Novel Roles of the Greatwall Kinase Rim15 in Yeast Oxidative Stress Tolerance through Mediating Antioxidant Systems and Transcriptional Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids, Strains, and Culture Media
2.2. Plasmid and Strain Construction
2.3. Estimation of Yeast Growth
2.4. Antioxidant Indicator Measurement
2.5. Transcriptome Analysis
2.6. Real-Time Quantitative PCR Analysis
2.7. Yeast Two-Hybrid Assays
2.8. Statistical Analysis
3. Results
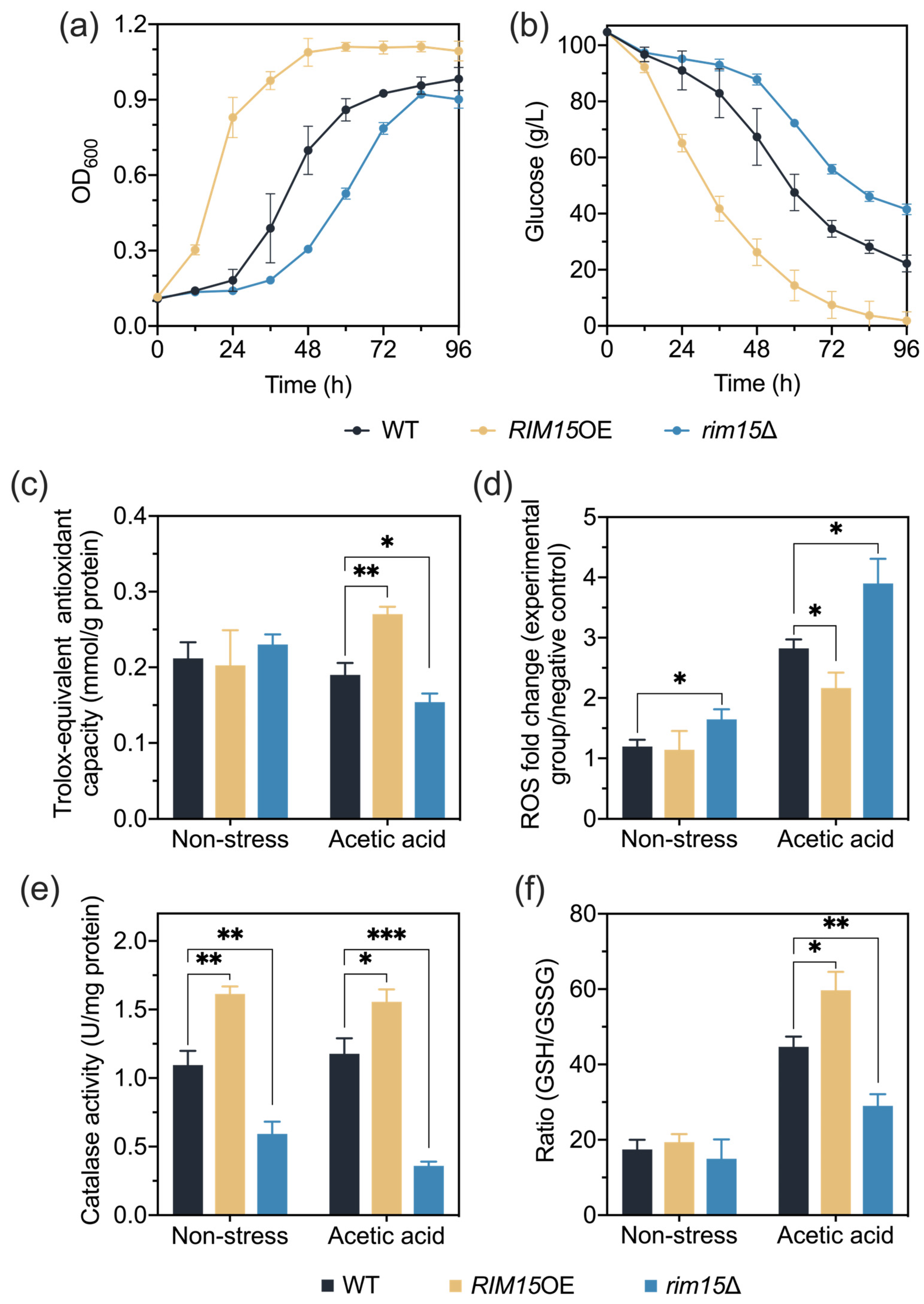
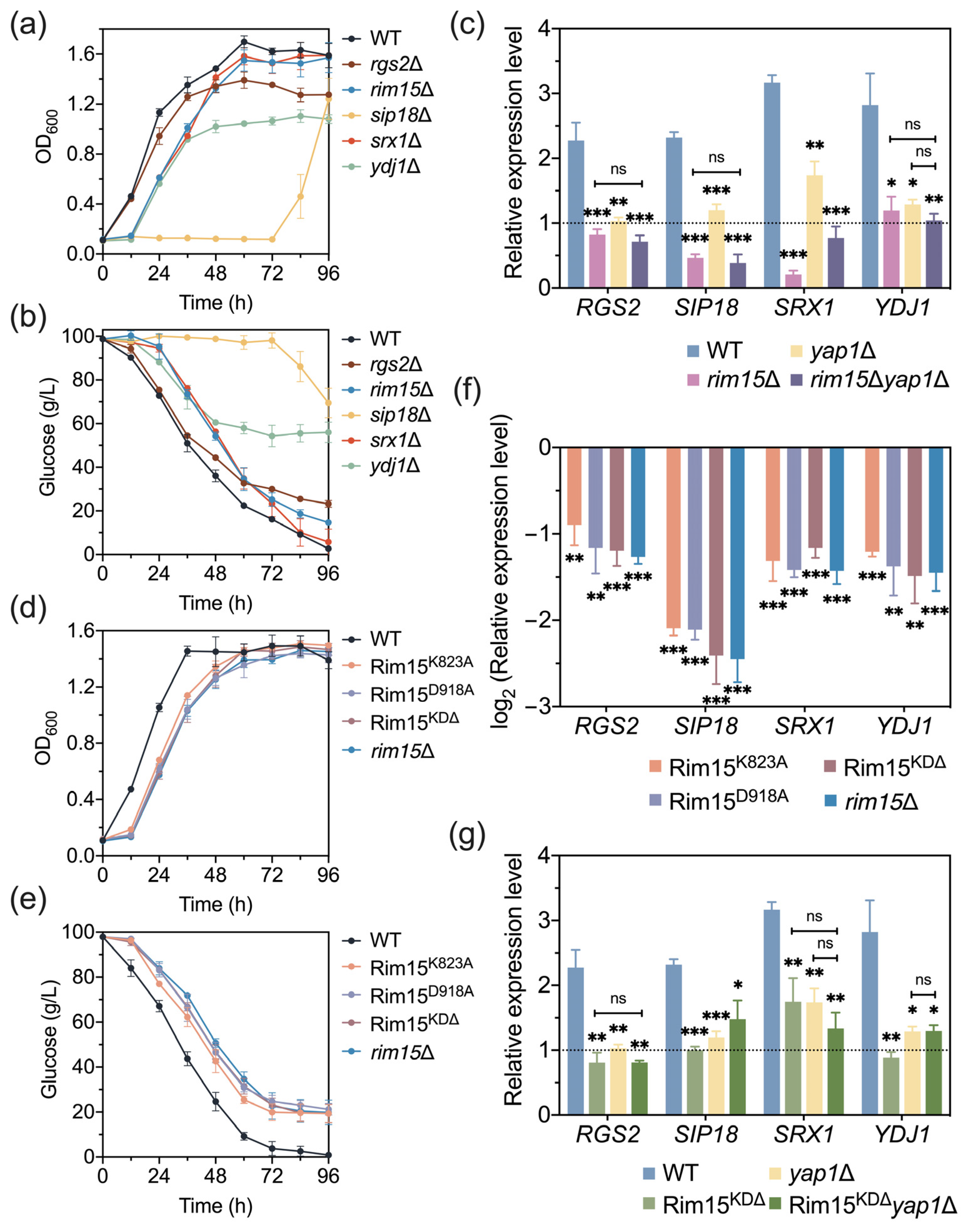
3.1. Rim15 Regulates Yeast Acetic Acid Stress Tolerance
3.2. Rim15 Regulates Acetic Acid Stress Tolerance through Mediating Antioxidant Systems
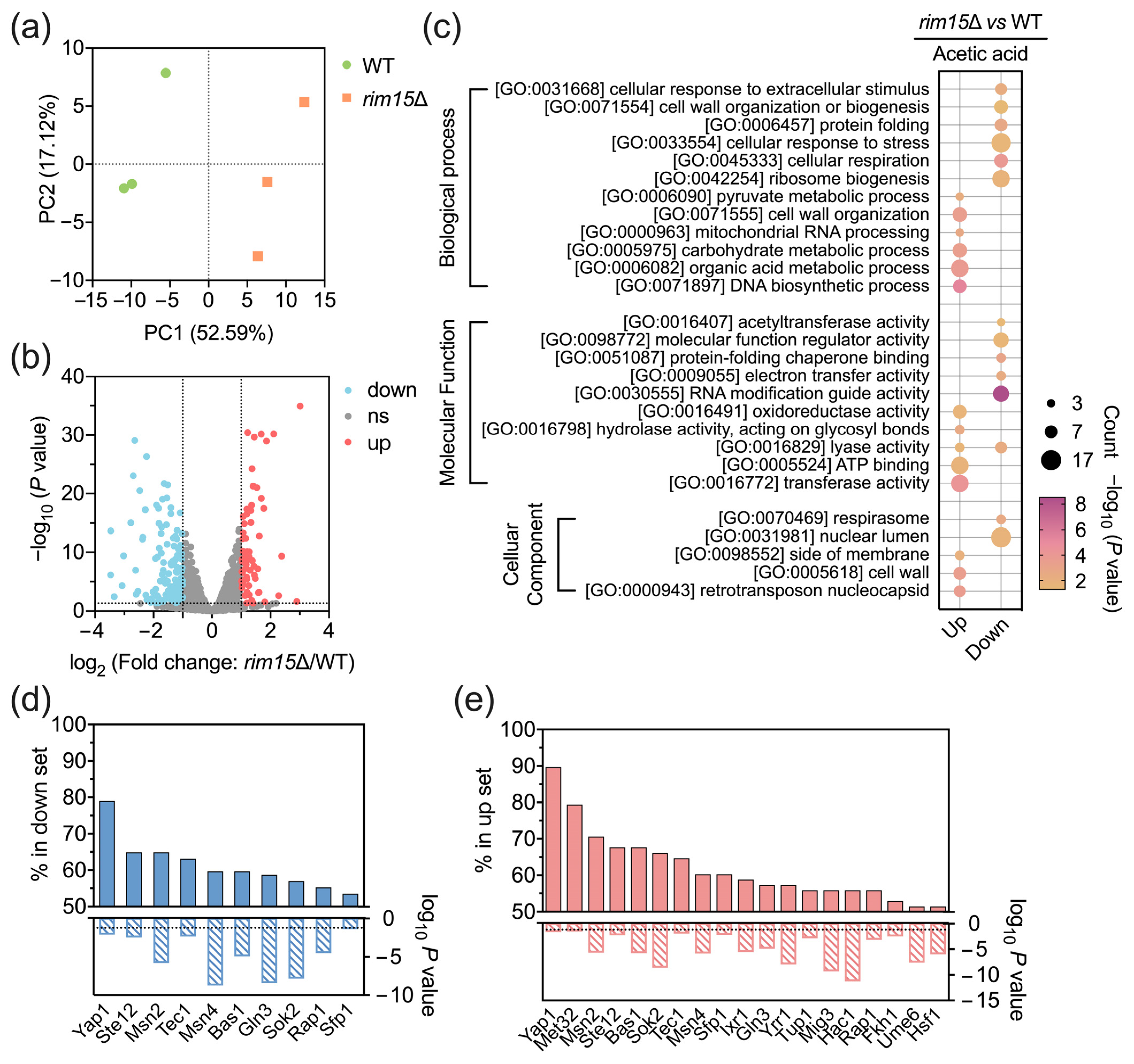
3.3. Transcriptomic Analysis Reveals Global Effects of Rim15 in Transcription
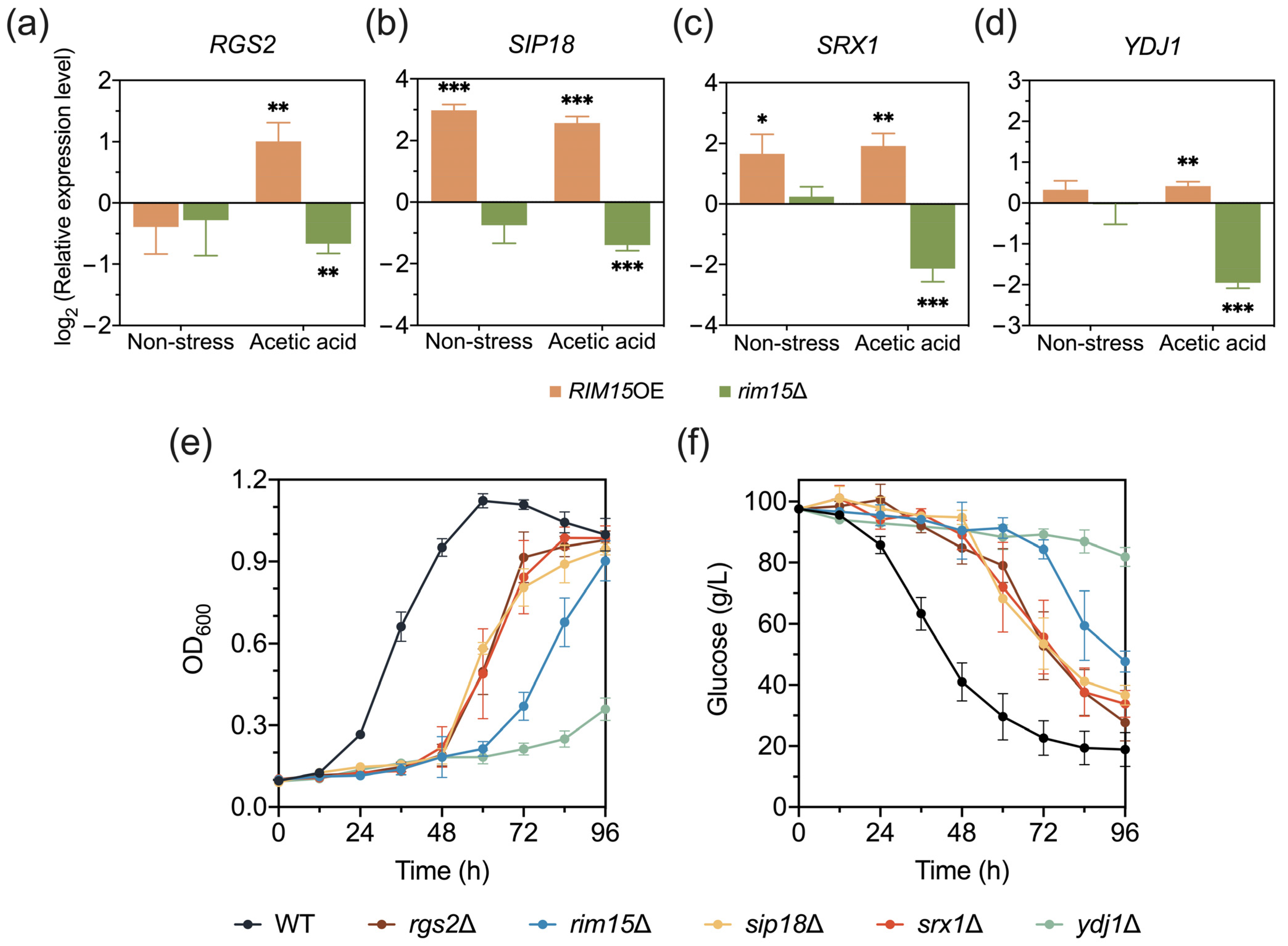
3.4. RGS2, SIP18, SRX1, and YDJ1 Are Novel Target Genes of Rim15 Which Affect Acetic Acid Tolerance in Yeast
3.5. Rim15 Functions with Yap1 in Transcriptional Regulation under Acetic Acid Stress
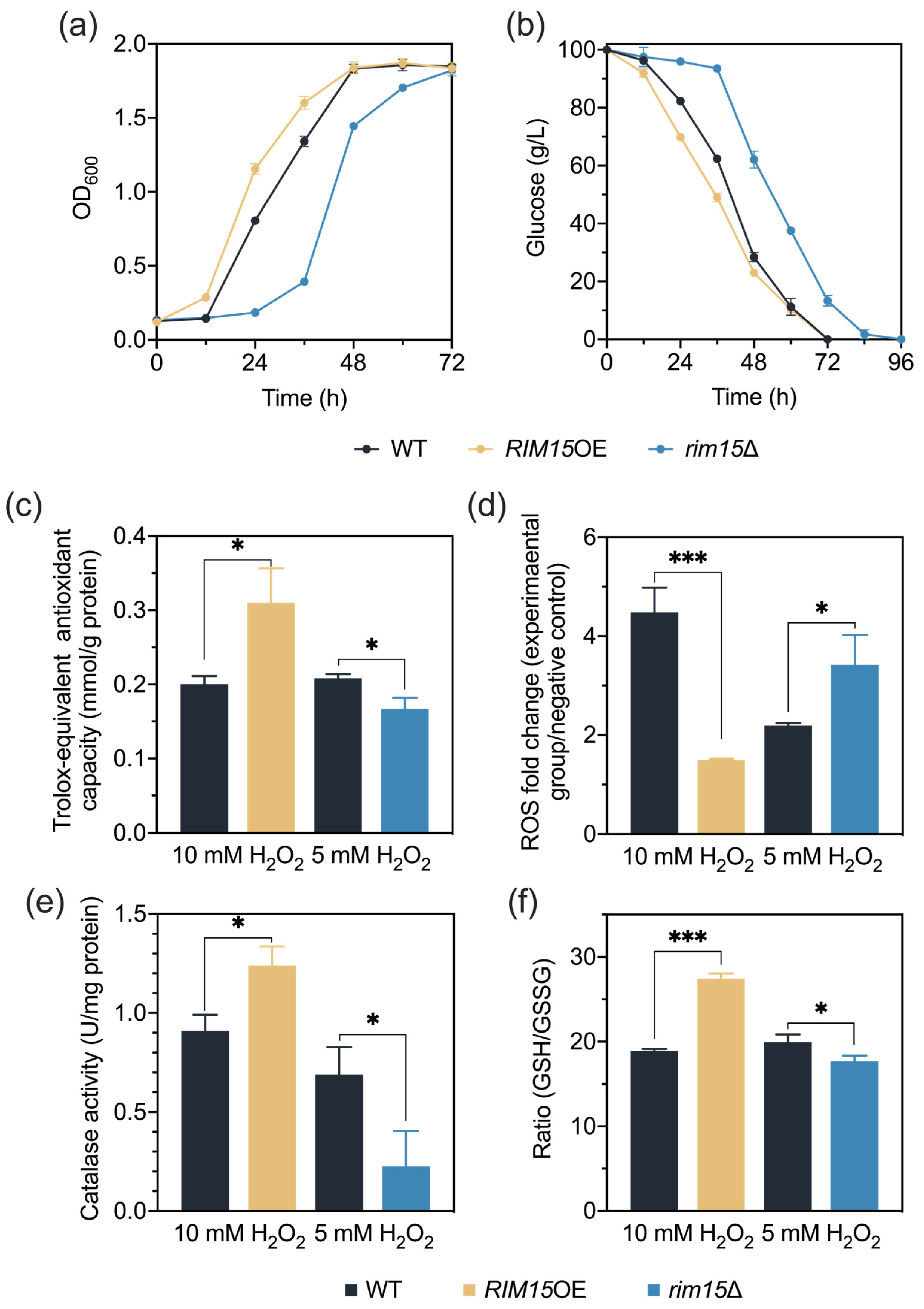
3.6. Rim15 Improves H2O2 Stress Tolerance through Antioxidant Systems
3.7. Rim15 Improves H2O2 Stress Tolerance through Transcriptional Regulation
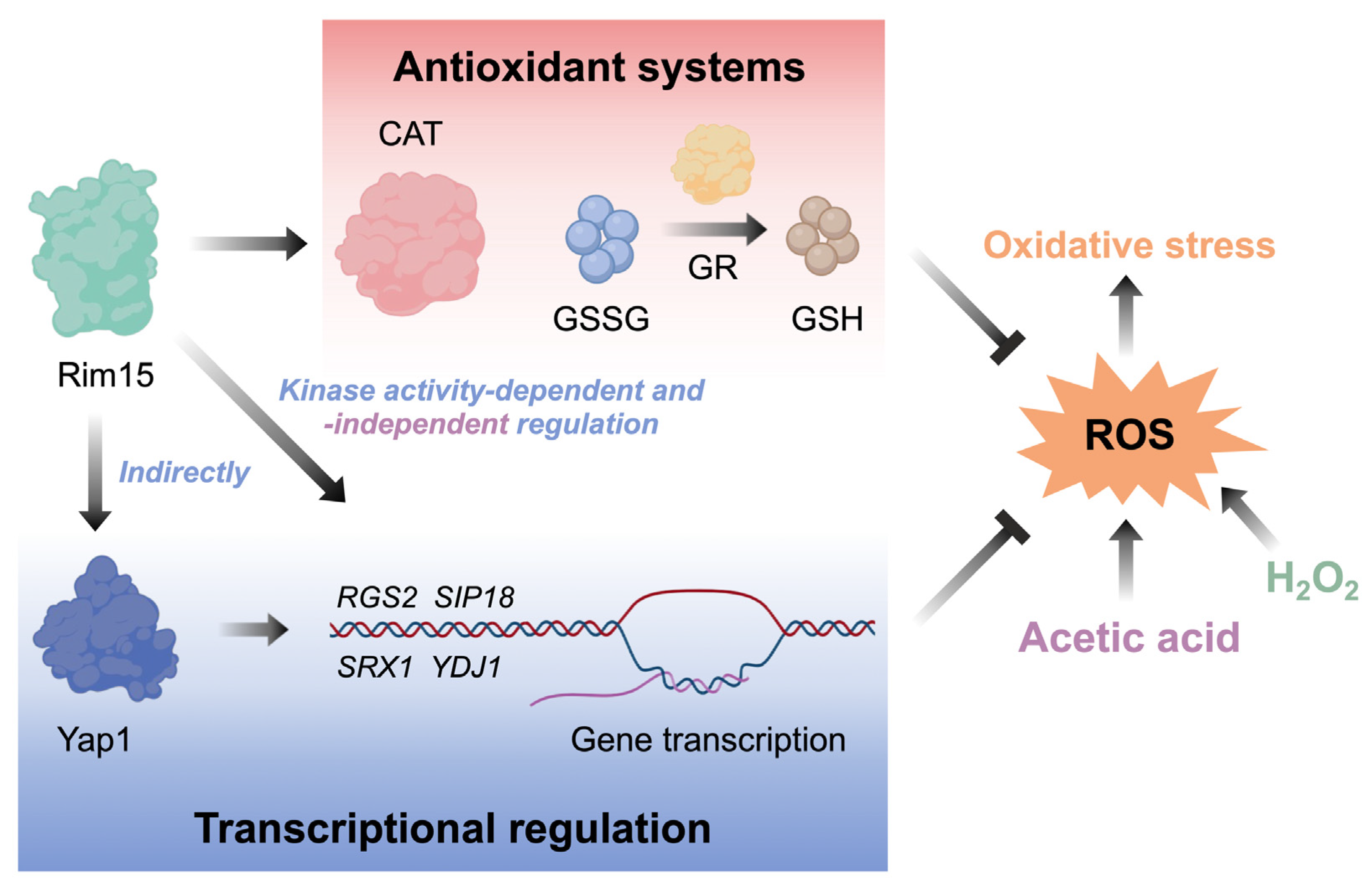
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro, A.; Lorca, T. Greatwall kinase at a glance. J. Cell Sci. 2018, 131, jcs222364. [Google Scholar] [CrossRef]
- Wei, M.; Fabrizio, P.; Hu, J.; Ge, H.; Cheng, C.; Li, L.; Longo, V.D. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008, 4, e13. [Google Scholar] [CrossRef]
- Bartholomew, C.R.; Suzuki, T.; Du, Z.; Backues, S.K.; Jin, M.; Lynch-Day, M.A.; Umekawa, M.; Kamath, A.; Zhao, M.; Xie, Z.; et al. Ume6 transcription factor is part of a signaling cascade that regulates autophagy. Proc. Natl. Acad. Sci. USA 2012, 109, 11206–11210. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Jin, M.; González-Rodríguez, P.; Füllgrabe, J.; Delorme-Axford, E.; Backues, S.K.; Joseph, B.; Klionsky, D.J. Rph1/KDM4 mediates nutrient-limitation signaling that leads to the transcriptional induction of autophagy. Curr. Biol. 2015, 25, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Delorme-Axford, E.; Klionsky, D.J. Transcriptional and post-transcriptional regulation of autophagy in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2018, 293, 5396–5403. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Tang, Y.; Quan, Z.; Zhang, Z.; Oliver, S.G.; Zhang, N. Chronological lifespan in yeast is dependent on the accumulation of storage carbohydrates mediated by Yak1, Mck1 and Rim15 kinases. PLoS Genet. 2016, 12, e1006458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wu, J.; Oliver, S.G. Gis1 is required for transcriptional reprogramming of carbon metabolism and the stress response during transition into stationary phase in yeast. Microbiology 2009, 155, 1690–1698. [Google Scholar] [CrossRef]
- Lee, P.; Kim, M.S.; Paik, S.M.; Choi, S.H.; Cho, B.R.; Hahn, J.S. Rim15-dependent activation of Hsf1 and Msn2/4 transcription factors by direct phosphorylation in Saccharomyces cerevisiae. FEBS Lett. 2013, 587, 3648–3655. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Yaakoub, H.; Mina, S.; Calenda, A.; Bouchara, J.P.; Papon, N. Oxidative stress response pathways in fungi. Cell Mol. Life Sci. 2022, 79, 333. [Google Scholar] [CrossRef]
- de Almeida, A.; de Oliveira, J.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic concepts, sources, cellular signaling, and its implications in aging pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Kim, D.; Hahn, J.S. Roles of the Yap1 transcription factor and antioxidants in Saccharomyces cerevisiae’s tolerance to furfural and 5-hydroxymethylfurfural, which function as thiol-reactive electrophiles generating oxidative stress. Appl. Environ. Microbiol. 2013, 79, 5069–5077. [Google Scholar] [CrossRef] [PubMed]
- Disasa, D.; Cheng, L.; Manzoor, M.; Liu, Q.; Wang, Y.; Xiang, L.; Qi, J. Amarogentin from Gentiana rigescens Franch exhibits antiaging and neuroprotective effects through antioxidative stress. Oxid. Med. Cell Longev. 2020, 2020, 3184019. [Google Scholar] [CrossRef]
- Korovila, I.; Hugo, M.; Castro, J.P.; Weber, D.; Höhn, A.; Grune, T.; Jung, T. Proteostasis, oxidative stress and aging. Redox Biol. 2017, 13, 550–567. [Google Scholar] [CrossRef]
- Hatem, E.; Berthonaud, V.; Dardalhon, M.; Lagniel, G.; Baudouin-Cornu, P.; Huang, M.E.; Labarre, J.; Chédin, S. Glutathione is essential to preserve nuclear function and cell survival under oxidative stress. Free Radic. Biol. Med. 2014, 67, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P. Environmental risk factors and their footprints in vivo—A proposal for the classification of oxidative stress biomarkers. Redox Biol. 2020, 34, 101442. [Google Scholar] [CrossRef]
- Doridot, L.; Jeljeli, M.; Chêne, C.; Batteux, F. Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol. 2019, 25, 101122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xiong, L.; Zhang, M.; Bai, F. Towards efficient bioethanol production from agricultural and forestry residues: Exploration of unique natural microorganisms in combination with advanced strain engineering. Bioresour. Technol. 2016, 215, 84–91. [Google Scholar] [CrossRef]
- Pérez-Díaz, I.M.; McFeeters, R.F. Microbiological preservation of cucumbers for bulk storage using acetic acid and food preservatives. J. Food Sci. 2008, 73, M287–M291. [Google Scholar] [CrossRef]
- Wan, C.; Zhang, M.; Fang, Q.; Xiong, L.; Zhao, X.; Hasunuma, T.; Bai, F.; Kondo, A. The impact of zinc sulfate addition on the dynamic metabolic profiling of Saccharomyces cerevisiae subjected to long term acetic acid stress treatment and identification of key metabolites involved in the antioxidant effect of zinc. Metallomics 2015, 7, 322–332. [Google Scholar] [CrossRef]
- Guaragnella, N.; Stirpe, M.; Marzulli, D.; Mazzoni, C.; Giannattasio, S. Acid stress triggers resistance to acetic acid-induced regulated cell death through Hog1 activation which requires RTG2 in Yeast. Oxid. Med. Cell Longev. 2019, 2019, 4651062. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, K.; Dakik, P.; Medkour, Y.; McAuley, M.; Mitrofanova, D.; Titorenko, V.I. Some metabolites act as second messengers in yeast chronological aging. Int. J. Mol. Sci. 2018, 19, 860. [Google Scholar] [CrossRef] [PubMed]
- Vall-Llaura, N.; Mir, N.; Garrido, L.; Vived, C.; Cabiscol, E. Redox control of yeast Sir2 activity is involved in acetic acid resistance and longevity. Redox Biol. 2019, 24, 101229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Xiong, L.; Tang, Y.J.; Mehmood, M.A.; Zhao, Z.K.; Bai, F.W.; Zhao, X.Q. Enhanced acetic acid stress tolerance and ethanol production in Saccharomyces cerevisiae by modulating expression of the de novo purine biosynthesis genes. Biotechnol. Biofuels 2019, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, K.; Mehmood, M.A.; Zhao, Z.K.; Bai, F.; Zhao, X. Deletion of acetate transporter gene ADY2 improved tolerance of Saccharomyces cerevisiae against multiple stresses and enhanced ethanol production in the presence of acetic acid. Bioresour. Technol. 2017, 245, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Zhao, X.Q.; Cheng, C.; Bai, F.W. Improved growth and ethanol fermentation of Saccharomyces cerevisiae in the presence of acetic acid by overexpression of SET5 and PPR1. Biotechnol. J. 2015, 10, 1903–1911. [Google Scholar] [CrossRef]
- Cheng, C.; Zhao, X.; Zhang, M.; Bai, F. Absence of Rtt109p, a fungal-specific histone acetyltransferase, results in improved acetic acid tolerance of Saccharomyces cerevisiae. FEMS Yeast Res. 2016, 16, fow010. [Google Scholar] [CrossRef]
- Guaragnella, N.; Bettiga, M. Acetic acid stress in budding yeast: From molecular mechanisms to applications. Yeast 2021, 38, 391–400. [Google Scholar] [CrossRef]
- de Nadal, E.; Posas, F. The HOG pathway and the regulation of osmoadaptive responses in yeast. FEMS Yeast Res. 2022, 22, foac013. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, L.; Shi, G.Y. Omics analysis of acetic acid tolerance in Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2017, 33, 94. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Xing, Q.; Cheng, C.; Zhang, M.M.; Liu, C.G.; Champreda, V.; Zhao, X.Q. Identification of Kic1p and Cdc42p as novel targets to engineer yeast acetic acid stress tolerance. Front. Bioeng. Biotechnol. 2022, 10, 837813. [Google Scholar] [CrossRef]
- Ye, P.L.; Wang, X.Q.; Yuan, B.; Liu, C.G.; Zhao, X.Q. Manipulating cell flocculation-associated protein kinases in Saccharomyces cerevisiae enables improved stress tolerance and efficient cellulosic ethanol production. Bioresour. Technol. 2022, 348, 126758. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Xu, X.; Wang, J.; Liu, C.; Niu, C.; Zheng, F.; Li, Q. Effect of environmental stresses during fermentation on brewing yeast and exploration on the novel flocculation-associated function of RIM15 gene. Bioresour. Technol. 2023, 379, 129004. [Google Scholar] [CrossRef] [PubMed]
- Pautasso, C.; Rossi, S. Transcriptional regulation of the protein kinase A subunits in Saccharomyces cerevisiae: Autoregulatory role of the kinase A activity. Biochim. Biophys. Acta 2014, 1839, 275–287. [Google Scholar] [CrossRef]
- Kim, H.S. Disruption of RIM15 confers an increased tolerance to heavy metals in Saccharomyces cerevisiae. Biotechnol. Lett. 2020, 42, 1193–1202. [Google Scholar] [CrossRef]
- Talarek, N.; Cameroni, E.; Jaquenoud, M.; Luo, X.; Bontron, S.; Lippman, S.; Devgan, G.; Snyder, M.; Broach, J.R.; De Virgilio, C. Initiation of the TORC1-regulated G0 program requires Igo1/2, which license specific mRNAs to evade degradation via the 5’-3’ mRNA decay pathway. Mol. Cell 2010, 38, 345–355. [Google Scholar] [CrossRef]
- DiCarlo, J.E.; Norville, J.E.; Mali, P.; Rios, X.; Aach, J.; Church, G.M. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013, 41, 4336–4343. [Google Scholar] [CrossRef]
- Zhang, G.C.; Kong, I.I.; Kim, H.; Liu, J.J.; Cate, J.H.; Jin, Y.S. Construction of a quadruple auxotrophic mutant of an industrial polyploid saccharomyces cerevisiae strain by using RNA-guided Cas9 nuclease. Appl. Environ. Microbiol. 2014, 80, 7694–7701. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zeng, Y.; Tang, R.Q.; Alper, H.S.; Bai, F.W.; Zhao, X.Q. Condition-specific promoter activities in Saccharomyces cerevisiae. Microb. Cell Fact. 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Teste, M.A.; Duquenne, M.; François, J.M.; Parrou, J.L. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol. Biol. 2009, 10, 99. [Google Scholar] [CrossRef]
- Pan, S.; Jia, B.; Liu, H.; Wang, Z.; Chai, M.Z.; Ding, M.Z.; Zhou, X.; Li, X.; Li, C.; Li, B.Z.; et al. Endogenous lycopene improves ethanol production under acetic acid stress in Saccharomyces cerevisiae. Biotechnol. Biofuels 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Bettray, W.; Slusarenko, A.J.; Gruhlke, M.C.H. S-allylmercaptoglutathione is a substrate for glutathione reductase (E.C. 1.8.1.7) from yeast (Saccharomyces cerevisiae). Antioxidants 2018, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Godinho, C.P.; Vitorino, M.V.; Robalo, T.T.; Fernandes, F.; Rodrigues, M.S.; Sá-Correia, I. Crosstalk between yeast cell plasma membrane ergosterol content and cell wall stiffness under acetic acid stress involving Pdr18. J. Fungi 2022, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hu, J.; Fan, L.; Chen, Q. RNA-Seq-based transcriptomic and metabolomic analysis reveal stress responses and programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 42659. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, M.; Masaki, K.; Fujii, T.; Iefuji, H. Yeast genes involved in response to lactic acid and acetic acid: Acidic conditions caused by the organic acids in Saccharomyces cerevisiae cultures induce expression of intracellular metal metabolism genes regulated by Aft1p. FEMS Yeast Res. 2006, 6, 924–936. [Google Scholar] [CrossRef]
- Lin, Y.R.; Kim, K.; Yang, Y.; Ivessa, A.; Sadoshima, J.; Park, Y. Regulation of longevity by regulator of G-protein signaling protein, Loco. Aging Cell 2011, 10, 438–447. [Google Scholar] [CrossRef]
- Rodríguez-Porrata, B.; Carmona-Gutierrez, D.; Reisenbichler, A.; Bauer, M.; Lopez, G.; Escoté, X.; Mas, A.; Madeo, F.; Cordero-Otero, R. Sip18 hydrophilin prevents yeast cell death during desiccation stress. J. Appl. Microbiol. 2012, 112, 512–525. [Google Scholar] [CrossRef]
- Gutiérrez-Escobedo, G.; Hernández-Carreón, O.; Morales-Rojano, B.; Revuelta-Rodríguez, B.; Vázquez-Franco, N.; Castaño, I.; De Las Peñas, A. Candida glabrata peroxiredoxins, Tsa1 and Tsa2, and sulfiredoxin, Srx1, protect against oxidative damage and are necessary for virulence. Fungal Genet. Biol. 2020, 135, 103287. [Google Scholar] [CrossRef]
- Auesukaree, C.; Damnernsawad, A.; Kruatrachue, M.; Pokethitiyook, P.; Boonchird, C.; Kaneko, Y.; Harashima, S. Genome-wide identification of genes involved in tolerance to various environmental stresses in Saccharomyces cerevisiae. J. Appl. Genet. 2009, 50, 301–310. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef]
- Lawenda, B.D.; Kelly, K.M.; Ladas, E.J.; Sagar, S.M.; Vickers, A.; Blumberg, J.B. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst. 2008, 100, 773–783. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef]
- Tasdogan, A.; Ubellacker, J.M.; Morrison, S.J. Redox regulation in cancer cells during metastasis. Cancer Discov. 2021, 11, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Wang, L.; Luong, V.Q.; Giannini, P.J.; Peng, A. Mastl kinase, a promising therapeutic target, promotes cancer recurrence. Oncotarget 2014, 5, 11479–11489. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Lartigue, L.; Vigneron, S.; Gadea, G.; Gire, V.; Del Rio, M.; Soubeyran, I.; Chibon, F.; Lorca, T.; Castro, A. Greatwall promotes cell transformation by hyperactivating AKT in human malignancies. Elife 2015, 4, e10115. [Google Scholar] [CrossRef] [PubMed]
- Leadsham, J.E.; Gourlay, C.W. cAMP/PKA signaling balances respiratory activity with mitochondria dependent apoptosis via transcriptional regulation. BMC Cell Biol. 2010, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Molin, M.; Yang, J.; Hanzén, S.; Toledano, M.B.; Labarre, J.; Nyström, T. Life span extension and H2O2 resistance elicited by caloric restriction require the peroxiredoxin Tsa1 in Saccharomyces cerevisiae. Mol. Cell 2011, 43, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowski, H.; Janta, A.; Sztangierska, W.; Obuchowski, I.; Chamera, T.; Kłosowska, A.; Liberek, K. Class-specific interactions between Sis1 J-domain protein and Hsp70 chaperone potentiate disaggregation of misfolded proteins. Proc. Natl. Acad. Sci. USA 2021, 118, e2108163118. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.W.; Muhlrad, D.; Garcia, J.; Parker, R. Differential effects of Ydj1 and Sis1 on Hsp70-mediated clearance of stress granules in Saccharomyces cerevisiae. RNA 2015, 21, 1660–1671. [Google Scholar] [CrossRef]
- Yang, S.; Sun, B.; Li, W.; Yang, H.; Li, N.; Zhang, X. Fatty acid metabolism is related to the immune microenvironment changes of gastric cancer and RGS2 is a new tumor biomarker. Front. Immunol. 2022, 13, 1065927. [Google Scholar] [CrossRef]
- Cho, J.; Min, H.Y.; Lee, H.J.; Hyun, S.Y.; Sim, J.Y.; Noh, M.; Hwang, S.J.; Park, S.H.; Boo, H.J.; Lee, H.J.; et al. RGS2-mediated translational control mediates cancer cell dormancy and tumor relapse. J. Clin. Investig. 2021, 131, e136779. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.I.; Choi, J.H.; Lee, K.H.; Kim, J.M. Bacteroides fragilis enterotoxin induces sulfiredoxin-1 expression in intestinal epithelial cell lines through a mitogen-activated protein kinases- and Nrf2-dependent pathway, leading to the suppression of apoptosis. Int. J. Mol. Sci. 2020, 21, 5383. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Guo, J.; Li, Z.; Wang, X.; Yang, C.; Cui, X. Sulfiredoxin-1 protects against simulated ischaemia/reperfusion injury in cardiomyocyte by inhibiting PI3K/AKT-regulated mitochondrial apoptotic pathways. Biosci. Rep. 2016, 36, e00325. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ling, G.; Suhasini, A.N.; Zhang, P.; Yamamoto, M.; Navas-Acien, A.; Cosgrove, G.; Tuder, R.M.; Kensler, T.W.; Watson, W.H.; et al. Nrf2-dependent sulfiredoxin-1 expression protects against cigarette smoke-induced oxidative stress in lungs. Free Radic. Biol. Med. 2009, 46, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Pousada, C.; Devaux, F.; Caetano, S.M.; Pimentel, C.; da Silva, S.; Cordeiro, A.C.; Amaral, C. Yeast AP-1 like transcription factors (Yap) and stress response: A current overview. Microb. Cell 2019, 6, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Z.; Zhang, X.; Yuan, L.; Dai, H.; Xiao, W. Transcriptomic profiling of chemical exposure reveals roles of Yap1 in protecting yeast cells from oxidative and other types of stresses. Yeast 2016, 33, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Haugen, A.C.; Kelley, R.; Collins, J.B.; Tucker, C.J.; Deng, C.; Afshari, C.A.; Brown, J.M.; Ideker, T.; Van Houten, B. Integrating phenotypic and expression profiles to map arsenic-response networks. Genome Biol. 2004, 5, R95. [Google Scholar] [CrossRef] [PubMed]
- Salin, H.; Fardeau, V.; Piccini, E.; Lelandais, G.; Tanty, V.; Lemoine, S.; Jacq, C.; Devaux, F. Structure and properties of transcriptional networks driving selenite stress response in yeasts. BMC Genom. 2008, 9, 333. [Google Scholar] [CrossRef]
- Okazaki, S.; Tachibana, T.; Naganuma, A.; Mano, N.; Kuge, S. Multistep disulfide bond formation in Yap1 is required for sensing and transduction of H2O2 stress signal. Mol. Cell 2007, 27, 675–688. [Google Scholar] [CrossRef]
- Avery, A.M.; Willetts, S.A.; Avery, S.V. Genetic dissection of the phospholipid hydroperoxidase activity of yeast gpx3 reveals its functional importance. J. Biol. Chem. 2004, 279, 46652–46658. [Google Scholar] [CrossRef]
- Delaunay, A.; Isnard, A.D.; Toledano, M.B. H2O2 sensing through oxidation of the Yap1 transcription factor. Embo J. 2000, 19, 5157–5166. [Google Scholar] [CrossRef]
- Kim, B.; Lee, Y.; Choi, H.; Huh, W.K. The trehalose-6-phosphate phosphatase Tps2 regulates ATG8 transcription and autophagy in Saccharomyces cerevisiae. Autophagy 2021, 17, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Deprez, M.A.; Maertens, J.M.; Olsson, L.; Bettiga, M.; Winderickx, J. The role of Sch9 and the V-ATPase in the adaptation response to acetic acid and the consequences for growth and chronological lifespan. Microorganisms 2021, 9, 1871. [Google Scholar] [CrossRef] [PubMed]
- Pedruzzi, I.; Dubouloz, F.; Cameroni, E.; Wanke, V.; Roosen, J.; Winderickx, J.; De Virgilio, C. TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol. Cell 2003, 12, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Mollapour, M.; Piper, P.W. Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol. Cell Biol. 2007, 27, 6446–6456. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-Q.; Yuan, B.; Zhang, F.-L.; Liu, C.-G.; Auesukaree, C.; Zhao, X.-Q. Novel Roles of the Greatwall Kinase Rim15 in Yeast Oxidative Stress Tolerance through Mediating Antioxidant Systems and Transcriptional Regulation. Antioxidants 2024, 13, 260. https://doi.org/10.3390/antiox13030260
Wang X-Q, Yuan B, Zhang F-L, Liu C-G, Auesukaree C, Zhao X-Q. Novel Roles of the Greatwall Kinase Rim15 in Yeast Oxidative Stress Tolerance through Mediating Antioxidant Systems and Transcriptional Regulation. Antioxidants. 2024; 13(3):260. https://doi.org/10.3390/antiox13030260
Chicago/Turabian StyleWang, Xue-Qing, Bing Yuan, Feng-Li Zhang, Chen-Guang Liu, Choowong Auesukaree, and Xin-Qing Zhao. 2024. "Novel Roles of the Greatwall Kinase Rim15 in Yeast Oxidative Stress Tolerance through Mediating Antioxidant Systems and Transcriptional Regulation" Antioxidants 13, no. 3: 260. https://doi.org/10.3390/antiox13030260
APA StyleWang, X.-Q., Yuan, B., Zhang, F.-L., Liu, C.-G., Auesukaree, C., & Zhao, X.-Q. (2024). Novel Roles of the Greatwall Kinase Rim15 in Yeast Oxidative Stress Tolerance through Mediating Antioxidant Systems and Transcriptional Regulation. Antioxidants, 13(3), 260. https://doi.org/10.3390/antiox13030260






