DPPH Measurements and Structure—Activity Relationship Studies on the Antioxidant Capacity of Phenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Synthesis
2.2. DPPH Radical Scavenging Activity Measurements
2.3. Food Component Dataset
2.4. Calculations
3. Results and Discussion
3.1. Results of DPPH Measurements
3.2. Relationships between the TEACs of DPPH and the BDE, IP, PDE, PA, and ETE
3.3. Comparison with the Literature Data
3.4. SAR of DPPH
3.4.1. Electron-Donating and Electron-Withdrawing Groups
3.4.2. Number and Position of Aromatic Attribute Hydroxy Groups
3.4.3. Phenols with Amino and Mercapto Groups
3.4.4. Effect of Dimerization on DPPH Activity
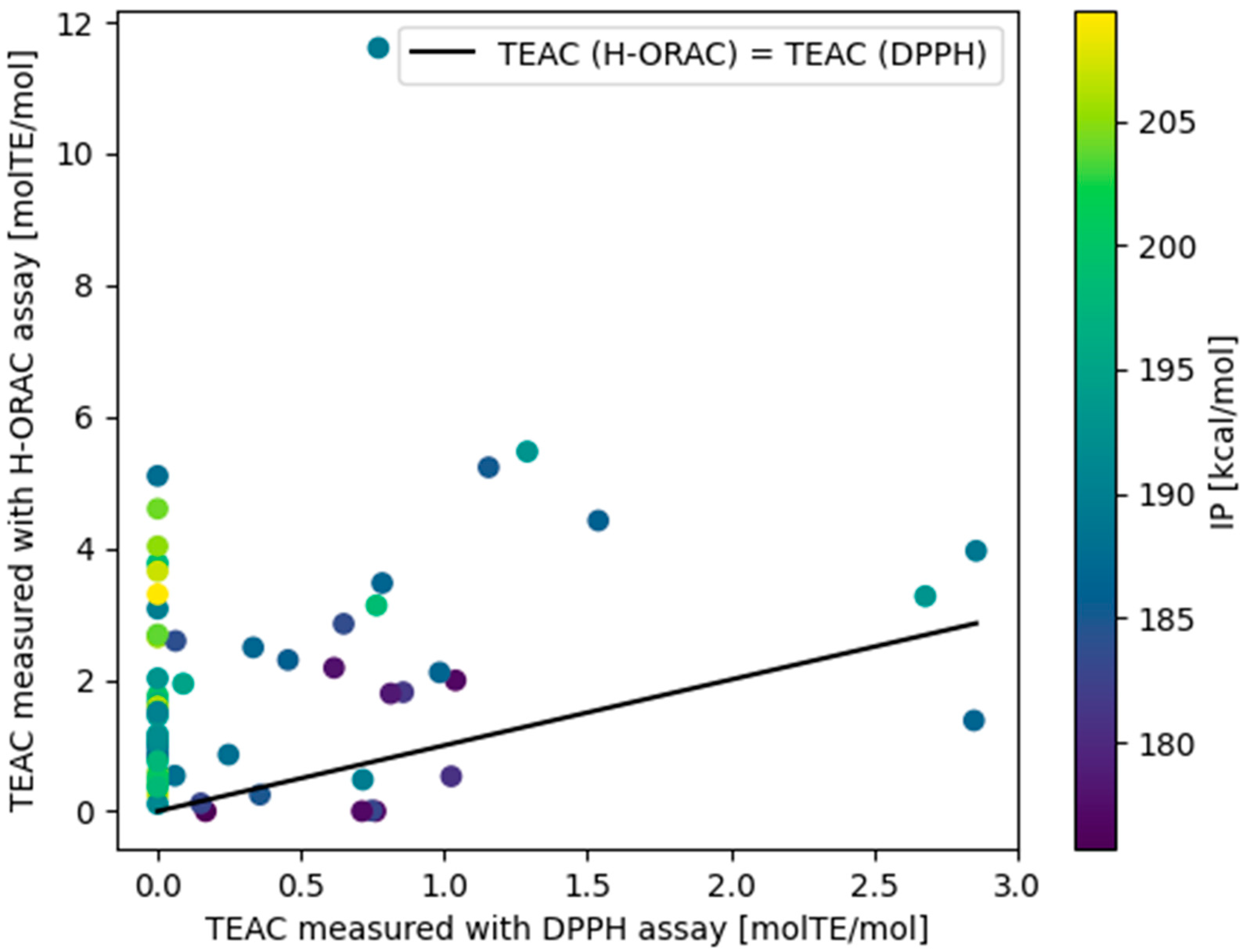
3.5. Comparison of H-ORAC and DPPH Assay Results
3.6. Comparison with FooDB Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant therapy in oxidative stress-induced neurodegenerative diseases: Role of nanoparticle-based drug delivery systems in clinical translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Kashino, I.; Mizoue, T.; Serafini, M.; Akter, S.; Sawada, N.; Ishihara, J.; Kotemori, A.; Inoue, M.; Yamaji, T.; Goto, A.; et al. Higher dietary non-enzymatic antioxidant capacity is associated with decreased risk of all-cause and cardiovascular disease mortality in Japanese adults. J. Nutr. 2019, 149, 1967–1976. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Iannone, M.; Ovidi, E.; Vitalini, S.; Laghezza Masci, V.; Marianelli, A.; Iriti, M.; Tiezzi, A.; Garzoli, S.M. From hops to craft beers: Production process, VOCs profile characterization, total polyphenol and flavonoid content determination and antioxidant activity evaluation. Processes 2022, 10, 517. [Google Scholar] [CrossRef]
- Takebayashi, J.; Oki, T.; Watanabe, J.; Yamasaki, K.; Chen, J.; Sato-Furukawa, M.; Tsubota-Utsugi, M.; Taku, K.; Goto, K.; Matsumoto, T.; et al. Hydrophilic antioxidant capacities of vegetables and fruits commonly consumed in Japan and estimated average daily intake of hydrophilic antioxidants from these foods. J. Food Compos. Anal. 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Kruzselyi, D.; Ott, P.G.; Moricz, A.M. Exploring antioxidant potential in two basidiomycetous mushrooms using high-performance thin-layer chromatography–DPPH–videodensitometry. J. Planar Chromatogr.—Mod. TLC 2023. [Google Scholar] [CrossRef]
- Wang, F.; Tan, J.; Jiang, R.; Li, F.; Zheng, R.; Yu, L.; Luo, L.; Zheng, Y.F. DPPH radical scavenging activity of new phenolics from the fermentation broth of mushroom Morehella importuna. Molecules 2023, 28, 4760. [Google Scholar] [CrossRef] [PubMed]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Mazzone, G.; Malaj, N.; Galano, A.; Russo, N.; Toscano, M. Antioxidant properties of several coumarin-chalcone hybrids from theoretical insights. RSC Adv. 2015, 5, 565–575. [Google Scholar] [CrossRef]
- Fujimoto, T.; Gotoh, H. Prediction and chemical interpretation of singlet-oxygen-scavenging activity of small molecule compounds by using machine learning. Antioxidants 2021, 10, 1751. [Google Scholar] [CrossRef]
- Fujimoto, T.; Gotoh, H. Feature selection for the interpretation of antioxidant mechanisms in plant phenolics. Molecules 2023, 28, 1454. [Google Scholar] [CrossRef] [PubMed]
- Ordoudi, S.A.; Tsimidou, M.Z.; Vafiadis, A.P.; Bakalbassis, E.G. Structure-DPPH• scavenging activity relationships: Parallel study of catechol and guaiacol acid derivatives. J. Agric. Food Chem. 2006, 54, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Laguna, O.; Durand, E.; Baréa, B.; Dauguet, S.; Fine, F.; Villeneuve, P.; Lecomte, J. Synthesis and evaluation of antioxidant activities of novel hydroxyalkyl esters and bis-aryl esters based on sinapic and caffeic acids. J. Agric. Food Chem. 2020, 68, 9308–9318. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.; Slavova-Kazakova, A.; Fabbri, D.; Angelova, S.; Dettori, M.A.; Nechev, J.; Delogu, G. Antiradical and antioxidant activities of new natural-like hydroxylated biphenyls of dehydrozingerone, zingerone and ferulic acid. C. R. Acad. Bulg. Sci. 2013, 66, 361–368. [Google Scholar] [CrossRef]
- Ogata, M.; Hoshi, M.; Urano, S.; Endo, T. Antioxidant activity of eugenol and related monomeric and dimeric compounds. Chem. Pharm. Bull. 2000, 48, 1467–1469. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- FooDB. Available online: https://foodb.ca/ (accessed on 16 February 2023).
- Naveja, J.J.; Rico-Hidalgo, M.P.; Medina-Franco, J.L. Analysis of a large food chemical database: Chemical space, diversity, and complexity. F1000Research 2018, 7, 993. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Sugimoto, N.; Akiyama, H.; Ukeda, H. Applicability of the DPPH assay for evaluating the antioxidant capacity of food additives—Inter-laboratory evaluation study. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef]
- DPPH Antioxidant Assay Kit. Available online: https://www.dojindo.com/JP-EN/products/D678/ (accessed on 11 January 2024).
- Shimamura, T.; Matsuura, R.; Tokuda, T.; Sugimoto, N.; Yamazaki, T.; Matsufuji, H.; Matsui, T.; Matsumoto, K.; Ukeda, H. Comparison of conventional antioxidants assays for evaluating potencies of natural antioxidants as food additives by collaborative study. Nippon Shokuhin Kagaku Kogaku Kaishi 2007, 54, 482–487. [Google Scholar] [CrossRef]
- Shimamura, T.; Ito, Y.; Kubo, Y.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Yamazaki, T.; Tada, A.; Sugimoto, N.; Akiyama, H.; et al. Relationship between catechin content and antioxidant capacity in natural food additive tea extract. Jpn. J. Food Chem. Saf. 2017, 24, 10–15. [Google Scholar]
- Muramatsu, D.; Uchiyama, H.; Higashi, H.; Kida, H.; Iwai, A. Effects of heat degradation of betanin in red beetroot (Beta vulgaris L.) on biological activity and antioxidant capacity. PLoS ONE 2023, 18, 0286255. [Google Scholar] [CrossRef]
- Sofian, F.F.; Kikuchi, N.; Koseki, T.; Kanno, Y.; Uesugi, S.; Shiono, Y.F. Antioxidant p-terphenyl compound, isolated from edible mushroom, Boletopsis leucomelas. Biosci. Biotechnol. Biochem. 2022, 86, 300–304. [Google Scholar] [CrossRef]
- Harunari, E.; Imada, C.; Igarashi, Y. Konamycins A and B and rubromycins CA1 and CA2, aromatic polyketides from the tunicate-derived streptomyces hyaluromycini MB-PO13T. J. Nat. Prod. 2019, 82, 1609–1615. [Google Scholar] [CrossRef]
- Ullah, A.; Sun, L.; Wang, F.F.; Nawaz, H.; Yamashita, K.; Cai, Y.; Anwar, F.; Khan, M.Q.; Mayakrishnan, G.; Kim, I.S. Eco-friendly bioactive β-caryophyllene/halloysite nanotubes loaded nanofibrous sheets for active food packaging. Food Packag. Shelf Life 2023, 35, 101028. [Google Scholar] [CrossRef]
- Kato, S.; Kuwata, K. Pro-/anti-oxidative properties of dopamine on membrane lipid peroxidation upon X-ray irradiation. Radiat. Phys. Chem. 2021, 185, 109518. [Google Scholar] [CrossRef]
- Sakurai, S.; Kikuchi, A.; Gotoh, H. Hydrophilic oxygen radical absorbance capacity values of low-molecular-weight phenolic compounds containing carbon, hydrogen, and oxygen. RSC Adv. 2022, 12, 4094–4100. [Google Scholar] [CrossRef]
- RDkit. Available online: https://www.rdkit.org/ (accessed on 11 January 2024).
- Riniker, S.; Landrum, G.A. Better informed distance geometry: Using what we know to improve conformation generation. J. Chem. Inf. Model. 2015, 55, 2562–2574. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Saito, S.; Kawabata, J. Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents. Tetrahedron 2005, 61, 8101–8108. [Google Scholar] [CrossRef]
- Takashima, M.; Shichiri, M.; Hagihara, Y.; Yoshida, Y.; Niki, E. Reactivity toward oxygen radicals and antioxidant action of thiol compounds. BioFactors 2012, 38, 240–248. [Google Scholar] [CrossRef]
- Bartmess, J.E. Thermodynamics of the electron and the proton. J. Phys. Chem. 1994, 98, 6420–6424. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Horton, W.; Peerannawar, S.; Török, B.; Török, M. Theoretical and experimental analysis of the antioxidant features of substituted phenol and aniline model compounds. Struct. Chem. 2019, 30, 23–35. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, Properties, and Applications of Hollow Micro-/Nanostructures. Chem. Rev. 2016, 11, 10983–11060. [Google Scholar] [CrossRef] [PubMed]
- Salum, M.L.; Robles, C.J.; Erra-Balsells, R. Photoisomerization of ionic liquid ammonium cinnamates: One-pot synthesis-isolation of Z-cinnamic acids. Org. Lett. 2010, 12, 4808–4811. [Google Scholar] [CrossRef]
- Wang, C.; Qian, C.; Roman, M.; Glasser, W.G.; Esker, A.R. Surface-initiated dehydrogenative polymerization of monolignols: A quartz crystal microbalance with dissipation monitoring and atomic force microscopy study. Biomacromolecules 2013, 14, 3964–3972. [Google Scholar] [CrossRef]
- Liu, J.; Ye, W.; Wang, S.; Zheng, J.; Tang, W.; Li, X. Synthesis of Lactams via Ir-Catalyzed C–H Amidation Involving Ir-Nitrene Intermediates. J. Org. Chem. 2020, 85, 4430–4440. [Google Scholar] [CrossRef]
- Yue, F.; Lu, F.; Regner, M.; Sun, R.; Ralph, J. Lignin-derived thioacidolysis dimers: Reevaluation, new products, authentication, and quantification. ChemSusChem 2017, 10, 830–835. [Google Scholar] [CrossRef]
- Li, J.; Liang, J.L.; Chan, P.W.H.; Che, C.M. Aziridination of alkenes with N-substituted hydrazines mediated by iodobenzene diacetate. Tetrahedron Lett. 2004, 45, 2685–2688. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; Barontini, M.; Tofani, D.; Balducci, V.; Gambacorta, A. Synthesis and Structure/Antioxidant Activity Relationship of Novel Catecholic Antioxidant Structural Analogues to Hydroxytyrosol and Its Lipophilic Esters. J. Agric. Food Chem. 2012, 60, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
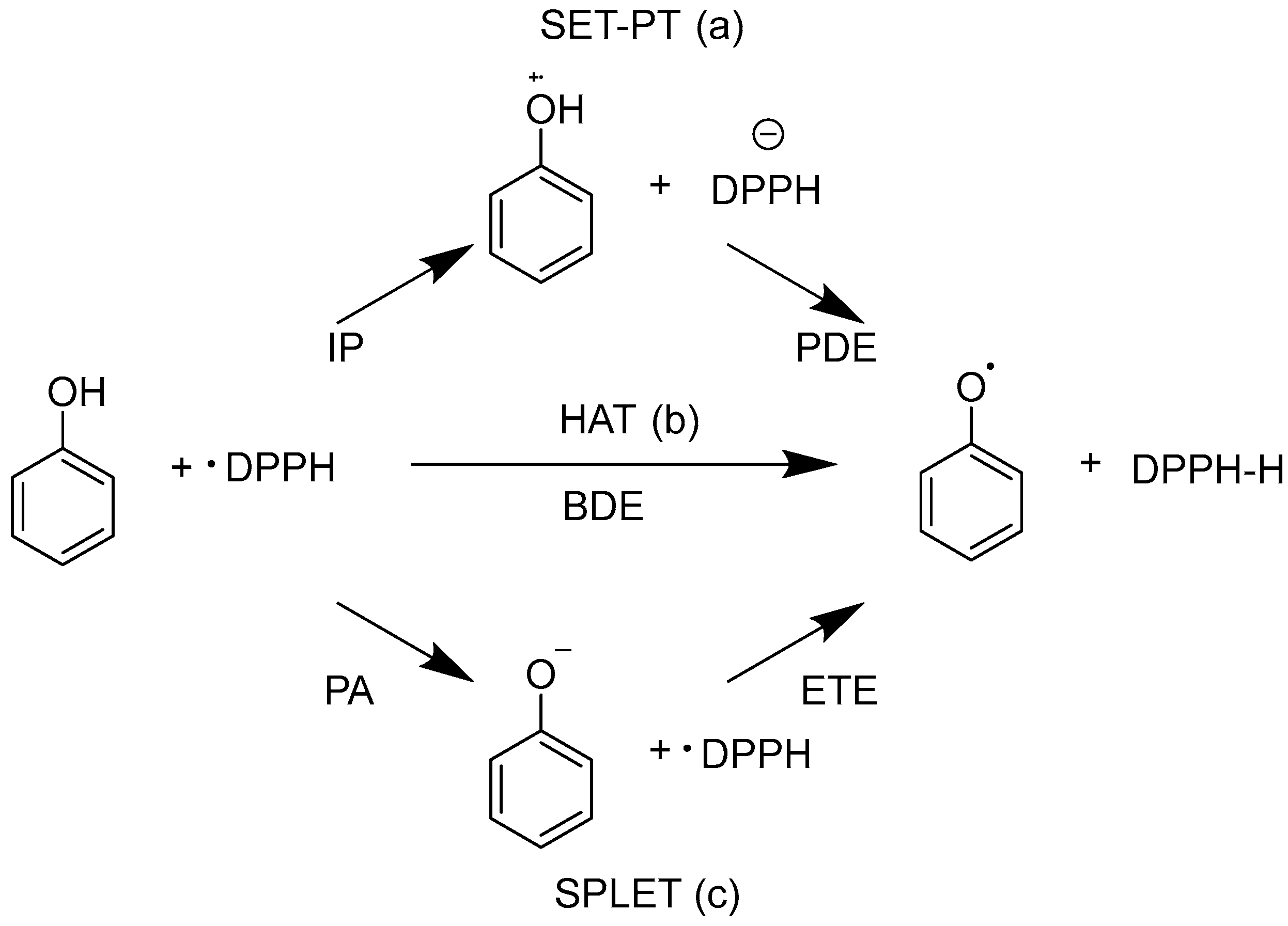
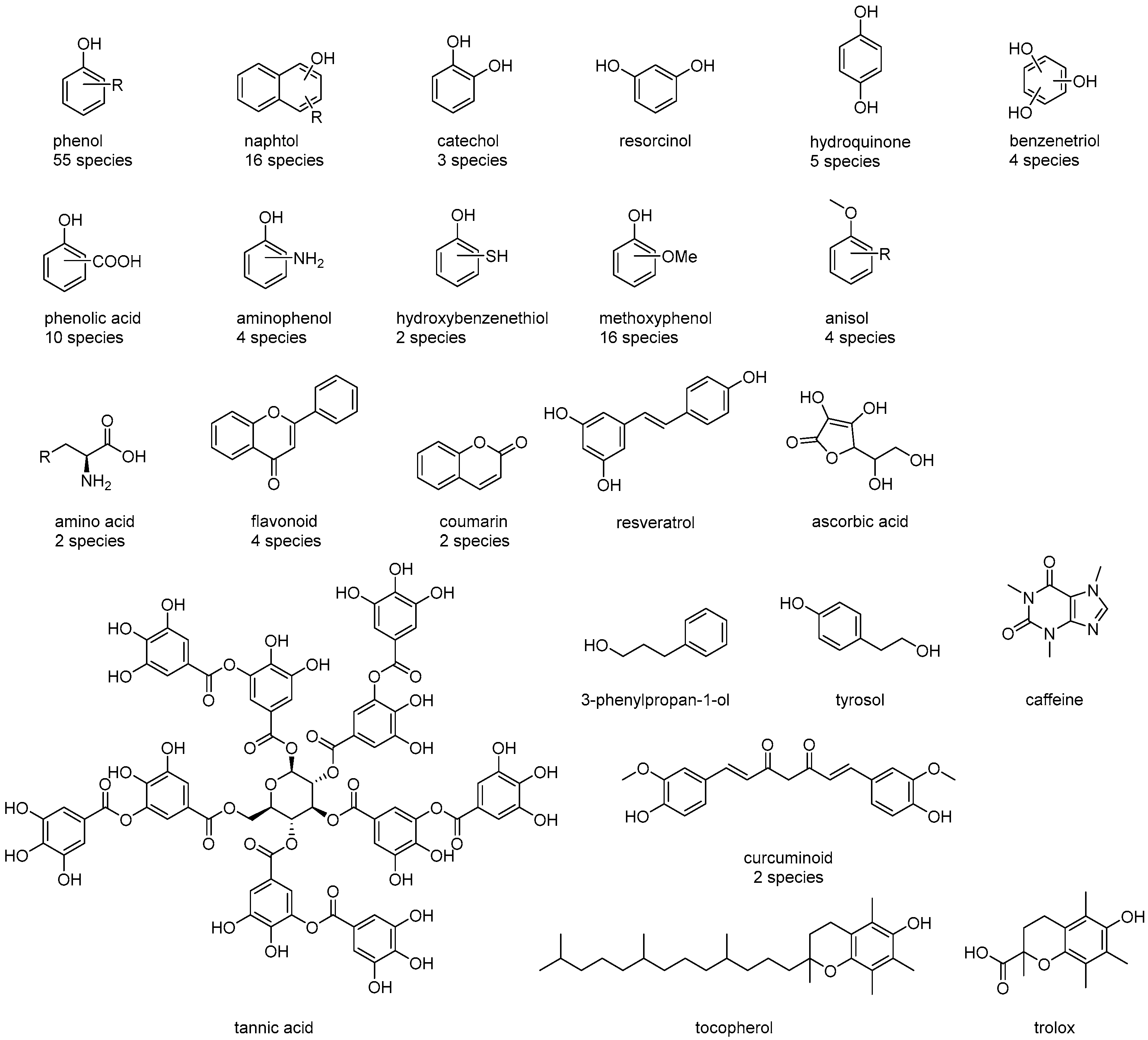

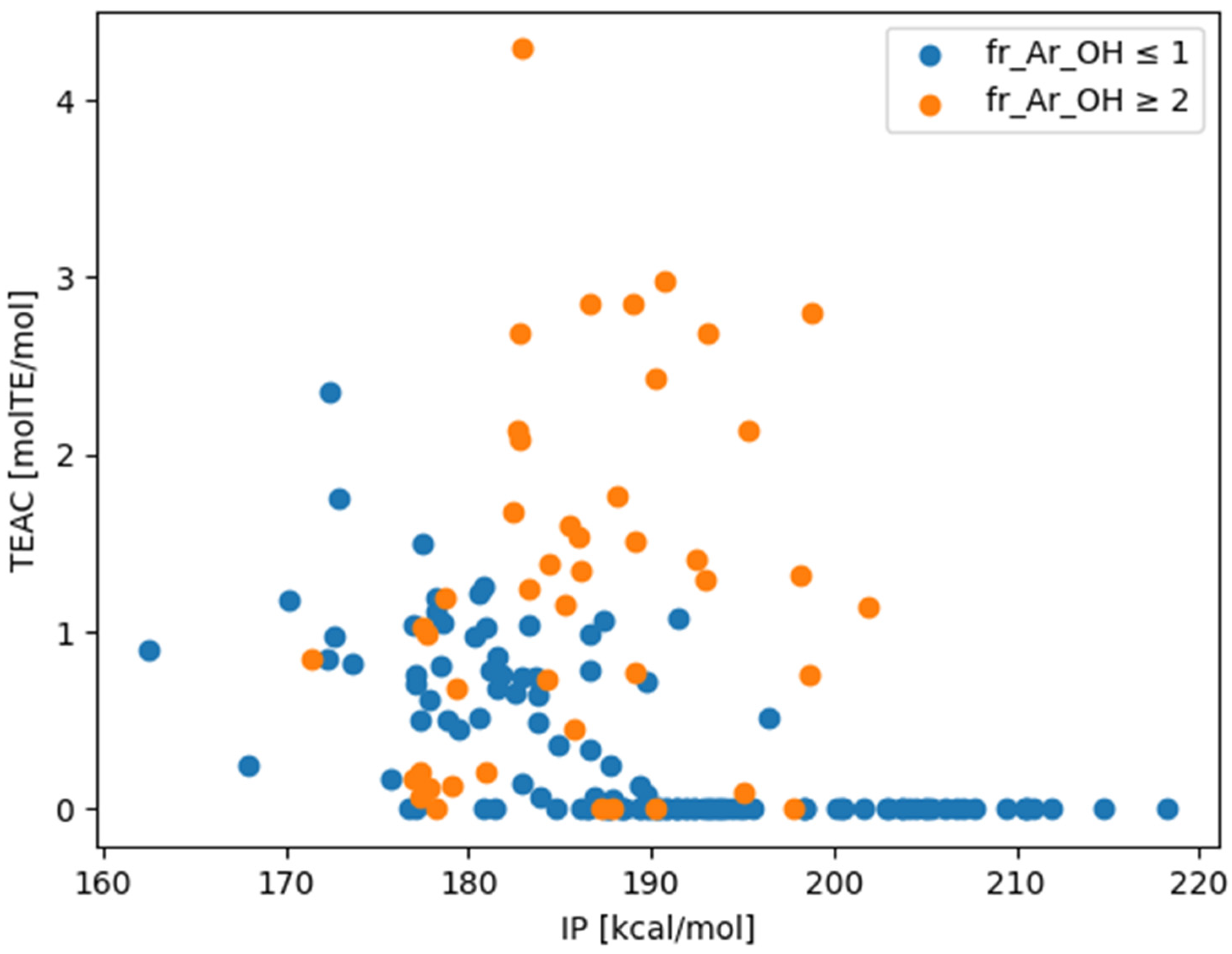
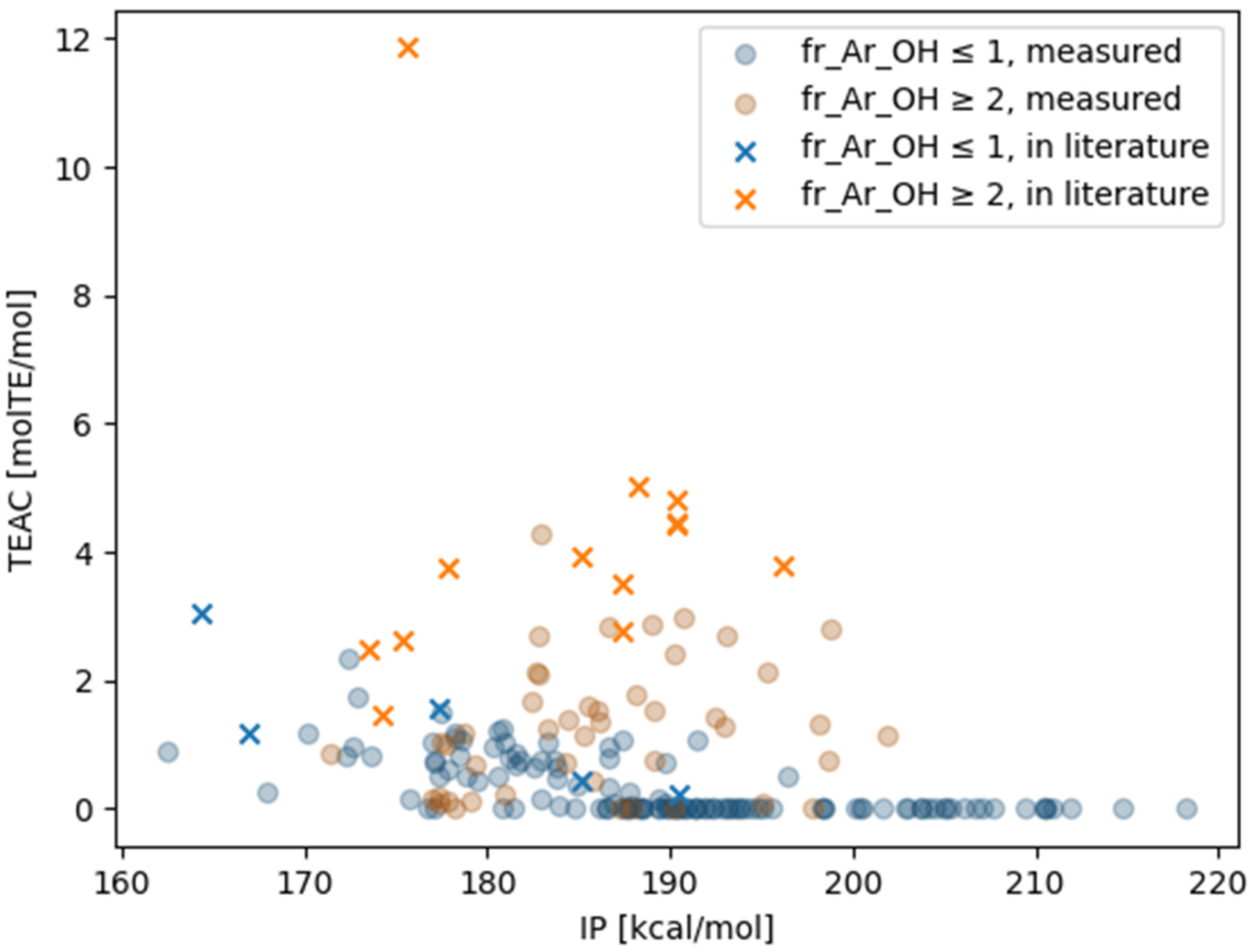

| Mean (fr_Ar_OH ≤ 1) | Std (fr_Ar_OH ≤ 1) | Mean (fr_Ar_OH ≥ 2) | Std (fr_Ar_OH ≥ 2) | |
|---|---|---|---|---|
| IP [kcal/mol] | 189.6 | 11.0 | 186.6 | 7.5 |
| BDE [kcal/mol] | 77.0 | 5.5 | 76.3 | 4.5 |
| PA [kcal/mol] | 272.9 | 10.8 | 266.5 | 7.4 |
| PDE [kcal/mol] | 143.4 | 9.4 | 145.8 | 7.1 |
| ETE [kcal/mol] | 60.2 | 12.2 | 65.9 | 9.2 |
| TEAC [molTE/mol] | 0.31 | 0.48 | 1.25 | 1.05 |
| Compound | TEAC [molTE/mol] (Measured) | Standard Deviation (Measured) | TEAC [molTE/mol] (Literature) | Ref. |
|---|---|---|---|---|
| L-ascorbic acid | 1.08 | 0.04 | 0.91 | [32] |
| Catechin | 2.43 | 0.07 | 3.08 | [30] |
| Catechin | 2.43 | 0.07 | 2.32 | [31] |
| Quercetin | 4.29 | 0.04 | 3.96 | [30] |
| Sesamol | 0.98 | 0.05 | 1.04 | [30] |
| Ferulic acid | 0.78 | 0.05 | 0.80 | [30] |
| Gallic acid | 2.81 | 0.07 | 3.07 | [30] |
| Morin | 0.99 | 0.12 | 1.33 | [30] |
| D-Alpha-tocopherol | 0.90 | 0.12 | 1.90 | [30] |
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | IUPAC Name (Common Name) | Category | X1 | X2 | X3 | X4 | X5 | TEAC [molTE/mol] | Standard Deviation |
| 0 | 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) | Trolox | - | - | - | - | - | 1.0 | - |
| 1 | 3-aminophenol | aminophenol | H | SH | H | H | H | 0.76 | 0.06 |
| 2 | 2-aminophenol | aminophenol | NH2 | H | H | H | H | 2.36 | 0.01 |
| 3 | 4-aminophenol | aminophenol | H | NH2 | H | H | H | 0.76 | 0.02 |
| 4 | benzene-1,2,4-triol | benzenetriol | OH | H | OH | H | H | 0.46 | 0.06 |
| 5 | benzene-1,2,3-triol | benzenetriol | OH | OH | H | H | H | 2.85 | 0.12 |
| 6 | 3,4,5-trihydroxybenzoic acid (gallic acid) | benzenetriol | OH | H | COOH | H | OH | 2.81 | 0.07 |
| 7 | benzene-1,3,5-triol | benzenetriol | H | OH | H | OH | H | 0.76 | 0.03 |
| 8 | 2-(2-hydroxy-5-methylphenyl)-4-methylphenol | biphenol | H | H | Me | H | coupled | 0.21 | 0.02 |
| 9 | 2-(2-hydroxy-3-methoxy-5-propylphenyl)-6-methoxy-4-propylphenol | biphenol | OMe | H | propyl | H | coupled | 0.85 | 0.13 |
| 10 | 4-butyl-2-(5-butyl-2-hydroxyphenyl)phenol | biphenol | H | H | butyl | H | coupled | 0.12 | - |
| 11 | 4-hexyl-2-(5-hexyl-2-hydroxyphenyl)phenol | biphenol | H | H | hexyl | H | coupled | 0.14 | 0.04 |
| 12 | benzene-1,2-diol (catechol) | catechol | OH | H | H | H | H | 2.86 | 0.03 |
| 13 | 2-chlorobenzene-1,4-diol | hydroquinone | Cl | H | OH | H | H | 1.41 | 0.04 |
| 14 | 2-bromobenzene-1,4-diol | hydroquinone | Br | H | OH | Br | H | 2.14 | 0.12 |
| 15 | 2-methylbenzene-1,4-diol | hydroquinone | Me | H | OH | H | H | 1.15 | 0.06 |
| 16 | hydroquinone | hydroquinone | H | H | OH | H | H | 0.77 | 0.02 |
| 17 | 2-mercaptophenol | hydroxybenzenethiol | SH | H | H | H | H | 0.77 | 0.27 |
| 18 | 4-mercaptophenol | hydroxybenzenethiol | H | H | SH | H | H | 0.74 | 0.04 |
| 19 | 2-methoxyphenol | methoxyphenol | OMe | H | H | H | H | 0.33 | 0.03 |
| 20 | 2-methoxy-4-propylphenol | methoxyphenol | OMe | H | propyl | H | H | 1.25 | 0.08 |
| 21 | 2,6-dimethoxyphenol | methoxyphenol | OMe | H | H | H | OMe | 0.61 | 0.05 |
| 22 | 2-methoxy-4-methylphenol | methoxyphenol | OMe | H | Me | H | H | 0.81 | 0.06 |
| 23 | 4-methoxyphenol | methoxyphenol | H | H | OMe | H | H | 0.65 | 0.06 |
| 24 | 4-hydroxy-3-methoxybenzaldehyde (vanillin) | methoxyphenol | OMe | H | CHO | H | H | 0.33 | 0.00 |
| 25 | 2,6-dimethylphenol | phenol | Me | H | H | H | Me | 0.71 | 0.06 |
| 26 | phenol | phenol | H | H | H | H | H | <0.03 | 0.00 |
| 27 | 2-caroboxybenzene-1,4-diol | phenolic acid | COOH | H | OH | H | H | 1.29 | 0.16 |
| 28 | 2-hydroxy-3-methylbenzoic acid (3-methylsalicylic acid) | phenolic acid | Me | H | H | H | COOH | <0.05 | - |
| 29 | benzene-1,3-diol (resorcinol) | resorcinol | H | OH | H | H | H | 0.09 | 0.06 |
| 30 | tannic acid | tannic acid | - | - | - | - | - | 15.4 | 1.30 |
| Entry | Compound | TEAC (H-ORAC) [molTE/mol] | Standard Deviation | TEAC (DPPH) [molTE/mol] | Standard Deviation |
|---|---|---|---|---|---|
| 0 | Trolox | 1.0 | - | 1.0 | - |
| 1 | Phenol | 1.76 | 0.160 | <0.03 | - |
| 2 | 2,6-dimethylphenol | 0.48 | 0.068 | 0.72 | 0.06 |
| 3 | 2,6-di-tert-butylphenol | <0.01 | - | 0.75 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, M.; Kitamura, Y.; Nagano, H.; Kawatsu, J.; Gotoh, H. DPPH Measurements and Structure—Activity Relationship Studies on the Antioxidant Capacity of Phenols. Antioxidants 2024, 13, 309. https://doi.org/10.3390/antiox13030309
Yamauchi M, Kitamura Y, Nagano H, Kawatsu J, Gotoh H. DPPH Measurements and Structure—Activity Relationship Studies on the Antioxidant Capacity of Phenols. Antioxidants. 2024; 13(3):309. https://doi.org/10.3390/antiox13030309
Chicago/Turabian StyleYamauchi, Moeka, Yukino Kitamura, Haruka Nagano, Junya Kawatsu, and Hiroaki Gotoh. 2024. "DPPH Measurements and Structure—Activity Relationship Studies on the Antioxidant Capacity of Phenols" Antioxidants 13, no. 3: 309. https://doi.org/10.3390/antiox13030309






