Redox Imbalance and Antioxidant Defenses Dysfunction: Key Contributors to Early Aging in Childhood Cancer Survivors
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
2.3. N-Acetyl Cysteine (NAC) CCS-MNCs Treatment
2.4. Oxidative Phosphorylation (OxPhos) Efficiency Evaluation
2.5. ROS Production Evaluation
2.6. Oxidative Damage Accumulation Assay
2.7. Evaluation of NADPH, NADP, GSH, and GSSG Intracellular Concentration
2.8. Antioxidant Enzymes Activity Assay
2.9. Western Blot Analysis
2.10. Statistical Analyses
3. Results
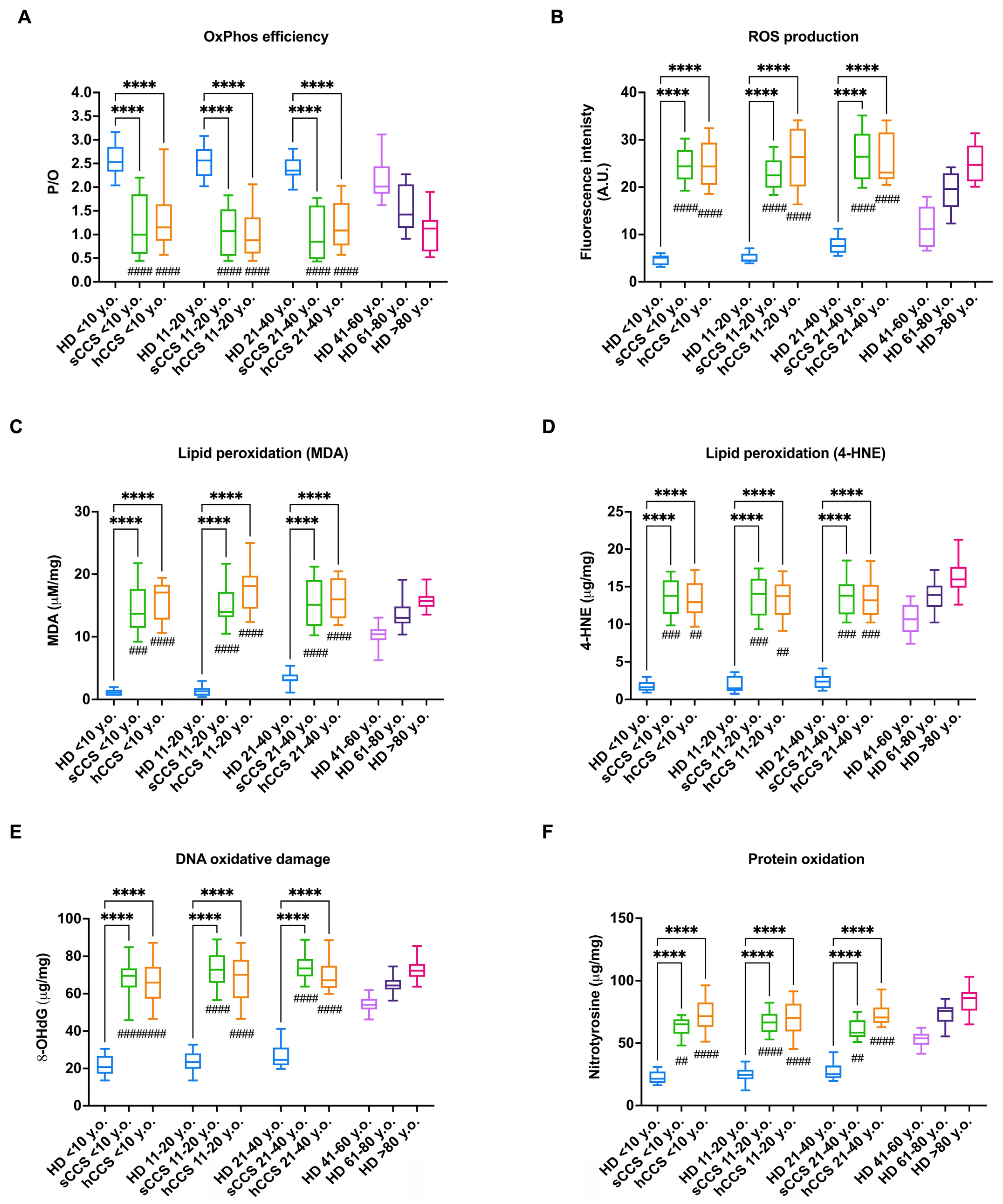
3.1. The CCS-MNCs Exhibit Impaired Energy Metabolism Efficiency and Increased ROS Production, Leading to the Accumulation of Oxidative Damage
3.2. NADPH and GSH Intracellular Levels Are Reduced in CCS-MNCs
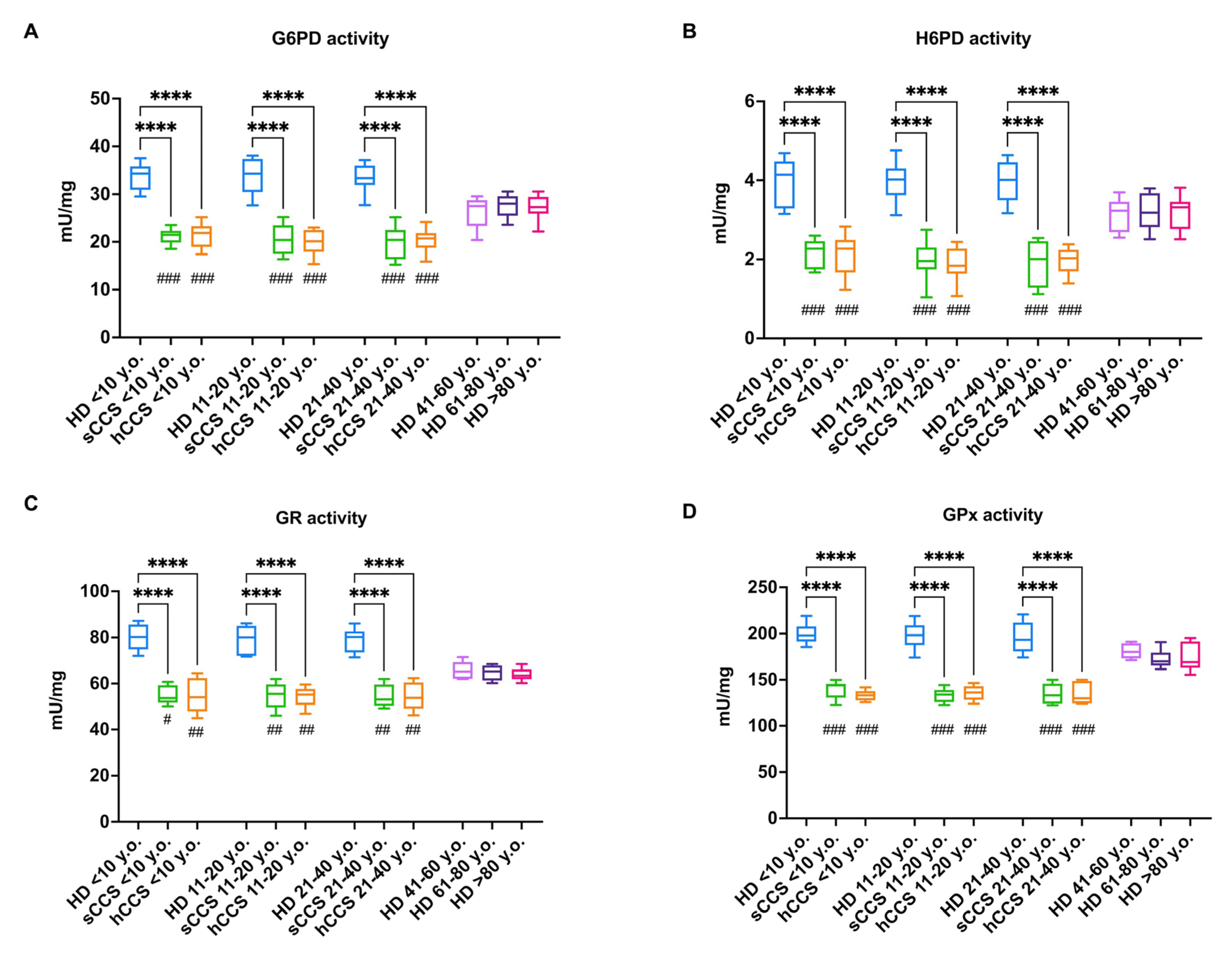
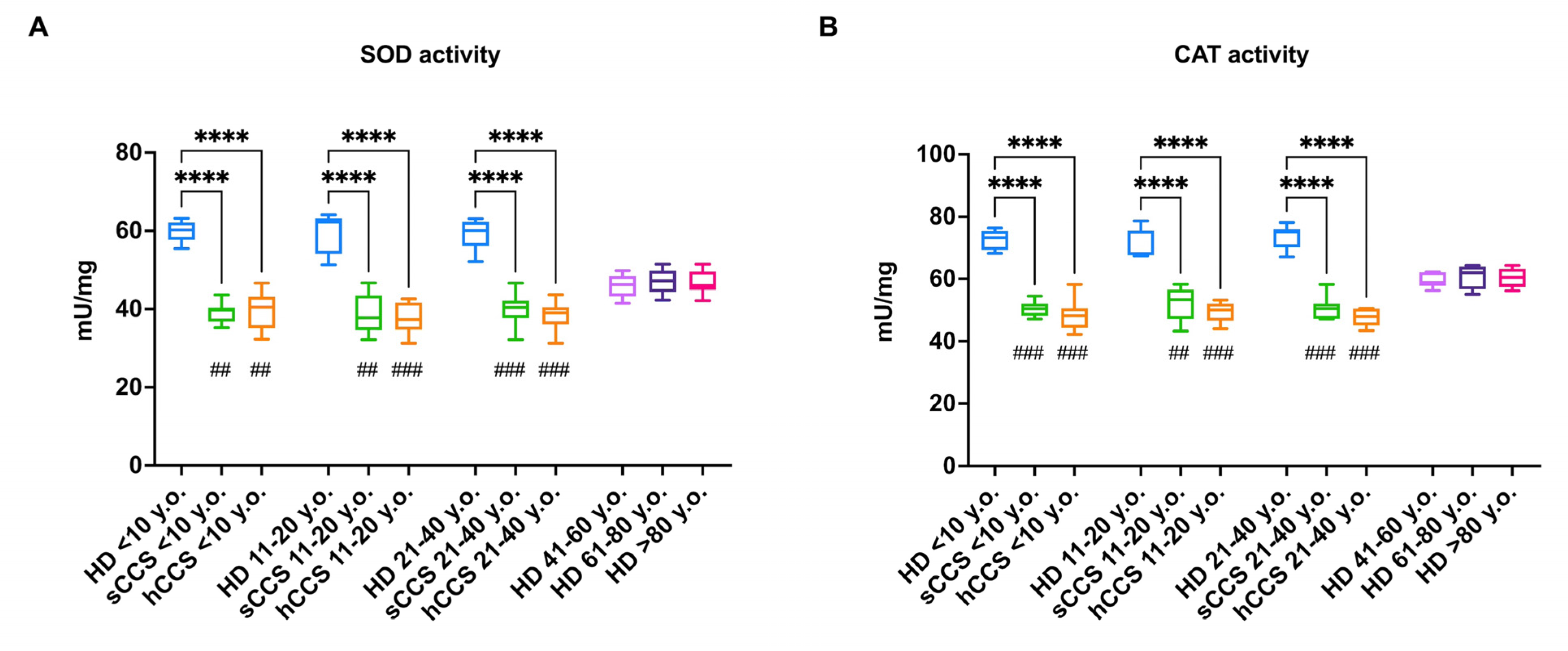
3.3. Oxidative Damage in CCS-MNCs Is Due to Reduced Expression and Activity of Enzymes Involved in Cellular Antioxidant Defenses
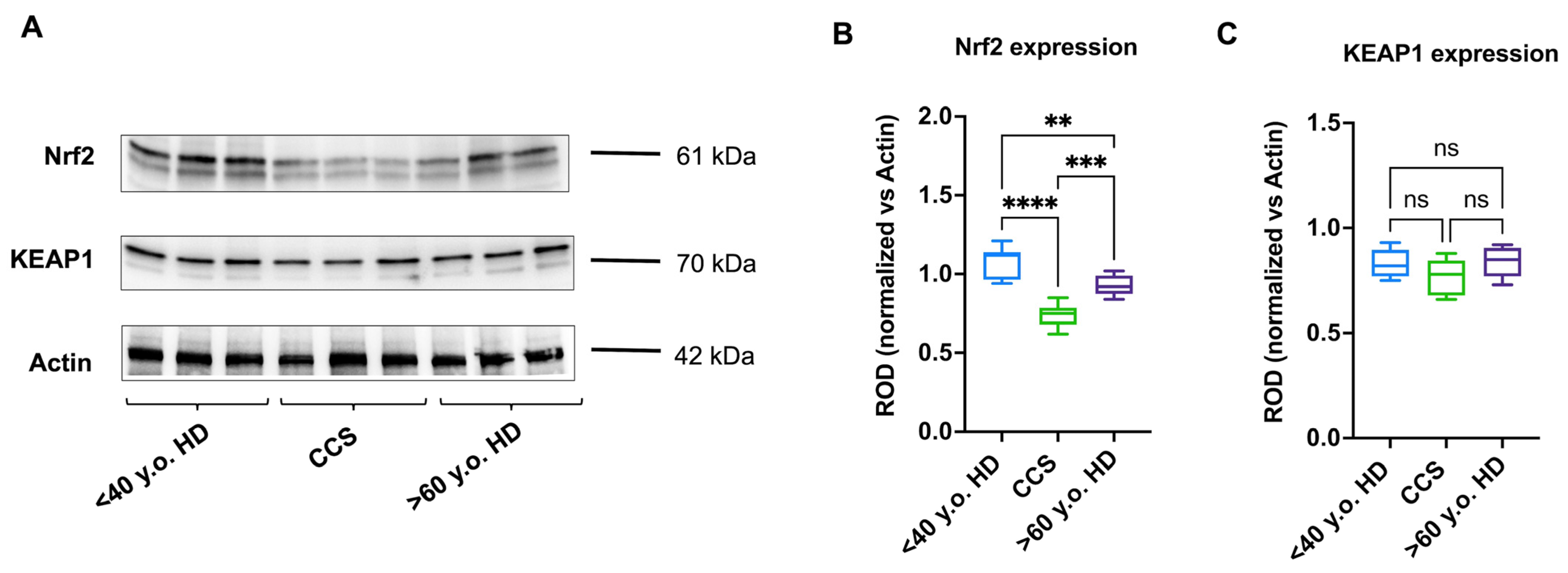
3.4. The Reduced Antioxidant Response in CCS-MNCs Is Due to Decreased Nrf2 Expression
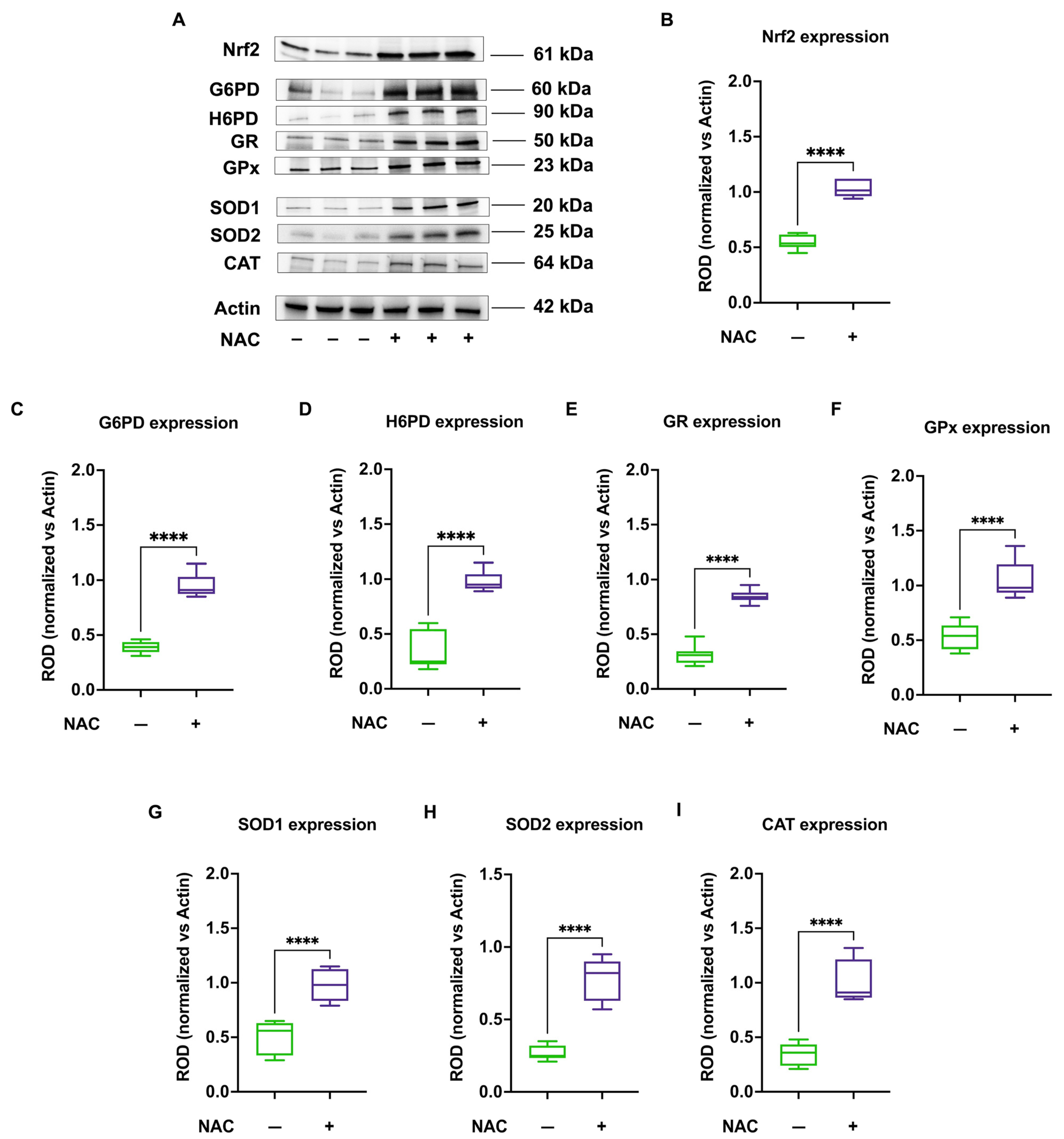
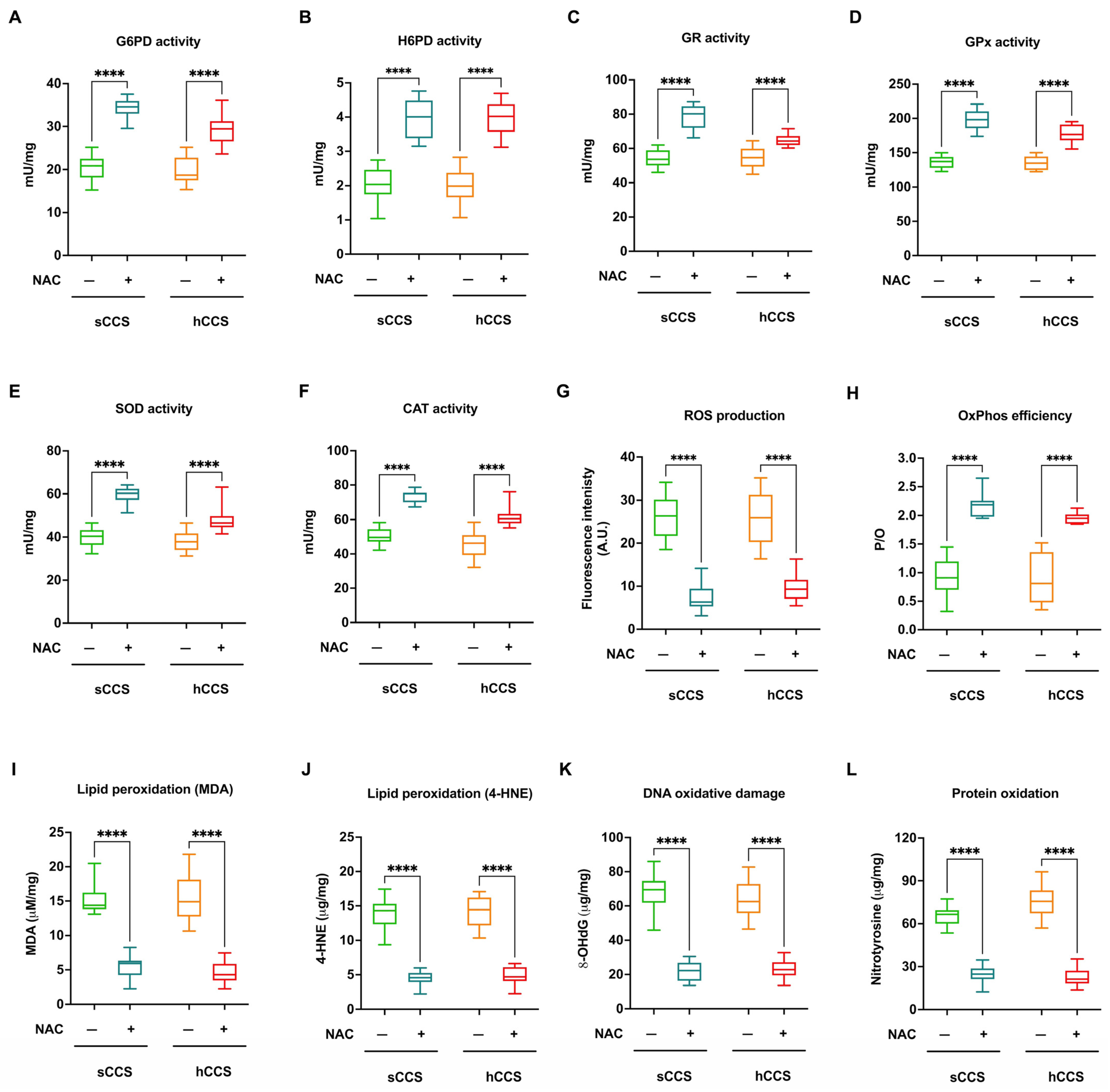
3.5. NAC Treatment Enhances Nrf2 Levels and Consequently Activates Antioxidant Defenses in CCS-MNCs, Improving OxPhos Efficiency, Reducing ROS Production, and Mitigating Oxidative Damage
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Botta, L.; Gatta, G.; Capocaccia, R.; Stiller, C.; Cañete, A.; Dal Maso, L.; Innos, K.; Mihor, A.; Erdmann, F.; Spix, C.; et al. Long-Term Survival and Cure Fraction Estimates for Childhood Cancer in Europe (EUROCARE-6): Results from a Population-Based Study. Lancet Oncol. 2022, 23, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.; Ludwig, K.; Pacenta, H.L.; Klesse, L.J.; Watt, T.C.; Laetsch, T.W. Recent Progress in the Treatment of Cancer in Children. CA Cancer J. Clin. 2021, 71, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Landier, W.; Skinner, R.; Wallace, W.H.; Hjorth, L.; Mulder, R.L.; Wong, F.L.; Yasui, Y.; Bhakta, N.; Constine, L.S.; Bhatia, S.; et al. Surveillance for Late Effects in Childhood Cancer Survivors. J. Clin. Oncol. 2018, 36, 2216–2222. [Google Scholar] [CrossRef]
- Meeske, K.A.; Nelson, M.B. The Role of the Long-Term Follow-up Clinic in Discovering New Emerging Late Effects in Adult Survivors of Childhood Cancer. J. Pediatr. Oncol. Nurs. 2008, 25, 213–219. [Google Scholar] [CrossRef]
- Mellblom, A.V.; Kiserud, C.E.; Rueegg, C.S.; Ruud, E.; Loge, J.H.; Fosså, S.D.; Lie, H.C. Self-Reported Late Effects and Long-Term Follow-up Care among 1889 Long-Term Norwegian Childhood, Adolescent, and Young Adult Cancer Survivors (the NOR-CAYACS Study). Support. Care Cancer 2021, 29, 2947–2957. [Google Scholar] [CrossRef]
- Ness, K.K.; Kirkland, J.L.; Gramatges, M.M.; Wang, Z.; Kundu, M.; McCastlain, K.; Li-Harms, X.; Zhang, J.; Tchkonia, T.; Pluijm, S.M.F.; et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J. Clin. Oncol. 2018, 36, 2206–2215. [Google Scholar] [CrossRef]
- Ariffin, H.; Azanan, M.S.; Abd Ghafar, S.S.; Oh, L.; Lau, K.H.; Thirunavakarasu, T.; Sedan, A.; Ibrahim, K.; Chan, A.; Chin, T.F.; et al. Young Adult Survivors of Childhood Acute Lymphoblastic Leukemia Show Evidence of Chronic Inflammation and Cellular Aging. Cancer 2017, 123, 4207–4214. [Google Scholar] [CrossRef]
- Ness, K.K.; Krull, K.R.; Jones, K.E.; Mulrooney, D.A.; Armstrong, G.T.; Green, D.M.; Chemaitilly, W.; Smith, W.A.; Wilson, C.L.; Sklar, C.A.; et al. Physiologic Frailty as a Sign of Accelerated Aging among Adult Survivors of Childhood Cancer: A Report from the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2013, 31, 4496–4503. [Google Scholar] [CrossRef]
- Ness, K.K.; Armstrong, G.T.; Kundu, M.; Wilson, C.L.; Tchkonia, T.; Kirkland, J.L. Frailty in Childhood Cancer Survivors. Cancer 2015, 121, 1540–1547. [Google Scholar] [CrossRef]
- Mainieri, F.; Giannini, C.; Chiarelli, F. Cardiovascular Risk in Childhood Cancer Survivors. Biomedicines 2022, 10, 3098. [Google Scholar] [CrossRef]
- Goldberg, J.F.; Hyun, G.; Ness, K.K.; Dixon, S.B.; Towbin, J.A.; Rhea, I.B.; Ehrhardt, M.J.; Srivastava, D.K.; Mulrooney, D.A.; Hudson, M.M.; et al. Dyslipidemia and Cardiovascular Disease among Childhood Cancer Survivors: A St. Jude Lifetime Cohort Report. JNCI J. Natl. Cancer Inst. 2024, 116, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Armenian, S.H.; McCormack, S.; Natarajan, R.; Mostoufi-Moab, S. Diabetes in Childhood Cancer Survivors: Emerging Concepts in Pathophysiology and Future Directions. Front. Med. 2023, 10, 1206071. [Google Scholar] [CrossRef]
- Söntgerath, R.; Eckert, K. Impairments of Lower Extremity Muscle Strength and Balance in Childhood Cancer Patients and Survivors: A Systematic Review. Pediatr. Hematol. Oncol. 2015, 32, 585–612. [Google Scholar] [CrossRef] [PubMed]
- Bratteteig, M.; Rueegg, C.S.; Raastad, T.; Grydeland, M.; Torsvik, I.K.; Schindera, C.; Ruud, E.; Anderssen, S.A. Physical Activity, Fitness, and Cardiovascular Disease Risk in Adolescent Childhood Cancer Survivors Compared to Controls: The Physical Activity in Childhood Cancer Survivors Study. J. Adolesc. Young Adult Oncol. 2024, 13, 338–346. [Google Scholar] [CrossRef]
- Castellino, S.M.; Ullrich, N.J.; Whelen, M.J.; Lange, B.J. Developing Interventions for Cancer-Related Cognitive Dysfunction in Childhood Cancer Survivors. JNCI J. Natl. Cancer Inst. 2014, 106, 186. [Google Scholar] [CrossRef]
- Kesler, S.R.; Sleurs, C.; McDonald, B.C.; Deprez, S.; van der Plas, E.; Nieman, B.J. Brain Imaging in Pediatric Cancer Survivors: Correlates of Cognitive Impairment. J. Clin. Oncol. 2021, 39, 1775–1785. [Google Scholar] [CrossRef]
- Ernst, M.; Hinz, A.; Brähler, E.; Merzenich, H.; Faber, J.; Wild, P.S.; Beutel, M.E. Quality of Life after Pediatric Cancer: Comparison of Long-Term Childhood Cancer Survivors’ Quality of Life with a Representative General Population Sample and Associations with Physical Health and Risk Indicators. Health Qual. Life Outcomes 2023, 21, 65. [Google Scholar] [CrossRef]
- Signorelli, C.; Wakefield, C.E.; McLoone, J.K.; Johnston, K.A.; Mertens, A.C.; Osborn, M.; Cohn, R.J.; Alvaro, F.; Cohn, R.; Corbett, R.; et al. Childhood Cancer Survivors’ Reported Late Effects, Motivations for Seeking Survivorship Care, and Patterns of Attendance. Oncologist 2023, 28, e276–e286. [Google Scholar] [CrossRef]
- Dixon, S.B.; Bjornard, K.L.; Alberts, N.M.; Armstrong, G.T.; Brinkman, T.M.; Chemaitilly, W.; Ehrhardt, M.J.; Fernandez-Pineda, I.; Force, L.M.; Gibson, T.M.; et al. Factors Influencing Risk-Based Care of the Childhood Cancer Survivor in the 21st Century. CA Cancer J. Clin. 2018, 68, 133–152. [Google Scholar] [CrossRef]
- Rossi, F.; Di Paola, A.; Pota, E.; Argenziano, M.; Di Pinto, D.; Marrapodi, M.M.; Di Leva, C.; Di Martino, M.; Tortora, C. Biological Aspects of Inflamm-Aging in Childhood Cancer Survivors. Cancers 2021, 13, 4933. [Google Scholar] [CrossRef]
- Ravera, S.; Vigliarolo, T.; Bruno, S.; Morandi, F.; Marimpietri, D.; Sabatini, F.; Dagnino, M.; Petretto, A.; Bartolucci, M.; Muraca, M.; et al. Identification of Biochemical and Molecular Markers of Early Aging in Childhood Cancer Survivors. Cancers 2021, 13, 5214. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kumar, N.; Sahun, M.; Smits, E.; Bogaerts, A.; Privat-Maldonado, A. Modulating the Antioxidant Response for Better Oxidative Stress-Inducing Therapies: How to Take Advantage of Two Sides of the Same Medal? Biomedicines 2022, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Gorni, D.; Finco, A. Oxidative Stress in Elderly Population: A Prevention Screening Study. Aging Med. 2020, 3, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. The Oxidative Damage Theory of Aging. Clin. Neurosci. Res. 2003, 2, 305–315. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Marceau, F.; Farci, S.; Ouchane, S.; Chauvat, F. The Glutathione System: A Journey from Cyanobacteria to Higher Eukaryotes. Antioxidants 2023, 12, 1199. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The Key Role of GSH in Keeping the Redox Balance in Mammalian Cells: Mechanisms and Significance of GSH in Detoxification via Formation of Conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The Role of Glutathione Reductase and Related Enzymes on Cellular Redox Homoeostasis Network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redox Potential of GSH/GSSG Couple: Assay and Biological Significance. Methods Enzymol. 2002, 348, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhang, Y.; Hao, S.; Sun, H.; Liu, B.; Zhou, H.; Wang, Y.; Xu, Z.X. Recent Findings in the Regulation of G6PD and Its Role in Diseases. Front. Pharmacol. 2022, 13, 932154. [Google Scholar] [CrossRef] [PubMed]
- Senesi, S.; Csala, M.; Marcolongo, P.; Fulceri, R.; Mandl, J.; Banhegyi, G.; Benedetti, A. Hexose-6-Phosphate Dehydrogenase in the Endoplasmic Reticulum. Biol. Chem. 2010, 391, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sambuceti, G.; Cossu, V.; Vitale, F.; Bianconi, E.; Carta, S.; Venturi, C.; Chiesa, S.; Lanfranchi, F.; Emionite, L.; Carlone, S.; et al. Mandatory Role of Endoplasmic Reticulum and Its Pentose Phosphate Shunt in the Myocardial Defense Mechanisms against the Redox Stress Induced by Anthracyclines. Mol. Cell. Biochem. 2023, 479, 2973–2987. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Song, M.Y.; Lee, D.Y.; Chun, K.S.; Kim, E.H. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Uruno, A.; Yamamoto, M. The KEAP1-NRF2 System and Neurodegenerative Diseases. Antioxid. Redox Signal. 2023, 38, 974–988. [Google Scholar] [CrossRef]
- Roberts, J.A.; Rainbow, R.D.; Sharma, P. Mitigation of Cardiovascular Disease and Toxicity through NRF2 Signalling. Int. J. Mol. Sci. 2023, 24, 6723. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Díaz-Rúa, R.; Keijer, J.; Caimari, A.; van Schothorst, E.M.; Palou, A.; Oliver, P. Peripheral Blood Mononuclear Cells as a Source to Detect Markers of Homeostatic Alterations Caused by the Intake of Diets with an Unbalanced Macronutrient Composition. J. Nutr. Biochem. 2015, 26, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Columbaro, M.; Ravera, S.; Capanni, C.; Panfoli, I.; Cuccarolo, P.; Stroppiana, G.; Degan, P.; Cappell, E. Treatment of FANCA Cells with Resveratrol and N-Acetylcysteine: A Comparative Study. PLoS ONE 2014, 9, e104857. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Podestà, M.; Sabatini, F.; Dagnino, M.; Cilloni, D.; Fiorini, S.; Barla, A.; Frassoni, F. Discrete Changes in Glucose Metabolism Define Aging. Sci. Rep. 2019, 9, 10347. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, E.; Bertola, N.; Bruno, S.; Degan, P.; Regis, S.; Corsolini, F.; Banelli, B.; Dufour, C.; Ravera, S. A Multidrug Approach to Modulate the Mitochondrial Metabolism Impairment and Relative Oxidative Stress in Fanconi Anemia Complementation Group A. Metabolites 2021, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Bertola, N.; Regis, S.; Bruno, S.; Mazzarello, A.N.; Serra, M.; Lupia, M.; Sabatini, F.; Corsolini, F.; Ravera, S.; Cappelli, E. Effects of Deacetylase Inhibition on the Activation of the Antioxidant Response and Aerobic Metabolism in Cellular Models of Fanconi Anemia. Antioxidants 2023, 12, 1100. [Google Scholar] [CrossRef]
- Marini, C.; Cossu, V.; Kumar, M.; Milanese, M.; Cortese, K.; Bruno, S.; Bellese, G.; Carta, S.; Zerbo, R.A.; Torazza, C.; et al. The Role of Endoplasmic Reticulum in the Differential Endurance against Redox Stress in Cortical and Spinal Astrocytes from the Newborn Sod1g93a Mouse Model of Amyotrophic Lateral Sclerosis. Antioxidants 2021, 10, 1392. [Google Scholar] [CrossRef]
- Ravera, S.; Bertola, N.; Puddu, A.; Bruno, S.; Maggi, D.; Panfoli, I. Crosstalk between the Rod Outer Segments and Retinal Pigmented Epithelium in the Generation of Oxidative Stress in an In Vitro Model. Cells 2023, 12, 2173. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Marini, C.; Ravera, S.; Buschiazzo, A.; Bianchi, G.; Orengo, A.M.; Bruno, S.; Bottoni, G.; Emionite, L.; Pastorino, F.; Monteverde, E.; et al. Discovery of a Novel Glucose Metabolism in Cancer: The Role of Endoplasmic Reticulum beyond Glycolysis and Pentose Phosphate Shunt. Sci. Rep. 2016, 6, 25092. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J.A. Mitochondrial Free Radical Generation, Oxidative Stress, and Aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. Mitochondrial Uncoupling, ROS Generation and Cardioprotection. Biochim. Biophys. Acta (BBA)—Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. The Complex Interplay between Mitochondria, ROS and Entire Cellular Metabolism. Antioxidants 2022, 11, 1995. [Google Scholar] [CrossRef]
- Shrestha, R.; Johnson, E.; Byrne, F.L. Exploring the Therapeutic Potential of Mitochondrial Uncouplers in Cancer. Mol. Metab. 2021, 51, 101222. [Google Scholar] [CrossRef]
- Quarrie, R.; Lee, D.S.; Reyes, L.; Erdahl, W.; Pfeiffer, D.R.; Zweier, J.L.; Crestanello, J.A. Mitochondrial Uncoupling Does Not Decrease Reactive Oxygen Species Production after Ischemia-Reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H996–H1004. [Google Scholar] [CrossRef][Green Version]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; MacK, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Reichmann, D.; Voth, W.; Jakob, U. Maintaining a Healthy Proteome during Oxidative Stress. Mol. Cell 2018, 69, 203–213. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Qiu, J.; Papatsenko, D.; Niu, X.; Schaniel, C.; Moore, K. Divisional History and Hematopoietic Stem Cell Function during Homeostasis. Stem Cell Rep. 2014, 2, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Sleeping Beauty Wakes up the Clonal Succession Model for Homeostatic Hematopoiesis. Cell Stem Cell 2014, 15, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Natarajan, R. Epigenetic Modifications in Metabolic Memory: What Are the Memories, and Can We Erase Them? Am. J. Physiol. Cell Physiol. 2022, 323, C570–C582. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Y.; Zhou, B.; Bao, Y.; Li, Z.; Gong, C.; Yang, H.; Wang, S.; Xiao, Y. The Role of M6A Modification in Physiology and Disease. Cell Death Dis. 2020, 11, 960. [Google Scholar] [CrossRef]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-Acetylcysteine for Antioxidant Therapy: Pharmacology and Clinical Utility. Expert Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. The Effect of N-Acetyl-Cysteine on NRF2 Antioxidant Gene in Asthenoteratozoospermia Men: A Clinical Trial Study. Int. J. Fertil. Steril. 2020, 14, 171–175. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.; Liu, J.; Zhu, Z.; Hu, Z. Protective Effect of N-Acetylcysteine (NAC) on Renal Ischemia/Reperfusion Injury through Nrf2 Signaling Pathway. J. Recept. Signal Transduct. 2014, 34, 396–400. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen Sulfide Protects against Cellular Senescence via S-Sulfhydration of Keap1 and Activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen Sulfide Induces Keap1 S-Sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The Mechanism of Action of N-Acetylcysteine (NAC): The Emerging Role of H2S and Sulfane Sulfur Species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef]



| Childhood Cancer Survivors | |||||
| Solid Tumors | Hematological Tumors | ||||
| Age (Years) | Subjects n° | Female/Male | Subjects n° | Female/Male | |
| <10 | 27 | 16/11 | 25 | 9/16 | |
| 11–20 | 29 | 13/16 | 28 | 15/13 | |
| 21–40 | 18 | 8/10 | 22 | 12/10 | |
| Controls | |||||
| Age-Matched | Adult | ||||
| Age (Years) | Subjects n° | Female/Male | Age (Years) | Subjects n° | Female/Male |
| <10 | 18 | 7/11 | 41–60 | 19 | 8/11 |
| 11–20 | 32 | 18/14 | 61–80 | 25 | 15/10 |
| 21–40 | 20 | 11/9 | >80 | 22 | 15/7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossu, V.; Bertola, N.; Fresia, C.; Sabatini, F.; Ravera, S. Redox Imbalance and Antioxidant Defenses Dysfunction: Key Contributors to Early Aging in Childhood Cancer Survivors. Antioxidants 2024, 13, 1397. https://doi.org/10.3390/antiox13111397
Cossu V, Bertola N, Fresia C, Sabatini F, Ravera S. Redox Imbalance and Antioxidant Defenses Dysfunction: Key Contributors to Early Aging in Childhood Cancer Survivors. Antioxidants. 2024; 13(11):1397. https://doi.org/10.3390/antiox13111397
Chicago/Turabian StyleCossu, Vanessa, Nadia Bertola, Chiara Fresia, Federica Sabatini, and Silvia Ravera. 2024. "Redox Imbalance and Antioxidant Defenses Dysfunction: Key Contributors to Early Aging in Childhood Cancer Survivors" Antioxidants 13, no. 11: 1397. https://doi.org/10.3390/antiox13111397
APA StyleCossu, V., Bertola, N., Fresia, C., Sabatini, F., & Ravera, S. (2024). Redox Imbalance and Antioxidant Defenses Dysfunction: Key Contributors to Early Aging in Childhood Cancer Survivors. Antioxidants, 13(11), 1397. https://doi.org/10.3390/antiox13111397









