Effect of a Very Low-Calorie Diet on Oxidative Stress, Inflammatory and Metabolomic Profile in Metabolically Healthy and Unhealthy Obese Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Dietary Intervention
2.3. Biochemical, Inflammatory and Oxidative Stress Parameters
2.4. Metabolomic Analysis
2.5. Statistical Analysis
3. Results
3.1. Body Composition and Biochemical Markers
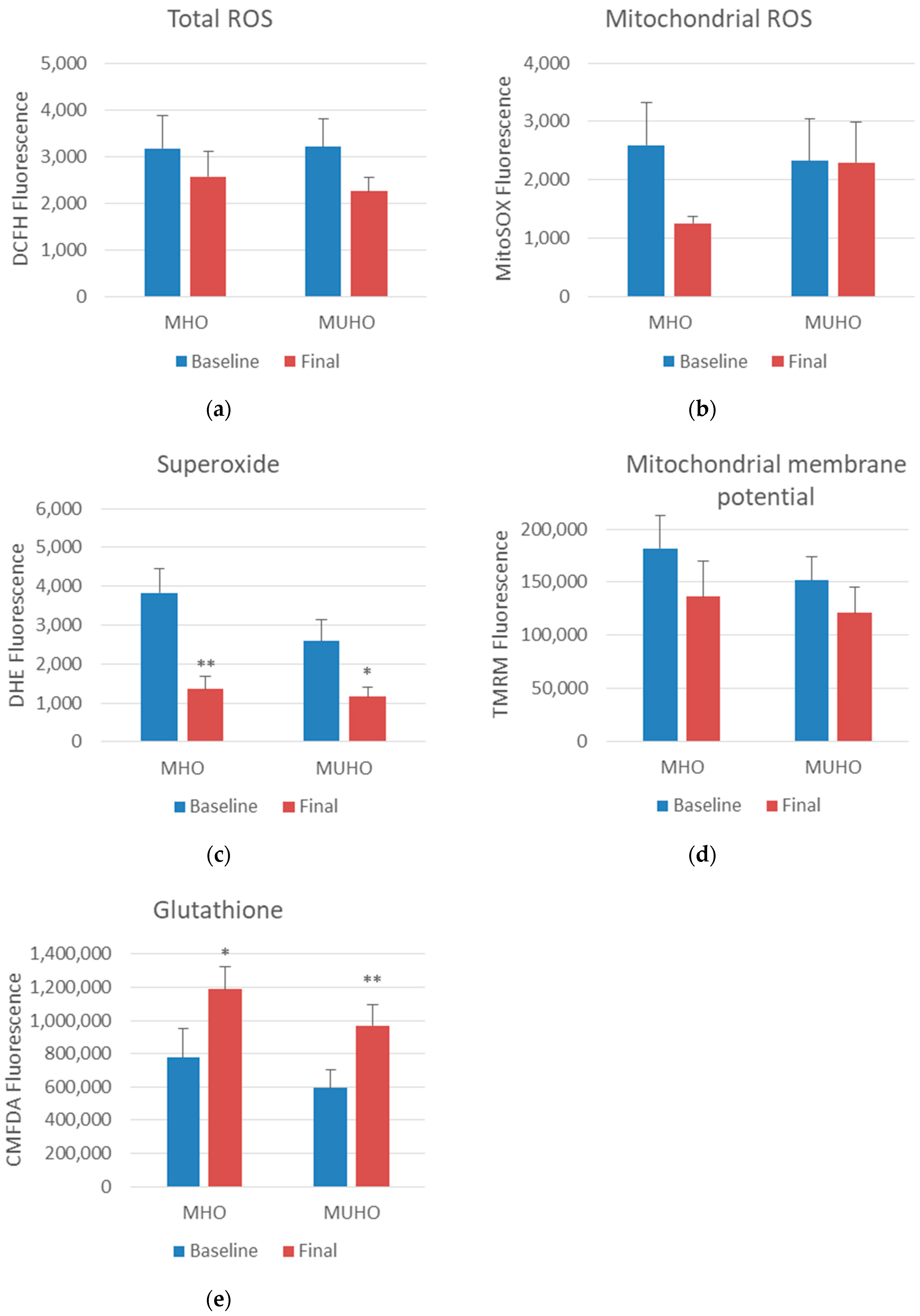
3.2. Oxidative Stress Parameters
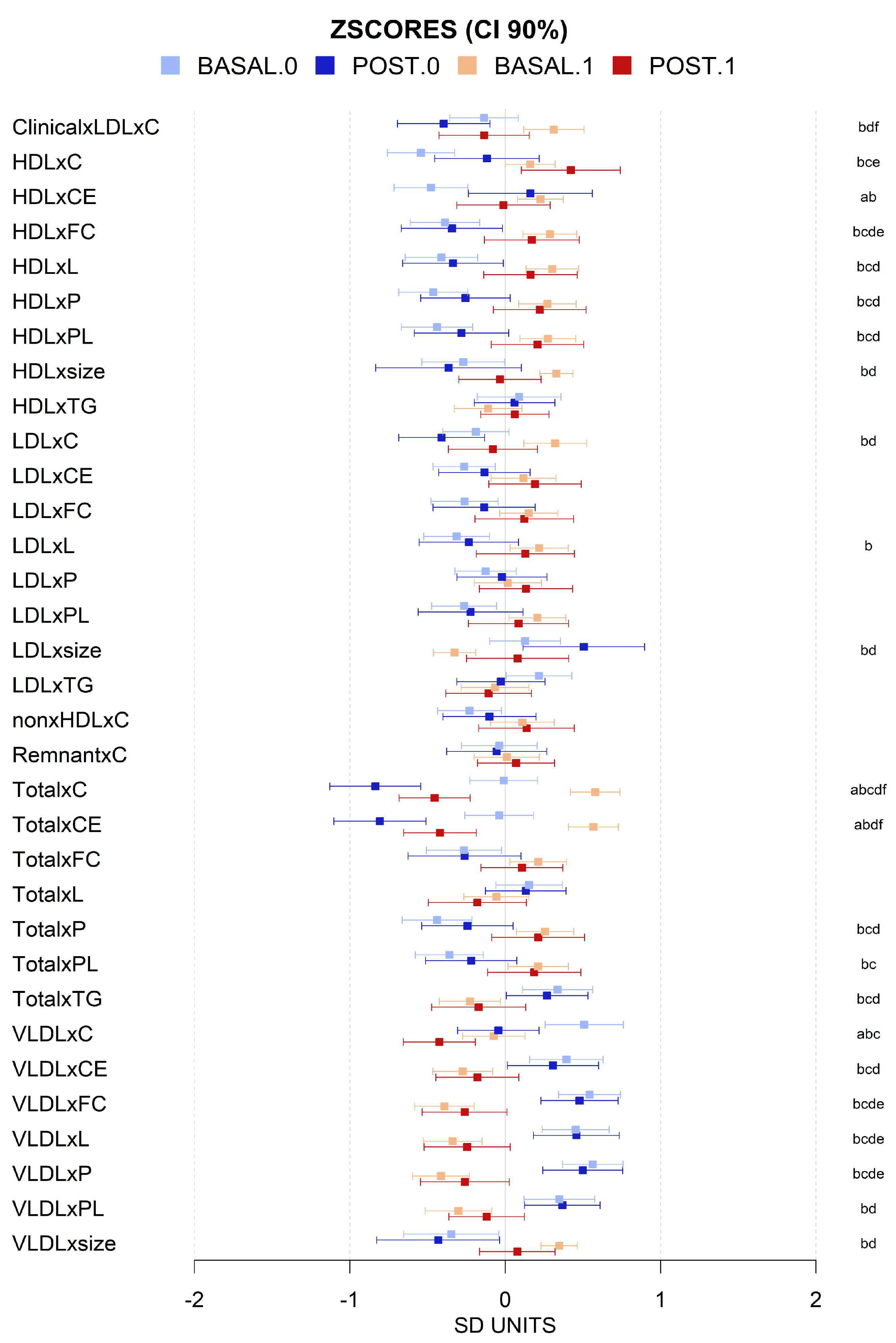
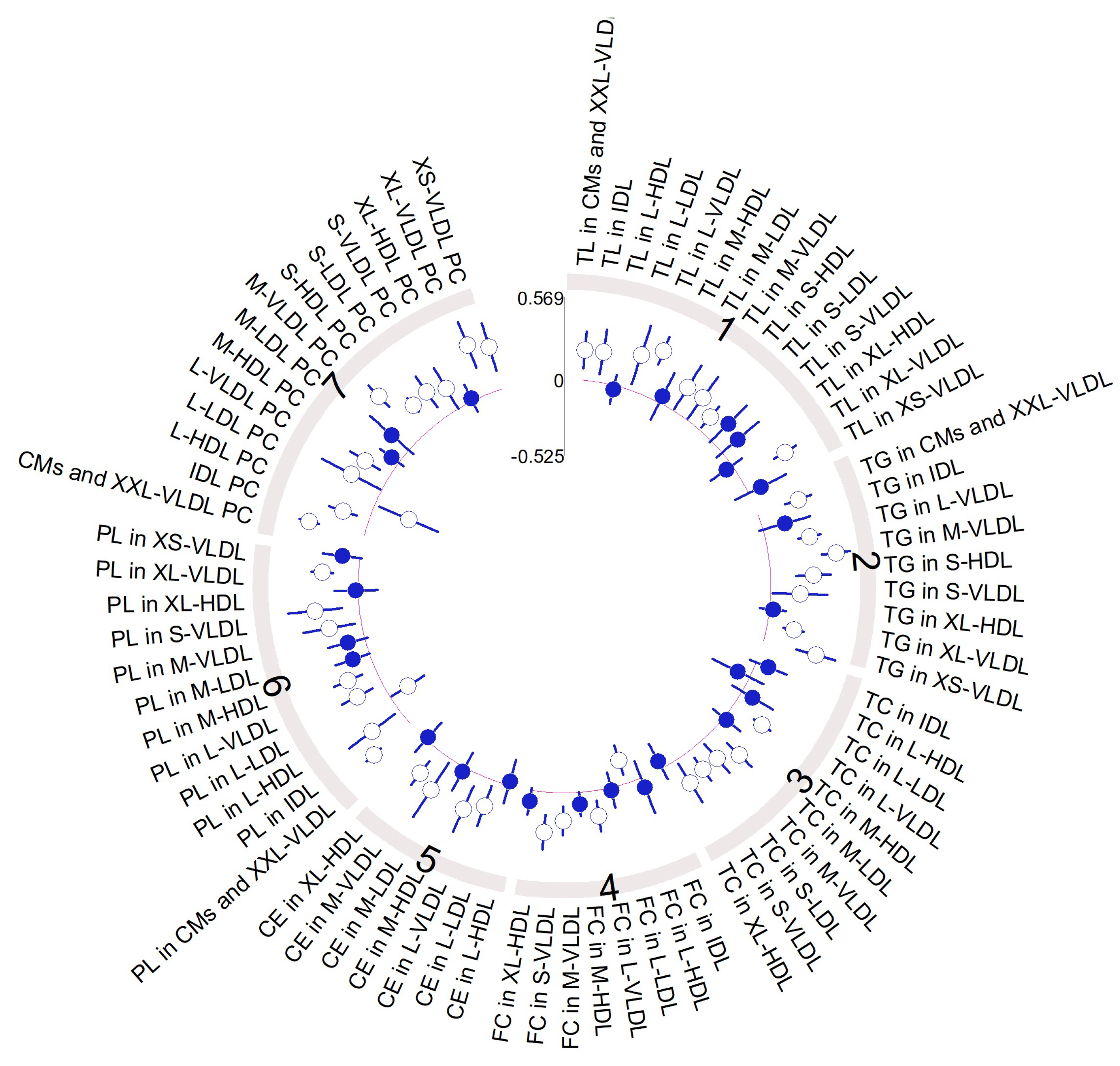
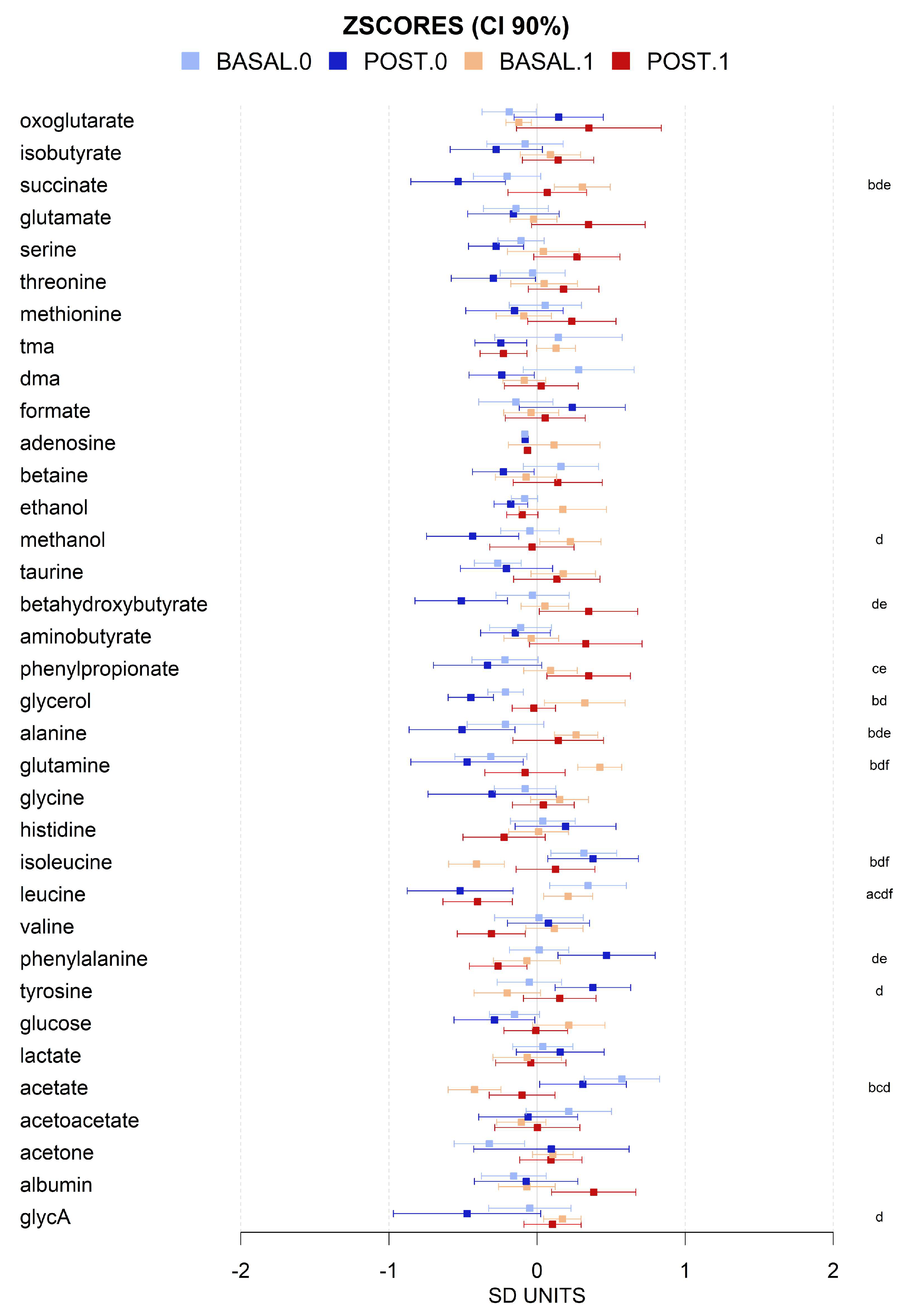
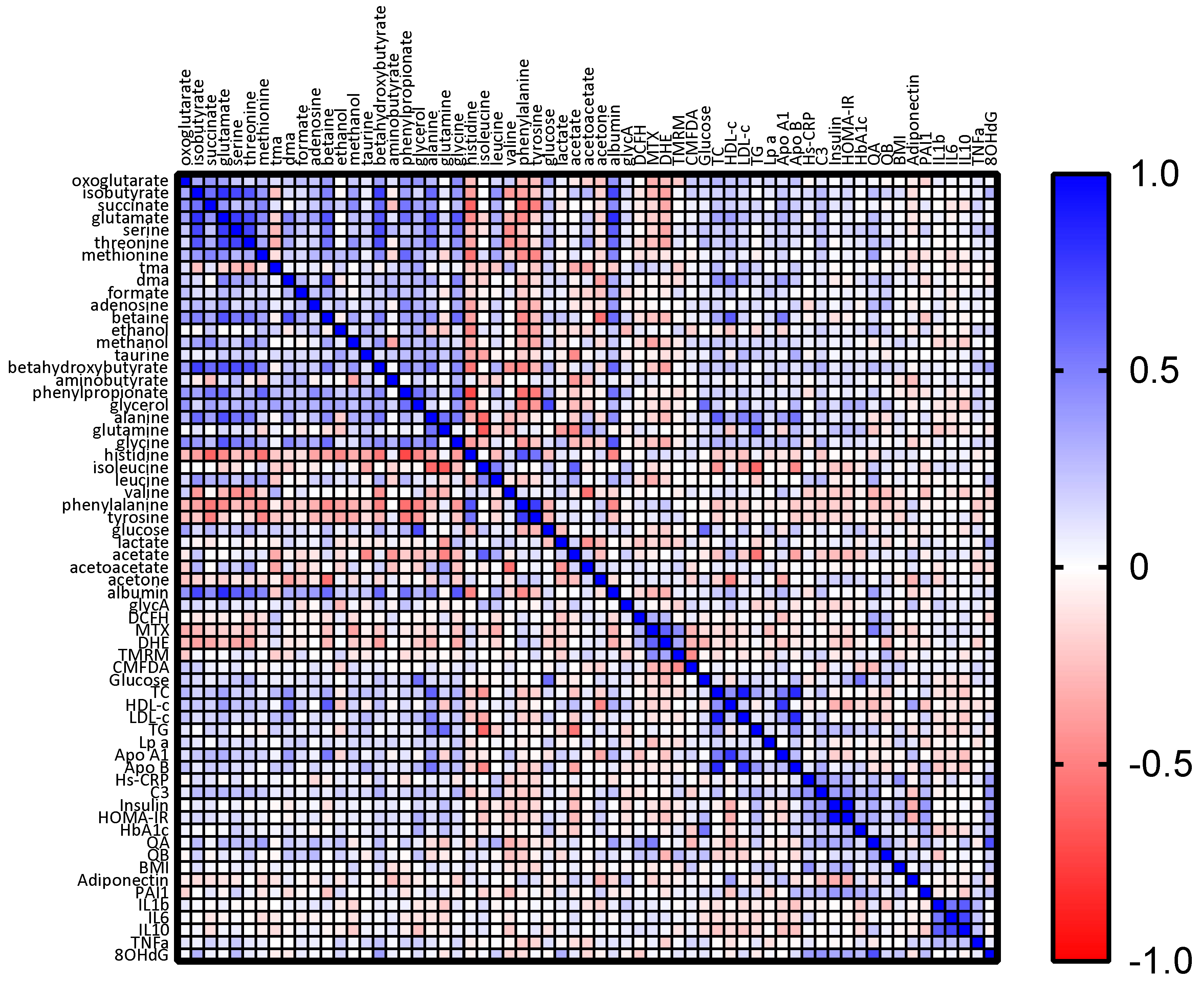
3.3. VLCD-Induced Metabolome Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefan, N.; Häring, H.U.; Hu, F.B.; Schulze, M.B. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013, 1, 152–162. [Google Scholar] [CrossRef]
- Tanriover, C.; Copur, S.; Gaipov, A.; Ozlusen, B.; Akcan, R.E.; Kuwabara, M.; Hornum, M.; Van Raalte, D.H.; Kanbay, M. Metabolically healthy obesity: Misleading phrase or healthy phenotype? Eur. J. Intern. Med. 2023, 111, 5–20. [Google Scholar] [CrossRef]
- Opio, J.; Croker, E.; Odongo, G.S.; Attia, J.; Wynne, K.; McEvoy, M. Metabolically healthy overweight/obesity are associated with increased risk of cardiovascular disease in adults, even in the absence of metabolic risk factors: A systematic review and meta-analysis of prospective cohort studies. Obes. Rev. 2020, 21, e13127. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Panic, A.; Stanimirovic, J.; Sudar-Milovanovic, E.; Isenovic, E.R. Oxidative stress in obesity and insulin resistance. Explor. Med. 2022, 3, 58–70. [Google Scholar] [CrossRef]
- McMurray, F.; Patten, D.A.; Harper, M.E. Reactive Oxygen Species and Oxidative Stress in Obesity-Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Mohanty, P.; Ghanim, H.; Aljada, A.; Browne, R.; Hamouda, W.; Prabhala, A.; Afzal, A.; Garg, R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J. Clin. Endocrinol. Metab. 2001, 86, 355–362. [Google Scholar] [CrossRef] [PubMed]
- López-Domènech, S.; Martínez-Herrera, M.; Abad-Jiménez, Z.; Morillas, C.; Escribano-López, I.; Díaz-Morales, N.; Bañuls, C.; Víctor, V.M.; Rocha, M. Dietary weight loss intervention improves subclinical atherosclerosis and oxidative stress markers in leukocytes of obese humans. Int. J. Obes. 2019, 43, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Skrha, J.; Kunesová, M.; Hilgertová, J.; Weiserová, H.; Krízová, J.; Kotrlíkova, E. Short-term very low calorie diet reduces oxidative stress in obese type 2 diabetic patients. Physiol. Res. 2005, 54, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tumova, E.; Sun, W.; Jones, P.H.; Vrablik, M.; Ballantyne, C.M.; Hoogeveen, R.C. The impact of rapid weight loss on oxidative stress markers and the expression of the metabolic syndrome in obese individuals. J. Obes. 2013, 2013, 729515. [Google Scholar] [CrossRef] [PubMed]
- Chashmniam, S.; Hashemi Madani, N.; Nobakht Mothlagh Ghoochani, B.F.; Safari-Alighiarloo, N.; Khamseh, M.E. The metabolome profiling of obese and non-obese individuals: Metabolically healthy obese and unhealthy non-obese paradox. Iran. J. Basic. Med. Sci. 2020, 23, 186–194. [Google Scholar] [CrossRef]
- Menni, C.; Migaud, M.; Kastenmüller, G.; Pallister, T.; Zierer, J.; Peters, A.; Mohney, R.P.; Spector, T.D.; Bagnardi, V.; Gieger, C.; et al. Metabolomic Profiling of Long-Term Weight Change: Role of Oxidative Stress and Urate Levels in Weight Gain. Obesity 2017, 25, 1618–1624. [Google Scholar] [CrossRef]
- Kim, Y.J.; Huh, I.; Kim, J.Y.; Park, S.; Ryu, S.H.; Kim, K.B.; Kim, S.; Park, T.; Kwon, O. Integration of Traditional and Metabolomics Biomarkers Identifies Prognostic Metabolites for Predicting Responsiveness to Nutritional Intervention against Oxidative Stress and Inflammation. Nutrients 2017, 9, 233. [Google Scholar] [CrossRef]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Lecube, A.; Monereo, S.; Rubio, M.Á.; Martínez-de-Icaya, P.; Martí, A.; Salvador, J.; Masmiquel, L.; Goday, A.; Bellido, D.; Lurbe, E.; et al. Prevention, diagnosis, and treatment of obesity 2016 position statement of the Spanish Society for the Study of Obesity. Endocrinol. Diabetes Nutr. 2017, 64, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Tukiainen, T.; Tynkkynen, T.; Laatikainen, R.; Järvelin, M.R.; Kähönen, M.; Lehtimäki, T.; Viikari, J. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 2009, 134, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Martín-Grau, M.; Pardo-Tendero, M.; Casanova, P.; Dromant, M.; Marrachelli, V.G.; Morales, J.M.; Borrás, C.; Pisoni, S.; Maestrini, S.; Di Blasio, A.M.; et al. Altered Lipid Moieties and Carbonyls in a Wistar Rat Dietary Model of Subclinical Fatty Liver: Potential Sex-Specific Biomarkers of Early Fatty Liver Disease? Antioxidants 2023, 12, 1808. [Google Scholar] [CrossRef] [PubMed]
- Maslov, L.N.; Naryzhnaya, N.V.; Boshchenko, A.A.; Popov, S.V.; Ivanov, V.V.; Oeltgen, P.R. Is oxidative stress of adipocytes a cause or consequence of the metabolic syndrome? J. Clin. Transl. Endocrinol. 2018, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.S.; Paik, J.K.; Kang, R.; Kim, M.; Choi, Y.; Lee, S.H.; Lee, J.H. Mild weight loss reduces inflammatory cytokines, leukocyte count, and oxidative stress in overweight and moderately obese participants treated for 3 years with dietary modification. Nutr. Res. 2013, 33, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Kanikowska, D.; Kanikowska, A.; Swora-Cwynar, E.; Grzymisławski, M.; Sato, M.; Bręborowicz, A.; Witowski, J.; Korybalska, K. Moderate Caloric Restriction Partially Improved Oxidative Stress Markers in Obese Humans. Antioxidants 2021, 10, 1018. [Google Scholar] [CrossRef]
- Bañuls, C.; Rovira-Llopis, S.; Lopez-Domenech, S.; Diaz-Morales, N.; Blas-Garcia, A.; Veses, S.; Morillas, C.; Victor, V.M.; Rocha, M.; Hernandez-Mijares, A. Oxidative and endoplasmic reticulum stress is impaired in leukocytes from metabolically unhealthy vs healthy obese individuals. Int. J. Obes. 2017, 41, 1556–1563. [Google Scholar] [CrossRef]
- Lorenzo, P.M.; Sajoux, I.; Izquierdo, A.G.; Gomez-Arbelaez, D.; Zulet, M.A.; Abete, I.; Castro, A.I.; Baltar, J.; Portillo, M.P.; Tinahones, F.J.; et al. Immunomodulatory effect of a very-low-calorie ketogenic diet compared with bariatric surgery and a low-calorie diet in patients with excessive body weight. Clin. Nutr. 2022, 41, 1566–1577. [Google Scholar] [CrossRef]
- Oberhauser, F.; Schulte, D.M.; Faust, M.; Güdelhöfer, H.; Hahn, M.; Müller, N.; Neumann, K.; Krone, W.; Laudes, M. Weight loss due to a very low calorie diet differentially affects insulin sensitivity and interleukin-6 serum levels in nondiabetic obese human subjects. Horm. Metab. Res. 2012, 44, 465–470. [Google Scholar] [CrossRef]
- Asghari, S.; Rezaei, M.; Rafraf, M.; Taghizadeh, M.; Asghari-Jafarabadi, M.; Ebadi, M. Effects of Calorie Restricted Diet on Oxidative/Antioxidative Status Biomarkers and Serum Fibroblast Growth Factor 21 Levels in Nonalcoholic Fatty Liver Disease Patients: A Randomized, Controlled Clinical Trial. Nutrients 2022, 14, 2509. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ožvald, I.; Božičević, D.; Duh, L.; Vinković Vrček, I.; Domijan, A.M.; Milić, M. Changes in anthropometric, biochemical, oxidative, and DNA damage parameters after 3-weeks-567-kcal-hospital-controlled-VLCD in severely obese patients with BMI ≥ 35 kgm−2. Clin. Nutr. ESPEN 2022, 49, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; de Jonge, L.; Frisard, M.I.; DeLany, J.P.; Larson-Meyer, D.E.; Rood, J.; Nguyen, T.; Martin, C.K.; Volaufova, J.; Most, M.M.; et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 2006, 295, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N. 8-Hydroxy-2-Deoxyguanosine Levels and Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Literature. Antioxid. Redox Signal. 2016, 24, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Tseng, Y.J.; Wang, S.Y.; Tsai, Y.S.; Chang, C.S.; Kuo, T.C.; Yao, W.J.; Shieh, C.C.; Wu, C.H.; Kuo, P.H. The metabolome profiling and pathway analysis in metabolic healthy and abnormal obesity. Int. J. Obes. 2015, 39, 1241–1248. [Google Scholar] [CrossRef]
- Wei, D.; González-Marrachelli, V.; Melgarejo, J.D.; Liao, C.T.; Hu, A.; Janssens, S.; Verhamme, P.; Van Aelst, L.; Vanassche, T.; Redon, J.; et al. Cardiovascular risk of metabolically healthy obesity in two european populations: Prevention potential from a metabolomic study. Cardiovasc. Diabetol. 2023, 22, 82. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 93. [Google Scholar] [CrossRef]
- Quesada, J.A.; Bertomeu-González, V.; Orozco-Beltrán, D.; Cordero, A.; Gil-Guillén, V.F.; López-Pineda, A.; Nouni-García, R.; Carratalá-Munuera, C. The benefits of measuring the size and number of lipoprotein particles for cardiovascular risk prediction: A systematic review and meta-analysis. Clin. Investig. Arterioscler. 2023, 35, 165–177. [Google Scholar] [CrossRef]
- Mills, H.L.; Patel, N.; White, S.L.; Pasupathy, D.; Briley, A.L.; Santos Ferreira, D.L.; Seed, P.T.; Nelson, S.M.; Sattar, N.; Tilling, K.; et al. The effect of a lifestyle intervention in obese pregnant women on gestational metabolic profiles: Findings from the UK Pregnancies Better Eating and Activity Trial (UPBEAT) randomised controlled trial. BMC Med. 2019, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Prieto, F.J.; Martinez-Tellez, B.; Ortiz-Alvarez, L.; Di, X.; Jurado-Fasoli, L.; Xu, H.; Ceperuelo-Mallafré, V.; Núñez-Roa, C.; Kohler, I.; Segura-Carretero, A.; et al. Elevated plasma succinate levels are linked to higher cardiovascular disease risk factors in young adults. Cardiovasc. Diabetol. 2021, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Ceperuelo-Mallafré, V.; Keiran, N.; Queipo-Ortuño, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Pérez-Brocal, V.; Andrés-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef]
- Astiarraga, B.; Martínez, L.; Ceperuelo-Mallafré, V.; Llauradó, G.; Terrón-Puig, M.; Rodríguez, M.M.; Casajoana, A.; Pellitero, S.; Megía, A.; Vilarrasa, N.; et al. Impaired Succinate Response to a Mixed Meal in Obesity and Type 2 Diabetes Is Normalized After Metabolic Surgery. Diabetes Care 2020, 43, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [PubMed]
- De Bandt, J.P.; Coumol, X.; Barouki, R. Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity. Nutrients 2022, 15, 68. [Google Scholar] [CrossRef]
- Yao, H.; Li, K.; Wei, J.; Lin, Y.; Liu, Y. The contradictory role of branched-chain amino acids in lifespan and insulin resistance. Front. Nutr. 2023, 10, 1189982. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Wu, Y.; Guo, Y.; Wang, X. Baseline Serum BCAAs are Related to the Improvement in Insulin Resistance in Obese People After a Weight Loss Intervention. Diabetes Metab. Syndr. Obes. 2023, 16, 179–186. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Giardina, S.; Cañueto, D.; Salas-Salvadó, J.; Cañellas, N.; Bulló, M. Changes in Plasma Metabolite Concentrations after a Low-Glycemic Index Diet Intervention. Mol. Nutr. Food 2019, 63, e1700975. [Google Scholar] [CrossRef]
- Sayda, M.H.; Abdul-Aziz, M.H.; Gharahdaghi, N.; Wilkinson, D.J.; Greenhaff, P.L.; Phillips, B.E.; Smith, K.; Idris, I.; Atherton, P.J. Caloric restriction improves glycaemic control without reducing plasma branched-chain amino acids or keto-acids in obese men. Sci. Rep. 2022, 12, 19273. [Google Scholar] [CrossRef]
- Ahmed, K.; Choi, H.N.; Yim, J.E. The impact of Taurine on Obesity-Induced Diabetes Mellitus: Mechanisms Underlying Its Effect. Endocrinol. Metab. 2023, 38, 482–492. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Bonfleur, M.L.; Batista, T.M.; Borck, P.V.; Carneiro, E.M. Regulation of glucose and lipid metabolism by the pancreatic and extra-pancreatic actions of taurine. Amino Acids 2018, 50, 1511–1524. [Google Scholar] [CrossRef]
- Rosa, F.T.; Freitas, E.C.; Deminice, R.; Jordao, A.A.; Marchini, J.S. Oxidative stress and inflammation in obesity after taurine supplementation: A double-blind, placebo-controlled study. Eur. J. Nutr. 2014, 53, 823–830. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Santos, J.L.; Martínez-González, M.A.; Clish, C.B.; Razquin, C.; Wang, D.; Liang, L.; Li, J.; Dennis, C.; Corella, D.; et al. Glycolysis/gluconeogenesis- and tricarboxylic acid cycle-related metabolites, Mediterranean diet, and type 2 diabetes. Am. J. Clin. Nutr. 2020, 111, 835–844. [Google Scholar] [CrossRef]
- Geidenstam, N.; Al-Majdoub, M.; Ekman, M.; Spégel, P.; Ridderstråle, M. Metabolite profiling of obese individuals before and after a one year weight loss program. Int. J. Obes. 2017, 41, 1369–1378. [Google Scholar] [CrossRef]
- Collins, K.A.; Kraus, W.E.; Rogers, R.J.; Hauser, E.R.; Lang, W.; Jiang, R.; Schelbert, E.B.; Huffman, K.M.; Jakicic, J.M. Effect of behavioral weight-loss program on biomarkers of cardiometabolic disease risk; Heart Health Study randomized trial. Obesity 2023, 31, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Parker, D.C.; Bhapkar, M.; Racette, S.B.; Martin, C.K.; Redman, L.M.; Das, S.K.; Connelly, M.A.; Pieper, C.F.; Orenduff, M.; et al. Calorie restriction improves lipid-related emerging cardiometabolic risk factors in healthy adults without obesity: Distinct influences of BMI and sex from CALERIETM a multicentre, phase 2, randomised controlled trial. EClinicalMedicine 2022, 43, 101261. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L.; Lou, P.; Zhao, M.; Wang, Y.; Tang, M.; Gong, M.; Liao, G.; Yuan, Y.; Li, L.; et al. Elevated branched-chain α-keto acids exacerbate macrophage oxidative stress and chronic inflammatory damage in type 2 diabetes mellitus. Free Radic. Biol. Med. 2021, 175, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef]
- Wang, Z.; Ohata, Y.; Watanabe, Y.; Yuan, Y.; Yoshii, Y.; Kondo, Y.; Nishizono, S.; Chiba, T. Taurine Improves Lipid Metabolism and Increases Resistance to Oxidative Stress. J. Nutr. Sci. Vitaminol. 2020, 66, 347–356. [Google Scholar] [CrossRef]
- Caetano, L.C.; Bonfleur, M.L.; Ribeiro, R.A.; Nardelli, T.R.; Lubaczeuski, C.; do Nascimento da Silva, J.; Carneiro, E.M.; Balbo, S.L. Taurine supplementation regulates Iκ-Bα protein expression in adipose tissue and serum IL-4 and TNF-α concentrations in MSG obesity. Eur. J. Nutr. 2017, 56, 705–713. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Li, B.Y.; Peng, W.Q.; Guo, L.; Tang, Q.Q. Taurine-mediated browning of white adipose tissue is involved in its anti-obesity effect in mice. J. Biol. Chem. 2019, 294, 15014–15024. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Toolabi, K.; Jannat Ali Pour, N.; Mohassel Azadi, S.; Bahiraee, A.; Zamani-Garmsiri, F.; Emamgholipour, S. Adipose tissue gene expression of long non-coding RNAs; MALAT1, TUG1 in obesity: Is it associated with metabolic profile and lipid homeostasis-related genes expression? Diabetol. Metab. Syndr. 2020, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective role of taurine against oxidative stress (Review). Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Asadi, M.; Mohammadi-Asl, J.; Ahmadi-Angali, K. Effect of weight-loss diet combined with taurine supplementation on body composition and some biochemical markers in obese women: A randomized clinical trial. Amino Acids 2020, 52, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Samadpour Masouleh, S.; Bagheri, R.; Ashtary-Larky, D.; Cheraghloo, N.; Wong, A.; Yousefi Bilesvar, O.; Suzuki, K.; Siahkouhian, M. The Effects of TRX Suspension Training Combined with Taurine Supplementation on Body Composition, Glycemic and Lipid Markers in Women with Type 2 Diabetes. Nutrients 2021, 13, 3958. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, N.; Zhang, X.; Horng, T. Mitochondrial metabolism regulates macrophage biology. J. Biol. Chem. 2021, 297, 100904. [Google Scholar] [CrossRef] [PubMed]
- Khoder-Agha, F.; Kietzmann, T. The glyco-redox interplay: Principles and consequences on the role of reactive oxygen species during protein glycosylation. Redox Biol. 2021, 42, 101888. [Google Scholar] [CrossRef] [PubMed]



| MHO | MUHO | |||
|---|---|---|---|---|
| Baseline | Final | Baseline | Final | |
| N (females %) | 34 (76.4) | 40 (62.5) | ||
| Weight (kg) | 112.1 ± 23.5 | 99.3 ± 21.7 *** | 115.9 ± 20.7 | 102.9 ± 19.6 *** |
| BMI (kg/m2) | 39.0 (35.5; 44.6) | 33.9 (30.7; 39.8) *** | 40.5 (36.9; 43.0) | 36.3 (30.7; 39.6) *** |
| Waist (cm) | 117.0 ± 14.9 | 105.5 ± 15.8 *** | 121.9 ± 15.8 | 108.7 ± 16.0 *** |
| Fat mass index (kg/m2) | 18.9 (15.8; 23.7) | 15.3 (12.3; 19.6) *** | 19.0 (16.8; 21.4) | 16.1 (12.5; 18.9) *** |
| Free fat mass index (kg/m2) | 21.0 ± 3.5 | 19.9 ± 3.1 *** | 21.0 ± 2.5 | 20.2 ± 2.4 *** |
| Visceral fat (L) | 4.1 (3.0; 6.2) | 2.8 (1.7; 4.4) *** | 4.9 (3.7; 8.5) | 3.1 (2.0; 4.8) *** |
| Resistance (Ω) | 526.5 ± 81.1 | 552.3 ± 87.7 *** | 526.5 ± 66.1 | 546.2 ± 71.8 *** |
| Reactance (Ω) | 50.0 ± 9.8 | 51.1 ± 9.3 | 50.7 ± 7.5 | 51.1 ± 9.0 |
| Phase angle (°) | 5.4 ± 0.5 | 5.3 ± 0.5 * | 5.5 ± 0.6 | 5.3 ± 0.6 ** |
| SBP (mmHg) | 128 ± 10 | 119 ± 13 ** | 132 ± 15 | 120 ± 14 *** |
| DBP (mmHg) | 78 ± 10 | 76 ± 9 | 83 ± 10 # | 76 ± 9 *** |
| Glucose (mg/dL) | 92.3 ± 10.0 | 90.5 ± 9.3 | 110.5 ± 35.6 ## | 98.3 ± 12.6 * |
| Insulin (μU/mL) | 17.8 ± 10.7 | 12.5 ± 6.0 *** | 26.5 ± 17.6 # | 15.6 ± 8.9 *** |
| HOMA-IR | 3.5 (2.1; 6.0) | 4.1 (3.6; 5.3) * | 5.6 (4.4; 8.1) ### | 4.8 (4.1; 5.7) ** |
| A1c (%) | 5.4 (5.1; 5.7) | 5.3 (5.0; 5.6) * | 5.6 (5.4; 6.0) # | 5.4 (5.2; 5.6) *** |
| TC (mg/dL) | 182.4 ± 39.6 | 173.8 ± 41.7 * | 192.2 ± 33.5 | 177.5 ± 39.4 ** |
| HDLc (mg/dL) | 49.5 (41.8; 56.0) | 46.0 (39.8; 55.3) | 41.0 ± (36.3; 49.8) ## | 41.0 ± 11.7 |
| LDLc (mg/dL) | 113.7 ± 31.5 | 108.4 ± 33.4 * | 114.8 ± 26.6 | 111.4 ± 35.3 |
| TG (mg/dL) | 96.5 (76.5; 106.5) | 79.5 (65.2; 96.5) | 162.5 (127.0; 222.5) ### | 113.0 (80.3; 156.5) *** |
| Apo A1 (mg/dL) | 141.0 (131.3; 164.0) | 128.0 (118.0; 149.0) * | 136.5 (123.0; 158.5) | 123.5 (111.5; 142.8) ** |
| Apo B (mg/dL) | 94.3 ± 23.0 | 86.8 ± 25.4 ** | 108.0 ± 24.5 # | 93.1 ± 30.6 ** |
| Lp(a) (mg/dL) | 10.0 (4.8; 28.5) | 11.5 (6.0; 29.0) | 8.5 (3.0; 29.8) | 11.0 (5.0; 27.0) |
| Uric acid (mg/dL) | 5.5 (5.0; 6.0) | 4.8 (4.2; 5.6) * | 5.7 (4.9; 6.8) | 5.2 (4.7; 6.3) * |
| hs-CRP (mg/L) | 8.2 (4.3; 16.9) | 7.0 (2.6; 13.2) ** | 8.8 (4.6; 13.9) | 5.0 (3.1; 11.3) * |
| C3 Protein (mg/dL) | 137.4 ± 23.4 | 124.5 ± 17.8 *** | 143.6 ± 17.4 | 131.9 ± 17.5 *** |
| Adiponectin (μg/mL) | 23,495.5 ± 14,729.1 | 31,306.1 ± 27,093.7 ** | 21,001.1 ± 12,412.5 | 24,674.9 ±14,965.5 ** |
| PAI-1 (ng/mL) | 114.3 (77.2; 133.2) | 89.1 (71.4; 122.7) * | 116.4 (100.3; 177.2) | 107.8 (77.6; 136.7) *** |
| IL-1b (pg/mL) | 1.5 (0.9; 2.4) | 1.4 (0.8; 2.6) | 1.3 (0.8; 2.3) | 1.3 (0.8; 2.4) |
| IL-6 (pg/mL) | 0.6 (0.2; 4.5) | 0.3 (0.2; 6.6) | 0.3 (0.2; 1.0) | 0.2 (0.2; 1.3) |
| IL-10 (pg/mL) | 12.3 (2.0; 32.9) | 14.6 (3.0; 52.5) | 9.7 (2.1; 20.4) | 9.8 (2.3; 18.3) |
| TNF-α (pg/mL) | 11.0 (9.5; 14.4) | 10.9 (8.7; 14.8) | 12.4 (11.3; 15.0) | 12.7 (11.1; 15.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch-Sierra, N.; Grau-del Valle, C.; Salom, C.; Zaragoza-Villena, B.; Perea-Galera, L.; Falcón-Tapiador, R.; Rovira-Llopis, S.; Morillas, C.; Monleón, D.; Bañuls, C. Effect of a Very Low-Calorie Diet on Oxidative Stress, Inflammatory and Metabolomic Profile in Metabolically Healthy and Unhealthy Obese Subjects. Antioxidants 2024, 13, 302. https://doi.org/10.3390/antiox13030302
Bosch-Sierra N, Grau-del Valle C, Salom C, Zaragoza-Villena B, Perea-Galera L, Falcón-Tapiador R, Rovira-Llopis S, Morillas C, Monleón D, Bañuls C. Effect of a Very Low-Calorie Diet on Oxidative Stress, Inflammatory and Metabolomic Profile in Metabolically Healthy and Unhealthy Obese Subjects. Antioxidants. 2024; 13(3):302. https://doi.org/10.3390/antiox13030302
Chicago/Turabian StyleBosch-Sierra, Neus, Carmen Grau-del Valle, Christian Salom, Begoña Zaragoza-Villena, Laura Perea-Galera, Rosa Falcón-Tapiador, Susana Rovira-Llopis, Carlos Morillas, Daniel Monleón, and Celia Bañuls. 2024. "Effect of a Very Low-Calorie Diet on Oxidative Stress, Inflammatory and Metabolomic Profile in Metabolically Healthy and Unhealthy Obese Subjects" Antioxidants 13, no. 3: 302. https://doi.org/10.3390/antiox13030302






