ‘canine’, ‘disease’, ‘disorder’, and ‘pathology’. Each selected article was carefully reviewed, and the final version of the manuscript includes relevant literature published up to November 2024. The main sections of this manuscript examine the role of OS in cardiovascular, respiratory, oncologic, gastrointestinal, hepatobiliary, endocrine, hematologic, infectious, parasitic, neurologic, renal, dermatologic, ophthalmologic, orthopedic, reproductive, dental, and other canine diseases (Table 2). This review aims to consolidate current evidence and provide a comprehensive overview of this expanding field.
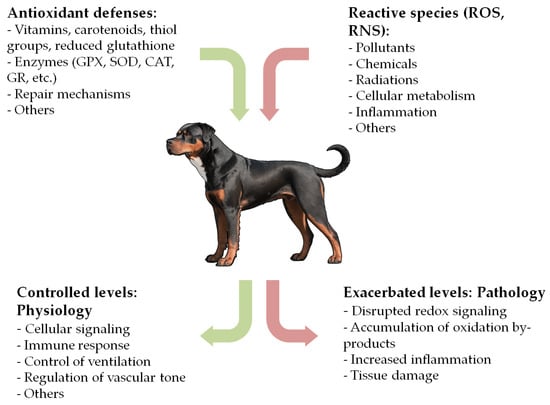
Figure 1.
Simplified mechanisms of oxidative stress.
2. OS in Canine Diseases
2.1. Cardiovascular, Respiratory, and Related Diseases
Cardiovascular diseases are likely among the most extensively studied OS-related pathologies in both human and canine medicine. Cardiac tissue has several sources of reactive species, primarily the mitochondrial electron transport chain, followed by various enzymatic sources such as xanthine oxidase, NADPH oxidase (NOX), and nitric oxide (NO) synthase, among others [12,29,30]. At moderate levels, reactive species play physiological roles, such as regulating vascular tone and signaling cascades in cardiac myocytes. However, increased ROS formation damages subcellular organelles, leading to myocyte contractile dysfunction, loss of functional myocardium, and a decrease in cardiac output. As a result, OS has been linked to various pathological conditions in humans, including hypertension, atherosclerosis, myocardial infarction, ischemia/reperfusion, and heart failure [8,11,12,29,31].
Consistent evidence supports that OS is also present in canine cardiovascular pathology. Several circulating biomarkers of oxidation, mainly lipid peroxidation markers such as MDA and IsoP, as well as antioxidant defense markers, including vitamins, enzymes, and total antioxidant capacity indexes, have been studied in dogs with myxomatous mitral valve disease (MMVD) and dilated cardiomyopathy (DCM) [32,33,34,35,36,37,38,39,40,41,42]. While some studies show discrepancies in results, most report significant differences in OS biomarkers between dogs with MMVD or DCM and healthy controls, suggesting increased oxidative assault and a decline in the efficacy of the antioxidant forces [32,33,34,35,36,37,38,40,41]. Additionally, some studies have shown significant correlations between OS parameters and cytokines, natriuretic peptides, other inflammatory markers, or echocardiographic measurements in dogs with heart failure. This suggests a combined effect of oxidative and inflammatory processes in these patients [38,40,42]. A few studies have failed to show such OS changes in dogs with cardiac conditions, possibly due to the specific biomarkers studied, non-linear changes in oxidation across stages of MMVD or DCM, or potential antioxidant effects of some therapeutic agents such as benazepril or sildenafil, which have yet to be conclusively studied [32,39,42].
To address potential complications arising from OS in dogs with heart disease, several studies have evaluated the efficacy of nutrients and antioxidant administration, especially in dogs with MMVD. Supplementation with specific lipids (omega-3 PUFAs or medium-chain triglycerides) and other compounds (e.g., magnesium, methionine, or lysine) seems to provide benefits by reducing mitochondrial ROS production and supporting other metabolic functions [43]. Boosting antioxidant defenses through the administration of vitamins, taurine, melatonin, and atorvastatin has also shown cardioprotective effects by attenuating OS in dogs with heart disease [43,44,45]. In contrast, Coenzyme Q10 has not demonstrated similar benefits [46].
Additionally, canine models have been extensively used to study induced atrial fibrillation [47,48,49,50,51,52], cardiac arrest [53,54], and heart failure [55,56]. Recent studies have demonstrated increased levels of oxidation markers (ROS, 8-OHdG) and decreased antioxidant enzymes (GPX, SOD) in the blood and cardiac tissue of dogs with induced atrial fibrillation, suggesting an important role of OS in promoting atrial tissue fibrosis, conduction disturbances, and therefore, atrial arrhythmias [47,48,49,50,51,52]. These negative effects have been shown to be attenuated by antihypertensive [52] and antidiabetic [47,49] drugs, among others [48,50,51].
Some cardiorespiratory diseases in dogs, such as tracheal collapse, Brachycephalic Obstructive Airway Syndrome, and exposure to pollutants, have also been linked to OS, possibly associated with inflammatory stages or recurrent hypoxia/reoxygenation events [57,58,59,60,61]. Oxidation markers (MDAs) have been reported to decrease in dogs with tracheal collapse receiving acupuncture and fatty acid supplementation [57,61], and increased antioxidant enzymes (SODs) have been found in dogs with Brachycephalic Obstructive Airway Syndrome after corrective surgery [58]. Conversely, one study did not detect significant differences in lipid (MDA) and protein oxidation (PC) markers in a canine model of hypoxia-induced neurogenic pulmonary edema [62]. Exposure to chromium and petrol generator exhaust fumes, simulating highly polluted environments, has also been linked to increased oxidation (MDA, ROS) and inflammatory biomarkers, along with a decrease in certain antioxidant enzymes (SOD, CAT) [59,60].
2.2. Oncologic Diseases
As demonstrated in various types of human cancers, reactive species are involved in multiple stages of carcinogenesis, including preneoplastic events driven by chronic inflammation, oxidative DNA mutations, proto-oncogene activation, neoplastic cell proliferation, invasion, angiogenesis, and metastasis [3,12,18,63,64,65]. The relationship between OS and cancer is complex, as ROS can both contribute to and result from tumorigenesis. Additionally, ROS can trigger cell death pathways, such as apoptosis and ferroptosis, which may prevent neoplastic events [3,18,66]. In light of these intricate phenomena, a therapeutic approach can be challenging. While enhancing antioxidant defenses might seem appropriate, many chemotherapeutic drugs and radiation therapies actually work by increasing OS in neoplastic cells to induce apoptosis [3,18,67]. However, this strategy also carries the risk of inducing toxic effects in normal tissues [18].
The role of OS in canine oncology has been studied in dogs with various types of cancer, particularly mammary gland tumors [64,65,66,68,69,70,71,72,73], lymphoma [74,75,76,77,78], and mast cell tumors [63,71], among others [71,79,80,81].
OS has been evidenced in dogs with mammary gland tumors, although its manifestation in OS biomarkers appears to depend on the type of sample analyzed. Consistently elevated markers of lipid (i.e., MDA, LOOH) and DNA (i.e., 8-OHdG) oxidation, along with significant alterations in various enzymatic and non-enzymatic antioxidants, have been detected in neoplastic mammary tissue compared to normal mammary gland tissue [64,66,68,69]. Conversely, some studies have reported significant variations in serum or plasma biomarkers in these dogs [70,71], while others have not observed such changes in circulating markers [66,72,73]. Therefore, some researchers recommend direct measurement in target tissues or the collection of multiple blood samples at different time points to account for the detoxification effect on circulating levels [66]. Additionally, a recent study found that female dogs with mammary cancer who received ozone therapy alongside chemotherapy (carboplatin) had a better oxidative profile compared to those receiving standard chemotherapy alone [65].
The literature on OS in canine lymphoma is limited but has produced interesting findings. Studies have reported an altered antioxidant balance in dogs with multicentric lymphoma, as indicated by changes in circulating markers of oxidation (ROS, MDA, and AOPP) and antioxidant defense (antioxidant capacity indexes, vitamins, and enzymes) [71,74,76,77,78]. Notably, two studies observed a correlation between higher OS levels and more aggressive lymphoma characteristics, such as advanced stages (IV and V) and T immunophenotype [74,78]. The impact of treatment on OS remains unclear, potentially due to variations in chemotherapy protocols or the specific biomarkers studied. While some studies have reported a significant correlation between the improvement in OS markers and better clinical response [77,78], others have not found such a correlation [76]. Interestingly, Bottari and colleagues reported even higher circulating markers of oxidation (MDA and AOPP) after CHOP chemotherapy (cyclophosphamide, vincristine, doxorubicin, and prednisone) in dogs with multicentric lymphoma, suggesting that the treatment might exacerbate OS levels [74]. A transient increase in ROS concentrations has also been observed in canine lymphoma and leukemia cell cultures after treatment with benzyl isothiocyanate, suggesting a therapeutic approach targeting OS in these cancers [75].
Lastly, one study revealed increased circulating MDA concentrations in a heterogeneous group of cancer-bearing dogs compared to the control, and another study showed elevated d-ROMs levels and decreased antioxidant capacity in dogs with mast cell tumors [63]. Conversely, another study dismissed the diagnostic value of IsoP for detecting canine urothelial carcinoma [81]. Recent research has also explored the potential of compounds like tepoxalin and myricetin to induce ROS generation and subsequent apoptosis in canine osteosarcoma cell lines [79,80].
2.3. Gastrointestinal and Exocrine Pancreatic Diseases
Remarkable evidence suggests that OS plays a significant part in both acute and chronic canine enteropathies, particularly in Inflammatory Bowel Disease (IBD). This is due to the release of reactive species by leukocytes in the inflamed intestinal mucosa and impaired tissue perfusion. Such OS can lead to further cellular damage, the perpetuation of inflammation, and delayed recovery time [82,83,84,85,86,87]. Consequently, OS-derived molecules have been proposed as promising biomarkers for canine enteropathies. Studies have consistently reported elevated levels of various oxidation biomarkers in the serum or plasma of dogs with IBD and acute enteropathies, including ROS, d-ROMs, MDA, and IsoP, with some also correlating with the severity of clinical presentation [82,85]. Notably, dogs with IBD often exhibit lower levels of several antioxidant biomarkers, such as TAS, CUPRAC, FRAP, and thiol groups [85,86,87]. Interestingly, Minamoto et al. [84] employed a comprehensive untargeted metabolomic approach and identified a significant impact of OS in canine IBD, which persisted even in dogs with apparent clinical improvement. A recent study investigating the response to treatment with allogeneic mesenchymal stem cells in these dogs did not observe changes in MDA levels but proposed albumin as an alternative antioxidant marker in IBD [83]; this has also been supported by the recently reported association between TAS and albumin in dogs [23]. These observations highlight the potential therapeutic value of antioxidant supplementation as a supportive or alternative approach to antimicrobial treatment in canine enteropathies, although further clinical trials are warranted [82,83].
OS has also been proposed to participate in the pathogenesis of canine pancreatitis, although research in this area has been more limited [88,89]. A recent study found elevated levels of reactive metabolites in dogs with acute pancreatitis and identified a significant correlation between urinary IsoP, C-reactive protein, and canine-specific pancreatic lipase [89]. These findings suggest a potential link between OS, pathological calcium signaling, mitochondrial dysfunction, and the amplification of inflammation in canine pancreatitis through ROS. However, the exact mechanisms underlying this relationship require further investigation [88,89].
2.4. Hepatobiliary Diseases
The liver plays a central role in redox regulation, making it both a major producer of reactive species and a target for their damaging effects. This vulnerability arises from the liver’s crucial functions in metabolism and toxin biotransformation, which contribute significantly to reactive species production [90,91]. Notably, copper metabolism is a significant source of ROS in the liver, and its dysregulation can contribute to the development of hepatitis and cirrhosis [92]. Additionally, the liver is the primary site for synthesizing GSH, considered the major intracellular antioxidant [90,93].
The implications of OS in hepatic diseases in dogs have been studied through the quantification of oxidants and antioxidants in various samples, such as blood, urine, and liver tissue, using a range of methods [90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. Elevated urinary IsoP levels have been found in dogs with liver disease of various origins, with a particularly pronounced increase in those with congenital portosystemic shunts [94,100]. Increased levels of plasmatic reactive metabolites and higher immunohistochemical expression of MDA in liver tissue have been reported in dogs with chronic hepatitis and copper-associated hepatitis. These markers have also shown a significant correlation with copper accumulation, necroinflammatory activity, and fibrosis scores [102,103]. The literature on impaired antioxidant defense due to the hepatic depletion of GSH dogs is extensive. Low reduced and oxidized glutathione ratios (GSH/GSSG) are often found in dogs with various hepatopathies (i.e., necroinflammatory liver disorders, extrahepatic bile duct obstruction, copper toxicosis, chronic extrahepatic cholestasis, and chronic hepatitis), along with decreased values of antioxidant enzymes and total antioxidant capacity indexes [90,91,92,102,104]. Further support for these findings comes from transcriptome and gene array analyses of liver tissue from dogs with hepatitis and age-related hepatic changes, which suggest the enhanced expression of genes related to OS and inflammation in hepatic dysfunction [95,99]. Overall, OS biomarkers appear to be promising for assessing canine liver disease of various origins, with only a few studies reporting differently [94,97,102].
Consequently, several authors advocate for antioxidant therapeutic interventions in canine hepatopathies, and various supplements have traditionally been included in their medical management [91,93,94,96,98]. However, the efficacy of antioxidant administration in these patients remains a topic of debate [91,94,100]. Given the complexity of redox homeostasis, supplementing with a single antioxidant may not sufficiently alter OS biomarkers to be detectable by statistical analysis [91]. Traditionally, therapeutic approaches have focused on replenishing depleted GSH [93,94] by administering glutathione precursors like S-adenosylmethionine (SAMe), which is more readily available for clinical practice. Other antioxidant products with evidence of efficacy in canine hepatopathies include vitamin E, ursodeoxycholic acid, and extracts of the milk thistle plant (Silymarin, Silybin, and Silybinin), among others [91,93,96,98]. Webb and Twedt’s review provides recommended dosages for these products in dogs and suggests the potential benefits of combination therapy [91].
2.5. Endocrine Diseases and Obesity
The contribution of OS to canine endocrinopathies has been studied mainly in dogs with hypothyroidism [105,106,107], Cushing’s syndrome [108,109,110], diabetes [111,112,113,114], obesity [115,116,117,118,119], and hyperlipidemia [120].
Data on hypothyroidism and OS, both in humans and dogs, are particularly conflicting. Thyroid hormones significantly influence redox homeostasis, yet their specific effects in hyper- and hypothyroidism remain intriguing, as discussed in a previous review [121]. Various studies have examined comprehensive panels of biomarkers in hypothyroid dogs, suggesting that increased OS is present, although the results vary widely depending on the biomarker studied and the sample type. Some studies have reported elevated d-ROMs and MDA levels in the blood and saliva of hypothyroid dogs [105,106,107], while others have found lower AOPP levels [105,106]. Similarly, the literature shows both increased [105,107] and decreased [106] levels of several antioxidant indexes. In this context, sample type seems to be relevant, with whole-blood samples potentially providing a better reflection of the altered redox homeostasis in these patients [106].
Unlike the well-established link between increased OS and diabetes in humans, which is associated with dysfunctional mitochondria and glucose auto-oxidation [3,111], studies in dogs have produced variable results. Some studies have reported increased markers of lipid and DNA oxidation, particularly in poorly controlled diabetic dogs, suggesting an association with disease severity [111,113]. Additionally, significant improvements in OS biomarkers have been observed following antioxidant supplementation with N-acetylcysteine [113] and Fibroblast growth factor-21 [112]. Conversely, a recent study found no differences in MDA or SOD levels between diabetic and control dogs and did not observe a significant benefit of another antioxidant, Andrographis paniculata [114].
Regarding Cushing’s syndrome, research in canine species has been limited but consistent. Two studies reported increased markers of lipid and protein oxidation in dogs with hypercortisolism, particularly in poorly controlled patients. These studies also noted a significant reduction in oxidation markers following treatment, highlighting both the presence of increased OS and the significant benefits of medical control in these patients [108,109].
A reasonable question arises when evaluating OS, particularly lipid peroxidation, in dogs with endocrinopathies (e.g., Cushing’s syndrome and hypothyroidism): the potential influence of the patient’s body condition score in the results [107,109]. Since obesity in humans has been associated with chronic inflammation and increased OS, several studies have studied this relationship in dogs [115,116,117,118,119]. Although this aspect remains unclear [118,119], significant variations in OS biomarkers have been detected in the blood, saliva, and adipose tissue of obese dogs [115,116,117,119]. Additionally, the impact of hyperlipidemia [120] and the significant interference of lipemia with OS biomarkers such as MDA and TAS have been reported [122]. This interference should be considered when interpreting results from dogs with endocrine diseases.
2.6. Hematologic Diseases
Red blood cells (RBCs) are particularly vulnerable to ROS attacks due to their ubiquity, proximity to oxygen molecules, and elevated iron concentrations. In humans, OS has been proposed as both a cause and consequence of anemia through mechanisms such as reducing the mean lifespan of RBCs or increasing tissue oxygen demand and ROS production [123,124,125]. While the literature on this topic in dogs is scarce, it has provided some interesting data on anemia of various causes: hemolytic, non-hemolytic, secondary to kidney, infectious, and oncologic diseases, among others [124,125,126,127,128]. Studies have shown variable changes in oxidation markers (ROS, IsoP, and MDA) [124,125,126], but substantial evidence suggests that OS and antioxidant depletion are involved in the development of anemia in dogs. This link is likely due to decreased antioxidant defenses, both enzymatic and non-enzymatic (GPX, Vitamin E, and GSH) [125,126,127,128]. Further investigation is needed to determine whether OS is a primary cause or a secondary effect of anemia and explore if antioxidant therapy could improve survival and overall outcomes in dogs with anemia from various causes [125,126,127]. It is also important to consider that hemolysis and icterus can be potential sources of interference, frequently affecting plasma or serum samples from these patients [122].
Additionally, two studies have highlighted that OS could be a significant concern in canine hemotherapy. The accumulation of oxidation products (MDA, PC) and depletion of natural cellular antioxidants (TAS, SOD, GPX, and CAT) have been detected in stored canine whole blood, along with increased hemolysis. These findings suggest that prolonged storage periods (>28 days) might discourage the use of stored blood in certain cases [129]. Furthermore, the therapeutic efficacy of canine bone marrow mesenchymal stem cell transplantation may be compromised by OS-mediated senescence, as indicated by increased levels of ROS and decreased antioxidant enzymes. This effect has been mitigated by adding antioxidants, such as mitoquinone, to cell cultures [130].
2.7. Infectious and Parasitic Diseases
2.7.1. Vector-Borne Diseases
Leishmaniosis
The role of OS in the pathogenesis of canine leishmaniosis (CanL) has been extensively studied, and substantial evidence has been gathered on the relevance of OS and its association with the clinical stages of CanL [131,132,133,134,135,136,137,138,139,140,141,142,143]. The interplay between the host’s immune response, the parasites, and OS creates a complex pathogenic landscape.
While Leishmania spp. can initially evade the immune system by suppressing ROS production by phagocytes, the subsequent development of inflammation in CanL is characterized by an increased influx of activated neutrophils and macrophages that generate high levels of oxidants. This contributes to the progression of the disease and a concomitant weakening of antioxidant defenses [131,133,134,139,140]. These aspects are supported by several studies that have found increased circulating oxidation markers (e.g., ROS, MDA, and total oxidant status) [132,133,134,135,137,138] and variable changes in antioxidant markers (e.g., TAS, CUPRAC, FRAP, GSH, and thiol groups) depending on the clinical stage [133,134,137,140,141,142]. Almeida and colleagues also found that OS in CanL causes neutrophil dysfunction, leading to their apoptosis, particularly in severe stages and in association with uremia [132,133]. More recent findings have suggested that increased OS impairs the lymphoproliferative response and, therefore, cellular immunity in dogs with CanL [134]. Additionally, correlations between OS biomarkers and parasite load have been observed [142], as well as improvements in antioxidant defense following successful therapy, indicating that OS may be a useful tool for monitoring the treatment and clinical follow-up of sick dogs [140].
Despite the established link between OS and disease progression in CanL, therapeutic strategies targeting the redox state have not been extensively explored. While some authors advocate for tailoring CanL treatment plans based on the patient’s redox status [139], research on the efficacy of enhancing the antioxidant defense system in this disease remains limited. A recent study reported a decrease in circulating MDA and PC, along with an increase in GSH, after the addition of nutritional adjuvants (omega-3 PUFAs and B vitamins) to standard anti-Leishmania treatment [136]. However, further research on this topic is needed.
Ehrlichiosis
Significant alterations in redox status have been documented in canine ehrlichiosis [144,145,146,147,148,149,150,151,152]. Increased levels of ROS, MDA, and AOPP have been observed in both naturally and experimentally infected dogs [144,145,146,147,149,151], while a decrease in nitric oxide (NO) and MDA was noted following doxycycline treatment [149]. Antioxidant markers (e.g., TAS, CUPRAC, FRAP, GPX, thiol groups, and others) have shown either increases or decreases depending on the disease stage (acute versus subclinical). These fluctuations likely reflect the complex interplay between OS and infectious agents [144,146,147,150,151].
Babesiosis
Studies on OS canine babesiosis have consistently found elevated levels of reactive species (NO), lipid (MDA), and DNA oxidation markers (8-OHdG), along with variable alterations in antioxidant enzymes and indexes (e.g., TAS, SOD, CAT, and GPX) in infected dogs [145,148,153,154,155,156,157,158]. Various authors have proposed that OS could be one of the mechanisms leading to anemia in dogs with babesiosis, as a result of oxidative damage to erythrocytes, favoring their destruction [155,156,158]. Additionally, infected dogs with secondary multiple organ dysfunction have shown more pronounced redox alterations, suggesting OS biomarkers could serve as indicators of disease severity and outcomes in canine babesiosis [155].
Other Vector-Borne Diseases
Similar trends of increased DNA and lipid oxidation have been observed in dogs with heartworm disease, alongside variable findings in antioxidant markers [159,160,161]. Additionally, OS has been proposed to play a role in the pathogenesis of canine hepatozoonosis and trypanosomosis and may be related to the development of anemia due to increased lipid peroxidation in erythrocytes [162,163].
2.7.2. Infectious and Parasitic Gastrointestinal Diseases
Canine parvoviral enteritis is associated with OS, as evidenced by increased circulating MDA and NO levels, along with alterations in enzymatic and non-enzymatic antioxidant markers, likely due to the virus-induced release of pro-inflammatory cytokines [164,165,166,167]. The addition of antioxidants such as N-acetylcysteine, resveratrol, and vitamin C to standard therapy can reduce the concentrations of MDA and NO and enhance the activity of certain antioxidant enzymes. However, a clear improvement in clinical scores or survival rates following antioxidant therapy has not been consistently demonstrated, and further research is necessary to determine the optimal selection and dosage of antioxidants for this purpose [164,165].
Regarding gastrointestinal parasites, the role of OS in their pathogenesis remains unclear. A study found significant changes in ROS metabolites and thiol levels in dogs with gastrointestinal nematodosis [168], while another study failed to demonstrate alterations in antioxidant markers in parasitized dogs [169].
2.7.3. Ectoparasites and Dermal Fungal Diseases
Canine demodicosis [170,171,172,173,174] and sarcoptic mange [175,176,177,178] are associated with increased OS. This is believed to result from the presence of parasites in the skin, which release antigenic material and trigger the production of pro-inflammatory cytokines. These factors may contribute to pathological changes in the tissue, such as erythema, edema, hypersensitivity, pruritus, and hyperkeratosis [173,174,175,176]. Elevated levels of peripheral oxidation biomarkers (MDA and other lipid hydroperoxides) have been consistently observed in dogs with both localized and generalized demodicosis, as well as in those with sarcoptic mange [170,171,173,175,176,177,178]. Markers of antioxidant defense have shown variable changes: while most of the studies have reported significant depletions in antioxidants like SOD, CAT, GPX, and vitamins [170,175,176,178], others have found no change or even increased levels in infested dogs compared to control [171,172,173,177]. This variability might be explained by an initial upregulation of antioxidant defenses, followed by their overutilization or sequestration in the skin as the disease progresses [170]. Interestingly, some authors have identified a relationship between OS, the severity of the infestation, and the rate of apoptosis in peripheral leukocytes in dogs with sarcoptic mange [176,178]. Moreover, treatment with ivermectin appears to normalize OS markers in both demodicosis and sarcoptic mange, especially when antioxidants such as vitamin E and selenium are added to standard therapy [172,175]. Additionally, a study reported increased OS in canine dermatophytosis, specifically noting a rise in circulating MDA and a decrease in both enzymatic and non-enzymatic antioxidants [179].
2.8. Neurologic Diseases
The nervous system, particularly the brain, is highly vulnerable to oxidative damage due to its high energy and oxygen consumption, the large concentration of PUFAs in myelin membranes, and its relatively low antioxidant defenses [180,181]. While OS has been linked to the etiopathology of several neurologic diseases in humans [181], research in dogs remains limited. A recent study in dogs with idiopathic epilepsy, experiencing either focal or generalized seizures, revealed significant alterations in circulating OS biomarkers, including higher levels of AOPP and lower levels of GSH, thiol groups, and other antioxidants [182]. These findings may be attributed to neuroinflammation and accelerated ROS-mediated neuronal deterioration, which could induce subsequent seizures [182,183]. Furthermore, Marquis et al. [180] evaluated IsoP, acrolein, and GSH levels in urine, cerebrospinal fluid, and spinal cord tissue of dogs with ascending–descending myelomalacia following spinal cord injury, finding exacerbated OS and a potential association with neurodegeneration and necrosis. In contrast, while the role of OS in canine motor neuron disease and degenerative myelopathy in Pembroke Welsh Corgi dogs has been studied, it has not been fully clarified [184,185,186].
2.9. Renal Diseases
Renal cells, particularly tubular epithelial cells, are significant sources of endogenous ROS due to their high mitochondrial activity, arterial blood flow, and the activity of ROS-producing NOX family enzymes [187,188]. Increases in renal ROS production can lead to the release of pro-inflammatory cytokines, and, if persistent, to inflammation and renal fibrosis, making OS a proven contributing factor to Chronic Kidney Disease (CKD) in both human and animal models [187,188,189,190]. This issue becomes even more concerning when the few remaining nephrons become hyperfunctional, further increasing mitochondrial oxidative phosphorylation and ROS production [187,189,190]. Additionally, several factors commonly present in humans and animals with CKD can exacerbate OS, including the activation of the renin–angiotensin system, systemic hypertension, chronic inflammation, proteinuria, anemia, and advanced age [187,190].
Various authors have studied the implications of OS in dogs with renal disease [124,187,189,190,191,192,193,194,195,196]. With very few exceptions in some circulating antioxidant indexes [189], most studies on canine CKD have evidenced significant alterations in OS biomarkers, especially in MDA, IsoP, ROS, TAS, and antioxidant enzymes [124,191,195,196]. Some studies have also found significant correlations between MDA, creatinine concentration [124], and the degree of renal dysfunction [192]. The role of OS has also been observed in nephrotoxicity caused by hemoglobinuria [193], chemotherapeutic drugs (cisplatin) [194], and uremic toxins (methylguanidine) in dogs [191]. Additionally, it has been shown that OS accelerates neutrophil apoptosis in canine CKD, potentially affecting their innate immune response [191,195]. Protecting the kidney from OS through antioxidant supplementation and other therapeutic actions has been suggested in dogs, as summarized in Brown’s review [187]. However, further clinical investigations are warranted due to the limited research in this area [187,190].
2.10. Dermatologic Diseases
The skin is continuously exposed to reactive species from both endogenous and environmental sources, necessitating robust enzymatic and non-enzymatic antioxidants, such as vitamins and carotenes [197,198]. Similar to humans, altered dermal redox homeostasis in dogs has been linked to certain skin diseases, particularly atopic dermatitis [198,199,200,201,202]. Despite some discrepancies existing depending on the specific biomarkers used, several studies have demonstrated a correlation between clinical scores (i.e., Canine Atopic Dermatitis Extent and Severity Index, CADESI) and OS biomarkers like MDA, antioxidant enzymes, and vitamin E [198,199,200]. The contribution of OS to atopic dermatitis is likely related to the infiltration of the skin with inflammatory cells and cytokines, which promote ROS formation and disrupt the skin’s antioxidant barrier [197,199,200]. Consequently, OS biomarkers have been proposed as useful tools for precision medicine in dogs with atopic dermatitis [199]. Furthermore, various researchers have advocated for a multimodal therapeutic approach that includes nutritional interventions and antioxidant supplementation (e.g., vitamins and carotenes) alongside standard therapies [198,199,202]. Limited data suggest that OS might also play a role in canine zinc-responsive dermatosis, although further investigation is warranted [203].
2.11. Ophthalmologic Diseases
OS is considered a risk factor for eye diseases [204] and has been studied primarily in two ophthalmologic disorders in dogs: cataracts [205,206,207,208,209,210,211] and glaucoma [212,213,214,215].
Lenses are chronically exposed to photo-oxidation of their proteins and lipids due to UV radiation, leading to protein aggregation and, ultimately, lens opacification. Despite the presence of antioxidant agents within the lens (such as vitamins and antioxidant enzymes), OS is widely recognized as a major contributor to cataract development, alongside other environmental and endogenous factors [205,206,210,211]. Significant alterations in oxidative biomarkers (MDA) and antioxidant biomarkers (TAS, SOD, CAT, and GPX) have been detected in the blood and aqueous humor of cataractous dogs [207,209]. Additionally, decreased antioxidant capacity and vitamin C levels have been observed in the aqueous humor of dogs following extracapsular lens extraction and experimental phacoemulsification, suggesting that these surgical procedures initially induce an OS condition in the eye [206,208]. In attempting to prevent or delay cataract formation, both oral antioxidant supplements and topical antioxidant eye drops have been used in humans and dogs [210,211]. Examples of antioxidant agents demonstrating protective effects in dogs, particularly in incipient cataracts, include grape seed extracts, vitamins C and E, curcuminoids, and others [205,210].
Similarly, OS appears to be a major contributor to retinal ganglion cell degeneration and glaucoma development [212,214,215]. Increased immunolabeling for OS biomarkers has been observed in the retinal tissue of dogs with acute glaucoma [213], and lower antioxidant enzymes (GPX) have been related to an increased risk of inherited glaucoma in Euraiser dogs [212]. Although antioxidants have been proposed to protect canine retinal membranes under experimental conditions [204], the literature on this topic remains limited.
2.12. Orthopedic Diseases
Reactive species are considered important mediators in the pathophysiology of osteoarthritis. Chondrocytes and activated inflammatory cells in this condition release increased amounts of ROS, which further damage collagen, proteoglycans, and hyaluronic acid and enhance chondrocyte senescence and cartilage degradation [216,217,218,219,220]. This redox imbalance has been documented in dogs with both naturally occurring and experimentally induced osteoarthritis, as evidenced by OS biomarkers in blood and canine chondrocyte cell cultures [217,218,219,220,221]. Enhanced oxidative processes have also been observed in circulating OS biomarkers in dogs suffering from hip dysplasia, likely due to similar mechanisms of cartilage inflammation and degradation [216,222].
Dietary composition, particularly the lipid profile with a focus on omega-3 PUFAs and eicosapentaenoic acid (EPA), appears to play a critical role in mitigating these processes. Both pharmaceutical interventions (e.g., N-acetylcysteine) [218] and nutraceutical products (e.g., fish oil, corn oil, and other plant-derived compounds) [217,220,221] have demonstrated protective effects against OS in canine osteoarthritis.
2.13. Reproductive System Diseases
Recent studies have shown consistent alterations in OS biomarkers measured in blood, urine, and uterine tissue in bitches with cystic endometrial hyperplasia and pyometra. These findings suggest that excessive ROS production may be a significant factor contributing to uterine damage by weakening local antioxidant defenses and exacerbating these disorders [223,224,225]. Additionally, as summarized in Domosławska-Wyderska and colleagues’ recent review [226], various studies indicate that OS may play a relevant role in the pathogenesis of canine benign prostatic hyperplasia. This association could be linked to age-related hormonal changes and chronic inflammation of the prostate. However, further research is needed to evaluate the potential benefits of antioxidants in this condition [226,227].
2.14. Dental Diseases
Studies investigating OS markers in canine periodontal disease have yielded mixed results [228,229]. While one recent study found no changes in salivary MDA concentrations [229], a previous study detected a significant accumulation of MDA and 8-OHdG in the saliva of dogs with periodontal disease, along with an increase in salivary SOD activity [228]. This earlier study also found correlations between OS biomarkers and the severity of gum and teeth clinical signs, which were attributed to the inflammatory processes in the oral cavity [228].
2.15. Others
Additionally, other studies have demonstrated that OS is present in dogs with ischemia-reperfusion injury [230], as well as in systemically ill dogs undergoing hospitalization due to various underlying disorders (e.g., infectious, inflammatory, immune-mediated, metabolic, and neoplastic) [231,232]. It has been observed that hospitalized dogs exhibit increased lipid peroxidation (elevated urinary IsoP levels) and antioxidant depletion, particularly in GSH and vitamin E. While N-acetylcysteine supplementation did not appear to improve overall redox state in these dogs, further research is needed to explore other antioxidant therapeutic options and their impact on longer-term outcomes [231,232].
3. Conclusions
Solid evidence demonstrates the role of OS in a multitude of canine diseases, impacting diverse organs and systems. In some conditions, it remains unclear whether reactive species are significant causative agents or merely byproducts of the inflammatory processes involved. Inconsistencies across studies may arise from differences in sample selection, the specific OS biomarkers used, and variations in analytical methods. Moreover, interpreting increased antioxidant defenses as a response to OS or antioxidant depletion as a sign of imbalance can be challenging.
Therapeutic approaches to managing OS vary widely among canine diseases. Certain antioxidants are commonly used in some diseases (e.g., hepatopathies), while pro-oxidant drugs are employed in others (e.g., oncology). In some areas, this issue remains underexplored.
To our knowledge, this is the first comprehensive review summarizing the current understanding of OS in canine pathology, with the aim of paving the way for further research in such a broad and evolving field.

Table 2.
Selected studies on oxidative stress in canine diseases.
Table 2.
Selected studies on oxidative stress in canine diseases.
| Group | Sub-Group | Disease * | Biomarkers of Oxidation* | Biomarkers of Antioxidant Defense * | Sample Type | Reference |
|---|---|---|---|---|---|---|
| Cardiovascular, respiratory, and related diseases | Cardiovascular | MMVD | MDA, mtDNA | - | Blood | [32] |
| MMVD | MDA, OxLDL | Vitamin E | Blood | [39] | ||
| MMVD and DCM | MDA | GPX, Vitamin E | Blood | [42] | ||
| MMVD and DCM | MDA | GPX | Blood | [38] | ||
| MMVD | - | CUPRAC, SOD, CAT, GR | Blood | [36] | ||
| MMVD and DCM | - | TAS (ABTS), CUPRAC, Thiol | Blood | [40] | ||
| MMVD, DCM, and others (Heart Failure) | - | TAS (ABTS), SOD, CAT, GPX | Blood | [37] | ||
| MMVD stage B1 | MDA | SOD, GPX, Vitamin E | Blood | [41] | ||
| MMVD and DCM (Heart Failure) | MDA, IsoP, PC | GSH:GSSG, vitamins A, C, and E, ORAC | Blood | [35] | ||
| DCM | MDA | GPX, SOD, Vitamins A, C, E | Blood | [34] | ||
| DCM | - | GPX, SOD, Vitamins A, C, E | Blood | [33] | ||
| MMVD and Heart Failure | - | - | Review | [43] | ||
| MMVD | MDA | - | Blood | [44] | ||
| MMVD | IsoP | GPX | Blood | [46] | ||
| MMVD | IsoP | - | Blood | [45] | ||
| Experimental cardiac models | Induced atrial fibrillation | ROS, XO | GPX, SOD | Blood | [52] | |
| Induced atrial fibrillation | ROS | - | Cardiac tissue | [49] | ||
| Induced atrial fibrillation | ROS, XO | - | Blood | [51] | ||
| Induced atrial fibrillation | ROS | - | Cardiac tissue | [47] | ||
| Induced atrial fibrillation | ROS | - | Cardiac tissue | [50] | ||
| Induced atrial fibrillation | ROS, 8-OHdG | - | Cardiac tissue | [48] | ||
| Induced heart failure | Panel of aldehydes | - | Cardiac tissue | [55] | ||
| Induced cardiac arrest | IsoP | Panel of enzymes | Cardiac tissue | [54] | ||
| Induced heart failure | Panel of aldehydes | - | Cardiac tissue | [56] | ||
| Induced cardiac arrest | IsoP | - | Coronary sinus plasma | [53] | ||
| Respiratory | Tracheal collapse | MDA | - | Blood | [57] | |
| Tracheal collapse | MDA | - | Blood | [61] | ||
| Air pollution | MDA, NO | SOD, CAT, GSH, SOD | Blood | [59] | ||
| Brachycephalic Obstructive Airway Syndrome | MDA | SOD, GPX | Blood | [58] | ||
| Chromium pollution | MDA | SOD, CAT | Tissues | [60] | ||
| Hypoxia-induced neurogenic pulmonary edema | MDA, PC | - | Tissues | [62] | ||
| Oncologic diseases | Mammary gland tumors | Mammary gland tumors | MDA | - | Blood | [72] |
| Mammary gland tumors | MDA | TAS (ABTS) | Blood | [65] | ||
| Mammary gland tumors | MDA, 8-OHdG | - | Mammary gland tissue | [68] | ||
| Mammary gland tumors | MDA | GSH, G6PD | Mammary gland tissue | [64] | ||
| Mammary gland tumors | NO, AOPP | FRAP | Blood | [70] | ||
| Mammary gland tumors | MDA | Vitamin E | Blood and mammary gland tissue | [66] | ||
| Mammary gland tumors | MDA, LOOH | SOD, CAT, GSH, GST, Vitamin C | Mammary gland tissue | [69] | ||
| Mammary gland tumors | MDA | SOD, GPX, Thiol | Blood | [73] | ||
| Lymphoma and leukemia | Lymphoma and lymphoid leukemia | ROS | - | Cell culture | [75] | |
| Lymphoma | d-ROMs | BAP | Blood | [76] | ||
| Lymphoma | MDA, AOPP | FRAP | Blood | [74] | ||
| Lymphoma | MDA, IsoP | ORAC, GPX, Vitamin C, Vitamin E | Blood | [78] | ||
| Lymphoma | MDA, ROS | GSH:GSSG, GPX, FRAP, SOD | Blood and lymph node tissue | [77] | ||
| Other oncologic diseases | Osteosarcoma | ROS | - | Neoplastic cells | [80] | |
| Osteosarcoma | ROS | - | Neoplastic cells | [79] | ||
| Mast cell tumor | d-ROMs | BAP, Vitamin E | Blood | [63] | ||
| Urothelial carcinoma | IsoP | - | Urine | [81] | ||
| Various cancer types: Mammary gland carcinoma, mast cell tumor, osteosarcoma, and others. | MDA | - | Blood | [71] | ||
| Gastrointestinal and exocrine pancreatic diseases | Gastrointestinal diseases | Chronic inflammatory enteropathy | MDA | GSH, Albumin | Blood | [83] |
| Acute Diarrhea (non-specific acute enteropathies) | d-ROMs, OSI | SAC | Blood | [82] | ||
| IBD | ROS, MDA, FOX | TAS, CUPRAC, FRAP, Thiol, PON-1 | Blood | [85] | ||
| IBD | - | CUPRAC | Blood | [87] | ||
| IBD | - | TAS (ABTS) | Blood | [86] | ||
| IBD | Metabolomic profile | Metabolomic profile | Blood | [84] | ||
| Exocrine pancreatic diseases | Acute Pancreatitis | RM, IsoP | AOP | Blood, urine | [89] | |
| Pancreatitis | - | - | Review | [88] | ||
| Hepatobiliary diseases | Acute liver injury | MDA, H2O2, 8-OHdG | G6PD, TrxR, CAT, SOD, GPX, GR, GSH | Liver tissue | [98] | |
| Liver disease (various origins) | - | GSH | Blood | [93] | ||
| Liver disease (various origins) | IsoP | - | Urine | [100] | ||
| Liver disease | d-ROMs | Thiol | Blood | [96] | ||
| Liver injury | ROS | CAT, GPX | Liver tissue | [104] | ||
| Chronic hepatitis | MDA, 4-HNE | - | Liver tissue | [103] | ||
| Cooper-associated hepatitis | RM, IsoP | TAS (ABTS) | Blood and urine | [102] | ||
| Liver disease (various origins) | IsoP | GSH, Vitamin E | Blood, urine, and liver tissue. | [94] | ||
| Cooper-associated hepatitis | Transcriptome and gene Arrays | Transcriptome and gene Arrays | Liver tissue | [95] | ||
| Chronic liver disease | MDA | - | Liver tissue | [101] | ||
| Age-related hepatic alterations | Genome Arrays | Genome Arrays | Liver tissue | [99] | ||
| Liver disease | - | - | Review | [91] | ||
| Portosystemic Shunt | - | Vitamin C | Blood | [97] | ||
| Liver disease (various origins) | - | GSH/GSSG and antioxidants gene expression | Liver tissue | [92] | ||
| Liver disease (various origins) | - | GSH/GSSG | Liver tissue | [90] | ||
| Endocrine diseases and obesity | Hypothyroidism | Hypothyroidism | TOS, POX-Act, d-ROMs, AOPP, MDA | CUPRAC, FRAP, TAS (ABTS), PON-1 | Blood | [106] |
| Hypothyroidism | MDA | TAC | Blood | [107] | ||
| Hypothyroidism | MDA, d-ROMs, TOS, POX-Act, AOPP | CUPRAC, FRAP, TAS (ABTS), Thiol, PON-1, GPX, FRAS | Blood and saliva | [105] | ||
| Cushing’s syndrome | Cardiac fibrosis-Cushing’s syndrome | 8-OHdG, NADPH oxidase | SOD | Blood and cardiac tissue | [110] | |
| Cushing’s syndrome | MDA | - | Blood | [109] | ||
| Cushing’s syndrome | PC | - | Blood | [108] | ||
| Diabetes | Diabetes | MDA | SOD | Blood | [114] | |
| Diabetes | H2O2, 8-OHdG, MDA | CAT, SOD, GPX, GSH-GSSG, TrxR, NADPH-NADP+, Thiol | Cerebrum tissue | [113] | ||
| Diabetes | ROS, MDA | CAT, GPX, GR, SOD | Pancreatic tissue | [112] | ||
| Diabetes | MDA | CAT, GSH | Blood | [111] | ||
| Hyperlipidemia | Hyperlipidemia | MDA | - | Blood | [120] | |
| Obesity | Obesity | MDA | - | Blood | [115] | |
| Obesity | MDA, ROS, FOX | CUPRAC, FRAP, TAS (ABTS), Thiol, PON-1 | Blood | [119] | ||
| Obesity-related metabolic dysfunction | - | Proteomics | Saliva | [117] | ||
| Obesity | MDA | FRAP, Ceruloplasmin | Blood | [118] | ||
| Obesity | - | Transcriptomics | Blood, adipose tissue | [116] | ||
| Hematologic diseases | Hemotherapy | Stored blood (transfusion medicine) | MDA, PC | TAS (ABTS), SOD, GPX, CAT | Blood | [129] |
| Bone marrow mesenchymal stem cells (BMSCs) transplantation | ROS, MDA | SOD, CAT, GPX | BMSCs culture | [130] | ||
| Anemia (various origins) | Anemia (hemolytic and nonhemolytic) | ROS | GSH, Vitamin E | Blood | [125] | |
| Anemia (various origins) | IsoP | TAS (ABTS), GPX | Blood and urine | [126] | ||
| Anemia (CKD) | MDA | GSH-GSSH, GPX, GR, SOD | Blood | [124] | ||
| Immune-mediated hemolytic anemia | - | Peroxiredoxin-2 | Blood | [128] | ||
| Immune-mediated hemolytic anemia | MDA | Vitamin E | Blood | [127] | ||
| Infectious and parasitic diseases | Vector-borne diseases | Leishmaniosis | MDA, PC | GSH/GSSG | Blood | [136] |
| Leishmaniosis | ROS, RNS, Hydroperoxides | SOD, FRAP | Blood | [139] | ||
| Leishmaniosis | - | PON-1 | Blood | [143] | ||
| Leishmaniosis | MDA | GSH/GSSG, Thiol | Blood | [138] | ||
| Leishmaniosis | TOC, MDA | TAS (ABTS) | Blood | [134] | ||
| Leishmaniosis | TOC, MDA | TAC | Blood and tissues | [142] | ||
| Leishmaniosis | - | SOD | Blood | [141] | ||
| Leishmaniosis | TOS | TAS (ABTS), FRAP, CUPRAC, PON-1, Thiol | Blood | [140] | ||
| Leishmaniosis | - | - | Review | [131] | ||
| Leishmaniosis | ROS | - | Blood | [132] | ||
| Leishmaniosis | TOC, MDA | TAS (ABTS), GSH | Blood | [133] | ||
| Leishmaniosis | MDA | TAS (ABTS) | Blood | [137] | ||
| Leishmaniosis | MDA | GSH, Vitamin C | Blood | [135] | ||
| Ehrlichiosis | R-OOHs | OXY, Thiol | Blood | [150] | ||
| Ehrlichiosis | MDA, NO | - | Blood | [149] | ||
| Ehrlichiosis | MDA, NO | TAC, SOD, GPX | Blood | [146] | ||
| Ehrlichiosis | ROS, MDA, FOX | TAS (ABTS), CUPRAC, FRAP | Blood | [151] | ||
| Ehrlichiosis | AOPP | FRAP | Blood | [144] | ||
| Ehrlichiosis | - | TAS (ABTS), PON-1 | Blood | [152] | ||
| Ehrlichiosis | MDA, NO, AOPP | GR | Blood | [147] | ||
| Ehrlichiosis and Babesiosis | MDA, NO | - | Blood | [145] | ||
| Ehrlichiosis and Babesiosis | MDA | - | Blood | [148] | ||
| Babesiosis | - | GSH, SOD, CAT | Blood | [158] | ||
| Babesiosis | LPO | SOD, CAT, TAS (ABTS) | Blood | [156] | ||
| Babesiosis | MDA | TAS (ABTS), SOD, CAT, GPX | Blood | [155] | ||
| Babesiosis | 8-OHdG, NO | TAS (ABTS) | Blood | [153] | ||
| Babesiosis | MDA | - | Blood | [154] | ||
| Babesiosis | MDA | - | Blood | [157] | ||
| Heartworm disease | Comet assay (DNA oxidation) | - | Blood | [160] | ||
| Heartworm disease | - | TAS (ABTS), GPX, PON-1 | Blood | [159] | ||
| Heartworm disease | MDA | SOD, CAT | Blood | [161] | ||
| Hepatozoonosis | MDA, NO | GSH | Blood | [162] | ||
| Trypanosomosis | LPO | TAS (ABTS), SOD, GSH | Blood | [163] | ||
| Infectious and parasitic gastrointestinal diseases | Parvoviral enteritis | MDA, NO | GST | Blood | [164] | |
| Parvoviral enteritis | MDA, NO | GST | Blood | [165] | ||
| Parvoviral enteritis | - | TAS (ABTS), PON-1 | Blood | [166] | ||
| Parvoviral enteritis | MDA | SOD, CAT | Blood | [167] | ||
| Gastrointestinal helminthiasis | R-OOHs | OXY, Thiol | Blood | [168] | ||
| Gastrointestinal helminthiasis | - | TAS (ABTS), PON-1 | Blood | [169] | ||
| Ectoparasites and dermal fungal diseases | Demodicosis | MDA | SOD, GPX, TAC, CAT | Blood | [173] | |
| Sarcoptic mange | MDA | SOD, CAT, vitamin A, vitamin C | Blood | [176] | ||
| Demodicosis | - | - | Review | [174] | ||
| Demodicosis | - | PON-1, TAS (ABTS) | Blood | [172] | ||
| Demodicosis | MDA | SOD, CAT, β-carotene, vitamin C | Blood | [170] | ||
| Sarcoptic mange | MDA | SOC, CAT, GPX, GSH, GST | Blood | [178] | ||
| Sarcoptic mange | MDA | GSH, SOD, CAT | Blood | [175] | ||
| Sarcoptic mange | TOS, LOOH | TAS (ABTS), Thiol | Blood | [177] | ||
| Demodicosis | MDA | GSH, SOD, CAT | Blood | [171] | ||
| Dermatophytosis | MDA | SOD, CAT, β-carotene, vitamin C | Blood | [179] | ||
| Neurologic diseases | Epilepsy | - | - | Review | [183] | |
| Epilepsy | MDA, AOPP | GSH, PON-1, Thiol | Blood | [182] | ||
| Myelomalacia | IsoP, Acrolein | GSH | Urine, cerebrospinal fluid, and spinal cord tissue samples | [180] | ||
| Degenerative Myelopathy, Pembroke Welsh Corgi | NO | SOD | Spinal cord tissue samples | [186] | ||
| Degenerative Myelopathy, Pembroke Welsh Corgi | IsoP | - | Cerebrospinal fluid | [184] | ||
| Hereditary canine spinal muscular atrophy | - | SOD, GPX, Vitamin E | Blood | [185] | ||
| Renal diseases | Chronic Kidney Disease | IsoP | - | Urine | [196] | |
| Nephrotoxicity | MDA, ROS | SOD, CAT | Madin–Darby canine kidney cell culture | [194] | ||
| Chronic Kidney Disease | d-ROMS | - | Blood | [190] | ||
| Chronic Kidney Disease | - | CUPRAC | Blood | [189] | ||
| Chronic Kidney Disease and Nephrotoxicity | MDA, ROS | TAS (ABTS) | Plasma and canine neutrophils | [191] | ||
| Nephrotoxicity | 4-HNE, Hb-oxidation products | - | Renal tissue | [193] | ||
| Chronic Kidney Disease | MDA | GSH-GSSH, GPX, GR, SOD | Blood | [124] | ||
| Chronic Kidney Disease | MDA, ROS | TAS (ABTS) | Plasma and canine neutrophils | [195] | ||
| Renal azotemia | MDA | CAT, GSH | Blood and urine | [192] | ||
| Chronic Kidney Disease | - | - | Review | [187] | ||
| Dermatologic diseases | Atopic dermatitis | - | - | Clinical Scores | [202] | |
| Atopic dermatitis | FOX | TAS, CUPRAC, FRAP, Thiol | Blood | [199] | ||
| Atopic dermatitis | MDA | TAC, GPX, SOD, Vitamin E | Blood and skin tissue | [198] | ||
| Atopic dermatitis | - | Vitamin E | Blood and skin tissue | [201] | ||
| Atopic dermatitis | MDA | TAS, GPX, SOD | Blood | [200] | ||
| Zinc-responsive dermatosis | - | SOD, metallothionein, heat shock proteins | Skin tissue | [203] | ||
| Ophthalmologic diseases | Cataracts | - | - | Ophthalmologic clinical evaluation | [210] | |
| Cataracts | MDA | TAS (ABTS) | Blood | [209] | ||
| Cataracts | Western immunoblotting | - | Canine lens epithelial cells | [205] | ||
| Cataracts | - | - | Review | [211] | ||
| Cataracts | - | TAC, Vitamin C | Aqueous humor | [208] | ||
| Cataracts | - | SOD, CAT, GPX, G6PD, Vitamin C | Blood and aqueous humor | [207] | ||
| Cataracts | TAC, Vitamin C | Aqueous humor | [206] | |||
| Glaucoma | - | - | Review | [214] | ||
| Glaucoma | - | - | Review | [215] | ||
| Glaucoma | MDA, Nitrotyrosine | - | Retinal tissue | [213] | ||
| Glaucoma | - | GPX | Blood | [212] | ||
| Retinal oxidative damage | MDA | Vitamin E | Retinal tissue | [204] | ||
| Orthopaedic diseases | Osteoarthritis | - | GSH | Blood | [221] | |
| Osteoarthritis | d-ROMs | OXY, BAP | Blood | [220] | ||
| Osteoarthritis | MDA, 8-OHdG | GSH | Blood | [217] | ||
| Osteoarthritis | - | SOD, GSH | Canine chondrocyte cell culture | [218] | ||
| Osteoarthritis | MDA | CAT | Blood | [219] | ||
| Hip dysplasia | MDA | GSH, CAT, SOD, GPX | Blood | [222] | ||
| Hip dysplasia | MDA | GSH, GPX, SOD, Vitamin E | Blood | [216] | ||
| Reproductive system diseases | Cystic endometrial hyperplasia-Pyometra | MDA | SOD, CAT, GPX, GSH, FRAP, TAS (ABTS) | Blood, urine, and uterine tissue | [223] | |
| Cystic endometrial hyperplasia | TOS, OSI | TAS (ABTS) | Blood | [224] | ||
| Pyometra | - | GSH, Vitamin C | Uterine tissue | [225] | ||
| Benign prostatic hyperplasia | - | - | Review | [226] | ||
| Benign prostatic hyperplasia | Bityrosine, formylkynurenine | FRAP | Blood | [227] | ||
| Dental diseases | Periodontal Disease | MDA | - | Saliva | [229] | |
| Periodontal Disease | MDA, 8-OHdG | FRAP, SOD | Saliva | [228] | ||
| Others | Ischemia-reperfusion | - | - | Review | [230] | |
| Systemically ill hospitalized dogs (various causes) | IsoP | GSH, cysteine, vitamin E | Blood, urine | [231] | ||
| Systemically ill hospitalized dogs (various causes) | IsoP | GSH, cysteine, vitamin E | Blood, urine | [232] | ||
* ABTS: 2,2′-azinobis(3-ethylbenzthiazolin-6-sulfonic acid) test; AOP: antioxidant potential; AOPP: Advanced Oxidation Protein Products; BAP: Biological Antioxidant Potential; BMSCs: Bone marrow mesenchymal stem cells; CAT: Catalase; CKD: Chronic Kidney Disease; CUPRAC: Cupric-Reducing Antioxidant Power; DCM: Dilated Cardiomyopathy; d-ROMs: Reactive Oxygen Metabolites; FOX: Ferrous oxidation-xylenol orange; FRAP: Ferric-Reducing Antioxidant Power; FRAS: ferric-reducing ability of saliva; G6PD: Glucose-6-phosphate dehydrogenase; GPX: Glutathione peroxidase; GR: Glutathione reductase; GSH: Reduced glutathione; GSH:GSSG: Reduced–oxidized glutathione ratio; GST: Glutathione S-transferase; H2O2: Hydrogen peroxide; IBD: Inflammatory Bowel Disease; IsoP: Isoprostanes; LOOH: Lipid hydroperoxides; MDA: Malondialdehyde; MMVD: Myxomatous mitral valve disease; NO: Nitric oxide; ORAC: Oxygen Radical Antioxidant Capacity; OSI: Oxidative Stress Index; OxLDL: Oxidized low-density lipoprotein; OXY: antioxidant barrier; PC: Protein Carbonyls; PON-1: Paraoxonase 1; POX-Act: Peroxideactivity; RM: Reactive metabolites; R-OOHs: Reactive oxidative metabolites; ROS: Reactive Oxygen Species; SAC: Serum antioxidant capacity; SOD: Superoxide dismutase; TAC: Total Antioxidant Capacity; TAS: Total Antioxidant Status; TOC: Total Oxidant Capacity; TOS: Total Oxidant Status; TrxR: Thioredoxin reductase; XO: Xantine Oxidative; 4-HNE: 4-hydroxy-2-nonenal; 8-OHdG: 8-hydroxy-2′-deoxyguanosine.
Author Contributions
Conceptualization, P.-M.B. and C.-L.F.; methodology, P.-M.B. and C.-L.F.; software, P.-M.B. and C.-L.F.; validation, P.-M.B., F.-R.M.L., M.G. and C.-L.F.; formal analysis, P.-M.B., F.-R.M.L., M.G. and C.-L.F.; investigation, P.-M.B. and C.-L.F.; resources, P.-M.B., F.-R.M.L., M.G. and C.-L.F.; data curation, P.-M.B. and C.-L.F.; writing—original draft preparation, P.-M.B.; writing—review and editing, P.-M.B., F.-R.M.L., M.G. and C.-L.F.; visualization, P.-M.B. and C.-L.F.; supervision, M.G. and C.-L.F.; project administration, M.G. and C.-L.F.; funding acquisition, M.G. and C.-L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We are grateful to the IT department at the VISAVET Health Surveillance Centre for figure editing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Introductory Remarks. In Oxidative Stress; Sies, H., Ed.; Academic Press: London, UK, 1985; pp. 1–8. ISBN 978-0-12-642760-8. [Google Scholar]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Sies, H. On the History of Oxidative Stress: Concept and Some Aspects of Current Development. Curr. Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in Metal-Induced Oxidative Stress and Human Disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of Oxidative Damage in Human Disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Whiteman, M. Measuring Reactive Species and Oxidative Damage in Vivo and in Cell Culture: How Should You Do It and What Do the Results Mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- Tejchman, K.; Kotfis, K.; Sieńko, J. Biomarkers and Mechanisms of Oxidative Stress—Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Chao, M.-R.; Evans, M.D.; Hu, C.-W.; Ji, Y.; Møller, P.; Rossner, P.; Cooke, M.S. Biomarkers of Nucleic Acid Oxidation—A Summary State-of-the-Art. Redox Biol. 2021, 42, 101872. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxid. Med. Cell Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-Five Years of Total Antioxidant Capacity Measurement of Foods and Biological Fluids: Merits and Limitations. J. Sci. Food Agric. 2020, 100, 5064–5078. [Google Scholar] [CrossRef] [PubMed]
- Perez-Montero, B.; Fermin-Rodriguez, M.L.; Portero-Fuentes, M.; Sarquis, J.; Caceres, S.; Del Portal, J.C.I.; de Juan, L.; Miro, G.; Cruz-Lopez, F. Serum Total Antioxidant Status in Dogs: Reference Intervals and Influence of Multiple Biological and Analytical Factors. Vet. Clin. Pathol. 2024, 00, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Stefanon, B.; Gabai, G.; Gelain, M.E.; Bonsembiante, F. Oxidative Stress and Nutraceuticals in the Modulation of the Immune Function: Current Knowledge in Animals of Veterinary Interest. Antioxidants 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Passantino, A.; Quartarone, V.; Pediliggeri, M.C.; Rizzo, M.; Piccione, G. Possible Application of Oxidative Stress Parameters for the Evaluation of Animal Welfare in Sheltered Dogs Subjected to Different Environmental and Health Conditions. J. Vet. Behav. 2014, 9, 290–294. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Vasconcellos, R.S.; Pedreira, R.S.; Silva, F.L.; Sá, F.C.; Kroll, F.S.A.; Maria, A.P.J.; Venturini, K.S.; Carciofi, A.C. Alterations to Oxidative Stress Markers in Dogs after a Short-Term Stress during Transport. J. Nutr. Sci. 2014, 3, e27. [Google Scholar] [CrossRef]
- Juodžentė, D.; Karvelienė, B.; Riškevičienė, V. The Influence of the Duration of the Preoperative Time Spent in the Veterinary Clinic without the Owner on the Psychogenic and Oxidative Stress in Dogs. J. Vet. Med. Sci. 2018, 80, 1129–1133. [Google Scholar] [CrossRef]
- Varney, J.L.; Fowler, J.W.; Gilbert, W.C.; Coon, C.N. Utilisation of Supplemented L-Carnitine for Fuel Efficiency, as an Antioxidant, and for Muscle Recovery in Labrador Retrievers. J. Nutr. Sci. 2017, 6, e8. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef]
- Chirathanaphirom, S.; Chuammitri, P.; Pongkan, W.; Manachai, N.; Chantawong, P.; Boonsri, B.; Boonyapakorn, C. Differences in Levels of Mitochondrial DNA Content at Various Stages of Canine Myxomatous Mitral Valve Disease. Animals 2023, 13, 3850. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Brown, D.J.; Rush, J.E. Antioxidant Status in Dogs with Idiopathic Dilated Cardiomyopathy. J. Nutr. 1998, 128, 2768S–2770S. [Google Scholar] [CrossRef]
- Freeman, L.M.; Brown, D.J.; Rush, J.E. Assessment of Degree of Oxidative Stress and Antioxidant Concentrations in Dogs with Idiopathic Dilated Cardiomyopathy. J. Am. Vet. Med. Assoc. 1999, 215, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Rush, J.E.; Milbury, P.E.; Blumberg, J.B. Antioxidant Status and Biomarkers of Oxidative Stress in Dogs with Congestive Heart Failure. J. Vet. Intern. Med. 2005, 19, 537–541. [Google Scholar] [CrossRef]
- Michałek, M.; Tabiś, A.; Cepiel, A.; Noszczyk-Nowak, A. Antioxidative Enzyme Activity and Total Antioxidant Capacity in Serum of Dogs with Degenerative Mitral Valve Disease. Can. J. Vet. Res. 2020, 84, 67–73. [Google Scholar]
- Michałek, M.; Tabiś, A.; Noszczyk-Nowak, A. Serum Total Antioxidant Capacity and Enzymatic Defence of Dogs with Chronic Heart Failure and Atrial Fibrillation: A Preliminary Study. J. Vet. Res. 2020, 64, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Nemec Svete, A.; Verk, B.; Čebulj-Kadunc, N.; Salobir, J.; Rezar, V.; Domanjko Petrič, A. Inflammation and Its Association with Oxidative Stress in Dogs with Heart Failure. BMC Vet. Res. 2021, 17, 176. [Google Scholar] [CrossRef]
- Reimann, M.J.; Häggström, J.; Møller, J.E.; Lykkesfeldt, J.; Falk, T.; Olsen, L.H. Markers of Oxidative Stress in Dogs with Myxomatous Mitral Valve Disease Are Influenced by Sex, Neuter Status, and Serum Cholesterol Concentration. J. Vet. Intern. Med. 2017, 31, 295–302. [Google Scholar] [CrossRef]
- Rubio, C.P.; Saril, A.; Kocaturk, M.; Tanaka, R.; Koch, J.; Ceron, J.J.; Yilmaz, Z. Changes of Inflammatory and Oxidative Stress Biomarkers in Dogs with Different Stages of Heart Failure. BMC Vet. Res. 2020, 16, 433. [Google Scholar] [CrossRef]
- Tomsič, K.; Domanjko Petrič, A.; Nemec, A.; Pirman, T.; Rezar, V.; Seliškar, A.; Vovk, T.; Nemec Svete, A. Evaluation of Antioxidant Status and Lipid Peroxidation in Dogs with Myxomatous Mitral Valve Degeneration Stage B1. Front. Vet. Sci. 2023, 10, 1203480. [Google Scholar] [CrossRef]
- Verk, B.; Nemec Svete, A.; Salobir, J.; Rezar, V.; Domanjko Petrič, A. Markers of Oxidative Stress in Dogs with Heart Failure. J. Vet. Diagn. Investig. 2017, 29, 636–644. [Google Scholar] [CrossRef]
- Laflamme, D.P. Key Nutrients Important in the Management of Canine Myxomatous Mitral Valve Disease and Heart Failure. J. Am. Vet. Med. Assoc. 2022, 260, S61–S70. [Google Scholar] [CrossRef] [PubMed]
- Pongkan, W.; Piamsiri, C.; Dechvongya, S.; Punyapornwitthaya, V.; Boonyapakorn, C. Short-Term Melatonin Supplementation Decreases Oxidative Stress but Does Not Affect Left Ventricular Structure and Function in Myxomatous Mitral Valve Degenerative Dogs. BMC Vet. Res. 2022, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Thassakorn, P.; Patchanee, P.; Pongkan, W.; Chattipakorn, N.; Boonyapakorn, C. Effect of Atorvastatin on Oxidative Stress and Inflammation Markers in Myxomatous Mitral Valve Disease in Dogs: A Comparison of Subclinical and Clinical Stages. J. Vet. Pharmacol. Ther. 2019, 42, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Druzhaeva, N.; Nemec Svete, A.; Tavčar-Kalcher, G.; Babič, J.; Ihan, A.; Pohar, K.; Krapež, U.; Domanjko Petrič, A. Effects of Coenzyme Q10 Supplementation on Oxidative Stress Markers, Inflammatory Markers, Lymphocyte Subpopulations, and Clinical Status in Dogs with Myxomatous Mitral Valve Disease. Antioxidants 2022, 11, 1427. [Google Scholar] [CrossRef]
- Igarashi, T.; Niwano, S.; Niwano, H.; Yoshizawa, T.; Nakamura, H.; Fukaya, H.; Fujiishi, T.; Ishizue, N.; Satoh, A.; Kishihara, J.; et al. Linagliptin Prevents Atrial Electrical and Structural Remodeling in a Canine Model of Atrial Fibrillation. Heart Vessel. 2018, 33, 1258–1265. [Google Scholar] [CrossRef]
- Kishihara, J.; Niwano, S.; Niwano, H.; Aoyama, Y.; Satoh, A.; Oikawa, J.; Kiryu, M.; Fukaya, H.; Masaki, Y.; Tamaki, H.; et al. Effect of Carvedilol on Atrial Remodeling in Canine Model of Atrial Fibrillation. Cardiovasc. Diagn. Ther. 2014, 4, 28–35. [Google Scholar] [CrossRef]
- Nishinarita, R.; Niwano, S.; Niwano, H.; Nakamura, H.; Saito, D.; Sato, T.; Matsuura, G.; Arakawa, Y.; Kobayashi, S.; Shirakawa, Y.; et al. Canagliflozin Suppresses Atrial Remodeling in a Canine Atrial Fibrillation Model. J. Am. Heart Assoc. 2021, 10, e017483. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Niwano, S.; Niwano, H.; Tamaki, H.; Nakamura, H.; Igarashi, T.; Oikawa, J.; Satoh, A.; Kishihara, J.; Murakami, M.; et al. Antiremodeling Effect of Xanthine Oxidase Inhibition in a Canine Model of Atrial Fibrillation. Int. Heart J. 2018, 59, 1077–1085. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, R.; Wang, X.; Li, J.; Xu, X.; Liu, T.; Liu, E.; Li, G. Suppression of Experimental Atrial Fibrillation in a Canine Model of Rapid Atrial Pacing by the Phosphodiesterase 3 Inhibitor Cilostazol. J. Electrocardiol. 2020, 60, 151–158. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, R.; Wang, X.; Li, J.; Yuan, M.; Liu, E.; Liu, T.; Li, G. Attenuation of Atrial Remodeling by Aliskiren via Affecting Oxidative Stress, Inflammation and PI3K/Akt Signaling Pathway. Cardiovasc. Drugs Ther. 2021, 35, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Cox, C.S.; Allen, S.J.; Stewart, R.H.; Mehlhorn, U.; Laine, G.A. The Antioxidant N-Acetylcysteine Preserves Myocardial Function and Diminishes Oxidative Stress after Cardioplegic Arrest. J. Thorac. Cardiovasc. Surg. 2003, 126, 1483–1488. [Google Scholar] [CrossRef]
- Sharma, A.B.; Sun, J.; Howard, L.L.; Williams, A.G.; Mallet, R.T. Oxidative Stress Reversibly Inactivates Myocardial Enzymes during Cardiac Arrest. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H198–H206. [Google Scholar] [CrossRef]
- Moe, G.; Konig, A.; Liu, P.; Jugdutt, B.I. Selective Type 1 Angiotensin II Receptor Blockade Attenuates Oxidative Stress and Regulates Angiotensin II Receptors in the Canine Failing Heart. Mol. Cell Biochem. 2008, 317, 97–104. [Google Scholar] [CrossRef]
- Moe, G.W.; Marin-Garcia, J.; Konig, A.; Goldenthal, M.; Lu, X.; Feng, Q. In Vivo TNF-Alpha Inhibition Ameliorates Cardiac Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis in Experimental Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1813–H1820. [Google Scholar] [CrossRef] [PubMed]
- Chueainta, P.; Punyapornwithaya, V.; Tangjitjaroen, W.; Pongkan, W.; Boonyapakorn, C. Acupuncture Improves Heart Rate Variability, Oxidative Stress Level, Exercise Tolerance, and Quality of Life in Tracheal Collapse Dogs. Vet. Sci. 2022, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Erjavec, V.; Vovk, T.; Svete, A.N. Evaluation of Oxidative Stress Parameters in Dogs with Brachycephalic Obstructive Airway Syndrome Before and after Surgery. J. Vet. Res. 2021, 65, 201–208. [Google Scholar] [CrossRef]
- Eze, U.U.; Eke, I.G.; Anakwue, R.C.; Oguejiofor, C.F.; Onyejekwe, O.B.; Udeani, I.J.; Onunze, C.J.; Obed, U.J.; Eze, A.A.; Anaga, A.O.; et al. Effects of Controlled Generator Fume Emissions on the Levels of Troponin I, C-Reactive Protein and Oxidative Stress Markers in Dogs: Exploring Air Pollution-Induced Cardiovascular Disease in a Low-Resource Country. Cardiovasc. Toxicol. 2021, 21, 1019–1032. [Google Scholar] [CrossRef]
- Lu, J.; Liu, K.; Qi, M.; Geng, H.; Hao, J.; Wang, R.; Zhao, X.; Liu, Y.; Liu, J. Effects of Cr(VI) Exposure on Electrocardiogram, Myocardial Enzyme Parameters, Inflammatory Factors, Oxidative Kinase, and ATPase of the Heart in Chinese Rural Dogs. Environ. Sci. Pollut. Res. Int. 2019, 26, 30444–30451. [Google Scholar] [CrossRef]
- Mektrirat, R.; Rueangsri, T.; Keeratichandacha, W.; Soonsawat, S.; Boonyapakorn, C.; Pongkan, W. Polyunsaturated Fatty Acid EAB-277® Supplementation Improved Heart Rate Variability and Clinical Signs in Tracheal Collapse Dogs. Front. Vet. Sci. 2022, 9, 880952. [Google Scholar] [CrossRef]
- Khademi, S.; Frye, M.A.; Jeckel, K.M.; Schroeder, T.; Monnet, E.; Irwin, D.C.; Cole, P.A.; Bell, C.; Miller, B.F.; Hamilton, K.L. Hypoxia Mediated Pulmonary Edema: Potential Influence of Oxidative Stress, Sympathetic Activation and Cerebral Blood Flow. BMC Physiol. 2015, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Finotello, R.; Pasquini, A.; Meucci, V.; Lippi, I.; Rota, A.; Guidi, G.; Marchetti, V. Redox Status Evaluation in Dogs Affected by Mast Cell Tumour. Vet. Comp. Oncol. 2014, 12, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Jayasri, K.; Padmaja, K.; Saibaba, M. Altered Oxidative Stress and Carbohydrate Metabolism in Canine Mammary Tumors. Vet. World 2016, 9, 1489–1492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva, L.P.; Portela, R.W.; Machado, M.C.; Canuto, G.A.; Costa-Neto, J.M.; Carvalho, V.D.; Sá, H.C.; Damasceno, K.A.; Souza, V.R.; Coelho, C.S.; et al. Ozone Therapy in the Integrated Treatment of Female Dogs with Mammary Cancer: Oxidative Profile and Quality of Life. Antioxidants 2024, 13, 673. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Fytianou, A.; Assaloumidis, N.; Psalla, D.; Constantinidis, T.C.; Kaldrymidou, E.; Koutinas, A.F. Markers of Lipid Peroxidation and α-Tocopherol Levels in the Blood and Neoplastic Tissue of Dogs with Malignant Mammary Gland Tumors. Vet. Clin. Pathol. 2013, 42, 323–328. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef]
- Karakurt, E.; KURU, M.; Dağ, S.; Beytut, E.; ORAL, H.; Nuhoğlu, H.; Yıldız, A. Presence and Importance of Oxidative Stress Parameters in Malignant Mammary Gland Tumors in Dogs. Kafkas Univ. Vet. Fak. Derg. 2021, 27, 517–523. [Google Scholar] [CrossRef]
- Kumaraguruparan, R.; Balachandran, C.; Manohar, B.M.; Nagini, S. Altered Oxidant-Antioxidant Profile in Canine Mammary Tumours. Vet. Res. Commun. 2005, 29, 287–296. [Google Scholar] [CrossRef]
- Machado, V.S.; Crivellenti, L.Z.; Bottari, N.B.; Tonin, A.A.; Pelinson, L.P.; Borin-Crivellenti, S.; Santana, A.E.; Torbitz, V.D.; Moresco, R.N.; Duarte, T.; et al. Oxidative Stress and Inflammatory Response Biomarkers in Dogs with Mammary Carcinoma. Pathol. Res. Pr. 2015, 211, 677–681. [Google Scholar] [CrossRef]
- Macotpet, A.; Suksawat, F.; Sukon, P.; Pimpakdee, K.; Pattarapanwichien, E.; Tangrassameeprasert, R.; Boonsiri, P. Oxidative Stress in Cancer-Bearing Dogs Assessed by Measuring Serum Malondialdehyde. BMC Vet. Res. 2013, 9, 101. [Google Scholar] [CrossRef]
- Schroers, M.; Walter, B.; Fischer, S.; Cremer, J.; Bauer, E.-M.; Zablotzki, Y.; Majzoub-Altweck, M.; Meyer-Lindenberg, A. Studies on the Association of Malondialdehyde as a Biomarker for Oxidative Stress and Degree of Malignancy in Dogs with Mammary Adenocarcinomas. Vet. Med. Sci. 2024, 10, e1496. [Google Scholar] [CrossRef] [PubMed]
- Szczubiał, M.; Kankofer, M.; Łopuszyński, W.; Dabrowski, R.; Lipko, J. Oxidative Stress Parameters in Bitches with Mammary Gland Tumours. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 336–340. [Google Scholar] [CrossRef]
- Bottari, N.B.; Munhoz, T.D.; Torbitz, V.D.; Tonin, A.A.; Anai, L.A.; Semolin, L.M.S.; Jark, P.C.; Bollick, Y.S.; Moresco, R.N.; França, R.T.; et al. Oxidative Stress in Dogs with Multicentric Lymphoma: Effect of Chemotherapy on Oxidative and Antioxidant Biomarkers. Redox Rep. 2015, 20, 267–274. [Google Scholar] [CrossRef]
- Henklewska, M.; Pawlak, A.; Li, R.-F.; Yi, J.; Zbyryt, I.; Obmińska-Mrukowicz, B. Benzyl Isothiocyanate, a Vegetable-Derived Compound, Induces Apoptosis via ROS Accumulation and DNA Damage in Canine Lymphoma and Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 11772. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, A.; Gavazza, A.; Biagi, G.; Lubas, G. Oxidative Stress in Lymphoma: Similarities and Differences between Dog and Human. Comp. Clin. Pathol. 2015, 24, 69–73. [Google Scholar] [CrossRef]
- Vajdovich, P.; Kriska, T.; Mézes, M.; Szabó, P.R.; Balogh, N.; Bánfi, A.; Arany-Tóth, A.; Gaál, T.; Jakus, J. Redox Status of Dogs with Non-Hodgkin Lymphomas. An ESR Study. Cancer Lett. 2005, 224, 339–346. [Google Scholar] [CrossRef]
- Winter, J.L.; Barber, L.G.; Freeman, L.; Griessmayr, P.C.; Milbury, P.E.; Blumberg, J.B. Antioxidant Status and Biomarkers of Oxidative Stress in Dogs with Lymphoma. J. Vet. Intern. Med. 2009, 23, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Loftus, J.P.; Cavatorta, D.; Bushey, J.J.; Levine, C.B.; Sevier, C.S.; Wakshlag, J.J. The 5-Lipoxygenase Inhibitor Tepoxalin Induces Oxidative Damage and Altered PTEN Status Prior to Apoptosis in Canine Osteosarcoma Cell Lines. Vet. Comp. Oncol. 2016, 14, e17-30. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, S.; Bazer, F.W.; Lim, W.; Song, G. Myricetin Treatment Induces Apoptosis in Canine Osteosarcoma Cells by Inducing DNA Fragmentation, Disrupting Redox Homeostasis, and Mediating Loss of Mitochondrial Membrane Potential. J. Cell Physiol. 2018, 233, 7457–7466. [Google Scholar] [CrossRef]
- Woolcock, A.D.; Cheney, A.; Deshuillers, P.; Knapp, D.; Moore, G.E. Assessment of Urinary 15-F2-Isoprostanes in Dogs with Urothelial Carcinoma of the Urinary Bladder and Other Lower Urinary Tract Diseases. J. Vet. Intern. Med. 2020, 34, 2454–2459. [Google Scholar] [CrossRef]
- Candellone, A.; Girolami, F.; Badino, P.; Jarriyawattanachaikul, W.; Odore, R. Changes in the Oxidative Stress Status of Dogs Affected by Acute Enteropathies. Vet. Sci. 2022, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, J.I.; Duque, F.J.; Usón-Casaús, J.; Martínez, M.S.; Míguez, M.P.; Pérez-Merino, E.M. Oxidative Stress in Dogs with Chronic Inflammatory Enteropathy Treated with Allogeneic Mesenchymal Stem Cells. Vet. Res. Commun. 2024, 48, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the Fecal Microbiota and Serum Metabolite Profiles in Dogs with Idiopathic Inflammatory Bowel Disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.P.; Martínez-Subiela, S.; Hernández-Ruiz, J.; Tvarijonaviciute, A.; Cerón, J.J.; Allenspach, K. Serum Biomarkers of Oxidative Stress in Dogs with Idiopathic Inflammatory Bowel Disease. Vet. J. 2017, 221, 56–61. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Arnao, M.B.; Ceron, J.J. Validation of Three Automated Assays for Total Antioxidant Capacity Determination in Canine Serum Samples. J. Vet. Diagn. Investig. 2016, 28, 693–698. [Google Scholar] [CrossRef]
- Rubio, C.P.; Tvarijonaviciute, A.; Martinez-Subiela, S.; Hernández-Ruiz, J.; Cerón, J.J. Validation of an Automated Assay for the Measurement of Cupric Reducing Antioxidant Capacity in Serum of Dogs. BMC Vet. Res. 2016, 12, 137. [Google Scholar] [CrossRef]
- Cridge, H.; Lim, S.Y.; Algül, H.; Steiner, J.M. New Insights into the Etiology, Risk Factors, and Pathogenesis of Pancreatitis in Dogs: Potential Impacts on Clinical Practice. J. Vet. Intern. Med. 2022, 36, 847–864. [Google Scholar] [CrossRef]
- Tusa, N.V.; Abuelo, A.; Levy, N.A.; Gandy, J.C.; Langlois, D.K.; Cridge, H. Peripheral Biomarkers of Oxidative Stress in Dogs with Acute Pancreatitis. J. Vet. Intern. Med. 2022, 36, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Center, S.A.; Warner, K.L.; Erb, H.N. Liver Glutathione Concentrations in Dogs and Cats with Naturally Occurring Liver Disease. Am. J. Vet. Res. 2002, 63, 1187–1197. [Google Scholar] [CrossRef]
- Webb, C.; Twedt, D. Oxidative Stress and Liver Disease. Vet. Clin. N. Am. Small Anim. Pr. 2008, 38, 125–135. [Google Scholar] [CrossRef]
- Spee, B.; Arends, B.; van den Ingh, T.S.G.A.M.; Penning, L.C.; Rothuizen, J. Copper Metabolism and Oxidative Stress in Chronic Inflammatory and Cholestatic Liver Diseases in Dogs. J. Vet. Intern. Med. 2006, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Martello, E.; Perondi, F.; Bisanzio, D.; Lippi, I.; Meineri, G.; Gabriele, V. Antioxidant Effect of a Dietary Supplement Containing Fermentative S-Acetyl-Glutathione and Silybin in Dogs with Liver Disease. Vet. Sci. 2023, 10, 131. [Google Scholar] [CrossRef]
- Barry-Heffernan, C.; Ekena, J.; Dowling, S.; Pinkerton, M.E.; Viviano, K. Biomarkers of Oxidative Stress as an Assessment of the Redox Status of the Liver in Dogs. J. Vet. Intern. Med. 2019, 33, 611–617. [Google Scholar] [CrossRef]
- Dirksen, K.; Spee, B.; Penning, L.C.; van den Ingh, T.S.G.A.M.; Burgener, I.A.; Watson, A.L.; Groot Koerkamp, M.; Rothuizen, J.; van Steenbeek, F.G.; Fieten, H. Gene Expression Patterns in the Progression of Canine Copper-Associated Chronic Hepatitis. PLoS ONE 2017, 12, e0176826. [Google Scholar] [CrossRef]
- Giannetto, C.; Arfuso, F.; Giudice, E.; Rizzo, M.; Piccione, G.; Mhalhel, K.; Levanti, M. Antioxidant and Hepatoprotective Effect of a Nutritional Supplement with Silymarin Phytosome, Choline Chloride, l-Cystine, Artichoke, and Vitamin E in Dogs. Antioxidants 2022, 11, 2339. [Google Scholar] [CrossRef]
- Hishiyama, N.; Kayanuma, H.; Matsui, T.; Yano, H.; Suganuma, T.; Funaba, M.; Fujise, H. Plasma Concentration of Vitamin C in Dogs with a Portosystemic Shunt. Can. J. Vet. Res. 2006, 70, 305–307. [Google Scholar]
- Huang, J.; Bai, Y.; Xie, W.; Wang, R.; Qiu, W.; Zhou, S.; Tang, Z.; Liao, J.; Su, R. Lyciumbarbarum Polysaccharides Ameliorate Canine Acute Liver Injury by Reducing Oxidative Stress, Protecting Mitochondrial Function, and Regulating Metabolic Pathways. J. Zhejiang Univ. Sci. B 2023, 24, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Kil, D.Y.; Vester Boler, B.M.; Apanavicius, C.J.; Schook, L.B.; Swanson, K.S. Age and Diet Affect Gene Expression Profiles in Canine Liver Tissue. PLoS ONE 2010, 5, e13319. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.K.; Steiner, J.M.; Suchodolski, J.S.; Lidbury, J.A. Urinary 15-F2t-Isoprostane Concentrations in Dogs with Liver Disease. Vet. Sci. 2023, 10, 82. [Google Scholar] [CrossRef]
- Vince, A.R.; Hayes, M.A.; Jefferson, B.J.; Stalker, M.J. Hepatic Injury Correlates with Apoptosis, Regeneration, and Nitric Oxide Synthase Expression in Canine Chronic Liver Disease. Vet. Pathol. 2014, 51, 932–945. [Google Scholar] [CrossRef]
- Vincent, A.M.; Sordillo, L.M.; Smedley, R.C.; Gandy, J.C.; Brown, J.L.; Langlois, D.K. Peripheral Markers of Oxidative Stress in Labrador Retrievers with Copper-Associated Hepatitis. J. Small Anim. Pr. 2021, 62, 866–873. [Google Scholar] [CrossRef]
- Yamkate, P.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S.; Giaretta, P.R. Immunohistochemical Expression of Oxidative Stress and Apoptosis Markers in Archived Liver Specimens from Dogs with Chronic Hepatitis. J. Comp. Pathol. 2022, 193, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Li, Y.; Mai, Q.; Li, Y.; Lin, Y.; Weng, X.; Ai, Z.; Li, M.; Shang, P.; Iqbal, M.; et al. Hepatotoxicity and the Role of the Gut-Liver Axis in Dogs after Oral Administration of Zinc Oxide Nanoparticles. Metallomics 2022, 14, mfac066. [Google Scholar] [CrossRef]
- Arostegui, L.G.G.; Prieto, A.M.; Marín, L.P.; López, G.G.; Tvarijonaviciute, A.; Madrigal, J.J.C.; Rubio, C.P. Changes in Biomarkers of Redox Status in Serum and Saliva of Dogs with Hypothyroidism. BMC Vet. Res. 2023, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- González-Arostegui, L.G.; Muñoz-Prieto, A.; García-López, G.; Cerón, J.J.; Tvarijonaviciute, A.; Rubio, C.P. Changes in Biomarkers of the Redox Status in Whole Blood and Red Blood Cell Lysates in Canine Hypothyroidism. Vet. Res. Commun. 2024, 48, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Ryad, N.M.; Ramadan, E.S.; Salem, N.Y.; Saleh, I.A.E.-S. Oxidative Biomarkers and Lipid Alterations in Euthyroid and Hypothyroid Dogs. Comp. Clin. Pathol. 2021, 30, 571–576. [Google Scholar] [CrossRef]
- Kim, H.; Yonezawa, T.; Maeda, S.; Tamahara, S.; Matsuki, N. Increases in Serum Carbonylated Protein Levels of Dogs with Hypercortisolism. Endocr. J. 2022, 69, 1387–1394. [Google Scholar] [CrossRef]
- Soares, F.A.C.; Filho, N.A.K.; Beretta, B.F.S.; Linden, T.S.; Pöppl, A.G.; González, F.H.D. Thiobarbituric Acid Reactive Substances in Dogs with Spontaneous Hypercortisolism. Domest. Anim. Endocrinol. 2021, 77, 106634. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Suzuki, S.; Soeta, S.; Kaneda, T.; Hara, A.Y. Mechanism of Long-Term High-Dose Prednisolone Administration Producing Myocardial Fibrosis in Beagle Dogs. Open Vet. J. 2023, 13, 1708–1717. [Google Scholar] [CrossRef]
- Chansaisakorn, W.; Sriphavatsarakorn, P.; Sopakdittapong, P.; Trisiriroj, M.; Pondeenana, S.; Buranakarl, C. Oxidative Stress and Intraerythrocytic Concentrations of Sodium and Potassium in Diabetic Dogs. Vet. Res. Commun. 2009, 33, 67–75. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, S.; Wang, Y.; Zhang, R.; Opoku, Y.K.; Xie, Y.; Li, D.; Ren, G. Fibroblast Growth Factor 21: A Novel Long-Acting Hypoglycemic Drug for Canine Diabetes. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, H.; Huo, H.; Ma, F.; Zhao, M.; Han, Q.; Hu, L.; Li, Y.; Zhang, H.; Pan, J.; et al. N-Acetylcysteine Combined with Insulin Alleviates the Oxidative Damage of Cerebrum via Regulating Redox Homeostasis in Type 1 Diabetic Mellitus Canine. Life Sci. 2022, 308, 120958. [Google Scholar] [CrossRef] [PubMed]
- Suemanotham, N.; Phochantachinda, S.; Chatchaisak, D.; Sakcamduang, W.; Chansawhang, A.; Pitchakarn, P.; Chantong, B. Antidiabetic Effects of Andrographis Paniculata Supplementation on Biochemical Parameters, Inflammatory Responses, and Oxidative Stress in Canine Diabetes. Front. Pharmacol. 2023, 14, 1077228. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, C.Z.; Michelotto, P.V.; Capriglione, L.G.A.; Roncoski, A.T.; Nishiyama, A. Weight Loss Modifies Lipid Peroxidation and Symmetric Dimethylarginine Levels in Obese Dogs. Can. J. Vet. Res. 2023, 87, 29–34. [Google Scholar]
- Grant, R.W.; Vester Boler, B.M.; Ridge, T.K.; Graves, T.K.; Swanson, K.S. Adipose Tissue Transcriptome Changes during Obesity Development in Female Dogs. Physiol. Genom. 2011, 43, 295–307. [Google Scholar] [CrossRef]
- Lucena, S.; Varela Coelho, A.; Anjo, S.I.; Manadas, B.; Mrljak, V.; Capela E Silva, F.; Lamy, E.; Tvarijonaviciute, A. Comparative Proteomic Analysis of Saliva from Dogs with and without Obesity-Related Metabolic Dysfuntion. J. Proteom. 2019, 201, 65–72. [Google Scholar] [CrossRef]
- Van de Velde, H.; Janssens, G.P.J.; Stuyven, E.; Cox, E.; Buyse, J.; Hesta, M. Short-Term Increase of Body Weight Triggers Immunological Variables in Dogs. Vet. Immunol. Immunopathol. 2012, 145, 431–437. [Google Scholar] [CrossRef]
- Vecchiato, C.G.; Golinelli, S.; Pinna, C.; Pilla, R.; Suchodolski, J.S.; Tvarijonaviciute, A.; Rubio, C.P.; Dorato, E.; Delsante, C.; Stefanelli, C.; et al. Fecal Microbiota and Inflammatory and Antioxidant Status of Obese and Lean Dogs, and the Effect of Caloric Restriction. Front. Microbiol. 2022, 13, 1050474. [Google Scholar] [CrossRef]
- Li, G.; Kawasumi, K.; Okada, Y.; Ishikawa, S.; Yamamoto, I.; Arai, T.; Mori, N. Comparison of Plasma Lipoprotein Profiles and Malondialdehyde between Hyperlipidemia Dogs with/without Treatment. BMC Vet. Res. 2014, 10, 67. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Perez-Montero, B.; Fermin-Rodriguez, M.L.; Miro, G.; de Juan, L.; Cruz-Lopez, F. Hemolysis, Icterus and Lipemia Interfere with the Determination of Two Oxidative Stress Biomarkers in Canine Serum. BMC Vet. Res. 2023, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Fibach, E.; Rachmilewitz, E. The Role of Oxidative Stress in Hemolytic Anemia. Curr. Mol. Med. 2008, 8, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Kogika, M.M.; Lustoza, M.D.; Hagiwara, M.K.; Caragelasco, D.S.; Martorelli, C.R.; Mori, C.S. Evaluation of Oxidative Stress in the Anemia of Dogs with Chronic Kidney Disease. Vet. Clin. Pathol. 2015, 44, 70–78. [Google Scholar] [CrossRef]
- Woolcock, A.D.; Serpa, P.B.S.; Santos, A.P.; Christian, J.A.; Moore, G.E. Reactive Oxygen Species, Glutathione, and Vitamin E Concentrations in Dogs with Hemolytic or Nonhemolytic Anemia. J. Vet. Intern. Med. 2020, 34, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.; Woolcock, A.; Brooks, A.; Moore, G.E. Glutathione Peroxidase Activity, Plasma Total Antioxidant Capacity, and Urinary F2- Isoprostanes as Markers of Oxidative Stress in Anemic Dogs. J. Vet. Intern. Med. 2017, 31, 1700–1707. [Google Scholar] [CrossRef]
- Pesillo, S.A.; Freeman, L.M.; Rush, J.E. Assessment of Lipid Peroxidation and Serum Vitamin E Concentration in Dogs with Immune-Mediated Hemolytic Anemia. Am. J. Vet. Res. 2004, 65, 1621–1624. [Google Scholar] [CrossRef]
- Tan, E.; Bienzle, D.; Shewen, P.; Kruth, S.; Wood, D. Potentially Antigenic RBC Membrane Proteins in Dogs with Primary Immune-Mediated Hemolytic Anemia. Vet. Clin. Pathol. 2012, 41, 45–55. [Google Scholar] [CrossRef]
- Bujok, J.; Wajman, E.; Trochanowska-Pauk, N.; Walski, T. Evaluation of Selected Hematological, Biochemical and Oxidative Stress Parameters in Stored Canine CPDA-1 Whole Blood. BMC Vet. Res. 2022, 18, 255. [Google Scholar] [CrossRef]
- Zhong, L.; Deng, J.; Gu, C.; Shen, L.; Ren, Z.; Ma, X.; Yan, Q.; Deng, J.; Zuo, Z.; Wang, Y.; et al. Protective Effect of MitoQ on Oxidative Stress-Mediated Senescence of Canine Bone Marrow Mesenchymal Stem Cells via Activation of the Nrf2/ARE Pathway. Vitr. Cell Dev. Biol. Anim. 2021, 57, 685–694. [Google Scholar] [CrossRef]
- Paltrinieri, S. Oxidative Stress and Canine Leishmaniasis: More than a Simple Consequence of Host-Parasite Interaction. Vet. J. 2013, 198, 547–548. [Google Scholar] [CrossRef]
- Almeida, B.F.M.; Narciso, L.G.; Bosco, A.M.; Pereira, P.P.; Braga, E.T.; Avanço, S.V.; Marcondes, M.; Ciarlini, P.C. Neutrophil Dysfunction Varies with the Stage of Canine Visceral Leishmaniosis. Vet. Parasitol. 2013, 196, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.F.M.; Narciso, L.G.; Melo, L.M.; Preve, P.P.; Bosco, A.M.; Lima, V.M.F.; Ciarlini, P.C. Leishmaniasis Causes Oxidative Stress and Alteration of Oxidative Metabolism and Viability of Neutrophils in Dogs. Vet. J. 2013, 198, 599–605. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, B.F.; Silva, K.L.; Chiku, V.M.; Leal, A.A.; Venturin, G.L.; Narciso, L.G.; Fink, M.F.; de Rezende Eugênio, F.; Dos Santos, P.S.; Ciarlini, P.C.; et al. The Effects of Increased Heme Oxygenase-1 on the Lymphoproliferative Response in Dogs with Visceral Leishmaniasis. Immunobiology 2017, 222, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Bildik, A.; Kargin, F.; Seyrek, K.; Pasa, S.; Ozensoy, S. Oxidative Stress and Non-Enzymatic Antioxidative Status in Dogs with Visceral Leishmaniasis. Res. Vet. Sci. 2004, 77, 63–66. [Google Scholar] [CrossRef]
- de Sousa Gonçalves, R.; de Pinho, F.A.; Dinis-Oliveira, R.J.; Mendes, M.O.; de Andrade, T.S.; da Silva Solcà, M.; Larangeira, D.F.; Silvestre, R.; Barrouin-Melo, S.M. Nutritional Adjuvants with Antioxidant Properties in the Treatment of Canine Leishmaniasis. Vet. Parasitol. 2021, 298, 109526. [Google Scholar] [CrossRef]
- Heidarpour, M.; Soltani, S.; Mohri, M.; Khoshnegah, J. Canine Visceral Leishmaniasis: Relationships between Oxidative Stress, Liver and Kidney Variables, Trace Elements, and Clinical Status. Parasitol. Res. 2012, 111, 1491–1496. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Cavallaro, M.; Taormina, A.; La Spada, G.; Marino, A. Anion Exchange through Band 3 Protein in Canine Leishmaniasis at Different Stages of Disease. Pflug. Arch. 2017, 469, 713–724. [Google Scholar] [CrossRef]
- Quintavalla, F.; Basini, G.; Bussolati, S.; Carrozzo, G.G.; Inglese, A.; Ramoni, R. Redox Status in Canine Leishmaniasis. Animals 2021, 11, 119. [Google Scholar] [CrossRef]
- Rubio, C.P.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Hernández-Ruiz, J.; Pardo-Marin, L.; Segarra, S.; Ceron, J.J. Changes in Serum Biomarkers of Oxidative Stress after Treatment for Canine Leishmaniosis in Sick Dogs. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 51–57. [Google Scholar] [CrossRef]
- Solcà, M.S.; Andrade, B.B.; Abbehusen, M.M.C.; Teixeira, C.R.; Khouri, R.; Valenzuela, J.G.; Kamhawi, S.; Bozza, P.T.; Fraga, D.B.M.; Borges, V.M.; et al. Circulating Biomarkers of Immune Activation, Oxidative Stress and Inflammation Characterize Severe Canine Visceral Leishmaniasis. Sci. Rep. 2016, 6, 32619. [Google Scholar] [CrossRef]
- Torrecilha, R.B.P.; Utsunomiya, Y.T.; Bosco, A.M.; Almeida, B.F.; Pereira, P.P.; Narciso, L.G.; Pereira, D.C.M.; Baptistiolli, L.; Calvo-Bado, L.; Courtenay, O.; et al. Correlations between Peripheral Parasite Load and Common Clinical and Laboratory Alterations in Dogs with Visceral Leishmaniasis. Prev. Vet. Med. 2016, 132, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Ibba, F.; Barbè, F.; Rossi, G. Influence of Domperidone Supplementation on Short-Term Changes in C-Reactive Protein and Paraoxonase-1 in Dogs with Leishmaniasis Undergoing Meglumine Antimoniate and Allopurinol Therapy. Vet. Clin. Pathol. 2020, 49, 618–623. [Google Scholar] [CrossRef]
- Bottari, N.B.; Crivellenti, L.Z.; Borin-Crivellenti, S.; Oliveira, J.R.; Coelho, S.B.; Contin, C.M.; Tatsch, E.; Moresco, R.N.; Santana, A.E.; Tonin, A.A.; et al. Iron Metabolism and Oxidative Profile of Dogs Naturally Infected by Ehrlichia Canis: Acute and Subclinical Disease. Microb. Pathog. 2016, 92, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Chethan, G.E.; Garkhal, J.; De, U.K. Disturbance of Thyroid Function in Canine Ehrlichiosis and Babesiosis Associated with Oxidative Stress. Comp. Clin. Pathol. 2016, 25, 987–992. [Google Scholar] [CrossRef]
- Çiftci, G.; Pekmezci, D.; Güzel, M.; Çenesiz, S.; Ural, K.; Aysul, N.; Kazak, F. Determination of Serum Oxidative Stress, Antioxidant Capacity and Protein Profiles in Dogs Naturally Infected with Ehrlichia Canis. Acta Parasitol. 2021, 66, 1341–1348. [Google Scholar] [CrossRef]
- Da Silva, A.S.; Munhoz, T.D.; Faria, J.L.M.; Vargas-Hérnandez, G.; Machado, R.Z.; Almeida, T.C.; Moresco, R.N.; Stefani, L.M.; Tinucci-Costa, M. Increase Nitric Oxide and Oxidative Stress in Dogs Experimentally Infected by Ehrlichia Canis: Effect on the Pathogenesis of the Disease. Vet. Microbiol. 2013, 164, 366–369. [Google Scholar] [CrossRef]
- Kumar, A.; Varshney, J.P.; Patra, R.C. A Comparative Study on Oxidative Stress in Dogs Infected with Ehrlichia Canis with or without Concurrent Infection with Babesia Gibsoni. Vet. Res. Commun. 2006, 30, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Pedreañez, A.; Mosquera-Sulbaran, J.; Muñoz, N. Increased Plasma Levels of Nitric Oxide and Malondialdehyde in Dogs Infected by Ehrlichia Canis: Effect of Doxycycline Treatment. Rev. Vet. Clin. 2021, 56, 185–190. [Google Scholar] [CrossRef]
- Pugliese, M.; Biondi, V.; Merola, G.; Landi, A.; Passantino, A. Oxidative Stress Evaluation in Dogs Affected with Canine Monocytic Ehrlichiosis. Antioxidants 2022, 11, 328. [Google Scholar] [CrossRef]
- Rubio, C.P.; Yilmaz, Z.; Martínez-Subiela, S.; Kocaturk, M.; Hernández-Ruiz, J.; Yalcin, E.; Tvarijonaviciute, A.; Escribano, D.; Ceron, J.J. Serum Antioxidant Capacity and Oxidative Damage in Clinical and Subclinical Canine Ehrlichiosis. Res. Vet. Sci. 2017, 115, 301–306. [Google Scholar] [CrossRef]
- Rudoler, N.; Harrus, S.; Martinez-Subiela, S.; Tvarijonaviciute, A.; van Straten, M.; Cerón, J.J.; Baneth, G. Comparison of the Acute Phase Protein and Antioxidant Responses in Dogs Vaccinated against Canine Monocytic Ehrlichiosis and Naive-Challenged Dogs. Parasit. Vectors 2015, 8, 175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ciftci, G.; Ural, K.; Aysul, N.; Cenesiz, S.; Guzel, M.; Pekmezci, D.; Sogut, M.Ü. Investigation of the 8-Hydroxy-2′-Deoxyguanosine, Total Antioxidant and Nitric Oxide Levels of Serum in Dogs Infected with Babesia Vogeli. Vet. Parasitol. 2014, 204, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Crnogaj, M.; Petlevski, R.; Mrljak, V.; Kis, I.; Torti, M.; Kucer, N.; Matijatko, V.; Sacer, I.; Stokovic, I. Malondialdehyde Levels in Serum of Dogs Infected with Babesia Canis. Vet. Med. 2010, 55, 163–171. [Google Scholar] [CrossRef]
- Crnogaj, M.; Cerón, J.J.; Šmit, I.; Kiš, I.; Gotić, J.; Brkljačić, M.; Matijatko, V.; Rubio, C.P.; Kučer, N.; Mrljak, V. Relation of Antioxidant Status at Admission and Disease Severity and Outcome in Dogs Naturally Infected with Babesia Canis Canis. BMC Vet. Res. 2017, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Gonmei, C.; Sarma, K.; Roychoudhury, P.; Ali, M.A.; Singh, D.; Prasad, H.; Ahmed, F.A.; Lalmuanpuii, R.; Shah, N.; Singh, N.S.; et al. Molecular Diagnosis and Clinico-Hemato-Biochemical Alterations and Oxidant-Antioxidant Biomarkers in Babesia-Infected Dogs of Mizoram, India. J. Vector Borne Dis. 2020, 57, 226–233. [Google Scholar] [CrossRef]
- Murase, T.; Ueda, T.; Yamato, O.; Tajima, M.; Maede, Y. Oxidative Damage and Enhanced Erythrophagocytosis in Canine Erythrocytes Infected with Babesia Gibsoni. J. Vet. Med. Sci. 1996, 58, 259–261. [Google Scholar] [CrossRef]
- Teodorowski, O.; Winiarczyk, S.; Tarhan, D.; Dokuzeylül, B.; Ercan, A.M.; Or, M.E.; Staniec, M.; Adaszek, Ł. Antioxidant Status, and Blood Zinc and Copper Concentrations in Dogs with Uncomplicated Babesiosis Due to Babesia Canis Infections. J. Vet. Res. 2021, 65, 169–174. [Google Scholar] [CrossRef]
- Carretón, E.; Cerón, J.J.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Caro-Vadillo, A.; Montoya-Alonso, J.A. Acute Phase Proteins and Markers of Oxidative Stress to Assess the Severity of the Pulmonary Hypertension in Heartworm-Infected Dogs. Parasit. Vectors 2017, 10, 477. [Google Scholar] [CrossRef]
- Rajković, M.; Glavinić, U.; Bogunović, D.; Vejnović, B.; Davitkov, D.; Đelić, N.; Stanimirović, Z. “Slow Kill” Treatment Reduces DNA Damage in Leukocytes of Dogs Naturally Infected with Dirofilaria Immitis. Vet. Parasitol. 2023, 322, 110008. [Google Scholar] [CrossRef]
- Rath, P.K.; Panda, S.; Mishra, B.; Patra, R.; Nath, I. Thoracic Radiography and Oxidative Stress Indices in Heartworm Affected Dogs. Vet. World 2014, 7, 689–692. [Google Scholar] [CrossRef]
- Kiral, F.; Karagenc, T.; Pasa, S.; Yenisey, C.; Seyrek, K. Dogs with Hepatozoon Canis Respond to the Oxidative Stress by Increased Production of Glutathione and Nitric Oxide. Vet. Parasitol. 2005, 131, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sarma, K.; Eregowda, C.G.; Roychoudhury, P.; Borthakur, S.K.; Jawalagatti, V.; Prasad, H.; Behera, S.K.; Thakur, N.; Bora, N.; Das, D. A 5-Year Prospective Study on Incidence and Clinico-Pathological Changes Associated with Naturally Occurring Trypanosomosis in Dogs of Mizoram, India. Acta Parasitol. 2022, 67, 61–71. [Google Scholar] [CrossRef]
- Chethan, G.E.; De, U.K.; Singh, M.K.; Chander, V.; Raja, R.; Paul, B.R.; Choudhary, O.P.; Thakur, N.; Sarma, K.; Prasad, H. Antioxidant Supplementation during Treatment of Outpatient Dogs with Parvovirus Enteritis Ameliorates Oxidative Stress and Attenuates Intestinal Injury: A Randomized Controlled Trial. Vet. Anim. Sci. 2023, 21, 100300. [Google Scholar] [CrossRef]
- Gaykwad, C.; Garkhal, J.; Chethan, G.E.; Nandi, S.; De, U.K. Amelioration of Oxidative Stress Using N-Acetylcysteine in Canine Parvoviral Enteritis. J. Vet. Pharmacol. Ther. 2018, 41, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kocaturk, M.; Tvarijonaviciute, A.; Martinez-Subiela, S.; Tecles, F.; Eralp, O.; Yilmaz, Z.; Ceron, J.J. Inflammatory and Oxidative Biomarkers of Disease Severity in Dogs with Parvoviral Enteritis. J. Small Anim. Pract. 2015, 56, 119–124. [Google Scholar] [CrossRef]
- Panda, D.; Patra, R.C.; Nandi, S.; Swarup, D. Oxidative Stress Indices in Gastroenteritis in Dogs with Canine Parvoviral Infection. Res. Vet. Sci. 2009, 86, 36–42. [Google Scholar] [CrossRef]
- Pugliese, M.; Napoli, E.; Monti, S.; Biondi, V.; Zema, E.; Passantino, A. Oxidative Stress and High-Mobility Group Box 1 Assay in Dogs with Gastrointestinal Parasites. Antioxidants 2022, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.M.S.; Tvarijonaviciute, A.; Martinez-Subiela, S.; Cerón, J.J.; Eckersall, P.D. Changes in Biochemical Analytes in Female Dogs with Subclinical Ancylostoma Spp. Infection. BMC Vet. Res. 2016, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Beigh, S.A.; Soodan, J.S.; Singh, R.; Khan, A.M. Trace Minerals Status and Antioxidative Enzyme Activity in Dogs with Generalized Demodecosis. Vet. Parasitol. 2013, 198, 180–186. [Google Scholar] [CrossRef]
- Dimri, U.; Ranjan, R.; Kumar, N.; Sharma, M.C.; Swarup, D.; Sharma, B.; Kataria, M. Changes in Oxidative Stress Indices, Zinc and Copper Concentrations in Blood in Canine Demodicosis. Vet. Parasitol. 2008, 154, 98–102. [Google Scholar] [CrossRef]
- Martínez-Subiela, S.; Bernal, L.J.; Tvarijonaviciute, A.; Garcia-Martinez, J.D.; Tecles, F.; Cerón, J.J. Canine Demodicosis: The Relationship between Response to Treatment of Generalised Disease and Markers for Inflammation and Oxidative Status. Vet. Dermatol. 2014, 25, 72-e24. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.Y.; Abdel-Saeed, H.; Farag, H.S.; Ghandour, R.A. Canine Demodicosis: Hematological and Biochemical Alterations. Vet. World 2020, 13, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Dimri, U. The Immuno-Pathological Conversions of Canine Demodicosis. Vet. Parasitol. 2014, 203, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Dimri, U.; Singh, S.K.; Mohanta, R.K. The Curative and Antioxidative Efficiency of Ivermectin and Ivermectin + Vitamin E-Selenium Treatment on Canine Sarcoptes Scabiei Infestation. Vet. Res. Commun. 2011, 35, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Beigh, S.A.; Soodan, J.S.; Bhat, A.M. Sarcoptic Mange in Dogs: Its Effect on Liver, Oxidative Stress, Trace Minerals and Vitamins. Vet. Parasitol. 2016, 227, 30–34. [Google Scholar] [CrossRef]
- Camkerten, I.; Sahin, T.; Borazan, G.; Gokcen, A.; Erel, O.; Das, A. Evaluation of Blood Oxidant/Antioxidant Balance in Dogs with Sarcoptic Mange. Vet. Parasitol. 2009, 161, 106–109. [Google Scholar] [CrossRef]
- Singh, S.K.; Dimri, U.; Sharma, M.C.; Swarup, D.; Sharma, B. Determination of Oxidative Status and Apoptosis in Peripheral Blood of Dogs with Sarcoptic Mange. Vet. Parasitol. 2011, 178, 330–338. [Google Scholar] [CrossRef]
- Beigh, S.A.; Soodan, J.S.; Singh, R.; Khan, A.M.; Dar, M.A. Evaluation of Trace Elements, Oxidant/Antioxidant Status, Vitamin C and β-Carotene in Dogs with Dermatophytosis. Mycoses 2014, 57, 358–365. [Google Scholar] [CrossRef]
- Marquis, A.; Packer, R.A.; Borgens, R.B.; Duerstock, B.S. Increase in Oxidative Stress Biomarkers in Dogs with Ascending-Descending Myelomalacia Following Spinal Cord Injury. J. Neurol. Sci. 2015, 353, 63–69. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Radaković, M.; Andrić, J.F.; Spariosu, K.; Vejnović, B.; Filipović, M.K.; Andrić, N. Serum Oxidant-Antioxidant Status and Butyrylcholinesterase Activity in Dogs with Idiopathic Epilepsy—A Pilot Study. Res. Vet. Sci. 2023, 165, 105076. [Google Scholar] [CrossRef] [PubMed]
- Peek, S.I.; Twele, F.; Meller, S.; Packer, R.M.A.; Volk, H.A. Epilepsy Is More than a Simple Seizure Disorder: Causal Relationships between Epilepsy and Its Comorbidities. Vet. J. 2024, 303, 106061. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.R.; March, P.A.; Oglesbee, M.; Ruaux, C.G.; Olby, N.J.; Berghaus, R.D.; O’Brien, D.P.; Keating, J.H.; Johnson, G.S.; Williams, D.A. Clinical Characterization of a Familial Degenerative Myelopathy in Pembroke Welsh Corgi Dogs. J. Vet. Intern. Med. 2007, 21, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Green, S.L.; Bouley, D.M.; Pinter, M.J.; Cork, L.C.; Vatassery, G.T. Canine Motor Neuron Disease: Clinicopathologic Features and Selected Indicators of Oxidative Stress. J. Vet. Intern. Med. 2001, 15, 112–119. [Google Scholar] [CrossRef]
- Ogawa, M.; Uchida, K.; Park, E.-S.; Kamishina, H.; Sasaki, J.; Chang, H.-S.; Yamato, O.; Nakayama, H. Immunohistochemical Observation of Canine Degenerative Myelopathy in Two Pembroke Welsh Corgi Dogs. J. Vet. Med. Sci. 2011, 73, 1275–1279. [Google Scholar] [CrossRef]
- Brown, S.A. Oxidative Stress and Chronic Kidney Disease. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 157–166. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef]
- Halfen, D.P.; Caragelasco, D.S.; Nogueira, J.P.; Jeremias, J.T.; Pedrinelli, V.; Oba, P.M.; Ruberti, B.; Pontieri, C.F.; Kogika, M.M.; Brunetto, M.A. Evaluation of Electrolyte Concentration and Pro-Inflammatory and Oxidative Status in Dogs with Advanced Chronic Kidney Disease under Dietary Treatment. Toxins 2019, 12, 3. [Google Scholar] [CrossRef]
- Martello, E.; Perondi, F.; Bruni, N.; Bisanzio, D.; Meineri, G.; Lippi, I. Chronic Kidney Disease and Dietary Supplementation: Effects on Inflammation and Oxidative Stress. Vet. Sci. 2021, 8, 277. [Google Scholar] [CrossRef]
- Bosco, A.M.; Almeida, B.F.M.; Pereira, P.P.; Dos Santos, D.B.; Neto, Á.J.S.; Ferreira, W.L.; Ciarlini, P.C. The Uremic Toxin Methylguanidine Increases the Oxidative Metabolism and Accelerates the Apoptosis of Canine Neutrophils. Vet. Immunol. Immunopathol. 2017, 185, 14–19. [Google Scholar] [CrossRef]
- Buranakarl, C.; Trisiriroj, M.; Pondeenana, S.; Tungjitpeanpong, T.; Jarutakanon, P.; Penchome, R. Relationships between Oxidative Stress Markers and Red Blood Cell Characteristics in Renal Azotemic Dogs. Res. Vet. Sci. 2009, 86, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Deuel, J.W.; Schaer, C.A.; Boretti, F.S.; Opitz, L.; Garcia-Rubio, I.; Baek, J.H.; Spahn, D.R.; Buehler, P.W.; Schaer, D.J. Hemoglobinuria-Related Acute Kidney Injury Is Driven by Intrarenal Oxidative Reactions Triggering a Heme Toxicity Response. Cell Death Dis. 2016, 7, e2064. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, L.; Zhai, H.; Wang, D.; Li, X.; Wang, Y.; Song, M.; Xu, C. Exploration of the Protective Mechanisms of Icariin against Cisplatin-Induced Renal Cell Damage in Canines. Front. Vet. Sci. 2024, 11, 1331409. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.R.A.; de Almeida, B.F.M.; Soeiro, C.S.; Ferreira, W.L.; de Lima, V.M.F.; Ciarlini, P.C. Oxidative Stress, Superoxide Production, and Apoptosis of Neutrophils in Dogs with Chronic Kidney Disease. Can. J. Vet. Res. 2013, 77, 136–141. [Google Scholar] [PubMed]
- Chen, H.; Segev, G. Evaluation of Oxidative Stress in Dogs and Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2024, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Picardo, M. Antioxidant Activity, Lipid Peroxidation and Skin Diseases. What’s New. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Plevnik Kapun, A.; Salobir, J.; Levart, A.; Tavčar Kalcher, G.; Nemec Svete, A.; Kotnik, T. Vitamin E Supplementation in Canine Atopic Dermatitis: Improvement of Clinical Signs and Effects on Oxidative Stress Markers. Vet. Rec. 2014, 175, 560. [Google Scholar] [CrossRef]
- Almela, R.M.; Rubio, C.P.; Cerón, J.J.; Ansón, A.; Tichy, A.; Mayer, U. Selected Serum Oxidative Stress Biomarkers in Dogs with Non-Food-Induced and Food-Induced Atopic Dermatitis. Vet. Dermatol. 2018, 29, 229-e82. [Google Scholar] [CrossRef]
- Plevnik Kapun, A.; Salobir, J.; Levart, A.; Kotnik, T.; Svete, A.N. Oxidative Stress Markers in Canine Atopic Dermatitis. Res. Vet. Sci. 2012, 92, 469–470. [Google Scholar] [CrossRef]
- Plevnik Kapun, A.; Salobir, J.; Levart, A.; Tavčar Kalcher, G.; Nemec Svete, A.; Kotnik, T. Plasma and Skin Vitamin E Concentrations in Canine Atopic Dermatitis. Vet. Q. 2013, 33, 2–6. [Google Scholar] [CrossRef]
- Witzel-Rollins, A.; Murphy, M.; Becvarova, I.; Werre, S.R.; Cadiergues, M.-C.; Meyer, H. Non-Controlled, Open-Label Clinical Trial to Assess the Effectiveness of a Dietetic Food on Pruritus and Dermatologic Scoring in Atopic Dogs. BMC Vet. Res. 2019, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, M.; Bongiovanni, L.; Russo, A.; Capuccini, S.; Mechelli, L.; Ordeix, L.; Della Salda, L. Oxidative Stress in the Pathogenesis of Canine Zinc-Responsive Dermatosis. Vet. Dermatol. 2011, 22, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zapata, G.L.; Guajardo, M.H.; Terrasa, A.M. The in Vitro Protective Effect of Alpha-Tocopherol on Oxidative Injury in the Dog Retina. Vet. J. 2008, 177, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Barden, C.A.; Chandler, H.L.; Lu, P.; Bomser, J.A.; Colitz, C.M.H. Effect of Grape Polyphenols on Oxidative Stress in Canine Lens Epithelial Cells. Am. J. Vet. Res. 2008, 69, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Barros, P.S.M.; Padovani, C.F.; Silva, V.V.; Queiroz, L.; Barros, S.B.M. Antioxidant Status of Dog Aqueous Humor after Extracapsular Lens Extraction. Braz. J. Med. Biol. Res. 2003, 36, 1491–1494. [Google Scholar] [CrossRef]
- Barros, P.S.M.; Safatle, A.M.V.; Queiroz, L.; Silva, V.V.; Barros, S.B.M. Blood and Aqueous Humour Antioxidants in Cataractous Poodles. Can. J. Ophthalmol. 2004, 39, 19–24. [Google Scholar] [CrossRef]
- De Biaggi, C.P.; Barros, P.S.M.; Silva, V.V.; Brooks, D.E.; Barros, S.B.M. Ascorbic Acid Levels of Aqueous Humor of Dogs after Experimental Phacoemulsification. Vet. Ophthalmol. 2006, 9, 299–302. [Google Scholar] [CrossRef]
- Madany, J. Serum Malondialdehyde Level and Activity of Total Antioxidant Status of Dogs with Age-Related Cataract. Pol. J. Vet. Sci. 2016, 19, 429–431. [Google Scholar] [CrossRef][Green Version]
- Park, S.; Kang, S.; Yoo, S.; Park, Y.; Seo, K. Effect of Oral Antioxidants on the Progression of Canine Senile Cataracts: A Retrospective Study. J. Vet. Sci. 2022, 23, e43. [Google Scholar] [CrossRef]
- Williams, D.L. Oxidation, Antioxidants and Cataract Formation: A Literature Review. Vet. Ophthalmol. 2006, 9, 292–298. [Google Scholar] [CrossRef]
- Boillot, T.; Rosolen, S.G.; Dulaurent, T.; Goulle, F.; Thomas, P.; Isard, P.-F.; Azoulay, T.; Lafarge-Beurlet, S.; Woods, M.; Lavillegrand, S.; et al. Determination of Morphological, Biometric and Biochemical Susceptibilities in Healthy Eurasier Dogs with Suspected Inherited Glaucoma. PLoS ONE 2014, 9, e111873. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Gionfriddo, J.R.; Tai, P.-Y.; Novakowski, A.N.; Alyahya, K.; Madl, J.E. Oxidative Stress Increases in Retinas of Dogs in Acute Glaucoma but Not in Chronic Glaucoma. Vet. Ophthalmol. 2015, 18, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.L.; McCowan, C.; White, A. Genetic and Biochemical Biomarkers in Canine Glaucoma. Vet. Pathol. 2017, 54, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Pizzirani, S. Definition, Classification, and Pathophysiology of Canine Glaucoma. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 1127–1157. [Google Scholar] [CrossRef] [PubMed]
- Ajadi, A.; Sanni, J.; Sobayo, E.; Ijaopo, O. Evaluation of Plasma Trace Elements and Oxidant/Antioxidant Status in Boerboel Dogs with Hip Dysplasia. Bulg. J. Vet. Med. 2020, 23, 237–247. [Google Scholar] [CrossRef]
- Barrouin-Melo, S.M.; Anturaniemi, J.; Sankari, S.; Griinari, M.; Atroshi, F.; Ounjaijean, S.; Hielm-Björkman, A.K. Evaluating Oxidative Stress, Serological- and Haematological Status of Dogs Suffering from Osteoarthritis, after Supplementing Their Diet with Fish or Corn Oil. Lipids Health Dis. 2016, 15, 139. [Google Scholar] [CrossRef]
- Dycus, D.L.; Au, A.Y.; Grzanna, M.W.; Wardlaw, J.L.; Frondoza, C.G. Modulation of Inflammation and Oxidative Stress in Canine Chondrocytes. Am. J. Vet. Res. 2013, 74, 983–989. [Google Scholar] [CrossRef][Green Version]
- Goranov, N.V. Serum Markers of Lipid Peroxidation, Antioxidant Enzymatic Defense, and Collagen Degradation in an Experimental (Pond-Nuki) Canine Model of Osteoarthritis. Vet. Clin. Pathol. 2007, 36, 192–195. [Google Scholar] [CrossRef]
- Musco, N.; Vassalotti, G.; Mastellone, V.; Cortese, L.; Della Rocca, G.; Molinari, M.L.; Calabrò, S.; Tudisco, R.; Cutrignelli, M.I.; Lombardi, P. Effects of a Nutritional Supplement in Dogs Affected by Osteoarthritis. Vet. Med. Sci. 2019, 5, 325–335. [Google Scholar] [CrossRef]
- Gabriele, V.; Bisanzio, D.; Riva, A.; Meineri, G.; Adami, R.; Martello, E. Long-Term Effects of a Diet Supplement Containing Cannabis Sativa Oil and Boswellia Serrata in Dogs with Osteoarthritis Following Physiotherapy Treatments: A Randomised, Placebo-Controlled and Double-Blind Clinical Trial. Nat. Prod. Res. 2023, 37, 1782–1786. [Google Scholar] [CrossRef]
- Polat, E.; Han, M.C.; Kaya, E.; Yilmaz, S.; Kayapinar, S.D.; Coskun, S.; Yildirim, A.; Can, U.K. The Effect of Hip Dysplasia on Some Biochemical Parameters, Oxidative Stress Factors and Hematocrit Levels in Dogs. Pol. J. Vet. Sci. 2021, 24, 473–478. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, J.K.; Verma, S.; Gattani, A.; Singh, G.D.; Singh, V.K. Evaluation of Uterine Antioxidants in Bitches Suffering from Cystic Endometrial Hyperplasia-Pyometra Complex. Pol. J. Vet. Sci. 2024, 27, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kurt, S.; Eski, F.; Mis, L. Investigation of the Usability of Kisspeptin and Oxidative Stress Parameters in the Early Diagnosis of Asymptomatic Cystic Endometrial Hyperplasia in Dogs. Reprod. Domest. Anim. 2021, 56, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Szczubiał, M.; Dąbrowski, R.; Bochniarz, M.; Brodzki, P. Uterine Non-Enzymatic Antioxidant Defence Mechanisms (Glutathione, Vitamin C, Copper and Zinc) in Diagnosis of Canine Pyometra. Pol. J. Vet. Sci. 2019, 22, 549–555. [Google Scholar] [CrossRef]
- Domosławska-Wyderska, A.; Zduńczyk, S.; Rafalska, A. Potential Role of Oxidative Stress in Pathogenesis of Benign Prostatic Hyperplasia in Male Dogs. Reprod. Domest. Anim. 2024, 59, e14580. [Google Scholar] [CrossRef]
- Domoslawska, A.; Zduńczyk, S.; Kankofer, M.; Bielecka, A. Oxidative Stress Biomarkers in Dogs with Benign Prostatic Hyperplasia. Ir. Vet. J. 2022, 75, 21. [Google Scholar] [CrossRef] [PubMed]
- Peștean, C.P.; Pocquet, H.; Dumitraș, D.A.; Morohoschi, A.G.; Ștefănuț, L.C.; Andrei, S. Correlation between Oxidative Stress Markers and Periodontal Disease in Dogs. Vet. Sci. 2024, 11, 99. [Google Scholar] [CrossRef]
- Schroers, M.; Reiser, K.; Alexander, T.; Zablotski, Y.; Meyer-Lindenberg, A. Saliva Malondialdehyde Concentration of Dogs With and Without Periodontal Disease. J. Vet. Dent. 2024, 1–7, Online ahead of print. [Google Scholar] [CrossRef]
- Vajdovich, P. Free Radicals and Antioxidants in Inflammatory Processes and Ischemia-Reperfusion Injury. Vet. Clin. N. Am. Small Anim. Pr. 2008, 38, 31–123. [Google Scholar] [CrossRef]
- Hagen, D.M.; Ekena, J.L.; Geesaman, B.M.; Viviano, K.R. Antioxidant Supplementation during Illness in Dogs: Effect on Oxidative Stress and Outcome, an Exploratory Study. J. Small Anim. Pr. 2019, 60, 543–550. [Google Scholar] [CrossRef]
- Viviano, K.R.; VanderWielen, B. Effect of N-Acetylcysteine Supplementation on Intracellular Glutathione, Urine Isoprostanes, Clinical Score, and Survival in Hospitalized Ill Dogs. J. Vet. Intern. Med. 2013, 27, 250–258. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).