The Skeletal Muscle, the Heart, and the Liver Are the Major Organs of the Accumulation of Nitric Oxide Metabolites after Oral Nitrite Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Drug Treatment
2.2. Measurement of Gastric pH
2.3. Tissue Harvesting and Homogenization
2.4. Measurement of Nitrite and Nitrosylated Species (RXNO) Concentrations
2.5. Measurement of Nitrate Concentrations
2.6. Statistics
3. Results
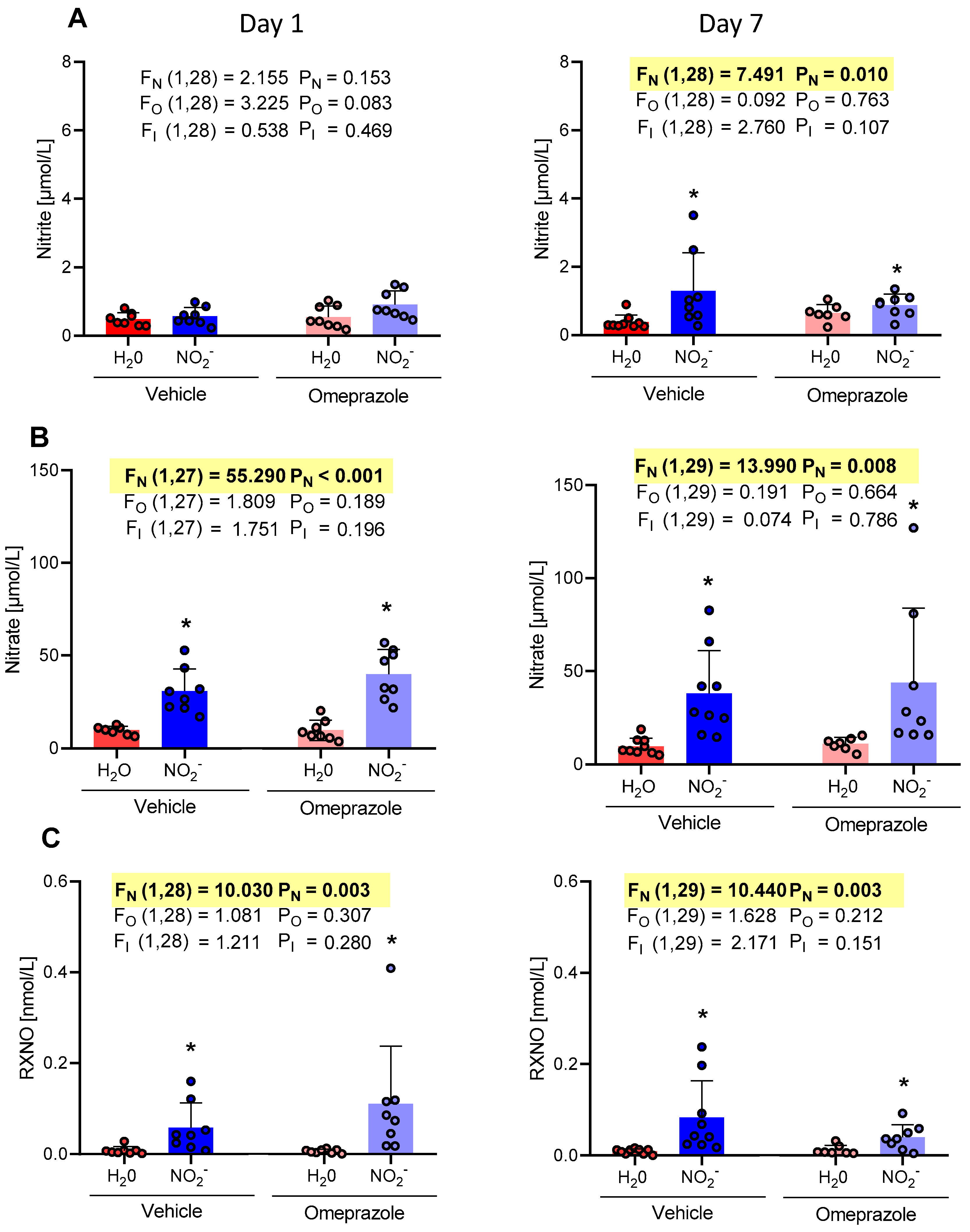
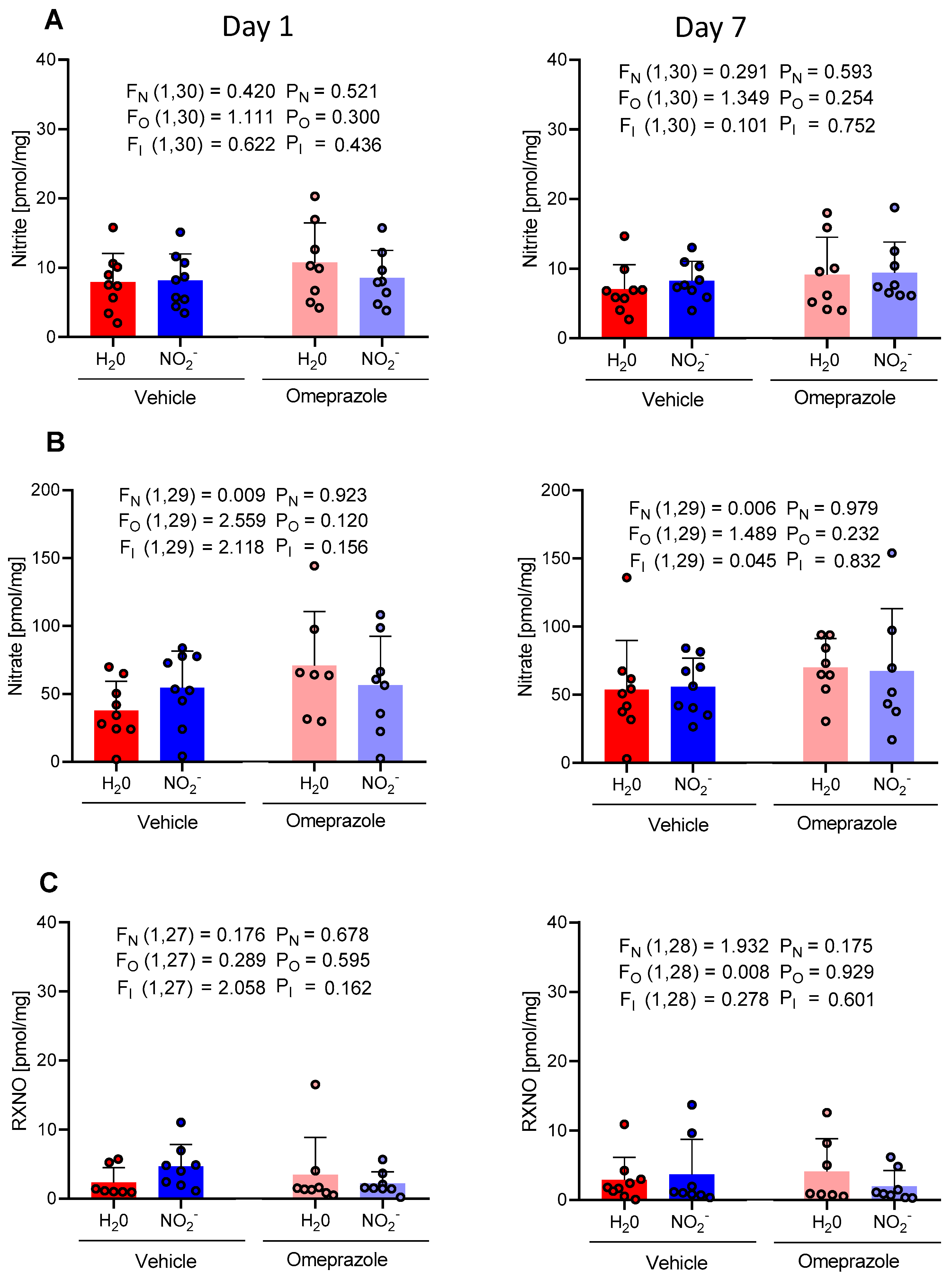
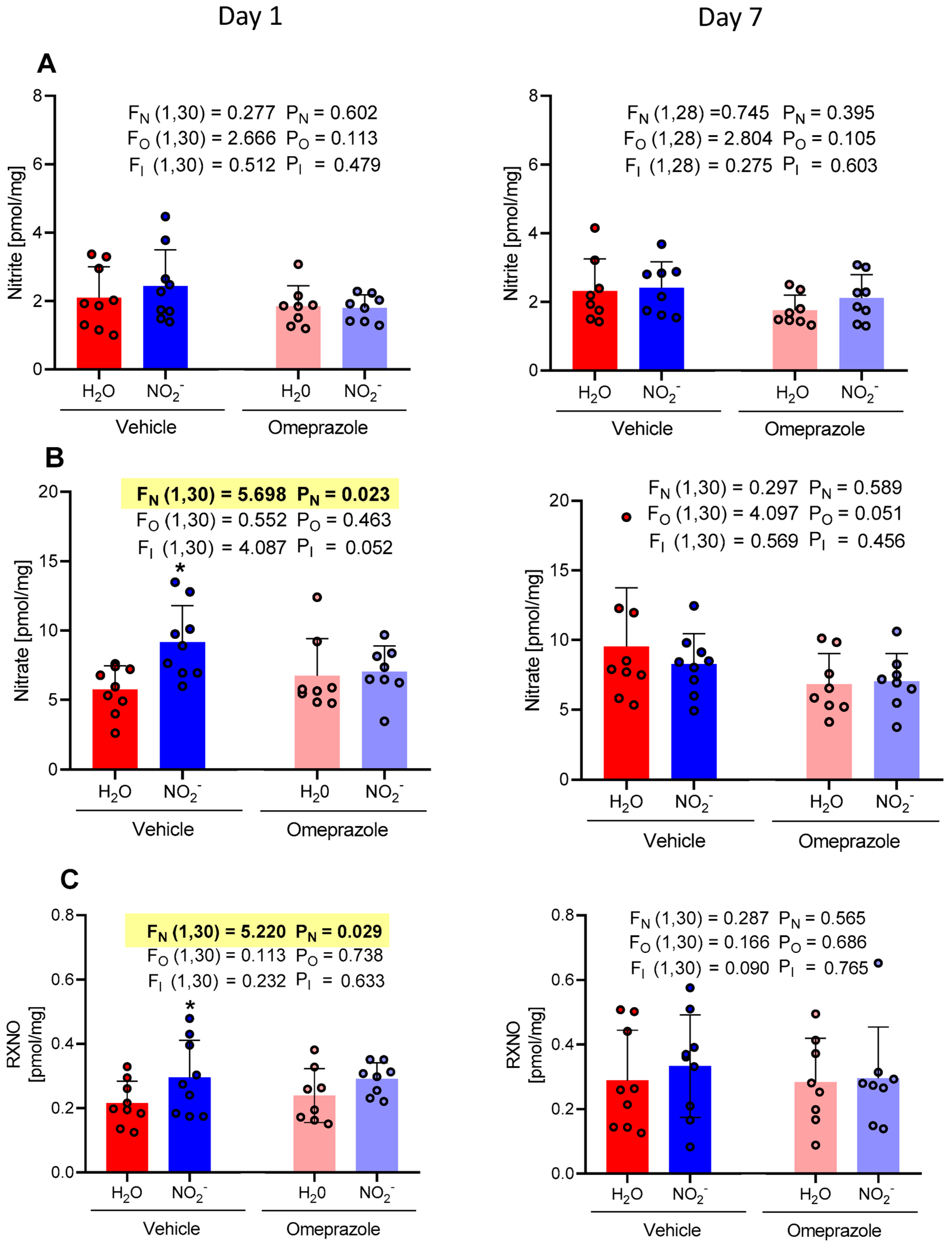
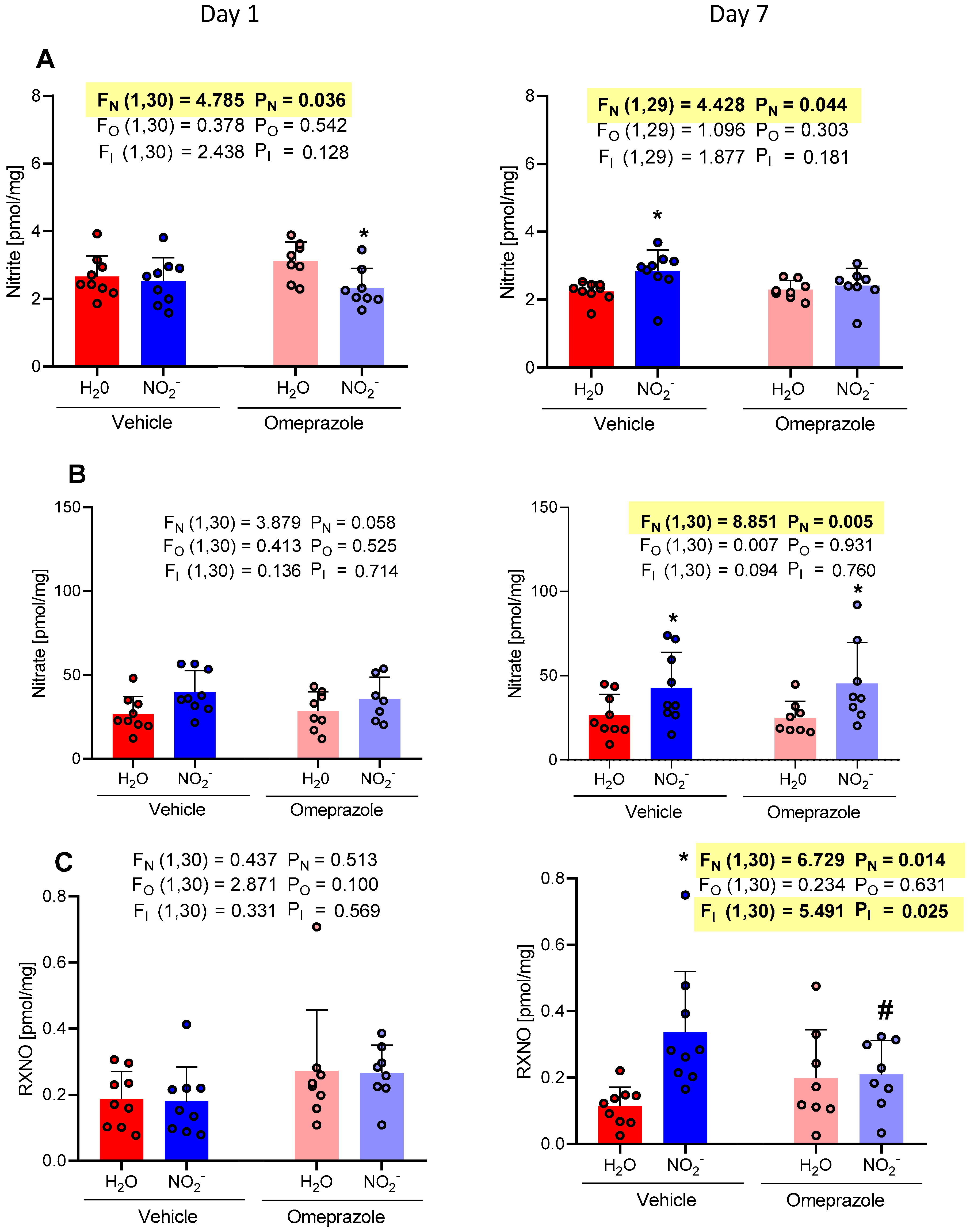
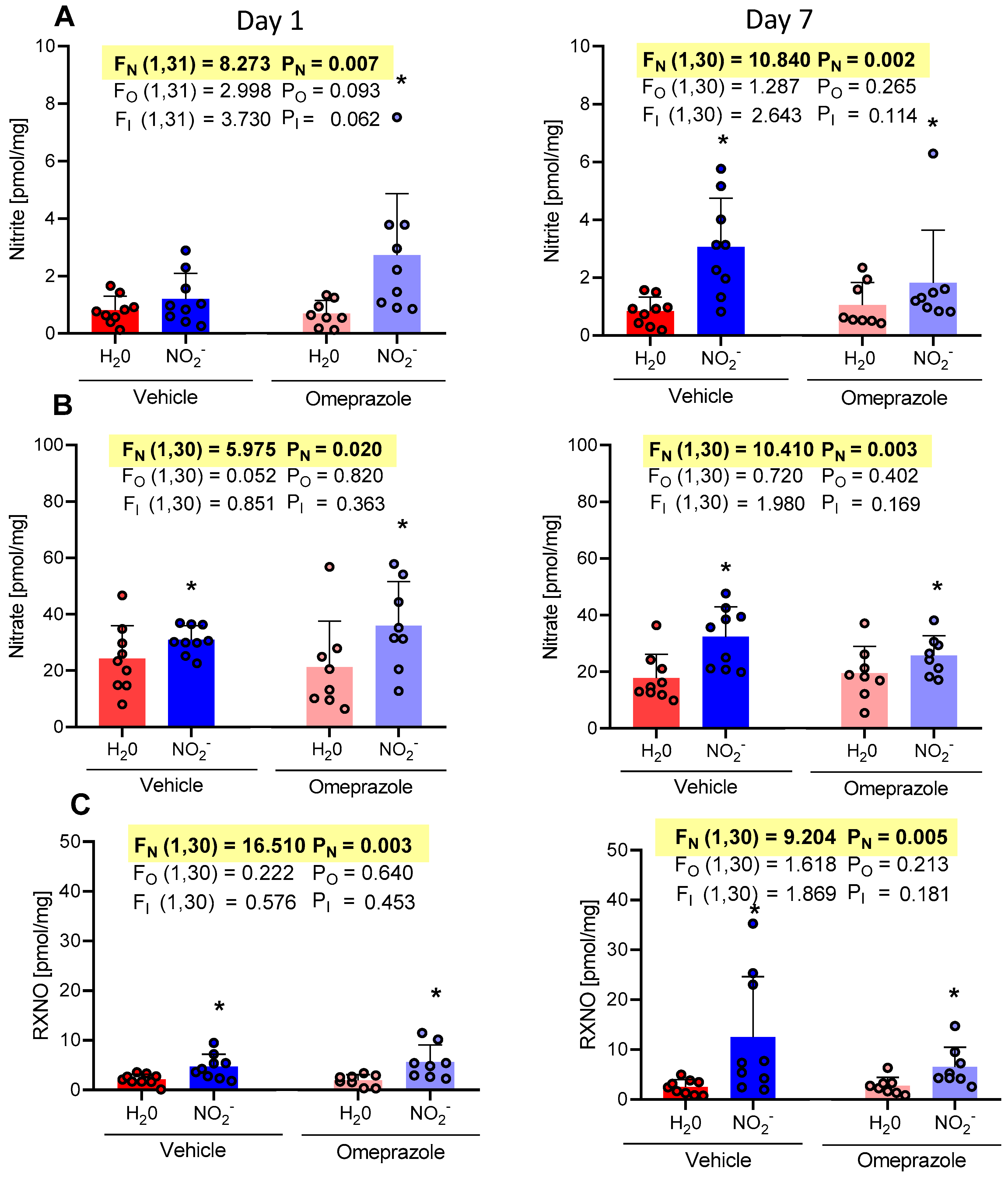
3.1. Time-Dependent Changes in Plasma NO Metabolite Concentrations
3.2. Time-Dependent Changes in Tissue Aortic NO Metabolite Concentrations
3.3. Time-Dependent Changes in Brain NO Metabolite Concentrations
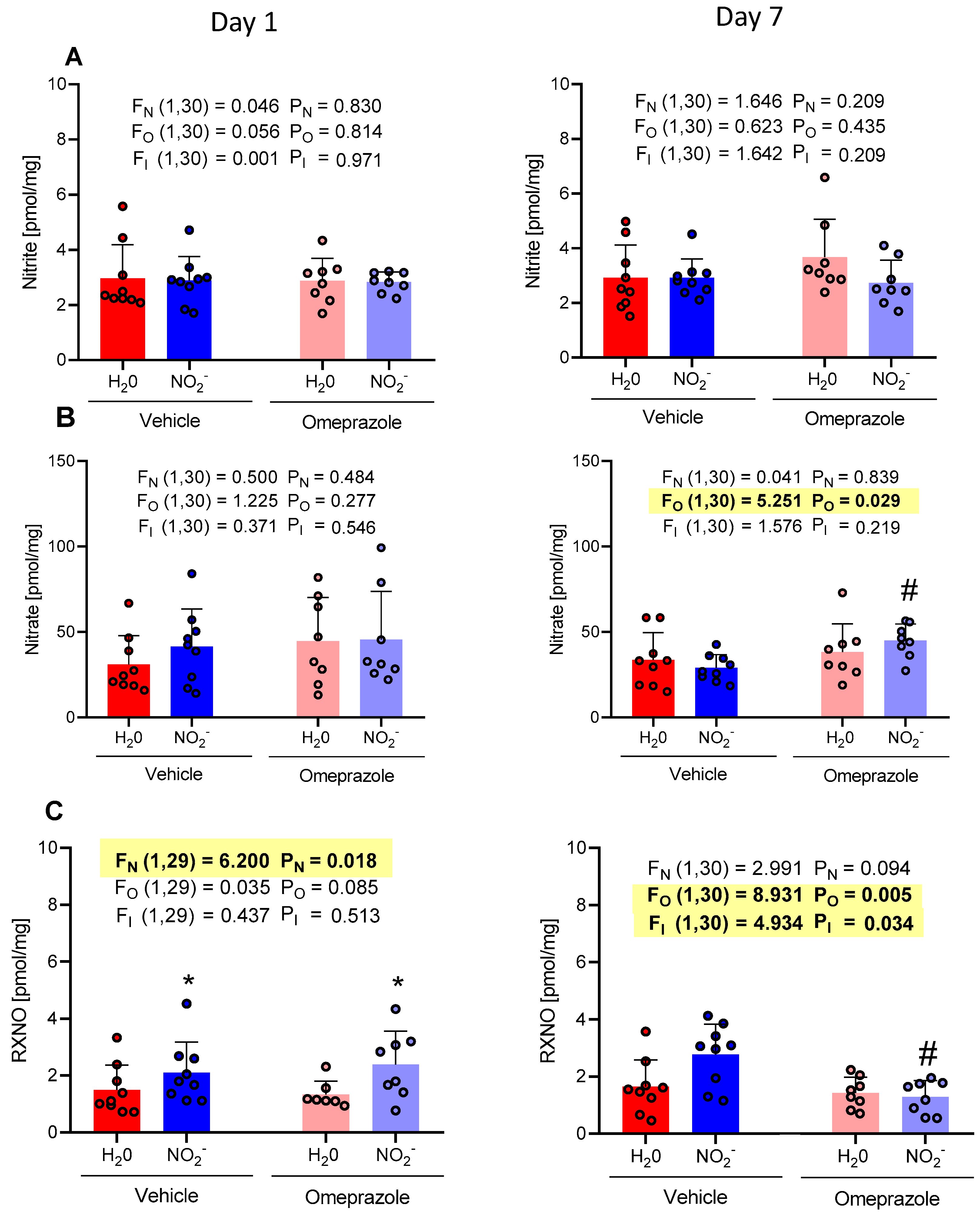
3.4. Time-Dependent Changes in Heart NO Metabolite Concentrations
3.5. Time-Dependent Changes in Gastrocnemius Muscle NO Metabolite Concentrations
3.6. Time-Dependent Changes in Liver NO Metabolite Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Moncada, S.; Higgs, A. The L-Arginine-Nitric Oxide Pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric Oxide Signaling in Health and Disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate-Nitrite-Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Govoni, M. Inorganic Nitrate Is a Possible Source for Systemic Generation of Nitric Oxide. Free Radic. Biol. Med. 2004, 37, 395–400. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Amaral, J.H.; Ferreira, G.C.; Portella, R.L.; Ceron, C.S.; Montenegro, M.F.; Toledo, J.C.; Tanus-Santos, J.E. Gastric S-Nitrosothiol Formation Drives the Antihypertensive Effects of Oral Sodium Nitrite and Nitrate in a Rat Model of Renovascular Hypertension. Free Radic. Biol. Med. 2015, 87, 252–262. [Google Scholar] [CrossRef]
- Bryan, N.S.; Rassaf, T.; Maloney, R.E.; Rodriguez, C.M.; Saijo, F.; Rodriguez, J.R.; Feelisch, M. Cellular Targets and Mechanisms of Nitros(Yl)Ation: An Insight into Their Nature and Kinetics in Vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 4308–4313. [Google Scholar] [CrossRef]
- Rodriguez, J.; Maloney, R.E.; Rassaf, T.; Bryan, N.S.; Feelisch, M. Chemical Nature of Nitric Oxide Storage Forms in Rat Vascular Tissue. Proc. Natl. Acad. Sci. USA 2003, 100, 336–341. [Google Scholar] [CrossRef]
- Ling, W.C.; Mustafa, M.R.; Vanhoutte, P.M.; Murugan, D.D. Chronic Administration of Sodium Nitrite Prevents Hypertension and Protects Arterial Endothelial Function by Reducing Oxidative Stress in Angiotensin II-Infused Mice. Vascul. Pharmacol. 2018, 102, 11–20. [Google Scholar] [CrossRef]
- Ling, W.C.; Mustafa, M.R.; Murugan, D.D. Therapeutic Implications of Nitrite in Hypertension. J. Cardiovasc. Pharmacol. 2020, 75, 123–134. [Google Scholar] [CrossRef]
- Ferreira, G.C.; Pinheiro, L.C.; Oliveira-Paula, G.H.; Angelis, C.D.; Portella, R.L.; Tanus-Santos, J.E. Antioxidant Tempol Modulates the Increases in Tissue Nitric Oxide Metabolites Concentrations after Oral Nitrite Administration. Chem. Biol. Interact. 2021, 349, 109658. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Ferreira, G.C.; Damacena de Angelis, C.; Toledo, J.C.; Tanus-Santos, J.E. A Comprehensive Time Course Study of Tissue Nitric Oxide Metabolites Concentrations after Oral Nitrite Administration. Free Radic. Biol. Med. 2020, 152, 43–51. [Google Scholar] [CrossRef]
- Shiva, S. Nitrite: A Physiological Store of Nitric Oxide and Modulator of Mitochondrial Function. Redox. Biol. 2013, 1, 40–44. [Google Scholar] [CrossRef]
- Li, H.; Cui, H.; Kundu, T.K.; Alzawahra, W.; Zweier, J.L. Nitric Oxide Production from Nitrite Occurs Primarily in Tissues Not in the Blood. J. Biol. Chem. 2008, 283, 17855–17863. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Schechter, A.N.; Kim-Shapiro, D.B.; Patel, R.P.; Hogg, N.; Shiva, S.; Cannon, R.O.; Kelm, M.; Wink, D.A.; Espey, M.G.; et al. The Emerging Biology of the Nitrite Anion. Nat. Chem. Biol. 2005, 1, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S. Mitochondria as Metabolizers and Targets of Nitrite. Nitric Oxide 2010, 22, 64–74. [Google Scholar] [CrossRef]
- Rassaf, T.; Feelisch, M.; Kelm, M. Circulating No Pool: Assessment of Nitrite and Nitroso Species in Blood and Tissues. Free Radic. Biol. Med. 2004, 36, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.C.; Oliveira-Paula, G.H.; Ferreira, G.C.; Dal-Cin de Paula, T.; Duarte, D.A.; Costa-Neto, C.M.; Tanus-Santos, J.E. Oral Nitrite Treatment Increases S-Nitrosylation of Vascular Protein Kinase C and Attenuates the Responses to Angiotensin II. Redox. Biol. 2021, 38, 101769. [Google Scholar] [CrossRef]
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling. Antioxid. Redox. Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef]
- Shih, C.J.; Chen, Y.T.; Ou, S.M.; Li, S.Y.; Chen, T.J.; Wang, S.J. Proton Pump Inhibitor Use Represents an Independent Risk Factor for Myocardial Infarction. Int. J. Cardiol. 2014, 177, 292–297. [Google Scholar] [CrossRef]
- Ghebremariam, Y.T.; LePendu, P.; Lee, J.C.; Erlanson, D.A.; Slaviero, A.; Shah, N.H.; Leiper, J.; Cooke, J.P. Unexpected Effect of Proton Pump Inhibitors. Circulation 2013, 128, 845–853. [Google Scholar] [CrossRef]
- Nogueira, R.C.; Pinheiro, L.C.; Sanches-Lopes, J.M.; Parente, J.M.; Oliveira-Paula, G.H.; Conde, S.O.; Castro, M.M.; Tanus-Santos, J.E. Omeprazole Induces Vascular Remodeling by Mechanisms Involving Xanthine Oxidoreductase and Matrix Metalloproteinase Activation. Biochem. Pharmacol. 2021, 190, 114633. [Google Scholar] [CrossRef]
- Nolde, M.; Bahls, M.; Friedrich, N.; Dörr, M.; Dreischulte, T.; Felix, S.B.; Rückert-Eheberg, I.; Ahn, N.; Amann, U.; Schwedhelm, E.; et al. Association of Proton Pump Inhibitor Use with Endothelial Function and Metabolites of the Nitric Oxide Pathway: A Cross-sectional Study. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 41, 198–204. [Google Scholar] [CrossRef]
- Montenegro, M.F.; Amaral, J.H.; Pinheiro, L.C.; Sakamoto, E.K.; Ferreira, G.C.; Reis, R.I.; Marçal, D.M.O.; Pereira, R.P.; Tanus-Santos, J.E. Sodium Nitrite Downregulates Vascular NADPH Oxidase and Exerts Antihypertensive Effects in Hypertension. Free Radic. Biol. Med. 2011, 51, 144–152. [Google Scholar] [CrossRef]
- Feelisch, M.; Rassaf, T.; Mnaimneh, S.; Singh, N.; Bryan, N.S.; Jourd’Heuil, D.; Kelm, M. Concomitant S-, N-, and Heme-nitros(Yl)Ation in Biological Tissues and Fluids: Implications for the Fate of NO in Vivo. FASEB J. 2002, 16, 1775–1785. [Google Scholar] [CrossRef]
- Bryan, N.S.; Fernandez, B.O.; Bauer, S.M.; Garcia-Saura, M.F.; Milsom, A.B.; Rassaf, T.; Maloney, R.E.; Bharti, A.; Rodriguez, J.; Feelisch, M. Nitrite Is a Signaling Molecule and Regulator of Gene Expression in Mammalian Tissues. Nat. Chem. Biol. 2005, 1, 290–297. [Google Scholar] [CrossRef]
- Carlström, M.; Persson, A.E.G.; Larsson, E.; Hezel, M.; Scheffer, P.G.; Teerlink, T.; Weitzberg, E.; Lundberg, J.O. Dietary Nitrate Attenuates Oxidative Stress, Prevents Cardiac and Renal Injuries, and Reduces Blood Pressure in Salt-Induced Hypertension. Cardiovasc. Res. 2011, 89, 574–585. [Google Scholar] [CrossRef]
- Sindler, A.L.; Devan, A.E.; Fleenor, B.S.; Seals, D.R. Inorganic Nitrite Supplementation for Healthy Arterial Aging. J. Appl. Physiol. 2014, 116, 463–477. [Google Scholar] [CrossRef]
- Broniowska, K.A.; Hogg, N. The Chemical Biology of S-Nitrosothiols. Antioxid. Redox. Signal. 2012, 17, 969–980. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Terry, M.H.; Schroeder, H.; Wilson, S.M.; Power, G.G.; Li, Q.; Tipple, T.E.; Borchardt, D.; Blood, A.B. Nitrite Potentiates the Vasodilatory Signaling of S-Nitrosothiols. Nitric Oxide 2018, 75, 60–69. [Google Scholar] [CrossRef]
- Tian, R.; Peng, R.; Yang, Z.; Peng, Y.-Y.; Lu, N. Supplementation of Dietary Nitrate Attenuated Oxidative Stress and Endothelial Dysfunction in Diabetic Vasculature through Inhibition of NADPH Oxidase. Nitric Oxide 2020, 96, 54–63. [Google Scholar] [CrossRef]
- Saijo, F.; Milsom, A.B.; Bryan, N.S.; Bauer, S.M.; Vowinkel, T.; Ivanovic, M.; Andry, C.; Granger, D.N.; Rodriguez, J.; Feelisch, M. On the Dynamics of Nitrite, Nitrate and Other Biomarkers of Nitric Oxide Production in Inflammatory Bowel Disease. Nitric Oxide 2010, 22, 155–167. [Google Scholar] [CrossRef]
- Piknova, B.; Kocharyan, A.; Schechter, A.N.; Silva, A.C. The Role of Nitrite in Neurovascular Coupling. Brain Res. 2011, 1407, 62–68. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Thomas, S.M.; Tunau-Spencer, K.J.; Schechter, A.N. Nitrate and Nitrite Metabolism in Aging Rats: A Comparative Study. Nutrients 2023, 15, 2490. [Google Scholar] [CrossRef]
- Bryan, N.S.; Calvert, J.W.; Elrod, J.W.; Gundewar, S.; Ji, S.Y.; Lefer, D.J. Dietary Nitrite Supplementation Protects against Myocardial Ischemia-Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2007, 104, 19144–19149. [Google Scholar] [CrossRef]
- Bryan, N.S.; Calvert, J.W.; Gundewar, S.; Lefer, D.J. Dietary Nitrite Restores NO Homeostasis and Is Cardioprotective in Endothelial Nitric Oxide Synthase-Deficient Mice. Free Radic. Biol. Med. 2008, 45, 468–474. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, X.-M.; Tarnawski, L.; Peleli, M.; Zhuge, Z.; Terrando, N.; Harris, R.A.; Olofsson, P.S.; Larsson, E.; Persson, A.E.G.; et al. Dietary Nitrate Attenuates Renal Ischemia-Reperfusion Injuries by Modulation of Immune Responses and Reduction of Oxidative Stress. Redox. Biol. 2017, 13, 320–330. [Google Scholar] [CrossRef]
- Choi, E.K.; Jung, H.; Kim, K.-J.; Kang, S.J.; Kim, H.J.; Lim, J.A.; Kwak, K.H.; Lim, D.G. Sodium Nitrite Attenuates Hepatic Ischemia-Reperfusion Injury in Rats. Exp. Clin. Transplant. 2019, 17, 348–354. [Google Scholar] [CrossRef]
- Piknova, B.; Schechter, A.N.; Park, J.W.; Vanhatalo, A.; Jones, A.M. Skeletal Muscle Nitrate as a Regulator of Systemic Nitric Oxide Homeostasis. Exerc. Sport Sci. Rev. 2022, 50, 2–13. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Swanson, K.M.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Skeletal Muscle as an Endogenous Nitrate Reservoir. Nitric Oxide 2015, 47, 10–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima-Silva, A.K.; Rebelo, M.A.; Barros, A.C.; Conde-Tella, S.O.; Tanus-Santos, J.E. The Skeletal Muscle, the Heart, and the Liver Are the Major Organs of the Accumulation of Nitric Oxide Metabolites after Oral Nitrite Treatment. Antioxidants 2024, 13, 255. https://doi.org/10.3390/antiox13030255
Lima-Silva AK, Rebelo MA, Barros AC, Conde-Tella SO, Tanus-Santos JE. The Skeletal Muscle, the Heart, and the Liver Are the Major Organs of the Accumulation of Nitric Oxide Metabolites after Oral Nitrite Treatment. Antioxidants. 2024; 13(3):255. https://doi.org/10.3390/antiox13030255
Chicago/Turabian StyleLima-Silva, Ana K., Macario A. Rebelo, Alessandra C. Barros, Sandra O. Conde-Tella, and Jose E. Tanus-Santos. 2024. "The Skeletal Muscle, the Heart, and the Liver Are the Major Organs of the Accumulation of Nitric Oxide Metabolites after Oral Nitrite Treatment" Antioxidants 13, no. 3: 255. https://doi.org/10.3390/antiox13030255




