Dietary Polyphenols Support Akkermansia muciniphila Growth via Mediation of the Gastrointestinal Redox Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Identification of Relationships between Luminal ROS, Metabolic Health and the Gut Microbiome
2.2.1. Animals and Protocols
2.2.2. Analysis of Microbial DNA
2.3. Influence of Dietary Supplementation of GI ROS and A. muciniphila Bloom
2.3.1. Animals and Protocols
2.3.2. Evaluation of Metabolic Status
2.3.3. Near-Infrared Fluorescence Imaging of GI ROS
2.3.4. Fecal Antioxidant Activity
2.3.5. Extraction and Quantification of Microbial DNA
2.4. Statistical Analysis
3. Results
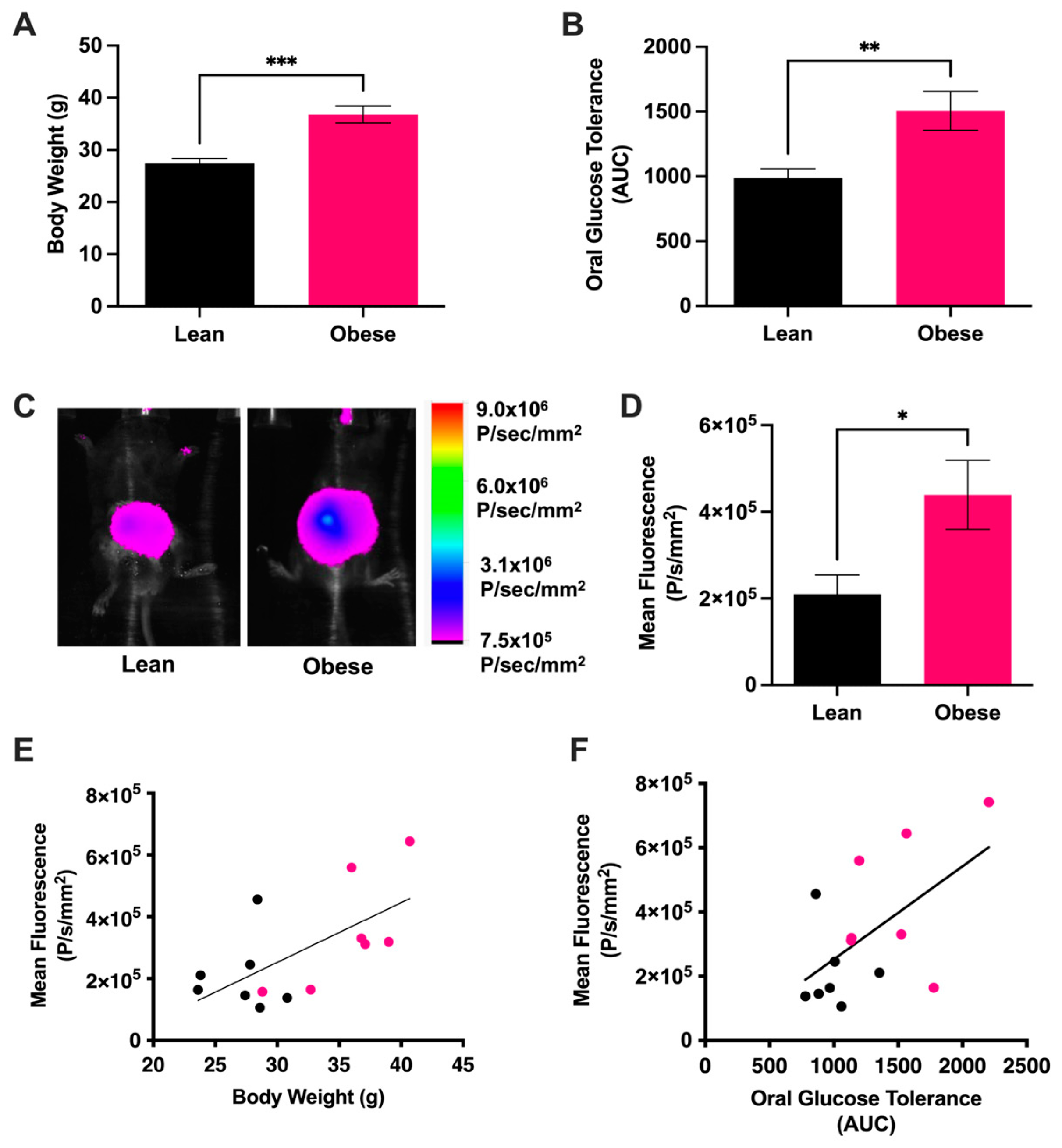
3.1. Intraluminal Redox Status Strongly Correlates with Metabolic Status
3.2. Mouse Phenotype Influences Microbial Community Structure
3.3. Level of GI ROS, OGT Is Inversely Correlated with Relative Abundance of the Beneficial Anaerobe Akkermansia muciniphila
3.4. Physical and Chemical Properties of Antioxidants Influence Intraluminal ROS and Akkermansia muciniphila Bloom
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, 160287. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic Syndrome: A Closer Look at the Growing Epidemic and Its Associated Pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Ruiz-Leon, A.; Sierra-Perez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Su, L.; Wang, J.; Duan, X.; Jiang, X. Fruit and Vegetable Consumption and Risk of the Metabolic Syndrome: A Meta-Analysis. Public Health Nutr. 2018, 21, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxid. Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed]
- John, G.K.; Mullin, G.E. The Gut Microbiome and Obesity. Curr. Oncol. Rep. 2016, 18, 2–8. [Google Scholar] [CrossRef]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and Malnutrition in Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kurnia, P.; Henning, S.M.; Lee, R.; Huang, J.; Garcia, M.C.; Surampudi, V.; Heber, D.; Li, Z. Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study. Nutrients 2021, 13, 3965. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia Muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Anhe, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with Dietary Polyphenols: A New Weapon to Combat the Metabolic Syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia Muciniphila: Paradigm for next Generation Beneficial Microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. ROS in Gastrointestinal Inflammation: Rescue or Sabotage? Br. J. Pharmacol. 2017, 174, 1704–1718. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Intestinal Redox Biology and Oxidative Stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef]
- Albenberg, L.; Esipova, T.; Judge, C.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.; Lewis, J.; Li, H.; et al. Correlation Between Intraluminal Oxygen Gradient and Radial Partitioning of Intestinal Microbiota in Humans and Mice. Gastroenterology 2014, 147, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Kalariya, H.M.; Poulev, A.; Ribnicky, D.M.; Jaja-Chimedza, A.; Roopchand, D.E. Grape Polyphenols Reduce Gut-Localized Reactive Oxygen Species Associated with the Development of Metabolic Syndrome in Mice. PLoS ONE 2018, 13, e0198716. [Google Scholar] [CrossRef] [PubMed]
- Kundu, K.; Knight, S.F.; Willett, N.; Lee, S.; Taylor, W.R.; Murthy, N. Hydrocyanines: A Class of Fluorescent Sensors That Can Image Reactive Oxygen Species in Cell Culture, Tissue, and in Vivo. Angew. Chem. Int. Ed. 2009, 48, 299–303. [Google Scholar] [CrossRef]
- Van Buiten, C.B.; Wu, G.; Lam, Y.Y.; Zhao, L.; Raskin, I. Elemental Iron Modifies the Redox Environment of the Gastrointestinal Tract: A Novel Therapeutic Target and Test for Metabolic Syndrome. Free Radic. Biol. Med. 2021, 168, 203–213. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 54670067, Ascorbic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ascorbic-Acid (accessed on 4 January 2024).
- Chungchunlam, S.M.S.; Moughan, P.J. Comparative Bioavailability of Vitamins in Human Foods Sourced from Animals and Plants. Crit. Rev. Food Sci. Nutr. 2023, 1–36. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 5280489, Beta-Carotene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Beta-Carotene (accessed on 4 January 2024).
- PubChem Compound Summary for CID 122738, Procyanidin B2, (+)−. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Procyanidin-B2 (accessed on 4 January 2024).
- Stoupi, S.; Williamson, G.; Viton, F.; Barron, D.; King, L.J.; Brown, J.E.; Clifford, M.N. In Vivo Bioavailability, Absorption, Excretion, and Pharmacokinetics of [14C]Procyanidin B2 in Male Rats. Drug Metab. Dispos. Biol. Fate Chem. 2010, 38, 287–291. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.; Rodrigo-García, J.; Martínez-Ruiz, N.; Cárdenas-Robles, A.; Mendoza-Díaz, S.; Álvarez-Parrilla, E.; González-Aguilar, G.; De La Rosa, L.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human Metabolism and Elimination of the Anthocyanin, Cyanidin-3-Glucoside: A (13)C-Tracer Study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 5280343, Quercetin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Quercetin (accessed on 4 January 2024).
- Li, H.; Li, M.; Fu, J.; Ao, H.; Wang, W.; Wang, X. Enhancement of Oral Bioavailability of Quercetin by Metabolic Inhibitory Nanosuspensions Compared to Conventional Nanosuspensions. Drug Deliv. 2021, 28, 1226–1236. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 445154, Resveratrol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/445154 (accessed on 4 January 2024).
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Sadlowski, C.M.; Maity, S.; Kundu, K.; Murthy, N. Hydrocyanines: A Versatile Family of Probes for Imaging Radical Oxidants in Vitro and in Vivo. Mol. Syst. Eng. 2017, 2, 191–200. [Google Scholar] [CrossRef]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Paynef, M.J.; Reed, J. Multi-Laboratory Validation of a Standard Method for Quantifying Proanthocyanidins in Cranberry Powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Honarbakhsh, M.; Ericsson, A.; Zhong, G.; Isoherranen, N.; Chengseng, Z.; Bromberg, Y.; Van Buiten, C.; Malta, K.; Joseph, L.; Vetriani, C.; et al. Impact of Vitamin A Transport and Storage on Intestinal Retinoid Homeostasis and Functions. J. Lipid Res. 2021, 62, 100046. [Google Scholar] [CrossRef]
- Carmody, R.; Gerber, G.; Leuvano, J.J.; Gatti, D.; Somes, L.; Svenson, K.; Turnbaugh, P. Diet Dominates Host Genotype in Shaping the Murine Gut Microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 26 February 2024).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 26 February 2024).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Carmody, R.N.; Kalariya, H.M.; Duran, R.M.; Moskal, K.; Poulev, A.; Kuhn, P.; Tveter, K.M.; Turnbaugh, P.J.; Raskin, I.; et al. Grape Proanthocyanidin-Induced Intestinal Bloom of Akkermansia Muciniphila Is Dependent on Its Baseline Abundance and Precedes Activation of Host Genes Related to Metabolic Health. J. Nutr. Biochem. 2018, 56, 142–151. [Google Scholar] [CrossRef]
- Million, M.; Raoult, D. Linking Gut Redox to Human Microbiome. Hum. Microbiome J. 2018, 10, 27–32. [Google Scholar] [CrossRef]
- Nallathambi, R.; Poulev, A.; Zuk, J.B.; Raskin, I. Proanthocyanidin-Rich Grape Seed Extract Reduces Inflammation and Oxidative Stress and Restores Tight Junction Barrier Function in Caco-2 Colon Cells. Nutrients 2020, 12, 1623. [Google Scholar] [CrossRef]
- Tamura, T.; Ozawa, M.; Tanaka, N.; Arai, S.; Mura, K. Bacillus Cereus Response to a Proanthocyanidin Trimer, a Transcriptional and Functional Analysis. Curr. Microbiol. 2016, 73, 115–123. [Google Scholar] [CrossRef]
- Morrison, K.E.; Jašarević, E.; Howard, C.D.; Bale, T.L. It’s the Fiber, Not the Fat: Significant Effects of Dietary Challenge on the Gut Microbiome. Microbiome 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric Obesity Is Associated with an Altered Gut Microbiota and Discordant Shifts in Firmicutes Populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef]
- Ecklu-Mensah, G.; Choo-Kang, C.; Maseng, M.G.; Donato, S.; Bovet, P.; Viswanathan, B.; Bedu-Addo, K.; Plange-Rhule, J.; Oti Boateng, P.; Forrester, T.E.; et al. Gut Microbiota and Fecal Short Chain Fatty Acids Differ with Adiposity and Country of Origin: The METS-Microbiome Study. Nat. Commun. 2023, 14, 5160. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Heber, D. Dietary Pomegranate Extract and Inulin Affect Gut Microbiome Differentially in Mice Fed an Obesogenic Diet. Anaerobe 2017, 48, 184–193. [Google Scholar] [CrossRef]
- Harrison, C.A.; Laubitz, D.; Ohland, C.L.; Midura-Kiela, M.T.; Patil, K.; Besselsen, D.G.; Jamwal, D.R.; Jobin, C.; Ghishan, F.K.; Kiela, P.R. Microbial Dysbiosis Associated with Impaired Intestinal Na+/H+ Exchange Accelerates and Exacerbates Colitis in Ex-Germ Free Mice. Mucosal Immunol. 2018, 11, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zhu, H.; Chen, J.; Kwek, E.; He, Z.; Liu, J.; Ma, N.; Ma, K.Y.; Chen, Z.-Y. Wild Melon Seed Oil Reduces Plasma Cholesterol and Modulates Gut Microbiota in Hypercholesterolemic Hamsters. J. Agric. Food Chem. 2020, 68, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Silva, S.; Falony, G.; Belda, E.; Nielsen, T.; Aron-Wisnewsky, J.; Chakaroun, R.; Forslund, S.K.; Assmann, K.; Valles-Colomer, M.; Nguyen, T.T.D.; et al. Statin Therapy Is Associated with Lower Prevalence of Gut Microbiota Dysbiosis. Nature 2020, 581, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Zhang, C.; Zhao, L.; Li, X.; Yu, Y.; Zhou, Y.; Xue, Y. Lactobacillus and Allobaculum Mediates the Improvement of Vascular Endothelial Dysfunction during Hypertension with TaohongSiwu Decoction Combined with Dubosiella Newyorkensis. Heliyon 2023, 9, e22572. [Google Scholar] [CrossRef]
- Liu, T.H.; Wang, J.; Zhang, C.Y.; Zhao, L.; Sheng, Y.Y.; Tao, G.S.; Xue, Y.Z. Gut Microbial Characteristical Comparison Reveals Potential Anti-Aging Function of Dubosiella Newyorkensis in Mice. Front. Endocrinol. 2023, 14, 1133167. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2016, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.X.; Jiang, X.M.; Wang, H.W.; Ge, L.; Lai, Y.T.; Jiang, X.Y.; Chen, F.; Huang, P.P. Probiotic Supplements Alleviate Gestational Diabetes Mellitus by Restoring the Diversity of Gut Microbiota: A Study Based on 16S rRNA Sequencing. J. Microbiol. 2021, 59, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, S.; Zhang, T.; Wang, W.; Li, J.; Chen, Y.Q.; Zhu, S. long Akkermansia Muciniphila Supplementation Improves Glucose Tolerance in Intestinal Ffar4 Knockout Mice during the Daily Light to Dark Transition. mSystems 2023, 8, e0057323. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.L.; Dong, X.; Moraes, A.C.F.; Zielke, R.A.; Fernandes, G.R.; Peremyslova, E.; Vasquez-Perez, S.; Schoenborn, A.A.; Gomes, E.P.; Pereira, A.C.; et al. Akkermansia Muciniphila Mediates Negative Effects of IFNγ on Glucose Metabolism. Nat. Commun. 2016, 7, 13329. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia Muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The Gut Microbiota: A Key Factor in the Therapeutic Effects of (Poly)Phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Atanassova, M.; Martorell, M.; Sharopov, F.; Atanassov, L.; Kumar, P.; Sharifi-Rad, J.; Tejada-Gavela, S.; Iriti, M.; Pezzani, R.; Varoni, E.M. Cocoa as Immunomodulatory Agent: An Update. Nat. Prod. Res. 2023, 1–12. [Google Scholar] [CrossRef]
- Crompton, D.W.; Shrimpton, D.H.; Silver, I.A. Measurements of the Oxygen Tension in the Lumen of the Small Intestine of the Domestic Duck. J. Exp. Biol. 1965, 43, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Lind Due, V.; Bonde, J.; Kann, T.; Perner, A. Extremely Low Oxygen Tension in the Rectal Lumen of Normal Human Subjects. Acta Anaesthesiol. Scand. 2003, 47, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Brune, A.; Emerson, D.; Breznak, J.A. The Termite Gut Microflora as an Oxygen Sink: Microelectrode Determination of Oxygen and pH Gradients in Guts of Lower and Higher Termites. Appl. Environ. Microbiol. 1995, 61, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Goda, F.; Liu, K.J.; Walczak, T.; O’Hara, J.A.; Jiang, J.; Swartz, H.M. In Vivo Oximetry Using EPR and India Ink. Magn. Reson. Med. 1995, 33, 237–245. [Google Scholar] [CrossRef]



| Antioxidant Compound | LogP | Bioavailability |
|---|---|---|
| Ascorbic Acid | −1.6 [19] | 76% 1 [20] |
| β-carotene | 13.5 [21] | 15.6% 1 [20] |
| Grape Pomace Extract | ||
| Procyanidin B2 | 2.4 [22] | 8–11% 2 [23] |
| Cyanidin-3-O-glucoside | 0.39 [24] | 12.4% 1 [25] |
| Quercetin | 1.5 [26] | 3.6% 2 [27] |
| Resveratrol | 3.1 [28] | <1% 1 [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Buiten, C.B.; Seitz, V.A.; Metcalf, J.L.; Raskin, I. Dietary Polyphenols Support Akkermansia muciniphila Growth via Mediation of the Gastrointestinal Redox Environment. Antioxidants 2024, 13, 304. https://doi.org/10.3390/antiox13030304
Van Buiten CB, Seitz VA, Metcalf JL, Raskin I. Dietary Polyphenols Support Akkermansia muciniphila Growth via Mediation of the Gastrointestinal Redox Environment. Antioxidants. 2024; 13(3):304. https://doi.org/10.3390/antiox13030304
Chicago/Turabian StyleVan Buiten, Charlene B., Valerie A. Seitz, Jessica L. Metcalf, and Ilya Raskin. 2024. "Dietary Polyphenols Support Akkermansia muciniphila Growth via Mediation of the Gastrointestinal Redox Environment" Antioxidants 13, no. 3: 304. https://doi.org/10.3390/antiox13030304
APA StyleVan Buiten, C. B., Seitz, V. A., Metcalf, J. L., & Raskin, I. (2024). Dietary Polyphenols Support Akkermansia muciniphila Growth via Mediation of the Gastrointestinal Redox Environment. Antioxidants, 13(3), 304. https://doi.org/10.3390/antiox13030304







