Effects of Contagious Respiratory Pathogens on Breath Biomarkers
Abstract
:1. Introduction
2. Methods
2.1. Experimental Setup, Ethics, and Human Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Multiplex PCR Test for Respiratory Pathogens
2.4. Reporting of Disease Symptoms
2.5. Infection Safety Measures and Breath Sampling Protocol
2.6. PTR-ToF-MS Measurements of Breath Composition and VOC Data Processing
2.7. Quantification of VOCs
2.8. Statistical Analysis
2.9. Q1–Q6 (All Mono-Pathogens)
2.10. Q7–Q8 (Asymptomatic Mono-Pathogens)
2.11. Q9–Q10 (Symptomatic Mono-Pathogens)
2.12. Q11–Q13 (Co-Pathogens)
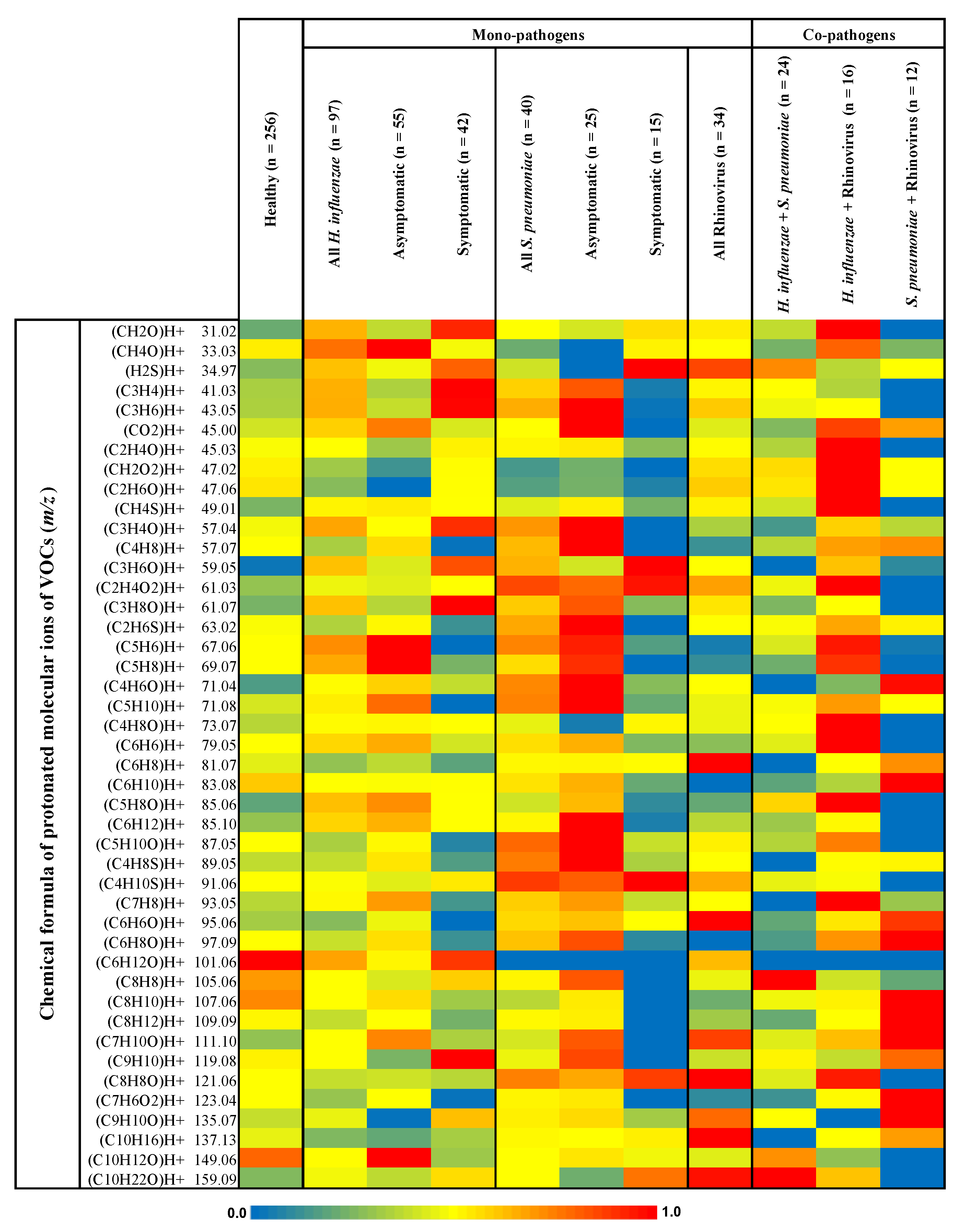
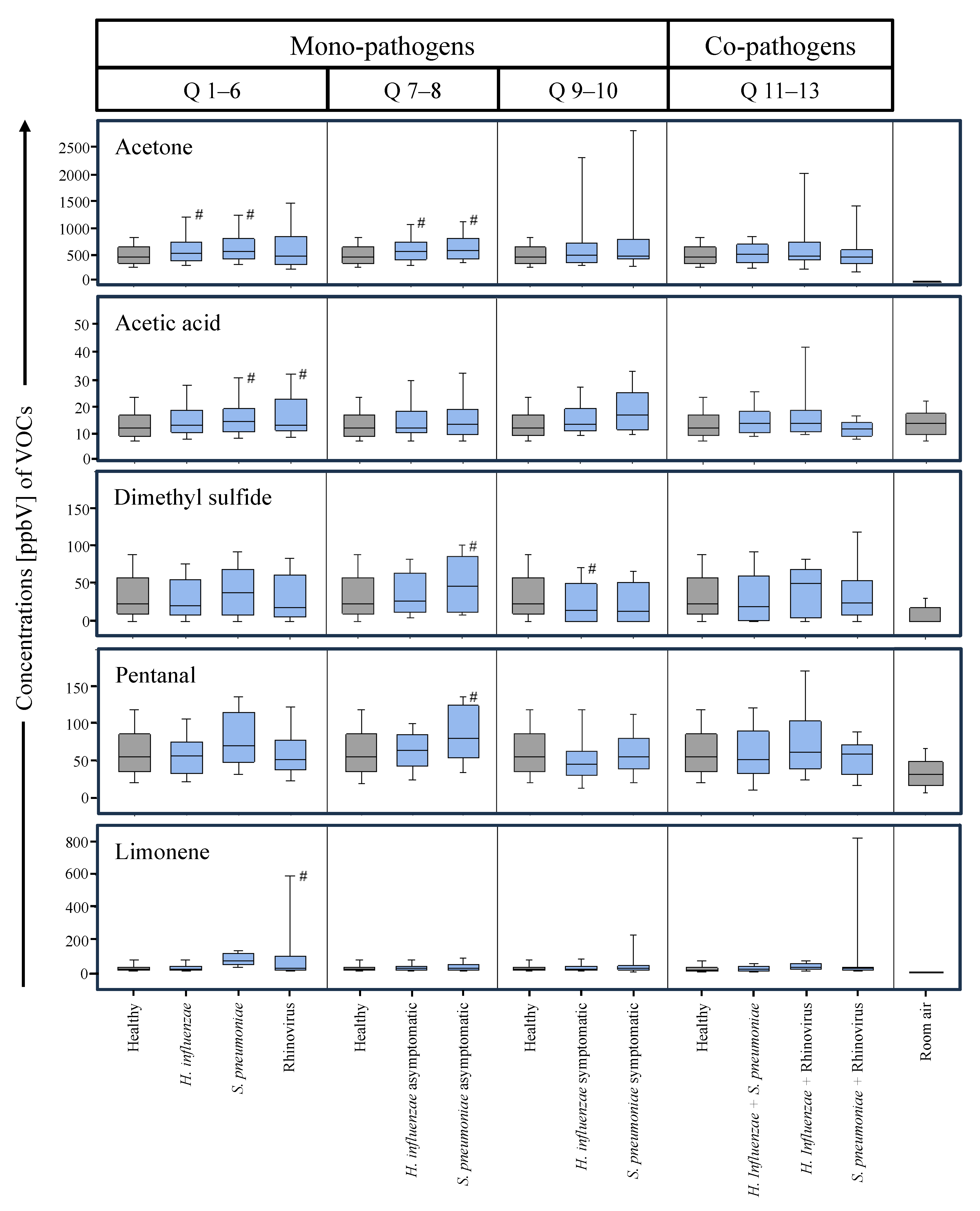
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Prim. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- DeMuri, G.P.; Gern, J.E.; Eickhoff, J.C.; Lynch, S.V.; Wald, E.R. Dynamics of Bacterial Colonization with Streptococcus Pneumoniae, Haemophilus Influenzae, and Moraxella Catarrhalis During Symptomatic and Asymptomatic Viral Upper Respiratory Tract Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The Effects of the COVID-19 Pandemic on Community Respiratory Virus Activity. Nat. Rev. Microbiol. 2022, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Natalini, J.G.; Singh, S.; Segal, L.N. The Dynamic Lung Microbiome in Health and Disease. Nat. Rev. Microbiol. 2022, 21, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Zaas, A.K.; Chen, M.; Varkey, J.; Veldman, T.; Hero, A.O.; Lucas, J.; Huang, Y.; Turner, R.; Gilbert, A.; Lambkin-Williams, R.; et al. Gene Expression Signatures Diagnose Influenza and Other Symptomatic Respiratory Viral Infections in Humans. Cell Host Microbe 2009, 6, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Zaas, A.K.; Garner, B.H.; Tsalik, E.L.; Burke, T.; Woods, C.W.; Ginsburg, G.S. The Current Epidemiology and Clinical Decisions Surrounding Acute Respiratory Infections. Trends Mol. Med. 2014, 20, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Message, S.D.; Laza-Stanca, V.; Mallia, P.; Parker, H.L.; Zhu, J.; Kebadze, T.; Contoli, M.; Sanderson, G.; Kon, O.M.; Papi, A.; et al. Rhinovirus-Induced Lower Respiratory Illness Is Increased in Asthma and Related to Virus Load and Th1/2 Cytokine and IL-10 Production. Proc. Natl. Acad. Sci. USA 2008, 105, 13562–13567. [Google Scholar] [CrossRef]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e14. [Google Scholar] [CrossRef]
- Traxler, S.; Barkowsky, G.; Saß, R.; Klemenz, A.-C.; Patenge, N.; Kreikemeyer, B.; Schubert, J.K.; Miekisch, W. Volatile Scents of Influenza A and S. Pyogenes (Co-)Infected Cells. Sci. Rep. 2019, 9, 18894. [Google Scholar] [CrossRef]
- Traxler, S.; Bischoff, A.-C.; Saß, R.; Trefz, P.; Gierschner, P.; Brock, B.; Schwaiger, T.; Karte, C.; Blohm, U.; Schröder, C.; et al. VOC Breath Profile in Spontaneously Breathing Awake Swine during Influenza A Infection. Sci. Rep. 2018, 8, 14857. [Google Scholar] [CrossRef]
- Gonzalez-Juarbe, N.; Riegler, A.N.; Jureka, A.S.; Gilley, R.P.; Brand, J.D.; Trombley, J.E.; Scott, N.R.; Platt, M.P.; Dube, P.H.; Petit, C.M.; et al. Influenza-Induced Oxidative Stress Sensitizes Lung Cells to Bacterial-Toxin-Mediated Necroptosis. Cell Rep. 2020, 32, 108062. [Google Scholar] [CrossRef] [PubMed]
- Tsalik, E.L.; Henao, R.; Nichols, M.; Burke, T.; Ko, E.R.; McClain, M.T.; Hudson, L.L.; Mazur, A.; Freeman, D.H.; Veldman, T.; et al. Host Gene Expression Classifiers Diagnose Acute Respiratory Illness Etiology. Sci. Transl. Med. 2016, 8, 322ra11. [Google Scholar] [CrossRef]
- Remy, R.; Kemnitz, N.; Trefz, P.; Fuchs, P.; Bartels, J.; Klemenz, A.-C.; Rührmund, L.; Sukul, P.; Miekisch, W.; Schubert, J.K. Profiling of Exhaled Volatile Organics in the Screening Scenario of a COVID-19 Test Center. iScience 2022, 25, 105195. [Google Scholar] [CrossRef]
- Di Simone, S.K.; Rudloff, I.; Nold-Petry, C.A.; Forster, S.C.; Nold, M.F. Understanding Respiratory Microbiome–Immune System Interactions in Health and Disease. Sci. Transl. Med. 2023, 15, eabq5126. [Google Scholar] [CrossRef]
- Miekisch, W.; Sukul, P.; Schubert, J.K. Chapter 2 Origin and Emission of Volatile Biomarkers in Breath: Basics and Dynamic Aspects. In Volatile Biomarkers for Human Health: From Nature to Artificial Senses; The Royal Society of Chemistry: London, UK, 2023; pp. 22–38. ISBN 978-1-83916-430-9. [Google Scholar]
- Sukul, P.; Bartels, J.; Fuchs, P.; Trefz, P.; Remy, R.; Rührmund, L.; Kamysek, S.; Schubert, J.K.; Miekisch, W. Effects of COVID-19 Protective Face-Masks and Wearing Durations onto Respiratory-Haemodynamic Physiology and Exhaled Breath Constituents. Eur. Respir. J. 2022, 60, 2200009. [Google Scholar] [CrossRef] [PubMed]
- Sukul, P.; Schubert, J.K.; Zanaty, K.; Trefz, P.; Sinha, A.; Kamysek, S.; Miekisch, W. Exhaled Breath Compositions under Varying Respiratory Rhythms Reflects Ventilatory Variations: Translating Breathomics towards Respiratory Medicine. Sci. Rep. 2020, 10, 14109. [Google Scholar] [CrossRef]
- Sukul, P.; Schubert, J.K.; Oertel, P.; Kamysek, S.; Taunk, K.; Trefz, P.; Miekisch, W. FEV Manoeuvre Induced Changes in Breath VOC Compositions: An Unconventional View on Lung Function Tests. Sci. Rep. 2016, 6, 28029. [Google Scholar] [CrossRef]
- Sukul, P.; Trefz, P.; Kamysek, S.; Schubert, J.K.; Miekisch, W. Instant Effects of Changing Body Positions on Compositions of Exhaled Breath. J. Breath Res. 2015, 9, 047105. [Google Scholar] [CrossRef]
- Sukul, P.; Trefz, P. Physio-Metabolic Monitoring via Breath Employing Real-Time Mass Spectrometry: Importance, Challenges, Potentials, and Pitfalls. In Bioanalytical Reviews; Springer: Berlin/Heidelberg, Germany, 2022; Volume 4, pp. 1–18. [Google Scholar]
- Sukul, P.; Grzegorzewski, S.; Broderius, C.; Trefz, P.; Mittlmeier, T.; Fischer, D.-C.; Miekisch, W.; Schubert, J.K. Physiological and Metabolic Effects of Healthy Female Aging on Exhaled Breath Biomarkers. iScience 2022, 25, 103739. [Google Scholar] [CrossRef]
- Sukul, P.; Schubert, J.K.; Trefz, P.; Miekisch, W. Natural Menstrual Rhythm and Oral Contraception Diversely Affect Exhaled Breath Compositions. Sci. Rep. 2018, 8, 10838. [Google Scholar] [CrossRef]
- Trefz, P.; Schmidt, S.C.; Sukul, P.; Schubert, J.K.; Miekisch, W.; Fischer, D.-C. Non-Invasive Assessment of Metabolic Adaptation in Paediatric Patients Suffering from Type 1 Diabetes Mellitus. J. Clin. Med. 2019, 8, 1797. [Google Scholar] [CrossRef]
- Sukul, P.; Richter, A.; Schubert, J.K.; Miekisch, W. Deficiency and Absence of Endogenous Isoprene in Adults, Disqualified Its Putative Origin. Heliyon 2021, 7, e05922. [Google Scholar] [CrossRef]
- Pugliese, G.; Trefz, P.; Weippert, M.; Pollex, J.; Bruhn, S.; Schubert, J.K.; Miekisch, W.; Sukul, P. Real-Time Metabolic Monitoring under Exhaustive Exercise and Evaluation of Ventilatory Threshold by Breathomics: Independent Validation of Evidence and Advances. Front. Physiol. 2022, 13, 946401. [Google Scholar] [CrossRef]
- Sukul, P.; Richter, A.; Junghanss, C.; Schubert, J.K.; Miekisch, W. Origin of Breath Isoprene in Humans Is Revealed via Multi-Omic Investigations. Commun. Biol. 2023, 6, 999. [Google Scholar] [CrossRef]
- Löser, B.; Grabenschröer, A.; Pugliese, G.; Sukul, P.; Trefz, P.; Schubert, J.K.; Miekisch, W. Changes of Exhaled Volatile Organic Compounds in Postoperative Patients Undergoing Analgesic Treatment: A Prospective Observational Study. Metabolites 2020, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Brock, B.; Kamysek, S.; Silz, J.; Trefz, P.; Schubert, J.K.; Miekisch, W. Monitoring of Breath VOCs and Electrical Impedance Tomography under Pulmonary Recruitment in Mechanically Ventilated Patients. J. Breath Res. 2017, 11, 016005. [Google Scholar] [CrossRef] [PubMed]
- Grassin-Delyle, S.; Roquencourt, C.; Moine, P.; Saffroy, G.; Carn, S.; Heming, N.; Fleuriet, J.; Salvator, H.; Naline, E.; Couderc, L.-J.; et al. Metabolomics of Exhaled Breath in Critically Ill COVID-19 Patients: A Pilot Study. EBioMedicine 2021, 63, 103154. [Google Scholar] [CrossRef] [PubMed]
- Sukul, P.; Trefz, P.; Schubert, J.K.; Miekisch, W. Advanced Setup for Safe Breath Sampling and Patient Monitoring under Highly Infectious Conditions in the Clinical Environment. Sci. Rep. 2022, 12, 17926. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.; Cordell, R.L.; Wilde, M.J.; Richardson, M.; Carr, L.; Dasi, A.S.D.; Hargadon, B.; Free, R.C.; Monks, P.S.; Brightling, C.E.; et al. Diagnosis of COVID-19 by Exhaled Breath Analysis Using Gas Chromatography–Mass Spectrometry. ERJ Open Res. 2021, 7, 00139-2021. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, D.M.; Sanders, D.; O’Brien, R.; Hempel, F.; Reed, M.J.; Riepe, A.C.; Bailie, K.; Brodrick, E.; Darnley, K.; Ellerkmann, R.; et al. Diagnosis of COVID-19 by Analysis of Breath with Gas Chromatography-Ion Mobility Spectrometry—A Feasibility Study. EclinicalMedicine 2020, 29, 100609. [Google Scholar] [CrossRef] [PubMed]
- Schwoebel, H.; Schubert, R.; Sklorz, M.; Kischkel, S.; Zimmermann, R.; Schubert, J.K.; Miekisch, W. Phase-Resolved Real-Time Breath Analysis during Exercise by Means of Smart Processing of PTR-MS Data. Anal. Bioanal. Chem. 2011, 401, 2079–2091. [Google Scholar] [CrossRef] [PubMed]
- Cappellin, L.; Karl, T.; Probst, M.; Ismailova, O.; Winkler, P.M.; Soukoulis, C.; Aprea, E.; Märk, T.D.; Gasperi, F.; Biasioli, F. On Quantitative Determination of Volatile Organic Compound Concentrations Using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. Environ. Sci. Technol. 2012, 46, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Trefz, P.; Schubert, J.K.; Miekisch, W. Effects of Humidity, CO2 and O2 on Real-Time Quantitation of Breath Biomarkers by Means of PTR-ToF-MS. J. Breath Res. 2018, 12, 026016. [Google Scholar] [CrossRef]
- Veskoukis, A.S. Chapter 8—Redox Signaling and Antioxidant Defense in Pathogenic Microorganisms: A Link to Disease and Putative Therapy. In Pathology; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 87–95. ISBN 978-0-12-815972-9. [Google Scholar]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Grimsrud, P.A.; Xie, H.; Griffin, T.J.; Bernlohr, D.A. Oxidative Stress and Covalent Modification of Protein with Bioactive Aldehydes. J. Biol. Chem. 2008, 283, 21837–21841. [Google Scholar] [CrossRef]
- Bortoni, M.E.; Terra, V.S.; Hinds, J.; Andrew, P.W.; Yesilkaya, H. The Pneumococcal Response to Oxidative Stress Includes a Role for Rgg. Microbiology 2009, 155, 4123–4134. [Google Scholar] [CrossRef] [PubMed]
- Zahlten, J.; Kim, Y.-J.; Doehn, J.-M.; Pribyl, T.; Hocke, A.C.; García, P.; Hammerschmidt, S.; Suttorp, N.; Hippenstiel, S.; Hübner, R.-H. Streptococcus Pneumoniae-Induced Oxidative Stress in Lung Epithelial Cells Depends on Pneumococcal Autolysis and Is Reversible by Resveratrol. J. Infect. Dis. 2015, 211, 1822–1830. [Google Scholar] [CrossRef]
- Yesilkaya, H.; Andisi, V.F.; Andrew, P.W.; Bijlsma, J.J.E. Streptococcus Pneumoniae and Reactive Oxygen Species: An Unusual Approach to Living with Radicals. Trends Microbiol. 2013, 21, 187–195. [Google Scholar] [CrossRef]
- Wark, P.; Pathinyake, P.; Hsu, A.; Parsons, K.; Wood, L. Effect of Oxidative Stress and Rhinovirus Infection on Mitochondrial/Endoplasmic Reticular Function in Human Primary Bronchial Epithelial Cells. Eur. Respir. J. 2016, 48, PA3989. [Google Scholar] [CrossRef]
- Biagioli, M.C.; Kaul, P.; Singh, I.; Turner, R.B. The Role of Oxidative Stress in Rhinovirus Induced Elaboration of IL-8 by Respiratory Epithelial Cells. Free Radic. Biol. Med. 1999, 26, 454–462. [Google Scholar] [CrossRef]
- Kaul, P.; Biagioli, M.C.; Singh, I.; Turner, R.B. Rhinovirus-Induced Oxidative Stress and Interleukin-8 Elaboration Involves P47-Phox but Is Independent of Attachment to Intercellular Adhesion Molecule-1 and Viral Replication. J. Infect. Dis. 2000, 181, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.C.; Vieira, A.J.; Beserra, F.P.; Pellizzon, C.H.; Nóbrega, R.H.; Rozza, A.L. Gastroprotective Effect of Limonene in Rats: Influence on Oxidative Stress, Inflammation and Gene Expression. Phytomedicine 2019, 53, 37–42. [Google Scholar] [CrossRef]
- Santana, H.S.R.; de Carvalho, F.O.; Silva, E.R.; Santos, N.G.L.; Shanmugam, S.; Santos, D.N.; Wisniewski, J.O.; Junior, J.S.C.; Nunes, P.S.; Araujo, A.A.S.; et al. Anti-Inflammatory Activity of Limonene in the Prevention and Control of Injuries in the Respiratory System: A Systematic Review. Curr. Pharm. Des. 2020, 26, 2182–2191. [Google Scholar] [CrossRef]
- Bacanlı, M. Limonene and Ursolic Acid in the Treatment of Diabetes: Citrus Phenolic Limonene, Triterpenoid Ursolic Acid, Antioxidants and Diabetes. In Diabetes; Academic Press: Cambridge, MA, USA, 2020; pp. 275–283. [Google Scholar] [CrossRef]
- Kalapos, M.P. On the Mammalian Acetone Metabolism: From Chemistry to Clinical Implications. Biochim. Biophys. Acta 2003, 1621, 122–139. [Google Scholar] [CrossRef]
- Othman, D.S.M.P.; Schirra, H.; McEwan, A.G.; Kappler, U. Metabolic Versatility in Haemophilus Influenzae: A Metabolomic and Genomic Analysis. Front. Microbiol. 2014, 5, 69. [Google Scholar] [CrossRef]
- Fan, F.; Ma, Y.; Ai, R.; Ding, Z.; Li, D.; Zhu, Y.; He, Q.; Zhang, X.; Dong, Y.; He, Y. Glycolytic Metabolism Is Critical for the Innate Antibacterial Defense in Acute Streptococcus Pneumoniae Otitis Media. Front. Immunol. 2021, 12, 624775. [Google Scholar] [CrossRef]
- Bacterial Growth Laws Reflect the Evolutionary Importance of Energy Efficiency—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4299221/ (accessed on 1 February 2023).
- Chen, S.L. Energy Requirement for Microbial Growth. Nature 1964, 202, 1135–1136. [Google Scholar] [CrossRef]
- Blaas, D.; Fuchs, R. Mechanism of Human Rhinovirus Infections. Mol. Cell. Pediatr. 2016, 3, 21. [Google Scholar] [CrossRef]
- Bochkov, Y.A.; Gern, J.E. Rhinoviruses and Their Receptors: Implications for Allergic Disease. Curr. Allergy Asthma Rep. 2016, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Gualdoni, G.A.; Mayer, K.A.; Kapsch, A.-M.; Kreuzberg, K.; Puck, A.; Kienzl, P.; Oberndorfer, F.; Frühwirth, K.; Winkler, S.; Blaas, D.; et al. Rhinovirus Induces an Anabolic Reprogramming in Host Cell Metabolism Essential for Viral Replication. Proc. Natl. Acad. Sci. USA 2018, 115, E7158–E7165. [Google Scholar] [CrossRef]
- Cope, E.K.; Goldstein-Daruech, N.; Kofonow, J.M.; Christensen, L.; McDermott, B.; Monroy, F.; Palmer, J.N.; Chiu, A.G.; Shirtliff, M.E.; Cohen, N.A.; et al. Regulation of Virulence Gene Expression Resulting from Streptococcus Pneumoniae and Nontypeable Haemophilus Influenzae Interactions in Chronic Disease. PLoS ONE 2011, 6, e28523. [Google Scholar] [CrossRef]
- Tikhomirova, A.; Kidd, S.P. Haemophilus Influenzae and Streptococcus Pneumoniae: Living Together in a Biofilm. Pathog. Dis. 2013, 69, 114–126. [Google Scholar] [CrossRef]
- Pericone, C.D.; Overweg, K.; Hermans, P.W.M.; Weiser, J.N. Inhibitory and Bactericidal Effects of Hydrogen Peroxide Production by Streptococcus Pneumoniae on Other Inhabitants of the Upper Respiratory Tract. Infect. Immun. 2000, 68, 3990–3997. [Google Scholar] [CrossRef]
- Shakhnovich, E.A.; King, S.J.; Weiser, J.N. Neuraminidase Expressed by Streptococcus Pneumoniae Desialylates the Lipopolysaccharide of Neisseria Meningitidis and Haemophilus Influenzae: A Paradigm for Interbacterial Competition among Pathogens of the Human Respiratory Tract. Infect. Immun. 2002, 70, 7161–7164. [Google Scholar] [CrossRef]
- Swords, W.E.; Moore, M.L.; Godzicki, L.; Bukofzer, G.; Mitten, M.J.; VonCannon, J. Sialylation of Lipooligosaccharides Promotes Biofilm Formation by Nontypeable Haemophilus Influenzae. Infect. Immun. 2004, 72, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W. Acetic Acid and Butyric Acid Released in Large Intestine Play Different Roles in the Alleviation of Constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Tangerman, A. Measurement and Biological Significance of the Volatile Sulfur Compounds Hydrogen Sulfide, Methanethiol and Dimethyl Sulfide in Various Biological Matrices. J. Chromatogr. B 2009, 877, 3366–3377. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Sánchez-Alcoholado, L.; Cabrera-Mulero, A.; Lopez-Dominguez, R.; Carmona-Saez, P.; Garcia-Fuentes, E.; Moreno-Indias, I.; Tinahones, F.J. Gut Microbiota Composition Is Associated with the Global DNA Methylation Pattern in Obesity. Front. Genet. 2019, 10, 613. [Google Scholar] [CrossRef]
- Singhal, R.; Shah, Y.M. Oxygen Battle in the Gut: Hypoxia and Hypoxia-Inducible Factors in Metabolic and Inflammatory Responses in the Intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut Microbiota-Derived Bile Acids in Intestinal Immunity, Inflammation, and Tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Zhu, J.; Fang, S.; Meng, L.; Zhou, C. Effects of Gut Microbiota on Host Adaptive Immunity Under Immune Homeostasis and Tumor Pathology State. Front. Immunol. 2022, 13, 844335. [Google Scholar] [CrossRef] [PubMed]
- Piters, W.A.A.d.S.; Binkowska, J.; Bogaert, D. Early Life Microbiota and Respiratory Tract Infections. Cell Host Microbe 2020, 28, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Sencio, V.; Barthelemy, A.; Tavares, L.P.; Machado, M.G.; Soulard, D.; Cuinat, C.; Queiroz-Junior, C.M.; Noordine, M.-L.; Salomé-Desnoulez, S.; Deryuter, L.; et al. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020, 30, 2934–2947.e6. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, Y.; Gui, J.; Hoen, A.G.; Morrison, H.G.; Baker, E.R.; Nadeau, K.C.; Li, H.; Li, Z.; Madan, J.C.; Karagas, M.R. The Relationship between the Gut Microbiome and the Risk of Respiratory Infections among Newborns. Commun. Med. 2022, 2, 87. [Google Scholar] [CrossRef] [PubMed]
- Antunes, K.H.; Singanayagam, A.; Williams, L.; Faiez, T.S.; Farias, A.; Jackson, M.M.; Faizi, F.K.; Aniscenko, J.; Kebadze, T.; Veerati, P.C.; et al. Airway-Delivered Short-Chain Fatty Acid Acetate Boosts Antiviral Immunity during Rhinovirus Infection. J. Allergy Clin. Immunol. 2023, 151, 447–457.e5. [Google Scholar] [CrossRef] [PubMed]
- Antunes, K.H.; Stein, R.T.; Franceschina, C.; da Silva, E.F.; de Freitas, D.N.; Silveira, J.; Mocellin, M.; Leitão, L.; Fachi, J.L.; Pral, L.P.; et al. Short-Chain Fatty Acid Acetate Triggers Antiviral Response Mediated by RIG-I in Cells from Infants with Respiratory Syncytial Virus Bronchiolitis. EBioMedicine 2022, 77, 103891. [Google Scholar] [CrossRef]
- López-López, N.; Euba, B.; Hill, J.; Dhouib, R.; Caballero, L.; Leiva, J.; Hosmer, J.; Cuesta, S.; Ramos-Vivas, J.; Díez-Martínez, R.; et al. Haemophilus Influenzae Glucose Catabolism Leading to Production of the Immunometabolite Acetate Has a Key Contribution to the Host Airway–Pathogen Interplay. ACS Infect. Dis. 2020, 6, 406–421. [Google Scholar] [CrossRef]
- Machado, M.G.; Patente, T.A.; Rouillé, Y.; Heumel, S.; Melo, E.M.; Deruyter, L.; Pourcet, B.; Sencio, V.; Teixeira, M.M.; Trottein, F. Acetate Improves the Killing of Streptococcus Pneumoniae by Alveolar Macrophages via NLRP3 Inflammasome and Glycolysis-HIF-1α Axis. Front. Immunol. 2022, 13, 773261. [Google Scholar] [CrossRef]
- Ji, J.; Xu, Y.; Zheng, M.; Luo, C.; Lei, H.; Qu, H.; Shu, D. Methionine Attenuates Lipopolysaccharide-Induced Inflammatory Responses via DNA Methylation in Macrophages. ACS Omega 2019, 4, 2331–2336. [Google Scholar] [CrossRef]
- Wu, X.; Han, Z.; Liu, B.; Yu, D.; Sun, J.; Ge, L.; Tang, W.; Liu, S. Gut Microbiota Contributes to the Methionine Metabolism in Host. Front. Microbiol. 2022, 13, 1065668. [Google Scholar] [CrossRef]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.T.H.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The Gut Microbiota Plays a Protective Role in the Host Defence against Pneumococcal Pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef]
- Sencio, V.; Machado, M.G.; Trottein, F. The Lung–Gut Axis during Viral Respiratory Infections: The Impact of Gut Dysbiosis on Secondary Disease Outcomes. Mucosal Immunol. 2021, 14, 296–304. [Google Scholar] [CrossRef]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef]
- Heaney, L.M.; Kang, S.; Turner, M.A.; Lindley, M.R.; Thomas, C.L.P. The Impact of a Graded Maximal Exercise Protocol on Exhaled Volatile Organic Compounds: A Pilot Study. Molecules 2022, 27, 370. [Google Scholar] [CrossRef]
- Dragonieri, S.; Quaranta, V.N.; Portacci, A.; Ahroud, M.; Di Marco, M.; Ranieri, T.; Carpagnano, G.E. Effect of Food Intake on Exhaled Volatile Organic Compounds Profile Analyzed by an Electronic Nose. Molecules 2023, 28, 5755. [Google Scholar] [CrossRef]
- Dragonieri, S.; Quaranta, V.N.; Buonamico, E.; Battisti, C.; Ranieri, T.; Carratu, P.; Carpagnano, G.E. Short-Term Effect of Cigarette Smoke on Exhaled Volatile Organic Compounds Profile Analyzed by an Electronic Nose. Biosensors 2022, 12, 520. [Google Scholar] [CrossRef]
- Zella, D.; Giovanetti, M.; Cella, E.; Borsetti, A.; Ciotti, M.; Ceccarelli, G.; D’Ettorre, G.; Pezzuto, A.; Tambone, V.; Campanozzi, L.; et al. The Importance of Genomic Analysis in Cracking the Coronavirus Pandemic. Expert Rev. Mol. Diagn. 2021, 21, 547–562. [Google Scholar] [CrossRef]
| All | Men | Women | |
|---|---|---|---|
| N° of subjects (%) | 479 | 249 (52.0) | 230 (48.0) |
| Age [years] (mean ± SD) | 39.1 ± 14.2 | 40.3 ± 13.8 | 37.9 ± 14.6 |
| N° of respiratory pathogen-positive tested patients (%) | 223 | 115 (51.6) | 108 (48.4) |
| Age of respiratory pathogen-positive tested patients [years] (mean ± SD) | 37.3 ± 12.4 | 38.3± 12.0 | 36.1 ± 12.8 |
| N° of healthy volunteers (%) | 256 | 134 (52.3) | 122 (47.7) |
| Age of healthy volunteers [years] (mean ± SD) | 40.7 ± 15.5 | 41.9± 15.0 | 39.5 ± 15.9 |
| Mono-Pathogens | Co-Pathogens | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Positive Cases | Asymptomatic | Symptomatic | All Positive Cases | ||||||||||
| Positive Cases | Positive Cases | ||||||||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | |
| 97 vs. 256 | 40 vs. 256 | 34 vs. 256 | 97 vs. 34 | 40 vs. 34 | 97 vs. 40 | 55 vs. 256 | 25 vs. 256 | 42 vs. 256 | 15 vs. 256 | 24 vs. 256 | 16 vs. 256 | 12 vs. 256 | |
| Kruskal–Wallis ANOVA on Ranks (Bonferroni Correction for Pairwise Multiple Comparisons) with Asymptotic Significance at p-Value ≤ 0.05 | H. influenzae vs. Healthy | S. pneumoniae vs. Healthy | Rhinovirus vs. Healthy | H. influenzae vs. Rhinovirus | S. pneumoniae vs. Rhinovirus | H. influenzae vs. S. pneumoniae | H. influenzae asymptomatic vs. Healthy | S. pneumoniae asymptomatic vs. Healthy | H. influenzae symptomatic vs. Healthy | S. pneumoniae symptomatic vs. Healthy | H. influenzae + S. pneumoniae vs. Healthy | H. influenzae + Rhinovirus vs. Healthy | S. pneumoniae + Rhinovirus vs. Healthy |
| Acetone | 0.009 | 0.013 | >0.05 | >0.05 | >0.05 | >0.05 | 0.016 | 0.027 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
| Acetic Acid | >0.05 | 0.040 | 0.029 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
| Dimethyl sulfide | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | 0.016 | 0.028 | >0.05 | >0.05 | >0.05 | >0.05 |
| Pentanal | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | 0.002 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
| Limonene | >0.05 | >0.05 | 0.016 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
| Healthy (n = 256) | All H. influenzae (n = 97) | Asymptomatic (n = 55) | Symptomatic (n = 42) | All S. pneumoniae (n = 40) | Asymptomatic (n = 25) | Symptomatic (n = 15) | All Rhinovirus (n = 34) | H. influenzae + S. pneumoniae (n = 24) | H. influenzae + Rhinovirus (n = 16) | S. pneumoniae + Rhinovirus (n = 12) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VOCs | RSDs (%) | ||||||||||
| Acetone | 57.4 | 81.7 | 59.7 | 95.3 | 76.9 | 41.5 | 97.2 | 72.0 | 41.5 | 100 | 69.9 |
| Acetic acid | 62.6 | 63.6 | 72.8 | 50.9 | 72.3 | 85.7 | 46.9 | 50.9 | 59.8 | 76.2 | 25.9 |
| Dimethyl sulfide | 94.8 | 95.0 | 82.4 | 116 | 83.7 | 66.9 | 112 | 103 | 118 | 75.6 | 106 |
| Pentanal | 59.8 | 57.5 | 47.9 | 70.8 | 53.3 | 48.9 | 53.9 | 61.9 | 59.6 | 68.3 | 46.9 |
| Limonene | 164 | 110 | 85.2 | 134 | 120 | 87.7 | 143 | 279 | 81.3 | 56.9 | 255 |
| Correlation Matrix (Dimension Reduction via Factor Analysis of Principal Components) | Healthy (n = 256) | All H. influenzae (n = 97) | Asymptomatic (n = 55) | Symptomatic (n = 42) | All S. pneumoniae (n = 40) | Asymptomatic (n = 25) | Symptomatic (n = 15) | All Rhinovirus (n = 34) | H. influenzae + S. pneumoniae (n = 24) | H. influenzae + Rhinovirus (n = 16) | S. pneumoniae + Rhinovirus (n = 12) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOCs | |||||||||||||
| Acetone | Acetic acid | R-value | 0.005 | 0.212 | 0.044 | 0.415 | 0.028 | −0.134 | 0.191 | 0.363 | 0.163 | 0.831 | 0.134 |
| p-value | 0.466 | 0.019 | 0.376 | 0.003 | 0.433 | 0.262 | 0.247 | 0.018 | 0.223 | 0.000 | 0.339 | ||
| Acetone | Dimethyl sulfide | R-value | 0.293 | 0.138 | 0.147 | 0.190 | 0.225 | 0.309 | 0.527 | 0.507 | 0.139 | 0.283 | 0.137 |
| p-value | 0.000 | 0.088 | 0.142 | 0.114 | 0.082 | 0.067 | 0.022 | 0.001 | 0.258 | 0.144 | 0.336 | ||
| Acetone | Pentanal | R-value | 0.282 | 0.421 | 0.473 | 0.453 | 0.289 | 0.279 | 0.637 | 0.472 | 0.267 | 0.305 | 0.112 |
| p-value | 0.000 | 0.000 | 0.000 | 0.001 | 0.035 | 0.088 | 0.005 | 0.002 | 0.104 | 0.125 | 0.365 | ||
| Acetone | Limonene | R-value | −0.003 | −0.024 | −0.069 | −0.011 | 0.256 | −0.265 | 0.365 | −0.009 | 0.008 | 0.140 | −0.047 |
| p-value | 0.480 | 0.409 | 0.308 | 0.474 | 0.055 | 0.100 | 0.091 | 0.481 | 0.485 | 0.302 | 0.442 | ||
| Acetic acid | Dimethyl sulfide | R-value | −0.028 | −0.124 | −0.206 | 0.028 | 0.124 | 0.227 | −0.172 | −0.152 | −0.096 | 0.368 | 0.629 |
| p-value | 0.329 | 0.114 | 0.066 | 0.431 | 0.223 | 0.138 | 0.27 | 0.195 | 0.328 | 0.081 | 0.014 | ||
| Acetic acid | Pentanal | R-value | 0.198 | 0.296 | 0.267 | 0.381 | 0.257 | 0.323 | 0.122 | 0.166 | 0.065 | 0.566 | 0.583 |
| p-value | 0.001 | 0.002 | 0.024 | 0.006 | 0.055 | 0.058 | 0.332 | 0.175 | 0.381 | 0.011 | 0.023 | ||
| Acetic acid | Limonene | R-value | −0.005 | −0.008 | 0.062 | −0.088 | 0.186 | 0.293 | 0.137 | −0.189 | 0.000 | −0.114 | 0.474 |
| p-value | 0.470 | 0.470 | 0.326 | 0.291 | 0.125 | 0.078 | 0.313 | 0.142 | 0.500 | 0.337 | 0.060 | ||
| Dimethyl sulfide | Pentanal | R-value | 0.621 | 0.483 | 0.321 | 0.632 | 0.590 | 0.560 | 0.425 | 0.582 | 0.459 | 0.776 | 0.675 |
| p-value | 0.000 | 0.000 | 0.008 | 0.000 | 0.000 | 0.002 | 0.057 | 0.000 | 0.012 | 0.000 | 0.008 | ||
| Dimethyl sulfide | Limonene | R-value | 0.110 | −0.028 | −0.082 | 0.019 | −0.031 | −0.172 | 0.233 | 0.100 | −0.122 | 0.189 | 0.766 |
| p-value | 0.040 | 0.394 | 0.277 | 0.452 | 0.424 | 0.206 | 0.202 | 0.286 | 0.285 | 0.242 | 0.002 | ||
| Pentanal | Limonene | R-value | 0.161 | −0.039 | −0.087 | −0.004 | 0.265 | 0.253 | 0.506 | −0.027 | 0.095 | −0.129 | 0.351 |
| p-value | 0.005 | 0.354 | 0.265 | 0.490 | 0.049 | 0.111 | 0.027 | 0.439 | 0.329 | 0.316 | 0.132 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemnitz, N.; Fuchs, P.; Remy, R.; Ruehrmund, L.; Bartels, J.; Klemenz, A.-C.; Trefz, P.; Miekisch, W.; Schubert, J.K.; Sukul, P. Effects of Contagious Respiratory Pathogens on Breath Biomarkers. Antioxidants 2024, 13, 172. https://doi.org/10.3390/antiox13020172
Kemnitz N, Fuchs P, Remy R, Ruehrmund L, Bartels J, Klemenz A-C, Trefz P, Miekisch W, Schubert JK, Sukul P. Effects of Contagious Respiratory Pathogens on Breath Biomarkers. Antioxidants. 2024; 13(2):172. https://doi.org/10.3390/antiox13020172
Chicago/Turabian StyleKemnitz, Nele, Patricia Fuchs, Rasmus Remy, Leo Ruehrmund, Julia Bartels, Ann-Christin Klemenz, Phillip Trefz, Wolfram Miekisch, Jochen K. Schubert, and Pritam Sukul. 2024. "Effects of Contagious Respiratory Pathogens on Breath Biomarkers" Antioxidants 13, no. 2: 172. https://doi.org/10.3390/antiox13020172
APA StyleKemnitz, N., Fuchs, P., Remy, R., Ruehrmund, L., Bartels, J., Klemenz, A.-C., Trefz, P., Miekisch, W., Schubert, J. K., & Sukul, P. (2024). Effects of Contagious Respiratory Pathogens on Breath Biomarkers. Antioxidants, 13(2), 172. https://doi.org/10.3390/antiox13020172






