Mitochondria-Targeted Antioxidant Therapeutics for Traumatic Brain Injury
Abstract
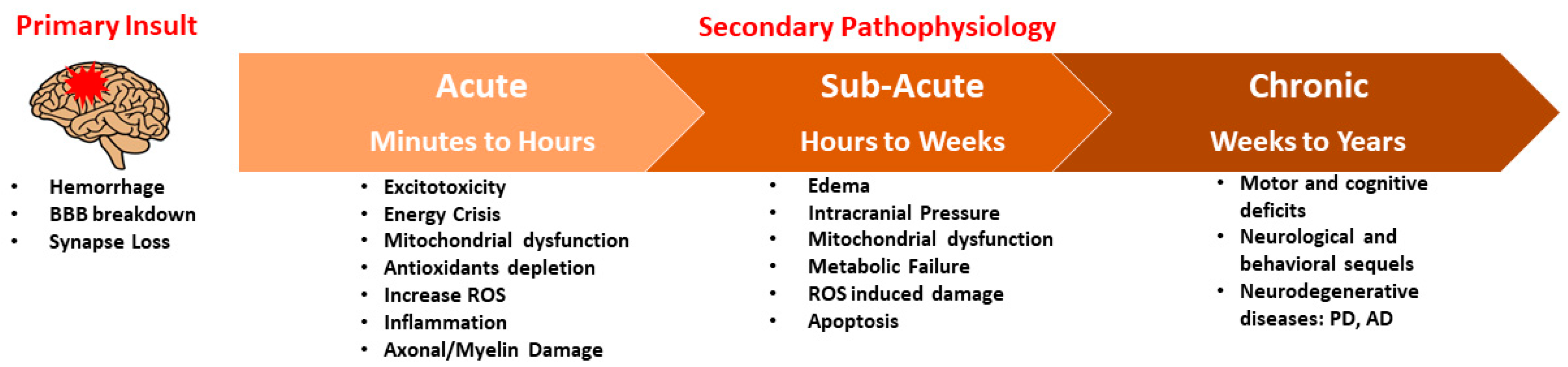
1. Introduction
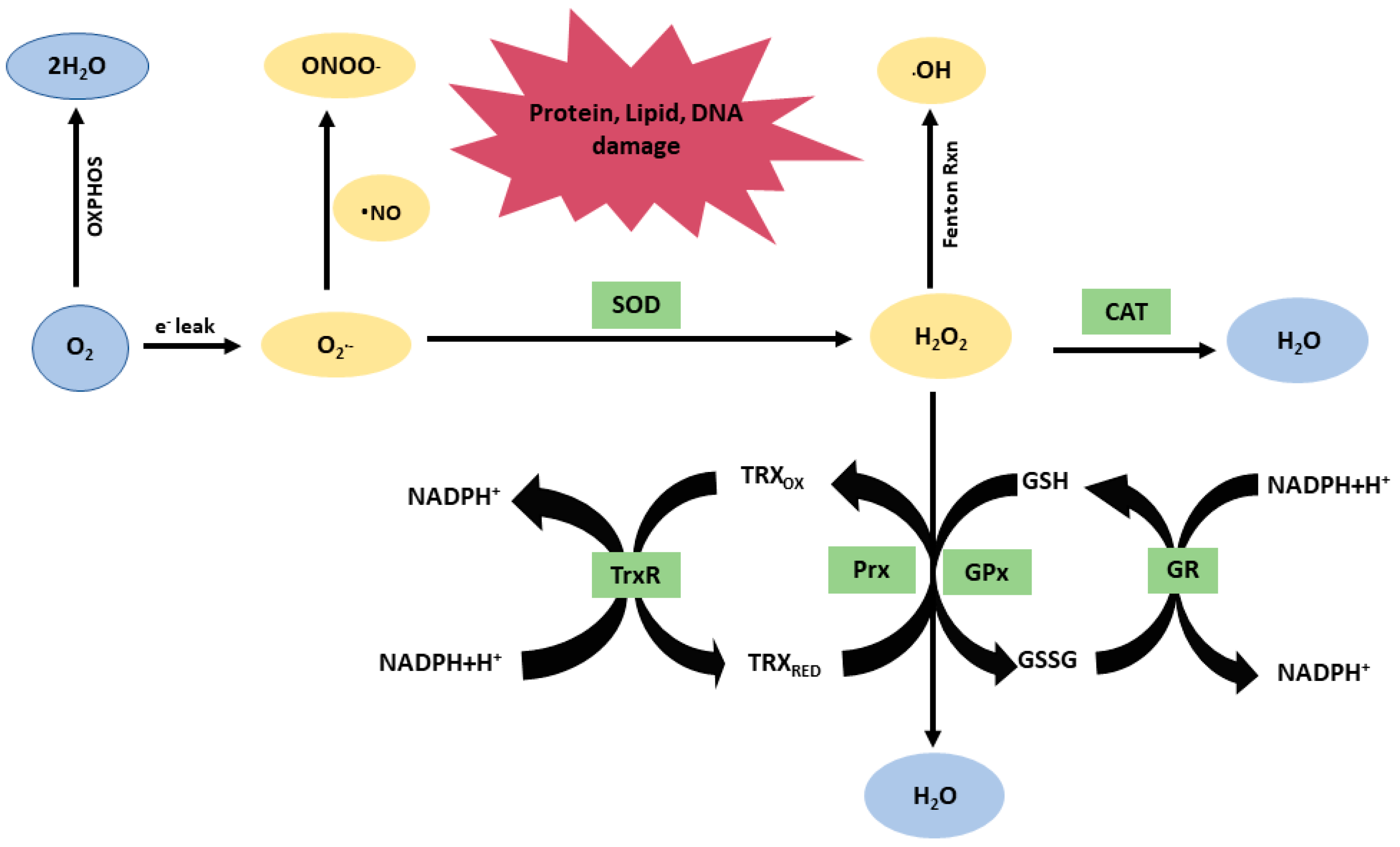
2. Mitochondrial Redox Mechanisms in TBI
3. Mitochondrial Antioxidants in TBI
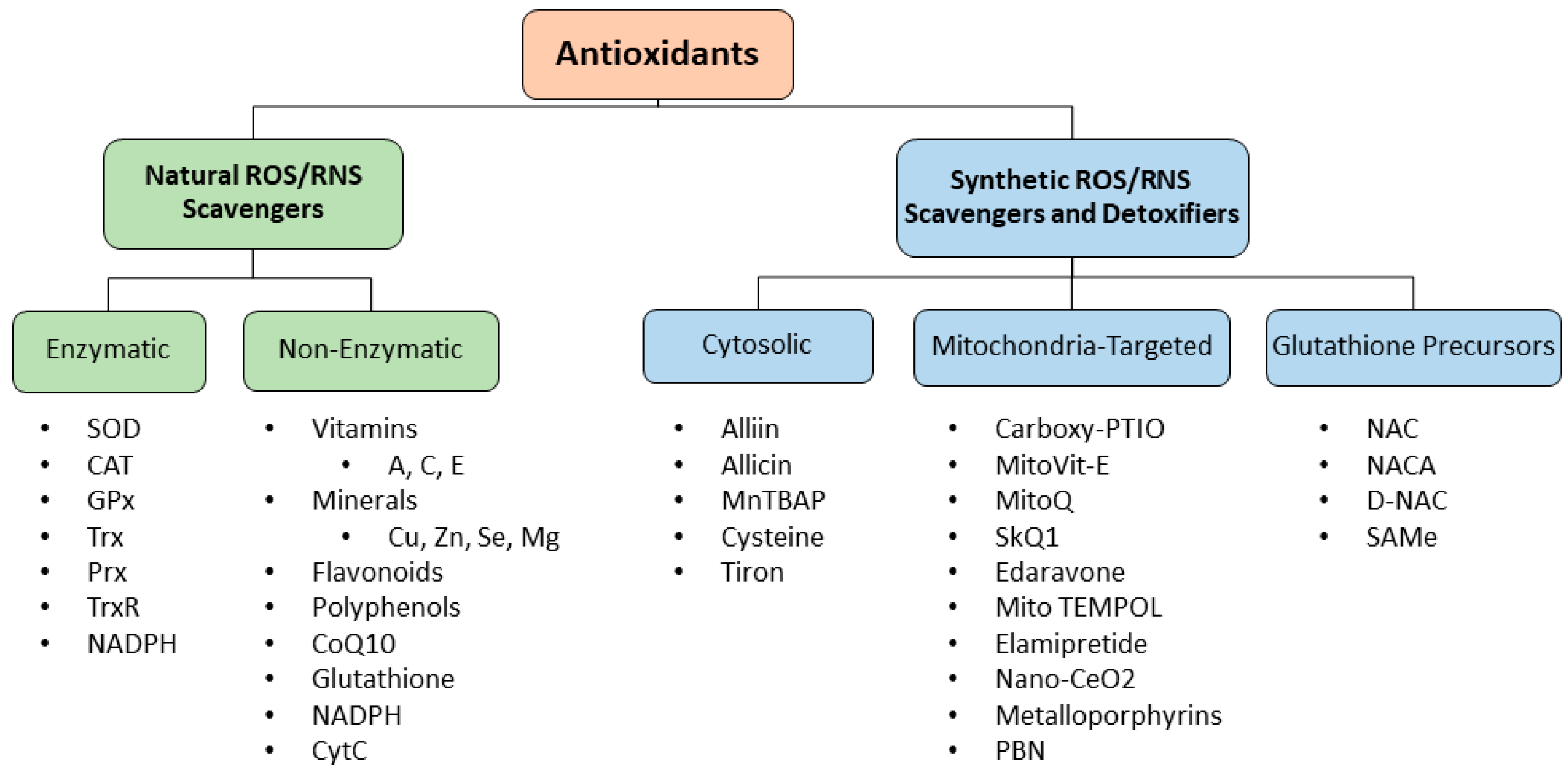
4. ROS–RNS Scavengers
4.1. Natural ROS–RNS Scavengers
4.2. Synthetic ROS–RNS Scavengers
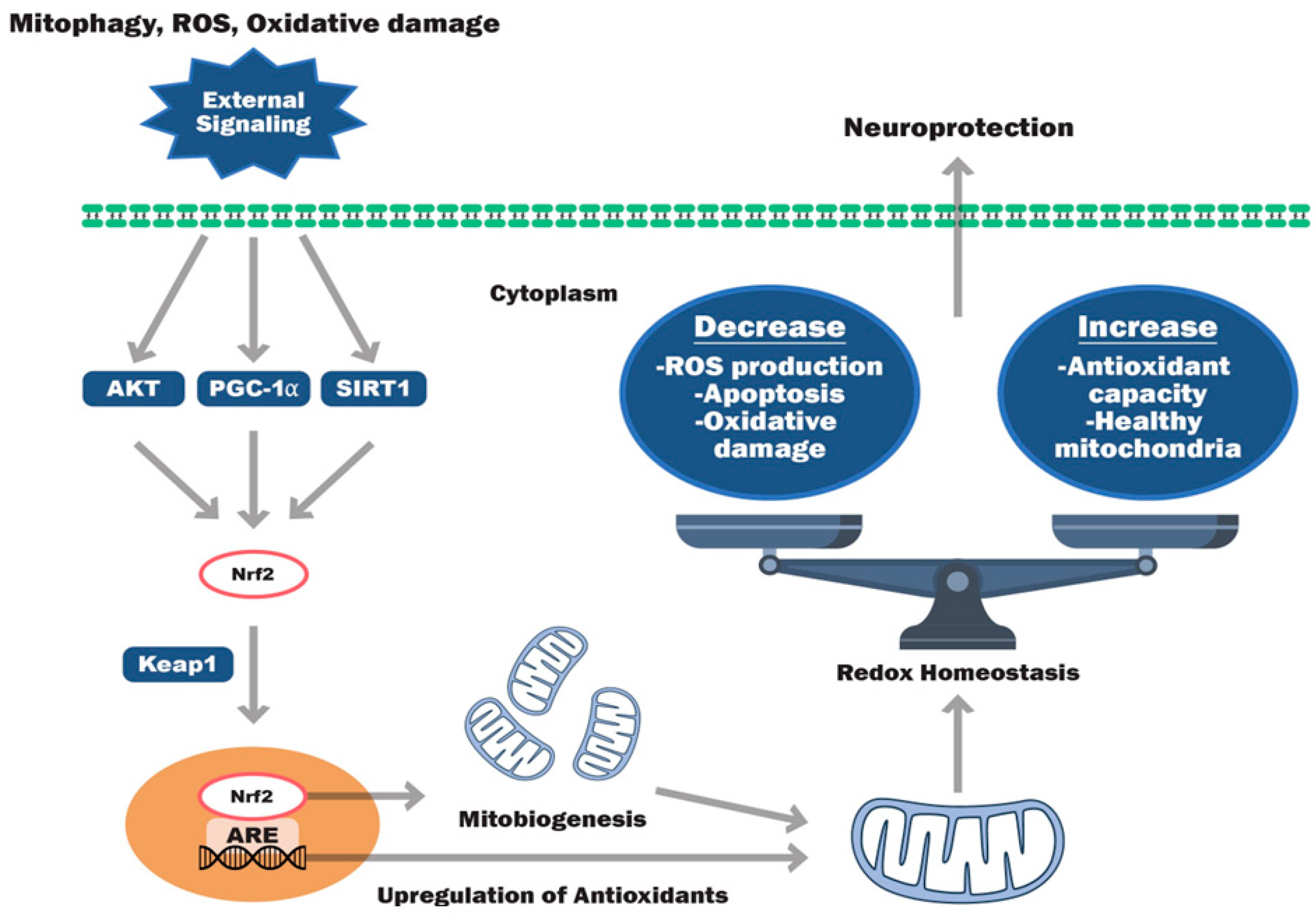
5. Signaling Pathway Modulators for Cellular Antioxidant Synthesis
5.1. Nrf2 Activators
5.2. SIRT, PGC-1α, AKT, and mTOR Modulators
6. Challenges and Future Approach
7. Holistic Approach to Improve TBI Outcomes
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- CDC. National Center for Health Statistics. 2022. Available online: https://wonder.cdc.gov/mcd.html (accessed on 8 September 2023).
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Corso, P.S.; Miller, T.R. The Incidence and Economic Burden of Injuries in the United States; Oxford University Press: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Coronado, V.G.; McGuire, L.C.; Faul, M.; Sugerman, D.E.; Pearson, W.S. Traumatic brain injury epidemiology and public health issues. Brain Inj. Med. Princ. Pract. 2012, 84, 84–100. [Google Scholar]
- Nagalakshmi; Sagarkar, S.; Sakharkar, A.J. Epigenetic mechanisms of traumatic brain injuries. Prog. Mol. Biol. Transl. Sci. 2018, 157, 263–298. [Google Scholar]
- Mena, J.H.; Sanchez, A.I.; Rubiano, A.M.; Peitzman, A.B.; Sperry, J.L.; Gutierrez, M.I.M.; Puyana, J.C. Effect of the Modified Glasgow Coma Scale Score Criteria for Mild Traumatic Brain Injury on Mortality Prediction: Comparing Classic and Modified Glasgow Coma Scale Score Model Scores of 13. J. Trauma 2011, 71, 1185–1192; Discussion 1193. [Google Scholar] [CrossRef] [PubMed]
- Alves, W.M.; Marshall, L.F. Traumatic brain injury. In Handbook of Neuroemergency Clinical Trials; Elsevier: Amsterdam, The Netherlands, 2006; pp. 61–79. [Google Scholar]
- Başkaya, M.K.; Doğan, A.; Rao, A.M.; Dempsey, R.J. Neuroprotective effects of citicoline on brain edema and blood—Brain barrier breakdown after traumatic brain injury. J. Neurosurg. 2000, 92, 448–452. [Google Scholar] [CrossRef]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood–Brain Barrier Pathophysiology in Traumatic Brain Injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef]
- Pandya, J.D.; Leung, L.Y.; Hwang, H.M.; Yang, X.; Deng-Bryant, Y.; Shear, D.A. Time-Course Evaluation of Brain Regional Mitochondrial Bioenergetics in a Pre-Clinical Model of Severe Penetrating Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2323–2334. [Google Scholar] [CrossRef]
- Pandya, J.D.; Leung, L.Y.; Flerlage, W.J.; Gilsdorf, J.S.; Bryant, Y.D.; Shear, D. Comprehensive profile of acute mitochondrial dysfunction in a preclinical model of severe penetrating TBI. Front. Neurol. 2019, 10, 605. [Google Scholar] [CrossRef]
- Li, J.; O, W.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative Stress and Neurodegenerative Disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.; Havlik, R.; Steffens, D.; Helms, M.; Newman, T.; Drosdick, D.; Phillips, C.; Gau, B.; Welsh–Bohmer, K.; Burke, J.; et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000, 55, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ou, S.; Cui, H.; Li, X.; Yin, Z.; Gu, D.; Wang, Z. Head Injury and Amyotrophic Lateral Sclerosis: A Meta-Analysis. Neuroepidemiology 2021, 55, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Lin, H.-C. Increased Risk of Multiple Sclerosis after Traumatic Brain Injury: A Nationwide Population-Based Study. J. Neurotrauma 2012, 29, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, W.B.; Joseph, B.; Spry, M.; Vekaria, H.J.; Saatman, K.E.; Sullivan, P.G. Acute Mitochondrial Impairment Underlies Prolonged Cellular Dysfunction after Repeated Mild Traumatic Brain Injuries. J. Neurotrauma 2019, 36, 1252–1263. [Google Scholar] [CrossRef]
- Kilbaugh, T.J.; Karlsson, M.; Byro, M.; Bebee, A.; Ralston, J.; Sullivan, S.; Duhaime, A.-C.; Hansson, M.J.; Elmér, E.; Margulies, S.S. Mitochondrial bioenergetic alterations after focal traumatic brain injury in the immature brain. Exp. Neurol. 2015, 271, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.D.; Pauly, J.R.; Nukala, V.N.; Sebastian, A.H.; Day, K.M.; Korde, A.S.; Maragos, W.F.; Hall, E.D.; Sullivan, P.G. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. J. Neurotrauma 2007, 24, 798–811. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Rabchevsky, A.G.; Keller, J.N.; Lovell, M.; Sodhi, A.; Hart, R.P.; Scheff, S.W. Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J. Comp. Neurol. 2004, 474, 524–534. [Google Scholar] [CrossRef]
- Andriessen, T.M.J.C.; Jacobs, B.; Vos, P.E. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 2010, 14, 2381–2392. [Google Scholar] [CrossRef]
- Bullock, M.R.; Povlishock, J.T. Guidelines for the management of severe traumatic brain injury. Editor’s Commentary. J. Neurotrauma 2007, 24 (Suppl. S1), 2 p preceding S1. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.K.; Michel, M.E.; Ansell, B.; Baethmann, A.; Biegon, A.; Bracken, M.B.; Bullock, M.R.; Choi, S.C.; Clifton, G.L.; Contant, C.F.; et al. Clinical Trials in Head Injury. J. Neurotrauma 2002, 19, 503–557. [Google Scholar] [CrossRef] [PubMed]
- Povlishock, J.T.; Katz, D.I. Update of Neuropathology and Neurological Recovery After Traumatic Brain Injury. J. Head Trauma Rehabilit. 2005, 20, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.; Greco, T.; Alexander, D.; Giza, C.C. The pathophysiology of traumatic brain injury at a glance. Dis. Model. Mech. 2013, 6, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.; Bergsneider, M.; Hattori, N.; Wu, H.-M.; Huang, S.-C.; Martin, N.A.; Glenn, T.C.; McArthur, D.L.; Hovda, D.A. Metabolic Crisis without Brain Ischemia is Common after Traumatic Brain Injury: A Combined Microdialysis and Positron Emission Tomography Study. J. Cereb. Blood Flow Metab. 2005, 25, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A.; Hovda, D.A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991, 561, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, J.; Sullivan, P.G.; Hovda, D.A.; Wieloch, T.; McIntosh, T.K. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 2004, 4, 705–713. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Krishnamurthy, S.; Patel, S.P.; Pandya, J.D.; Rabchevsky, A.G. Temporal Characterization of Mitochondrial Bioenergetics after Spinal Cord Injury. J. Neurotrauma 2007, 24, 991–999. [Google Scholar] [CrossRef]
- Singh, I.N.; Sullivan, P.G.; Deng, Y.; Mbye, L.H.; Hall, E.D. Time Course of Post-Traumatic Mitochondrial Oxidative Damage and Dysfunction in a Mouse Model of Focal Traumatic Brain Injury: Implications for Neuroprotective Therapy. J. Cereb. Blood Flow Metab. 2006, 26, 1407–1418. [Google Scholar] [CrossRef]
- Xiong, Y.; Gu, Q.; Peterson, P.; Muizelaar, J.; Lee, C. Mitochondrial Dysfunction and Calcium Perturbation Induced by Traumatic Brain Injury. J. Neurotrauma 1997, 14, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gilmer, L.K.; Roberts, K.N.; Sullivan, P.G.; Miller, K.; Scheff, S. Early mitochondrial dysfunction after cortical contusion injury. J. Neurotrauma 2009, 219, 1. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.D.; Pauly, J.R.; Sullivan, P.G. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp. Neurol. 2009, 218, 381–389. [Google Scholar] [CrossRef]
- Pandya, J.D.; Musyaju, S.; Modi, H.R.; Cao, Y.; Flerlage, W.J.; Huynh, L.; Kociuba, B.; Visavadiya, N.P.; Kobeissy, F.; Wang, K.; et al. Comprehensive evaluation of mitochondrial redox profile, calcium dynamics, membrane integrity and apoptosis markers in a preclinical model of severe penetrating traumatic brain injury. Free. Radic. Biol. Med. 2023, 198, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Crompton, M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999, 341 Pt 2, 233–249. [Google Scholar] [CrossRef]
- Halestrap, A.P. Mitochondrial calcium in health and disease. Biochim. Biophys. Acta (BBA)—Bioenerg. 2009, 1787, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 2009, 46, 821–831. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Rabchevsky, A.G.; Waldmeier, P.C.; Springer, J.E. Mitochondrial permeability transition in CNS trauma: Cause or effect of neuronal cell death? J. Neurosci. Res. 2005, 79, 231–239. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Walker, C.L.; Pomatto, L.C.D.; Tripathi, D.N.; Davies, K.J.A.; Ninsontia, C.; Phiboonchaiyanan, P.P.; Kiratipaiboon, C.; Chanvorachote, P.; et al. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W. H. Freeman and Company: New York, NY, USA, 2002. [Google Scholar]
- Chance, B.; Williams, G.R. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 1956, 17, 65–134. [Google Scholar] [PubMed]
- Deng, Y.; Thompson, B.M.; Gao, X.; Hall, E.D. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 2007, 205, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.N.; Sullivan, P.G.; Hall, E.D. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J. Neurosci. Res. 2007, 85, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Bayir, H.; E Kagan, V.; Tyurina, Y.Y.; Tyurin, V.; A Ruppel, R.; Adelson, P.D.; Graham, S.H.; Janesko, K.; Clark, R.S.B.; Kochanek, P.M. Assessment of Antioxidant Reserves and Oxidative Stress in Cerebrospinal Fluid after Severe Traumatic Brain Injury in Infants and Children. Pediatr. Res. 2002, 51, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free. Radic. Biol. Med. 2008, 45, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Detloff, M.R.; Johnson, K.; Kupina, N.C. Peroxynitrite-Mediated Protein Nitration and Lipid Peroxidation in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2004, 21, 9–20. [Google Scholar] [CrossRef]
- Hall, E.D.; Vaishnav, R.A.; Mustafa, A.G. Antioxidant Therapies for Traumatic Brain Injury. Neurotherapeutics 2010, 7, 51–61. [Google Scholar] [CrossRef]
- Hall, E.D.; Wang, J.A.; Miller, D.M. Relationship of nitric oxide synthase induction to peroxynitrite-mediated oxidative damage during the first week after experimental traumatic brain injury. Exp. Neurol. 2012, 238, 176–182. [Google Scholar] [CrossRef]
- Hill, R.L.; Kulbe, J.R.; Singh, I.N.; Wang, J.A.; Hall, E.D. Synaptic Mitochondria are More Susceptible to Traumatic Brain Injury-induced Oxidative Damage and Respiratory Dysfunction than Non-synaptic Mitochondria. Neuroscience 2018, 386, 265–283. [Google Scholar] [CrossRef]
- Abdul-Muneer, P.M.; Chandra, N.; Haorah, J. Interactions of Oxidative Stress and Neurovascular Inflammation in the Pathogenesis of Traumatic Brain Injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Petronilho, F.; Feier, G.; de Souza, B.; Guglielmi, C.; Constantino, L.S.; Walz, R.; Quevedo, J.; Dal-Pizzol, F. Oxidative Stress in Brain According to Traumatic Brain Injury Intensity. J. Surg. Res. 2010, 164, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.; Crupi, R.; Calabrese, V.; Graziano, A.; Milone, P.; Pennisi, G.; Radak, Z.; Calabrese, E.J.; Cuzzocrea, S. Traumatic Brain Injury: Oxidative Stress and Neuroprotection. Antioxid. Redox Signal. 2013, 19, 836–853. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gajardo, R.; Matamala, J.M.; Carrasco, R.; Gutiérrez, R.; Melo, R.; Rodrigo, R. Novel Therapeutic Strategies for Traumatic Brain Injury: Acute Antioxidant Reinforcement. CNS Drugs 2014, 28, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Xu, J.; Ogura, Y.; Monno, I.; Koya, D. Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease. Front. Physiol. 2020, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Suthammarak, W.; Somerlot, B.H.; Opheim, E.; Sedensky, M.; Morgan, P.G. Novel interactions between mitochondrial superoxide dismutases and the electron transport chain. Aging Cell 2013, 12, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.K.; Clair, D.K.S.; Huang, C.-L.; Yokomise, H.; Miyatake, A.; Setyawati, M.I.; Tay, C.Y.; Leong, D.T.; Schumacher, B.; Gartner, A.; et al. Watching the watcher: Regulation of p53 by mitochondria. Futur. Oncol. 2009, 5, 117–130. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Taffe, K.M.; Abrahamson, E.E.; Dixon, C.E.; Kochanek, P.M.; Ikonomovic, M.D. Time Course Analysis of Hippocampal Nerve Growth Factor and Antioxidant Enzyme Activity following Lateral Controlled Cortical Impact Brain Injury in the Rat. J. Neurotrauma 2004, 21, 491–500. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Omar, R.A.; Kim, K.S.; Robakis, N.K. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer’s disease. Am. J. Pathol. 1992, 140, 621–628. [Google Scholar]
- Zemlan, F.P.; Thienhaus, O.J.; Bosmann, H.B. Superoxide dismutase activity in Alzheimer’s disease: Possible mechanism for paired helical filament formation. Brain Res. 1989, 476, 160–162. [Google Scholar] [CrossRef]
- Massaad, C.A.; Washington, T.M.; Pautler, R.G.; Klann, E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13576–13581. [Google Scholar] [CrossRef] [PubMed]
- Palace, V.P.; Khaper, N.; Qin, Q.; Singal, P.K. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free. Radic. Biol. Med. 1999, 26, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.J.; Miranda-Massari, J.R.; Olalde, J. Vitamin C and mitochondrial function in health and exercise. In Molecular Nutrition and Mitochondria; Elsevier: Amsterdam, The Netherlands, 2023; pp. 225–242. [Google Scholar]
- Eleff, S.; Kennaway, N.G.; Buist, N.R.; Darley-Usmar, V.M.; A Capaldi, R.; Bank, W.J.; Chance, B. 31P NMR study of improvement in oxidative phosphorylation by vitamins K3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proc. Natl. Acad. Sci. USA 1984, 81, 3529–3533. [Google Scholar] [CrossRef] [PubMed]
- Kc, S.; Càrcamo, J.M.; Golde, D.W. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Gluti) and confers mitochondrial protection against oxidative injury. FASEB J. 2005, 19, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- González, M.J.; Miranda, J.R.; Riordan, H.D. Vitamin C as an Ergogenic Aid. J. Orthomol. Med. 2005, 20, 100–102. [Google Scholar]
- U.S. Department of Health & Human Services. Vitamin A and Carotenoids. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ (accessed on 29 April 2022).
- Hayashi, T.; Sawa, K.; Kawasaki, M.; Arisawa, M.; Shimizu, M.; Morita, N. Inhibition of cow’s milk xanthine oxidase by flavonoids. J. Nat. Prod. 1988, 51, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Dose- and Time-Dependent Neuroprotective Effects of Pycnogenol® following Traumatic Brain Injury. J. Neurotrauma 2013, 30, 1542–1549. [Google Scholar] [CrossRef]
- Chung, L.Y. The Antioxidant Properties of Garlic Compounds: Allyl Cysteine, Alliin, Allicin, and Allyl Disulfide. J. Med. Food 2006, 9, 205–213. [Google Scholar] [CrossRef]
- Schwartz, I.F.; Hershkovitz, R.; Iaina, A.; Gnessin, E.; Wollman, Y.; Chernichowski, T.; Blum, M.; Levo, Y.; Schwartz, D. Garlic attenuates nitric oxide production in rat cardiac myocytes through inhibition of inducible nitric oxide synthase and the arginine transporter CAT-2 (cationic amino acid transporter-2). Clin. Sci. 2002, 102, 487–493. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.L.; Adenuga, G.A.; Sandhir, R. Selenium and zinc protect brain mitochondrial antioxidants and electron transport chain enzymes following postnatal protein malnutrition. Life Sci. 2016, 152, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J. Glutathione! Integr. Med. 2014, 13, 8–12. [Google Scholar]
- Korshunov, S.S.; Krasnikov, B.F.; O Pereverzev, M.; Skulachev, V.P. The antioxidant functions of cytochrome c. FEBS Lett. 1999, 462, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Pereverzev, M.O.; Vygodina, T.V.; Konstantinov, A.A.; Skulachev, V.P. Cytochrome c, an ideal antioxidant. Biochem. Soc. Trans. 2003, 31 Pt 6, 1312–1315. [Google Scholar] [CrossRef] [PubMed]
- Sokol, R.J.; McKim, J.M.; Goff, M.; Ruyle, S.Z.; Devereaux, M.W.; Han, D.; Packer, L.; Everson, G. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology 1998, 114, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Cuzzocrea, S.; Rebouças, J.S.; Ferrer-Sueta, G.; Mazzon, E.; Di Paola, R.; Radi, R.; Spasojević, I.; Benov, L.; Salvemini, D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: Comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two different models of oxidative stress injuries, SOD-specific E. coli model and carrageenan-induced pleurisy. Free. Radic. Biol. Med. 2009, 46, 192. [Google Scholar] [CrossRef]
- Zahmatkesh, M.; Kadkhodaee, M.; Moosavi, S.M.S.; Jorjani, M.; Kajbafzadeh, A.; Golestani, A.; Ghaznavi, R. Beneficial effects of MnTBAP, a broad-spectrum reactive species scavenger, in rat renal ischemia/reperfusion injury. Clin. Exp. Nephrol. 2005, 9, 212–218. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jang, H.-J.; Cho, W.-Y.; Yeon, S.-J.; Lee, C.-H. In vitro antioxidant actions of sulfur-containing amino acids. Arab. J. Chem. 2020, 13, 1678–1684. [Google Scholar] [CrossRef]
- Nandi, A.; Chatterjee, I.B. Scavenging of superoxide radical by ascorbic acid. J. Biosci. 1987, 11, 435–441. [Google Scholar] [CrossRef]
- E Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, S1119–S1124. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Slikker, W., Jr.; Ali, S.F. Role of Metallothionein and other Antioxidants in Scavenging Superoxide Radicals and their Possible Role in Neuroprotection. Neurochem. Int. 1996, 29, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.P.; Delles, C.; Schmidt, B.M.; Oehmer, S.; Schwarz, T.K.; Schmieder, R.E.; John, S. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am. J. Hypertens. 2005, 18, 1111–1117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taiwo, F.A. Mechanism of tiron as scavenger of superoxide ions and free electrons. Spectroscopy 2008, 22, 491–498. [Google Scholar] [CrossRef]
- Wright, V.P.; Klawitter, P.F.; Iscru, D.F.; Merola, A.J.; Clanton, T.L. Superoxide scavengers augment contractile but not energetic responses to hypoxia in rat diaphragm. J. Appl. Physiol. 2005, 98, 1753–1760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pfeiffer, S.; Leopold, E.; Hemmens, B.; Schmidt, K.; Werner, E.R.; Mayer, B. Interference of Carboxy-PTIO with Nitric Oxide- and Peroxynitrite-Mediated Reactions. Free. Radic. Biol. Med. 1997, 22, 787–794. [Google Scholar] [CrossRef]
- Lilley, E.; Gibson, A. Antioxidant protection of NO-induced relaxations of the mouse anococcygeus against inhibition by superoxide anions, hydroquinone and carboxy-PTIO. Br. J. Pharmacol. 1996, 119, 432–438. [Google Scholar] [CrossRef]
- Yang, J.; Su, Y.; Richmond, A. Antioxidants tiron and N-acetyl-L-cysteine differentially mediate apoptosis in melanoma cells via a reactive oxygen species-independent NF-kappaB pathway. Free Radic. Biol. Med. 2007, 42, 1369–1380. [Google Scholar] [CrossRef]
- Hill, R.L.; Singh, I.N.; Wang, J.A.; Kulbe, J.R.; Hall, E.D. Protective effects of phenelzine administration on synaptic and non-synaptic cortical mitochondrial function and lipid peroxidation-mediated oxidative damage following TBI in young adult male rats. Exp. Neurol. 2020, 330, 113322. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Porteous, C.M.; Coulter, C.V.; Murphy, M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999, 263, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M. Endothelial mitochondria and heart disease. Cardiovasc. Res. 2010, 88, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta (BBA)—Bioenerg. 2008, 1777, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Smith, R.A. Targeting Antioxidants to Mitochondria by Conjugation to Lipophilic Cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cochemé, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, H.; Shen, R.; Fang, J.; Yang, Y.; Dai, W.; Zhu, Y.; Zhou, M. Mitochondrial-targeted antioxidant MitoQ provides neuroprotection and reduces neuronal apoptosis in experimental traumatic brain injury possibly via the Nrf2-ARE pathway. Am. J. Transl. Res. 2018, 10, 1887–1899. [Google Scholar] [PubMed]
- Tabet, M.; El-Kurdi, M.; Haidar, M.A.; Nasrallah, L.; Reslan, M.A.; Shear, D.; Pandya, J.D.; El-Yazbi, A.F.; Sabra, M.; Mondello, S.; et al. Mitoquinone supplementation alleviates oxidative stress and pathologic outcomes following repetitive mild traumatic brain injury at a chronic time point. Exp. Neurol. 2022, 351, 113987. [Google Scholar] [CrossRef] [PubMed]
- Liberman, E.A.; Topaly, V.P.; Tsofina, L.M.; Jasaitis, A.A.; Skulachev, V.P. Mechanism of Coupling of Oxidative Phosphorylation and the Membrane Potential of Mitochondria. Nature 1969, 222, 1076–1078. [Google Scholar] [CrossRef]
- Skulachev, V.P.; Vyssokikh, M.Y.; Chernyak, B.V.; Averina, O.A.; Andreev-Andrievskiy, A.A.; Zinovkin, R.A.; Lyamzaev, K.G.; Marey, M.V.; Egorov, M.V.; Frolova, O.J.; et al. Mitochondrion-targeted antioxidant SkQ1 prevents rapid animal death caused by highly diverse shocks. Sci. Rep. 2023, 13, 4326. [Google Scholar] [CrossRef]
- Amemiya, S.; Kamiya, T.; Nito, C.; Inaba, T.; Kato, K.; Ueda, M.; Shimazaki, K.; Katayama, Y. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur. J. Pharmacol. 2005, 516, 125–130. [Google Scholar] [CrossRef]
- Toyoda, K.; Fujii, K.; Kamouchi, M.; Nakane, H.; Arihiro, S.; Okada, Y.; Ibayashi, S.; Iida, M. Free radical scavenger, edaravone, in stroke with internal carotid artery occlusion. J. Neurol. Sci. 2004, 221, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Yuki, S.; Kogure, K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke 1988, 19, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yuki, S.; Watanabe, T.; Mitsuka, M.; Saito, K.-I.; Kogure, K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997, 762, 240–242. [Google Scholar] [CrossRef]
- Homma, T.; Kobayashi, S.; Sato, H.; Fujii, J. Edaravone, a free radical scavenger, protects against ferroptotic cell death in vitro. Exp. Cell Res. 2019, 384, 111592. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.J.; Yasuhara, T.; Shingo, T.; Muraoka, K.; Agari, T.; Kameda, M.; Uozumi, T.; Tajiri, N.; Morimoto, T.; Jing, M.; et al. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008, 9, 75. [Google Scholar] [CrossRef]
- Jiao, S.-S.; Yao, X.-Q.; Liu, Y.-H.; Wang, Q.-H.; Zeng, F.; Lu, J.-J.; Liu, J.; Zhu, C.; Shen, L.-L.; Liu, C.-H.; et al. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc. Natl. Acad. Sci. USA 2015, 112, 5225–5230. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Akishita, M.; Nagai, K.; Yu, W.; Hasegawa, H.; Eto, M.; Kozaki, K.; Toba, K. Potent free radical scavenger, edaravone, suppresses oxidative stress-induced endothelial damage and early atherosclerosis. Atherosclerosis 2007, 191, 281–289. [Google Scholar] [CrossRef]
- Higashi, Y.; Jitsuiki, D.; Chayama, K.; Yoshizumi, M. Edaravone (3-Methyl-1-Phenyl-2-Pyrazolin-5-one), A Novel Free Radical Scavenger, for Treatment of Cardiovascular Diseases. Recent Pat. Cardiovasc. Drug Discov. 2006, 1, 85–93. [Google Scholar] [CrossRef]
- Shetty, S.; Anushree, U.; Kumar, R.; Bharati, S. Mitochondria-targeted antioxidant, mito-TEMPO mitigates initiation phase of N-Nitrosodiethylamine-induced hepatocarcinogenesis. Mitochondrion 2021, 58, 123–130. [Google Scholar] [CrossRef]
- Nhu, N.T.; Xiao, S.-Y.; Liu, Y.; Kumar, V.B.; Cui, Z.-Y.; Lee, S.-D. Neuroprotective Effects of a Small Mitochondrially-Targeted Tetrapeptide Elamipretide in Neurodegeneration. Front. Integr. Neurosci. 2021, 15, 747901. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-P.; Waldbaum, S.; Rowley, S.; Huang, T.-T.; Day, B.J.; Patel, M. Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: Attenuation by a lipophilic metalloporphyrin. Neurobiol. Dis. 2012, 45, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.L.; Purvis, R.S.; Griffith, J.W. Comparison of Neuroprotective Effects Induced by α-Phenyl-N-tert-butyl nitrone (PBN) and N-tert-Butyl-α-(2 sulfophenyl) nitrone (S-PBN) in Lithium-Pilocarpine Status Epilepticus. NeuroToxicology 2005, 26, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Deletraz, A.; Zéamari, K.; Hua, K.; Combes, M.; Villamena, F.A.; Tuccio, B.; Callizot, N.; Durand, G. Substituted α-phenyl and α-naphthlyl-N-tert-butyl nitrones: Synthesis, spin-trapping, and neuroprotection evaluation. J. Org. Chem. 2020, 85, 6073–6085. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, B.; Diez-Iriepa, D.; Merás-Sáiz, B.; Chioua, M.; García-Vieira, D.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Martínez-Murillo, R.; Gonzàlez-Nieto, D.; et al. Synthesis, antioxidant properties and neuroprotection of α-phenyl-tert-butylnitrone derived HomoBisNitrones in in vitro and in vivo ischemia models. Sci. Rep. 2020, 10, 14150. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Yakoub, K.M.; Caruso, G.; Lazzarino, G.; Signoretti, S.; Barbey, A.K.; Tavazzi, B.; Lazzarino, G.; Belli, A.; Amorini, A.M. Antioxidant Therapies in Traumatic Brain Injury. Antioxidants 2020, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.K.; Vemuganti, R. Antioxidant therapies in traumatic brain injury. Neurochem. Int. 2022, 152, 105255. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Hergenroeder, G.W.; Jeter, C.B.; Choi, H.A.; Kobori, N.; Moore, A.N. Traumatic Brain Injury Alters Methionine Metabolism: Implications for Pathophysiology. Front. Syst. Neurosci. 2016, 10, 36. [Google Scholar] [CrossRef]
- Sonthalia, S.; Daulatabad, D.; Sarkar, R. Glutathione as a skin whitening agent: Facts, myths, evidence and controversies. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 262–272. [Google Scholar] [CrossRef]
- Holmay, M.J.B.; Terpstra, M.; Coles, L.D.; Mishra, U.; Ahlskog, M.B.; Öz, G.; Cloyd, J.C.; Tuite, P.J. N-acetylcysteine Boosts Brain and Blood Glutathione in Gaucher and Parkinson Diseases. Clin. Neuropharmacol. 2013, 36, 103–106. [Google Scholar] [CrossRef]
- Alkandari, A.F.; Madhyastha, S.; Rao, M.S. N-Acetylcysteine Amide against Aβ-Induced Alzheimer’s-like Pathology in Rats. Int. J. Mol. Sci. 2023, 24, 12733. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.D.; Readnower, R.D.; Patel, S.P.; Yonutas, H.M.; Pauly, J.R.; Goldstein, G.A.; Rabchevsky, A.G.; Sullivan, P.G. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol. 2014, 257, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Sullivan, P.G.; Pandya, J.D.; Goldstein, G.A.; VanRooyen, J.L.; Yonutas, H.M.; Eldahan, K.C.; Morehouse, J.; Magnuson, D.S.; Rabchevsky, A.G. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp. Neurol. 2014, 257, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Malhi, G.S.; Gray, L.J.; Dean, O.M. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol. Sci. 2013, 34, 167–177. [Google Scholar] [CrossRef]
- Baki, S.G.A.; Schwab, B.; Haber, M.; Fenton, A.A.; Bergold, P.J. Minocycline Synergizes with N-Acetylcysteine and Improves Cognition and Memory Following Traumatic Brain Injury in Rats. PLoS ONE 2010, 5, e12490. [Google Scholar] [CrossRef]
- Hicdonmez, T.; Kanter, M.; Tiryaki, M.; Parsak, T.; Cobanoglu, S. Neuroprotective Effects of N-acetylcysteine on Experimental Closed Head Trauma in Rats. Neurochem. Res. 2006, 31, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Thomale, U.-W.; Griebenow, M.; Kroppenstedt, S.-N.; Unterberg, A.W.; Stover, J.F. The effect of N-acetylcysteine on posttraumatic changes after controlled cortical impact in rats. Intensiv. Care Med. 2006, 32, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Peterson, P.; Lee, C. Effect of N-Acetylcysteine on Mitochondrial Function Following Traumatic Brain Injury in Rats. J. Neurotrauma 1999, 16, 1067–1082. [Google Scholar] [CrossRef]
- Thomale, U.W.; Griebenow, M.; Kroppenstedt, S.N.; Unterberg, A.W.; Stover, J.F. The antioxidant effect of N-acethylcysteine on experimental contusion in rats. Acta Neurochir. Suppl. 2005, 95, 429–431. [Google Scholar]
- Hagos, F.T.; Empey, P.E.; Wang, P.; Ma, X.; Poloyac, S.M.; Bayir, H.; Kochanek, P.M.; Bell, M.J.; Clark, R.S.B. Exploratory Application of Neuropharmacometabolomics in Severe Childhood Traumatic Brain Injury*. Crit. Care Med. 2018, 46, 1471–1479. [Google Scholar] [CrossRef]
- Clark, R.S.B.; Empey, P.E.; Bayır, H.; Rosario, B.L.; Poloyac, S.M.; Kochanek, P.M.; Nolin, T.D.; Au, A.K.; Horvat, C.M.; Wisniewski, S.R.; et al. Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children. PLoS ONE 2017, 12, e0180280. [Google Scholar] [CrossRef]
- Clark, R.S.B.; Empey, P.E.; Kochanek, P.M.; Bell, M.J. N-Acetylcysteine and Probenecid Adjuvant Therapy for Traumatic Brain Injury. Neurotherapeutics 2023, 20, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, M.E.; Balaban, C.; Slade, M.D.; Tsao, J.W.; Hoffer, B. Amelioration of Acute Sequelae of Blast Induced Mild Traumatic Brain Injury by N-Acetyl Cysteine: A Double-Blind, Placebo Controlled Study. PLoS ONE 2013, 8, e54163. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sekhon, B.; Jatana, M.; Giri, S.; Gilg, A.G.; Sekhon, C.; Singh, I.; Singh, A.K. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J. Neurosci. Res. 2004, 76, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.; Sekhon, C.; Khan, M.; Patel, S.J.; Singh, I.; Singh, A.K. N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res. 2003, 971, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Rosenbaum, Z.; Melamed, E.; Offen, D. Antioxidant Therapy in Acute Central Nervous System Injury: Current State. Pharmacol. Rev. 2002, 54, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Pahan, K.; Sheikh, F.G.; Namboodiri, A.M.; Singh, I. N-Acetyl Cysteine Inhibits Induction of No Production By Endotoxin or Cytokine Stimulated Rat Peritoneal Macrophages, C6 Glial Cells and Astrocytes. Free. Radic. Biol. Med. 1998, 24, 39–48. [Google Scholar] [CrossRef]
- Aitio, M.L. N-acetylcysteine—Passe-partout or much ado about nothing? Br. J. Clin. Pharmacol. 2006, 61, 5–15. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.D.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Chen, W.; Ercal, N.; Huynh, T.; Volkov, A.; Chusuei, C.C. Characterizing N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) binding for lead poisoning treatment. J. Colloid Interface Sci. 2012, 371, 144–149. [Google Scholar] [CrossRef]
- Atlas, D. Emerging therapeutic opportunities of novel thiol-amides, NAC-amide (AD4/NACA) and thioredoxin mimetics (TXM-Peptides) for neurodegenerative-related disorders. Free Radic. Biol. Med. 2021, 176, 120–141. [Google Scholar] [CrossRef] [PubMed]
- Offen, D.; Gilgun-Sherki, Y.; Barhum, Y.; Benhar, M.; Grinberg, L.; Reich, R.; Melamed, E.; Atlas, D. A low molecular weight copper chelator crosses the blood–brain barrier and attenuates experimental autoimmune encephalomyelitis. J. Neurochem. 2004, 89, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, L.; Fibach, E.; Amer, J.; Atlas, D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free. Radic. Biol. Med. 2005, 38, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Bahat-Stroomza, M.; Gilgun-Sherki, Y.; Offen, D.; Panet, H.; Saada, A.; Krool-Galron, N.; Barzilai, A.; Atlas, D.; Melamed, E. A novel thiol antioxidant that crosses the blood brain barrier protects dopaminergic neurons in experimental models of Parkinson’s disease. Eur. J. Neurosci. 2005, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.; Nascimento, B.; Akhtar, U.; Rhind, S.G.; Tien, H.; Nathens, A.; Da Luz, L.T. Systematic Review of Human and Animal Studies Examining the Efficacy and Safety of N-Acetylcysteine (NAC) and N-Acetylcysteine Amide (NACA) in Traumatic Brain Injury: Impact on Neurofunctional Outcome and Biomarkers of Oxidative Stress and Inflammation. Front. Neurol. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Kurano, T.; Kanazawa, T.; Iioka, S.; Kondo, H.; Kosuge, Y.; Suzuki, T. Intranasal Administration of N-acetyl-L-cysteine Combined with Cell-Penetrating Peptide-Modified Polymer Nanomicelles as a Potential Therapeutic Approach for Amyotrophic Lateral Sclerosis. Pharmaceutics 2022, 14, 2590. [Google Scholar] [CrossRef]
- Kannan, S.; Balakrishnan, B.; Nance, E.; Johnston, M.V.; Rangaramanujam, K. Nanomedicine in cerebral palsy. Int. J. Nanomed. 2013, 8, 4183–4195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nance, E.; Zhang, F.; Mishra, M.K.; Zhang, Z.; Kambhampati, S.P.; Kannan, R.M.; Kannan, S. Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials 2016, 101, 96–107. [Google Scholar] [CrossRef]
- Zhang, F.; Nance, E.; Alnasser, Y.; Kannan, R.; Kannan, S. Microglial migration and interactions with dendrimer nanoparticles are altered in the presence of neuroinflammation. J. Neuroinflamm. 2016, 13, 65. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Mishra, M.K.; Jyoti, A.; Balakrishnan, B.; Zhang, F.; Nance, E.; Romero, R.; Kannan, S.; Kannan, R.M. Biodistribution of Fluorescently Labeled PAMAM Dendrimers in Neonatal Rabbits: Effect of Neuroinflammation. Mol. Pharm. 2013, 10, 4560–4571. [Google Scholar] [CrossRef]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci. Transl. Med. 2012, 4, 130ra46. [Google Scholar] [CrossRef] [PubMed]
- Nance, E.; Porambo, M.; Zhang, F.; Mishra, M.K.; Buelow, M.; Getzenberg, R.; Johnston, M.; Kannan, R.M.; Fatemi, A.; Kannan, S. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J. Control. Release 2015, 214, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.K.; Beaty, C.A.; Lesniak, W.G.; Kambhampati, S.P.; Zhang, F.; Wilson, M.A.; Blue, M.E.; Troncoso, J.C.; Kannan, S.; Johnston, M.V.; et al. Dendrimer Brain Uptake and Targeted Therapy for Brain Injury in a Large Animal Model of Hypothermic Circulatory Arrest. ACS Nano 2014, 8, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- Modi, H.R.; Wang, Q.; Olmstead, S.J.; Khoury, E.S.; Sah, N.; Guo, Y.; Gharibani, P.; Sharma, R.; Kannan, R.M.; Kannan, S.; et al. Systemic administration of dendrimer N-acetyl cysteine improves outcomes and survival following cardiac arrest. Bioeng. Transl. Med. 2022, 7, e10259. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, S.P.; Bhutto, I.A.; Wu, T.; Ho, K.; McLeod, D.S.; Lutty, G.A.; Kannan, R.M. Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J. Control. Release 2021, 335, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A. S-Adenosyl Methionine (SAMe) for Depression in Adults. Issues Ment. Heal. Nurs. 2019, 40, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Chang, L.; Xiao, Y.; Liu, Q. S-Adenosyl-L-Methionine for the Treatment of Chronic Liver Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0122124. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shu, R.; Yu, C.; Fu, Z.; Li, Z. Mammalian AKT, the Emerging Roles on Mitochondrial Function in Diseases. Aging Dis. 2022, 13, 157–174. [Google Scholar] [CrossRef]
- Li, H.; Tang, Z.; Chu, P.; Song, Y.; Yang, Y.; Sun, B.; Niu, M.; Qaed, E.; Shopit, A.; Han, G.; et al. Neuroprotective effect of phosphocreatine on oxidative stress and mitochondrial dysfunction induced apoptosis in vitro and in vivo: Involvement of dual PI3K/Akt and Nrf2/HO-1 pathways. Free Radic. Biol. Med. 2018, 120, 228–238. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2–ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; D’mello, V.; Caruso, D.; Abdul-Muneer, P.M. Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J. Mol. Med. 2019, 97, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Muneer, P.M. Nrf2 as a Potential Therapeutic Target for Traumatic Brain Injury. J. Integr. Neurosci. 2023, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-G.; Yong, Y.-Y.; Pan, Y.-R.; Zhang, L.; Wu, J.-M.; Zhang, Y.; Tang, Y.; Wei, J.; Yu, L.; Law, B.Y.-K.; et al. Targeting Nrf2-Mediated Oxidative Stress Response in Traumatic Brain Injury: Therapeutic Perspectives of Phytochemicals. Oxidative Med. Cell. Longev. 2022, 2022, 1015791. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yang, B.; Wang, L.; Li, B.; Guo, X.; Zhang, M.; Jiang, Z.; Fu, J.; Pi, J.; Guan, D.; et al. Curcumin plays neuroprotective roles against traumatic brain injury partly via Nrf2 signaling. Toxicol. Appl. Pharmacol. 2018, 346, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, A.; Nanjundeshwari, G.; Swetha, M.; Swetha, R. A Review On Omaveloxolone. Int. J. Pharm. Sci. 2023, 1, 447–456. [Google Scholar] [CrossRef]
- Lynch, D.R.; Farmer, J.; Hauser, L.; Blair, I.A.; Wang, Q.Q.; Mesaros, C.; Snyder, N.; Boesch, S.; Chin, M.; Delatycki, M.B.; et al. Safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia. Ann. Clin. Transl. Neurol. 2019, 6, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.R.; Chin, M.P.; Delatycki, M.B.; Subramony, S.H.; Corti, M.; Hoyle, J.C.; Boesch, S.; Nachbauer, W.; Mariotti, C.; Mathews, K.D.; et al. Safety and Efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study). Ann. Neurol. 2021, 89, 212–225. [Google Scholar] [CrossRef]
- Abeti, R.; Baccaro, A.; Esteras, N.; Giunti, P. Novel Nrf2-Inducer Prevents Mitochondrial Defects and Oxidative Stress in Friedreich’s Ataxia Models. Front. Cell. Neurosci. 2018, 12, 188. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Carter, J.L. Multiple Sclerosis: Current and Emerging Disease-Modifying Therapies and Treatment Strategies. Mayo Clin. Proc. 2014, 89, 225–240. [Google Scholar] [CrossRef]
- Maldonado, P.P.; Guevara, C.; Olesen, M.A.; Orellana, J.A.; Quintanilla, R.A.; Ortiz, F.C. Neurodegeneration in Multiple Sclerosis: The Role of Nrf2-Dependent Pathways. Antioxidants 2022, 11, 1146. [Google Scholar] [CrossRef]
- Abd El-Fatah, I.M.; Abdelrazek, H.M.; Ibrahim, S.M.; Abdallah, D.M.; El-Abhar, H.S. Dimethyl fumarate abridged tauo-/amyloidopathy in a D-Galactose/ovariectomy-induced Alzheimer’s-like disease: Modulation of AMPK/SIRT-1, AKT/CREB/BDNF, AKT/GSK-3β, adiponectin/Adipo1R, and NF-κB/IL-1β/ROS trajectories. Neurochem. Int. 2021, 148, 105082. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; Manai, F.; Davinelli, S.; Tucci, P.; Saso, L.; Amadio, M. Novel potential pharmacological applications of dimethyl fumarate—An overview and update. Front. Pharmacol. 2023, 14, 1264842. [Google Scholar] [CrossRef] [PubMed]
- Kunze, R.; Urrutia, A.; Hoffmann, A.; Liu, H.; Helluy, X.; Pham, M.; Reischl, S.; Korff, T.; Marti, H.H. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood–brain barrier integrity. Exp. Neurol. 2015, 266, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Campolo, M.; Paterniti, I.; Lanza, M.; Filippone, A.; Cuzzocrea, S.; Esposito, E. Dimethyl Fumarate Attenuates Neuroinflammation and Neurobehavioral Deficits Induced by Experimental Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, G.; Zhang, J.; Ting, S.-M.; Gonzales, N.; Aronowski, J. Dimethyl Fumarate Protects Brain From Damage Produced by Intracerebral Hemorrhage by Mechanism Involving Nrf2. Stroke 2015, 46, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Krämer, T.; Grob, T.; Menzel, L.; Hirnet, T.; Griemert, E.; Radyushkin, K.; Thal, S.C.; Methner, A.; Schaefer, M.K.E. Dimethyl fumarate treatment after traumatic brain injury prevents depletion of antioxidative brain glutathione and confers neuroprotection. J. Neurochem. 2017, 143, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Arnold, D.L.; Bar-Or, A.; Fox, R.J.; Kappos, L.; Mokliatchouk, O.; Jiang, X.; Lyons, J.; Kapadia, S.; Miller, C. Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: Final ENDORSE study results. Mult. Scler. J. 2022, 28, 801–816. [Google Scholar] [CrossRef]
- Hayashi, G.; Jasoliya, M.; Sahdeo, S.; Saccà, F.; Pane, C.; Filla, A.; Marsili, A.; Puorro, G.; Lanzillo, R.; Brescia Morra, V.; et al. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum. Mol. Genet. 2017, 26, 2864–2873. [Google Scholar] [CrossRef]
- Majkutewicz, I. Dimethyl fumarate: A review of preclinical efficacy in models of neurodegenerative diseases. Eur. J. Pharmacol. 2022, 926, 175025. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [PubMed]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and neurodegenerative diseases. BioFactors 2013, 39, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017, 69 Pt B, 257–269. [Google Scholar] [CrossRef]
- Schepici, G.; Bramanti, P.; Mazzon, E. Efficacy of Sulforaphane in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8637. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Mishra, G.; Ilgün, S.; Samarghandian, S. New insights into the role of the Nrf2 signaling pathway in green tea catechin applications. Phytother. Res. 2021, 35, 3078–3112. [Google Scholar] [CrossRef]
- Afzal, O.; Dalhat, M.H.; Altamimi, A.S.A.; Rasool, R.; Alzarea, S.I.; Almalki, W.H.; Murtaza, B.N.; Iftikhar, S.; Nadeem, S.; Nadeem, M.S.; et al. Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits. Molecules 2022, 27, 7604. [Google Scholar] [CrossRef]
- Chiang, M.-C.; Tsai, T.-Y.; Wang, C.-J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Lakshmi, S. Role of Quercetin Benefits in Neurodegeneration. Adv. Neurobiol. 2016, 12, 229–245. [Google Scholar]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Zhang, D.D. Bardoxolone Brings Nrf2-Based Therapies to Light. Antioxidants Redox Signal. 2013, 19, 517–518. [Google Scholar] [CrossRef]
- Hisamichi, M.; Kamijo-Ikemori, A.; Sugaya, T.; Hoshino, S.; Kimura, K.; Shibagaki, Y. Role of bardoxolone methyl, a nuclear factor erythroid 2-related factor 2 activator, in aldosterone- and salt-induced renal injury. Hypertens. Res. 2018, 41, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; He, H.; Zuo, Z.; Xu, Z.; Wei, Z.; Deng, J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell. Mol. Biol. Lett. 2019, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; Galtieri, A.; Russo, A.; Giardina, B.; Ficarra, S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2015, 2015, 392169. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a Therapeutic Agent for Neurodegenerative Diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef]
- Sönmez, Ü.; Sönmez, A.; Erbil, G.; Tekmen, I.; Baykara, B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci. Lett. 2007, 420, 133–137. [Google Scholar] [CrossRef]
- Singleton, R.H.; Yan, H.Q.; Fellows-Mayle, W.; Dixon, C.E. Resveratrol Attenuates Behavioral Impairments and Reduces Cortical and Hippocampal Loss in a Rat Controlled Cortical Impact Model of Traumatic Brain Injury. J. Neurotrauma 2010, 27, 1091–1099. [Google Scholar] [CrossRef]
- Salberg, S.; Yamakawa, G.; Christensen, J.; Kolb, B.; Mychasiuk, R. Assessment of a nutritional supplement containing resveratrol, prebiotic fiber, and omega-3 fatty acids for the prevention and treatment of mild traumatic brain injury in rats. Neuroscience 2017, 365, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Cong, P.; Wang, T.; Tong, C.; Liu, Y.; Shi, L.; Mao, S.; Shi, X.; Jin, H.; Liu, Y.; Hou, M. Resveratrol ameliorates thoracic blast exposure-induced inflammation, endoplasmic reticulum stress and apoptosis in the brain through the Nrf2/Keap1 and NF-κB signaling pathway. Injury 2021, 52, 2795–2802. [Google Scholar] [CrossRef] [PubMed]
- Ateş, O.; Çayli, S.; Altinoz, E.; Gurses, I.; Yucel, N.; Sener, M.; Kocak, A.; Yologlu, S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol. Cell. Biochem. 2007, 294, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Z.; Shen, R.; Zhong, W.; Zheng, H.; Chen, Z.; Tang, J.; Zhu, J. Resveratrol Improves Mitochondrial Biogenesis Function and Activates PGC-1α Pathway in a Preclinical Model of Early Brain Injury Following Subarachnoid Hemorrhage. Front. Mol. Biosci. 2021, 8, 620683. [Google Scholar] [CrossRef]
- Jiang, W.; Li, S.; Li, X. Therapeutic potential of berberine against neurodegenerative diseases. Sci. China Life Sci. 2015, 58, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Javadipour, M.; Rezaei, M.; Keshtzar, E.; Khodayar, M.J. Metformin in contrast to berberine reversed arsenic-induced oxidative stress in mitochondria from rat pancreas probably via Sirt3-dependent pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22368. [Google Scholar] [CrossRef] [PubMed]
- Rotermund, C.; Machetanz, G.; Fitzgerald, J.C. The Therapeutic Potential of Metformin in Neurodegenerative Diseases. Front. Endocrinol. 2018, 9, 400. [Google Scholar] [CrossRef]
- Wang, Y.; An, H.; Liu, T.; Qin, C.; Sesaki, H.; Guo, S.; Radovick, S.; Hussain, M.; Maheshwari, A.; Wondisford, F.E.; et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019, 29, 1511–1523.e5. [Google Scholar] [CrossRef]
- Fanoudi, S.; Hosseini, M.; Alavi, M.S.; Boroushaki, M.T.; Hosseini, A.; Sadeghnia, H.R. Everolimus, a mammalian target of rapamycin inhibitor, ameliorated streptozotocin-induced learning and memory deficits via neurochemical alterations in male rats. EXCLI J. 2018, 17, 999–1017. [Google Scholar] [CrossRef]
- Shiau, J.-P.; Chuang, Y.-T.; Cheng, Y.-B.; Tang, J.-Y.; Hou, M.-F.; Yen, C.-Y.; Chang, H.-W. Impacts of Oxidative Stress and PI3K/AKT/mTOR on Metabolism and the Future Direction of Investigating Fucoidan-Modulated Metabolism. Antioxidants 2022, 11, 911. [Google Scholar] [CrossRef]
- Stein, D.G. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015, 29, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidou, C.; Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002, 1, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K. Unique challenges in clinical trials in traumatic brain injury. Crit. Care Med. 2009, 37, S129–S135. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Roozenbeek, B.; Manley, G.T. Clinical Trials in Traumatic Brain Injury: Past Experience and Current Developments. Neurotherapeutics 2010, 7, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.; Hicks, R. Combination therapies for traumatic brain injury: Prospective considerations. J. Neurotrauma 2009, 26, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.G.; Narayan, R.K.; Li, C. Multi-Mechanistic Approaches to the Treatment of Traumatic Brain Injury: A Review. J. Clin. Med. 2023, 12, 2179. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, M.R.; Przekwas, A.J.; Gupta, R.K. Combination Therapy for Multi-Target Manipulation of Secondary Brain Injury Mechanisms. Curr. Neuropharmacol. 2018, 16, 484–504. [Google Scholar] [CrossRef]
- Arun, P.; Ariyannur, P.S.; Moffett, J.R.; Xing, G.; Hamilton, K.; Grunberg, N.E.; Ives, J.A.; Namboodiri, A.M. Metabolic Acetate Therapy for the Treatment of Traumatic Brain Injury. J. Neurotrauma 2010, 27, 293–298. [Google Scholar] [CrossRef]
- Scafidi, S.; Racz, J.; Hazelton, J.; McKenna, M.C.; Fiskum, G. Neuroprotection by Acetyl-L-Carnitine after Traumatic Injury to the Immature Rat Brain. Dev. Neurosci. 2010, 32, 480–487. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rassu, G.; Gavini, E.; Sorrenti, M.; Catenacci, L.; Giunchedi, P. Nose-to-Brain Delivery of Antioxidants as a Potential Tool for the Therapy of Neurological Diseases. Pharmaceutics 2020, 12, 1246. [Google Scholar] [CrossRef]
- Pandya, J.D.; Musyaju, S.; Modi, H.R.; Okada-Rising, S.L.; Bailey, Z.S.; Scultetus, A.H.; Shear, D.A. Intranasal delivery of mitochondria targeted neuroprotective compounds for traumatic brain injury: Screening based on pharmacological and physiological properties. J. Transl. Med. 2024, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, A.; Reddi, S.; Thakker-Varia, S.; Alder, J. Status of precision medicine approaches to traumatic brain injury. Neural Regen. Res. 2022, 17, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.S.; Schuster, J.M.; Smith, D.H.; Stein, S.C. Cost-Effectiveness of Biomarker Screening for Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.W.; Pandya, J.D.; Shear, D.A. Gut Microbiota as a Therapeutic Target to Ameliorate the Biochemical, Neuroanatomical, and Behavioral Effects of Traumatic Brain Injuries. Front. Neurol. 2019, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.S.; Buell, T.; Robertson, C.S. Systemic manifestations of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 205–218. [Google Scholar] [PubMed]
- Taraskina, A.; Ignatyeva, O.; Lisovaya, D.; Ivanov, M.; Ivanova, L.; Golovicheva, V.; Baydakova, G.; Silachev, D.; Popkov, V.; Ivanets, T.; et al. Effects of Traumatic Brain Injury on the Gut Microbiota Composition and Serum Amino Acid Profile in Rats. Cells 2022, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.S.; Grandhi, R.; Patterson, T.T.; Nicholson, S.E. A Review of Traumatic Brain Injury and the Gut Microbiome: Insights into Novel Mechanisms of Secondary Brain Injury and Promising Targets for Neuroprotection. Brain Sci. 2018, 8, 113. [Google Scholar] [CrossRef]
- Celorrio, M.; Abellanas, M.A.; Rhodes, J.; Goodwin, V.; Moritz, J.; Vadivelu, S.; Wang, L.; Rodgers, R.; Xiao, S.; Anabayan, I.; et al. Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol. Commun. 2021, 9, 40. [Google Scholar] [CrossRef]
- Ullah, H.; Arbab, S.; Tian, Y.; Liu, C.-Q.; Chen, Y.; Li, Q.; Khan, M.I.U.; Hassan, I.U.; Li, K. The gut microbiota–brain axis in neurological disorder. Front. Neurosci. 2023, 17, 1225875. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Microbes, Immunity, and Behavior: Psychoneuroimmunology Meets the Microbiome. Neuropsychopharmacology 2017, 42, 178–192. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Gangwar, S.K.; Devanarayanan, T.N.; Hegde, M.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A. Interplay of dietary antioxidants and gut microbiome in human health: What has been learnt thus far? J. Funct. Foods 2023, 100, 105365. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants 2021, 10, 1563. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
| ROS Scavengers | Properties and Mechanisms of Action |
|---|---|
| Enzymatic ROS scavengers | |
| Superoxide dismutase (SOD) | Enzyme. Converts superoxide radicals into oxygen and H2O2. |
| Catalase (CAT) | Enzyme in the peroxisomes. Neutralizes H2O2 in water. |
| Glutathione peroxidase (GPx) Thioredoxin system: Thioredoxin (Trx), Peroxiredoxin (Prx), Thioredoxin reductase (TrxR) | Thiol-dependent enzymatic antioxidants. Neutralize H2O2 and are recycled by nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. |
| Non-enzymatic ROS scavengers | |
| Vitamin A (retinol) or carotenoids | Fat-soluble antioxidant. Donates electrons to neutralize free radicals. |
| Vitamin E (tocopherols and tocotrienols) | Fat-soluble antioxidant. Scavenges lipid peroxyl radicals. |
| Vitamin C (ascorbic acid) | Water-soluble antioxidant. Donates electrons to neutralize free radicals. Scavenge superoxide. |
| Carotenoids | Found in various fruits and vegetables. Of the ~600 types of carotenoids, some can synthesize Vitamin A. Neutralizers of ROS. |
| Polyphenols | Ubiquitously present in fruits and vegetables. Free-radical scavenger. |
| Flavonoids | Phytochemicals present in plants, fruits, and vegetables. Scavengers of ROS. |
| Pycnogenol (PYC) | Combination of bioflavonoids with robust capacity to scavenge free radicals. |
| Alliin | Found in both natural and synthetic compounds. A bioactive compound derived from garlic. Superoxide scavenger. |
| Allicin | Synthesized from alliin. Inhibits superoxide, nitric oxide (NO) and hydroxyl radicals. |
| Minerals (copper, zinc and selenium, magnesium) | Precursors to antioxidants that help regulate free radicals. |
| Coenzyme Q10 (CoQ10), coenzyme Q (CoQ) | Lipid antioxidant. Essential component of the ETC. Protects cells from oxidative damage. |
| Glutathione | Tripeptide. Detoxifies ROS. Maintains redox balance. |
| NADPH | NADPH, as a cofactor independently and with redoxins, plays a crucial role in ROS detoxification. |
| Cytochrome C | Endogenous heme protein located in mitochondria. Oxidized cytochrome C is able to scavenge superoxide radicals. |
| ROS Scavengers and Detoxifiers | Properties and Mechanisms of Action |
|---|---|
| Non-targeted compounds | |
| MnTBAP | O2•− scavenger. Possesses SOD- and catalase-like activity. Also scavenges ONOO−. |
| Cysteine | Amino acid. O2•− scavenger. |
| Tiron | Reduced and oxidized Tiron species. Reacts with O2•− radical. |
| Carboxy-PTIO | Specific NO scavenger. Reacts with O2•− radical. |
| Phenelzine | FDA-approved drug. MAO inhibitor. Aldehyde-scavenging properties partially protect against oxidative damage. |
| Mitochondria-targeted compounds | |
| MitoVit-E | Vitamin E attached to TPP. Reduces mitochondrial oxidative damage. |
| MitoQ | CoQ10 derivative linked with TPP. Scavenges mitochondrial ROS. |
| Plastoquinone (SkQ1) | Targeted antioxidant. Scavenges mitochondrial ROS. |
| Edaravone | Used clinically as a neuroprotective compound. Reduces oxidative damage and lipid peroxidation. |
| Mito TEMPOL | Cell permeable, stable nitroxide. SOD mimetic. |
| Elamipretide (SS-31) | Cationic tetrapeptide freely permeable to the mitochondria. Reduces the production of toxic ROS. |
| Cerium oxide nanoparticles (Nano-CeO2) | Cerium atoms linked by oxygen atoms. Scavengers of ROS. |
| Metalloporphyrins | Manganese and iron complexes. Synthetic catalytic antioxidants that mimic the body’s own antioxidant enzymes. |
| Phenyl-tert-butylnitrone (PBN) | Nitroxide radical. ROS-scavenging properties. |
| Glutathione precursors | |
| NAC | A cysteine prodrug. Replenishes intracellular glutathione level. |
| NACA | N-acetyl cysteine (NAC) analog.Glutathione precursor. |
| D-NAC | Dendrimer-tagged NAC. Serves as a prodrug to synthesize glutathione. |
| S-adenosyl methionine (SAMe) | SAMe is processed stepwise into cysteine synthesis, and ultimately synthesize glutathione. |
| Pathway Modulators | Properties and Mechanisms of Action |
|---|---|
| Nrf2 activators | |
| Omaveloxolone (RTA-408) | Synthetic compound. FDA-approved for the treatment of FA. Prevents Nrf2 degradation. |
| Dimethyl fumarate (DMF) | Synthetic compound. Activates the Nrf2 pathway and AKT pathway. |
| Curcumin | Derived from turmeric. Activates the Nrf2 pathway. |
| Sulforaphane | Naturally found in cruciferous vegetables. Activates Nrf2 by inhibiting Keap1. |
| Epigallocatechin gallate (EGCG) | Abundant in green tea. Activates the Nrf2 pathway and has antioxidant and anti-inflammatory properties. |
| Quercetin | Present in various fruits, vegetables and grains. Activates Nrf2 and SIRT1. |
| Oltipraz | Synthetic compound. Activates Nrf2 by modifying Keap1. |
| Bardoxolone methyl | Synthetic compound. Activates the Nrf2 pathway. |
| SIRT1, PGC-1α, and mTOR modulators | |
| Resveratrol | Natural polyphenol compound. Most-relevant SIRT1 and mTOR modulator, AKT activator, Nrf2 activator and PGC-1α activator. |
| Naringenin | Natural citrus flavonoid. Modulates SIRT1. |
| SRT2104 | Synthetic compound. SIRT1 activator. |
| 1,4-dihydropyridine derivative | Synthetic compound. SIRT1 activator. |
| Naphthofuran derivative | Synthetic compound. SIRT1 activator. |
| Bisarylaniline derivative | New synthetic analog. SIRT1 activator. |
| Berberine | Small molecule isolated from various plants, mainly used in Chinese traditional medicine. PGC-1α activator. |
| Metformin | Anti-diabetic drug. Activator of AMPK, which further regulates PGC-1α. |
| Rapamycin/Sirolimus | Bacterial origin natural product. mTOR inhibitor and increases antioxidant defense. |
| Everolimus | Newly developed mTOR inhibitor. Rapamycin analog. |
| Temsirolimus | Newly developed mTOR inhibitor. Rapamycin analog. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modi, H.R.; Musyaju, S.; Ratcliffe, M.; Shear, D.A.; Scultetus, A.H.; Pandya, J.D. Mitochondria-Targeted Antioxidant Therapeutics for Traumatic Brain Injury. Antioxidants 2024, 13, 303. https://doi.org/10.3390/antiox13030303
Modi HR, Musyaju S, Ratcliffe M, Shear DA, Scultetus AH, Pandya JD. Mitochondria-Targeted Antioxidant Therapeutics for Traumatic Brain Injury. Antioxidants. 2024; 13(3):303. https://doi.org/10.3390/antiox13030303
Chicago/Turabian StyleModi, Hiren R., Sudeep Musyaju, Meaghan Ratcliffe, Deborah A. Shear, Anke H. Scultetus, and Jignesh D. Pandya. 2024. "Mitochondria-Targeted Antioxidant Therapeutics for Traumatic Brain Injury" Antioxidants 13, no. 3: 303. https://doi.org/10.3390/antiox13030303
APA StyleModi, H. R., Musyaju, S., Ratcliffe, M., Shear, D. A., Scultetus, A. H., & Pandya, J. D. (2024). Mitochondria-Targeted Antioxidant Therapeutics for Traumatic Brain Injury. Antioxidants, 13(3), 303. https://doi.org/10.3390/antiox13030303






