Integrative Multiomics Profiling Unveils the Protective Function of Ulinastatin against Dextran Sulfate Sodium-Induced Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
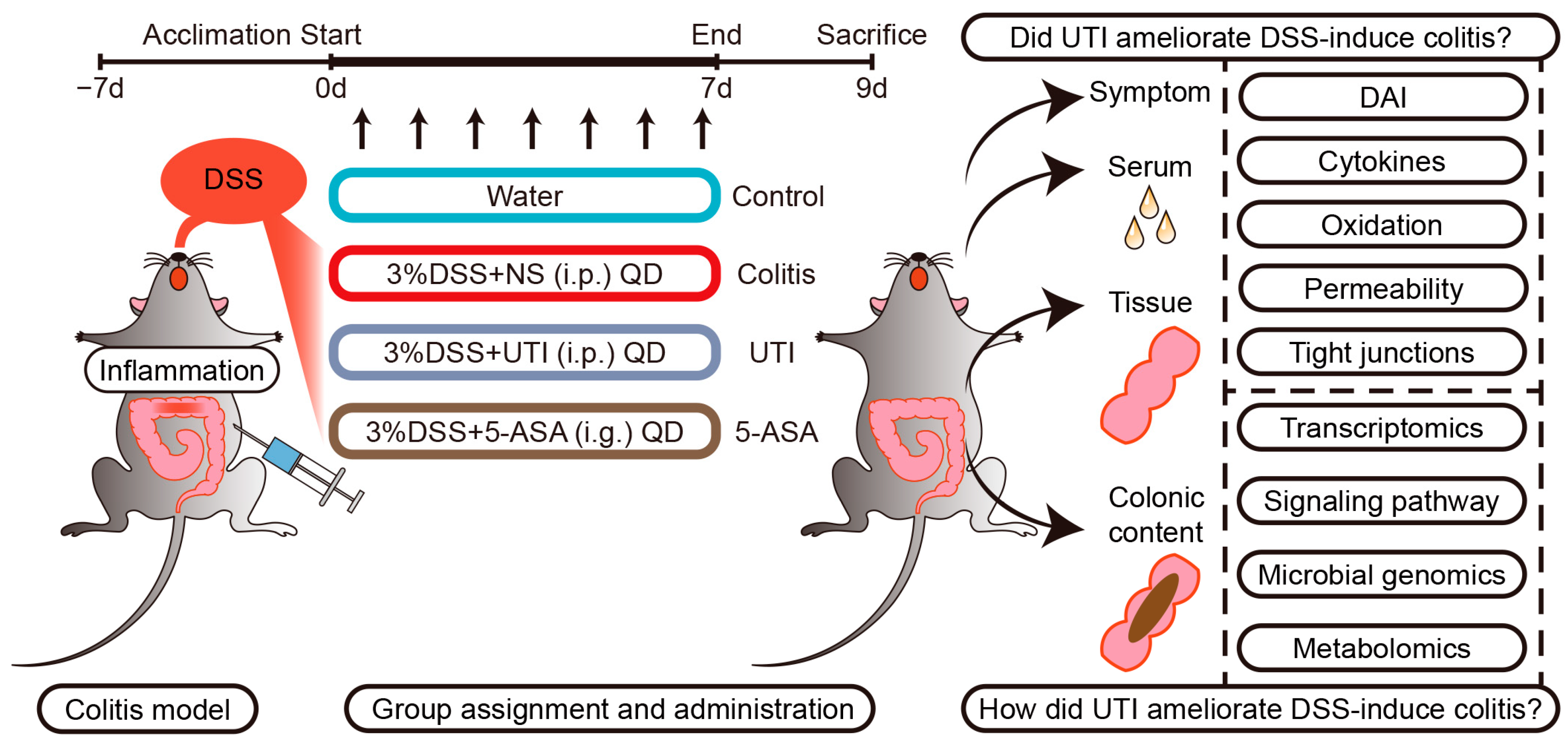
2.2. Animals and Colitis Model Establishment
2.3. Specimen Collection and Evaluation of Colitis
2.4. Histopathological Analysis
2.5. In Vivo Imaging of ROS
2.6. Biochemical Analysis
2.7. Intestinal Barrier Function Detection
2.8. Immunohistochemistry and Immunofluorescence
2.9. Quantitative Real-Time PCR
2.10. Western Blotting Analysis
2.11. RNA Sequencing
2.12. 16S rDNA Gene High-Throughput Sequencing
2.13. Intestinal Metabolomics Analysis
2.14. Statistical Analysis
3. Results
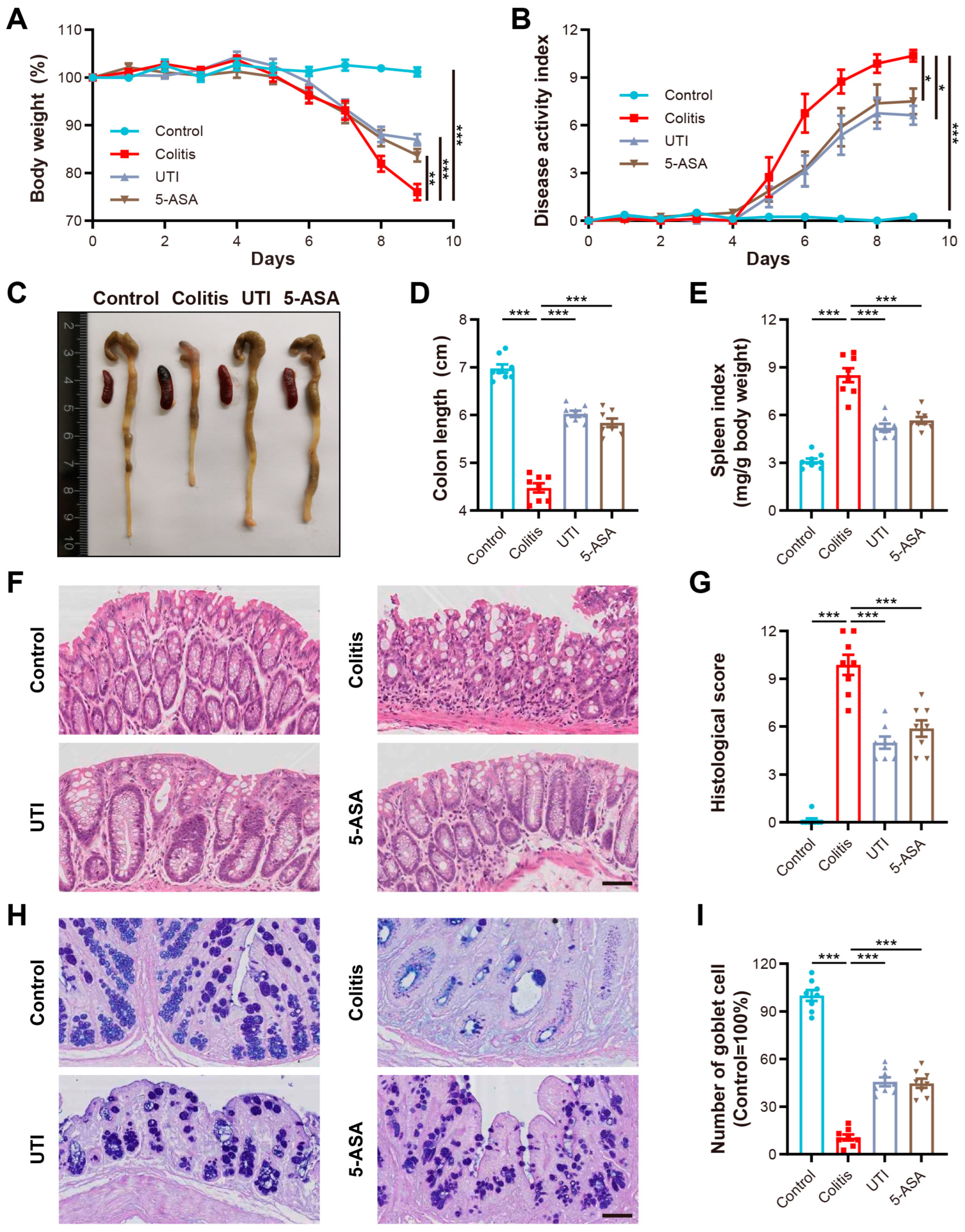
3.1. UTI Administration Ameliorated Pathological Features in DSS-Induced Colitis
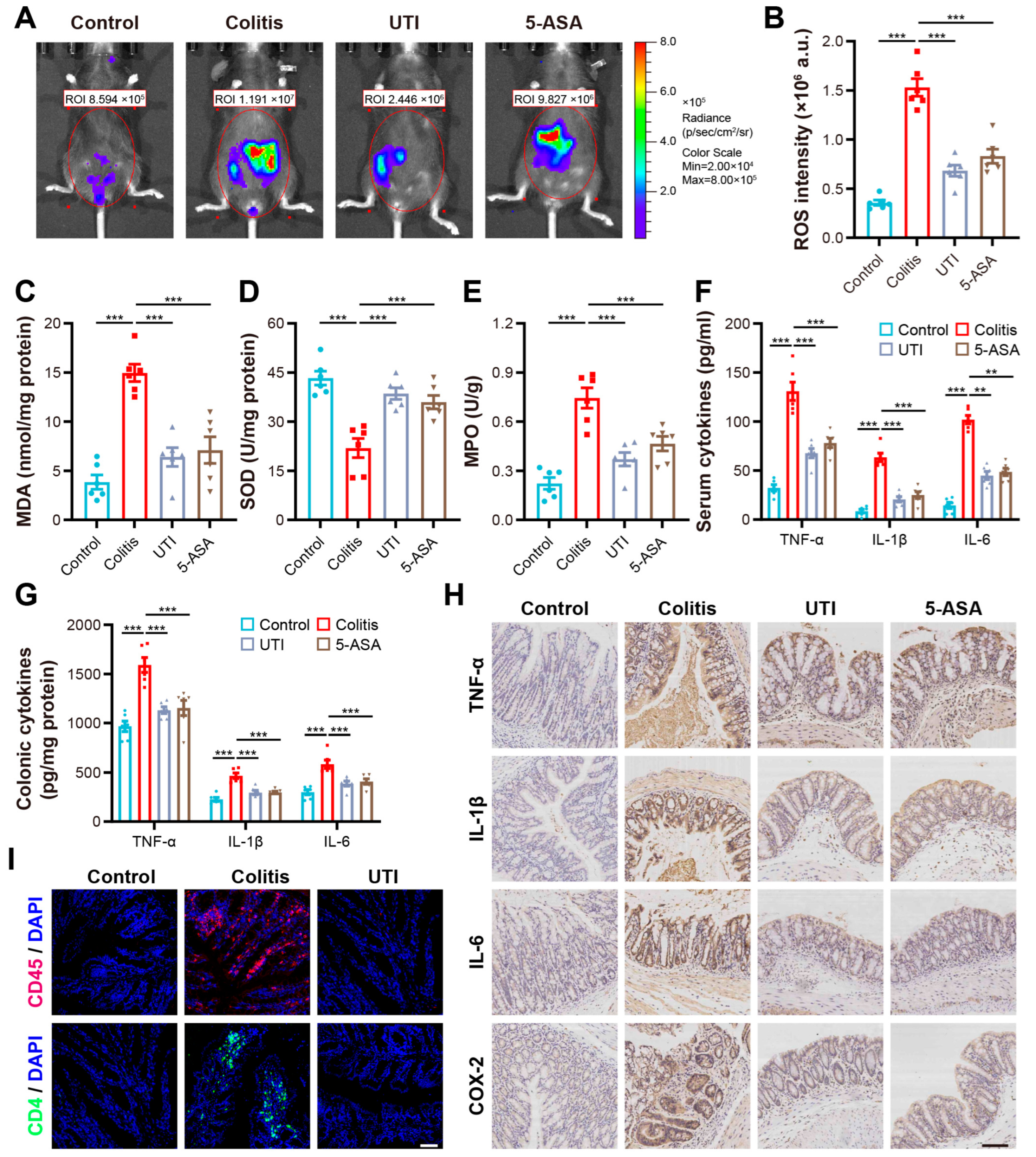
3.2. UTI Reduced Oxidative Stress and Modulated Colonic Inflammation in DSS-Induced Colitis
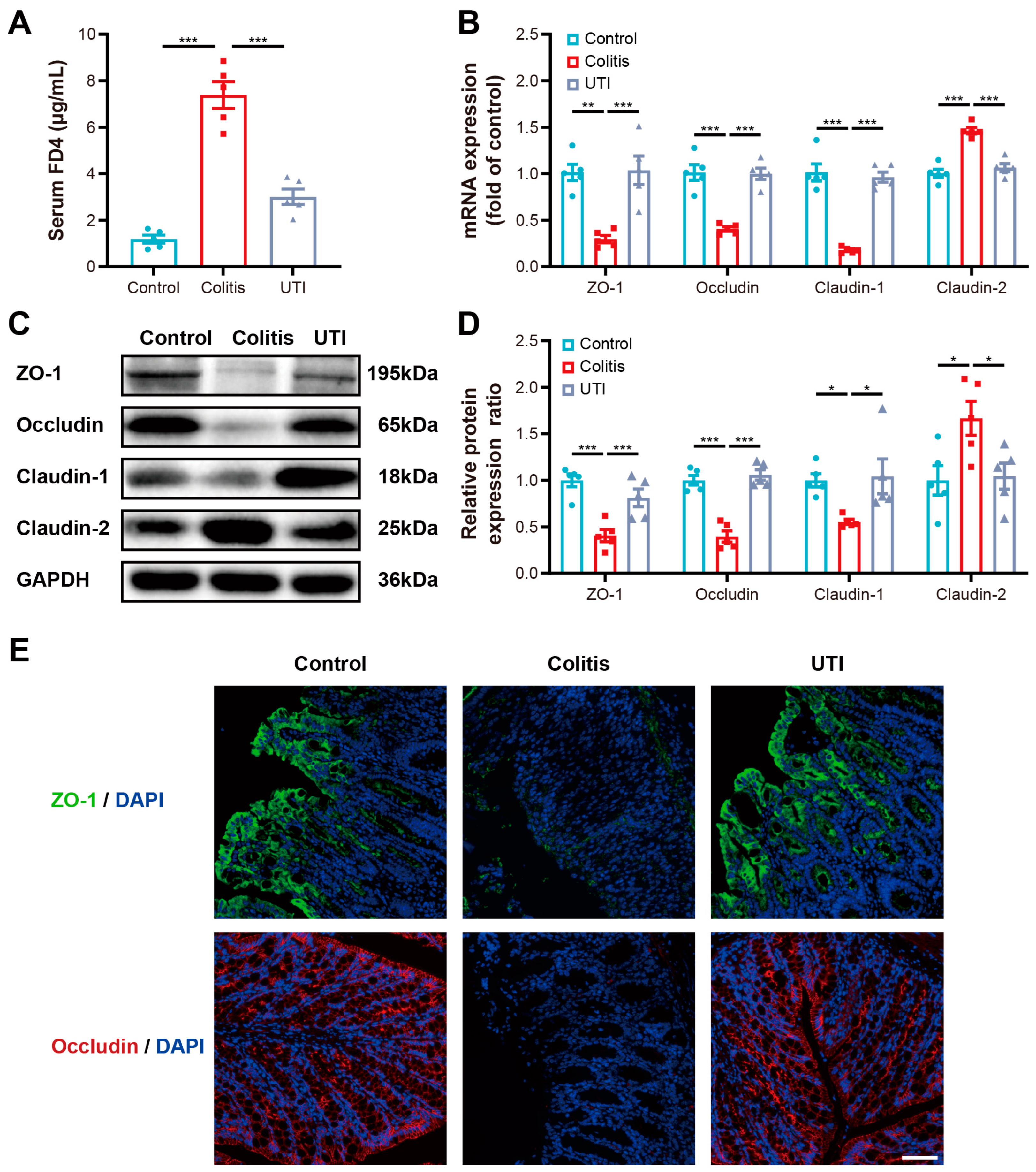
3.3. UTI Maintained Intestinal Barrier Function and Integrity in DSS-Induced Colitis
3.4. UTI-Mediated Gut Barrier Protection Is Associated with JAK2/STAT3/SOCS1 Signaling Pathway
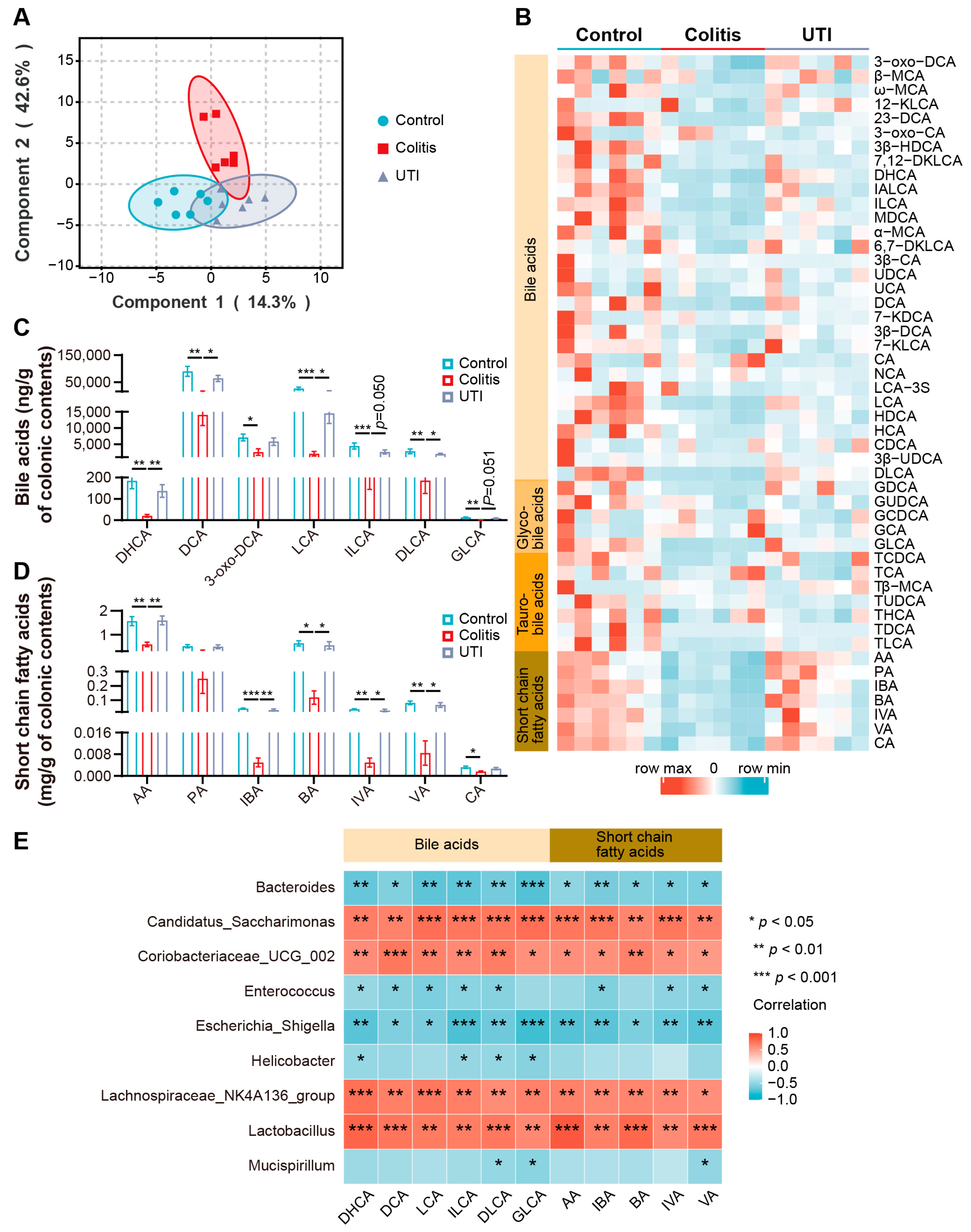
3.5. UTI Alleviates Gut Microbiota Dysbiosis in DSS-Induced Colitis Mice
3.6. UTI Improved Microbial BA and SCFA Dysmetabolism in DSS-Induced Colitis
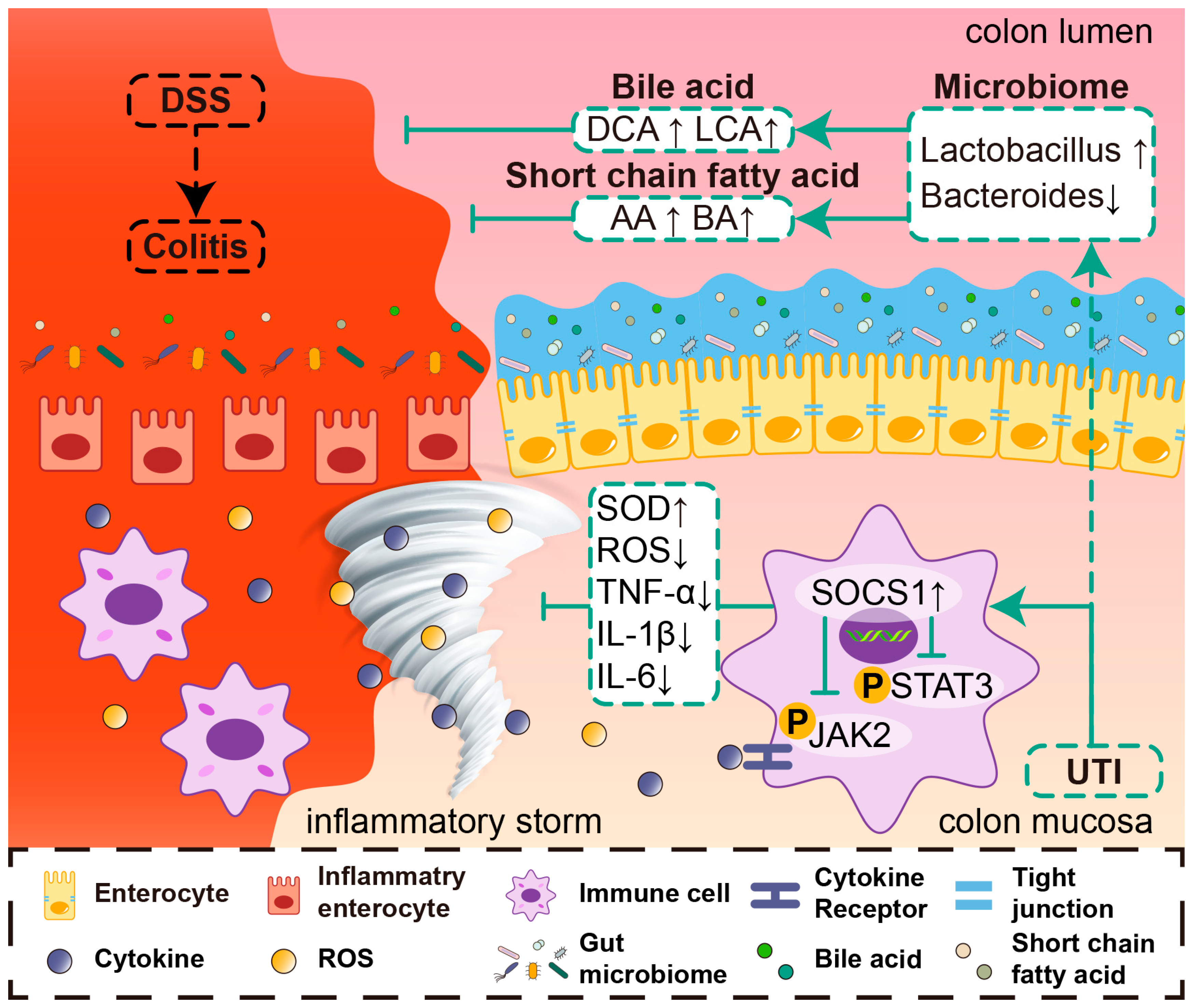
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Turpin, W.; Goethel, A.; Bedrani, L.; Croitoru, M.K. Determinants of IBD Heritability: Genes, Bugs, and More. Inflamm. Bowel Dis. 2018, 24, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Eliakim, R.; Magro, F.; Maaser, C.; Chowers, Y.; Geboes, K.; Mantzaris, G.; Reinisch, W.; Colombel, J.-F.; Vermeire, S.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 1: Definitions and diagnosis. J. Crohn’s Colitis 2012, 6, 965–990. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Brun, L.; Ballabeni, P.; Pittet, V.; Vavricka, B.M.P.; Zeitz, J.; Rogler, G.; Schoepfer, A.M.; Swiss IBD Cohort Study Group. Frequency and Risk Factors for Extraintestinal Manifes-tations in the Swiss Inflammatory Bowel Disease Cohort. Am. J. Gastroenterol. 2011, 106, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Lasa, J.S.; Olivera, P.A.; Danese, S.; Peyrin-Biroulet, L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Knights, D.; Lassen, K.G.; Xavier, R.J. Advances in inflammatory bowel disease pathogenesis: Linking host genetics and the micro-biome. Gut 2013, 62, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.; Mu, C.; Sun, Y.; Gu, Y.; Chen, D.; Liu, T.; Cao, H. Gut microbial metabolome in inflammatory bowel disease: From association to therapeutic perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 2402–2414. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Liu, F.; Zhu, X.-R.; Suo, F.-Y.; Jia, Z.-J.; Yao, S.-K. Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J. Gastroenterol. 2021, 27, 3609–3629. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Richard, D.; Faïs, T.; Vazeille, E.; Chervy, M.; Bonnin, V.; Dalmasso, G.; Denizot, J.; Billard, E.; Bonnet, R.; et al. Propionate catabolism by CD-associated adherent-invasive E. coli counteracts its anti-inflammatory effect. Gut Microbes 2021, 13, 1839318. [Google Scholar] [CrossRef] [PubMed]
- Ooijevaar, R.E.; Terveer, E.M.; Verspaget, H.W.; Kuijper, E.J.; Keller, J.J. Clinical Application and Potential of Fecal Microbiota Trans-plantation. Annu. Rev. Med. 2019, 70, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Casteele, N.V. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Sopjani, M.; Morina, R.; Uka, V.; Xuan, N.T.; Dërmaku-Sopjani, M. JAK2-mediated Intracellular Signaling. Curr. Mol. Med. 2021, 21, 417–425. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.C.S.; Wildenberg, M.E.; De Jonge, W.J.; D’haens, G.R. The Future of Janus Kinase Inhibitors in Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 885–893. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Janus kinase (JAK) inhibitors in the treatment of neoplastic and inflammatory disorders. Pharmacol. Res. 2022, 183, 10632. [Google Scholar] [CrossRef]
- Siegmund, B. Janus Kinase inhibitors in the New Treatment Paradigms of Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, S761–S766. [Google Scholar] [CrossRef]
- Inoue, K.-I.; Takano, H. Urinary trypsin inhibitor as a therapeutic option for endotoxin-related inflammatory disorders. Expert Opin. Investig. Drugs 2010, 19, 513–520. [Google Scholar] [CrossRef]
- Karnad, D.R.; Bhadade, R.; Verma, P.K.; Moulick, N.D.; Daga, M.K.; Chafekar, N.D.; Iyer, S. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: A multicenter randomized controlled study. Intensiv. Care Med. 2014, 40, 830–838. [Google Scholar] [CrossRef]
- He, Q.-L.; Zhong, F.; Ye, F.; Wei, M.; Liu, W.-F.; Li, M.-N.; Li, Q.-B.; Huang, W.-Q.; Sun, L.-B.; Shu, H.-H. Does Intraoperative Ulinastatin Improve Postoperative Clinical Outcomes in Patients Undergoing Cardiac Surgery: A Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2014, 2014, 630835. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, L.; Zhang, M.; Fang, X.; Huang, Z.; Yang, Z.; Zhou, T. Beneficial effects of ulinastatin on gut barrier function in sepsis. Indian J. Med. Res. 2013, 138, 904–911. [Google Scholar]
- Asghar, M.N.; Emani, R.; Alam, C.; Helenius, T.O.; Grönroos, T.J.; Sareila, O.; Din, M.U.; Holmdahl, R.; Hänninen, A.; Toivola, D.M. In Vivo Imaging of Reactive Oxygen and Nitrogen Species in Murine Colitis. Inflamm. Bowel Dis. 2014, 20, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Hanash, A.M.; Dudakov, J.A.; Hua, G.; O’connor, M.H.; Young, L.F.; Singer, N.V.; West, M.L.; Jenq, R.R.; Holland, A.M.; Kappel, L.W.; et al. Interleukin-22 Protects Intestinal Stem Cells from Immune-Mediated Tissue Damage and Regulates Sensitivity to Graft versus Host Disease. Immunity 2012, 37, 339–350. [Google Scholar] [CrossRef]
- Li, B.R.; Wu, J.; Li, H.S.; Jiang, Z.H.; Zhou, X.M.; Xu, C.H.; Ding, N.; Zha, J.M.; He, W.Q. In Vitro and In Vivo Approaches to Determine Intestinal Epithelial Cell Permeability. J. Vis. Exp. 2018, 140, e57032. [Google Scholar]
- Choi, S.-J.; Nagar, H.; Lee, J.W.; Kim, S.; Lee, I.; Piao, S.; Jeon, B.H.; Kim, C.-S. Effect of Ulinastatin on Syndecan-2-Mediated Vascular Damage in IDH2-Deficient Endothelial Cells. Biomedicines 2022, 10, 187. [Google Scholar] [CrossRef]
- Feng, X.; Ma, W.; Chen, J.; Jiao, W.; Wang, Y. Ulinastatin alleviates early brain injury after traumatic brain injury by inhibiting oxidative stress and apoptosis. Acta Cir. Bras. 2022, 37, e370108. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, X.; Jin, G. Ulinastatin protects rats from sepsis-induced acute lung injury by suppressing the JAK-STAT3 pathway. J. Cell. Biochem. 2019, 120, 2554–2559. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Cannatelli, R.; Monico, M.C.; Maconi, G.; Ardizzone, S. An Update on Current Pharmacotherapeutic Options for the Treatment of Ulcerative Colitis. J. Clin. Med. 2022, 11, 2302. [Google Scholar] [CrossRef]
- Ji, M.; Chen, T.; Wang, B.; Chen, M.; Ding, Q.; Chen, L.; Fang, Y.; Yu, X.; Chen, Y.; Wang, X.; et al. Effects of ulinastatin combined with mechanical ventilation on oxygen metabolism, inflammation and stress response and antioxidant capacity of ARDS. Exp. Ther. Med. 2018, 15, 4665–4670. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, Y.; Zhang, Y.; Cui, J.; Dong, Y.; Zhang, L.; Liu, P.; Hao, Y.; Xu, Y.; Wang, J. Ulinastatin ameliorates preeclampsia induced by N(gamma)-nitro-l-arginine methyl ester in a rat model via inhibition of the systemic and placental inflammatory response. J. Hypertens. 2023, 41, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ji, X.; Liu, J.; Guo, X.; Pang, R.; Zhuang, H.; Dong, L.; Duan, M.; Li, A. Ulinastatin Improves Renal Microcirculation by Protecting Endothelial Cells and Inhibiting Autophagy in a Septic Rat Model. Kidney Blood Press. Res. 2022, 47, 256–269. [Google Scholar] [CrossRef]
- Liu, M.; Shen, J.; Zou, F.; Zhao, Y.; Li, B.; Fan, M. Effect of ulinastatin on the permeability of the blood-brain barrier on rats with global cerebral ischemia/reperfusion injury as assessed by MRI. Biomed. Pharmacother. 2017, 85, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Zhu, G. Ulinastatin Attenuates Brain Edema After Traumatic Brain Injury in Rats. Cell Biochem. Biophys. 2015, 71, 595–600. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, C.; Zhang, Z.; Shi, J.; Lin, Y.; Gu, L.; Ma, X.; Li, H. Intravenous infusion of ulinastatin attenuates acute kidney injury after cold ischemia/reperfusion. Int. Urol. Nephrol. 2019, 51, 1873–1881. [Google Scholar] [CrossRef]
- Yu, L.; Luo, Q.; Fang, H. Mechanism of ulinastatin protection against lung injury caused by lower limb ischemia-reperfusion. Panminerva Medica 2014, 56, 49–55. [Google Scholar]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1 beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J. Exp. Med. 2012, 209, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Nalle, S.C.; Shen, L.; Turner, E.S.; Singh, G.; Breskin, L.A.; Khramtsova, E.A.; Khramtsova, G.; Tsai, P.Y.; Fu, Y.-X.; et al. TNFR2 Activates MLCK-Dependent Tight Junction Dysregulation to Cause Apoptosis-Mediated Barrier Loss and Experimental Colitis. Gastroenterology 2013, 145, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, S.R. Mechanistic insights into Regulation of JAK2 Tyrosine Kinase. Front. Endocrinol. 2018, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Hillmer, E.J.; Zhang, H.Y.; Li, H.S.; Watowich, S.S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol. Res. 2016, 111, 784–803. [Google Scholar] [CrossRef] [PubMed]
- Carow, B.; Rottenberg, M.E. SOCS3, a major regulato of infection and inflammation. Front Immunol. 2014, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Babon, J.J.; Sabo, J.K.; Zhang, J.-G.; Nicola, N.A.; Norton, R.S. The SOCS Box Encodes a Hierarchy of Affinities for Cullin5: Implications for Ubiquitin Ligase Formation and Cytokine Signalling Suppression. J. Mol. Biol. 2009, 387, 162–174. [Google Scholar] [CrossRef]
- Liu, W.; Pang, G.; Wang, S.; Sun, A. Protective effect of ulinastatin on severe pulmonary infection under immunosuppression and its molecular mechanism. Exp. Ther. Med. 2017, 14, 3583–3588. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome—Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Cai, Z.; Chai, J.; Liu, J.; Liu, B.; Yu, Y.; Liu, J.; Zhang, T. Egg white peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition. Food Chem. 2021, 360, 129981. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Guan, X.X.; Tang, Y.J.; Sun, J.F.; Wang, X.K.; Wang, W.D.; Fan, J.M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef] [PubMed]
- Ahrne, S.; Hagslatt, M.-L.J. Effect of Lactobacilli on Paracellular Permeability in the Gut. Nutrients 2011, 3, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Ishikawa, D.; Okahara, K.; Ito, S.; Haga, K.; Takahashi, M.; Arakawa, A.; Shibuya, T.; Osada, T.; Kuwahara-Arai, K.; et al. Bacteroidetes Species Are Correlated with Disease Activity in Ulcerative Colitis. J. Clin. Med. 2021, 10, 1749. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.-J.; Goeser, L.; Lu, K.; Faith, J.J.; Hansen, J.J. Enterococcus faecalis Glucosamine Metabolism Exacerbates Experimental Colitis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1373–1389. [Google Scholar] [CrossRef]
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the role of gut microbiome–host metabolic signal disruption in health and disease. Trends Microbiol. 2011, 19, 349–359. [Google Scholar] [CrossRef]
- Proctor, L.M. and Integrative HMPiRN. The Integrative Human Microbiome Project: Dynamic Analysis of Microbiome-Host Omics Profiles during Periods of Human Health and Disease. Cell Host Microbe 2014, 16, 276–289. [Google Scholar]
- Schaap, F.G.; Trauner, M.; Jansen, P.L.M. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y.L. Bile Acid Signaling in Metabolic Disease and Drug Therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? Ebiomedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Vojinovic, D.; Radjabzadeh, D.; Kurilshikov, A.; Amin, N.; Wijmenga, C.; Franke, L.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat. Commun. 2019, 10, 5813. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Yan, J.; Wang, R.; Zhang, L.; Hu, X.; Xu, J.; Li, F.; Sun, Q. Integrative Multiomics Profiling Unveils the Protective Function of Ulinastatin against Dextran Sulfate Sodium-Induced Colitis. Antioxidants 2024, 13, 214. https://doi.org/10.3390/antiox13020214
Yu T, Yan J, Wang R, Zhang L, Hu X, Xu J, Li F, Sun Q. Integrative Multiomics Profiling Unveils the Protective Function of Ulinastatin against Dextran Sulfate Sodium-Induced Colitis. Antioxidants. 2024; 13(2):214. https://doi.org/10.3390/antiox13020214
Chicago/Turabian StyleYu, Tianyu, Jun Yan, Ruochen Wang, Lei Zhang, Xiake Hu, Jiaxi Xu, Fanni Li, and Qi Sun. 2024. "Integrative Multiomics Profiling Unveils the Protective Function of Ulinastatin against Dextran Sulfate Sodium-Induced Colitis" Antioxidants 13, no. 2: 214. https://doi.org/10.3390/antiox13020214
APA StyleYu, T., Yan, J., Wang, R., Zhang, L., Hu, X., Xu, J., Li, F., & Sun, Q. (2024). Integrative Multiomics Profiling Unveils the Protective Function of Ulinastatin against Dextran Sulfate Sodium-Induced Colitis. Antioxidants, 13(2), 214. https://doi.org/10.3390/antiox13020214







