Long-Term Pentoxifylline Therapy Is Associated with a Reduced Risk of Atherosclerotic Cardiovascular Disease by Inhibiting Oxidative Stress and Cell Apoptosis in Diabetic Kidney Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data and Sample Source
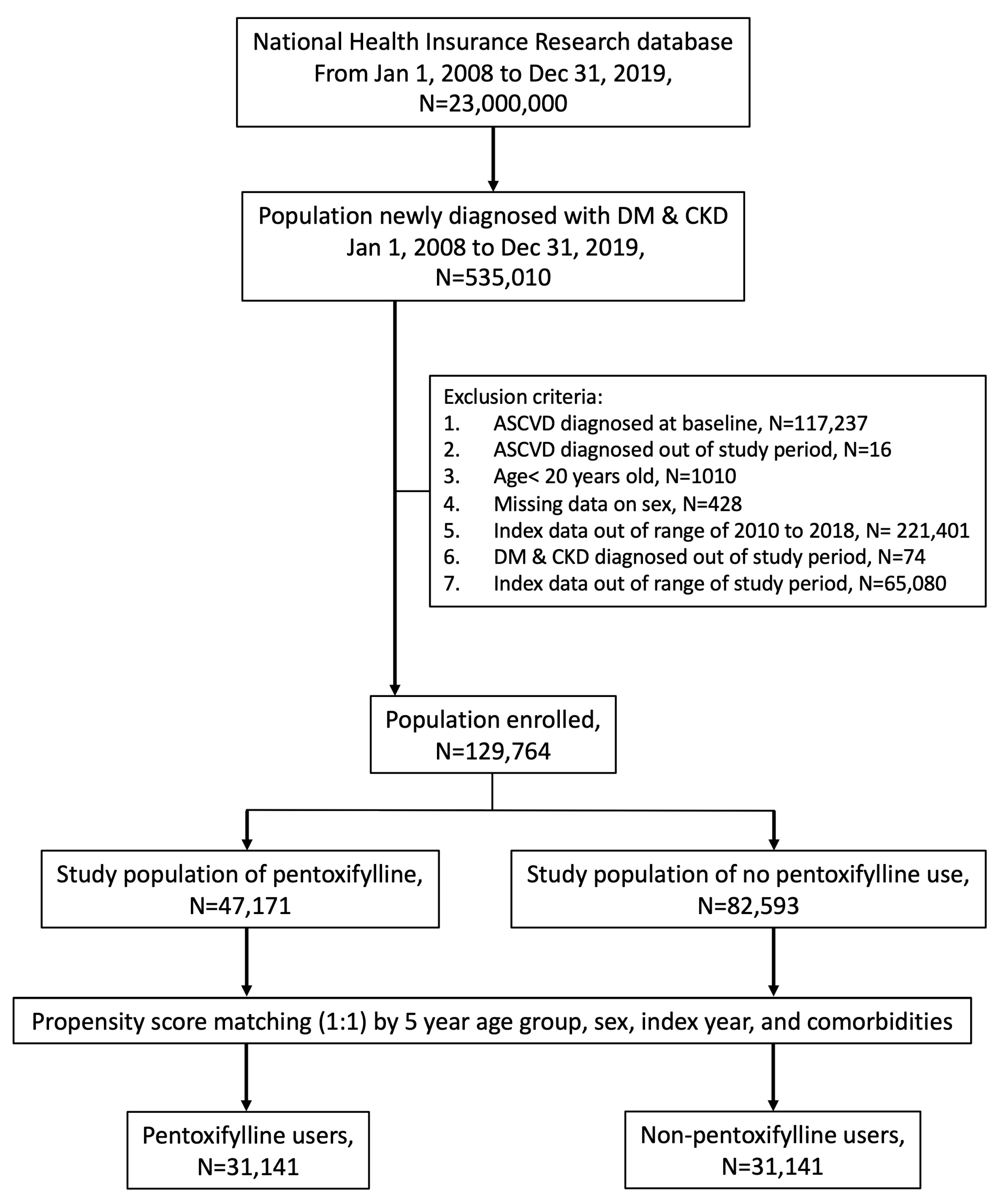
2.2. Study Participants
2.3. Outcome and Variables
2.4. In Vitro Cell Culture and Assay for Cellular Viability and Apoptosis
2.5. Measurement of Intracellular ROS Levels
2.6. Forecasting Interactions Between Chemicals and Proteins
2.7. Protein Extraction and Western Blotting
2.8. SiRNA Transfection
2.9. Quantitative Polymerase Chain Reaction in Real Time
2.10. Experimental Animals and Study Design
2.11. Assay for Lipid Peroxidation, Oil Red O Staining, and Histological Staining of Aortic Sections
2.12. Statistical Analyses
3. Results
3.1. Incidence and Hazard Ratios of ASCVD
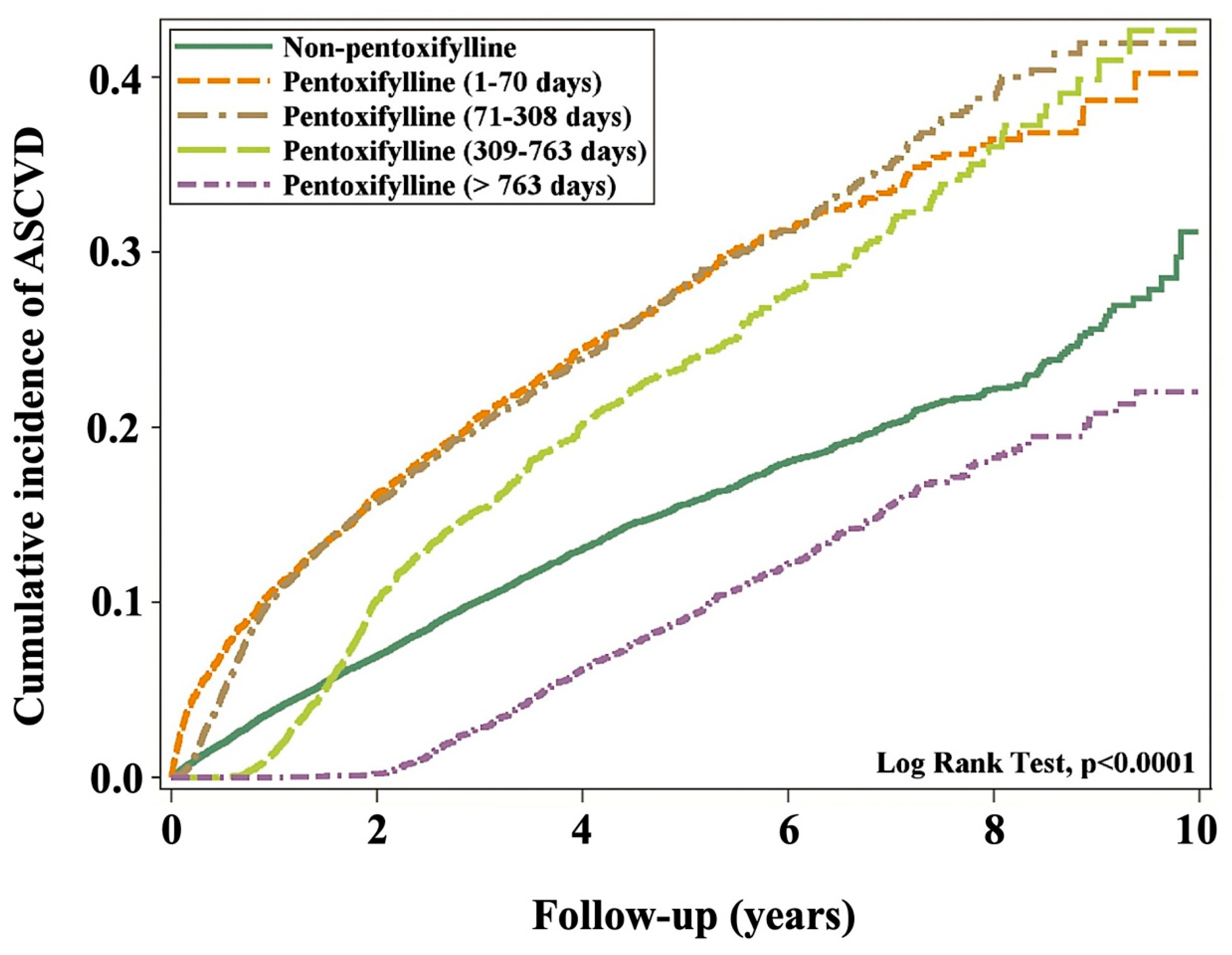
3.2. Cumulative ASCVD Incidence
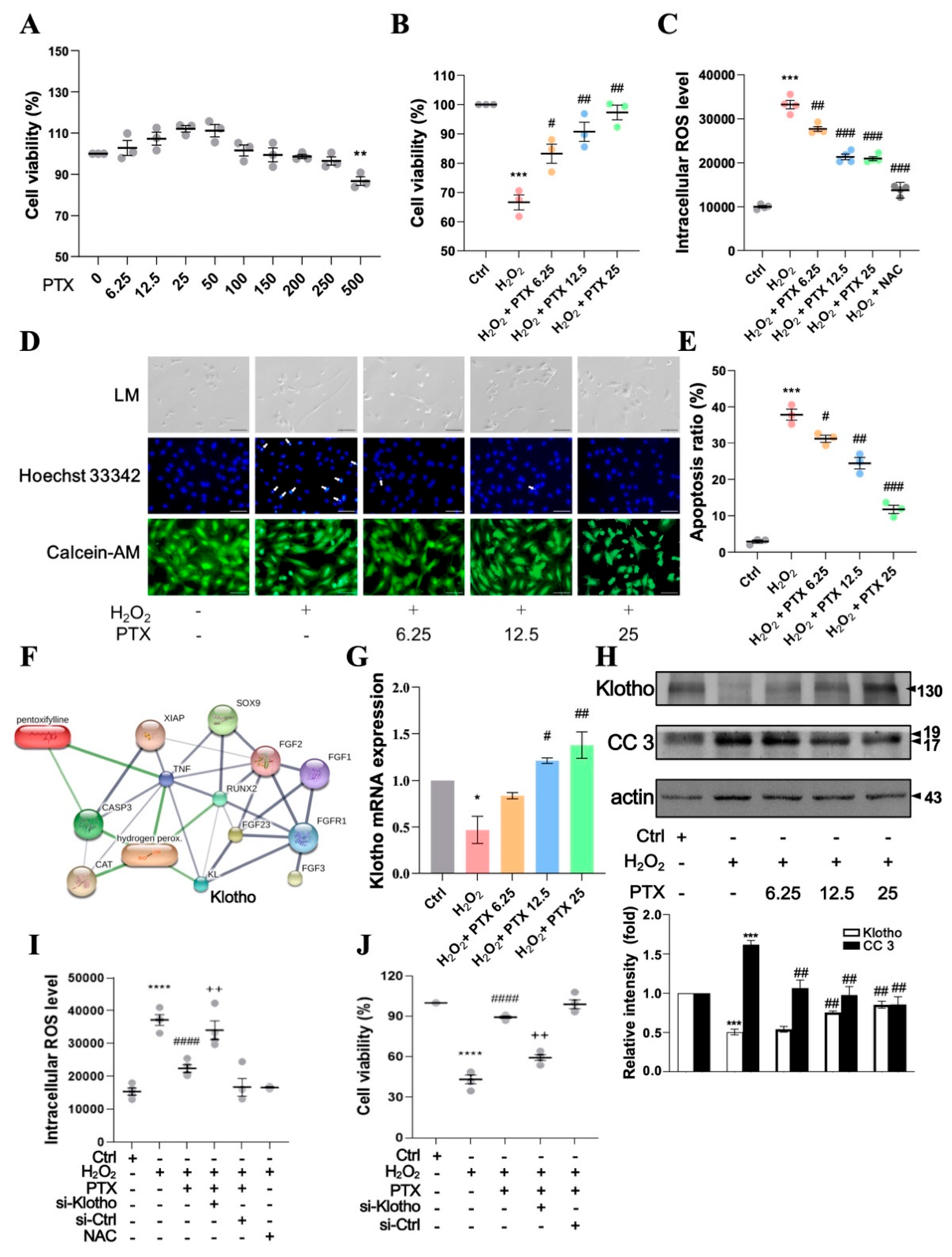
3.3. Pentoxifylline Inhibits H2O2-Induced Cellular Apoptosis in HAECs
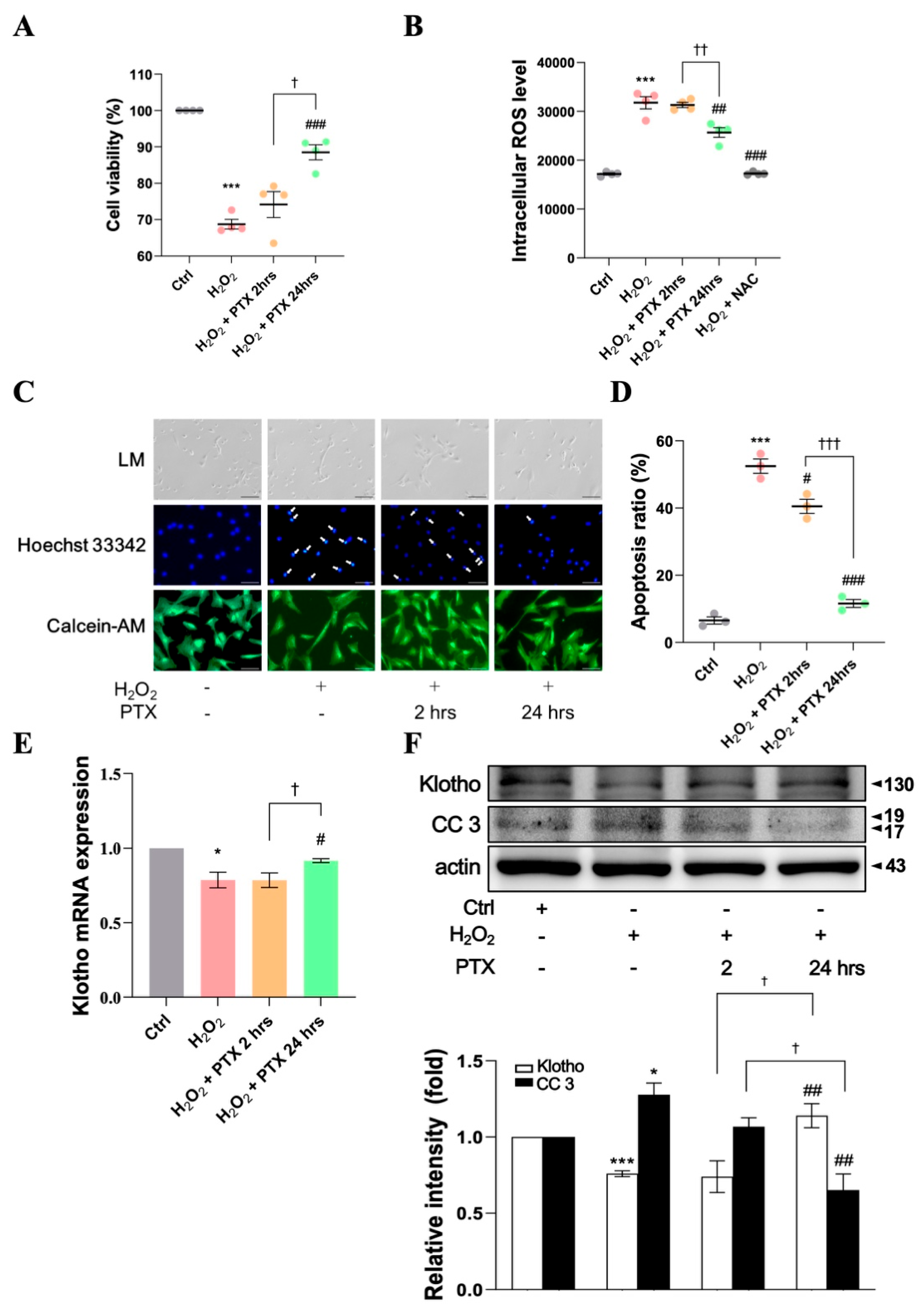
3.4. Protective Effect of Short- or Long-Term Pentoxifylline Exposure Against H2O2-Induced ECs
3.5. PTX Inhibits Atherosclerosis in a DKD Mouse Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.S.; Tsai, P.H.; Tseng, K.F.; Chen, F.Y.; Yang, W.C.; Shen, M.Y. Sesamol ameliorates renal injury-mediated atherosclerosis via inhibition of oxidative stress/IKKα/p53. Antioxidants 2021, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Bakris, G.L. Diabetic kidney disease: A determinant of cardiovascular risk in type 1 diabetes. Diabetes Care 2018, 41, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Matsushita, K.; Woodward, M.; Bilo, H.J.G.; Chalmers, J.; Heerspink, H.J.L.; Lee, B.J.; Perkins, R.M.; Rossing, P.; Sairenchi, T.; et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 2012, 380, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on Cardiovascular Health and Diseases in China 2021: An Updated Summary. Biomed. Environ. Sci. 2022, 35, 573–603. [Google Scholar] [CrossRef]
- Bi, L.; Yi, J.; Wu, C.; Hu, S.; Zhang, X.; Lu, J.; Liu, J.; Zhang, H.; Yang, Y.; Cui, J.; et al. Atherosclerotic Cardiovascular Disease Risk and Lipid-Lowering Therapy Requirement in China. Front. Cardiovasc. Med. 2022, 9, 839571. [Google Scholar] [CrossRef]
- Vasan, R.S.; Enserro, D.M.; Xanthakis, V.; Beiser, A.S.; Seshadri, S. Temporal Trends in the Remaining Lifetime Risk of Cardiovascular Disease Among Middle-Aged Adults Across 6 Decades: The Framingham Study. Circulation 2022, 145, 1324–1338. [Google Scholar] [CrossRef]
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A.; Global Burden of Cardiovascular. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef]
- Leopold, J.A.; Loscalzo, J. Oxidative mechanisms and atherothrombotic cardiovascular disease. Drug Discov. Today Ther. Strateg. 2008, 5, 5–13. [Google Scholar] [CrossRef]
- Sugiura, H.; Yoshida, T.; Mitobe, M.; Yoshida, S.; Shiohira, S.; Nitta, K.; Tsuchiya, K. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol. Dial. Transplant. 2010, 25, 60–68. [Google Scholar] [CrossRef]
- Wang, N.; Ma, J.; Ren, Y.; Xiang, S.; Jia, R. Secreted klotho from exosomes alleviates inflammation and apoptosis in acute pancreatitis. Am. J. Transl. Res. 2019, 11, 3375–3383. [Google Scholar] [PubMed]
- Donate-Correa, J.; Ferri, C.M.; Mora-Fernandez, C.; Perez-Delgado, N.; Gonzalez-Luis, A.; Navarro-Gonzalez, J.F. Pentoxifylline ameliorates subclinical atherosclerosis progression in patients with type 2 diabetes and chronic kidney disease: A randomized pilot trial. Cardiovasc. Diabetol. 2024, 23, 314. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Gao, Y.; Zhu, S.; Cui, Q.; Du, J. Klotho improves cardiac function by suppressing reactive oxygen species (ROS) mediated apoptosis by modulating Mapks/Nrf2 signaling in doxorubicin-induced cardiotoxicity. Med. Sci. Monit. 2017, 23, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Lee, P. Suppression of hypercholesterolemic atherosclerosis by pentoxifylline and its mechanism. Atherosclerosis 2007, 192, 313–322. [Google Scholar] [CrossRef]
- Crouch, S.P.; Fletcher, J. Effect of ingested pentoxifylline on neutrophil superoxide anion production. Infect. Immun. 1992, 60, 4504–4509. [Google Scholar] [CrossRef]
- Lehr, H.A.; Krombach, F.; Münzing, S.; Bodlaj, R.; Glaubitt, S.I.; Seiffge, D.; Hübner, C.; von Andrian, U.H.; Messmer, K. In vitro effects of oxidized low density lipoprotein on CD11b/CD18 and L-selectin presentation on neutrophils and monocytes with relevance for the in vivo situation. Am. J. Pathol. 1995, 146, 218–227. [Google Scholar]
- Chen, Y.M.; Chiang, W.C.; Lin, S.L.; Tsai, T.J. Therapeutic efficacy of pentoxifylline on proteinuria and renal progression: An update. J. Biomed. Sci. 2017, 24, 84. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; Chahin, J.; Méndez, M.L.; Gallego, E.; Macía, M.; del Castillo, N.; Rivero, A.; Getino, M.A.; et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: The PREDIAN trial. J. Am. Soc. Nephrol. 2015, 26, 220–229. [Google Scholar] [CrossRef]
- Chen, P.M.; Lai, T.S.; Chen, P.Y.; Lai, C.F.; Wu, V.; Chiang, W.C.; Chen, Y.M.; Wu, K.D.; Tsai, T.J. Renoprotective effect of combining pentoxifylline with angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in advanced chronic kidney disease. J. Formos. Med. Assoc. 2014, 113, 219–226. [Google Scholar] [CrossRef]
- McCormick, B.B.; Sydor, A.; Akbari, A.; Fergusson, D.; Doucette, S.; Knoll, G. The effect of pentoxifylline on proteinuria in diabetic kidney disease: A meta-analysis. Am. J. Kidney Dis. 2008, 52, 454–463. [Google Scholar] [CrossRef]
- Mostafa-Hedeab, G.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Jeandet, P.; Saad, H.M.; Batiha, G.E.S. A raising dawn of pentoxifylline in management of inflammatory disorders in COVID-19. Inflammopharmacology 2022, 30, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Ueng, K.C.; Jeng, J.S.; Charng, M.J.; Lin, T.H.; Chien, K.L.; Wang, C.Y.; Chao, T.H.; Liu, P.Y.; Su, C.H.; et al. 2017 Taiwan lipid guidelines for high risk patients. J. Formos. Med. Assoc. 2017, 116, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Chiang, J.H.; Hsu, H.J. Lower risk of musculoskeletal pain among patients with end-stage renal disease treated by hemodialysis: A frequency-matched retrospective cohort study. Medicine 2018, 97, e11935. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Goff, D.C.; Lloyd-Jones, D.M.; Smith, S.C.; Blum, C.; Schwartz, J.S.; 2013 ACC/AHA Cholesterol Guideline Panel. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: Synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann. Intern. Med. 2014, 160, 339–343. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chen, F.Y.; Lee, A.S.; Ting, K.H.; Chang, C.M.; Hsu, J.F.; Lee, W.S.; Sheu, J.R.; Chen, C.H.; Shen, M.Y. Sesamol reduces the atherogenicity of electronegative L5 LDL in vivo and in vitro. J. Nat. Prod. 2015, 78, 225–233. [Google Scholar] [CrossRef]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. STITCH: Interaction networks of chemicals and proteins. Nucleic Acids Res. 2008, 36, D684–D688. [Google Scholar] [CrossRef]
- Tsai, P.H.; Chen, L.Z.; Tseng, K.F.; Chen, F.Y.; Shen, M.Y. Apolipoprotein C3-rich low-density lipoprotein induces endothelial cell senescence via FBXO31 and its inhibition by sesamol in vitro and in vivo. Biomedicines 2022, 10, 854. [Google Scholar] [CrossRef]
- Tseng, K.F.; Tsai, P.H.; Wang, J.S.; Chen, F.Y.; Shen, M.Y. Sesamol attenuates renal inflammation and arrests reactive-oxygen-species-mediated IL-1beta secretion via the HO-1-induced inhibition of the IKKα/NFκB pathway in vivo and in vitro. Antioxidants 2022, 11, 2461. [Google Scholar] [CrossRef]
- Giralt-López, A.; Molina-Van den Bosch, M.; Vergara, A.; García-Carro, C.; Seron, D.; Jacobs-Cachá, C.; Soler, M.J. Revisiting experimental models of diabetic nephropathy. Int. J. Mol. Sci. 2020, 21, 3587. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Hsu, J.F.; Chen, F.Y.; Lu, J.; Chang, C.M.; Madjid, M.; Dean, J.; Dixon, R.A.F.; Shayani, S.; Chou, T.C.; et al. Combined LDL and VLDL electronegativity correlates with coronary heart disease risk in asymptomatic individuals. J. Clin. Med. 2019, 8, 1193. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Szklarczyk, D.; Pletscher-Frankild, S.; Blicher, T.H.; von Mering, C.; Jensen, L.J.; Bork, P.; STITCH. STITCH 4: Integration of protein-chemical interactions with user data. Nucleic Acids Res. 2014, 42, D401–D407. [Google Scholar] [CrossRef]
- Ortiz, A.; Covic, A.; Fliser, D.; Fouque, D.; Goldsmith, D.; Kanbay, M.; Mallamaci, F.; Massy, Z.A.; Rossignol, P.; Vanholder, R.; et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014, 383, 1831–1843. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Sánchez-Niño, M.D.; Donate-Correa, J.; Martín-Núñez, E.; Ferri, C.; Pérez-Delgado, N.; Górriz, J.L.; Martínez-Castelao, A.; Ortiz, A.; Mora-Fernández, C. Effects of pentoxifylline on soluble klotho concentrations and renal tubular cell expression in diabetic kidney disease. Diabetes Care 2018, 41, 1817–1820. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martín-Carro, B.; Cannata-Andía, J.B.; Mora-Fernández, C.; Navarro-González, J.F. Klotho, oxidative stress, and mitochondrial damage in kidney disease. Antioxidants 2023, 12, 239. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular disease in chronic kidney disease: Pathophysiological insights and therapeutic options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Wang, G.J.; Chang, C.T.; Yang, C.Y.; Chen, C.H. Negatively charged L5 as a naturally occurring atherogenic low-density lipoprotein. BioMedicine 2012, 2, 147–154. [Google Scholar] [CrossRef]
- Atabek, M.E.; Kurtoglu, S.; Selver, B.; Baykara, M. Effectiveness of pentoxifylline on the cross-sectional area of intima media thickness and functions of the common carotid artery in adolescents with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2011, 24, 945–951. [Google Scholar] [CrossRef]
- Fernandes, J.L.; de Oliveira, R.T.D.; Mamoni, R.L.; Coelho, O.R.; Nicolau, J.C.; Blotta, M.H.S.L.; Serrano, C.V. Pentoxifylline reduces pro-inflammatory and increases anti-inflammatory activity in patients with coronary artery disease—A randomized placebo-controlled study. Atherosclerosis 2008, 196, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Herskovits, E.; Famulari, A.; Tamaroff, L.; Gonzalez, A.M.; Vzquez, A.; Dominguez, R.; Fraiman, H.; Vila, J.; Benjamin, V.; Matera, V. Comparative study of pentoxifylline vs antiaggregants in patients with transient ischaemic attacks. Acta Neurol. Scand. Suppl. 1989, 127, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Herskovits, E.; Famulari, A.; Tamaroff, L.; Gonzalez, A.M.; Vázquez, A.; Dominguez, R.; Fraiman, H.; Vila, J. Preventive treatment of cerebral transient ischemia: Comparative randomized trial of pentoxifylline versus conventional antiaggregants. Eur. Neurol. 1985, 24, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Kleber, M.E.; Rohrer, L.; Lehmann, M.; Triem, S.; Jennings, R.T.; Petrakis, I.; Dressel, A.; Lepper, P.M.; Scharnagl, H.; et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur. Heart J. 2017, 38, 1597–1607. [Google Scholar] [CrossRef]

| Variable | Non-Pentoxifylline Users | Pentoxifylline Users | p-Value |
|---|---|---|---|
| n (%)/Mean ± SD | n (%)/Mean ± SD | ||
| All | 31,141 | 31,141 | |
| Sex | 0.9232 | ||
| Female | 14,529 (46.66) | 14,541 (46.69) | |
| Male | 16,612 (53.34) | 16,600 (53.31) | |
| Age (y) | 0.9992 | ||
| <50 | 2642 (8.48) | 2649 (8.51) | |
| 50–59 | 5508 (17.69) | 5491 (17.63) | |
| 60–69 | 8536 (27.41) | 8560 (27.49) | |
| 70–79 | 8186 (26.29) | 8171 (26.24) | |
| ≥80 | 6269 (20.13) | 6270 (20.13) | |
| Mean age | 67.75 ± 13.00 | 67.75 ± 12.96 | 0.9914 |
| Comorbidities | |||
| AMI | 0.2974 | ||
| No | 30,884 (99.17) | 30,907 (99.25) | |
| Yes | 257 (0.83) | 234 (0.75) | |
| Hypertension | 0.8354 | ||
| No | 4352 (13.98) | 4370 (14.03) | |
| Yes | 26,789 (86.02) | 26,771 (85.97) | |
| COPD | 0.7386 | ||
| No | 26,352 (84.62) | 26,382 (84.72) | |
| Yes | 4789 (15.38) | 4759 (15.28) | |
| LC | 0.5868 | ||
| No | 29,631 (95.15) | 29,660 (95.24) | |
| Yes | 1510 (4.85) | 1481 (4.76) | |
| Autoimmune diseases | 0.2023 | ||
| No | 30,884 (99.17) | 30,912 (99.26) | |
| Yes | 257 (0.83) | 229 (0.74) | |
| Follow-up period (y) | 3.09 ± 2.13 | 3.18 ± 2.20 | <0.0001 |
| Variable | Event | Person-y | IR | Crude | Adjusted | ||
|---|---|---|---|---|---|---|---|
| N = 8134 | 100 Person-y | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Pentoxifylline | |||||||
| No | 3321 | 96,190 | 3.45 | 1 (Reference) | 1 (Reference) | ||
| Yes | 4813 | 99,149 | 4.85 | 1.41 (1.35, 1.47) | <0.0001 | 1.41 (1.34, 1.47) | <0.0001 |
| Sex | |||||||
| Female | 3587 | 91,169 | 3.93 | 1 (Reference) | 1 (Reference) | ||
| Male | 4547 | 104,170 | 4.36 | 1.11 (1.06, 1.16) | <0.0001 | 1.12 (1.07, 1.17) | <0.0001 |
| Age (y) | |||||||
| <50 | 672 | 19,274 | 3.49 | 1 (Reference) | 1 (Reference) | ||
| 50–59 | 1585 | 38,989 | 4.07 | 1.16 (1.06, 1.27) | 0.0010 | 1.14 (1.04, 1.24) | 0.0058 |
| 60–69 | 2385 | 55,353 | 4.31 | 1.22 (1.12, 1.33) | <0.0001 | 1.16 (1.07, 1.27) | 0.0007 |
| 70–79 | 2213 | 50,785 | 4.36 | 1.24 (1.13, 1.35) | <0.0001 | 1.14 (1.04, 1.24) | 0.0042 |
| ≥80 | 1279 | 30,938 | 4.13 | 1.15 (1.05, 1.27) | 0.0030 | 1.04 (0.94, 1.14) | 0.4763 |
| Mean age | |||||||
| Comorbidities | |||||||
| AMI | |||||||
| No | 8013 | 194,346 | 4.12 | 1 (Reference) | 1 (Reference) | ||
| Yes | 121 | 993 | 12.18 | 2.88 (2.41, 3.44) | <0.0001 | 2.75 (2.30, 3.30) | <0.0001 |
| Hypertension | |||||||
| No | 792 | 30,352 | 2.61 | 1 (Reference) | 1 (Reference) | ||
| Yes | 7342 | 164,987 | 4.45 | 1.69 (1.57, 1.82) | <0.0001 | 1.66 (1.54, 1.79) | <0.0001 |
| COPD | |||||||
| No | 6909 | 169,267 | 4.08 | 1 (Reference) | 1 (Reference) | ||
| Yes | 1225 | 26,072 | 4.70 | 1.14 (1.07, 1.21) | <0.0001 | 1.10 (1.03, 1.17) | 0.0025 |
| LC | |||||||
| No | 7866 | 187,425 | 4.20 | 1 (Reference) | 1 (Reference) | ||
| Yes | 268 | 7913 | 3.39 | 0.80 (0.71, 0.90) | 0.0003 | 0.79 (0.70, 0.90) | 0.0002 |
| Autoimmune diseases | |||||||
| No | 8065 | 193,904 | 4.16 | 1 (Reference) | 1 (Reference) | ||
| Yes | 69 | 1435 | 4.81 | 1.15 (0.91, 1.46) | 0.2445 | 1.19 (0.94, 1.50) | 0.1588 |
| Variable | Event | Person-y | IR | Crude | Adjusted | ||
|---|---|---|---|---|---|---|---|
| N = 8134 | 100 Person-y | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Pentoxifylline | |||||||
| No | 3321 | 96,190 | 3.45 | 1 (Reference) | 1 (Reference) | ||
| 1–70 days | 1546 | 20,890 | 7.40 | 2.14 (2.01, 2.27) | <0.0001 | 2.16 (2.03, 2.29) | <0.0001 |
| 71–308 days | 1578 | 21,636 | 7.29 | 2.10 (1.98, 2.23) | <0.0001 | 2.11 (1.99, 2.24) | <0.0001 |
| 309–763 days | 1040 | 20,932 | 4.97 | 1.42 (1.33, 1.53) | <0.0001 | 1.41 (1.32, 1.51) | <0.0001 |
| >763 days | 649 | 35,690 | 1.82 | 0.53 (0.49, 0.58) | <0.0001 | 0.53 (0.49, 0.58) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-S.; Tsai, P.-H.; Tseng, K.-F.; Lin, C.-L.; Chen, F.-Y.; Chang, C.-T.; Shen, M.-Y. Long-Term Pentoxifylline Therapy Is Associated with a Reduced Risk of Atherosclerotic Cardiovascular Disease by Inhibiting Oxidative Stress and Cell Apoptosis in Diabetic Kidney Disease Patients. Antioxidants 2024, 13, 1471. https://doi.org/10.3390/antiox13121471
Wang J-S, Tsai P-H, Tseng K-F, Lin C-L, Chen F-Y, Chang C-T, Shen M-Y. Long-Term Pentoxifylline Therapy Is Associated with a Reduced Risk of Atherosclerotic Cardiovascular Disease by Inhibiting Oxidative Stress and Cell Apoptosis in Diabetic Kidney Disease Patients. Antioxidants. 2024; 13(12):1471. https://doi.org/10.3390/antiox13121471
Chicago/Turabian StyleWang, Jie-Sian, Ping-Hsuan Tsai, Kuo-Feng Tseng, Cheng-Li Lin, Fang-Yu Chen, Chiz-Tzung Chang, and Ming-Yi Shen. 2024. "Long-Term Pentoxifylline Therapy Is Associated with a Reduced Risk of Atherosclerotic Cardiovascular Disease by Inhibiting Oxidative Stress and Cell Apoptosis in Diabetic Kidney Disease Patients" Antioxidants 13, no. 12: 1471. https://doi.org/10.3390/antiox13121471
APA StyleWang, J.-S., Tsai, P.-H., Tseng, K.-F., Lin, C.-L., Chen, F.-Y., Chang, C.-T., & Shen, M.-Y. (2024). Long-Term Pentoxifylline Therapy Is Associated with a Reduced Risk of Atherosclerotic Cardiovascular Disease by Inhibiting Oxidative Stress and Cell Apoptosis in Diabetic Kidney Disease Patients. Antioxidants, 13(12), 1471. https://doi.org/10.3390/antiox13121471






