Oxidative Stress in Kidney Injury and Hypertension
Abstract
1. Introduction
2. Hypertension and Nephrosclerosis
2.1. Hypertension Disrupts Renal Structure and Function
2.2. Oxidative Stress Impairs Renal Function to Promote the Development of Hypertension
2.3. Reactive Oxygen Species and Cell Signaling
2.4. Summary
3. Renal Mechanisms Contributing to the Development of Hypertension
3.1. Resetting of the Pressure–Natriuresis Relation
3.2. Importance of the Intrarenal Renin-Angiotensin System and Endothelin
3.3. Gut Microbiota
3.4. Summary
4. NADPH Oxidases and Reactive Oxygen Species in the Kidney
4.1. Normotensive Animals
4.2. Spontaneously Hypertensive Rats
4.2.1. The Model
4.2.2. Oxidative Stress in the Kidney
4.3. Ang II-Induced Hypertension
4.3.1. The Model
4.3.2. Oxidative Stress in the Kidney
4.4. Dahl Salt-Sensitive Hypertension
4.4.1. The Model
4.4.2. Oxidative Stress in the Kidney
4.5. Summary
5. Role of SGLT2 and MR in Chronic Kidney Disease and Hypertension
5.1. Clinical Evidence from Human Studies
5.2. Animal Experimental Models of Hypertension
5.3. Summary
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| HTN | Hypertension |
| BP | Blood pressure |
| ROS | Reactive oxygen species |
| CKD | Chronic kidney disease |
| ESRD | End-stage renal disease |
| RAAS | renin–angiotensin–aldosterone system |
| SGLT2 | Sodium–glucose cotransporter 2 |
| NOX | NADPH oxidase |
| MR | Mineralocorticoid receptor |
| SS HTN | Salt-sensitive hypertension |
| GFR | Glomerular filtration rate |
| SHR | spontaneously hypertensive rats |
| Ang II | Angiotensin II |
| SNS | Sympathetic nervous system |
| NO | Nitric oxide |
| ET-1 | Endothelin-1 |
| TxA2 | Thromboxane A2 |
| PKC | Protein kinase C |
| MAPK | Mitogen-activated protein kinase |
| VSMC | Vascular smooth muscle cells |
| ECM | Extracellular matrix |
| TGF-β1 | Transforming growth factor beta 1 |
| EMT | Epithelial-mesenchymal transition |
| DKD | Diabetic kidney disease |
| ARBs | Angiotensin receptor blockers |
References
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e426–e483. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Qiu, Y.; Wang, C.; Bakris, G. Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999–2004. Am. J. Kidney Dis. 2008, 51, S30–S37. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Williams, G.H.; Hollenberg, N.K. Sodium-sensitive essential hypertension: Emerging insights into an old entity. J. Am. Coll. Nutr. 1989, 8, 490–494. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hebert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Girerd, S.; Jaisser, F. Mineralocorticoid receptor antagonists and kidney diseases: Pathophysiological basis. Kidney Int. 2019, 96, 302–319. [Google Scholar] [CrossRef]
- Bernard, K.; Thannickal, V.J. NADPH Oxidase Inhibition in Fibrotic Pathologies. Antioxid. Redox Signal. 2020, 33, 455–479. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Rodicio, J.L. The kidney in arterial hypertension. Nephrol. Dial. Transplant. 2001, 16, 50–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.; Qu, X.; Caruana, G.; Li, J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 2016, 92, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Caetano, E.R.; Zatz, R.; Saldanha, L.B.; Praxedes, J.N. Hypertensive nephrosclerosis as a relevant cause of chronic renal failure. Hypertension 2001, 38, 171–176. [Google Scholar] [CrossRef]
- Buday, A.; Orsy, P.; Godo, M.; Mozes, M.; Kokeny, G.; Lacza, Z.; Koller, A.; Ungvari, Z.; Gross, M.L.; Benyo, Z.; et al. Elevated systemic TGF-beta impairs aortic vasomotor function through activation of NADPH oxidase-driven superoxide production and leads to hypertension, myocardial remodeling, and increased plaque formation in apoE−/− mice. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H386–H395. [Google Scholar] [CrossRef]
- Sharma, R.; Khanna, A.; Sharma, M.; Savin, V.J. Transforming growth factor-beta1 increases albumin permeability of isolated rat glomeruli via hydroxyl radicals. Kidney Int. 2000, 58, 131–136. [Google Scholar] [CrossRef]
- Goettsch, C.; Goettsch, W.; Muller, G.; Seebach, J.; Schnittler, H.J.; Morawietz, H. Nox4 overexpression activates reactive oxygen species and p38 MAPK in human endothelial cells. Biochem. Biophys. Res. Commun. 2009, 380, 355–360. [Google Scholar] [CrossRef]
- Rhyu, D.Y.; Yang, Y.; Ha, H.; Lee, G.T.; Song, J.S.; Uh, S.T.; Lee, H.B. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 2005, 16, 667–675. [Google Scholar] [CrossRef]
- Abe, M.; Okada, K.; Soma, M. T-type Ca channel blockers in patients with chronic kidney disease in clinical practice. Curr. Hypertens. Rev. 2013, 9, 202–209. [Google Scholar] [CrossRef]
- Hayashi, K.; Homma, K.; Wakino, S.; Tokuyama, H.; Sugano, N.; Saruta, T.; Itoh, H. T-type Ca channel blockade as a determinant of kidney protection. Keio J. Med. 2010, 59, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Tomino, Y. Renoprotective effects of the L-/T-type calcium channel blocker benidipine in patients with hypertension. Curr. Hypertens. Rev. 2013, 9, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Schneider, M.P.; Raff, U.; Ritt, M.; Striepe, K.; Alberici, M.; Schmieder, R.E. Effects of manidipine vs. amlodipine on intrarenal haemodynamics in patients with arterial hypertension. Br. J. Clin. Pharmacol. 2013, 75, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Rodriguez-Iturbe, B. Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin Pract. Nephrol 2006, 2, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Touyz, R.M. Oxidative stress, Noxs, and hypertension: Experimental evidence and clinical controversies. Ann. Med. 2012, 44 (Suppl. S1), S2–S16. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.; Campo, C.; Gil, P.; Roldan, C.; Vigil, L.; Rodicio, J.L.; Ruilope, L.M. Development of chronic kidney disease and cardiovascular prognosis in essential hypertensive patients. J. Am. Soc. Nephrol. 2004, 15, 1616–1622. [Google Scholar] [CrossRef]
- Boulestreau, R.; van den Born, B.H.; Lip, G.Y.H.; Gupta, A. Malignant Hypertension: Current Perspectives and Challenges. J. Am. Heart Assoc. 2022, 11, e023397. [Google Scholar] [CrossRef]
- Mule, G.; Castiglia, A.; Cusumano, C.; Scaduto, E.; Geraci, G.; Altieri, D.; Di Natale, E.; Cacciatore, O.; Cerasola, G.; Cottone, S. Subclinical Kidney Damage in Hypertensive Patients: A Renal Window Opened on the Cardiovascular System. Focus on Microalbuminuria. Adv. Exp. Med. Biol. 2017, 956, 279–306. [Google Scholar] [CrossRef]
- Nlandu, K.S.; Dizin, E.; Sossauer, G.; Szanto, I.; Martin, P.Y.; Feraille, E.; Krause, K.H.; de Seigneux, S. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 2012, 23, 1967–1976. [Google Scholar] [CrossRef]
- Jha, J.C.; Dai, A.; Garzarella, J.; Charlton, A.; Urner, S.; Ostergaard, J.A.; Okabe, J.; Holterman, C.E.; Skene, A.; Power, D.A.; et al. Independent of Renox, NOX5 Promotes Renal Inflammation and Fibrosis in Diabetes by Activating ROS-Sensitive Pathways. Diabetes 2022, 71, 1282–1298. [Google Scholar] [CrossRef]
- Jha, J.C.; Dai, A.; Holterman, C.E.; Cooper, M.E.; Touyz, R.M.; Kennedy, C.R.; Jandeleit-Dahm, K.A.M. Endothelial or vascular smooth muscle cell-specific expression of human NOX5 exacerbates renal inflammation, fibrosis and albuminuria in the Akita mouse. Diabetologia 2019, 62, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Reactive oxygen species: Roles in blood pressure and kidney function. Curr. Hypertens. Rep 2002, 4, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Tan, R.J.; Liu, Y. Myofibroblast in Kidney Fibrosis: Origin, Activation, and Regulation. Adv. Exp. Med. Biol. 2019, 1165, 253–283. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Mueller, E.; Stahl, R.A.; Ziyadeh, F.N. Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J. Clin. Investig. 1993, 92, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Deelman, L.; Sharma, K. Mechanisms of kidney fibrosis and the role of antifibrotic therapies. Curr. Opin. Nephrol. Hypertens. 2009, 18, 85–90. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Zhang, J.Q.; Ramires, F.J. Local angiotensin II and transforming growth factor-beta1 in renal fibrosis of rats. Hypertension 2000, 35, 1078–1084. [Google Scholar] [CrossRef]
- Saito, S.; Shimizu, H.; Yisireyili, M.; Nishijima, F.; Enomoto, A.; Niwa, T. Indoxyl sulfate-induced activation of (pro)renin receptor is involved in expression of TGF-beta1 and alpha-smooth muscle actin in proximal tubular cells. Endocrinology 2014, 155, 1899–1907. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Karalliedde, J.; Viberti, G. Evidence for renoprotection by blockade of the renin-angiotensin-aldosterone system in hypertension and diabetes. J. Hum. Hypertens. 2006, 20, 239–253. [Google Scholar] [CrossRef]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 2014, 28, 74–79. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, S.S.; Chen, Y.; Ahokas, R.A.; Sun, Y. Kidney fibrosis in hypertensive rats: Role of oxidative stress. Am. J. Nephrol. 2008, 28, 548–554. [Google Scholar] [CrossRef]
- Nishiyama, A.; Yoshizumi, M.; Hitomi, H.; Kagami, S.; Kondo, S.; Miyatake, A.; Fukunaga, M.; Tamaki, T.; Kiyomoto, H.; Kohno, M.; et al. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J. Am. Soc. Nephrol. 2004, 15, 306–315. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, X.; Song, Y.; Qu, L.; Tang, J.; Meng, L.; Wang, Y. Apocynin attenuates renal fibrosis via inhibition of NOXs-ROS-ERK-myofibroblast accumulation in UUO rats. Free. Radic. Res. 2016, 50, 840–852. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Jones, D.; Textor, S.; Goff, D.C.; Murphy, T.P.; Toto, R.D.; White, A.; Cushman, W.C.; White, W.; Sica, D.; et al. Resistant hypertension: Diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008, 51, 1403–1419. [Google Scholar] [CrossRef]
- Gomes, S.A.; Hare, J.M.; Rangel, E.B. Kidney-Derived c-Kit(+) Cells Possess Regenerative Potential. Stem Cells Transl. Med. 2018, 7, 317–324. [Google Scholar] [CrossRef]
- Kaissling, B.; Lehir, M.; Kriz, W. Renal epithelial injury and fibrosis. Biochim. Biophys. Acta 2013, 1832, 931–939. [Google Scholar] [CrossRef]
- Fujii, S.; Zhang, L.; Kosaka, H. Albuminuria, expression of nicotinamide adenine dinucleotide phosphate oxidase and monocyte chemoattractant protein-1 in the renal tubules of hypertensive Dahl salt-sensitive rats. Hypertens. Res. 2007, 30, 991–998. [Google Scholar] [CrossRef][Green Version]
- Qi, W.; Niu, J.; Qin, Q.; Qiao, Z.; Gu, Y. Glycated albumin triggers fibrosis and apoptosis via an NADPH oxidase/Nox4-MAPK pathway-dependent mechanism in renal proximal tubular cells. Mol. Cell. Endocrinol. 2015, 405, 74–83. [Google Scholar] [CrossRef]
- Cho, S.; Yu, S.L.; Kang, J.; Jeong, B.Y.; Lee, H.Y.; Park, C.G.; Yu, Y.B.; Jin, D.C.; Hwang, W.M.; Yun, S.R.; et al. NADPH oxidase 4 mediates TGF-beta1/Smad signaling pathway induced acute kidney injury in hypoxia. PLoS ONE 2019, 14, e0219483. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Vascular endothelin in hypertension. Vasc. Pharmacol. 2005, 43, 19–29. [Google Scholar] [CrossRef]
- Ohshiro, Y.; Ma, R.C.; Yasuda, Y.; Hiraoka-Yamamoto, J.; Clermont, A.C.; Isshiki, K.; Yagi, K.; Arikawa, E.; Kern, T.S.; King, G.L. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes 2006, 55, 3112–3120. [Google Scholar] [CrossRef]
- Cottone, S.; Mule, G.; Guarneri, M.; Palermo, A.; Lorito, M.C.; Riccobene, R.; Arsena, R.; Vaccaro, F.; Vadala, A.; Nardi, E.; et al. Endothelin-1 and F2-isoprostane relate to and predict renal dysfunction in hypertensive patients. Nephrol. Dial. Transplant. 2009, 24, 497–503. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Sukkar, M.B.; Khorasani, N.M.; Bhavsar, P.K.; Chung, K.F. TGF-beta regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L295–L304. [Google Scholar] [CrossRef]
- Das, R.; Xu, S.; Quan, X.; Nguyen, T.T.; Kong, I.D.; Chung, C.H.; Lee, E.Y.; Cha, S.K.; Park, K.S. Upregulation of mitochondrial Nox4 mediates TGF-beta-induced apoptosis in cultured mouse podocytes. Am. J. Physiol. Ren. Physiol. 2014, 306, F155–F167. [Google Scholar] [CrossRef]
- Rajaram, R.D.; Dissard, R.; Faivre, A.; Ino, F.; Delitsikou, V.; Jaquet, V.; Cagarelli, T.; Lindenmeyer, M.; Jansen-Duerr, P.; Cohen, C.; et al. Tubular NOX4 expression decreases in chronic kidney disease but does not modify fibrosis evolution. Redox Biol. 2019, 26, 101234. [Google Scholar] [CrossRef]
- Ponnuchamy, B.; Khalil, R.A. Cellular mediators of renal vascular dysfunction in hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1001–R1018. [Google Scholar] [CrossRef]
- Weinberger, M.H. Pathogenesis of salt sensitivity of blood pressure. Curr. Hypertens. Rep. 2006, 8, 166–170. [Google Scholar] [CrossRef]
- Lavoie, J.L.; Bianco, R.A.; Sakai, K.; Keen, H.L.; Ryan, M.J.; Sigmund, C.D. Transgenic mice for studies of the renin-angiotensin system in hypertension. Acta Physiol. Scand. 2004, 181, 571–577. [Google Scholar] [CrossRef]
- Bianchi, G.; Fox, U.; Di Francesco, G.F.; Bardi, U.; Radice, M. The hypertensive role of the kidney in spontaneously hypertensive rats. Clin. Sci. Mol. Med. Suppl. 1973, 45 (Suppl. S1), 135s–139s. [Google Scholar] [CrossRef]
- Madrid, M.I.; Garcia-Salom, M.; Tornel, J.; De Gasparo, M.; Fenoy, F.J. Effect of interactions between nitric oxide and angiotensin II on pressure diuresis and natriuresis. Am. J. Physiol. 1997, 273, R1676–R1682. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Stevenson, M.D.; Lozhkin, A.; Hayami, T.; Holland, N.A.; Yang, X.; Moss, N.; Pan, H.; Wickline, S.A.; Stockand, J.D.; et al. Renal NOXA1/NOX1 Signaling Regulates Epithelial Sodium Channel and Sodium Retention in Angiotensin II-induced Hypertension. Antioxid. Redox Signal. 2022, 36, 550–566. [Google Scholar] [CrossRef]
- Cowley, A.W., Jr. Role of the renal medulla in volume and arterial pressure regulation. Am. J. Physiol. 1997, 273, R1–R15. [Google Scholar] [CrossRef]
- Rettig, R. Does the kidney play a role in the aetiology of primary hypertension? Evidence from renal transplantation studies in rats and humans. J. Hum. Hypertens. 1993, 7, 177–180. [Google Scholar]
- Crowley, S.D.; Gurley, S.B.; Herrera, M.J.; Ruiz, P.; Griffiths, R.; Kumar, A.P.; Kim, H.S.; Smithies, O.; Le, T.H.; Coffman, T.M. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. USA 2006, 103, 17985–17990. [Google Scholar] [CrossRef]
- Meneton, P.; Jeunemaitre, X.; de Wardener, H.E.; MacGregor, G.A. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol. Rev. 2005, 85, 679–715. [Google Scholar] [CrossRef]
- Arendshorst WJ, N.L. Renal Circulation and Glomerular Hemodynamics; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Carlstrom, M.; Wilcox, C.S.; Arendshorst, W.J. Renal autoregulation in health and disease. Physiol. Rev. 2015, 95, 405–511. [Google Scholar] [CrossRef]
- Navar, L.G.; Arendshorst, W.J.; Pallone, T.L.; Inscho, E.W.; Imig, J.D.; Bell, P.D. Chapter 13—The Renal Microcirculation. In Microcirculation, 2nd ed.; Tuma, R.F., Durán, W.N., Ley, K., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 550–683. [Google Scholar]
- Khalil, R.A. Protein Kinase C Inhibitors as Modulators of Vascular Function and their Application in Vascular Disease. Pharmaceuticals 2013, 6, 407–439. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef]
- Araujo, M.; Wilcox, C.S. Oxidative stress in hypertension: Role of the kidney. Antioxid. Redox Signal. 2014, 20, 74–101. [Google Scholar] [CrossRef]
- Kobori, H.; Nangaku, M.; Navar, L.G.; Nishiyama, A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 2007, 59, 251–287. [Google Scholar] [CrossRef]
- Neves, L.A.; Santos, R.A.; Khosla, M.C.; Milsted, A. Angiotensin-(1–7) regulates the levels of angiotensin II receptor subtype AT1 mRNA differentially in a strain-specific fashion. Regul. Pept. 2000, 95, 99–107. [Google Scholar] [CrossRef]
- Gonzalez-Villalobos, R.A.; Janjoulia, T.; Fletcher, N.K.; Giani, J.F.; Nguyen, M.T.; Riquier-Brison, A.D.; Seth, D.M.; Fuchs, S.; Eladari, D.; Picard, N.; et al. The absence of intrarenal ACE protects against hypertension. J. Clin. Investig. 2013, 123, 2011–2023. [Google Scholar] [CrossRef]
- Navar, L.G.; Harrison-Bernard, L.M.; Nishiyama, A.; Kobori, H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 2002, 39, 316–322. [Google Scholar] [CrossRef]
- Chappell, M.C.; Modrall, J.G.; Diz, D.I.; Ferrario, C.M. Novel aspects of the renal renin-angiotensin system: Angiotensin-(1–7), ACE2 and blood pressure regulation. Contrib. Nephrol. 2004, 143, 77–89. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Dikalova, A.; Clempus, R.; Lassegue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; San Martin, A.; Lyle, A.; Weber, D.S.; Weiss, D.; et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 2005, 112, 2668–2676. [Google Scholar] [CrossRef]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Vascular oxidative stress upregulates angiotensin II type I receptors via mechanisms involving nuclear factor kappa B. Clin. Exp. Hypertens. 2014, 36, 367–373. [Google Scholar] [CrossRef]
- Christensen, H.R.; Nielsen, H.; Christensen, K.L.; Baandrup, U.; Jespersen, L.T.; Mulvany, M.J. Long-term hypotensive effects of an angiotensin converting enzyme inhibitor in spontaneously hypertensive rats: Is there a role for vascular structure? J. Hypertens. Suppl. 1988, 6, S27–S31. [Google Scholar]
- Dukacz, S.A.; Adams, M.A.; Kline, R.L. Short- and long-term enalapril affect renal medullary hemodynamics in the spontaneously hypertensive rat. Am. J. Physiol. 1999, 276, R10–R16. [Google Scholar] [CrossRef]
- Kohan, D.E.; Rossi, N.F.; Inscho, E.W.; Pollock, D.M. Regulation of blood pressure and salt homeostasis by endothelin. Physiol. Rev. 2011, 91, 1–77. [Google Scholar] [CrossRef]
- Speed, J.S.; LaMarca, B.; Berry, H.; Cockrell, K.; George, E.M.; Granger, J.P. Renal medullary endothelin-1 is decreased in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R519–R523. [Google Scholar] [CrossRef]
- Cediel, E.; Vázquez-Cruz, B.; Navarro-Cid, J.; de las Heras, N.; Sanz-Rosa, D.; Cachofeiro, V.; Lahera, V. Role of endothelin-1 and thromboxane A2 in renal vasoconstriction induced by angiotensin II in diabetes and hypertension. Kidney Int. Suppl. 2002, 62, S2–S7. [Google Scholar] [CrossRef]
- Granger, J.P.; Abram, S.; Stec, D.; Chandler, D.; LaMarca, B. Endothelin, the kidney, and hypertension. Curr. Hypertens. Rep. 2006, 8, 298–303. [Google Scholar] [CrossRef]
- Kirchengast, M.; Witte, K.; Stolpe, K.; Schilling, L.; Nedvetsky, P.I.; Schmidt, H.H.; Lemmer, B. Effects of chronic endothelin ET(A) receptor blockade on blood pressure and vascular formation of cyclic guanosine-3′,5′-monophosphate in spontaneously hypertensive rats. Arzneimittelforschung 2005, 55, 498–504. [Google Scholar] [CrossRef]
- Romero, M.; Jimenez, R.; Sanchez, M.; Lopez-Sepulveda, R.; Zarzuelo, A.; Tamargo, J.; Perez-Vizcaino, F.; Duarte, J. Vascular superoxide production by endothelin-1 requires Src non-receptor protein tyrosine kinase and MAPK activation. Atherosclerosis 2010, 212, 78–85. [Google Scholar] [CrossRef]
- Hocher, B.; Thone-Reineke, C.; Rohmeiss, P.; Schmager, F.; Slowinski, T.; Burst, V.; Siegmund, F.; Quertermous, T.; Bauer, C.; Neumayer, H.H.; et al. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J. Clin. Investig. 1997, 99, 1380–1389. [Google Scholar] [CrossRef]
- Kassab, S.; Miller, M.T.; Novak, J.; Reckelhoff, J.; Clower, B.; Granger, J.P. Endothelin-A receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertension 1998, 31, 397–402. [Google Scholar] [CrossRef]
- Bugaj, V.; Mironova, E.; Kohan, D.E.; Stockand, J.D. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am. J. Physiol. Cell Physiol. 2012, 302, C188–C194. [Google Scholar] [CrossRef]
- Bird, J.E.; Giancarli, M.R. Cardiovascular and renal effects of endothelin B receptor selective agonists in spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 1996, 28, 381–384. [Google Scholar] [CrossRef]
- Jose, P.A.; Raj, D. Gut microbiota in hypertension. Curr. Opin. Nephrol. Hypertens. 2015, 24, 403–409. [Google Scholar] [CrossRef]
- Galla, S.; Chakraborty, S.; Cheng, X.; Yeo, J.; Mell, B.; Zhang, H.; Mathew, A.V.; Vijay-Kumar, M.; Joe, B. Disparate effects of antibiotics on hypertension. Physiol. Genom. 2018, 50, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Toral, M.; de la Visitacion, N.; Sanchez, M.; Gomez-Guzman, M.; Munoz, R.; Algieri, F.; Vezza, T.; Jimenez, R.; Galvez, J.; et al. Changes to the gut microbiota induced by losartan contributes to its antihypertensive effects. Br. J. Pharmacol. 2020, 177, 2006–2023. [Google Scholar] [CrossRef]
- Souders, C.L., 2nd; Zubcevic, J.; Martyniuk, C.J. Tumor Necrosis Factor Alpha and the Gastrointestinal Epithelium: Implications for the Gut-Brain Axis and Hypertension. Cell. Mol. Neurobiol. 2022, 42, 419–437. [Google Scholar] [CrossRef]
- Yang, T.; Magee, K.L.; Colon-Perez, L.M.; Larkin, R.; Liao, Y.S.; Balazic, E.; Cowart, J.R.; Arocha, R.; Redler, T.; Febo, M.; et al. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiol. 2019, 226, e13256. [Google Scholar] [CrossRef]
- Abboud, F.M.; Cicha, M.Z.; Ericsson, A.; Chapleau, M.W.; Singh, M.V. Altering Early Life Gut Microbiota Has Long-Term Effect on Immune System and Hypertension in Spontaneously Hypertensive Rats. Front. Physiol. 2021, 12, 752924. [Google Scholar] [CrossRef]
- Yang, T.; Mei, X.; Tackie-Yarboi, E.; Akere, M.T.; Kyoung, J.; Mell, B.; Yeo, J.Y.; Cheng, X.; Zubcevic, J.; Richards, E.M.; et al. Identification of a Gut Commensal That Compromises the Blood Pressure-Lowering Effect of Ester Angiotensin-Converting Enzyme Inhibitors. Hypertension 2022, 79, 1591–1601. [Google Scholar] [CrossRef]
- Gonzalez-Correa, C.; Moleon, J.; Minano, S.; Robles-Vera, I.; Toral, M.; Martin-Morales, N.; O’Valle, F.; Sanchez, M.; Gomez-Guzman, M.; Jimenez, R.; et al. Mineralocorticoid receptor blockade improved gut microbiota dysbiosis by reducing gut sympathetic tone in spontaneously hypertensive rats. Biomed. Pharmacother. 2023, 158, 114149. [Google Scholar] [CrossRef]
- Gill, P.S.; Wilcox, C.S. NADPH oxidases in the kidney. Antioxid. Redox Signal. 2006, 8, 1597–1607. [Google Scholar] [CrossRef]
- Holterman, C.E.; Read, N.C.; Kennedy, C.R. Nox and renal disease. Clin. Sci. 2015, 128, 465–481. [Google Scholar] [CrossRef]
- Sinha, N.; Dabla, P.K. Oxidative stress and antioxidants in hypertension—A current review. Curr. Hypertens. Rev. 2015, 11, 132–142. [Google Scholar] [CrossRef]
- Lassegue, B.; San, M.A.; Griendling, K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef] [PubMed]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef] [PubMed]
- Wolin, M.S. Reactive oxygen species and the control of vascular function. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H539–H549. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Jandeleit-Dahm, K.A. The role of NADPH oxidase in vascular disease--hypertension, atherosclerosis & stroke. Curr. Pharm. Des. 2015, 21, 5933–5944. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Munzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef]
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011, 34, 5–14. [Google Scholar] [CrossRef]
- Majid, D.S.; Kopkan, L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin. Exp. Pharmacol. Physiol. 2007, 34, 946–952. [Google Scholar] [CrossRef]
- Rivera, J.; Sobey, C.G.; Walduck, A.K.; Drummond, G.R. Nox isoforms in vascular pathophysiology: Insights from transgenic and knockout mouse models. Redox Rep. 2010, 15, 50–63. [Google Scholar] [CrossRef]
- Small, D.M.; Coombes, J.S.; Bennett, N.; Johnson, D.W.; Gobe, G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 2012, 17, 311–321. [Google Scholar] [CrossRef]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef] [PubMed]
- Salman, I.M.; Ameer, O.Z.; Sattar, M.A.; Abdullah, N.A.; Yam, M.F.; Najim, H.S.; Abdulkarim, M.F.; Abdullah, G.Z.; Kaur, G.; Khan, M.A.; et al. Characterization of renal hemodynamic and structural alterations in rat models of renal impairment: Role of renal sympathoexcitation. J. Nephrol. 2011, 24, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Peixoto, E.B.; Souza, D.S.; de Faria, J.B. Hypertension increases pro-oxidant generation and decreases antioxidant defense in the kidney in early diabetes. Am. J. Nephrol. 2008, 28, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Iturbe, B.; Vaziri, N.D.; Johnson, R.J. Inflammation, angiotensin II, and hypertension. Hypertension 2008, 52, e135–e136. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef]
- Ambasta, R.K.; Kumar, P.; Griendling, K.K.; Schmidt, H.H.; Busse, R.; Brandes, R.P. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 2004, 279, 45935–45941. [Google Scholar] [CrossRef]
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Forro, L.; Schlegel, W.; Krause, K.H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Oliveira-Paula, G.H. Sources and Effects of Oxidative Stress in Hypertension. Curr. Hypertens. Rev. 2020, 16, 166–180. [Google Scholar] [CrossRef]
- Lyle, A.N.; Deshpande, N.N.; Taniyama, Y.; Seidel-Rogol, B.; Pounkova, L.; Du, P.; Papaharalambus, C.; Lassegue, B.; Griendling, K.K. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009, 105, 249–259. [Google Scholar] [CrossRef]
- Prior, K.K.; Leisegang, M.S.; Josipovic, I.; Lowe, O.; Shah, A.M.; Weissmann, N.; Schroder, K.; Brandes, R.P. CRISPR/Cas9-mediated knockout of p22phox leads to loss of Nox1 and Nox4, but not Nox5 activity. Redox Biol. 2016, 9, 287–295. [Google Scholar] [CrossRef]
- Knock, G.A. NADPH oxidase in the vasculature: Expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic. Biol. Med. 2019, 145, 385–427. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Howitt, L.; Matthaei, K.I.; Drummond, G.R.; Hill, C.E. Nox1 upregulates the function of vascular T-type calcium channels following chronic nitric oxide deficit. Pflügers Arch. Eur. J. Physiol. 2015, 467, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Neves, K.B.; Rios, F.J.; van der Mey, L.; Alves-Lopes, R.; Cameron, A.C.; Volpe, M.; Montezano, A.C.; Savoia, C.; Touyz, R.M. VEGFR (Vascular Endothelial Growth Factor Receptor) Inhibition Induces Cardiovascular Damage via Redox-Sensitive Processes. Hypertension 2018, 71, 638–647. [Google Scholar] [CrossRef]
- Zimmerman, M.C.; Takapoo, M.; Jagadeesha, D.K.; Stanic, B.; Banfi, B.; Bhalla, R.C.; Miller, F.J., Jr. Activation of NADPH oxidase 1 increases intracellular calcium and migration of smooth muscle cells. Hypertension 2011, 58, 446–453. [Google Scholar] [CrossRef]
- Barton, M.; Meyer, M.R.; Prossnitz, E.R. Nox1 downregulators: A new class of therapeutics. Steroids 2019, 152, 108494. [Google Scholar] [CrossRef]
- Liu, M.; Grigoryev, D.N.; Crow, M.T.; Haas, M.; Yamamoto, M.; Reddy, S.P.; Rabb, H. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009, 76, 277–285. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N.; Yamamoto, M. Targeting the KEAP1-NRF2 System to Prevent Kidney Disease Progression. Am. J. Nephrol. 2017, 45, 473–483. [Google Scholar] [CrossRef]
- Feng, Y.L.; Chen, H.; Chen, D.Q.; Vaziri, N.D.; Su, W.; Ma, S.X.; Shang, Y.Q.; Mao, J.R.; Yu, X.Y.; Zhang, L.; et al. Activated NF-kappaB/Nrf2 and Wnt/beta-catenin pathways are associated with lipid metabolism in CKD patients with microalbuminuria and macroalbuminuria. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2317–2332. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Z.; Carter, G.; Wellstein, A.; Jose, P.A.; Tomlinson, J.; Leiper, J.; Welch, W.J.; Wilcox, C.S.; Wang, D. NRF2 prevents hypertension, increased ADMA, microvascular oxidative stress, and dysfunction in mice with two weeks of ANG II infusion. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R399–R406. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Huang, L.; Zhu, X.; Sha, J.; Li, G.; Ma, G.; Zhang, W.; Gu, M.; Guo, Y. Nrf2 signaling attenuates epithelial-to-mesenchymal transition and renal interstitial fibrosis via PI3K/Akt signaling pathways. Exp. Mol. Pathol. 2019, 111, 104296. [Google Scholar] [CrossRef] [PubMed]
- Potteti, H.R.; Noone, P.M.; Tamatam, C.R.; Ankireddy, A.; Noel, S.; Rabb, H.; Reddy, S.P. Nrf2 mediates hypoxia-inducible HIF1alpha activation in kidney tubular epithelial cells. Am. J. Physiol. Ren. Physiol. 2021, 320, F464–F474. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, Y.; Liu, M.; Chen, H. Hydrogen sulfide attenuates renal I/R-induced activation of the inflammatory response and apoptosis via regulating Nrf2-mediated NLRP3 signaling pathway inhibition. Mol. Med. Rep. 2021, 24, 518. [Google Scholar] [CrossRef] [PubMed]
- Tastan, B.; Arioz, B.I.; Genc, S. Targeting NLRP3 Inflammasome With Nrf2 Inducers in Central Nervous System Disorders. Front. Immunol. 2022, 13, 865772. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, R.D.; Dissard, R.; Jaquet, V.; de Seigneux, S. Potential benefits and harms of NADPH oxidase type 4 in the kidneys and cardiovascular system. Nephrol. Dial. Transplant. 2019, 34, 567–576. [Google Scholar] [CrossRef]
- Younis, N.N.; Elsherbiny, N.M.; Shaheen, M.A.; Elseweidy, M.M. Modulation of NADPH oxidase and Nrf2/HO-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J. Pharm. Pharmacol. 2020, 72, 1546–1555. [Google Scholar] [CrossRef]
- Sedeek, M.; Callera, G.; Montezano, A.; Gutsol, A.; Heitz, F.; Szyndralewiez, C.; Page, P.; Kennedy, C.R.; Burns, K.D.; Touyz, R.M.; et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: Implications in type 2 diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2010, 299, F1348–F1358. [Google Scholar] [CrossRef]
- Holterman, C.E.; Thibodeau, J.F.; Kennedy, C.R. NADPH oxidase 5 and renal disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 81–87. [Google Scholar] [CrossRef]
- Yu, P.; Han, W.; Villar, V.A.; Yang, Y.; Lu, Q.; Lee, H.; Li, F.; Quinn, M.T.; Gildea, J.J.; Felder, R.A.; et al. Unique role of NADPH oxidase 5 in oxidative stress in human renal proximal tubule cells. Redox Biol. 2014, 2, 570–579. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Sadegh, S.; Anastasi, E.; Guney, E.; Nogales, C.; Kacprowski, T.; Hassan, A.A.; Teubner, A.; Huang, P.H.; Hsu, C.Y.; et al. NOX5-induced uncoupling of endothelial NO synthase is a causal mechanism and theragnostic target of an age-related hypertension endotype. PLoS Biol. 2020, 18, e3000885. [Google Scholar] [CrossRef]
- Touyz, R.M.; Anagnostopoulou, A.; Rios, F.; Montezano, A.C.; Camargo, L.L. NOX5: Molecular biology and pathophysiology. Exp. Physiol. 2019, 104, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Holterman, C.E.; Boisvert, N.C.; Thibodeau, J.F.; Kamto, E.; Novakovic, M.; Abd-Elrahman, K.S.; Ferguson, S.S.G.; Kennedy, C.R.J. Podocyte NADPH Oxidase 5 Promotes Renal Inflammation Regulated by the Toll-Like Receptor Pathway. Antioxid. Redox Signal. 2019, 30, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Jay, D.B.; Papaharalambus, C.A.; Seidel-Rogol, B.; Dikalova, A.E.; Lassegue, B.; Griendling, K.K. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic. Biol. Med. 2008, 45, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; De Lucca Camargo, L.; Persson, P.; Rios, F.J.; Harvey, A.P.; Anagnostopoulou, A.; Palacios, R.; Gandara, A.C.P.; Alves-Lopes, R.; Neves, K.B.; et al. NADPH Oxidase 5 Is a Pro-Contractile Nox Isoform and a Point of Cross-Talk for Calcium and Redox Signaling-Implications in Vascular Function. J. Am. Heart Assoc. 2018, 7, e009388. [Google Scholar] [CrossRef] [PubMed]
- Gole, H.K.; Tharp, D.L.; Bowles, D.K. Upregulation of intermediate-conductance Ca2+-activated K+ channels (KCNN4) in porcine coronary smooth muscle requires NADPH oxidase 5 (NOX5). PLoS ONE 2014, 9, e105337. [Google Scholar] [CrossRef]
- Callera, G.E.; Touyz, R.M.; Teixeira, S.A.; Muscara, M.N.; Carvalho, M.H.; Fortes, Z.B.; Nigro, D.; Schiffrin, E.L.; Tostes, R.C. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension 2003, 42, 811–817. [Google Scholar] [CrossRef]
- Yura, T.; Fukunaga, M.; Khan, R.; Nassar, G.N.; Badr, K.F.; Montero, A. Free-radical-generated F2-isoprostane stimulates cell proliferation and endothelin-1 expression on endothelial cells. Kidney Int. 1999, 56, 471–478. [Google Scholar] [CrossRef]
- Daou, G.B.; Srivastava, A.K. Reactive oxygen species mediate Endothelin-1-induced activation of ERK1/2, PKB, and Pyk2 signaling, as well as protein synthesis, in vascular smooth muscle cells. Free Radic. Biol. Med. 2004, 37, 208–215. [Google Scholar] [CrossRef]
- Banes-Berceli, A.K.; Ogobi, S.; Tawfik, A.; Patel, B.; Shirley, A.; Pollock, D.M.; Fulton, D.; Marrero, M.B. Endothelin-1 activation of JAK2 in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Vasc. Pharmacol. 2005, 43, 310–319. [Google Scholar] [CrossRef]
- Chen, F.; Qian, L.H.; Deng, B.; Liu, Z.M.; Zhao, Y.; Le, Y.Y. Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress. CNS Neurosci. Ther. 2013, 19, 675–681. [Google Scholar] [CrossRef]
- Tong, X.; Schroder, K. NADPH oxidases are responsible for the failure of nitric oxide to inhibit migration of smooth muscle cells exposed to high glucose. Free Radic. Biol. Med. 2009, 47, 1578–1583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Selemidis, S.; Dusting, G.J.; Peshavariya, H.; Kemp-Harper, B.K.; Drummond, G.R. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc. Res. 2007, 75, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; de Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef] [PubMed]
- Yahr, J.; Thomas, G.; Calle, J.; Taliercio, J.J. Resistant hypertension: A stepwise approach. Clevel. Clin. J. Med. 2023, 90, 115–125. [Google Scholar] [CrossRef]
- Rettig, R.; Folberth, C.G.; Graf, C.; Kopf, D.; Stauss, H.; Unger, T. Are renal mechanisms involved in primary hypertension? Evidence from kidney transplantation studies in rats. Klin. Wochenschr. 1991, 69, 597–602. [Google Scholar] [CrossRef]
- Grisk, O.; Kloting, I.; Exner, J.; Spiess, S.; Schmidt, R.; Junghans, D.; Lorenz, G.; Rettig, R. Long-term arterial pressure in spontaneously hypertensive rats is set by the kidney. J. Hypertens. 2002, 20, 131–138. [Google Scholar] [CrossRef]
- Rettig, R.; Grisk, O. The kidney as a determinant of genetic hypertension: Evidence from renal transplantation studies. Hypertension 2005, 46, 463–468. [Google Scholar] [CrossRef]
- Rettig, R.; Bandelow, N.; Patschan, O.; Kuttler, B.; Frey, B.; Uber, A. The importance of the kidney in primary hypertension: Insights from cross-transplantation. J. Hum. Hypertens. 1996, 10, 641–644. [Google Scholar]
- Harrap, S.B.; Wang, B.Z.; MacLellan, D.G. Transplantation studies of the role of the kidney in long-term blood pressure reduction following brief ACE inhibitor treatment in young spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 1994, 21, 129–131. [Google Scholar] [CrossRef]
- Kawabe, K.; Watanabe, T.X.; Shiono, K.; Sokabe, H. Influence on blood pressure of renal isografts between spontaneously hypertensive and normotensive rats, utilizing the F1 hybrids. Jpn. Heart J. 1978, 19, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, H.; Lundin, S.; Henning, M.; Aurell, M.; Karlberg, B.E.; Berglund, G. Hormonal pattern during development of hypertension in spontaneously hypertensive rats (SHR). Clin. Exp. Hypertens. A 1982, 4, 915–935. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, H.; Lundin, S.; Ricksten, S.E.; Gothberg, G.; Aurell, M.; Hallback, M.; Berglund, G. Sodium balance and structural vascular changes in the kidney during development of hypertension in spontaneously hypertensive rats. Acta Medica Scand. Suppl. 1979, 625, 111–115. [Google Scholar] [CrossRef] [PubMed]
- McLennan, G.P.; Kline, R.L.; Mercer, P.F. Effect of enalapril treatment on the pressure-natriuresis curve in spontaneously hypertensive rats. Hypertension 1991, 17, 54–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roman, R.J. Altered pressure-natriuresis relationship in young spontaneously hypertensive rats. Hypertension 1987, 9, iii130. [Google Scholar] [CrossRef]
- Nagaoka, A.; Kakihana, M.; Shibota, M.; Fujiwara, K.; Shimakawa, K. Reduced sodium excretory ability in young spontaneously hypertensive rats. Jpn. J. Pharmacol. 1982, 32, 839–844. [Google Scholar] [CrossRef]
- Toal, C.B.; Leenen, F.H. Body fluid volumes during development of hypertension in the spontaneously hypertensive rat. J. Hypertens. 1983, 1, 345–350. [Google Scholar] [CrossRef]
- Roman, R.J. Alterations in renal medullary hemodynamics and the pressure-natriuretic response in genetic hypertension. Am. J. Hypertens. 1990, 3, 893–900. [Google Scholar] [CrossRef]
- Ferrone, R.A.; Antonaccio, M.J. Prevention of the development of spontaneous hypertension in rats by captopril (SQ 14,225). Eur. J. Pharmacol. 1979, 60, 131–137. [Google Scholar] [CrossRef]
- Lundie, M.J.; Friberg, P.; Kline, R.L.; Adams, M.A. Long-term inhibition of the renin-angiotensin system in genetic hypertension: Analysis of the impact on blood pressure and cardiovascular structural changes. J. Hypertens. 1997, 15, 339–348. [Google Scholar] [CrossRef]
- Harrap, S.B.; Doyle, A.E. Genetic co-segregation of renal haemodynamics and blood pressure in the spontaneously hypertensive rat. Clin Sci. 1988, 74, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.W., Jr. Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 2008, 52, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Harrap, S.B.; Doyle, A.E. Renal haemodynamics and total body sodium in immature spontaneously hypertensive and Wistar-Kyoto rats. J. Hypertens. Suppl. 1986, 4, S249–S252. [Google Scholar] [PubMed]
- Franco, M.; Tapia, E.; Bautista, R.; Pacheco, U.; Santamaria, J.; Quiroz, Y.; Johnson, R.J.; Rodriguez-Iturbe, B. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am. J. Physiol. Ren. Physiol. 2013, 304, F982–F990. [Google Scholar] [CrossRef]
- Nabha, L.; Garbern, J.C.; Buller, C.L.; Charpie, J.R. Vascular oxidative stress precedes high blood pressure in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2005, 27, 71–82. [Google Scholar] [CrossRef]
- Simao, S.; Gomes, P.; Pinto, V.; Silva, E.; Amaral, J.S.; Igreja, B.; Afonso, J.; Serrao, M.P.; Pinho, M.J.; Soares-da-Silva, P. Age-related changes in renal expression of oxidant and antioxidant enzymes and oxidative stress markers in male SHR and WKY rats. Exp. Gerontol. 2011, 46, 468–474. [Google Scholar] [CrossRef]
- Camargo, L.L.; Harvey, A.P.; Rios, F.J.; Tsiropoulou, S.; Da Silva, R.N.O.; Cao, Z.; Graham, D.; McMaster, C.; Burchmore, R.J.; Hartley, R.C.; et al. Vascular Nox (NADPH Oxidase) Compartmentalization, Protein Hyperoxidation, and Endoplasmic Reticulum Stress Response in Hypertension. Hypertension 2018, 72, 235–246. [Google Scholar] [CrossRef]
- Diez, J.; Fortuno, M.A.; Zalba, G.; Etayo, J.C.; Fortuno, A.; Ravassa, S.; Beaumont, J. Altered regulation of smooth muscle cell proliferation and apoptosis in small arteries of spontaneously hypertensive rats. Eur. Heart J. 1998, 19 (Suppl. G), G29–G33. [Google Scholar]
- Zhou, X.; Bohlen, H.G.; Miller, S.J.; Unthank, J.L. NAD(P)H oxidase-derived peroxide mediates elevated basal and impaired flow-induced NO production in SHR mesenteric arteries in vivo. Am. J. Physiol. Hear. Circ. Physiol. 2008, 295, H1008–H1016. [Google Scholar] [CrossRef]
- Park, J.B.; Touyz, R.M.; Chen, X.; Schiffrin, E.L. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am. J. Hypertens. 2002, 15, 78–84. [Google Scholar] [CrossRef]
- Spitler, K.M.; Webb, R.C. Endoplasmic reticulum stress contributes to aortic stiffening via proapoptotic and fibrotic signaling mechanisms. Hypertension 2014, 63, e40–e45. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Li, Y.; Anand-Srivastava, M.B. Reduced levels of cyclic AMP contribute to the enhanced oxidative stress in vascular smooth muscle cells from spontaneously hypertensive rats. Can. J. Physiol. Pharmacol. 2008, 86, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Tabet, F.; Callera, G.E.; Montezano, A.C.; Yogi, A.; He, Y.; Quinn, M.T.; Salaices, M.; Touyz, R.M. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J. Am. Soc. Hypertens. 2011, 5, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Cruzado, M.C.; Risler, N.R.; Miatello, R.M.; Yao, G.; Schiffrin, E.L.; Touyz, R.M. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension: Effect of angiotensin II and role of insulinlike growth factor-1 receptor transactivation. Am. J. Hypertens. 2005, 18, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Graton, M.E.; Potje, S.R.; Troiano, J.A.; Vale, G.T.; Perassa, L.A.; Nakamune, A.; Tirapelli, C.R.; Bendhack, L.M.; Antoniali, C. Apocynin alters redox signaling in conductance and resistance vessels of spontaneously hypertensive rats. Free Radic. Biol. Med. 2019, 134, 53–63. [Google Scholar] [CrossRef]
- Brosnan, M.J.; Hamilton, C.A.; Graham, D.; Lygate, C.A.; Jardine, E.; Dominiczak, A.F. Irbesartan lowers superoxide levels and increases nitric oxide bioavailability in blood vessels from spontaneously hypertensive stroke-prone rats. J. Hypertens. 2002, 20, 281–286. [Google Scholar] [CrossRef]
- Adler, S.; Huang, H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am. J. Physiol. Ren. Physiol. 2004, 287, F907–F913. [Google Scholar] [CrossRef]
- Chabrashvili, T.; Tojo, A.; Onozato, M.L.; Kitiyakara, C.; Quinn, M.T.; Fujita, T.; Welch, W.J.; Wilcox, C.S. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 2002, 39, 269–274. [Google Scholar] [CrossRef]
- Polizio, A.H.; Pena, C. Effects of angiotensin II type 1 receptor blockade on the oxidative stress in spontaneously hypertensive rat tissues. Regul. Pept. 2005, 128, 1–5. [Google Scholar] [CrossRef]
- Patinha, D.; Afonso, J.; Sousa, T.; Morato, M.; Albino-Teixeira, A. Diabetes-induced increase of renal medullary hydrogen peroxide and urinary angiotensinogen is similar in normotensive and hypertensive rats. Life Sci. 2014, 108, 71–79. [Google Scholar] [CrossRef]
- Zhan, C.D.; Sindhu, R.K.; Vaziri, N.D. Up-regulation of kidney NAD(P)H oxidase and calcineurin in SHR: Reversal by lifelong antioxidant supplementation. Kidney Int. 2004, 65, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Ito, O.; Guo, Q.; Ito, D.; Muroya, Y.; Rong, R.; Mori, T.; Ito, S.; Kohzuki, M. Endogenous hydrogen peroxide up-regulates the expression of nitric oxide synthase in the kidney of SHR. J. Hypertens. 2011, 29, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.J.; Lin, K.M.; Kuo, H.C.; Huang, C.F.; Lin, Y.J.; Huang, L.T.; Tain, Y.L. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: L-citrulline and nitrate. Transl. Res. 2014, 163, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.D.; Sindhu, R.K.; Pang, J.; Ehdaie, A.; Vaziri, N.D. Superoxide dismutase, catalase and glutathione peroxidase in the spontaneously hypertensive rat kidney: Effect of antioxidant-rich diet. J. Hypertens. 2004, 22, 2025–2033. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Ni, Z.; Oveisi, F.; Trnavsky-Hobbs, D.L. Effect of antioxidant therapy on blood pressure and NO synthase expression in hypertensive rats. Hypertension 2000, 36, 957–964. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Ni, Z.; Oveisi, F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension 1998, 31, 1248–1254. [Google Scholar] [CrossRef]
- Nava, E.; Llinas, M.T.; Gonzalez, J.D.; Salazar, F.J. Nitric oxide synthase activity in renal cortex and medulla of normotensive and spontaneously hypertensive rats. Am. J. Hypertens. 1996, 9, 1236–1239. [Google Scholar] [CrossRef]
- Welch, W.J.; Tojo, A.; Lee, J.U.; Kang, D.G.; Schnackenberg, C.G.; Wilcox, C.S. Nitric oxide synthase in the JGA of the SHR: Expression and role in tubuloglomerular feedback. Am. J. Physiol. 1999, 277, F130–F138. [Google Scholar] [CrossRef]
- Kim, S.W.; Moon, K.H.; Lee, S.C.; Kim, N.H.; Kang, D.G.; Lee, J.U.; Choi, K.C.; Kang, Y.J. Altered renal expression of nitric oxide synthase isozymes in spontaneously hypertensive rats. Korean J. Intern. Med. 1999, 14, 21–26. [Google Scholar] [CrossRef]
- Kumar, U.; Chen, J.; Sapoznikhov, V.; Canteros, G.; White, B.H.; Sidhu, A. Overexpression of inducible nitric oxide synthase in the kidney of the spontaneously hypertensive rat. Clin. Exp. Hypertens. 2005, 27, 17–31. [Google Scholar] [CrossRef]
- Kumar, U.; Shin, Y.; Wersinger, C.; Patel, Y.; Sidhu, A. Diminished expression of constitutive nitric oxide synthases in the kidney of spontaneously hypertensive rat. Clin. Exp. Hypertens. 2003, 25, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Schnackenberg, C.G.; Welch, W.J.; Wilcox, C.S. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: Role of nitric oxide. Hypertension 1998, 32, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.J.; Mendonca, M.; Blau, J.; Karber, A.; Dennehy, K.; Patel, K.; Lao, Y.S.; Jose, P.A.; Wilcox, C.S. Antihypertensive response to prolonged tempol in the spontaneously hypertensive rat. Kidney Int. 2005, 68, 179–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goodman, A.I.; Quan, S.; Yang, L.; Synghal, A.; Abraham, N.G. Functional expression of human heme oxygenase-1 gene in renal structure of spontaneously hypertensive rats. Exp. Biol. Med. 2003, 228, 454–458. [Google Scholar] [CrossRef] [PubMed]
- de Richelieu, L.T.; Sorensen, C.M.; Holstein-Rathlou, N.H.; Salomonsson, M. NO-independent mechanism mediates tempol-induced renal vasodilation in SHR. Am. J. Physiol. Ren. Physiol. 2005, 289, F1227–F1234. [Google Scholar] [CrossRef]
- Feng, M.G.; Dukacz, S.A.; Kline, R.L. Selective effect of tempol on renal medullary hemodynamics in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1420–R1425. [Google Scholar] [CrossRef]
- Cao, P.; Ito, O.; Ito, D.; Rong, R.; Zheng, Y.; Kohzuki, M. Combination of Exercise Training and SOD Mimetic Tempol Enhances Upregulation of Nitric Oxide Synthase in the Kidney of Spontaneously Hypertensive Rats. Int. J. Hypertens. 2020, 2020, 2142740. [Google Scholar] [CrossRef]
- Ono, H.; Ono, Y.; Frohlich, E.D. ACE inhibition prevents and reverses L-NAME-exacerbated nephrosclerosis in spontaneously hypertensive rats. Hypertension 1996, 27, 176–183. [Google Scholar] [CrossRef]
- Meng, X.M.; Ren, G.L.; Gao, L.; Yang, Q.; Li, H.D.; Wu, W.F.; Huang, C.; Zhang, L.; Lv, X.W.; Li, J. NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab. Investig. 2018, 98, 63–78. [Google Scholar] [CrossRef]
- Baumann, M.; Janssen, B.J.; Hermans, J.J.; Peutz-Kootstra, C.; Witzke, O.; Smits, J.F.; Struijker Boudier, H.A. Transient AT1 receptor-inhibition in prehypertensive spontaneously hypertensive rats results in maintained cardiac protection until advanced age. J. Hypertens. 2007, 25, 207–215. [Google Scholar] [CrossRef]
- Baumann, M.; Hermans, J.J.; Janssen, B.J.; Peutz-Kootstra, C.; Witzke, O.; Heemann, U.; Smits, J.F.; Boudier, H.A. Transient prehypertensive treatment in spontaneously hypertensive rats: A comparison of spironolactone and losartan regarding long-term blood pressure and target organ damage. J. Hypertens. 2007, 25, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Rodella, L.; Porteri, E.; Rezzani, R.; Sleiman, I.; Paiardi, S.; Guelfi, D.; De Ciuceis, C.; Boari, G.E.; Bianchi, R.; et al. Effects of losartan and enalapril at different doses on cardiac and renal interstitial matrix in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2003, 25, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Xu, X.; Dai, Y.; Xu, R.; Li, G.; Yao, Y.; Li, J.; Zheng, F. The effects of single and combined application of ramipril and losartan on renal structure and function in hypertensive rats. Clin. Exp. Hypertens. 2018, 40, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Harrap, S.B.; Nicolaci, J.A.; Doyle, A.E. Persistent effects on blood pressure and renal haemodynamics following chronic angiotensin converting enzyme inhibition with perindopril. Clin. Exp. Pharmacol. Physiol. 1986, 13, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Pang, Y.J.; Zhu, W.W.; Zhao, T.T.; Zheng, M.; Wang, Y.B.; Sun, Z.J.; Sun, S.J. Benazepril, an angiotensin-converting enzyme inhibitor, alleviates renal injury in spontaneously hypertensive rats by inhibiting advanced glycation end-product-mediated pathways. Clin. Exp. Pharmacol. Physiol. 2009, 36, 287–296. [Google Scholar] [CrossRef]
- Mizutani, K.; Ikeda, K.; Tsuda, K.; Yamori, Y. Inhibitor for advanced glycation end products formation attenuates hypertension and oxidative damage in genetic hypertensive rats. J. Hypertens. 2002, 20, 1607–1614. [Google Scholar] [CrossRef]
- Kemp, B.A.; Howell, N.L.; Keller, S.R.; Gildea, J.J.; Shao, W.; Navar, L.G.; Carey, R.M. Defective Renal Angiotensin III and AT(2) Receptor Signaling in Prehypertensive Spontaneously Hypertensive Rats. J. Am. Heart Assoc. 2019, 8, e012016. [Google Scholar] [CrossRef]
- Wu, L.; Wang, R.; De Champlain, J.; Wilson, T.W. Beneficial and deleterious effects of rosiglitazone on hypertension development in spontaneously hypertensive rats. Am. J. Hypertens. 2004, 17, 749–756. [Google Scholar] [CrossRef]
- Diep, Q.N.; Schiffrin, E.L. Increased expression of peroxisome proliferator-activated receptor-alpha and -gamma in blood vessels of spontaneously hypertensive rats. Hypertension 2001, 38, 249–254. [Google Scholar] [CrossRef]
- Efrati, S.; Berman, S.; Ilgiyeav, E.; Averbukh, Z.; Weissgarten, J. PPAR-gamma activation inhibits angiotensin II synthesis, apoptosis, and proliferation of mesangial cells from spontaneously hypertensive rats. Nephron Exp. Nephrol. 2007, 106, e107–e112. [Google Scholar] [CrossRef]
- Yousefipour, Z.; Newaz, M. PPARalpha ligand clofibrate ameliorates blood pressure and vascular reactivity in spontaneously hypertensive rats. Acta Pharmacol. Sin. 2014, 35, 476–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steckelings, U.M.; Widdop, R.E.; Sturrock, E.D.; Lubbe, L.; Hussain, T.; Kaschina, E.; Unger, T.; Hallberg, A.; Carey, R.M.; Sumners, C. The Angiotensin AT(2) Receptor: From a Binding Site to a Novel Therapeutic Target. Pharmacol. Rev. 2022, 74, 1051–1135. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Abdul Sattar, M.; Johns, E.J.; Eseyin, O.A. Renoprotective and haemodynamic effects of adiponectin and peroxisome proliferator-activated receptor agonist, pioglitazone, in renal vasculature of diabetic Spontaneously hypertensive rats. PLoS ONE 2020, 15, e0229803. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Umemoto, S.; Guo, F.; Umeji, K.; Itoh, S.; Kishi, H.; Kobayashi, S.; Matsuzaki, M. Nifedipine activates PPARgamma and exerts antioxidative action through Cu/ZnSOD independent of blood-pressure lowering in SHRSP. J. Atheroscler. Thromb. 2010, 17, 785–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shokoji, T.; Nishiyama, A.; Fujisawa, Y.; Hitomi, H.; Kiyomoto, H.; Takahashi, N.; Kimura, S.; Kohno, M.; Abe, Y. Renal sympathetic nerve responses to tempol in spontaneously hypertensive rats. Hypertension 2003, 41, 266–273. [Google Scholar] [CrossRef]
- Wyss, J.M.; Oparil, S.; Sripairojthikoon, W. Neuronal control of the kidney: Contribution to hypertension. Can. J. Physiol. Pharmacol. 1992, 70, 759–770. [Google Scholar] [CrossRef]
- Winternitz, S.R.; Oparil, S. Importance of the renal nerves in the pathogenesis of experimental hypertension. Hypertension 1982, 4, iii108. [Google Scholar] [CrossRef]
- Rudd, M.A.; Grippo, R.S.; Arendshorst, W.J. Acute renal denervation produces a diuresis and natriuresis in young SHR but not WKY rats. Am. J. Physiol. -Ren. Physiol. 1986, 251, F655–F661. [Google Scholar] [CrossRef]
- Katholi, R.E. Renal nerves and hypertension: An update. Fed. Proc. 1985, 44, 2846–2850. [Google Scholar]
- Shokoji, T.; Fujisawa, Y.; Kimura, S.; Rahman, M.; Kiyomoto, H.; Matsubara, K.; Moriwaki, K.; Aki, Y.; Miyatake, A.; Kohno, M.; et al. Effects of local administrations of tempol and diethyldithio-carbamic on peripheral nerve activity. Hypertension 2004, 44, 236–243. [Google Scholar] [CrossRef]
- Schluter, T.; Grimm, R.; Steinbach, A.; Lorenz, G.; Rettig, R.; Grisk, O. Neonatal sympathectomy reduces NADPH oxidase activity and vascular resistance in spontaneously hypertensive rat kidneys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R391–R399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez-Iturbe, B.; Ferrebuz, A.; Vanegas, V.; Quiroz, Y.; Mezzano, S.; Vaziri, N.D. Early and sustained inhibition of nuclear factor-kappaB prevents hypertension in spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 2005, 315, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Elks, C.M.; Mariappan, N.; Haque, M.; Guggilam, A.; Majid, D.S.; Francis, J. Chronic NF-kappaB blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am. J. Physiol. Ren. Physiol. 2009, 296, F298–F305. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Zicha, J.; Kojsova, S.; Dobesova, Z.; Jendekova, L.; Kunes, J. Effect of chronic N-acetylcysteine treatment on the development of spontaneous hypertension. Clin. Sci. 2006, 110, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, W.; Chen, Q.; Cao, Q.; Di, W.; Lan, R.; Chen, Z.; Bai, J.; Han, Z.; Xu, W. Inhibition of RAGE by FPS-ZM1 alleviates renal injury in spontaneously hypertensive rats. Eur. J. Pharmacol. 2020, 882, 173228. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 2009, 54, 810–817. [Google Scholar] [CrossRef]
- Takenaka, T.; Inoue, T.; Miyazaki, T.; Kobori, H.; Nishiyama, A.; Ishii, N.; Hayashi, M.; Suzuki, H. Klotho Ameliorates Medullary Fibrosis and Pressure Natriuresis in Hypertensive Rat Kidneys. Hypertension 2018, 72, 1151–1159. [Google Scholar] [CrossRef]
- Naruse, M.; Takagi, S.; Tanabe, A.; Naruse, K.; Adachi, C.; Yoshimoto, T.; Seki, T.; Takano, K. Augmented expression of tissue endothelin-1 messenger RNA is a common feature in hypertensive rats. J. Cardiovasc. Pharmacol. 2000, 36, S195–S197. [Google Scholar] [CrossRef]
- Kerr, S.; Brosnan, M.J.; McIntyre, M.; Reid, J.L.; Dominiczak, A.F.; Hamilton, C.A. Superoxide anion production is increased in a model of genetic hypertension: Role of the endothelium. Hypertension 1999, 33, 1353–1358. [Google Scholar] [CrossRef]
- Morawietz, H.; Weber, M.; Rueckschloss, U.; Lauer, N.; Hacker, A.; Kojda, G. Upregulation of vascular NAD(P)H oxidase subunit gp91phox and impairment of the nitric oxide signal transduction pathway in hypertension. Biochem. Biophys. Res. Commun. 2001, 285, 1130–1135. [Google Scholar] [CrossRef]
- Akasaki, T.; Ohya, Y.; Kuroda, J.; Eto, K.; Abe, I.; Sumimoto, H.; Iida, M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: Involvement of the renin-angiotensin system. Hypertens. Res. 2006, 29, 813–820. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, T.; Honma, H.; Ikeda, Y.; Kuroyanagi, R.; Takano, H.; Obata, J.; Sato, T.; Kimura, H.; Yoshida, Y.; Tamura, K. Renal protective effects of angiotensin II receptor I antagonist CV-11974 in spontaneously hypertensive stroke-prone rats (SHR-sp). Blood Press. Suppl. 1994, 5, 61–66. [Google Scholar] [PubMed]
- Camargo, M.J.; von Lutterotti, N.; Pecker, M.S.; James, G.D.; Timmermans, P.B.; Laragh, J.H. DuP 753 increases survival in spontaneously hypertensive stroke-prone rats fed a high sodium diet. Am. J. Hypertens. 1991, 4, 341S–345S. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, H.; Palmgren, E.; Widgren, B.; Aurell, M. Failure of angiotensin II to suppress plasma renin activity in normotensive subjects with a positive family history of hypertension. Clin. Sci. 2005, 109, 311–317. [Google Scholar] [CrossRef]
- Ljungman, S.; Aurell, M.; Hartford, M.; Wikstrand, J.; Berglund, G. Effects of subpressor doses of angiotensin II on renal hemodynamics in relation to blood pressure. Hypertension 1983, 5, 368–374. [Google Scholar] [CrossRef]
- Fitzgerald, S.M.; Stevenson, K.M.; Evans, R.G.; Anderson, W.P. Low dose angiotensin II infusions into the renal artery induce chronic hypertension in conscious dogs. Blood Press. 1997, 6, 52–61. [Google Scholar] [CrossRef]
- Luft, F.C.; Wilcox, C.S.; Unger, T.; Kuhn, R.; Demmert, G.; Rohmeiss, P.; Ganten, D.; Sterzel, R.B. Angiotensin-induced hypertension in the rat. Sympathetic nerve activity and prostaglandins. Hypertension 1989, 14, 396–403. [Google Scholar] [CrossRef]
- Navar, L.G.; Ichihara, A.; Chin, S.Y.; Imig, J.D. Nitric oxide-angiotensin II interactions in angiotensin II-dependent hypertension. Acta Physiol. Scand. 2000, 168, 139–147. [Google Scholar] [CrossRef]
- Gonzalez, A.A.; Prieto, M.C. Roles of collecting duct renin and (pro)renin receptor in hypertension: Mini review. Ther. Adv. Cardiovasc. Dis. 2015, 9, 191–200. [Google Scholar] [CrossRef]
- Gonzalez-Villalobos, R.A.; Satou, R.; Seth, D.M.; Semprun-Prieto, L.C.; Katsurada, A.; Kobori, H.; Navar, L.G. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension 2009, 53, 351–355. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.S.; Adam, A.G.; Jaimes, E.A.; Raij, L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension 2003, 42, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, Z.; Wang, X.; Jose, P.A.; Falck, J.R.; Welch, W.J.; Aslam, S.; Teerlink, T.; Wilcox, C.S. Impaired endothelial function and microvascular asymmetrical dimethylarginine in angiotensin II-infused rats: Effects of tempol. Hypertension 2010, 56, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Polichnowski, A.J.; Griffin, K.A.; Picken, M.M.; Licea-Vargas, H.; Long, J.; Williamson, G.A.; Bidani, A.K. Hemodynamic basis for the limited renal injury in rats with angiotensin II-induced hypertension. Am. J. Physiol. Ren. Physiol. 2015, 308, F252–F260. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Neves, M.F.; Amiri, F.; Touyz, R.M.; Schiffrin, E.L. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J. Hypertens. 2004, 22, 535–542. [Google Scholar] [CrossRef]
- Gavazzi, G.; Deffert, C.; Trocme, C.; Schappi, M.; Herrmann, F.R.; Krause, K.H. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension 2007, 50, 189–196. [Google Scholar] [CrossRef]
- Modlinger, P.; Chabrashvili, T.; Gill, P.S.; Mendonca, M.; Harrison, D.G.; Griendling, K.K.; Li, M.; Raggio, J.; Wellstein, A.; Chen, Y.; et al. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 2006, 47, 238–244. [Google Scholar] [CrossRef]
- Nouri, P.; Gill, P.; Li, M.; Wilcox, C.S.; Welch, W.J. p22phox in the macula densa regulates single nephron GFR during angiotensin II infusion in rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1685–H1689. [Google Scholar] [CrossRef][Green Version]
- Landmesser, U.; Cai, H.; Dikalov, S.; McCann, L.; Hwang, J.; Jo, H.; Holland, S.M.; Harrison, D.G. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 2002, 40, 511–515. [Google Scholar] [CrossRef]
- Saleem, M.; Wang, X.; Pokkunuri, I.; Asghar, M. Superoxide via Sp3 mechanism increases renal renin activity, renal AT1 receptor function, and blood pressure in rats. Am. J. Physiol. Ren. Physiol. 2018, 315, F1478–F1483. [Google Scholar] [CrossRef]
- Touyz, R.M.; Mercure, C.; He, Y.; Javeshghani, D.; Yao, G.; Callera, G.E.; Yogi, A.; Lochard, N.; Reudelhuber, T.L. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension 2005, 45, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Yogi, A.; Mercure, C.; Touyz, J.; Callera, G.E.; Montezano, A.C.; Aranha, A.B.; Tostes, R.C.; Reudelhuber, T.; Touyz, R.M. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension 2008, 51, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Touyz, R.M. Reactive oxygen species and endothelial function--role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin. Pharmacol. Toxicol. 2012, 110, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Lopez, A.; Llinas, M.T.; Rodriguez, F.; Lopez-Farre, A.; Nava, E.; Salazar, F.J. Changes in NOS activity and protein expression during acute and prolonged ANG II administration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R31–R37. [Google Scholar] [CrossRef] [PubMed]
- Mollnau, H.; Wendt, M.; Szocs, K.; Lassegue, B.; Schulz, E.; Oelze, M.; Li, H.; Bodenschatz, M.; August, M.; Kleschyov, A.L.; et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ. Res. 2002, 90, E58–E65. [Google Scholar] [CrossRef]
- Matsuno, K.; Yamada, H.; Iwata, K.; Jin, D.; Katsuyama, M.; Matsuki, M.; Takai, S.; Yamanishi, K.; Miyazaki, M.; Matsubara, H.; et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation 2005, 112, 2677–2685. [Google Scholar] [CrossRef]
- Garrido-Urbani, S.; Jemelin, S.; Deffert, C.; Carnesecchi, S.; Basset, O.; Szyndralewiez, C.; Heitz, F.; Page, P.; Montet, X.; Michalik, L.; et al. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS ONE 2011, 6, e14665. [Google Scholar] [CrossRef]
- Wei, H.; Mi, X.; Ji, L.; Yang, L.; Xia, Q.; Wei, Y.; Miyamori, I.; Fan, C. Protein kinase C-delta is involved in induction of NOX1 gene expression by aldosterone in rat vascular smooth muscle cells. Biochemistry 2010, 75, 304–309. [Google Scholar] [CrossRef]
- Samai, M.; Sharpe, M.A.; Gard, P.R.; Chatterjee, P.K. Comparison of the effects of the superoxide dismutase mimetics EUK-134 and tempol on paraquat-induced nephrotoxicity. Free Radic. Biol. Med. 2007, 43, 528–534. [Google Scholar] [CrossRef]
- Chabrashvili, T.; Kitiyakara, C.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R117–R124. [Google Scholar] [CrossRef]
- Carlstrom, M.; Lai, E.Y.; Ma, Z.; Steege, A.; Patzak, A.; Eriksson, U.J.; Lundberg, J.O.; Wilcox, C.S.; Persson, A.E. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertension 2010, 56, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.J.; Chabrashvili, T.; Solis, G.; Chen, Y.; Gill, P.S.; Aslam, S.; Wang, X.; Ji, H.; Sandberg, K.; Jose, P.; et al. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 2006, 48, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Whiting, C.; Castillo, A.; Haque, M.Z.; Majid, D.S. Protective role of the endothelial isoform of nitric oxide synthase in ANG II-induced inflammatory responses in the kidney. Am. J. Physiol. Ren. Physiol. 2013, 305, F1031–F1041. [Google Scholar] [CrossRef][Green Version]
- Manning, R.D., Jr.; Hu, L.; Mizelle, H.L.; Granger, J.P. Role of nitric oxide in long-term angiotensin II-induced renal vasoconstriction. Hypertension 1993, 21, 949–955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, J.; Yu, M.; Jiang, J.; Luo, Y.; Zhang, Q.; Wang, S.; Yang, F.; Wang, A.; Wang, L.; Zhuang, M.; et al. Angiotensin II Decreases Endothelial Nitric Oxide Synthase Phosphorylation via AT(1)R Nox/ROS/PP2A Pathway. Front. Physiol. 2020, 11, 566410. [Google Scholar] [CrossRef]
- Ling, W.C.; Mustafa, M.R.; Vanhoutte, P.M.; Murugan, D.D. Chronic administration of sodium nitrite prevents hypertension and protects arterial endothelial function by reducing oxidative stress in angiotensin II-infused mice. Vasc. Pharmacol. 2018, 102, 11–20. [Google Scholar] [CrossRef]
- Sasser, J.M.; Moningka, N.C.; Cunningham, M.W., Jr.; Croker, B.; Baylis, C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R740–R746. [Google Scholar] [CrossRef]
- Weber, D.S.; Rocic, P.; Mellis, A.M.; Laude, K.; Lyle, A.N.; Harrison, D.G.; Griendling, K.K. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H37–H42. [Google Scholar] [CrossRef]
- Bao, W.; Behm, D.J.; Nerurkar, S.S.; Ao, Z.; Bentley, R.; Mirabile, R.C.; Johns, D.G.; Woods, T.N.; Doe, C.P.; Coatney, R.W.; et al. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J. Cardiovasc. Pharmacol. 2007, 49, 362–368. [Google Scholar] [CrossRef]
- Fukui, T.; Ishizaka, N.; Rajagopalan, S.; Laursen, J.B.; Capers, Q.t.; Taylor, W.R.; Harrison, D.G.; de Leon, H.; Wilcox, J.N.; Griendling, K.K. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ. Res. 1997, 80, 45–51. [Google Scholar] [CrossRef]
- Laude, K.; Cai, H.; Fink, B.; Hoch, N.; Weber, D.S.; McCann, L.; Kojda, G.; Fukai, T.; Schmidt, H.H.; Dikalov, S.; et al. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H7–H12. [Google Scholar] [CrossRef][Green Version]
- Carlstrom, M.; Lai, E.Y.; Ma, Z.; Patzak, A.; Brown, R.D.; Persson, A.E. Role of NOX2 in the regulation of afferent arteriole responsiveness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R72–R79. [Google Scholar] [CrossRef]
- Wang, H.D.; Xu, S.; Johns, D.G.; Du, Y.; Quinn, M.T.; Cayatte, A.J.; Cohen, R.A. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 2001, 88, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.E.; Cifuentes, M.E.; Kiarash, A.; Quinn, M.T.; Pagano, P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ. Res. 2001, 89, 408–414. [Google Scholar] [CrossRef]
- Zimnol, A.; Spicker, N.; Balhorn, R.; Schroder, K.; Schupp, N. The NADPH Oxidase Isoform 1 Contributes to Angiotensin II-Mediated DNA Damage in the Kidney. Antioxidants 2020, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, G.; Banfi, B.; Deffert, C.; Fiette, L.; Schappi, M.; Herrmann, F.; Krause, K.H. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006, 580, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Mironova, E.; Archer, C.R.; Vendrov, A.E.; Runge, M.S.; Madamanchi, N.R.; Arendshorst, W.J.; Stockand, J.D.; Abd El-Aziz, T.M. NOXA1-dependent NADPH oxidase 1 signaling mediates angiotensin II activation of the epithelial sodium channel. Am. J. Physiol. Ren. Physiol. 2022, 323, F633–F641. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassegue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 2014, 20, 281–294. [Google Scholar] [CrossRef]
- Bendall, J.K.; Rinze, R.; Adlam, D.; Tatham, A.L.; de Bono, J.; Wilson, N.; Volpi, E.; Channon, K.M. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: Studies in endothelial-targeted Nox2 transgenic mice. Circ. Res. 2007, 100, 1016–1025. [Google Scholar] [CrossRef]
- Wang, Y.; Kuro-o, M.; Sun, Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 2012, 11, 410–417. [Google Scholar] [CrossRef]
- Haque, M.Z.; Majid, D.S. Reduced renal responses to nitric oxide synthase inhibition in mice lacking the gene for gp91phox subunit of NAD(P)H oxidase. Am. J. Physiol. Ren. Physiol. 2008, 295, F758–F764. [Google Scholar] [CrossRef][Green Version]
- Somanna, N.K.; Valente, A.J.; Krenz, M.; Fay, W.P.; Delafontaine, P.; Chandrasekar, B. The Nox1/4 Dual Inhibitor GKT137831 or Nox4 Knockdown Inhibits Angiotensin-II-Induced Adult Mouse Cardiac Fibroblast Proliferation and Migration. AT1 Physically Associates With Nox4. J. Cell. Physiol. 2016, 231, 1130–1141. [Google Scholar] [CrossRef]
- Cui, W.; Matsuno, K.; Iwata, K.; Ibi, M.; Katsuyama, M.; Kakehi, T.; Sasaki, M.; Ikami, K.; Zhu, K.; Yabe-Nishimura, C. NADPH oxidase isoforms and anti-hypertensive effects of atorvastatin demonstrated in two animal models. J. Pharmacol. Sci. 2009, 111, 260–268. [Google Scholar] [CrossRef]
- Lee, D.Y.; Wauquier, F.; Eid, A.A.; Roman, L.J.; Ghosh-Choudhury, G.; Khazim, K.; Block, K.; Gorin, Y. Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: Role of mitochondrial reactive oxygen species. J. Biol. Chem. 2013, 288, 28668–28686. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Furuyama, T.; Inoue, K.; Matsuda, D.; Matsubara, Y.; Okahara, A.; Ago, T.; Nakashima, Y.; Mori, M.; Matsumoto, T. Attenuation of Angiotensin II-Induced Hypertension in BubR1 Low-Expression Mice Via Repression of Angiotensin II Receptor 1 Overexpression. J. Am. Heart Assoc. 2019, 8, e011911. [Google Scholar] [CrossRef]
- Bouabout, G.; Ayme-Dietrich, E.; Jacob, H.; Champy, M.F.; Birling, M.C.; Pavlovic, G.; Madeira, L.; Fertak, L.E.; Petit-Demouliere, B.; Sorg, T.; et al. Nox4 genetic inhibition in experimental hypertension and metabolic syndrome. Arch. Cardiovasc. Dis. 2018, 111, 41–52. [Google Scholar] [CrossRef]
- Papinska, A.M.; Rodgers, K.E. Long-Term Administration of Angiotensin (1–7) to db/db Mice Reduces Oxidative Stress Damage in the Kidneys and Prevents Renal Dysfunction. Oxidative Med. Cell. Longev. 2018, 2018, 1841046. [Google Scholar] [CrossRef]
- Jaimes, E.A.; Tian, R.X.; Pearse, D.; Raij, L. Up-regulation of glomerular COX-2 by angiotensin II: Role of reactive oxygen species. Kidney Int. 2005, 68, 2143–2153. [Google Scholar] [CrossRef]
- Peshavariya, H.M.; Liu, G.S.; Chang, C.W.; Jiang, F.; Chan, E.C.; Dusting, G.J. Prostacyclin signaling boosts NADPH oxidase 4 in the endothelium promoting cytoprotection and angiogenesis. Antioxid. Redox Signal. 2014, 20, 2710–2725. [Google Scholar] [CrossRef]
- Jennings, B.L.; Anderson, L.J.; Estes, A.M.; Fang, X.R.; Song, C.Y.; Campbell, W.B.; Malik, K.U. Involvement of cytochrome P-450 1B1 in renal dysfunction, injury, and inflammation associated with angiotensin II-induced hypertension in rats. Am. J. Physiol. Ren. Physiol. 2012, 302, F408–F420. [Google Scholar] [CrossRef]
- Lara, L.S.; McCormack, M.; Semprum-Prieto, L.C.; Shenouda, S.; Majid, D.S.; Kobori, H.; Navar, L.G.; Prieto, M.C. AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II-salt hypertension. Am. J. Physiol. Ren. Physiol. 2012, 302, F85–F94. [Google Scholar] [CrossRef]
- Shimada, S.; Abais-Battad, J.M.; Alsheikh, A.J.; Yang, C.; Stumpf, M.; Kurth, T.; Mattson, D.L.; Cowley, A.W., Jr. Renal Perfusion Pressure Determines Infiltration of Leukocytes in the Kidney of Rats With Angiotensin II-Induced Hypertension. Hypertension 2020, 76, 849–858. [Google Scholar] [CrossRef]
- Shimada, S.; Yang, C.; Kumar, V.; Mattson, D.L.; Cowley, A.W., Jr. Acute Increase of Renal Perfusion Pressure Causes Rapid Activation of mTORC1 (Mechanistic Target Of Rapamycin Complex 1) and Leukocyte Infiltration. Hypertension 2022, 79, 1180–1189. [Google Scholar] [CrossRef]
- Kopkan, L.; Castillo, A.; Navar, L.G.; Majid, D.S. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am. J. Physiol. Ren. Physiol. 2006, 290, F80–F86. [Google Scholar] [CrossRef]
- Zhou, M.S.; Jaimes, E.A.; Raij, L. Inhibition of oxidative stress and improvement of endothelial function by amlodipine in angiotensin II-infused rats. Am. J. Hypertens. 2004, 17, 167–171. [Google Scholar] [CrossRef]
- Montanari, A.; Lazzeroni, D.; Pela, G.; Crocamo, A.; Lytvyn, Y.; Musiari, L.; Cabassi, A.; Cherney, D.Z.I. Calcium channel blockade blunts the renal effects of acute nitric oxide synthase inhibition in healthy humans. Am. J. Physiol. Ren. Physiol. 2017, 312, F870–F878. [Google Scholar] [CrossRef]
- Pech, V.; Sikka, S.C.; Sindhu, R.K.; Vaziri, N.D.; Majid, D.S. Oxidant stress and blood pressure responses to angiotensin II administration in rats fed varying salt diets. Am. J. Hypertens. 2006, 19, 534–540. [Google Scholar] [CrossRef]
- Ortiz, M.C.; Manriquez, M.C.; Romero, J.C.; Juncos, L.A. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension 2001, 38, 655–659. [Google Scholar] [CrossRef]
- Vera, T.; Kelsen, S.; Yanes, L.L.; Reckelhoff, J.F.; Stec, D.E. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1472–R1478. [Google Scholar] [CrossRef]
- Karbach, S.H.; Schonfelder, T.; Brandao, I.; Wilms, E.; Hormann, N.; Jackel, S.; Schuler, R.; Finger, S.; Knorr, M.; Lagrange, J.; et al. Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J. Am. Heart Assoc. 2016, 5, 9. [Google Scholar] [CrossRef]
- Avery, E.G.; Bartolomaeus, H.; Rauch, A.; Chen, C.Y.; N’Diaye, G.; Lober, U.; Bartolomaeus, T.U.P.; Fritsche-Guenther, R.; Rodrigues, A.F.; Yarritu, A.; et al. Quantifying the impact of gut microbiota on inflammation and hypertensive organ damage. Cardiovasc. Res. 2023, 119, 1441–1452. [Google Scholar] [CrossRef]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, F.; Zhou, Q.; Qiu, Y.; Zhang, J.; Tu, Q.; Zhou, Z.; Shao, Y.; Xu, S.; Wang, Y.; et al. Berberine Improves Vascular Dysfunction by Inhibiting Trimethylamine-N-oxide via Regulating the Gut Microbiota in Angiotensin II-Induced Hypertensive Mice. Front. Microbiol. 2022, 13, 814855. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.; Lu, A.; Liu, X.; Zhang, L.; Xu, C.; Liu, X.; Li, H.; Yang, T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J. Hypertens. 2017, 35, 1899–1908. [Google Scholar] [CrossRef]
- Armando, I.; Villar, V.A.; Jose, P.A. Genomics and Pharmacogenomics of Salt-sensitive Hypertension. Curr. Hypertens. Rev. 2015, 11, 49–56. [Google Scholar] [CrossRef]
- Majid, D.S.; Prieto, M.C.; Navar, L.G. Salt-Sensitive Hypertension: Perspectives on Intrarenal Mechanisms. Curr. Hypertens. Rev. 2015, 11, 38–48. [Google Scholar] [CrossRef]
- Tian, N.; Moore, R.S.; Braddy, S.; Rose, R.A.; Gu, J.W.; Hughson, M.D.; Manning, R.D., Jr. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3388–H3395. [Google Scholar] [CrossRef]
- Ando, K.; Fujita, T. Pathophysiology of salt sensitivity hypertension. Ann. Med. 2012, 44 (Suppl. S1), S119–S126. [Google Scholar] [CrossRef]
- Nishimoto, M.; Fujita, T. Renal mechanisms of salt-sensitive hypertension: Contribution of two steroid receptor-associated pathways. Am. J. Physiol. Ren. Physiol. 2015, 308, F377–F387. [Google Scholar] [CrossRef]
- Rapp, J.P. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 1982, 4, 753–763. [Google Scholar] [CrossRef]
- Dahl, L.K.; Heine, M.; Tassinari, L. Role of genetic factors in susceptibility to experimental hypertension due to chronic excess salt ingestion. Nature 1962, 194, 480–482. [Google Scholar] [CrossRef]
- Churchill, P.C.; Churchill, M.C.; Bidani, A.K. Kidney cross transplants in Dahl salt-sensitive and salt-resistant rats. Am. J. Physiol. Heart Circ. Physiol. 1992, 262, H1809–H1817. [Google Scholar] [CrossRef]
- Knudsen, K.D.; Dahl, L.K.; Thompson, K.; Iwai, J.; Heine, M.; Leitl, G. Effects of chronic excess salt ingestion. Inheritance of hypertension in the rat. J. Exp. Med. 1970, 132, 976–1000. [Google Scholar] [CrossRef]
- Rapp, J.P.; Dene, H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension 1985, 7, 340–349. [Google Scholar] [CrossRef]
- Chandramohan, G.; Bai, Y.; Norris, K.; Rodriguez-Iturbe, B.; Vaziri, N.D. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am. J. Nephrol. 2008, 28, 158–167. [Google Scholar] [CrossRef]
- Manning, R.D., Jr.; Tian, N.; Meng, S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am. J. Nephrol. 2005, 25, 311–317. [Google Scholar] [CrossRef]
- Jaimes, E.A.; Zhou, M.S.; Pearse, D.D.; Puzis, L.; Raij, L. Upregulation of cortical COX-2 in salt-sensitive hypertension: Role of angiotensin II and reactive oxygen species. Am. J. Physiol. Ren. Physiol. 2008, 294, F385–F392. [Google Scholar] [CrossRef]
- Tian, N.; Moore, R.S.; Phillips, W.E.; Lin, L.; Braddy, S.; Pryor, J.S.; Stockstill, R.L.; Hughson, M.D.; Manning, R.D., Jr. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1858–R1865. [Google Scholar] [CrossRef]
- Meng, S.; Roberts, L.J., 2nd; Cason, G.W.; Curry, T.S.; Manning, R.D., Jr. Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 2002, 283, R732–R738. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, J.H.; Noh, J.R.; Gang, G.T.; Tadi, S.; Yim, Y.H.; Jeoung, N.H.; Kwak, T.H.; Lee, S.H.; Kweon, G.R.; et al. Prevention of salt-induced renal injury by activation of NAD(P)H:quinone oxidoreductase 1, associated with NADPH oxidase. Free. Radic. Biol. Med. 2012, 52, 880–888. [Google Scholar] [CrossRef]
- Kobori, H.; Mori, H.; Masaki, T.; Nishiyama, A. Angiotensin II blockade and renal protection. Curr. Pharm. Des. 2013, 19, 3033–3042. [Google Scholar] [CrossRef]
- Gong, J.; Luo, M.; Yong, Y.; Zhong, S.; Li, P. Alamandine alleviates hypertension and renal damage via oxidative-stress attenuation in Dahl rats. Cell Death Discov. 2022, 8, 22. [Google Scholar] [CrossRef]
- Kawarazaki, H.; Ando, K.; Fujita, M.; Matsui, H.; Nagae, A.; Muraoka, K.; Kawarasaki, C.; Fujita, T. Mineralocorticoid receptor activation: A major contributor to salt-induced renal injury and hypertension in young rats. Am. J. Physiol. Ren. Physiol. 2011, 300, F1402–F1409. [Google Scholar] [CrossRef][Green Version]
- Bayorh, M.A.; Rollins-Hairston, A.; Adiyiah, J.; Lyn, D.; Eatman, D. Eplerenone suppresses aldosterone/ salt-induced expression of NOX-4. J. Renin Angiotensin Aldosterone Syst. 2011, 12, 195–201. [Google Scholar] [CrossRef]
- Takeda, Y. Effects of eplerenone, a selective mineralocorticoid receptor antagonist, on clinical and experimental salt-sensitive hypertension. Hypertens. Res. 2009, 32, 321–324. [Google Scholar] [CrossRef]
- Zhou, X.; Crook, M.F.; Sharif-Rodriguez, W.; Zhu, Y.; Ruben, Z.; Pan, Y.; Urosevic-Price, O.; Wang, L.; Flattery, A.M.; Forrest, G.; et al. Chronic antagonism of the mineralocorticoid receptor ameliorates hypertension and end organ damage in a rodent model of salt-sensitive hypertension. Clin. Exp. Hypertens. 2011, 33, 538–547. [Google Scholar] [CrossRef]
- Chade, A.R.; Rodriguez-Porcel, M.; Herrmann, J.; Zhu, X.; Grande, J.P.; Napoli, C.; Lerman, A.; Lerman, L.O. Antioxidant intervention blunts renal injury in experimental renovascular disease. J. Am. Soc. Nephrol. 2004, 15, 958–966. [Google Scholar] [CrossRef]
- Leibowitz, A.; Volkov, A.; Voloshin, K.; Shemesh, C.; Barshack, I.; Grossman, E. Melatonin prevents kidney injury in a high salt diet-induced hypertension model by decreasing oxidative stress. J. Pineal Res. 2016, 60, 48–54. [Google Scholar] [CrossRef]
- Huang, P.; Shen, Z.; Liu, J.; Huang, Y.; Chen, S.; Yu, W.; Wang, S.; Ren, Y.; Li, X.; Tang, C.; et al. Hydrogen Sulfide Inhibits High-Salt Diet-Induced Renal Oxidative Stress and Kidney Injury in Dahl Rats. Oxidative Med. Cell. Longev. 2016, 2016, 2807490. [Google Scholar] [CrossRef]
- Ikeda, Y.; Saito, K.; Kim, J.I.; Yokoyama, M. Nitric oxide synthase isoform activities in kidney of Dahl salt-sensitive rats. Hypertension 1995, 26, 1030–1034. [Google Scholar] [CrossRef]
- Barton, M.; Vos, I.; Shaw, S.; Boer, P.; D’Uscio, L.V.; Grone, H.J.; Rabelink, T.J.; Lattmann, T.; Moreau, P.; Luscher, T.F. Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension: Mechanisms of renal artery endothelial dysfunction and role of endothelin for vascular hypertrophy and Glomerulosclerosis. J. Am. Soc. Nephrol. 2000, 11, 835–845. [Google Scholar] [CrossRef]
- Castrop, H.; Kurtz, A. Differential nNOS gene expression in salt-sensitive and salt-resistant Dahl rats. J. Hypertens. 2001, 19, 1223–1231. [Google Scholar] [CrossRef]
- Bayorh, M.A.; Ganafa, A.A.; Eatman, D.; Walton, M.; Feuerstein, G.Z. Simvastatin and losartan enhance nitric oxide and reduce oxidative stress in salt-induced hypertension. Am. J. Hypertens. 2005, 18, 1496–1502. [Google Scholar] [CrossRef]
- Wen, Y.; Rudemiller, N.P.; Zhang, J.; Jeffs, A.D.; Griffiths, R.; Lu, X.; Ren, J.; Privratsky, J.; Crowley, S.D. Stimulating Type 1 Angiotensin Receptors on T Lymphocytes Attenuates Renal Fibrosis. Am. J. Pathol. 2019, 189, 981–988. [Google Scholar] [CrossRef]
- Chen, P.Y.; Sanders, P.W. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J. Clin. Investig. 1991, 88, 1559–1567. [Google Scholar] [CrossRef]
- Zhou, M.S.; Kosaka, H.; Tian, R.X.; Abe, Y.; Chen, Q.H.; Yoneyama, H.; Yamamoto, A.; Zhang, L. L-Arginine improves endothelial function in renal artery of hypertensive Dahl rats. J. Hypertens. 2001, 19, 421–429. [Google Scholar] [CrossRef]
- Fujii, S.; Zhang, L.; Igarashi, J.; Kosaka, H. L-arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension 2003, 42, 1014–1020. [Google Scholar] [CrossRef]
- Tashiro, Y.; Yogo, K.; Serizawa, K.; Endo, K. Nicorandil suppresses urinary protein excretion and activates eNOS in Dahl salt-sensitive hypertensive rats. Clin. Exp. Nephrol. 2015, 19, 343–349. [Google Scholar] [CrossRef]
- Kataoka, H.; Otsuka, F.; Ogura, T.; Yamauchi, T.; Kishida, M.; Takahashi, M.; Mimura, Y.; Makino, H. The role of nitric oxide and the renin-angiotensin system in salt-restricted Dahl rats. Am. J. Hypertens. 2001, 14, 276–285. [Google Scholar] [CrossRef][Green Version]
- Ni, Z.; Oveisi, F.; Vaziri, N.D. Nitric oxide synthase isotype expression in salt-sensitive and salt-resistant Dahl rats. Hypertension 1999, 34, 552–557. [Google Scholar] [CrossRef]
- Johns, E.J.; O’Shaughnessy, B.; O’Neill, S.; Lane, B.; Healy, V. Impact of elevated dietary sodium intake on NAD(P)H oxidase and SOD in the cortex and medulla of the rat kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R234–R240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Q.; Sun, N.; Liang, M.; Tian, Z. Mitochondrial Dysfunction and Altered Renal Metabolism in Dahl Salt-Sensitive Rats. Kidney Blood Press. Res. 2017, 42, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.; Asico, L.D.; Villar, V.A.M.; Zheng, X.; Cuevas, S.; Armando, I.; Jose, P.A.; Wang, X. Increased renal oxidative stress in salt-sensitive human GRK4gamma486V transgenic mice. Free. Radic. Biol. Med. 2017, 106, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.W., Jr.; Yang, C.; Zheleznova, N.N.; Staruschenko, A.; Kurth, T.; Rein, L.; Kumar, V.; Sadovnikov, K.; Dayton, A.; Hoffman, M.; et al. Evidence of the Importance of Nox4 in Production of Hypertension in Dahl Salt-Sensitive Rats. Hypertension 2016, 67, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kurth, T.; Zheleznova, N.N.; Yang, C.; Cowley, A.W., Jr. NOX4/H(2)O(2)/mTORC1 Pathway in Salt-Induced Hypertension and Kidney Injury. Hypertension 2020, 76, 133–143. [Google Scholar] [CrossRef]
- Pavlov, T.S.; Palygin, O.; Isaeva, E.; Levchenko, V.; Khedr, S.; Blass, G.; Ilatovskaya, D.V.; Cowley, A.W., Jr.; Staruschenko, A. NOX4-dependent regulation of ENaC in hypertension and diabetic kidney disease. FASEB J. 2020, 34, 13396–13408. [Google Scholar] [CrossRef]
- Tian, N.; Thrasher, K.D.; Gundy, P.D.; Hughson, M.D.; Manning, R.D., Jr. Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension 2005, 45, 934–939. [Google Scholar] [CrossRef]
- Adejare, A.; Oloyo, A.; Anigbogu, C.; Jaja, S. l-arginine Supplementation Increased Only Endothelium-Dependent Relaxation in Sprague-Dawley Rats Fed a High-Salt Diet by Enhancing Abdominal Aorta Endothelial Nitric Oxide Synthase Gene Expression. Clin. Med. Insights: Cardiol. 2020, 14, 1179546820902843. [Google Scholar] [CrossRef]
- Cappetta, D.; Ciuffreda, L.P.; Cozzolino, A.; Esposito, G.; Scavone, C.; Sapio, L.; Naviglio, S.; D’Amario, D.; Crea, F.; Rossi, F.; et al. Dipeptidyl Peptidase 4 Inhibition Ameliorates Chronic Kidney Disease in a Model of Salt-Dependent Hypertension. Oxidative Med. Cell. Longev. 2019, 2019, 8912768. [Google Scholar] [CrossRef]
- Manger, W.M.; Simchon, S.; Stier, C.T., Jr.; Loscalzo, J.; Jan, K.M.; Jan, R.; Haddy, F. Protective effects of dietary potassium chloride on hemodynamics of Dahl salt-sensitive rats in response to chronic administration of sodium chloride. J. Hypertens. 2003, 21, 2305–2313. [Google Scholar] [CrossRef]
- Zhou, M.S.; Schuman, I.H.; Jaimes, E.A.; Raij, L. Renoprotection by statins is linked to a decrease in renal oxidative stress, TGF-beta, and fibronectin with concomitant increase in nitric oxide bioavailability. Am. J. Physiol. Ren. Physiol. 2008, 295, F53–F59. [Google Scholar] [CrossRef] [PubMed]
- Abais-Battad, J.M.; Saravia, F.L.; Lund, H.; Dasinger, J.H.; Fehrenbach, D.J.; Alsheikh, A.J.; Zemaj, J.; Kirby, J.R.; Mattson, D.L. Dietary influences on the Dahl SS rat gut microbiota and its effects on salt-sensitive hypertension and renal damage. Acta Physiol. 2021, 232, e13662. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Braun, T.; Khasbab, R.; Di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients 2018, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Galla, S.; Cheng, X.; Yeo, J.Y.; Mell, B.; Singh, V.; Yeoh, B.; Saha, P.; Mathew, A.V.; Vijay-Kumar, M.; et al. Salt-Responsive Metabolite, beta-Hydroxybutyrate, Attenuates Hypertension. Cell Rep. 2018, 25, 677–689.e4. [Google Scholar] [CrossRef]
- Khanna, H.D.; Sinha, M.K.; Khanna, S.; Tandon, R. Oxidative stress in hypertension: Association with antihypertensive treatment. Indian J. Physiol. Pharmacol. 2008, 52, 283–287. [Google Scholar]
- Duni, A.; Dounousi, E.; Pavlakou, P.; Eleftheriadis, T.; Liakopoulos, V. Hypertension in Chronic Kidney Disease: Novel Insights. Curr. Hypertens. Rev. 2020, 16, 45–54. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Mistry, N.; Bakris, G.L. The changing trajectory of diabetic kidney disease. Curr. Opin. Nephrol. Hypertens. 2023, 32, 98–102. [Google Scholar] [CrossRef]
- Colbert, G.B.; Elrggal, M.E.; Gaddy, A.; Madariaga, H.M.; Lerma, E.V. Management of Hypertension in Diabetic Kidney Disease. J. Clin. Med. 2023, 12, 6868. [Google Scholar] [CrossRef]
- O’Hara, D.V.; Lam, C.S.P.; McMurray, J.J.V.; Yi, T.W.; Hocking, S.; Dawson, J.; Raichand, S.; Januszewski, A.S.; Jardine, M.J. Applications of SGLT2 inhibitors beyond glycaemic control. Nat. Rev. Nephrol. 2024, 20, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Shaheen, A.; Diab, R.A.; Desouki, M.T. MicroRNAs (miRNAs) role in hypertension: Pathogenesis and promising therapeutics. Ann. Med. Surg. 2024, 86, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Sowers, J.R. Hypertension in Diabetes: An Update of Basic Mechanisms and Clinical Disease. Hypertension 2021, 78, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, O.; Bohovyk, R.; Levchenko, V.; Palygin, O.; Klemens, C.A.; Rieg, T.; Staruschenko, A. SGLT2 inhibition effect on salt-induced hypertension, RAAS, and Na+ transport in Dahl SS rats. Am. J. Physiol. Ren. Physiol. 2022, 322, F692–F707. [Google Scholar] [CrossRef]
- Wilcox, C.S. Antihypertensive and Renal Mechanisms of SGLT2 (Sodium-Glucose Linked Transporter 2) Inhibitors. Hypertension 2020, 75, 894–901. [Google Scholar] [CrossRef]
- Damianaki, A.; Polychronopoulou, E.; Wuerzner, G.; Burnier, M. New Aspects in the Management of Hypertension in Patients with Chronic Kidney Disease not on Renal Replacement Therapy. High Blood Press. Cardiovasc. Prev. 2022, 29, 125–135. [Google Scholar] [CrossRef]
- Song, P.; Huang, W.; Onishi, A.; Patel, R.; Kim, Y.C.; van Ginkel, C.; Fu, Y.; Freeman, B.; Koepsell, H.; Thomson, S.; et al. Knockout of Na(+)-glucose cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase NOS1 in the macula densa and glomerular hyperfiltration. Am. J. Physiol. Ren. Physiol. 2019, 317, F207–F217. [Google Scholar] [CrossRef]
- Llorens-Cebria, C.; Molina-Van den Bosch, M.; Vergara, A.; Jacobs-Cacha, C.; Soler, M.J. Antioxidant Roles of SGLT2 Inhibitors in the Kidney. Biomolecules 2022, 12, 173. [Google Scholar] [CrossRef]
- Brown, E.; Wilding, J.P.H.; Alam, U.; Barber, T.M.; Karalliedde, J.; Cuthbertson, D.J. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann. Med. 2021, 53, 2072–2089. [Google Scholar] [CrossRef]
- Briasoulis, A.; Al Dhaybi, O.; Bakris, G.L. SGLT2 Inhibitors and Mechanisms of Hypertension. Curr. Cardiol. Rep. 2018, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Delic, D.; Cao, Y.; Shen, L.; Shao, Q.; Zhang, Z.; Wu, H.; Hasan, A.A.; Reichetzeder, C.; Gaballa, M.M.S.; et al. Renoprotective effects of empagliflozin are linked to activation of the tubuloglomerular feedback mechanism and blunting of the complement system. Am. J. Physiol. Cell Physiol. 2023, 324, C951–C962. [Google Scholar] [CrossRef] [PubMed]
- Oe, Y.; Vallon, V. The Pathophysiological Basis of Diabetic Kidney Protection by Inhibition of SGLT2 and SGLT1. Kidney Dial. 2022, 2, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Laursen, J.C.; Sondergaard-Heinrich, N.; de Melo, J.M.L.; Haddock, B.; Rasmussen, I.K.B.; Safavimanesh, F.; Hansen, C.S.; Storling, J.; Larsson, H.B.W.; Groop, P.H.; et al. Acute effects of dapagliflozin on renal oxygenation and perfusion in type 1 diabetes with albuminuria: A randomised, double-blind, placebo-controlled crossover trial. eClinicalMedicine 2021, 37, 100895. [Google Scholar] [CrossRef] [PubMed]
- Croteau, D.; Luptak, I.; Chambers, J.M.; Hobai, I.; Panagia, M.; Pimentel, D.R.; Siwik, D.A.; Qin, F.; Colucci, W.S. Effects of Sodium-Glucose Linked Transporter 2 Inhibition With Ertugliflozin on Mitochondrial Function, Energetics, and Metabolic Gene Expression in the Presence and Absence of Diabetes Mellitus in Mice. J. Am. Heart Assoc. 2021, 10, e019995. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Thomson, S.C.; Vallon, V. Effects of SGLT2 inhibitor and dietary NaCl on glomerular hemodynamics assessed by micropuncture in diabetic rats. Am. J. Physiol. Ren. Physiol. 2021, 320, F761–F771. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, S.H.; Kang, J.M.; Heo, J.H.; Kim, D.J.; Park, S.H.; Sung, M.; Kim, J.; Oh, J.; Yang, D.H.; et al. Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy. Am. J. Physiol. Ren. Physiol. 2019, 317, F767–F780. [Google Scholar] [CrossRef]
- Elkazzaz, S.K.; Khodeer, D.M.; El Fayoumi, H.M.; Moustafa, Y.M. Role of sodium glucose cotransporter type 2 inhibitors dapagliflozin on diabetic nephropathy in rats; Inflammation, angiogenesis and apoptosis. Life Sci. 2021, 280, 119018. [Google Scholar] [CrossRef]
- Das, N.A.; Carpenter, A.J.; Belenchia, A.; Aroor, A.R.; Noda, M.; Siebenlist, U.; Chandrasekar, B.; DeMarco, V.G. Empagliflozin reduces high glucose-induced oxidative stress and miR-21-dependent TRAF3IP2 induction and RECK suppression, and inhibits human renal proximal tubular epithelial cell migration and epithelial-to-mesenchymal transition. Cell. Signal. 2020, 68, 109506. [Google Scholar] [CrossRef]
- Zhou, Y.; Tai, S.; Zhang, N.; Fu, L.; Wang, Y. Dapagliflozin prevents oxidative stress-induced endothelial dysfunction via sirtuin 1 activation. Biomed. Pharmacother. 2023, 165, 115213. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.A.; Anghel, R.; Marcu, D.T.M.; Mitu, O.; Roca, M.; Mitu, F. Impact of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors on Arterial Stiffness and Vascular Aging-What Do We Know So Far? (A Narrative Review). Life 2022, 12, 803. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Zerin, F.; Menon, S.N.; Alam, M.A.; Hasan, R. Mechanism of canagliflozin-induced vasodilation in resistance mesenteric arteries and the regulation of systemic blood pressure. J. Pharmacol. Sci. 2022, 150, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sawami, K.; Tanaka, A.; Node, K. Recent understandings about hypertension management in type 2 diabetes: What are the roles of SGLT2 inhibitor, GLP-1 receptor agonist, and finerenone? Hypertens. Res. 2023, 46, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Kogot-Levin, A.; Hinden, L.; Riahi, Y.; Israeli, T.; Tirosh, B.; Cerasi, E.; Mizrachi, E.B.; Tam, J.; Mosenzon, O.; Leibowitz, G. Proximal Tubule mTORC1 Is a Central Player in the Pathophysiology of Diabetic Nephropathy and Its Correction by SGLT2 Inhibitors. Cell Rep. 2020, 32, 107954. [Google Scholar] [CrossRef]
- Tomita, I.; Kume, S.; Sugahara, S.; Osawa, N.; Yamahara, K.; Yasuda-Yamahara, M.; Takeda, N.; Chin-Kanasaki, M.; Kaneko, T.; Mayoux, E.; et al. SGLT2 Inhibition Mediates Protection from Diabetic Kidney Disease by Promoting Ketone Body-Induced mTORC1 Inhibition. Cell Metab. 2020, 32, 404–419.e6. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney disease—Mechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar] [CrossRef]
- Mende, C.W.; Samarakoon, R.; Higgins, P.J. Mineralocorticoid Receptor-Associated Mechanisms in Diabetic Kidney Disease and Clinical Significance of Mineralocorticoid Receptor Antagonists. Am. J. Nephrol. 2023, 54, 50–61. [Google Scholar] [CrossRef]
- Yao, L.; Liang, X.; Wang, P. Therapeutic perspective: Evolving evidence of nonsteroidal mineralocorticoid receptor antagonists in diabetic kidney disease. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E531–E541. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Agarwal, R. The Nonsteroidal Mineralocorticoid-Receptor-Antagonist Finerenone in Cardiorenal Medicine: A State-of-the-Art Review of the Literature. Am. J. Hypertens. 2023, 36, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Gohda, T.; Kantharidis, P.; Okabe, J.; Murakoshi, M.; Suzuki, Y. Potential of Modulating Aldosterone Signaling and Mineralocorticoid Receptor with microRNAs to Attenuate Diabetic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 869. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Long, X.; Du, J.; Yuan, X. RNA-Seq transcriptomic landscape profiling of spontaneously hypertensive rats treated with a sodium-glucose cotransporter 2 (SGLT2) inhibitor. Biomed. Pharmacother. 2023, 166, 115289. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Tan, F.; Long, X.; Fang, Q.; Wang, Y.; Wang, J.; He, J.; Yuan, X.; Du, J. RNA-Seq transcriptome analysis of renal tissue from spontaneously hypertensive rats revealed renal protective effects of dapagliflozin, an inhibitor of sodium-glucose cotransporter 2. Eur. J. Pharm. Sci. 2023, 189, 106531. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Nakano, D.; Nishiyama, A. Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System. Int. J. Mol. Sci. 2019, 20, 629. [Google Scholar] [CrossRef]
- Puglisi, S.; Rossini, A.; Poli, R.; Dughera, F.; Pia, A.; Terzolo, M.; Reimondo, G. Effects of SGLT2 Inhibitors and GLP-1 Receptor Agonists on Renin-Angiotensin-Aldosterone System. Front. Endocrinol. 2021, 12, 738848. [Google Scholar] [CrossRef]
- Castoldi, G.; Carletti, R.; Ippolito, S.; Colzani, M.; Pelucchi, S.; Zerbini, G.; Perseghin, G.; Zatti, G.; di Gioia, C.R.T. Cardioprotective Effects of Sodium Glucose Cotransporter 2 Inhibition in Angiotensin II-Dependent Hypertension Are Mediated by the Local Reduction of Sympathetic Activity and Inflammation. Int. J. Mol. Sci. 2023, 24, 10710. [Google Scholar] [CrossRef]
- Miyata, K.N.; Lo, C.S.; Zhao, S.; Liao, M.C.; Pang, Y.; Chang, S.Y.; Peng, J.; Kretzler, M.; Filep, J.G.; Ingelfinger, J.R.; et al. Angiotensin II up-regulates sodium-glucose co-transporter 2 expression and SGLT2 inhibitor attenuates Ang II-induced hypertensive renal injury in mice. Clin. Sci. 2021, 135, 943–961. [Google Scholar] [CrossRef]
- Bruckert, C.; Matsushita, K.; Mroueh, A.; Amissi, S.; Auger, C.; Houngue, U.; Remila, L.; Chaker, A.B.; Park, S.H.; Algara-Suarez, P.; et al. Empagliflozin prevents angiotensin II-induced hypertension related micro and macrovascular endothelial cell activation and diastolic dysfunction in rats despite persistent hypertension: Role of endothelial SGLT1 and 2. Vasc. Pharmacol. 2022, 146, 107095. [Google Scholar] [CrossRef]
- Reyes-Pardo, H.; Bautista, R.; Vargas-Robles, H.; Rios, A.; Sanchez, D.; Escalante, B. Role of sodium/glucose cotransporter inhibition on a rat model of angiotensin II-dependent kidney damage. BMC Nephrol. 2019, 20, 292. [Google Scholar] [CrossRef]
- Kolkhof, P.; Hartmann, E.; Freyberger, A.; Pavkovic, M.; Mathar, I.; Sandner, P.; Droebner, K.; Joseph, A.; Huser, J.; Eitner, F. Effects of Finerenone Combined with Empagliflozin in a Model of Hypertension-Induced End-Organ Damage. Am. J. Nephrol. 2021, 52, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Bao, P.; Yao, S.; Zhang, X.; Mu, J.J.; Hu, G.L.; Du, M.F.; Chu, C.; Zhang, X.Y.; Wang, L.; et al. Associations of SGLT2 genetic polymorphisms with salt sensitivity, blood pressure changes and hypertension incidence in Chinese adults. Hypertens. Res. 2023, 46, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, O.; Levchenko, V.; Klemens, C.A.; Rieg, T.; Liu, R.; Staruschenko, A. Effect of SGLT2 inhibition on salt-induced hypertension in female Dahl SS rats. Sci. Rep. 2023, 13, 19231. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, C.H.; Kim, G.H. Effects of empagliflozin on nondiabetic salt-sensitive hypertension in uninephrectomized rats. Hypertens. Res. 2019, 42, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Fujisawa, Y.; Kobara, H.; Masaki, T.; Nakano, D.; Rahman, A.; Nishiyama, A. Effects of an SGLT2 inhibitor on the salt sensitivity of blood pressure and sympathetic nerve activity in a nondiabetic rat model of chronic kidney disease. Hypertens. Res. 2020, 43, 492–499. [Google Scholar] [CrossRef]
- Fujita, M.; Ando, K.; Kawarazaki, H.; Kawarasaki, C.; Muraoka, K.; Ohtsu, H.; Shimizu, H.; Fujita, T. Sympathoexcitation by brain oxidative stress mediates arterial pressure elevation in salt-induced chronic kidney disease. Hypertension 2012, 59, 105–112. [Google Scholar] [CrossRef]
- Hattori, M.; Rahman, A.; Kidoguchi, S.; Jahan, N.; Fujisawa, Y.; Morisawa, N.; Ohsaki, H.; Kobara, H.; Masaki, T.; Hossain, A.; et al. Association of Antihypertensive Effects of Esaxerenone with the Internal Sodium Balance in Dahl Salt-Sensitive Hypertensive Rats. Int. J. Mol. Sci. 2022, 23, 8915. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, J.; Zhang, T.; He, L.; Ma, S.; Zuo, Q.; Zhang, G.; Wang, Y.; Guo, Y. Canagliflozin ameliorates epithelial-mesenchymal transition in high-salt diet-induced hypertensive renal injury through restoration of sirtuin 3 expression and the reduction of oxidative stress. Biochem. Biophys. Res. Commun. 2023, 653, 53–61. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, Z.; Zhang, T.; He, L.; Ma, S.; Zuo, Q.; Zhang, G.; Wang, X.; Guo, Y. Canagliflozin and irbesartan ameliorate renal fibrosis via the TGF-beta1/Smad signaling pathway in Dahl salt-sensitive rats. J. Int. Med Res. 2023, 51, 3000605231206289. [Google Scholar] [CrossRef]
- Ito, H.; Okamoto, R.; Ali, Y.; Zhe, Y.; Katayama, K.; Ito, M.; Dohi, K. Cardiorenal protective effects of sodium-glucose cotransporter 2 inhibition in combination with angiotensin II type 1 receptor blockade in salt-sensitive Dahl rats. J. Hypertens. 2022, 40, 956–968. [Google Scholar] [CrossRef]
- Patera, F.; Gatticchi, L.; Cellini, B.; Chiasserini, D.; Reboldi, G. Kidney Fibrosis and Oxidative Stress: From Molecular Pathways to New Pharmacological Opportunities. Biomolecules 2024, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akazawa, K.; Saito, Y.; Akagi, S.; Ohno, Y.; Kondo, M.; Miura, D.; et al. Inhibitory Effects of Tofogliflozin on Cardiac Hypertrophy in Dahl Salt-Sensitive and Salt-Resistant Rats Fed a High-Fat Diet. Int. Heart J. 2019, 60, 728–735. [Google Scholar] [CrossRef] [PubMed]


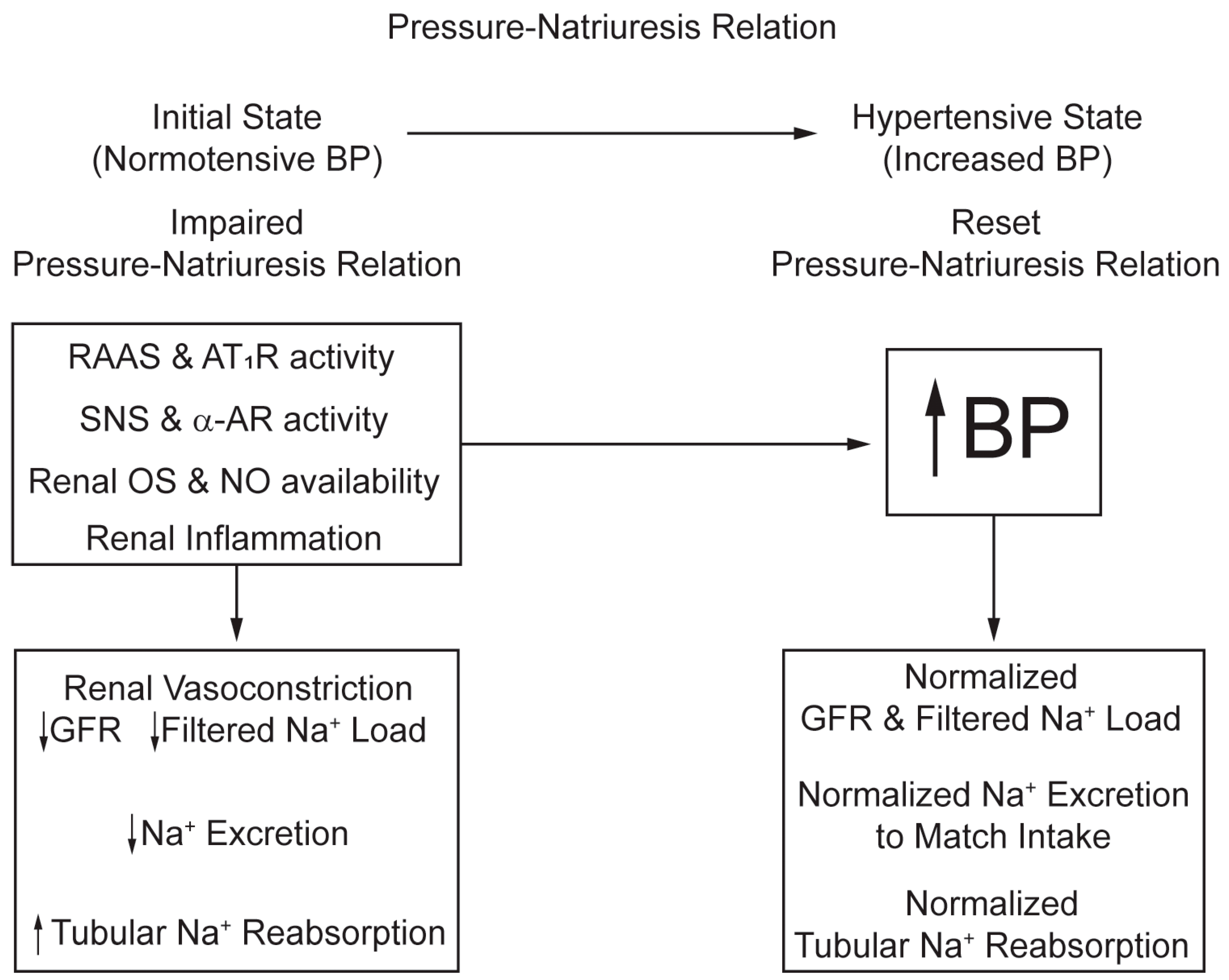
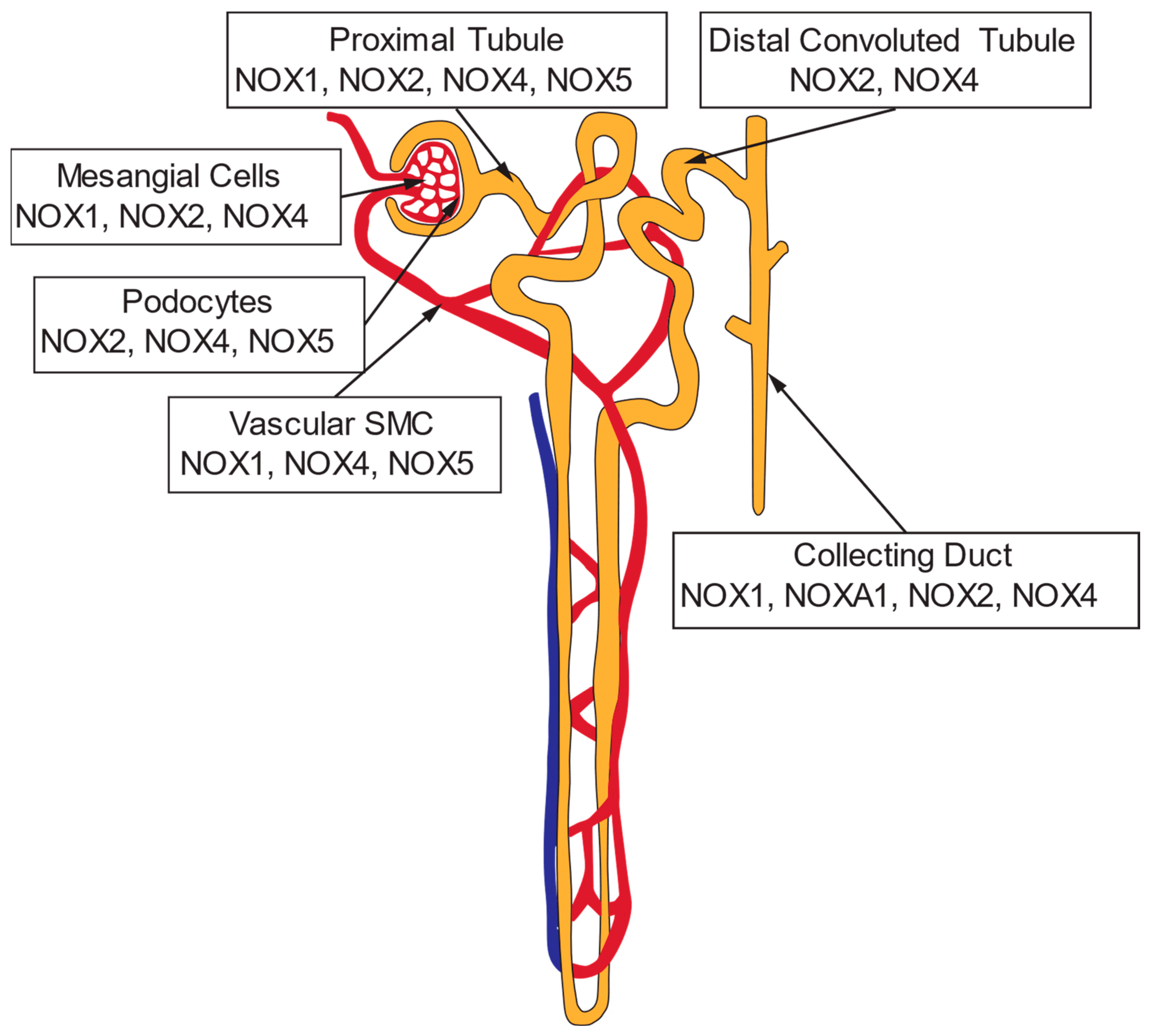
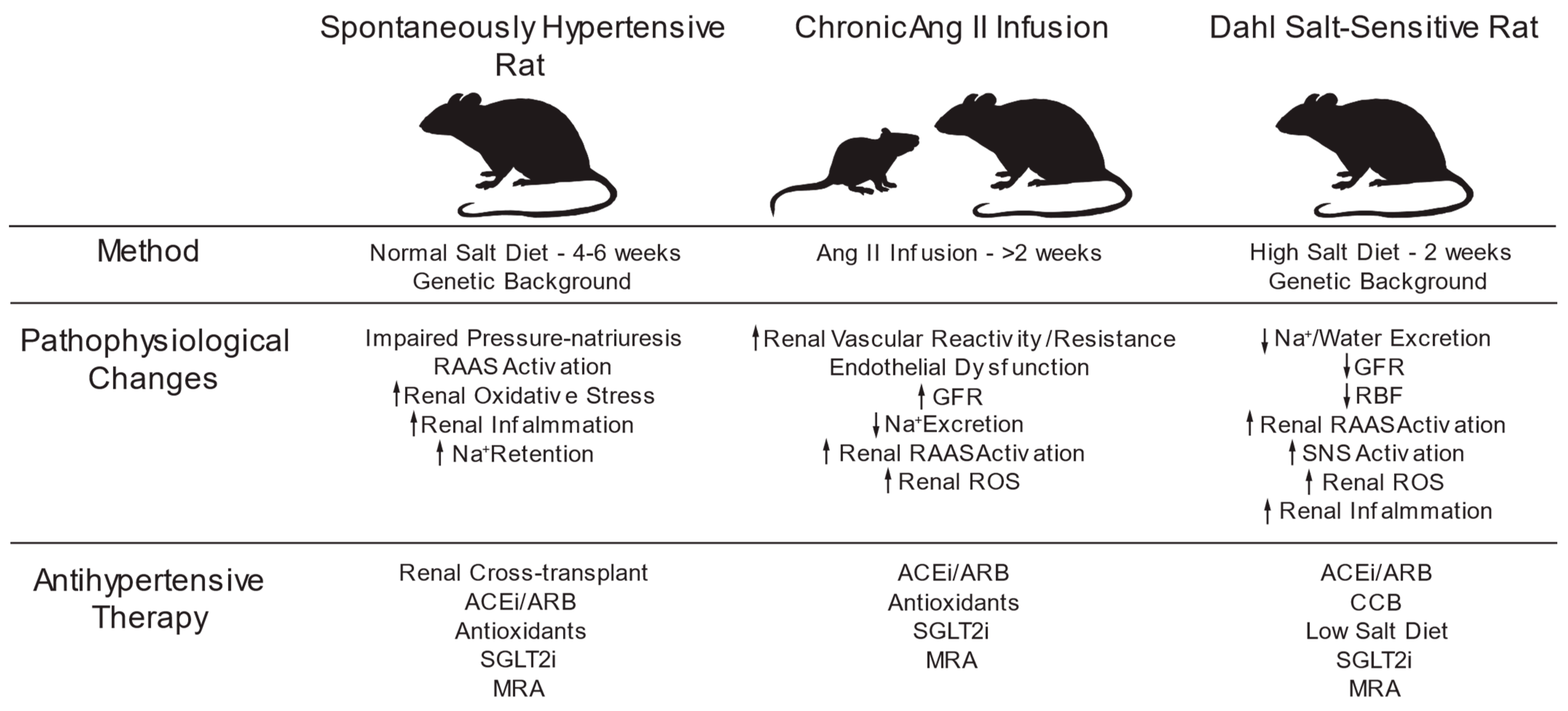

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arendshorst, W.J.; Vendrov, A.E.; Kumar, N.; Ganesh, S.K.; Madamanchi, N.R. Oxidative Stress in Kidney Injury and Hypertension. Antioxidants 2024, 13, 1454. https://doi.org/10.3390/antiox13121454
Arendshorst WJ, Vendrov AE, Kumar N, Ganesh SK, Madamanchi NR. Oxidative Stress in Kidney Injury and Hypertension. Antioxidants. 2024; 13(12):1454. https://doi.org/10.3390/antiox13121454
Chicago/Turabian StyleArendshorst, Willaim J., Aleksandr E. Vendrov, Nitin Kumar, Santhi K. Ganesh, and Nageswara R. Madamanchi. 2024. "Oxidative Stress in Kidney Injury and Hypertension" Antioxidants 13, no. 12: 1454. https://doi.org/10.3390/antiox13121454
APA StyleArendshorst, W. J., Vendrov, A. E., Kumar, N., Ganesh, S. K., & Madamanchi, N. R. (2024). Oxidative Stress in Kidney Injury and Hypertension. Antioxidants, 13(12), 1454. https://doi.org/10.3390/antiox13121454






