Fibrinogen Structural Changes and Their Potential Role in Endometriosis-Related Thrombosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Sample Collection and Fibrinogen Purification
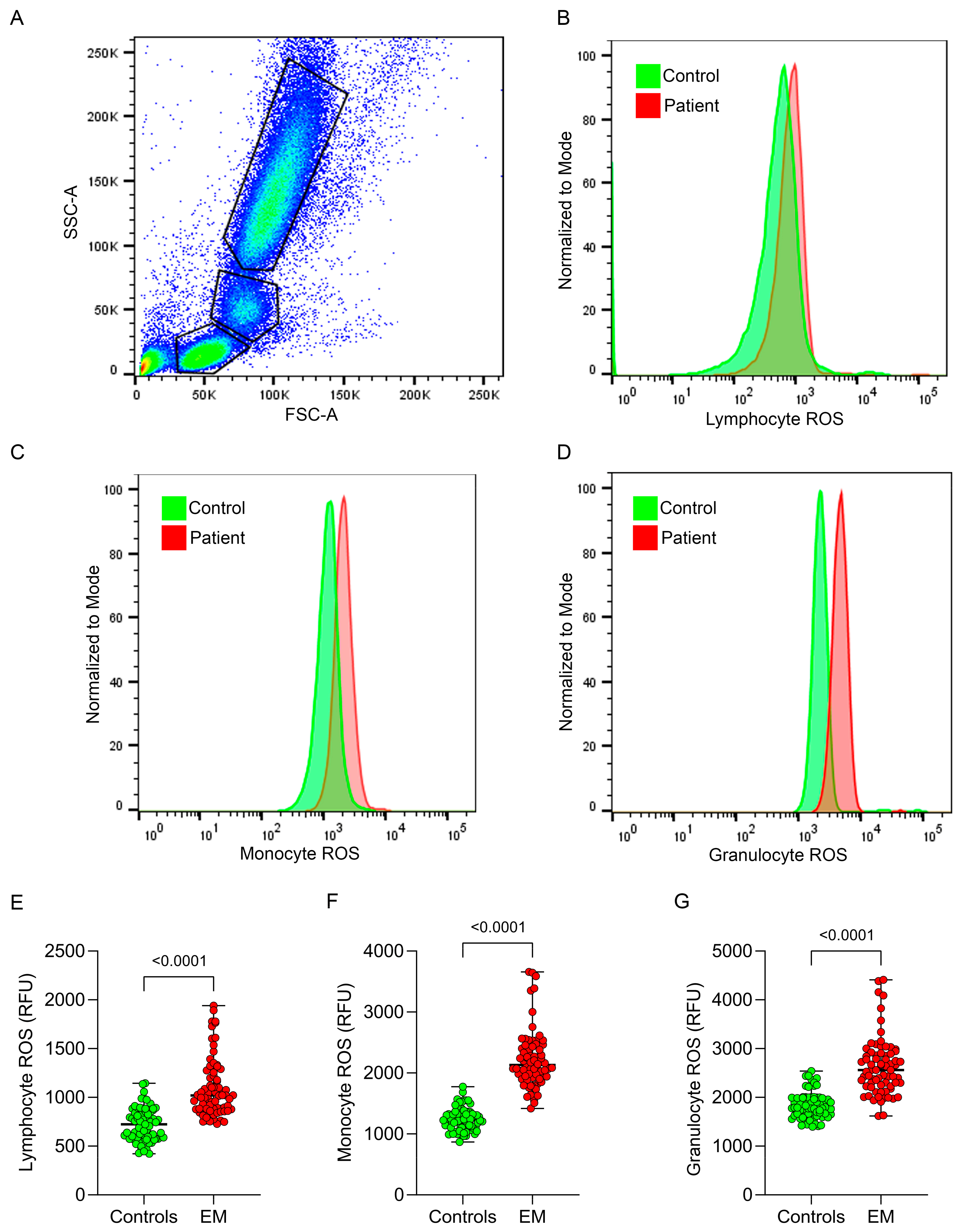
2.3. Measurement of ROS Production in Peripheral Leukocytes
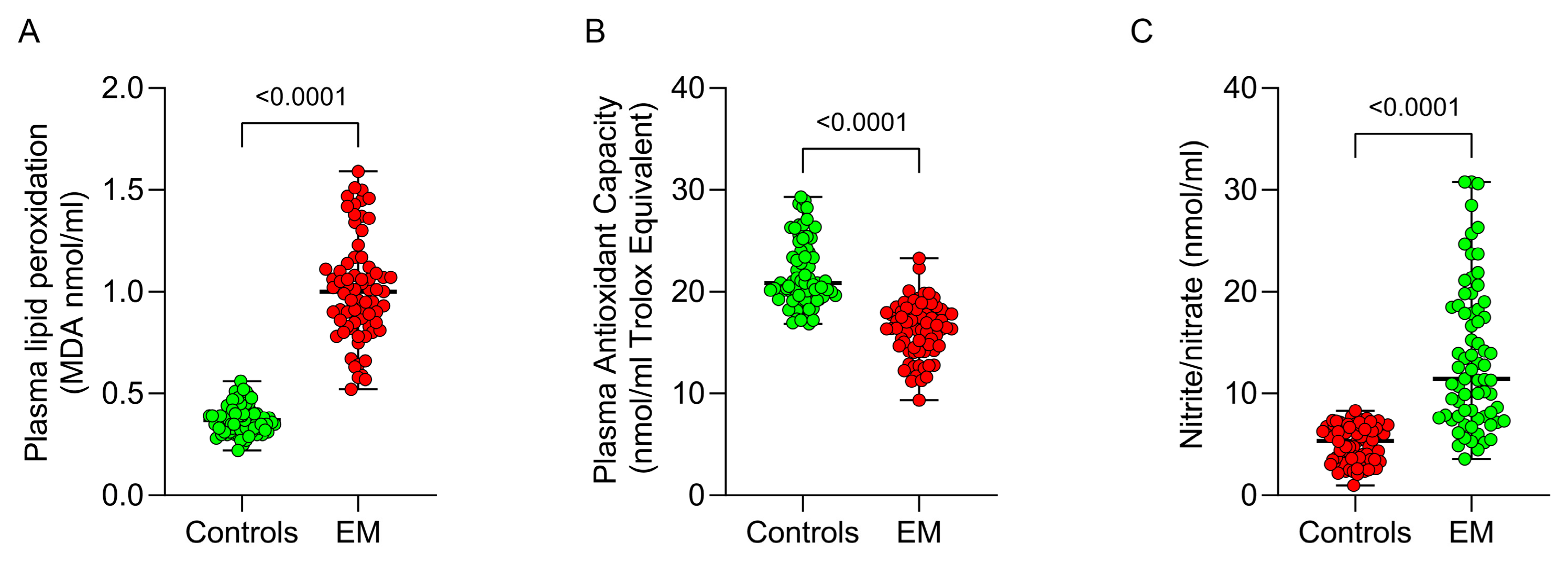
2.4. Plasma Lipid Peroxidation Assay
2.5. Plasma Nitrate/Nitrite Content Assay
2.6. Determination of Plasma Total Antioxidant Capacity
2.7. Fibrinogen Oxidation Assessment
2.8. Fibrinogen Structure Determination
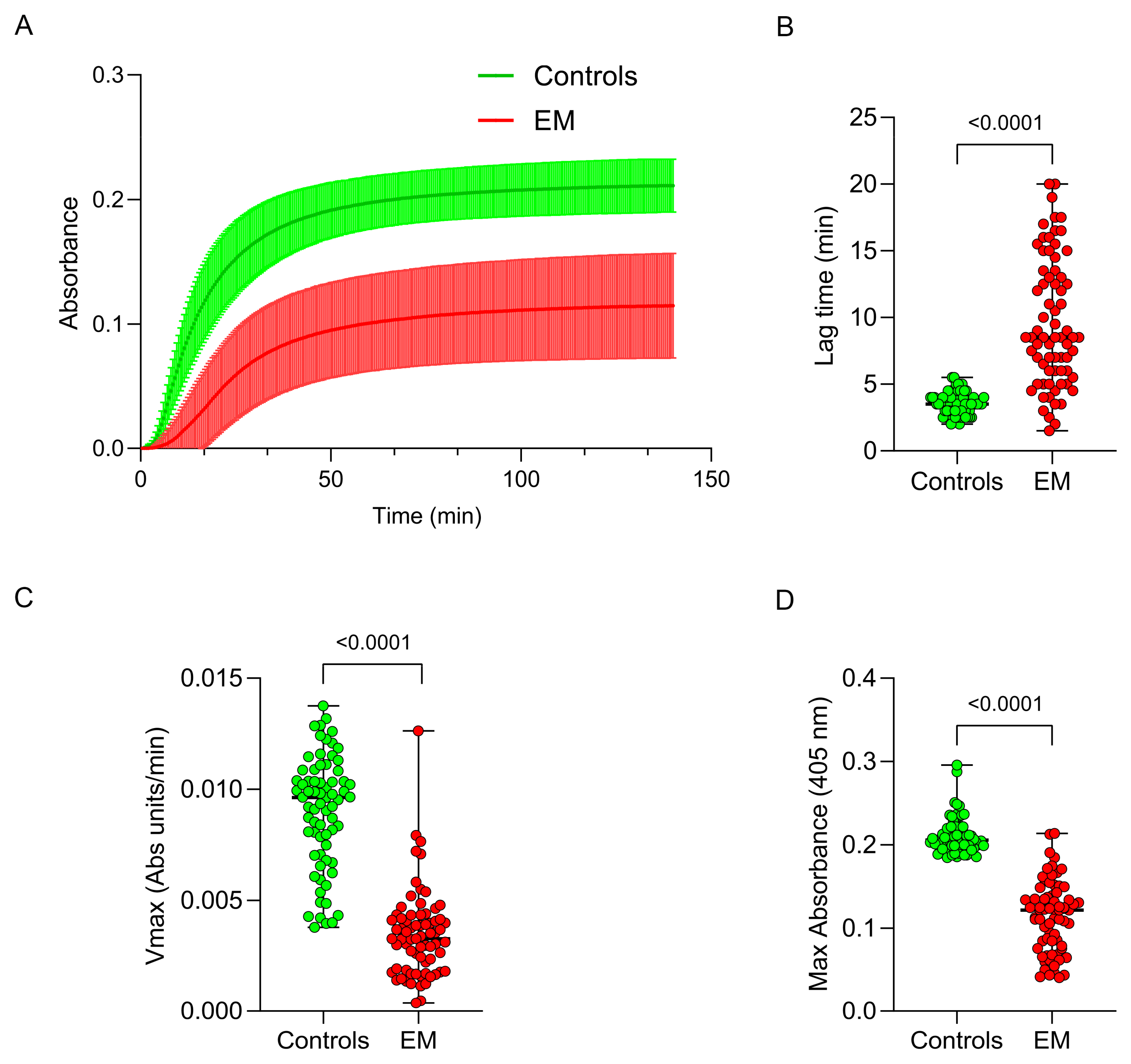
2.9. Fibrinogen Functional Analysis
2.10. Statistical Analysis
3. Results
3.1. Intracellular Leukocyte ROS Production
3.2. Systemic Redox Status
3.3. Fibrinogen Structural Changes and Fibrinogen Oxidation
3.4. Fibrinogen Functional Assessment
3.5. Correlation Between Redox Status and Fibrinogen Alterations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Imanaka, S.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H. Rethinking the pathogenesis of endometriosis: Complex interactions of genomic, epigenetic, and environmental factors. J. Obs. Gynaecol. Res. 2024, 50, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Shanoo, A.; Acharya, N. Endometriosis: A Comprehensive Exploration of Inflammatory Mechanisms and Fertility Implications. Cureus 2024, 16, e66128. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Wang, Y.; Nicholes, K.; Shih, I.M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obs. Gynecol. 1984, 64, 151–154. [Google Scholar]
- Mehedintu, C.; Plotogea, M.N.; Ionescu, S.; Antonovici, M. Endometriosis still a challenge. J. Med. Life 2014, 7, 349–357. [Google Scholar]
- The Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility. Fertil. Steril. 2006, 86, S156–S160. [Google Scholar] [CrossRef]
- Trinchant, R.; García-Velasco, J.A. Oocyte Quality in Women with Endometriosis. Gynecol. Obs. Investig. 2024, 1–9. [Google Scholar] [CrossRef]
- Augoulea, A.; Alexandrou, A.; Creatsa, M.; Vrachnis, N.; Lambrinoudaki, I. Pathogenesis of endometriosis: The role of genetics, inflammation and oxidative stress. Arch. Gynecol. Obs. 2012, 286, 99–103. [Google Scholar] [CrossRef]
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekhar, T.; Asemi, Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J. Cell. Physiol. 2019, 234, 19384–19392. [Google Scholar] [CrossRef]
- Cirillo, M.; Argento, F.R.; Attanasio, M.; Becatti, M.; Ladisa, I.; Fiorillo, C.; Coccia, M.E.; Fatini, C. Atherosclerosis and Endometriosis: The Role of Diet and Oxidative Stress in a Gender-Specific Disorder. Biomedicines 2023, 11, 450. [Google Scholar] [CrossRef]
- Cuffaro, F.; Russo, E.; Amedei, A. Endometriosis, Pain, and Related Psychological Disorders: Unveiling the Interplay among the Microbiome, Inflammation, and Oxidative Stress as a Common Thread. Int. J. Mol. Sci. 2024, 25, 6473. [Google Scholar] [CrossRef]
- Ansariniya, H.; Yavari, A.; Javaheri, A.; Zare, F. Oxidative stress-related effects on various aspects of endometriosis. Am. J. Reprod. Immunol. 2022, 88, e13593. [Google Scholar] [CrossRef]
- Bettiol, A.; Galora, S.; Argento, F.R.; Fini, E.; Emmi, G.; Mattioli, I.; Bagni, G.; Fiorillo, C.; Becatti, M. Erythrocyte oxidative stress and thrombosis. Expert. Rev. Mol. Med. 2022, 24, e31. [Google Scholar] [CrossRef]
- Becatti, M.; Mannucci, A.; Barygina, V.; Mascherini, G.; Emmi, G.; Silvestri, E.; Wright, D.; Taddei, N.; Galanti, G.; Fiorillo, C. Redox status alterations during the competitive season in élite soccer players: Focus on peripheral leukocyte-derived ROS. Intern. Emerg. Med. 2017, 12, 777–788. [Google Scholar] [CrossRef]
- Fiorillo, C.; Becatti, M.; Attanasio, M.; Lucarini, L.; Nassi, N.; Evangelisti, L.; Porciani, M.C.; Nassi, P.; Gensini, G.F.; Abbate, R.; et al. Evidence for oxidative stress in plasma of patients with Marfan syndrome. Int. J. Cardiol. 2010, 145, 544–546. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, X.; Lv, X.; Lin, A.; Li, Y. NADPH oxidase 4-mediating oxidative stress contributes to endometriosis. J. Appl. Genet. 2024, 65, 113–120. [Google Scholar] [CrossRef]
- Didziokaite, G.; Biliute, G.; Gudaite, J.; Kvedariene, V. Oxidative Stress as a Potential Underlying Cause of Minimal and Mild Endometriosis-Related Infertility. Int. J. Mol. Sci. 2023, 24, 3809. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wen, S.; Zeng, J.; Liu, J.; Ye, W.; Wu, J.; Huang, S.; Xie, W.; Wen, H.; Sun, Y.; et al. AOPPs induces EMT and fibrosis by activating oxidative stress through ERK/p38 MAPK signaling pathway in endometriosis. Reprod. Biol. 2024, 24, 100950. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Luo, Y.; Fu, J. Effects of Mn (II) on peroxynitrite nitrifying fibrinogen. Biomed. Mater. Eng. 2014, 24, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Arangia, A.; Marino, Y.; Fusco, R.; Siracusa, R.; Cordaro, M.; D’Amico, R.; Macrì, F.; Raffone, E.; Impellizzeri, D.; Cuzzocrea, S.; et al. Fisetin, a Natural Polyphenol, Ameliorates Endometriosis Modulating Mast Cells Derived NLRP-3 Inflammasome Pathway and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 5076. [Google Scholar] [CrossRef] [PubMed]
- Emmi, G.; Becatti, M.; Bettiol, A.; Hatemi, G.; Prisco, D.; Fiorillo, C. Behçet’s Syndrome as a Model of Thrombo-Inflammation: The Role of Neutrophils. Front. Immunol. 2019, 10, 1085. [Google Scholar] [CrossRef]
- Hong, C.; Li, X.; Zhang, K.; Huang, Q.; Li, B.; Xin, H.; Hu, B.; Meng, F.; Zhu, X.; Tang, D.; et al. Novel perspectives on autophagy-oxidative stress-inflammation axis in the orchestration of adipogenesis. Front. Endocrinol. 2024, 15, 1404697. [Google Scholar] [CrossRef]
- Nanda, A.K.T.; Banerjee, P.; Dutta, M.; Wangdi, T.; Sharma, P.; Chaudhury, K.; Jana, S.K. Cytokines, Angiogenesis, and Extracellular Matrix Degradation are Augmented by Oxidative Stress in Endometriosis. Ann. Lab. Med. 2020, 40, 390–397. [Google Scholar] [CrossRef]
- Mamillapalli, R.; Taylor, H.S. Endometriosis causes cardiovascular disease. Am. J. Obs. Gynecol. 2022, 227, 671. [Google Scholar] [CrossRef]
- Poeta do Couto, C.; Policiano, C.; Pinto, F.J.; Brito, D.; Caldeira, D. Endometriosis and cardiovascular disease: A systematic review and meta-analysis. Maturitas 2023, 171, 45–52. [Google Scholar] [CrossRef]
- Machin, N.; Ragni, M.V. Hormones and thrombosis: Risk across the reproductive years and beyond. Transl. Res. 2020, 225, 9–19. [Google Scholar] [CrossRef]
- Bettiol, A.; Argento, F.R.; Fini, E.; Bello, F.; Di Scala, G.; Taddei, N.; Emmi, G.; Prisco, D.; Becatti, M.; Fiorillo, C. ROS-driven structural and functional fibrinogen modifications are reverted by interleukin-6 inhibition in Giant Cell Arteritis. Thromb. Res. 2023, 230, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Fiorillo, C.; Argento, F.R.; Fini, E.; Borghi, S.; Falcini, M.; Roccarina, D.; La Delfa, R.; Lillo, L.; Zurli, T.; et al. Oxidative stress-induced fibrinogen modifications in liver transplant recipients: Unraveling a novel potential mechanism for cardiovascular risk. Res. Pract. Thromb. Haemost. 2024, 8, 102555. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Mannucci, A.; Argento, F.R.; Gitto, S.; Vizzutti, F.; Marra, F.; Taddei, N.; Fiorillo, C.; Laffi, G. Super-Resolution Microscopy Reveals an Altered Fibrin Network in Cirrhosis: The Key Role of Oxidative Stress in Fibrinogen Structural Modifications. Antioxidants 2020, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Undas, A. Fibrin clot properties and their modulation in thrombotic disorders. Thromb. Haemost. 2014, 112, 32–42. [Google Scholar] [CrossRef]
- Undas, A.; Zawilska, K.; Ciesla-Dul, M.; Lehmann-Kopydłowska, A.; Skubiszak, A.; Ciepłuch, K.; Tracz, W. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood 2009, 114, 4272–4278. [Google Scholar] [CrossRef]
- Natorska, J.; Ząbczyk, M.; Mastalerz, L.; Undas, A. Increased factor XI but not factor XII is associated with enhanced inflammation and impaired fibrin clot properties in patients with eosinophilic granulomatosis with polyangiitis. Clin. Exp. Rheumatol. 2024, 42, 822–827. [Google Scholar] [CrossRef]
- Varjú, I.; Sorvillo, N.; Cherpokova, D.; Farkas, Á.; Farkas, V.J.; Komorowicz, E.; Feller, T.; Kiss, B.; Kellermayer, M.Z.; Szabó, L.; et al. Citrullinated Fibrinogen Renders Clots Mechanically Less Stable, but Lysis-Resistant. Circ. Res. 2021, 129, 342–344. [Google Scholar] [CrossRef]
- Varjú, I.; Tóth, E.; Farkas, Á.; Farkas, V.J.; Komorowicz, E.; Feller, T.; Kiss, B.; Kellermayer, M.Z.; Szabó, L.; Wacha, A.; et al. Citrullinated fibrinogen forms densely packed clots with decreased permeability. J. Thromb. Haemost. 2022, 20, 2862–2872. [Google Scholar] [CrossRef]
- Vilar, R.; Fish, R.J.; Casini, A.; Neerman-Arbez, M. Fibrin(ogen) in human disease: Both friend and foe. Haematologica 2020, 105, 284–296. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Wang, H.; Liu, J. Study on the influence of oxidative stress on the fibrillization of fibrinogen. Biochem. J. 2016, 473, 4373–4384. [Google Scholar] [CrossRef]
- Weigandt, K.M.; White, N.; Chung, D.; Ellingson, E.; Wang, Y.; Fu, X.; Pozzo, D.C. Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen. Biophys. J. 2012, 103, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Mechanisms of fibrin polymerization and clinical implications. Blood 2013, 121, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Wang, Y.; Fu, X.; Cardenas, J.C.; Martin, E.J.; Brophy, D.F.; Wade, C.E.; Wang, X.; St John, A.E.; Lim, E.B.; et al. Post-translational oxidative modification of fibrinogen is associated with coagulopathy after traumatic injury. Free. Radic. Biol. Med. 2016, 96, 181–189. [Google Scholar] [CrossRef]
- Yurina, L.V.; Vasilyeva, A.D.; Bugrova, A.E.; Indeykina, M.I.; Kononikhin, A.S.; Nikolaev, E.N.; Rosenfeld, M.A. Hypochlorite-Induced Oxidative Modification of Fibrinogen. Dokl. Biochem. Biophys. 2019, 484, 37–41. [Google Scholar] [CrossRef]
- Yurina, L.; Vasilyeva, A.; Indeykina, M.; Bugrova, A.; Biryukova, M.; Kononikhin, A.; Nikolaev, E.; Rosenfeld, M. Ozone-induced damage of fibrinogen molecules: Identification of oxidation sites by high-resolution mass spectrometry. Free. Radic. Res. 2019, 53, 430–455. [Google Scholar] [CrossRef]
- Yurina, L.V.; Vasilyeva, A.D.; Vasserman, L.A.; Podoplelova, N.A.; Panteleev, M.A.; Rosenfeld, M.A. Effect of Hypochlorite- and Peroxide-Induced Oxidation of Fibrinogen on the Fibrin Structure. Dokl. Biochem. Biophys. 2021, 499, 242–246. [Google Scholar] [CrossRef]
- Yurina, L.V.; Vasilyeva, A.D.; Gavrilina, E.S.; Ivanov, V.S.; Obydennyi, S.I.; Chabin, I.A.; Indeykina, M.I.; Kononikhin, A.S.; Nikolaev, E.N.; Rosenfeld, M.A. A role of methionines in the functioning of oxidatively modified fibrinogen. Biochim. Biophys. Acta Proteins Proteom. 2024, 1872, 141013. [Google Scholar] [CrossRef]
- Ząbczyk, M.; Ariëns, R.A.S.; Undas, A. Fibrin clot properties in cardiovascular disease: From basic mechanisms to clinical practice. Cardiovasc. Res. 2023, 119, 94–111. [Google Scholar] [CrossRef]
- Tenopoulou, M. Fibrinogen post-translational modifications are biochemical determinants of fibrin clot properties and interactions. FEBS J. 2024. [Google Scholar] [CrossRef]
- Słaboszewski, M.; Kolec, R.; Paszek, E.; Baran, M.; Undas, A. Prothrombotic plasma fibrin clot phenotype is associated with spontaneous echo contrast in atrial fibrillation: The role of protein carbonylation. Thromb. Res. 2024, 240, 109065. [Google Scholar] [CrossRef]
- Konieczyńska, M.; Natorska, J.; Undas, A. Thrombosis and Aging: Fibrin Clot Properties and Oxidative Stress. Antioxid. Redox Signal. 2024, 41, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Sproul, E.P.; Hannan, R.T.; Brown, A.C. Controlling Fibrin Network Morphology, Polymerization, and Degradation Dynamics in Fibrin Gels for Promoting Tissue Repair. Protocol 2018, 1758, 85–99. [Google Scholar] [CrossRef]
- Azizova, O.A.; Piryazev, A.P.; Aseychev, A.V.; Shvachko, A.G. Oxidative modification of fibrinogen inhibits its transformation into fibrin under the effect of thrombin. Bull. Exp. Biol. Med. 2009, 147, 201–203. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.J.; Snoek, C.J.M.; Rijken, D.C.; de Maat, M.P.M. Effects of Post-Translational Modifications of Fibrinogen on Clot Formation, Clot Structure, and Fibrinolysis: A Systematic Review. Arter. Thromb. Vasc. Biol. 2020, 40, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Schuett, K.; Savvaidis, A.; Maxeiner, S.; Lysaja, K.; Jankowski, V.; Schirmer, S.H.; Dimkovic, N.; Boor, P.; Kaesler, N.; Dekker, F.W.; et al. Clot Structure: A Potent Mortality Risk Factor in Patients on Hemodialysis. J. Am. Soc. Nephrol. 2017, 28, 1622–1630. [Google Scholar] [CrossRef]
- Goodyear, M.D.; Krleza-Jeric, K.; Lemmens, T. The Declaration of Helsinki. BMJ 2007, 335, 624–625. [Google Scholar] [CrossRef]
- Haas, D.; Shebl, O.; Shamiyeh, A.; Oppelt, P. The rASRM score and the Enzian classification for endometriosis: Their strengths and weaknesses. Acta Obs. Gynecol. Scand. 2013, 92, 3–7. [Google Scholar] [CrossRef]
- Miniati, M.; Fiorillo, C.; Becatti, M.; Monti, S.; Bottai, M.; Marini, C.; Grifoni, E.; Formichi, B.; Bauleo, C.; Arcangeli, C.; et al. Fibrin resistance to lysis in patients with pulmonary hypertension other than thromboembolic. Am. J. Respir. Crit. Care Med. 2010, 181, 992–996. [Google Scholar] [CrossRef]
- Becatti, M.; Emmi, G.; Silvestri, E.; Bruschi, G.; Ciucciarelli, L.; Squatrito, D.; Vaglio, A.; Taddei, N.; Abbate, R.; Emmi, L.; et al. Neutrophil Activation Promotes Fibrinogen Oxidation and Thrombus Formation in Behçet Disease. Circulation 2016, 133, 302–311. [Google Scholar] [CrossRef]
- Becatti, M.; Fiorillo, C.; Gori, A.M.; Marcucci, R.; Paniccia, R.; Giusti, B.; Violi, F.; Pignatelli, P.; Gensini, G.F.; Abbate, R. Platelet and leukocyte ROS production and lipoperoxidation are associated with high platelet reactivity in Non-ST elevation myocardial infarction (NSTEMI) patients on dual antiplatelet treatment. Atherosclerosis 2013, 231, 392–400. [Google Scholar] [CrossRef]
- Becatti, M.; Fucci, R.; Mannucci, A.; Barygina, V.; Mugnaini, M.; Criscuoli, L.; Giachini, C.; Bertocci, F.; Picone, R.; Emmi, G.; et al. A Biochemical Approach to Detect Oxidative Stress in Infertile Women Undergoing Assisted Reproductive Technology Procedures. Int. J. Mol. Sci. 2018, 19, 592. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.; Sofi, F.; Luisi, M.L.; Rafanelli, E.; Fiorillo, C.; Becatti, M.; Abbate, R.; Casini, A.; Gensini, G.F.; Benedettelli, S. An organic khorasan wheat-based replacement diet improves risk profile of patients with acute coronary syndrome: A randomized crossover trial. Nutrients 2015, 7, 3401–3415. [Google Scholar] [CrossRef] [PubMed]
- Emmi, G.; Bettiol, A.; Niccolai, E.; Ramazzotti, M.; Amedei, A.; Pagliai, G.; Taddei, N.; Sofi, F.; Fiorillo, C.; Prisco, D.; et al. Butyrate-Rich Diets Improve Redox Status and Fibrin Lysis in Behçet’s Syndrome. Circ. Res. 2021, 128, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Chen, H.; Feng, W. Assessment of Risk Factors Associated with Severe Endometriosis and Establishment of Preoperative Prediction Model. Diagnostics 2022, 12, 2348. [Google Scholar] [CrossRef]
- Wiegers, H.M.G.; Scheres, L.J.J.; Tahir, L.; Hutten, B.A.; Middeldorp, S.; Mijatovic, V. Risk of venous thromboembolism in women with endometriosis. Thromb. Res. 2022, 217, 104–106. [Google Scholar] [CrossRef]
- Smyk, J.M.; Danielecka, Z.; Kotowska, M.; Zawadka, M.; Andruszkiewicz, P.; Grąt, M.; Główczyńska, R.; Grabowski, M.; Gąsecka, A.; Romejko-Wolniewicz, E. Cardiovascular risks and endothelial dysfunction in reproductive-age women with endometriosis. Sci. Rep. 2024, 14, 24127. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Marchandot, B.; Curtiaud, A.; Matsushita, K.; Trimaille, A.; Host, A.; Faller, E.; Garbin, O.; Akladios, C.; Jesel, L.; Morel, O. Endometriosis and cardiovascular disease. Eur. Heart J. Open 2022, 2, oeac001. [Google Scholar] [CrossRef]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Missmer, S.A. Endometriosis and Risk of Coronary Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 257–264. [Google Scholar] [CrossRef]
- Sherwani, S.; Khan, M.W.A.; Rajendrasozhan, S.; Al-Motair, K.; Husain, Q.; Khan, W.A. The vicious cycle of chronic endometriosis and depression-an immunological and physiological perspective. Front. Med. 2024, 11, 1425691. [Google Scholar] [CrossRef] [PubMed]
- Adilbayeva, A.; Kunz, J. Pathogenesis of Endometriosis and Endometriosis-Associated Cancers. Int. J. Mol. Sci. 2024, 25, 7624. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Cacciottola, L.; Donnez, J.; Dolmans, M.M. Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets? Int. J. Mol. Sci. 2021, 22, 7138. [Google Scholar] [CrossRef]
- Potere, N.; Bonaventura, A.; Abbate, A. Novel Therapeutics and Upcoming Clinical Trials Targeting Inflammation in Cardiovascular Diseases. Arter. Thromb. Vasc. Biol. 2024. [Google Scholar] [CrossRef]
- Viganò, P.; Ottolina, J.; Sarais, V.; Rebonato, G.; Somigliana, E.; Candiani, M. Coagulation Status in Women With Endometriosis. Reprod. Sci. 2018, 25, 559–565. [Google Scholar] [CrossRef]
- Rafi, U.; Ahmad, S.; Bokhari, S.S.; Iqbal, M.A.; Zia, A.; Khan, M.A.; Roohi, N. Association of Inflammatory Markers/Cytokines with Cardiovascular Risk Manifestation in Patients with Endometriosis. Mediat. Inflamm. 2021, 2021, 3425560. [Google Scholar] [CrossRef]
- Abramiuk, M.; Grywalska, E.; Małkowska, P.; Sierawska, O.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P. The Role of the Immune System in the Development of Endometriosis. Cells 2022, 11, 2028. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Wang, M.; Cheng, W. Effects of vitamin C on the outcome of in vitro fertilization-embryo transfer in endometriosis: A randomized controlled study. J. Int. Med. Res. 2018, 46, 4624–4633. [Google Scholar] [CrossRef]
- Nasiri, N.; Moini, A.; Eftekhari-Yazdi, P.; Karimian, L.; Salman-Yazdi, R.; Arabipoor, A. Oxidative Stress Statues in Serum and Follicular Fluid of Women with Endometriosis. Cell J. 2017, 18, 582–587. [Google Scholar] [CrossRef]
- Amreen, S.; Kumar, P.; Gupta, P.; Rao, P. Evaluation of Oxidative Stress and Severity of Endometriosis. J. Hum. Reprod. Sci. 2019, 12, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Emmi, G.; Bettiol, A.; Silvestri, E.; Di Scala, G.; Taddei, N.; Prisco, D.; Fiorillo, C. Behçet’s syndrome as a tool to dissect the mechanisms of thrombo-inflammation: Clinical and pathogenetic aspects. Clin. Exp. Immunol. 2019, 195, 322–333. [Google Scholar] [CrossRef]
- Roitman, E.V.; Azizova, O.A.; Morozov, Y.A.; Aseichev, A.V. Effect of oxidized fibrinogens on blood coagulation. Bull. Exp. Biol. Med. 2004, 138, 245–247. [Google Scholar] [CrossRef]
- Štikarová, J.; Kotlín, R.; Riedel, T.; Suttnar, J.; Pimková, K.; Chrastinová, L.; Dyr, J.E. The effect of reagents mimicking oxidative stress on fibrinogen function. Sci. World J. 2013, 2013, 359621. [Google Scholar] [CrossRef]
- Rosenfeld, M.A.; Wasserman, L.A.; Vasilyeva, A.D.; Podoplelova, N.A.; Panteleev, M.A.; Yurina, L.V. Hypochlorite-induced oxidation of fibrinogen: Effects on its thermal denaturation and fibrin structure. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129970. [Google Scholar] [CrossRef]
- Piróg, M.; Kacalska-Janssen, O.; Jach, R.; Ząbczyk, M.; Natorska, J. Fibrin clot properties among women with endometriosis and the impact of ovarian stimulation. Reprod. Biomed. Online 2021, 43, 81–90. [Google Scholar] [CrossRef]
- Becatti, M.; Marcucci, R.; Bruschi, G.; Taddei, N.; Bani, D.; Gori, A.M.; Giusti, B.; Gensini, G.F.; Abbate, R.; Fiorillo, C. Oxidative modification of fibrinogen is associated with altered function and structure in the subacute phase of myocardial infarction. Arter. Thromb. Vasc. Biol. 2014, 34, 1355–1361. [Google Scholar] [CrossRef]
- Nowak, P.; Zbikowska, H.M.; Ponczek, M.; Kolodziejczyk, J.; Wachowicz, B. Different vulnerability of fibrinogen subunits to oxidative/nitrative modifications induced by peroxynitrite: Functional consequences. Thromb. Res. 2007, 121, 163–174. [Google Scholar] [CrossRef]
- Nowak, W.; Treliński, J.; Chojnowski, K.; Matczak, J.; Robak, M.; Misiewicz, M.; Nowak, P. Assessment of oxidative/nitrative modifications of plasma proteins, selected ROTEM parameters and kinetics of fibrinogen polymerization in patients with multiple myeloma at diagnosis. Med. Oncol. 2017, 34, 4. [Google Scholar] [CrossRef]
- Ceznerová, E.; Kaufmanová, J.; Stikarová, J.; Pastva, O.; Loužil, J.; Chrastinová, L.; Suttnar, J.; Kotlín, R.; Dyr, J.E. Thrombosis-associated hypofibrinogenemia: Novel abnormal fibrinogen variant FGG c.8G>A with oxidative posttranslational modifications. Blood Coagul. Fibrinolysis 2022, 33, 228–237. [Google Scholar] [CrossRef]
- Torbitz, V.D.; Bochi, G.V.; de Carvalho, J.A.; de Almeida Vaucher, R.; da Silva, J.E.; Moresco, R.N. In vitro oxidation of fibrinogen promotes functional alterations and formation of advanced oxidation protein products, an inflammation mediator. Inflammation 2015, 38, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Isik, B.; Ceylan, A.; Isik, R. Oxidative stress in smokers and non-smokers. Inhal. Toxicol. 2007, 19, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.; Chung, W.; Liu, D.; Seagar, R.; O’Halloran, T.; Koshy, A.N.; Horrigan, M.; Farouque, O.; Gow, P.; Angus, P. Cardiac Risk Stratification in Liver Transplantation: Results of a Tiered Assessment Protocol Based on Traditional Cardiovascular Risk Factors. Liver Transpl. 2021, 27, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.B.; Hwang, E.A.; Han, S.Y.; Park, S.B.; Kim, H.C.; Ha, E.Y.; Suh, S.I.; Mun, K.C. Effects of tacrolimus on antioxidant status and oxidative stress in glioma cells. Transplant. Proc. 2008, 40, 2740–2741. [Google Scholar] [CrossRef] [PubMed]
- Kwasny-Krochin, B.; Gluszko, P.; Undas, A. Unfavorably altered fibrin clot properties in patients with active rheumatoid arthritis. Thromb. Res. 2010, 126, e11–e16. [Google Scholar] [CrossRef]
- Undas, A.; Kolarz, M.; Kopeć, G.; Tracz, W. Altered fibrin clot properties in patients on long-term haemodialysis: Relation to cardiovascular mortality. Nephrol. Dial. Transpl. 2008, 23, 2010–2015. [Google Scholar] [CrossRef]
- Czubkowski, P.; Socha, P.; Pawlowska, J. Oxidative stress in liver transplant recipients. Ann. Transplant. 2011, 16, 99–108. [Google Scholar]
- Tsai, Y.F.; Liu, F.C.; Sung, W.C.; Lin, C.C.; Chung, P.C.; Lee, W.C.; Yu, H.P. Ischemic reperfusion injury-induced oxidative stress and pro-inflammatory mediators in liver transplantation recipients. Transpl. Proc. 2014, 46, 1082–1086. [Google Scholar] [CrossRef]
- Jóźwik-Plebanek, K.; Prejbisz, A.; Wypasek, E.; Pręgowska-Chwała, B.; Hanus, K.; Kaszuba, A.M.; Januszewicz, M.; Bieleń, P.; Kabat, M.; Kruk, M.; et al. Altered plasma fibrin clot properties in hypertensive patients with obstructive sleep apnoea are improved by continuous positive airway pressure treatment. J. Hypertens. 2017, 35, 1035–1043. [Google Scholar] [CrossRef]
- Swanepoel, A.C.; Lindeque, B.G.; Swart, P.J.; Abdool, Z.; Pretorius, E. Estrogen causes ultrastructural changes of fibrin networks during the menstrual cycle: A qualitative investigation. Microsc. Res. Techol. 2014, 77, 594–601. [Google Scholar] [CrossRef]
- Swanepoel, A.C.; Visagie, A.; de Lange, Z.; Emmerson, O.; Nielsen, V.G.; Pretorius, E. The clinical relevance of altered fibrinogen packaging in the presence of 17β-estradiol and progesterone. Thromb. Res. 2016, 146, 23–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piróg, M.; Jach, R.; Undas, A. Effects of ultra-low-dose versus standard hormone therapy on fibrinolysis and thrombin generation in postmenopausal women. Eur. J. Obs. Gynecol. Reprod. Biol. 2017, 217, 77–82. [Google Scholar] [CrossRef] [PubMed]
- White, R.E.; Gerrity, R.; Barman, S.A.; Han, G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids 2010, 75, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex. Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal treatments for endometriosis: The endocrine background. Rev. Endocr. Metab. Disord. 2022, 23, 333–355. [Google Scholar] [CrossRef]
- Cagnacci, A.; Gazzo, I.; Stigliani, S.; Paoletti, A.M.; Anserini, P.; Londero, A.P.; Xholli, A. Oxidative Stress: The Role of Estrogen and Progesterone. J. Clin. Med. 2023, 12, 7304. [Google Scholar] [CrossRef]
- Oală, I.E.; Mitranovici, M.I.; Chiorean, D.M.; Irimia, T.; Crișan, A.I.; Melinte, I.M.; Cotruș, T.; Tudorache, V.; Moraru, L.; Moraru, R.; et al. Endometriosis and the Role of Pro-Inflammatory and Anti-Inflammatory Cytokines in Pathophysiology: A Narrative Review of the Literature. Diagnostics 2024, 14, 3112. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, Y.; Liu, Z.; Wang, Y. Inflammation and endometriosis. Front. Biosci. (Landmark Ed) 2016, 21, 941–948. [Google Scholar] [CrossRef]


| Variables | Patients (n = 71) | Controls (n = 71) | p Value |
|---|---|---|---|
| Age, y, median (range) | 30 (20–47) | 32 (20–39) | 0.4049 |
| Weight, kg, median (range) | 58 (44–87) | 56.5 (45–78) | 0.3470 |
| BMI, kg/m2, median (range) | 21 (16.2–29.5) | 22.5 (16.1–28.6) | 0.9246 |
| Underweight (<18.5 kg/m2), n (%) | 17 (23.9) | 10 (14.1) | 0.1989 |
| Overweight (25–29.99 kg/m2), n (%) | 9 (12.7) | 6 (8.5) | 0.5864 |
| Waist circumference ≥ 80 cm, n (%) | 17 (23.9) | N/A | - |
| WHR ≥ 0.85, n (%) | 11 (15.5) | N/A | - |
| Smoking habit, n (%) | 18 (23.4) | 24 (33.8) | 0.3580 |
| Migraine, n (%) | 31 (43.7) | N/A | - |
| Migraine with aura, n (%) | 7 (9.9) | N/A | - |
| Dyslipidaemia, n (%) | 36 (50.7) | 39 (54.9) | 0.7369 |
| Total cholesterol > 200 mg/dL, n (%) | 22 (31) | 24 (33.8) | 0.8578 |
| LDL cholesterol > 116 mg/dL, n (%) | 29 (40.8) | 31 (43.66) | 0.8652 |
| HDL cholesterol < 48 mg/dL, n (%) | 7 (9.9) | 6 (8.5) | 1 |
| Triglycerides > 150 mg/dL, n (%) | 3 (4.2) | 4 (5.6) | 1 |
| Lipoprotein (a) > 300 mg/L, n (%) | 18 (23.4) | N/A | - |
| History of negative obstetric events, n (%) | 2 (2.8) | N/A | - |
| Family history of endometriosis, n (%) | 14 (19.7) | N/A | - |
| Family history of CVD, n (%) | 19 (26.7) | N/A | - |
| Fibrinogen mg/dL, median (range) | 278 (150–550) | 322 (157–411) | 0.5702 |
| ROS Limpho | ROS Mono | ROS Granu | MDA | ORAC | Nitrate/ Nitrite | Fibrin Deg | Max Abs | Lag Time | Vmax | Dityr | IF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROS Lympho | 0.89 * | 0.80 * | 0.184 | −0.020 | 0.238 * | 0.100 | −0.053 | 0.113 | −0.035 | −0.026 | 0.033 | |
| ROS Mono | 0.89 * | 0.85 * | 0.122 | −0.012 | 0.176 | 0.134 | −0.057 | 0.088 | 0.068 | 0.003 | −0.008 | |
| ROS Granu | 0.80 * | 0.85 * | 0.092 | 0.117 | 0.210 | 0.370 | −0.058 | 0.030 | −0.027 | 0.049 | −0.007 | |
| MDA | 0.184 | 0.122 | 0.092 | −0.051 | 0.268 * | −0.177 | 0.051 | 0.021 | −0.054 | −0.13 | 0.015 | |
| ORAC | −0.020 | −0.012 | 0.117 | −0.051 | 0.182 | 0.101 | −0.143 | −0.150 | 0.107 | 0.006 | −0.085 | |
| Nitrate/Nitrite | 0.238 * | 0.176 | 0.210 | 0.268 * | 0.182 | 0.196 | −0.031 | −0.137 | −0.009 | 0.173 | −0.324 * | |
| Fibrin Deg | 0.100 | 0.134 | 0.37 * | −0.177 | 0.101 | 0.196 | 0.144 | −0.045 | 0.226 | 0.060 | −0.26 * | |
| Max Abs | −0.053 | −0.057 | −0.058 | 0.051 | −0.143 | −0.031 | 0.144 | 0.211 | 0.26 * | −0.006 | 0.151 | |
| Lag Time | 0.113 | 0.088 | 0.030 | 0.021 | −0.150 | −0.137 | −0.045 | 0.211 | −0.128 | 0.097 | −0.023 | |
| Vmax | −0.035 | 0.068 | −0.027 | −0.054 | 0.107 | −0.009 | 0.226 | 0.26 * | −0.128 | 0.100 | −0.198 | |
| Dityr | −0.026 | 0.003 | 0.049 | −0.13 | 0.006 | 0.173 | 0.060 | −0.006 | 0.097 | 0.100 | −0.42 * | |
| IF | 0.033 | −0.008 | −0.007 | 0.015 | −0.085 | −0.324 * | −0.26 * | 0.151 | −0.023 | −0.198 | −0.42 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fini, E.; Argento, F.R.; Borghi, S.; Giurranna, E.; Nencini, F.; Cirillo, M.; Fatini, C.; Taddei, N.; Coccia, M.E.; Fiorillo, C.; et al. Fibrinogen Structural Changes and Their Potential Role in Endometriosis-Related Thrombosis. Antioxidants 2024, 13, 1456. https://doi.org/10.3390/antiox13121456
Fini E, Argento FR, Borghi S, Giurranna E, Nencini F, Cirillo M, Fatini C, Taddei N, Coccia ME, Fiorillo C, et al. Fibrinogen Structural Changes and Their Potential Role in Endometriosis-Related Thrombosis. Antioxidants. 2024; 13(12):1456. https://doi.org/10.3390/antiox13121456
Chicago/Turabian StyleFini, Eleonora, Flavia Rita Argento, Serena Borghi, Elvira Giurranna, Francesca Nencini, Michela Cirillo, Cinzia Fatini, Niccolò Taddei, Maria Elisabetta Coccia, Claudia Fiorillo, and et al. 2024. "Fibrinogen Structural Changes and Their Potential Role in Endometriosis-Related Thrombosis" Antioxidants 13, no. 12: 1456. https://doi.org/10.3390/antiox13121456
APA StyleFini, E., Argento, F. R., Borghi, S., Giurranna, E., Nencini, F., Cirillo, M., Fatini, C., Taddei, N., Coccia, M. E., Fiorillo, C., & Becatti, M. (2024). Fibrinogen Structural Changes and Their Potential Role in Endometriosis-Related Thrombosis. Antioxidants, 13(12), 1456. https://doi.org/10.3390/antiox13121456








