VdCHS2 Overexpression Enhances Anthocyanin Biosynthesis, Modulates the Composition Ratio, and Increases Antioxidant Activity in Vitis davidii Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cell Growth
2.2. Construction of Plant Vector for Overexpression of VdCHS2 Gene
2.3. Agrobacterium-Mediated Genetic Transformation of Spine Grape Cells
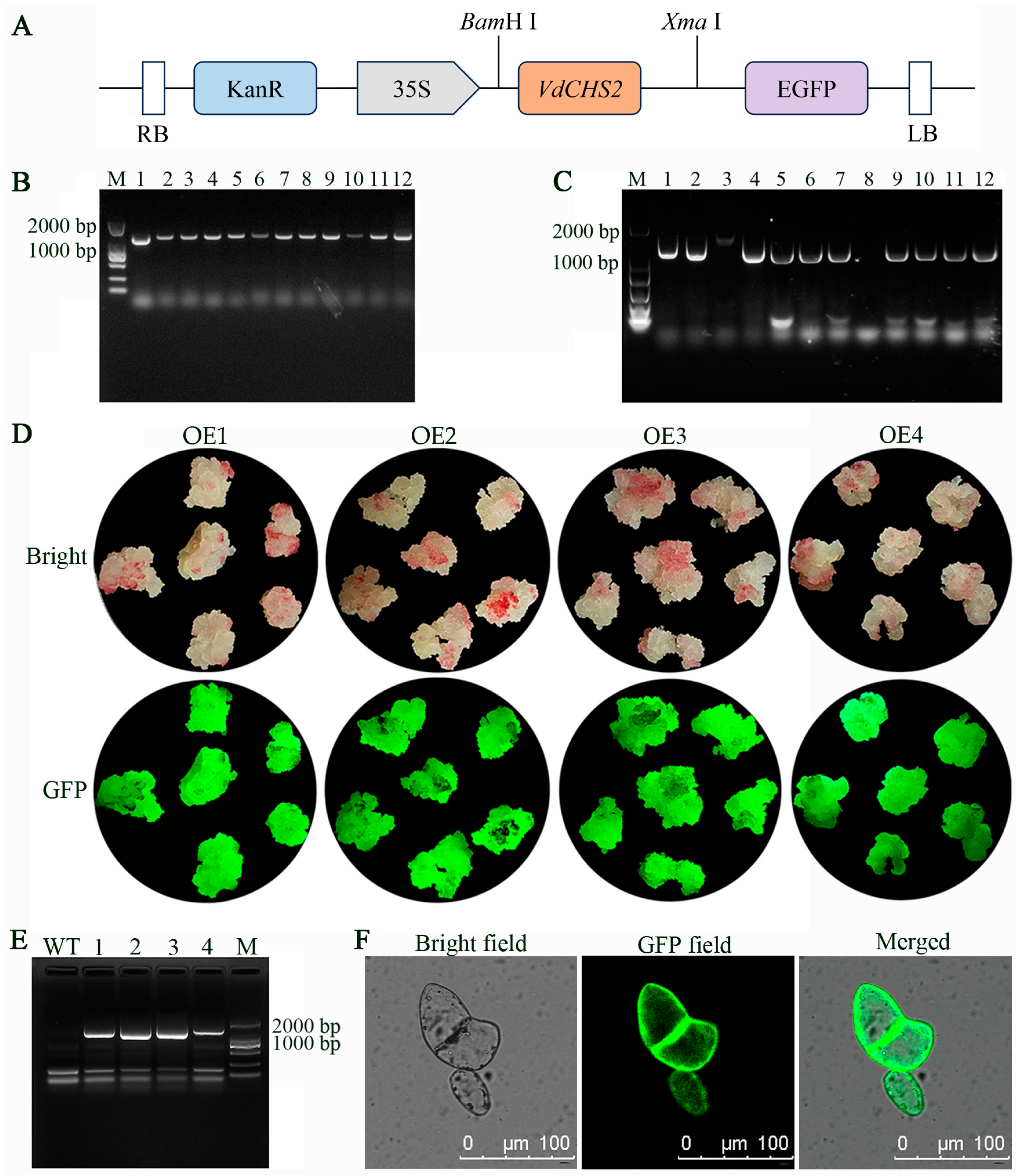
2.4. Screening and Identification of Transgenic Positive Cells
2.5. Subcellular Localization Analysis
2.6. Determination of Anthocyanin, Flavonoid, and Proanthocyanidin Contents
2.7. Metabolomic Analysis of Anthocyanins in Spine Grape Cells
2.8. RT-qPCR Analysis of Genes Associated with Anthocyanin Synthesis
2.9. Antioxidant Activity Analysis
2.10. VdCHS2 Suspension Cell Culture and Metabolite Determination
2.11. Statistical Analysis
3. Results
3.1. Construction of VdCHS2 Overexpression Vector and Genetic Transformation
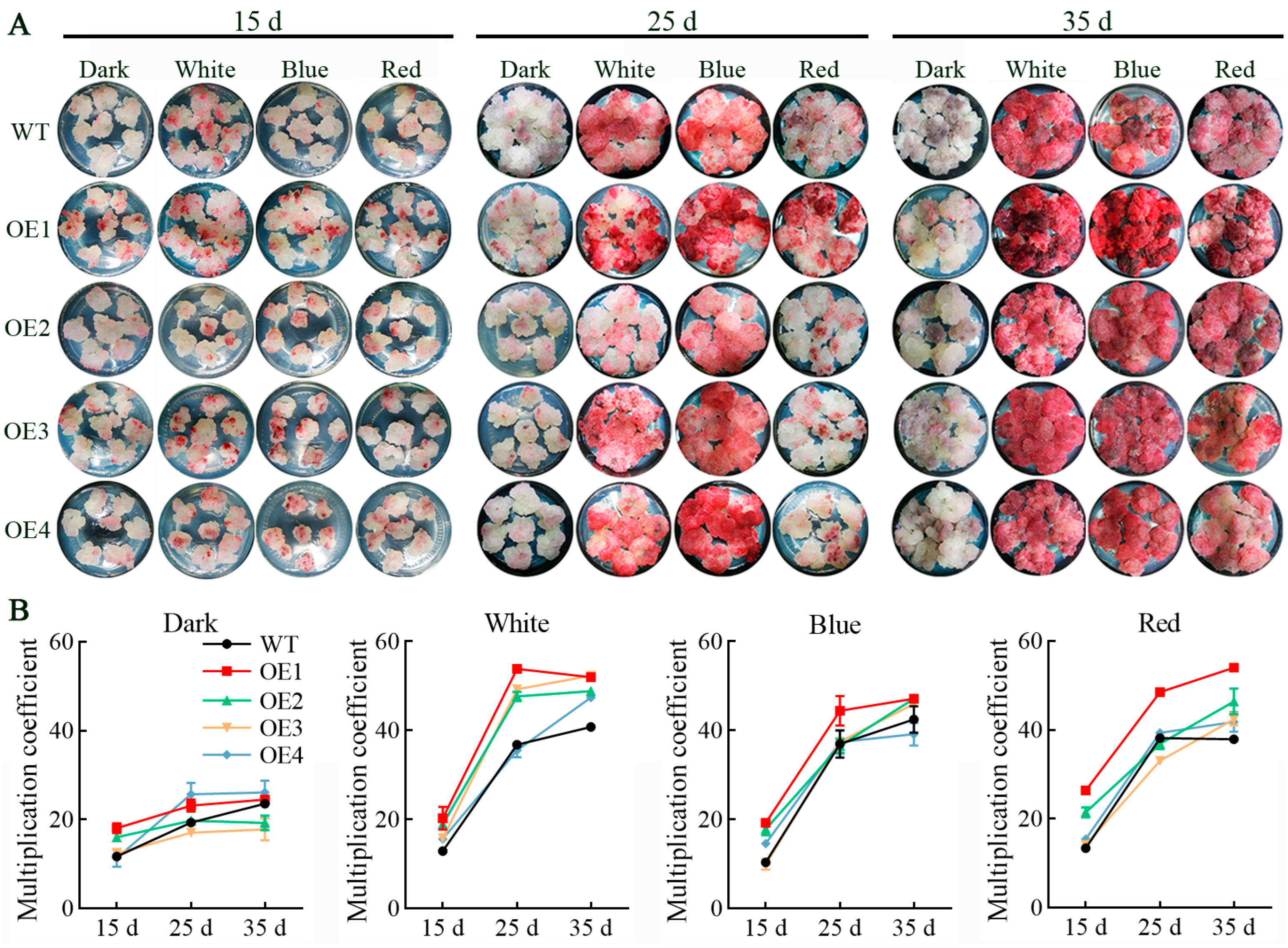
3.2. Light Spectrum Affects Growth and Proliferation of VdCHS2-Transformed Cells
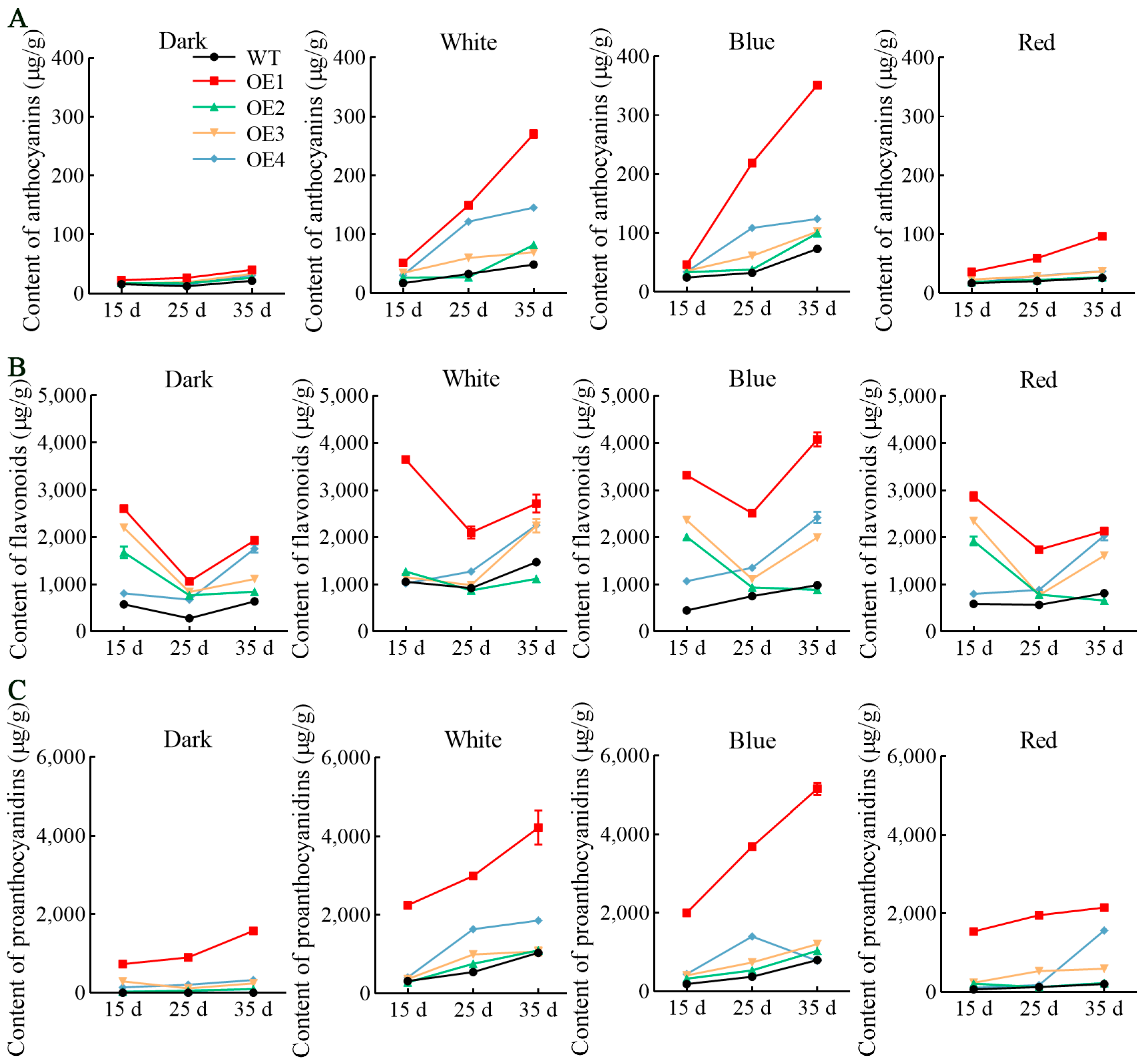
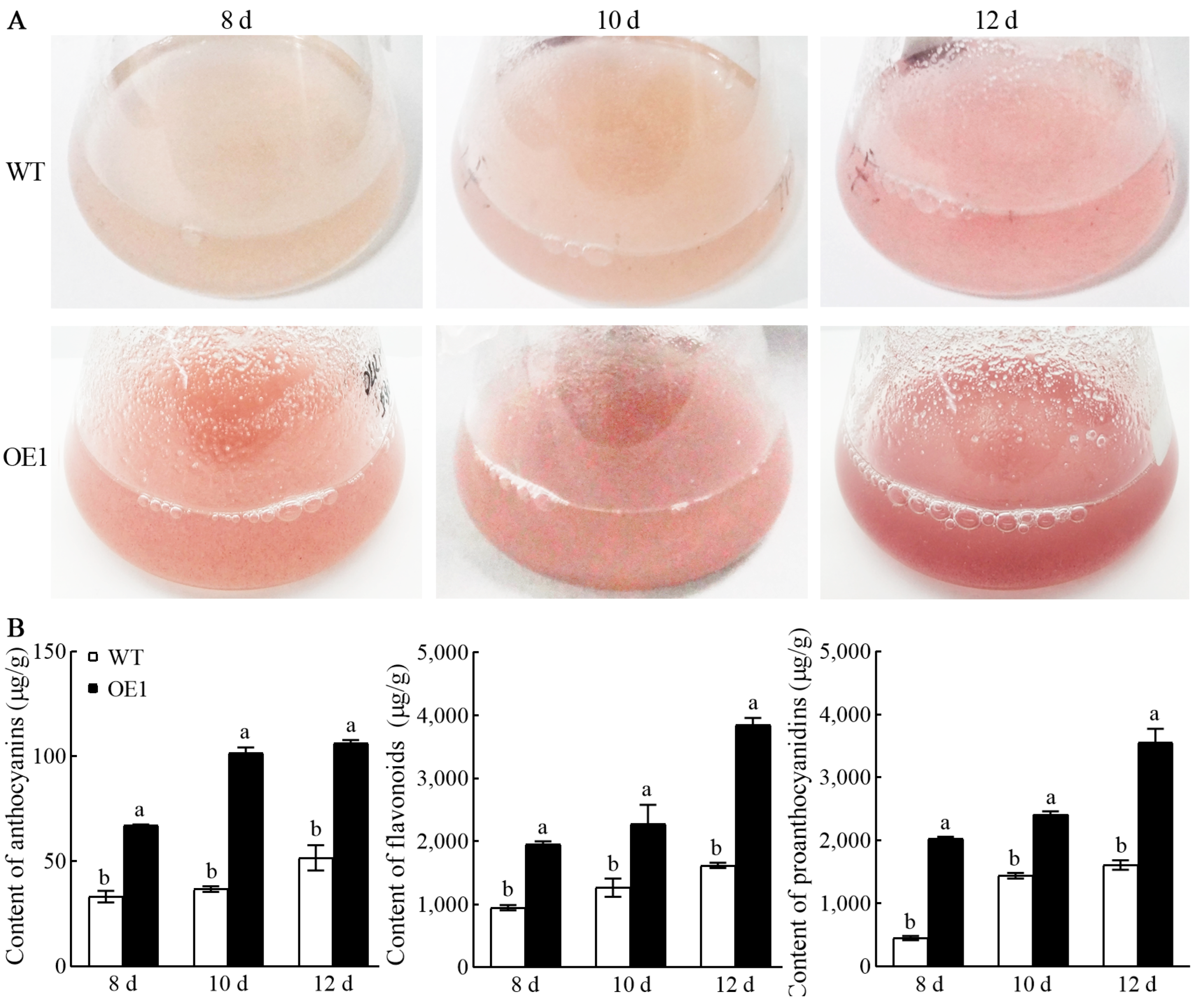
3.3. Light Spectrum Significantly Improved Accumulation of Anthocyanins, Flavonoids, and Proanthocyanidins in VdCHS2-Transformed Cell Lines
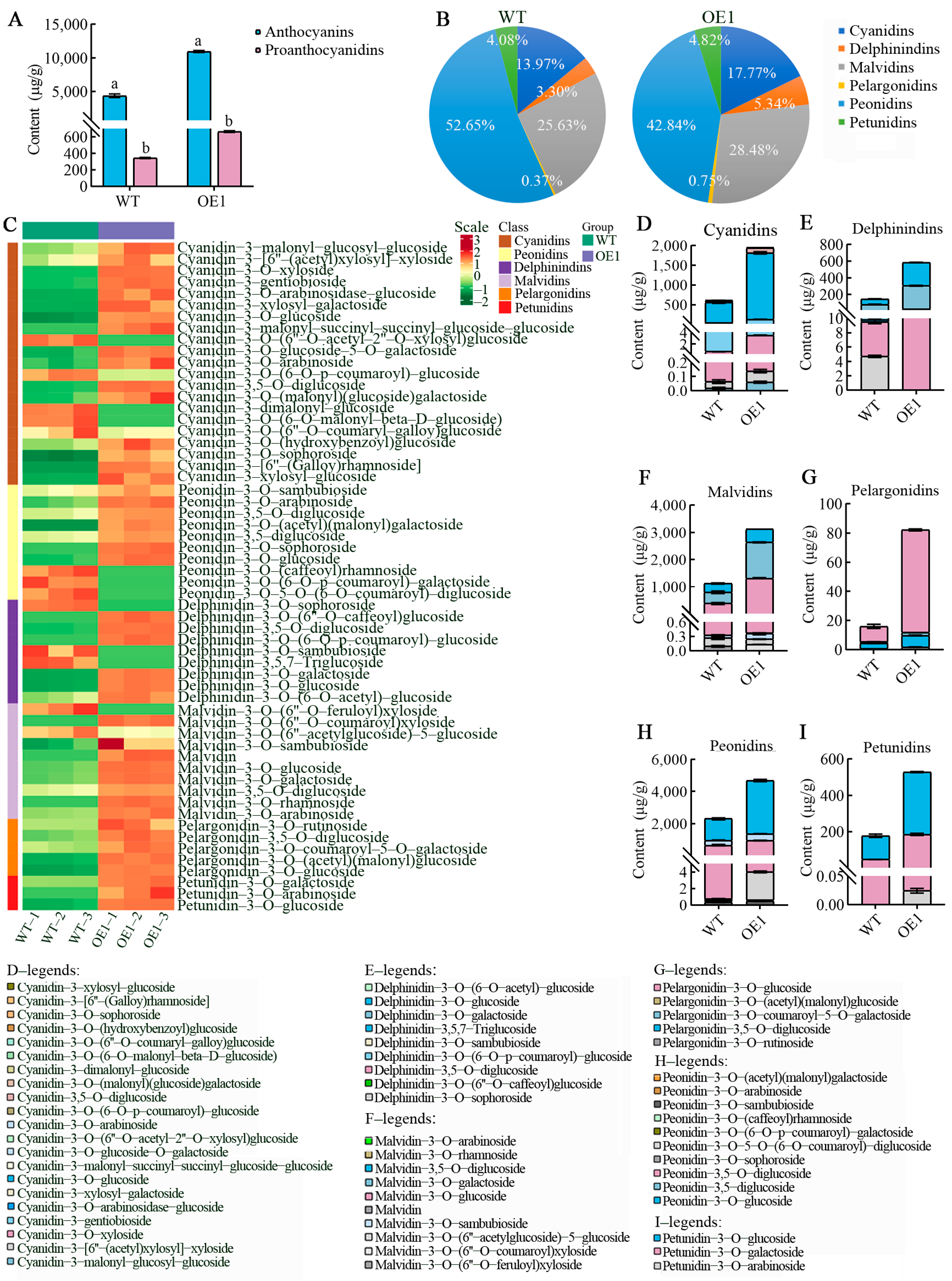
3.4. VdCHS2 Affected Anthocyanin Accumulation and Its Component Changes
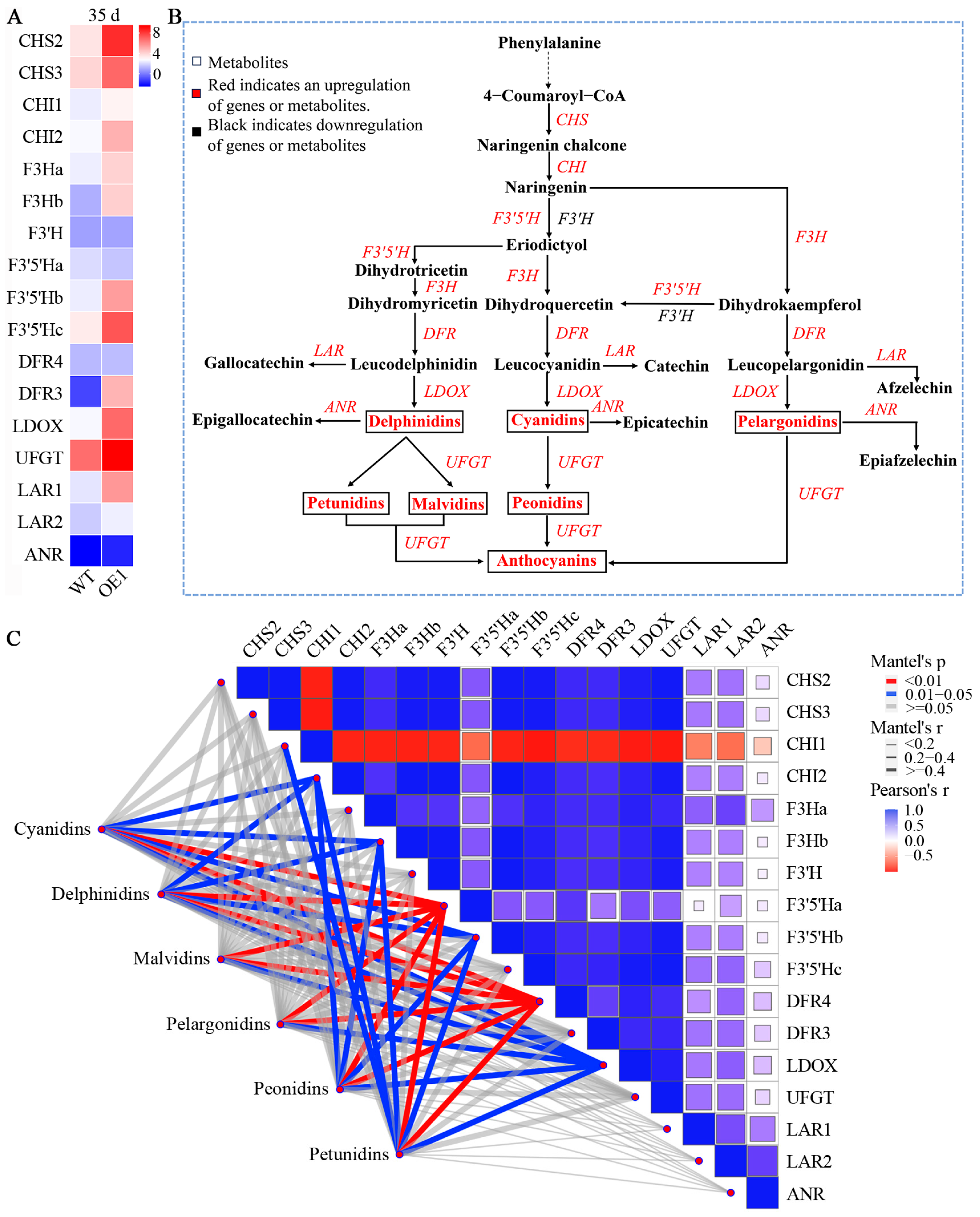
3.5. Overexpression of VdCHS2 Affects Expression of Anthocyanin Synthesis Genes and Metabolites
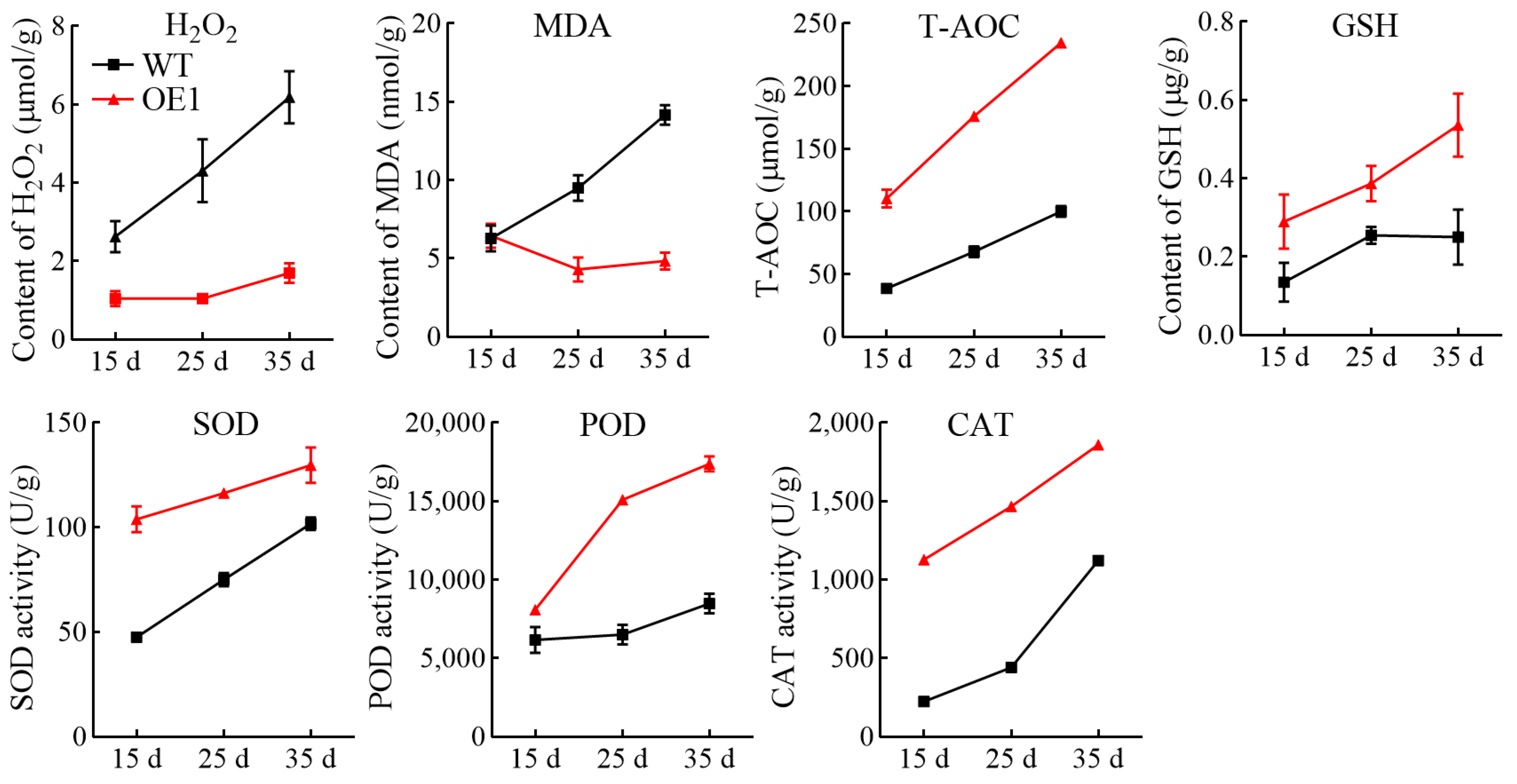
3.6. Overexpression of VdCHS2 Significantly Increased Antioxidant Activity in Spine Grape Cells
3.7. Construction of Anthocyanin Plant Cell Factory Based on CHS2 Overexpression
4. Discussion
4.1. The CHS Gene Promotes the Growth and Maturation of Spine Grape Cells
4.2. Short-Wavelength Light Promoted the Synthesis and Accumulation of Secondary Metabolites Such as Anthocyanins
4.3. The CHS Gene Increased Anthocyanin Accumulation and Affected the Composition of Anthocyanins
4.4. The CHS Gene Increased the Antioxidant Activity of Spine Grape Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, S.; Xu, X.; Gao, Q.; Huang, C.; Luo, Z.P.; Liu, P.P.; Wu, M.Z.; Huang, H.T.; Yang, J.; Zeng, J.M.; et al. NtERF4 promotes the biosynthesis of chlorogenic acid and flavonoids by targeting PAL genes in Nicotiana tabacum. Planta 2023, 259, 31. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Sun, L.X.; Xi, D.D.; Li, X.F.; Gao, L.; Miao, L.M.; Luo, Y.; Tian, M.M.; Zhu, H.F. Methyl jasmonate-induced bHLH42 mediates tissue-specific accumulation of anthocyanins via regulating flavonoid metabolism-related pathways in Caitai. Physiol. Plant. 2024, 176, e14434. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2023, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Y.; Zhong, M.Y.; Zhang, Q.S.; Zhao, W.; Wang, M.X.; Luo, C.Y. Phylogenetic Implications and Functional Disparity in the Chalcone synthase Gene Family of Common Seagrass Zostera marina. Front. Mar. Sci. 2021, 8, 760902. [Google Scholar] [CrossRef]
- Liu, W.Y.; Feng, Y.; Yu, S.H.; Fan, Z.Q.; Li, X.L.; Li, J.Y.; Yin, H.F. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Reimold, U.; Kröger, M.; Kreuzaler, F.; Hahlbrock, K. Coding and 3′ non-coding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J. 1983, 2, 1801–1805. [Google Scholar] [CrossRef]
- Oberholzer, V.; Durbin, M.L.; Clegg, M.T. Comparative genomics of chalcone synthase and Myb genes in the grass family. Genes Genet. Syst. 2000, 75, 1–16. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Xu, F.; Li, L.L.; Cheng, H.; Zhang, W.W. Seasonal pattern of flavonoid content and related enzyme activities in leaves of Ginkgo biloba L. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 98–106. [Google Scholar] [CrossRef]
- Hermes, V.S.; Dall’asta, P.; Amaral, F.P.; Anacleto, K.B.; Arisi, A.C.M. The regulation of transcription of genes related to oxidative stress and glutathione synthesis in Zea mays leaves by nitric oxide. Biologia Plantarum. 2013, 57, 620–626. [Google Scholar] [CrossRef]
- Schnitzler, J.; Jungblut, T.; Heller, W.; Köfferlein, M.; Hutzler, P.; Heinzmann, U.; Schmelzer, E.; Ernst, D.; Langebartels, C.; Sandermann, H. Tissue localization of U.V.-B-screening pigments and of chalcone synthase mRNA in needles of scots pine seedlings. New Phytol. 1996, 132, 247–258. [Google Scholar] [CrossRef]
- Zhou, X.W.; Li, J.Y.; Zhu, Y.L.; Ni, S.; Chen, J.L.; Feng, X.J.; Zhang, Y.F.; Li, S.Q.; Zhu, H.G.; Wen, Y.G. De novo assembly of the Camellia nitidissima transcriptome reveals key genes of flower pigment biosynthesis. Front. Plant Sci. 2017, 8, 1545–1560. [Google Scholar] [CrossRef]
- Gao, J.F.; Shen, L.; Yuan, J.L.; Zheng, H.L.; Su, Q.S.; Yang, W.G.; Zhang, L.Q.; Nnaemeka, V.E.; Sun, J.; Ke, L.; et al. Functional analysis of GhCHS, GhANR and GhLAR in colored fiber formation of Gossypium hirsutum L. BMC Plant Biol. 2020, 20, 28. [Google Scholar] [CrossRef]
- Ma, X.T.; Hou, Y.Y.; Umar, A.W.; Wang, Y.H.; Yu, L.L.; Ahmad, N.; Yao, N.; Zhang, M.; Liu, X.M. Safflower CtFLS1-Induced Drought Tolerance by Stimulating the Accumulation of Flavonols and Anthocyanins in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 5546. [Google Scholar] [CrossRef]
- Chen, L.J.; Guo, H.M.; Lin, Y.; Chen, H.M. Chalcone synthase EaCHS1 from Eupatorium adenophorum functions in salt stress tolerance in tobacco. Plant Cell Rep. 2015, 34, 885–894. [Google Scholar]
- Shi, N.; Pei, X.X.; Li, M.Y.; Chen, W.T.; Li, H.Q.; Yang, G.S.; Duan, C.Q.; Wang, J. Metabolomics of Vitis davidii Foëx. grapes from southern China: Flavonoids and volatiles reveal the flavor profiles of five spine grape varieties. Food Chem. 2024, 454, 139732. [Google Scholar] [CrossRef]
- Lai, C.C.; Que, Q.X.; Pan, R.; Wang, Q.; Gao, H.Y.; Guan, X.F.; Che, J.M.; Lai, G.T. The Single-Stranded DNA-Binding Gene Whirly (Why1) with a Strong Pathogen-Induced Promoter from Vitis pseudoreticulata Enhances Resistance to Phytophthora capsici. Int. J. Mol. Sci. 2022, 23, 8052. [Google Scholar] [CrossRef]
- Meng, J.F.; Fang, Y.L.; Qin, M.Y.; Zhuang, X.F.; Zhang, Z.W. Varietal differences among the phenolic profiles and antioxidant properties of four cultivars of spine grape (Vitis davidii Foex) in Chongyi County (China). Food Chem. 2012, 134, 2049–2056. [Google Scholar] [CrossRef]
- Han, F.L.; Ju, Y.L.; Ruan, X.R.; Zhao, X.F.; Yue, X.F.; Zhuang, X.F.; Qin, M.Y.; Fang, Y.L. Color, anthocyanin, and antioxidant characteristics of young wines produced from spine grapes (Vitis davidii Foex) in China. Food Nutr. Res. 2017, 61, 1339552. [Google Scholar] [CrossRef]
- Wei, H.R.; Chen, X.; Zong, X.J.; Shu, H.R.; Gao, D.S.; Liu, Q.Z. Comparative transcriptome analysis of genes involved in anthocyanin biosynthesis in the red and yellow fruits of sweet Cherry (Prunus avium L.). PLoS ONE 2015, 10, e0121164. [Google Scholar] [CrossRef]
- Lai, C.C.; Pan, H.; Huang, X.G.; Fan, L.H.; Duan, C.Q.; Li, S.Z. Validation of reference genes for gene expression analysis of response to anthocyanin induction in cell cultures of Vitis davidii (Rom. Caill.) Foëx. Vitr. Cell. Dev. Biol. Plant 2018, 54, 642–657. [Google Scholar] [CrossRef]
- Lai, C.C.; Zhang, J.; Lai, G.T.; He, L.Y.; Xu, h.; Li, S.Y.; Che, J.M.; Wang, Q.; Guan, X.F.; Huang, J.Q.; et al. Targeted regulation of 5-aminolevulinic acid enhances flavonoids, anthocyanins and proanthocyanidins accumulation in Vitis davidii callus. BMC Plant Biol. 2024, 24, 944. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Pan, H.; Zhang, J.; Wang, Q.; Que, Q.X.; Pan, R.; Lai, Z.X.; Lai, G.T. Light Quality Modulates Growth, Triggers Differential Accumulation of Phenolic Compounds, and Changes the Total Antioxidant Capacity in the Red Callus of Vitis davidii. J. Agric. Food Chem. 2022, 70, 13264–13278. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.T.; Fu, P.N.; He, L.Y.; Che, J.M.; Wang, Q.; Lai, P.F.; Lu, J.; Lai, C.C. CRISPR/Cas9 mediated CHS2 mutation provides a new insight into resveratrol biosynthesis by causing a metabolic pathway shift from flavonoids to stilbenoids in Vitis davidii cells. Hortic. Res. 2024, uhae268. [Google Scholar] [CrossRef]
- Ji, X.L.; Ren, J.; Zhang, Y.X.; Lang, S.Y.; Wang, D.; Song, X.S. Integrated Analysis of the Metabolome and Transcriptome on Anthocyanin Biosynthesis in Four Developmental Stages of Cerasus humilis Peel Coloration. Int. J. Mol. Sci. 2021, 22, 11880. [Google Scholar] [CrossRef]
- Che, J.M.; Lai, C.C.; Lai, G.T.; Chen, Q.Q.; Liu, G.H.; Liu, B. Effects of a Mixture of Brevibacillus brevis with Other Bacillus sp. Strains against Gray Mold and on Enzyme Activities of Grape. Agronomy 2023, 13, 1724. [Google Scholar] [CrossRef]
- Hu, H.G.; Hu, Y.L.; Pang, Z.C.; Cheng, J.J.; Duan, Y.J.; Li, W.M.; Sun, D.Q. Bioinformatics Analysis of Banana Chalcone Synthase Gene Family. Mol. Plant Breed. 2018, 16, 6931–6937. [Google Scholar]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zheng, X.T.; Sun, B.M.; Peng, C.; Chow, W. Over-expression of the CHS gene enhances resistance of Arabidopsis leaves to high light. Environ. Exp. Bot. 2018, 154, 33–43. [Google Scholar] [CrossRef]
- Hou, Q.D.; Li, S.; Shang, C.Q.; Wen, Z.; Cai, X.W.; Hong, Y.; Qiao, G. Genome-wide characterization of chalcone synthase genes in sweet cherry and functional characterization of CpCHS1 under drought stress. Front. Plant Sci. 2022, 13, 989959. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, L.X.; Tao, S.R.; Lin, H.Y.; Wang, J.B.; Tan, F.R.; Zhao, K.; Wu, X.; Li, P.; Pan, A.H.; et al. Validation of a carnation-specific gene ANS used as an endogenous reference gene in qualitative and real-time quantitative PCR for carnations. Jaoac. Int. 2011, 94, 1227–1232. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Li, S.G.; An, H.S.; Zhang, X.Y.; Zhou, B.Q. Integrated transcriptome and metabolome analysis reveals the anthocyanin biosynthesis mechanisms in blueberry (Vaccinium corymbosum L.) leaves under different light qualities. Front. Plant Sci. 2022, 13, 1073332. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Shakeel, M.; Wang, D.L.; Qu, C.P.; Yang, S.M.; Ahmad, S.; Song, Z.J. Metabolome and transcriptome profiling unveil the mechanisms of light-induced anthocyanin synthesis in rabbiteye blueberry (vaccinium ashei: Reade). BMC Plant Biol. 2022, 22, 223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schouten, R.E.; Tikunov, Y.; Liu, X.X.; Visser, R.G.F.; Tan, F.; Bovy, A.; Marcelis, L.F.M. Blue light increases anthocyanin content and delays fruit ripening in purple pepper fruit. Postharvest Biol. Technol. 2022, 192, 112024. [Google Scholar] [CrossRef]
- Ni, J.B.; Liao, Y.F.; Zhang, M.M.; Pan, C.; Yang, Q.S.; Bai, S.L.; Teng, Y.W. Blue Light Simultaneously Induces Peel Anthocyanin Biosynthesis and Flesh Carotenoid/Sucrose Biosynthesis in Mango Fruit. J. Agric. Food Chem. 2022, 70, 16021–16035. [Google Scholar] [CrossRef]
- Jiang, M.M.; Ren, L.; Lian, H.L.; Liu, Y.; Chen, H.Y. Novel insight into the mechanism underlying light-controlled anthocyanin accumulation in eggplant (Solanum melongena L.). Plant Sci. 2016, 249, 46–58. [Google Scholar] [CrossRef]
- Miao, L.X.; Zhang, Y.C.; Yang, X.F.; Xiao, J.P.; Zhang, H.Q.; Zhang, Z.F.; Wang, Y.Z.; Jiang, G.H. Colored light-quality selective plastic films affect anthocyanin content, enzyme activities, and the expression of flavonoid genes in strawberry (Fragaria × ananassa) fruit. Food Chem. 2016, 207, 93–100. [Google Scholar] [CrossRef]
- Tao, R.Y.; Bai, S.L.; Ni, J.B.; Yang, Q.S.; Zhao, Y.; Teng, Y.W. The blue light signal transduction pathway is involved in anthocyanin accumulation in ‘Red Zaosu’ pear. Planta 2018, 248, 37–48. [Google Scholar] [CrossRef]
- Liu, L.; Kong, J.H.; Fan, P.G.; Wang, Y.J.; Duan, W.; Liang, Z.C.; Matus, J.T.; Dai, Z.W. Supplementing with monochromatic blue LED light during the day, rather than at night, increases anthocyanins in the berry skin of grapevine (Vitis vinifera L.). Planta 2024, 260, 69. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.Q.; Lu, J.Y.; Sun, Y.H.; Cui, Y.Y. Research progress of proanthocyanidins and anthocyanidins. Phytother. Res. 2023, 37, 2552–2577. [Google Scholar] [CrossRef]
- Yoshida, K. Chemical and biological study of flavonoid-related plant pigment: Current findings and beyond. Biosci. Biotechnol. Biochem. 2024, 88, 705–718. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; Chu, D.K.; Wang, X.M. Exploring the evolution of CHS gene family in plants. Front. Genet. 2024, 15, 1368358. [Google Scholar] [CrossRef]
- Roslan, N.; Huy, T.S.; Ming, W.S.; Khalid, N.; Mohamed, Z. Tissue specific expression of chalcone synthase (chs) transcripts and overexpression of BrCHS in cell suspension cultures of Boesenbergia rotunda. Malays. J. Sci. 2020, 39, 92–110. [Google Scholar] [CrossRef]
- Yin, Y.C.; Hou, J.M.; Tian, S.K.; Yang, L.; Zhang, Z.X.; Li, W.D.; Liu, Y. Overexpressing chalcone synthase (CHS) gene enhanced flavonoids accumulation in Glycyrrhiza uralensis hairy roots. Bot. Lett. 2019, 167, 219–231. [Google Scholar] [CrossRef]
- Kasai, A.; Kasai, K.; Yumoto, S.; Senda, M. Structural features of GmIRCHS, candidate of the I gene inhibiting seed coat pigmentation in soybean: Implications for inducing endogenous RNA silencing of chalcone synthase genes. Plant Mol. Biol. 2007, 64, 467–479. [Google Scholar] [CrossRef]
- Wang, Y.; Dou, Y.; Wang, R.; Guan, X.L.; Hu, Z.H.; Zheng, J. Molecular characterization and functional analysis of chalcone synthase from Syringa oblata Lindl. in the flavonoid biosynthetic pathway. Gene 2017, 635, 16–23. [Google Scholar] [CrossRef]
- Wang, F.; Ji, G.S.; Xu, Z.B.; Feng, B.; Zhou, Q.; Fan, X.L.; Wang, T. Metabolomics and transcriptomics provide insights into anthocyanin biosynthesis in the developing grains of purple wheat (Triticum aestivum L.). J. Agric. Food Chem. 2021, 69, 11171–11184. [Google Scholar] [CrossRef]
- Abdel-Aal, E.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef]
- He, W.; Zeng, M.M.; Chen, J.; Jiao, Y.Z.; Niu, F.X.; Tao, G.J.; Zhang, S.; Qin, F.; He, Z.Y. Identification and quantitation of anthocyanins in purple-fleshed sweet potatoes cultivated in China by UPLC-PDA and UPLC-QTOF-MS/MS. J. Agric. Food Chem. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Jiang, L.Y.; Li, Y.L.; Chen, Q.; Ye, Y.T.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.R.; Tang, H.R. Effect of red and blue light on anthocyanin accumulation and differential gene expression in strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef]
- Yang, X.W.; Wang, X.Y.; Ji, L.; Yi, Z.L.; Fu, C.X.; Ran, J.C.; Hu, R.B.; Zhou, G.K. Overexpression of a Miscanthus lutarioriparius NAC gene MlNAC5 confers enhanced drought and cold tolerance in Arabidopsis. Plant Cell Rep. 2014, 34, 943–958. [Google Scholar] [CrossRef]
- Wang, F.B.; Ren, G.L.; Li, F.S.; Qi, S.T.; Xu, Y.; Wang, B.W.; Yang, Y.L.; Ye, Y.X.; Zhou, Q.; Chen, X.H. A chalcone synthase gene AeCHS from Abelmoschus esculentus regulates flavonoid accumulation and abiotic stress tolerance in transgenic Arabidopsis. Acta Physiol. Plant 2018, 40, 97. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Khalid, M.F.; Jawaid, M.Z.; Nawaz, M.; Shakoor, R.A.; Ahmed, T. Employing Titanium Dioxide Nanoparticles as Biostimulant against Salinity: Improving Antioxidative Defense and Reactive Oxygen Species Balancing in Eggplant Seedlings. Antioxidants 2024, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J. Plant Growth Regul. 2019, 39, 641–655. [Google Scholar] [CrossRef]
- Jiao, J.; Gaia, Q.Y.; Wang, X.; Qin, Q.P.; Wang, Z.Y.; Liu, J.; Fu, Y.J. Chitosan elicitation of Isatis tinctoria L. hairy root cultures for enhancing flavonoid productivity and gene expression and related antioxidant activity. Ind. Crops Prod. 2018, 124, 8–35. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Lai, G.; Lin, J.; Guo, A.; Yang, F.; Pan, R.; Che, J.; Lai, C. VdCHS2 Overexpression Enhances Anthocyanin Biosynthesis, Modulates the Composition Ratio, and Increases Antioxidant Activity in Vitis davidii Cells. Antioxidants 2024, 13, 1472. https://doi.org/10.3390/antiox13121472
He L, Lai G, Lin J, Guo A, Yang F, Pan R, Che J, Lai C. VdCHS2 Overexpression Enhances Anthocyanin Biosynthesis, Modulates the Composition Ratio, and Increases Antioxidant Activity in Vitis davidii Cells. Antioxidants. 2024; 13(12):1472. https://doi.org/10.3390/antiox13121472
Chicago/Turabian StyleHe, Liyuan, Gongti Lai, Junxuan Lin, Aolin Guo, Fangxue Yang, Ruo Pan, Jianmei Che, and Chengchun Lai. 2024. "VdCHS2 Overexpression Enhances Anthocyanin Biosynthesis, Modulates the Composition Ratio, and Increases Antioxidant Activity in Vitis davidii Cells" Antioxidants 13, no. 12: 1472. https://doi.org/10.3390/antiox13121472
APA StyleHe, L., Lai, G., Lin, J., Guo, A., Yang, F., Pan, R., Che, J., & Lai, C. (2024). VdCHS2 Overexpression Enhances Anthocyanin Biosynthesis, Modulates the Composition Ratio, and Increases Antioxidant Activity in Vitis davidii Cells. Antioxidants, 13(12), 1472. https://doi.org/10.3390/antiox13121472





