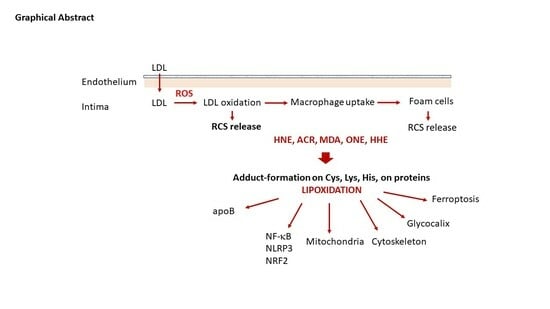
Reactive Carbonyl Species and Protein Lipoxidation in Atherogenesis
Abstract
:1. Introduction
2. Atherosclerosis from the Early Steps to Advanced Lesions: A Brief Overview
2.1. Endothelial Dysfunction
2.2. LDL Transcytosis
2.3. LDL Oxidation and Foam Cell Formation
2.4. Advanced Lesions
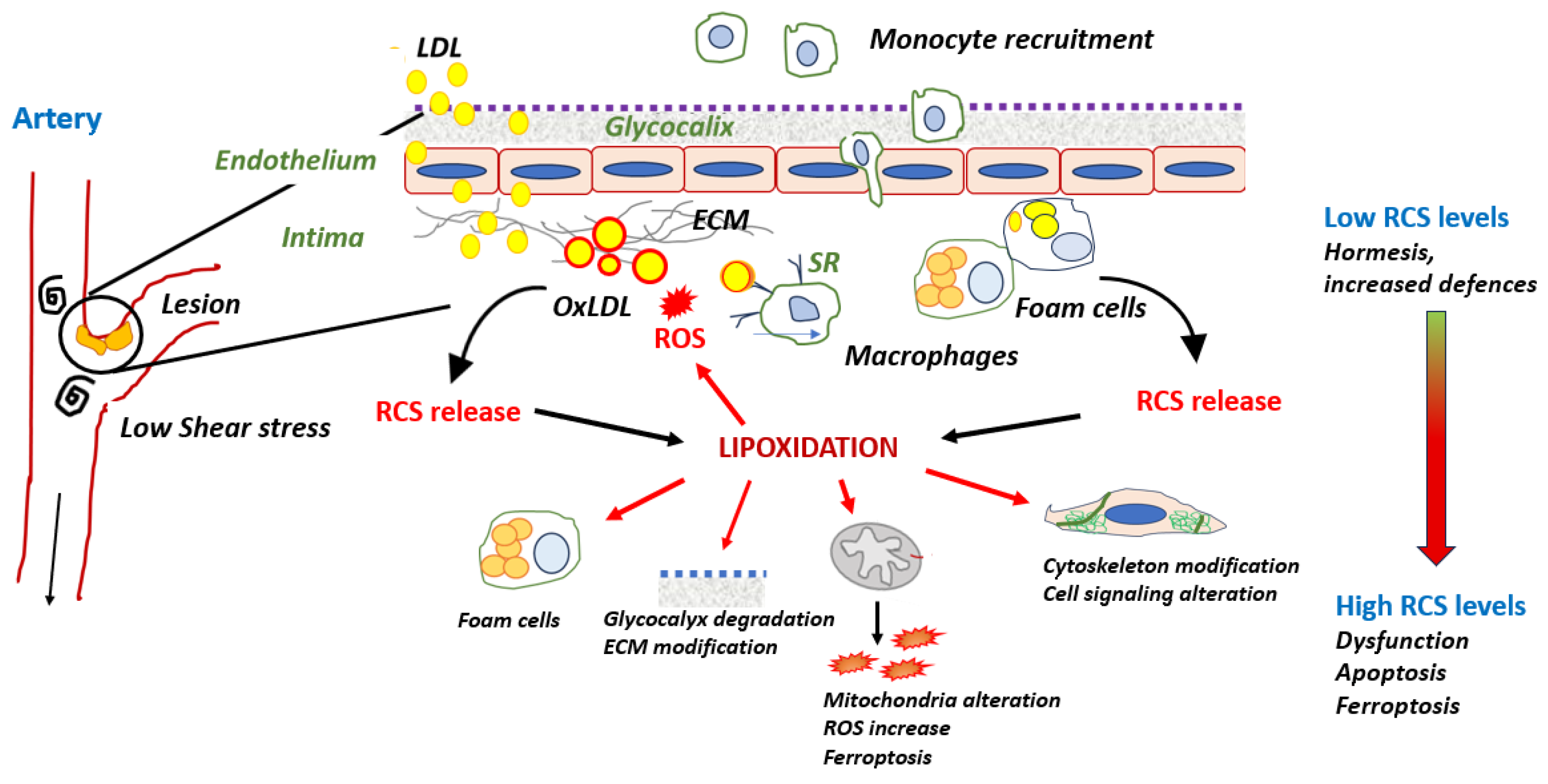
3. RCS in Early Atherosclerosis Lesions, from Hormesis to Dysfunctions
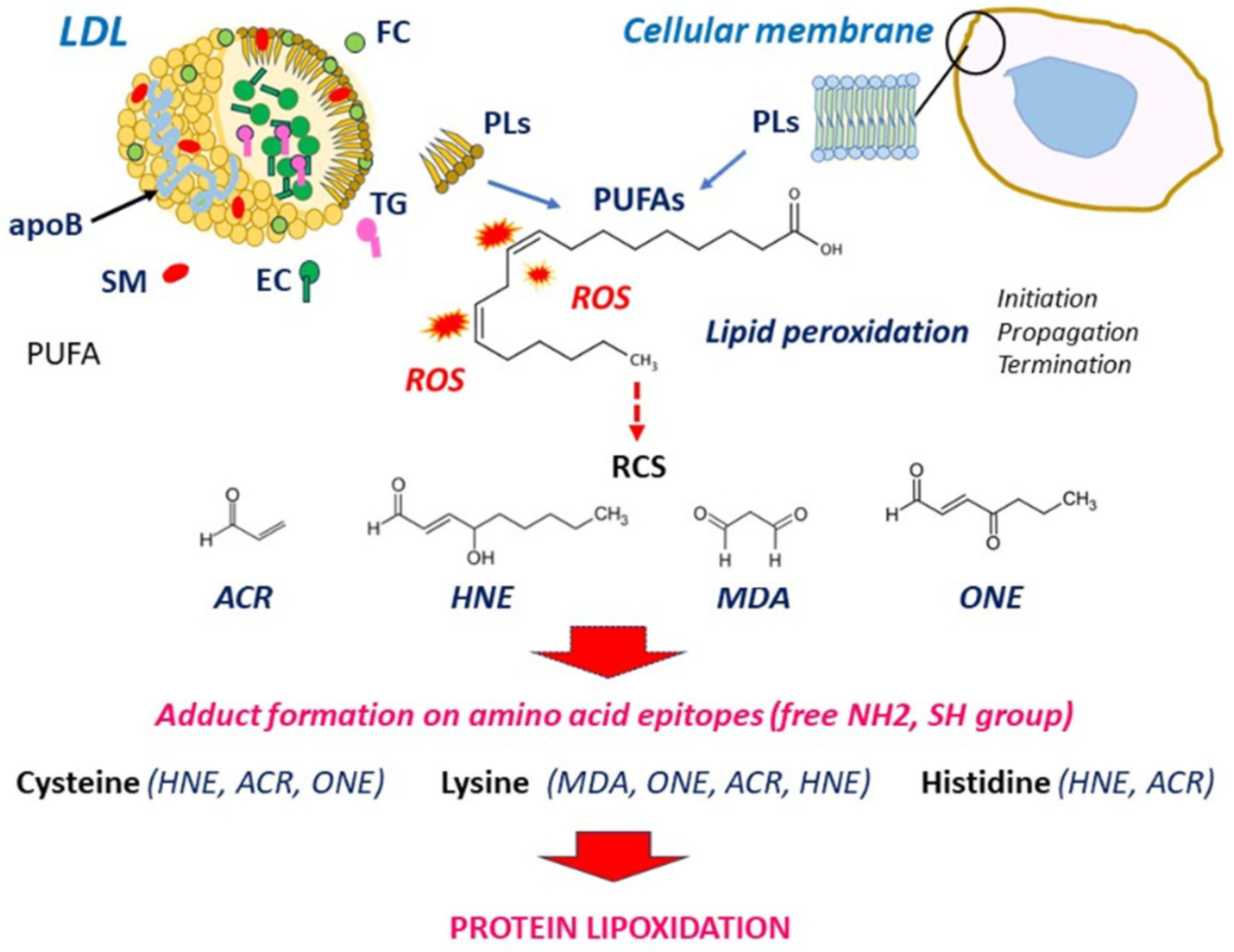
3.1. LDL Oxidation and Formation of RCS
| Systems | Targets | RCS | Epitopes | Consequences | References |
|---|---|---|---|---|---|
| LDL oxidation | apoB | MDA, HNE | Lys, His, Cys | Foam cells | [66,67] |
| ACR | Cys | [71] | |||
| Transcription factors in inflammation | |||||
| NF-kB | IkBα | HNE | Cys | Inhibition | [72,73] |
| IKK | HNE, ACR | Cys-179 | id. | [74,75,76,77,78] | |
| NLRP3 | HNE | Cys | Inhibition | [79] | |
| Nrf2 | Keap1 | HNE, RCS | Cys | Antioxidant | [80] |
| Mitochondria | Complex I | HNE, ONE | Decreased activity | [81] | |
| Complex II | ACR | ROS increase | [82,83,84,85,86,87,88,89,90] | ||
| ANT | HHE, HNE | Apoptosis | [91,92] | ||
| Antioxidant systems | |||||
| ALDH2 | HNE, ONE | ROS increase | [93] | ||
| GSTA4 | HNE | id. | [94] | ||
| TRX-1 | HNE, ACR | Cys73 | id. | [95] | |
| PRX6 | HNE, ONE | Cys-91, Lys-209 | Inhibition | [96] | |
| eNOS | ONE | Lys | Decreased activity | [97] | |
| GTPCH | HNE | id. | [98] | ||
| Endothelial barrier components | |||||
| Glycocalyx | HPSE | ACR, MDA | Lys | Degradation | [99] |
| Cytoskeleton | Actin | HNE | Cys-374 | Stress fibers | [100,101] |
| Vimentin | RCS | Cys-328 | Altered motility | [102,103] | |
| Growth factor receptors | |||||
| PDGFR, | HNE, ACR | Altered signaling | [104,105,106,107,108] | ||
| EGFR | - | ||||
| Endothelial senescence | |||||
| TXNIP | PPARγ | HNE | His-413 | Senescence | [109] |
| SIRT1 | HNE, ONE | Cys | id. | [110] | |
| 20S proteasome | HNE | Inhibition, ROS | [111,112,113,114,115] | ||
| ER stress | GRP78 | HNE, ONE | His, Lys | Apoptosis, | [116,117] |
| PDI HNE | Cys | Apoptosis | [118] | ||
| Cell death | CDR | HNE | Apoptosis | [119] | |
| ANT | HHE | Apoptosis | [92] | ||
| VDAC2 | HNE, ONE | Cys-210 | Ferroptosis | [120] | |
3.2. Protein Lipoxidation in the Vascular Wall
3.2.1. Modification of apoB by RCS in LDL: A Main Role in Foam Cell Formation
3.2.2. RCS and Inflammation
Lipoxidation of Transcription Factors and Inflammation
- NF-κB
- NLRP3
- Nrf2
Cyclooxygenase-2 Activation
RCS and ROS
- Effect of RCS on Mitochondrial ROS Production
- Effect of RCS on Antioxidant Systems
- -
- ALDH2
- -
- Glutathione-S Transferases (GSTs)
- -
- Thioredoxin 1
- -
- Peroxiredoxins
- Effect of RCS on eNOS
3.2.3. Lipoxidation of Endothelial Barrier Components
Glycocalyx
Extracellular Matrix Proteins
Cytoskeleton Proteins
- Actin
- Tubulin
- Vimentin
- Integrins and focal adhesions
Cell Signaling Kinases and Growth Factor Receptors
3.3. Lipoxidation in Advanced Lesions
3.3.1. Endothelial Senescence
- Sirtuins
- Proteasome and autophagy
3.3.2. Lipoxidation and ER Stress
3.3.3. Lipoxidation and Cell Death
- Apoptosis
- Ferroptosis
3.3.4. Lipoxidation and Angiogenesis
3.3.5. Lipoxidation and Vascular Calcifications
4. Pharmacological Interventions for Preventing and Neutralizing Lipoxidation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Nakano, M.; Virmani, R.; Fuster, V. Acute coronary events. Circulation 2012, 125, 1147–1156. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Figueiredo, C.S.; Roseira, E.S.; Viana, T.T.; Silveira, M.A.D.; de Melo, R.M.V.; Fernandez, M.G.; Lemos, L.M.G.; Passos, L.C.S. Inflammation in Coronary Atherosclerosis: Insights into Pathogenesis and Therapeutic Potential of Anti-Inflammatory Drugs. Pharmaceuticals 2023, 16, 1242. [Google Scholar] [CrossRef]
- Salekeen, R.; Haider, A.N.; Akhter, F.; Billah, M.M.; Islam, M.E.; Didarul Islam, K.M. Lipid oxidation in pathophysiology of atherosclerosis: Current understanding and therapeutic strategies. Int. J. Cardiol. Cardiovasc. Risk Prev. 2022, 14, 200143. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R. Advanced lipoxidation end-products. Chem. Biol. Interact. 2011, 192, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Naudí, A.; Jové, M.; Ayala, V.; Cabré, R.; Portero-Otín, M.; Pamplona, R. Non-enzymatic modification of aminophospholipids by carbonyl-amine reactions. Int. J. Mol. Sci. 2013, 14, 3285–3313. [Google Scholar] [CrossRef]
- Domingues, R.M.; Domingues, P.; Melo, T.; Pérez-Sala, D.; Reis, A.; Spickett, C.M. Lipoxidation adducts with peptides and proteins: Deleterious modifications or signaling mechanisms? J. Proteomics 2013, 92, 110–131. [Google Scholar] [CrossRef]
- Viedma-Poyatos, Á.; González-Jiménez, P.; Langlois, O.; Company-Marín, I.; Spickett, C.M.; Pérez-Sala, D. Protein Lipoxidation: Basic Concepts and Emerging Roles. Antioxidants 2021, 10, 295. [Google Scholar] [CrossRef]
- Spickett, C.M.; Pitt, A.R. Modification of proteins by reactive lipid oxidation products and biochemical effects of lipoxidation. Essays Biochem. 2020, 64, 19–31. [Google Scholar] [CrossRef]
- Milkovic, L.; Zarkovic, N.; Marusic, Z.; Zarkovic, K.; Jaganjac, M. The 4-Hydroxynonenal-Protein Adducts and Their Biological Relevance: Are Some Proteins Preferred Targets? Antioxidants 2023, 12, 856. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Ho, I.; Manzano-Pech, L.; Rubio-Ruíz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative Stress and Its Association with Cardiometabolic Disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.R.; Bäck, M.; Bochaton-Piallat, M.L.; Caligiuri, G.; Daemen, M.J.; Davies, P.F.; Hoefer, I.E.; Holvoet, P.; Jo, H.; Krams, R.; et al. Biomechanical factors in atherosclerosis: Mechanisms and clinical implications. Eur. Heart J. 2014, 35, 3013–3020, 3020a–3020d. [Google Scholar] [CrossRef]
- McQueen, A.; Warboys, C.M. Mechanosignalling pathways that regulate endothelial barrier function. Curr. Opin. Cell Biol. 2023, 84, 102213. [Google Scholar] [CrossRef]
- Ciccarelli, G.; Conte, S.; Cimmino, G.; Maiorano, P.; Morrione, A.; Giordano, A. Mitochondrial Dysfunction: The Hidden Player in the Pathogenesis of Atherosclerosis? Int. J. Mol. Sci. 2023, 24, 1086. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in Atherosclerosis-No Longer a Theory. Clin. Chem. 2021, 67, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fernández-Hernando, C. Transport of LDLs into the arterial wall: Impact in atherosclerosis. Curr. Opin. Lipidol. 2020, 31, 279–285. [Google Scholar] [CrossRef]
- Simionescu, M.; Popov, D.; Sima, A. Endothelial transcytosis in health and disease. Cell Tissue Res. 2009, 335, 27–40. [Google Scholar] [CrossRef]
- Ho, T.W.W.; Henry, A.; Lee, W.L. LDL Transcytosis by the Arterial Endothelium-Atherosclerosis by a Thousand Cuts? Curr. Atheroscler. Rep. 2023, 25, 457–465. [Google Scholar] [CrossRef]
- Scipione, C.A.; Cybulsky, M.I. Early atherogenesis: New insights from new approaches. Curr. Opin. Lipidol. 2022, 33, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.Y.; Fairn, G.D.; Lee, W.L. Transcellular vesicular transport in epithelial and endothelial cells: Challenges and opportunities. Traffic 2018, 19, 5–18. [Google Scholar] [CrossRef]
- Luchetti, F.; Crinelli, R.; Nasoni, M.G.; Benedetti, S.; Palma, F.; Fraternale, A.; Iuliano, L. LDL receptors, caveolae and cholesterol in endothelial dysfunction: OxLDLs accomplices or victims? Br. J. Pharmacol. 2021, 178, 3104–3114. [Google Scholar] [CrossRef]
- Shu, Y.; Jin, S. Caveolin-1 in endothelial cells: A potential therapeutic target for atherosclerosis. Heliyon 2023, 9, e18653. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Khan, M.A.; Mohammad, I.; Banerjee, S.; Tomar, A.; Varughese, K.I.; Mehta, J.L.; Chandele, A.; Arockiasamy, A. Oxidized LDL receptors: A recent update. Curr. Opin. Lipidol. 2023, 34, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, H. LOX-1 and atherosclerosis: Proof of concept in LOX-1-knockout mice. Circ. Res. 2007, 100, 1534–1536. [Google Scholar] [CrossRef]
- Zeya, B.; Arjuman, A.; Chandra, N.C. Lectin-like Oxidized Low-Density Lipoprotein (LDL) Receptor (LOX-1): A Chameleon Receptor for Oxidized LDL. Biochemistry 2016, 55, 4437–4444. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. LOX-1 and ROS, inseparable factors in the process of endothelial damage. Free Radic. Res. 2014, 48, 841–848. [Google Scholar] [CrossRef]
- Karasawa, T.; Takahashi, M. Role of NLRP3 Inflammasomes in Atherosclerosis. J. Atheroscler. Thromb. 2017, 24, 443–451. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.C.; David, S.G.; David, A.G.; Țarcă, V.; Pădureț, I.A.; Mîndru, D.E.; Roșu, S.T.; Roșu, E.V.; Adumitrăchioaiei, H.; Bernic, J.; et al. Atherosclerosis from Newborn to Adult-Epidemiology, Pathological Aspects, and Risk Factors. Life 2023, 13, 2056. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Dweck, M.R.; Narula, N.; Pisapia, D.; Narula, J.; Strauss, H.W. Coronary artery calcification: From mechanism to molecular imaging. JACC Cardiovasc. Imaging 2017, 10, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Freise, C.; Querfeld, U.; Ludwig, A.; Hamm, B.; Schnorr, J.; Taupitz, M. Uraemic extracellular vesicles augment osteogenic transdifferentiation of vascular smooth muscle cells via enhanced AKT signalling and PiT-1 expression. J. Cell Mol. Med. 2021, 5, 5602–5614. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, J.; Li, X.; Tan, R.; Chen, L.; Yang, L.; Dai, F.; Ma, L.; Xu, L.; Wang, Z.; et al. CXCR6 Mediates Pressure Overload-Induced Aortic Stiffness by Increasing Macrophage Recruitment and Reducing Exosome-miRNA29b. J. Cardiovasc. Transl. Res. 2023, 16, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Duan, Y.; Liu, C.; Huang, H.; Xiao, X.; He, Z. Extracellular vesicles in atherosclerosis and vascular calcification: The versatile non-coding RNAs from endothelial cells and vascular smooth muscle cells. Front. Med. 2023, 10, 1193660. [Google Scholar] [CrossRef]
- Kawtharany, L.; Bessueille, L.; Issa, H.; Hamade, E.; Zibara, K.; Magne, D. Inflammation and Microcalcification: A Never-Ending Vicious Cycle in Atherosclerosis? J. Vasc. Res. 2022, 59, 137–150. [Google Scholar] [CrossRef]
- Cretoiu, D.; Ionescu, R.F.; Enache, R.M.; Cretoiu, S.M.; Voinea, S.C. Gut Microbiome, Functional Food, Atherosclerosis, and Vascular Calcifications-Is There a Missing Link? Microorganisms 2021, 9, 1913. [Google Scholar] [CrossRef]
- De Meyer, G.R.; Grootaert, M.O.; Michiels, C.F.; Kurdi, A.; Schrijvers, D.M.; Martinet, W. Autophagy in vascular disease. Circ. Res. 2015, 116, 468–479. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rahbarghazi, R.; Nouri, M.; Aghamohammadzadeh, N.; Safaei, N.; Ahmadi, M. Role of autophagy in atherosclerosis: Foe or friend? J. Inflamm. 2019, 16, 8. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Interplay between efferocytosis and atherosclerosis. Arch. Cardiovasc. Dis. 2023, 116, 474–484. [Google Scholar] [CrossRef]
- De Meyer, G.R.Y.; Zurek, M.; Puylaert, P.; Martinet, W. Programmed death of macrophages in atherosclerosis: Mechanisms and therapeutic targets. Nat. Rev. Cardiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, S.; Sun, M.; Hua, M.; Liu, Z.; Mu, G.; Wang, Z.; Xiang, Q.; Cui, Y. Ferroptosis of Endothelial Cells in Vascular Diseases. Nutrients 2022, 14, 4506. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Osakabe, N.; Di Paola, R.; Siracusa, R.; Fusco, R.; D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Fritsch, T.; Abdelhameed, A.S.; et al. Hormesis defines the limits of lifespan. Ageing Res. Rev. 2023, 91, 102074. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.E. Nrf2-mediated redox signalling in vascular health and disease. Free Radic. Biol. Med. 2014, 75 (Suppl. S1), S1. [Google Scholar] [CrossRef] [PubMed]
- Nègre-Salvayre, A.; Garoby-Salom, S.; Swiader, A.; Rouahi, M.; Pucelle, M.; Salvayre, R. Proatherogenic effects of 4-hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Domingues, M.R.; Spickett, C.M.; Domingues, P.; Altomare, A.; Sánchez-Gómez, F.J.; Oeste, C.L.; Pérez-Sala, D. Protein lipoxidation: Detection strategies and challenges. Redox Biol. 2015, 5, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Martinez Fernandez, A.; Casalnuovo, F.; Altomare, A.; Aldini, G.; Banfi, C. Lipid Peroxidation in Atherosclerotic Cardiovascular Diseases. Antioxid. Redox Signal 2021, 34, 49–98. [Google Scholar] [CrossRef] [PubMed]
- Frangie, C.; Daher, J. Role of myeloperoxidase in inflammation and atherosclerosis. Biomed. Rep. 2022, 16, 53. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Guerby, P.; Gayral, S.; Laffargue, M.; Salvayre, R. Role of reactive oxygen species in atherosclerosis: Lessons from murine genetic models. Free Radic. Biol. Med. 2020, 149, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, Z.; Chandrakala, A.N.; Pressley, D.; Parthasarathy, S. Oxidized low density lipoproteins--do we know enough about them? Cardiovasc. Drugs Ther. 2011, 25, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Spickett, C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta 2012, 1818, 2374–2387. [Google Scholar] [CrossRef]
- Sousa, B.C.; Pitt, A.R.; Spickett, C.M. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 2017, 111, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.B.; Spickett, C.M. Lipoproteins as targets and markers of lipoxidation. Redox Biol. 2019, 23, 101066. [Google Scholar] [CrossRef] [PubMed]
- Winklhofer-Roob, B.M.; Faustmann, G.; Roob, J.M. Low-density lipoprotein oxidation biomarkers in human health and disease and effects of bioactive compounds. Free Radic. Biol. Med. 2017, 111, 38–86. [Google Scholar] [CrossRef] [PubMed]
- Schaur, R.J.; Siems, W.; Bresgen, N. Eckl PM. 4-Hydroxy-nonenal-A Bioactive Lipid Peroxidation Product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef]
- Lee, S.H.; Blair, I.A. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Toxicol. 2000, 13, 698–702. [Google Scholar] [CrossRef]
- Doorn, J.A.; Petersen, D.R. Covalent adduction of nucleophilic amino acids by 4-hydroxynonenal and 4-oxononenal. Chem. Biol. Interact. 2003, 143–144, 93–100. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakazato, Y.; Saiki, R.; Igarashi, K.; Kitada, M.; Ishii, I. Acrolein-conjugated low-density lipoprotein induces macrophage foam cell formation. Atherosclerosis 2013, 227, 51–57. [Google Scholar] [CrossRef]
- Page, S.; Fischer, C.; Baumgartner, B.; Haas, M.; Kreusel, U.; Loidl, G.; Hayn, M.; Ziegler-Heitbrock, H.W.; Neumeier, D.; Brand, K. 4-Hydroxynonenal prevents NF-kappaB activation and tumor necrosis factor expression by inhibiting IkappaB phosphorylation and subsequent proteolysis. J. Biol. Chem. 1999, 274, 11611–11618. [Google Scholar] [CrossRef]
- Dou, X.; Li, S.; Wang, Z.; Gu, D.; Shen, C.; Yao, T.; Song, Z. Inhibition of NF-κB activation by 4-hydroxynonenal contributes to liver injury in a mouse model of alcoholic liver disease. Am. J. Pathol. 2012, 181, 1702–1710. [Google Scholar] [CrossRef]
- Byun, M.S.; Choi, J.; Jue, D. Cysteine-179 of IkappaB kinase beta plays a critical role in enzyme activation by promoting phosphorylation of activation loop serines. Exp. Mol. Med. 2006, 38, 546–552. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Zavadskiy, S.P.; Astakhov, D.V.; Terentiev, A.A. Lipid peroxidation: Reactive carbonyl species, protein/DNA adducts, and signaling switches in oxidative stress and cancer. Biochem. Biophys. Res. Commun. 2023, 687, 149167. [Google Scholar] [CrossRef]
- Ji, C.; Kozak, K.R.; Marnett, L.J. IkappaB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J. Biol. Chem. 2001, 276, 18223–18228. [Google Scholar] [CrossRef]
- Tirumalai, R.; Rajesh Kumar, T.; Mai, K.H.; Biswal, S. Acrolein causes transcriptional induction of phase II genes by activation of Nrf2 in human lung type II epithelial (A549) cells. Toxicol. Lett. 2002, 132, 27–36. [Google Scholar] [CrossRef]
- Valacchi, G.; Pagnin, E.; Phung, A.; Nardini, M.; Schock, B.C.; Cross, C.E.; van der Vliet, A. Inhibition of NFkappaB activation and IL-8 expression in human bronchial epithelial cells by acrolein. Antioxid. Redox Signal 2005, 7, 25–31. [Google Scholar] [CrossRef]
- Hsu, C.G.; Chávez, C.L.; Zhang, C.; Sowden, M.; Yan, C.; Berk, B.C. The lipid peroxidation product 4-hydroxynonenal inhibits NLRP3 inflammasome activation and macrophage pyroptosis. Cell Death Differ. 2022, 29, 1790–1803. [Google Scholar] [CrossRef]
- Uruno, A.; Motohashi, H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 2011, 25, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Azzu, V.; Parker, N.; Brand, M.D. High membrane potential promotes alkenal-induced mitochondrial uncoupling and influences adenine nucleotide translocase conformation. Biochem. J. 2008, 413, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Picklo, M.J.; Amarnath, V.; McIntyre, J.O.; Graham, D.G.; Montine, T.J. 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J. Neurochem. 1999, 72, 1617–1624. [Google Scholar] [CrossRef]
- Landar, A.; Zmijewski, J.W.; Dickinson, D.A.; Le Goffe, C.; Johnson, M.S.; Milne, G.L.; Zanoni, G.; Vidari, G.; Morrow, J.D.; Darley-Usmar, V.M. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1777–H1787. [Google Scholar] [CrossRef]
- Long, J.; Wang, X.; Gao, H.; Liu, Z.; Liu, C.; Miao, M.; Liu, J. Malonaldehyde acts as a mitochondrial toxin: Inhibitory effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Life Sci. 2006, 79, 1466–1472. [Google Scholar] [CrossRef]
- Sun, L.; Luo, C.; Long, J.; Wei, D.; Liu, J. Acrolein is a mitochondrial toxin: Effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Mitochondrion 2006, 6, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Porter, N.A.; Brash, A.R. Autoxidative transformation of chiral omega6 hydroxy linoleic and arachidonic acids to chiral 4-hydroxy-2E-nonenal. Chem. Res. Toxicol. 2004, 17, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Singh, I.N.; Wang, J.A.; Hall, E.D. Administration of the Nrf2–ARE activators sulforaphane and carnosic acid attenuates 4-hydroxy-2-nonenal-induced mitochondrial dysfunction ex vivo. Free Radic. Biol. Medicine 2013, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, P.; Bailey, T.; Bhattarai, S.; Subedi, U.; Miller, C.; Ara, H.; Kidambi, S.; Sun, H.; Panchatcharam, M.; et al. Electrophilic Aldehyde 4-Hydroxy-2-Nonenal Mediated Signaling and Mitochondrial Dysfunction. Biomolecules 2022, 12, 1555. [Google Scholar] [CrossRef]
- Lashin, O.M.; Szweda, P.A.; Szweda, L.I.; Romani, A.M. Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Radic. Biol. Med. 2006, 40, 886–896. [Google Scholar] [CrossRef]
- Hwang, H.V.; Sandeep, N.; Paige, S.L.; Ranjbarvaziri, S.; Hu, D.Q.; Zhao, M.; Lan, I.S.; Coronado, M.; Kooiker, K.B.; Wu, S.M.; et al. 4HNE Impairs Myocardial Bioenergetics in Congenital Heart Disease-Induced Right Ventricular Failure. Circulation 2020, 142, 1667–1683. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jung, G.Y.; Heo, H.J.; Yun, M.R.; Park, J.Y.; Bae, S.S.; Hong, K.W.; Lee, W.S.; Kim, C.D. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol. Lett. 2006, 166, 212–221. [Google Scholar] [CrossRef]
- Kristal, B.S.; Park, B.K.; Yu, B.P. 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. J. Biol. Chem. 1996, 271, 6033–6038. [Google Scholar] [CrossRef]
- Doorn, J.A.; Hurley, T.D.; Petersen, D.R. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem. Res. Toxicol. 2006, 19, 102–110. [Google Scholar] [CrossRef]
- Balogh, L.M.; Atkins, W.M. Interactions of glutathione transferases with 4-hydroxynonenal. Drug Metab. Rev. 2011, 43, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Halvey, P.J.; Hansen, J.M.; Reed, M.; Pohl, J.; Jones, D.P. Reactive aldehyde modification of thioredoxin-1 activates early steps of inflammation and cell adhesion. Am. J. Pathol. 2007, 171, 1670–1681. [Google Scholar] [CrossRef]
- Roede, J.R.; Carbone, D.L.; Doorn, J.A.; Kirichenko, O.V.; Reigan, P.; Petersen, D.R. In vitro and in silico characterization of peroxiredoxin 6 modified by 4-hydroxynonenal and 4-oxononenal. Chem. Res. Toxicol. 2008, 21, 2289–2299. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.; Picklo, M.J., Sr.; Vasquez-Vivar, J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2340–2347. [Google Scholar] [CrossRef]
- Ko, K.; Suzuki, T.; Ishikawa, R.; Hattori, N.; Ito, R.; Umehara, K.; Furihata, T.; Dohmae, N.; Linhardt, R.J.; Igarashi, K.; et al. Ischemic stroke disrupts the endothelial glycocalyx through activation of proHPSE via acrolein exposure. J. Biol. Chem. 2020, 295, 18614–18624. [Google Scholar] [CrossRef]
- Aldini, G.; Dalle-Donne, I.; Vistoli, G.; Maffei Facino, R.; Carini, M. Covalent modification of actin by 4-hydroxy-trans-2-nonenal (HNE): LC-ESI-MS/MS evidence for Cys374 Michael adduction. J. Mass. Spectrom. 2005, 40, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, M.; Miyagawa-Hayashino, A.; Akatsuka, S.; Shirase, T.; Lee, W.H.; Uchida, K.; Toyokuni, S. Susceptibility of actin to modification by 4-hydroxy-2-nonenal. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2005, 827, 119–126. [Google Scholar] [CrossRef]
- Mónico, A.; Duarte, S.; Pajares, M.A.; Pérez-Sala, D. Vimentin disruption by lipoxidation and electrophiles: Role of the cysteine residue and filament dynamics. Redox Biol. 2019, 23, 101098. [Google Scholar] [CrossRef]
- Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2020, 21, 4675. [Google Scholar] [CrossRef]
- Escargueil-Blanc, I.; Salvayre, R.; Vacaresse, N.; Jürgens, G.; Darblade, B.; Arnal, J.F.; Parthasarathy, S.; Nègre-Salvayre, A. Mildly oxidized LDL induces activation of platelet-derived growth factor beta-receptor pathway. Circulation 2001, 104, 1814–1821. [Google Scholar] [CrossRef]
- Suc, I.; Meilhac, O.; Lajoie-Mazenc, I.; Vandaele, J.; Jürgens, G.; Salvayre, R.; Nègre-Salvayre, A. Activation of EGF receptor by oxidized LDL. FASEB J. 1998, 12, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef]
- Liu, W.; Akhand, A.A.; Kato, M.; Yokoyama, I.; Miyata, T.; Kurokawa, K.; Uchida, K.; Nakashima, I. 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J. Cell Sci. 1999, 112 Pt 14, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Auge, N.; Garcia, V.; Maupas-Schwalm, F.; Levade, T.; Salvayre, R.; Negre-Salvayre, A. Oxidized LDL-induced smooth muscle cell proliferation involves the EGF receptor/PI-3 kinase/Akt and the sphingolipid signaling pathways. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1990–1995. [Google Scholar] [CrossRef]
- Riahi, Y.; Kaiser, N.; Cohen, G.; Abd-Elrahman, I.; Blum, G.; Shapira, O.M.; Koler, T.; Simionescu, M.; Sima, A.V.; Zarkovic, N.; et al. Foam cell-derived 4-hydroxynonenal induces endothelial cell senescence in a TXNIP-dependent manner. J. Cell Mol. Med. 2015, 19, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Caito, S.; Rajendrasozhan, S.; Cook, S.; Chung, S.; Yao, H.; Friedman, A.E.; Brookes, P.S.; Rahman, I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010, 24, 3145–3159. [Google Scholar] [CrossRef]
- Friguet, B.; Szweda, L.I. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 1997, 405, 21–25. [Google Scholar] [CrossRef]
- Grune, T.; Davies, K.J. The proteasomal system and HNE-modified proteins. Mol. Aspects Med. 2003, 24, 195–204. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Petropoulos, I.; Grimm, S.; Georgila, K.; Catalgol, B.; Friguet, B.; Grune, T.; Gonos, E.S. Protein damage, repair and proteolysis. Mol. Aspects Med. 2014, 35, 1–71. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Kapphahn, R.J. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004, 578, 217–223. [Google Scholar] [CrossRef]
- Just, J.; Jung, T.; Friis, N.A.; Lykkemark, S.; Drasbek, K.; Siboska, G.; Grune, T.; Kristensen, P. Identification of an unstable 4-hydroxynoneal modification on the 20S proteasome subunit α7 by recombinant antibody technology. Free Radic. Biol. Med. 2015, 89, 786–792. [Google Scholar] [CrossRef]
- Galligan, J.J.; Fritz, K.S.; Backos, D.S.; Shearn, C.T.; Smathers, R.L.; Jiang, H.; MacLean, K.N.; Reigan, P.R.; Petersen, D.R. Oxidative stress-mediated aldehyde adduction of GRP78 in a mouse model of alcoholic liver disease: Functional independence of ATPase activity and chaperone function. Free Radic. Biol. Med. 2014, 73, 411–420. [Google Scholar] [CrossRef]
- Vladykovskaya, E.; Sithu, S.D.; Haberzettl, P.; Wickramasinghe, N.S.; Merchant, M.L.; Hill, B.G.; McCracken, J.; Agarwal, A.; Dougherty, S.; Gordon, S.A.; et al. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J. Biol. Chem. 2012, 287, 11398–11409. [Google Scholar] [CrossRef]
- Carbone, D.L.; Doorn, J.A.; Kiebler, Z.; Petersen, D.R. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem. Res. Toxicol. 2005, 18, 1324–1331. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, R.; Sharma, A.; Vatsyayan, R.; Yadav, S.; Singhal, S.S.; Rauniyar, N.; Prokai, L.; Awasthi, S.; Awasthi, Y.C. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry 2010, 49, 6263–6275. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Lan, T.; Qin, W.; Zhu, Y.; Qin, K.; Gao, J.; Wang, H.; Hou, X.; Chen, N.; et al. Quantitative Profiling of Protein Carbonylations in Ferroptosis by an Aniline-Derived Probe. J. Am. Chem. Soc. 2018, 140, 4712–4720. [Google Scholar] [CrossRef]
- Zadeh, M.; Mota-Martorell, N.; Pradas, I.; Martín-Gari, M.; Ayala, V.; Pamplona, R. The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity. Antioxidants 2020, 9, 1132. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K.; Melkumyants, A.M. Malondialdehyde as an Important Key Factor of Molecular Mechanisms of Vascular Wall Damage under Heart Diseases Development. Int. J. Mol. Sci. 2022, 24, 128. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef]
- Tian, K.; Xu, Y.; Sahebkar, A.; Xu, S. CD36 in Atherosclerosis: Pathophysiological Mechanisms and Therapeutic Implications. Curr. Atheroscler. Rep. 2020, 22, 59. [Google Scholar] [CrossRef]
- Levitan, I.; Volkov, S.; Subbaiah, P.V. Oxidized LDL: Diversity, patterns of recognition, and pathophysiology. Antioxid. Redox Signal 2010, 13, 39–75. [Google Scholar] [CrossRef] [PubMed]
- Qin, S. LDL and HDL Oxidative Modification and Atherosclerosis. Adv. Exp. Med. Biol. 2020, 1276, 157–169. [Google Scholar] [CrossRef]
- Goyal, T.; Mitra, S.; Khaidakov, M.; Wang, X.; Singla, S.; Ding, Z.; Liu, S.; Mehta, J.L. Current Concepts of the Role of Oxidized LDL Receptors in Atherosclerosis. Curr. Atheroscler. Rep. 2012, 14, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Hoff, H.F.; O’Neil, J.; Chisolm GM 3rd Cole, T.B.; Quehenberger, O.; Esterbauer, H.; Jürgens, G. Modification of low density lipoprotein with 4-hydroxynonenal induces uptake by macrophages. Arteriosclerosis 1989, 9, 538–549. [Google Scholar] [CrossRef]
- Jürgens, G.; Lang, J.; Esterbauer, H. Modification of human low-density lipoprotein by the lipid peroxidation product 4-hydroxynonenal. Biochim. Biophys. Acta 1986, 875, 103–114. [Google Scholar] [CrossRef]
- Bräsen, J.H.; Häkkinen, T.; Malle, E.; Beisiegel, U.; Ylä-Herttuala, S. Patterns of oxidized epitopes, but not NF-kappa B expression, change during atherogenesis in WHHL rabbits. Atherosclerosis 2003, 166, 13–21. [Google Scholar] [CrossRef]
- Yamada, S.; Funada, T.; Shibata, N.; Kobayashi, M.; Kawai, Y.; Tatsuda, E.; Furuhata, A.; Uchida, K. Protein-bound 4-hydroxy-2-hexenal as a marker of oxidized n-3 polyunsaturated fatty acids. J. Lipid Res. 2004, 45, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Toyokuni, S.; Nishikawa, K.; Kawakishi, S.; Oda, H.; Hiai, H.; Stadtman, E.R. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: Markers for atherosclerosis. Biochemistry 1994, 33, 12487–12494. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Williams, K.J.; Tabas, I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–561. [Google Scholar] [CrossRef]
- de Winther, M.P.; Kanters, E.; Kraal, G.; Hofker, M.H. Nuclear factor kappaB signaling in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 904–914. [Google Scholar] [CrossRef]
- Mimura, J.; Itoh, K. Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic. Biol. Med. 2015, 88 Pt B, 221–232. [Google Scholar] [CrossRef]
- Matsumori, A. Nuclear Factor-κB is a Prime Candidate for the Diagnosis and Control of Inflammatory Cardiovascular Disease. Eur. Cardiol. 2023, 18, e40. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Bornfeldt, K.E. Inflammasomes and Atherosclerosis: A Mixed Picture. Circ. Res. 2023, 132, 1505–1520. [Google Scholar] [CrossRef]
- Ruef, J.; Moser, M.; Bode, C.; Kübler, W.; Runge, M.S. 4-hydroxynonenal induces apoptosis, NF-kappaB-activation and formation of 8-isoprostane in vascular smooth muscle cells. Basic. Res. Cardiol. 2001, 96, 143–150. [Google Scholar] [CrossRef]
- Lee, S.J.; Seo, K.W.; Yun, M.R.; Bae, S.S.; Lee, W.S.; Hong, K.W.; Kim, C.D. 4-Hydroxynonenal enhances MMP-2 production in vascular smooth muscle cells via mitochondrial ROS-mediated activation of the Akt/NF-kappaB signaling pathways. Free Radic. Biol. Med. 2008, 45, 1487–1492. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, C.E.; Seo, K.W.; Kim, C.D. HNE-induced 5-LO expression is regulated by NF-{kappa}B/ERK and Sp1/p38 MAPK pathways via EGF receptor in murine macrophages. Cardiovasc. Res. 2010, 88, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, S.; Gamba, P.; Testa, G.; Rossin, D.; Biasi, F.; Poli, G.; Leonarduzzi, G. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 2015, 14, 569–581. [Google Scholar] [CrossRef]
- Je, J.H.; Lee, J.Y.; Jung, K.J.; Sung, B.; Go, E.K.; Yu, B.P.; Chung, H.Y. NF-kappaB activation mechanism of 4-hydroxyhexenal via NIK/IKK and p38 MAPK pathway. FEBS Lett. 2004, 566, 183–189. [Google Scholar] [CrossRef]
- Hattori, Y.; Hattori, S.; Kasai, K. 4-hydroxynonenal prevents NO production in vascular smooth muscle cells by inhibiting nuclear factor-kappaB-dependent transcriptional activation of inducible NO synthase. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1179–1183. [Google Scholar] [CrossRef]
- Chapple, S.J.; Cheng, X.; Mann, G.E. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013, 1, 319–331. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361, Erratum in Nature 2010, 466, 652. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, M.Y.; Yang, D.; Motro, B.; Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Ma, Q.; He, X. Molecular basis of electrophilic and oxidative defense: Promises and perils of Nrf2. Pharmacol. Rev. 2012, 64, 1055–1081. [Google Scholar] [CrossRef]
- Jakobs, P.; Serbulea, V.; Leitinger, N.; Eckers, A.; Haendeler, J. Nuclear Factor (Erythroid-Derived 2)-Like 2 and Thioredoxin-1 in Atherosclerosis and Ischemia/Reperfusion Injury in the Heart. Antioxid. Redox Signal 2017, 26, 630–644. [Google Scholar] [CrossRef]
- Pajares, M.; Jiménez-Moreno, N.; García-Yagüe, Á.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rábano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 2016, 12, 1902–1916. [Google Scholar] [CrossRef]
- da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a Potential Mediator of Cardiovascular Risk in Metabolic Diseases. Front. Pharmacol. 2019, 10, 382. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Ruiz, E.; Leake, D.S.; Unoki, H.; Yamamoto, M.; Mann, G.E. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: Activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004, 94, 609–616. [Google Scholar] [CrossRef]
- Harada, N.; Ito, K.; Hosoya, T.; Mimura, J.; Maruyama, A.; Noguchi, N.; Yagami, K.; Morito, N.; Takahashi, S.; Maher, J.M.; et al. Nrf2 in bone marrow-derived cells positively contributes to the advanced stage of atherosclerotic plaque formation. Free Radic. Biol. Med. 2012, 53, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K. HNE as an inducer of COX-2. Free Radic. Biol. Med. 2017, 111, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Wall, S.B.; Kramer, P.A.; Ravi, S.; Mitchell, T.; Johnson, M.S.; Wilson, L.; Barnes, S.; Landar, A.; Darley-Usmar, V.M. Pleiotropic effects of 4-hydroxynonenal on oxidative burst and phagocytosis in neutrophils. Redox Biol. 2016, 9, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; John, A. 4-hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4-4 and cytochrome P450 2E1 in PC12 cells. Toxicol. Appl. Pharmacol. 2006, 216, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Mali, V.R.; Ning, R.; Chen, J.; Yang, X.P.; Xu, J.; Palaniyandi, S.S. Impairment of aldehyde dehydrogenase-2 by 4-hydroxy-2-nonenal adduct formation and cardiomyocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin. Exp. Biol. Med. 2014, 239, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef]
- Echtay, K.S.; Pakay, J.L.; Esteves, T.C.; Brand, M.D. Hydroxynonenal and uncoupling proteins: A model for protection against oxidative damage. Biofactors 2005, 24, 119–130. [Google Scholar] [CrossRef]
- Sheehan, D.; Meade, G.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001, 360 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Röth, E.; Marczin, N.; Balatonyi, B.; Ghosh, S.; Kovács, V.; Alotti, N.; Borsiczky, B.; Gasz, B. Effect of a glutathione S-transferase inhibitor on oxidative stress and ischemia-reperfusion-induced apoptotic signalling of cultured cardiomyocytes. Exp. Clin. Cardiol. 2011, 16, 92–96. [Google Scholar]
- Mitchell, A.E.; Morin, D.; Lamé, M.W.; Jones, A.D. Purification, Mass Spectrometric Characterization, and Covalent Modification of Murine Glutathione S-Transferases. Chem. Res. Toxicol. 1995, 8, 1054–1062. [Google Scholar] [CrossRef]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef]
- Wong, C.M.; Marcocci, L.; Das, D.; Wang, X.; Luo, H.; Zungu-Edmondson, M.; Suzuki, Y.J. Mechanism of protein decarbonylation. Free Radic. Biol. Med. 2013, 65, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, I.; Liu, W.; Akhand, A.A.; Takeda, K.; Kawamoto, Y.; Kato, M.; Suzuki, H. 4-hydroxynonenal triggers multistep signal transduction cascades for suppression of cellular functions. Mol. Aspects Med. 2003, 24, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Shearn, C.T.; Reigan, P.; Petersen, D.R. Inhibition of hydrogen peroxide signaling by 4-hydroxynonenal due to differential regulation of Akt1 and Akt2 contributes to decreases in cell survival and proliferation in hepatocellular carcinoma cells. Free Radic. Biol. Med. 2012, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Cancel, L.M.; Fu, B.M.; Tarbell, J.M. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc. Eng. Technol. 2021, 12, 37–71. [Google Scholar] [CrossRef] [PubMed]
- Askari, H.; Sadeghinejad, M.; Fancher, I.S. Mechanotransduction and the endothelial glycocalyx: Interactions with membrane and cytoskeletal proteins to transduce force. Curr. Top. Membr. 2023, 91, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Curry, F.E.; Adamson, R.H. Endothelial glycocalyx: Permeability barrier and mechanosensor. Ann. Biomed. Eng. 2012, 40, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Paone, S.; Chan, E.; Poon, I.K.H.; Baxter, A.A.; Thomas, S.R.; Hulett, M.D. Heparanase: A Novel Therapeutic Target for the Treatment of Atherosclerosis. Cells 2022, 11, 3198. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.K.; Davis, G.E. Extracellular Matrix Remodeling in Vascular Disease: Defining Its Regulators and Pathological Influence. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1599–1616. [Google Scholar] [CrossRef]
- Halper, J. Basic Components of Vascular Connective Tissue and Extracellular Matrix. Adv. Pharmacol. 2018, 81, 95–127. [Google Scholar] [CrossRef]
- Xu, J.; Shi, G.P. Vascular wall extracellular matrix proteins and vascular diseases. Biochim. Biophys. Acta 2014, 1842, 2106–2119. [Google Scholar] [CrossRef]
- Greilberger, J.; Schmut, O.; Jürgens, G. In vitro interactions of oxidatively modified LDL with type I, II, III, IV, and V collagen, laminin, fibronectin, and poly-D-lysine. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2721–2728, Erratum in Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1197. [Google Scholar] [CrossRef]
- Dunér, P.; To, F.; Alm, R.; Gonçalves, I.; Fredrikson, G.N.; Hedblad, B.; Berglund, G.; Nilsson, J.; Bengtsson, E. Immune responses against fibronectin modified by lipoprotein oxidation and their association with cardiovascular disease. J. Intern. Med. 2009, 265, 593–603. [Google Scholar] [CrossRef]
- Dunér, P.; To, F.; Berg, K.; Alm, R.; Björkbacka, H.; Engelbertsen, D.; Fredrikson, G.N.; Nilsson, J.; Bengtsson, E. Immune responses against aldehyde-modified laminin accelerate atherosclerosis in Apoe−/− mice. Atherosclerosis 2010, 212, 457–465. [Google Scholar] [CrossRef]
- Dunér, P.; Gonçalves, I.; Grufman, H.; Edsfeldt, A.; To, F.; Nitulescu, M.; Nilsson, J.; Bengtsson, E. Increased aldehyde-modification of collagen type IV in symptomatic plaques--a possible cause of endothelial dysfunction. Atherosclerosis 2015, 240, 26–32. [Google Scholar] [CrossRef]
- Zarkovic, K.; Larroque-Cardoso, P.; Pucelle, M.; Salvayre, R.; Waeg, G.; Nègre-Salvayre, A.; Zarkovic, N. Elastin aging and lipid oxidation products in human aorta. Redox Biol. 2015, 4, 109–117. [Google Scholar] [CrossRef]
- Larroque-Cardoso, P.; Camaré, C.; Nadal-Wollbold, F.; Grazide, M.H.; Pucelle, M.; Garoby-Salom, S.; Bogdanowicz, P.; Josse, G.; Schmitt, A.M.; Uchida, K.; et al. Elastin Modification by 4-Hydroxynonenal in Hairless Mice Exposed to UV-A. Role in Photoaging and Actinic Elastosis. J. Investig. Dermatol. 2015, 135, 1873–1881. [Google Scholar] [CrossRef]
- Larroque-Cardoso, P.; Mucher, E.; Grazide, M.H.; Josse, G.; Schmitt, A.M.; Nadal-Wolbold, F.; Zarkovic, K.; Salvayre, R.; Nègre-Salvayre, A. 4-Hydroxynonenal impairs transforming growth factor-β1-induced elastin synthesis via epidermal growth factor receptor activation in human and murine fibroblasts. Free Radic. Biol. Med. 2014, 71, 427–436. [Google Scholar] [CrossRef]
- Yang, S.; Nugent, M.A.; Panchenko MPEGF antagonizes TGF-beta-induced tropoelastin expression in lung fibroblasts via stabilization of Smad corepressor, T. G.I.F. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L143–L151. [Google Scholar] [CrossRef]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef] [PubMed]
- Usatyuk, P.V.; Natarajan, V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J. Biol. Chem. 2004, 279, 11789–11797. [Google Scholar] [CrossRef] [PubMed]
- Usatyuk, P.V.; Natarajan, V. Hydroxyalkenals and oxidized phospholipids modulation of endothelial cytoskeleton, focal adhesion and adherens junction proteins in regulating endothelial barrier function. Microvasc. Res. 2012, 83, 45–55. [Google Scholar] [CrossRef]
- Stewart, B.J.; Doorn, J.A.; Petersen, D.R. Residue-specific adduction of tubulin by 4-hydroxynonenal and 4-oxononenal causes cross-linking and inhibits polymerization. Chem. Res. Toxicol. 2007, 20, 1111–1119. [Google Scholar] [CrossRef]
- Kokubo, J.; Nagatani, N.; Hiroki, K.; Kuroiwa, K.; Watanabe, N.; Arai, T. Mechanism of destruction of microtubule structures by 4-hydroxy-2-nonenal. Cell Struct. Funct. 2008, 33, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.D.; Sidell, K.R.; Graham, D.G.; Montine, T.J. The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J. Neurochem. 1999, 72, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.D.; Boutte, A.; Milatovic, D.; Montine, T.J. Mechanisms of 4-hydroxynonenal-induced neuronal microtubule dysfunction. Brain Res. 2005, 1037, 90–98. [Google Scholar] [CrossRef]
- Shakhov, A.S.; Alieva, I.B. The “Third Violin” in the Cytoskeleton Orchestra-The Role of Intermediate Filaments in the Endothelial Cell’s Life. Biomedicines 2022, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jeong, S.J.; Park, J.H.; Cho, W.; Ahn, Y.H.; Choi, Y.H.; Oh, G.T.; Silverstein, R.L.; Park, Y.M. Plasma Membrane Localization of CD36 Requires Vimentin Phosphorylation; A Mechanism by Which Macrophage Vimentin Promotes Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 792717. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Attiq, A.; Afzal, S.; Ahmad, W.; Kandeel, M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 2024, 17, 176338. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. 4-Hydroxynonenal activates Src through a non-canonical pathway that involves, E.G.F.R./.P.T.P.1.B. Free Radic. Biol. Med. 2015, 89, 701–707. [Google Scholar] [CrossRef]
- Cantero, A.V.; Portero-Otín, M.; Ayala, V.; Auge, N.; Sanson, M.; Elbaz, M.; Thiers, J.C.; Pamplona, R.; Salvayre, R.; Nègre-Salvayre, A. Methylglyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-beta: Implications for diabetic atherosclerosis. FASEB J. 2007, 21, 3096–3106. [Google Scholar] [CrossRef]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Wojtasińska, A.; Frąk, W.; Lisińska, W.; Sapeda, N.; Młynarska, E.; Rysz, J.; Franczyk, B. Novel Insights into the Molecular Mechanisms of Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 13434. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Goligorsky, M.S.; Chen, J.; Patschan, S. Stress-induced premature senescence of endothelial cells: A perilous state between recovery and point of no return. Curr. Opin. Hematol. 2009, 16, 215–219. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Forman, H.J. 4-hydroxynonenal-mediated signaling and aging. Free Radic. Biol. Med. 2017, 111, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.D.; Prabhu, K.S.; Thompson, J.T.; Reddy, P.S.; Peters, J.M.; Peterson, B.R.; Reddy, C.C.; Vanden Heuvel, J.P. The oxidative stress mediator 4-hydroxynonenal is an intracellular agonist of the nuclear receptor peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). Free Radic. Biol. Med. 2007, 42, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Y.; Sin-Malia, Y.; Cohen, G.; Alpert, E.; Gruzman, A.; Eckel, J.; Staels, B.; Guichardant, M.; Sasson, S. The natural protective mechanism against hyperglycemia in vascular endothelial cells: Roles of the lipid peroxidation product 4-hydroxydodecadienal and peroxisome proliferator-activated receptor delta. Diabetes 2010, 59, 808–818. [Google Scholar] [CrossRef]
- Zhao, K.; Harshaw, R.; Chai, X.; Marmorstein, R. Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD (+)-dependent Sir2 histone/protein deacetylases. Proc. Natl. Acad. Sci. USA 2004, 101, 8563–8568. [Google Scholar] [CrossRef]
- Begum, M.K.; Konja, D.; Singh, S.; Chlopicki, S.; Wang, Y. Endothelial SIRT1 as a Target for the Prevention of Arterial Aging: Promises and Challenges. J. Cardiovasc. Pharmacol. 2021, 78 (Suppl. S6), S63–S77. [Google Scholar] [CrossRef]
- Fenton, M.; Barker, S.; Kurz, D.J.; Erusalimsky, J.D. Cellular senescence after single and repeated balloon catheter denudations of rabbit carotid arteries. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 220–226. [Google Scholar] [CrossRef]
- Gómez-Virgilio, L.; Silva-Lucero, M.D.; Flores-Morelos, D.S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.M.; Zacapala-Gómez, A.E.; Luna-Muñoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O.; et al. Autophagy: A Key Regulator of Homeostasis and Disease: An Overview of Molecular Mechanisms and Modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, S.; Ni, B.; He, K.; Zhu, P.; Wu, X.; Shao, Y. Augmenting ATG14 alleviates atherosclerosis and inhibits inflammation via promotion of autophagosome-lysosome fusion in macrophages. Autophagy 2021, 17, 4218–4230. [Google Scholar] [CrossRef]
- Luo, S.; Jiang, L.; Li, Q.; Sun, X.; Liu, T.; Pei, F.; Zhang, T.; Liu, T.; Dong, L.; Liu, X.; et al. Acrolein-induced autophagy-dependent apoptosis via activation of the lysosomal-mitochondrial pathway in EAhy926 cells. Toxicol. In Vitro 2018, 52, 146–153. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Zhou, A.X.; Tabas, I. The UPR in atherosclerosis. Semin. Immunopathol. 2013, 35, 321–332. [Google Scholar] [CrossRef]
- Yang, S.; Wu, M.; Li, X.; Zhao, R.; Zhao, Y.; Liu, L.; Wang, S. Role of Endoplasmic Reticulum Stress in Atherosclerosis and Its Potential as a Therapeutic Target. Oxid. Med. Cell Longev. 2020, 2020, 9270107. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Pfaffenbach, K.T.; Lee, A.S. The critical role of GRP78 in physiologic and pathologic stress. Curr. Opin. Cell Biol. 2011, 23, 150–156. [Google Scholar] [CrossRef]
- Bu, L.L.; Yuan, H.H.; Xie, L.L.; Guo, M.H.; Liao, D.F.; Zheng, X.L. New Dawn for Atherosclerosis: Vascular Endothelial Cell Senescence and Death. Int. J. Mol. Sci. 2023, 24, 15160. [Google Scholar] [CrossRef]
- Salvayre, R.; Negre-Salvayre, A.; Camaré, C. Oxidative theory of atherosclerosis and antioxidants. Biochimie 2016, 125, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef] [PubMed]
- Sanson, M.; Augé, N.; Vindis, C.; Muller, C.; Bando, Y.; Thiers, J.C.; Marachet, M.A.; Zarkovic, K.; Sawa, Y.; Salvayre, R.; et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: Prevention by oxygen-regulated protein 150 expression. Circ. Res. 2009, 104, 328–336. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Cell death mechanisms: Cross-talk and role in disease. Exp. Cell Res. 2010, 316, 1374–1383. [Google Scholar] [CrossRef]
- Yang, J.K. Death effector domain for the assembly of death-inducing signaling complex. Apoptosis 2015, 20, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, Y.C.; Sharma, R.; Sharma, A.; Yadav, S.; Singhal, S.S.; Chaudhary, P.; Awasthi, S. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic. Biol. Med. 2008, 45, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.S.; Woo, S.M.; Choi, K.S.; Kwon, T.K. Acrolein sensitizes human renal cancer Caki cells to TRAIL-induced apoptosis via ROS-mediated up-regulation of death receptor-5 (DR5) and down-regulation of Bcl-2. Exp. Cell Res. 2011, 317, 2592–2601. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Wu, H.; Wang, F.; Ta, N.; Zhang, T.; Gao, W. The Multifaceted Regulation of Mitochondria in Ferroptosis. Life 2021, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Alvira, C.M.; Umesh, A.; Husted, C.; Ying, L.; Hou, Y.; Lyu, S.C.; Nowak, J.; Cornfield, D.N. Voltage-dependent anion channel-2 interaction with nitric oxide synthase enhances pulmonary artery endothelial cell nitric oxide production. Am. J. Respir. Cell Mol. Biol. 2012, 47, 669–678. [Google Scholar] [CrossRef]
- Amoscato, A.A.; Anthonymuthu, T.; Kapralov, O.; Sparvero, L.J.; Shrivastava, I.H.; Mikulska-Ruminska, K.; Tyurin, V.A.; Shvedova, A.A.; Tyurina, Y.Y.; Bahar, I.; et al. Formation of protein adducts with Hydroperoxy-PE electrophilic cleavage products during ferroptosis. Redox Biol. 2023, 63, 102758. [Google Scholar] [CrossRef]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef] [PubMed]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Ayalasomayajula, S.P.; Kompella, U.B. Induction of vascular endothelial growth factor by 4-hydroxynonenal and its prevention by glutathione precursors in retinal pigment epithelial cells. Eur. J. Pharmacol. 2002, 449, 213–220. [Google Scholar] [CrossRef]
- Camaré, C.; Vanucci-Bacqué, C.; Augé, N.; Pucelle, M.; Bernis, C.; Swiader, A.; Baltas, M.; Bedos-Belval, F.; Salvayre, R.; Nègre-Salvayre, A. 4-Hydroxynonenal Contributes to Angiogenesis through a Redox-Dependent Sphingolipid Pathway: Prevention by Hydralazine Derivatives. Oxid. Med. Cell Longev. 2017, 2017, 9172741. [Google Scholar] [CrossRef]
- Pugliese, G.; Iacobini, C.; Blasetti Fantauzzi, C.; Menini, S. The dark and bright side of atherosclerotic calcification. Atherosclerosis 2015, 238, 220–230. [Google Scholar] [CrossRef]
- Shioi, A.; Ikari, Y. Plaque Calcification During Atherosclerosis Progression and Regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef]
- Olsson, M.; Thyberg, J.; Nilsson, J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1218–1222. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Q.; Hao, H.; Flaker, G.C.; Liu, Z. N-Acetylcysteine and Atherosclerosis: Promises and Challenges. Antioxidants 2023, 12, 2073. [Google Scholar] [CrossRef]
- Colzani, M.; De Maddis, D.; Casali, G.; Carini, M.; Vistoli, G.; Aldini, G. Reactivity, Selectivity, and Reaction Mechanisms of Aminoguanidine, Hydralazine, Pyridoxamine, and Carnosine as Sequestering Agents of Reactive Carbonyl Species: A Comparative Study. ChemMedChem 2016, 11, 1778–1789. [Google Scholar] [CrossRef]
- Baye, E.; Ukropcova, B.; Ukropec, J.; Hipkiss, A.; Aldini, G.; de Courten, B. Physiological and therapeutic effects of carnosine on cardiometabolic risk and disease. Amino Acids 2016, 48, 1131–1149. [Google Scholar] [CrossRef]
- Barski, O.A.; Xie, Z.; Baba, S.P.; Sithu, S.D.; Agarwal, A.; Cai, J.; Bhatnagar, A.; Srivastava, S. Dietary carnosine prevents early atherosclerotic lesion formation in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1162–1170. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosinases, their substrates and diseases. Molecules 2014, 19, 2299–2329. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Ricci, C.; Scipioni, A.; Blasetti Fantauzzi, C.; Giaccari, A.; Salomone, E.; Canevotti, R.; Lapolla, A.; Orioli, M.; et al. D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br. J. Pharmacol. 2012, 166, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Feehan, J.; Hariharan, R.; Buckenham, T.; Handley, C.; Bhatnagar, A.; Baba, S.P.; de Courten, B. Carnosine as a potential therapeutic for the management of peripheral vascular disease. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Burcham, P.C.; Kerr, P.G.; Fontaine, F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000, 5, 47–49. [Google Scholar] [CrossRef]
- Galvani, S.; Coatrieux, C.; Elbaz, M.; Grazide, M.H.; Thiers, J.C.; Parini, A.; Uchida, K.; Kamar, N.; Rostaing, L.; Baltas, M.; et al. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic. Biol. Med. 2008, 45, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Gupta, A.; Chigurupati, S.; Singh, S.; Sehgal, A.; Badavath, V.N.; Alhowail, A.; Mani, V.; Bhatia, S.; Al-Harrasi, A.; et al. Natural and Synthetic Agents Targeting Reactive Carbonyl Species against Metabolic Syndrome. Molecules 2022, 27, 1583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nègre-Salvayre, A.; Salvayre, R. Reactive Carbonyl Species and Protein Lipoxidation in Atherogenesis. Antioxidants 2024, 13, 232. https://doi.org/10.3390/antiox13020232
Nègre-Salvayre A, Salvayre R. Reactive Carbonyl Species and Protein Lipoxidation in Atherogenesis. Antioxidants. 2024; 13(2):232. https://doi.org/10.3390/antiox13020232
Chicago/Turabian StyleNègre-Salvayre, Anne, and Robert Salvayre. 2024. "Reactive Carbonyl Species and Protein Lipoxidation in Atherogenesis" Antioxidants 13, no. 2: 232. https://doi.org/10.3390/antiox13020232
APA StyleNègre-Salvayre, A., & Salvayre, R. (2024). Reactive Carbonyl Species and Protein Lipoxidation in Atherogenesis. Antioxidants, 13(2), 232. https://doi.org/10.3390/antiox13020232