Selenomethionine Alleviates Deoxynivalenol-Induced Oxidative Injury in Porcine Intestinal Epithelial Cells Independent of MAPK Pathway Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Cell Culture
2.3. Cell Viability Analysis
2.4. Cell Proliferation Analysis
2.5. Lactate Dehydrogenase (LDH) Release Analysis
2.6. Intracellular Reactive Oxygen Species (ROS) Analysis
2.7. RNA Isolation and RT-qPCR Analysis
2.8. Western Blotting Analysis
2.9. Statistical Analysis
3. Results
3.1. The Cell Viability of IPEC-J2 Cells in Response to Toxic Dosages of DON and Physiological (or Supra-Physiological) Dosages of Selenium as SeMet
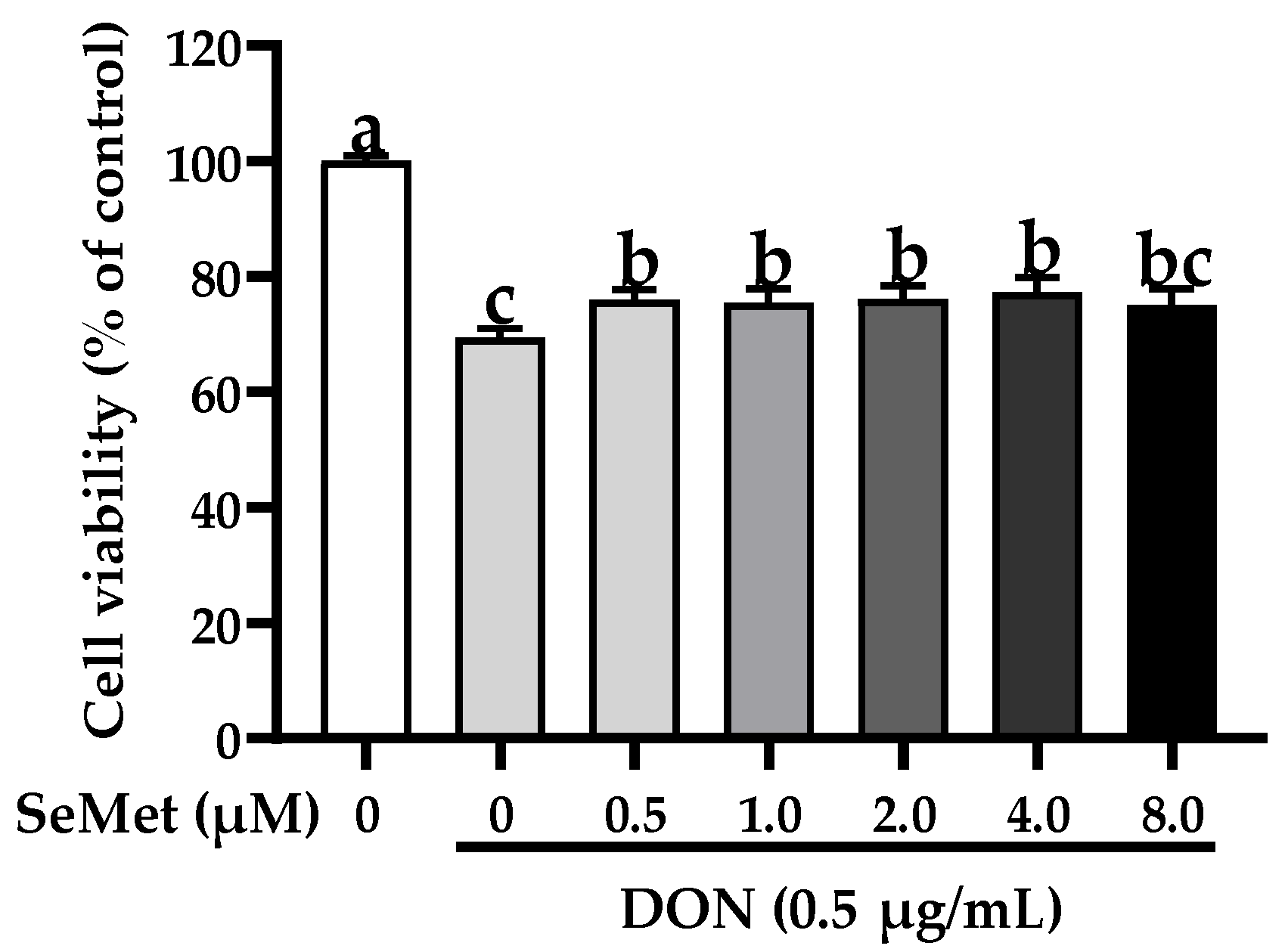
3.2. Effect of SeMet on Cell Viability of IPEC-J2 Cells under DON Exposure
3.3. Effect of SeMet on Cell Proliferation of IPEC-J2 Cells under DON Exposure
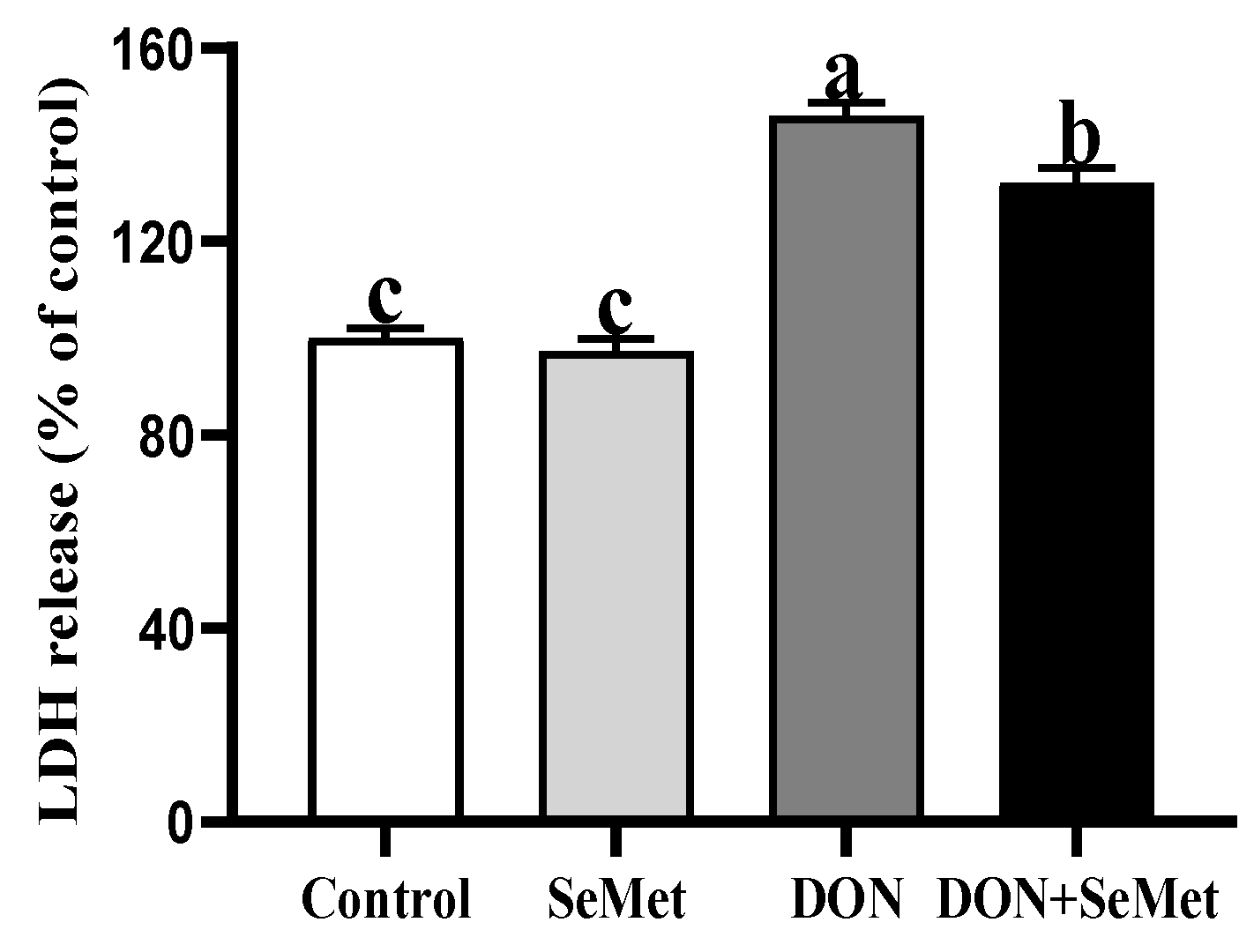
3.4. Effect of SeMet on LDH Release of IPEC-J2 Cells under DON Exposure
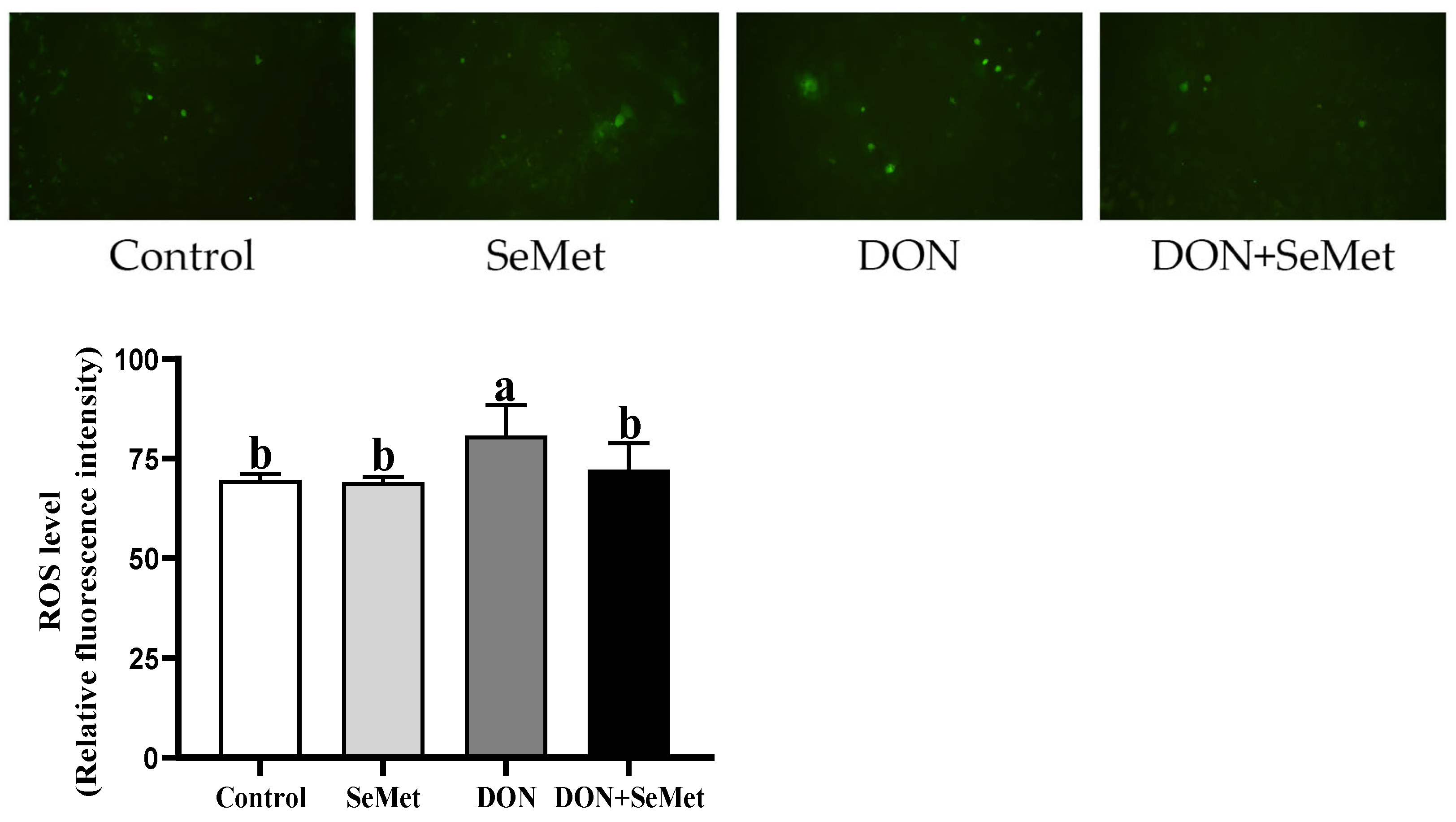
3.5. Effect of SeMet on ROS Level in IPEC-J2 Cells under DON Exposure
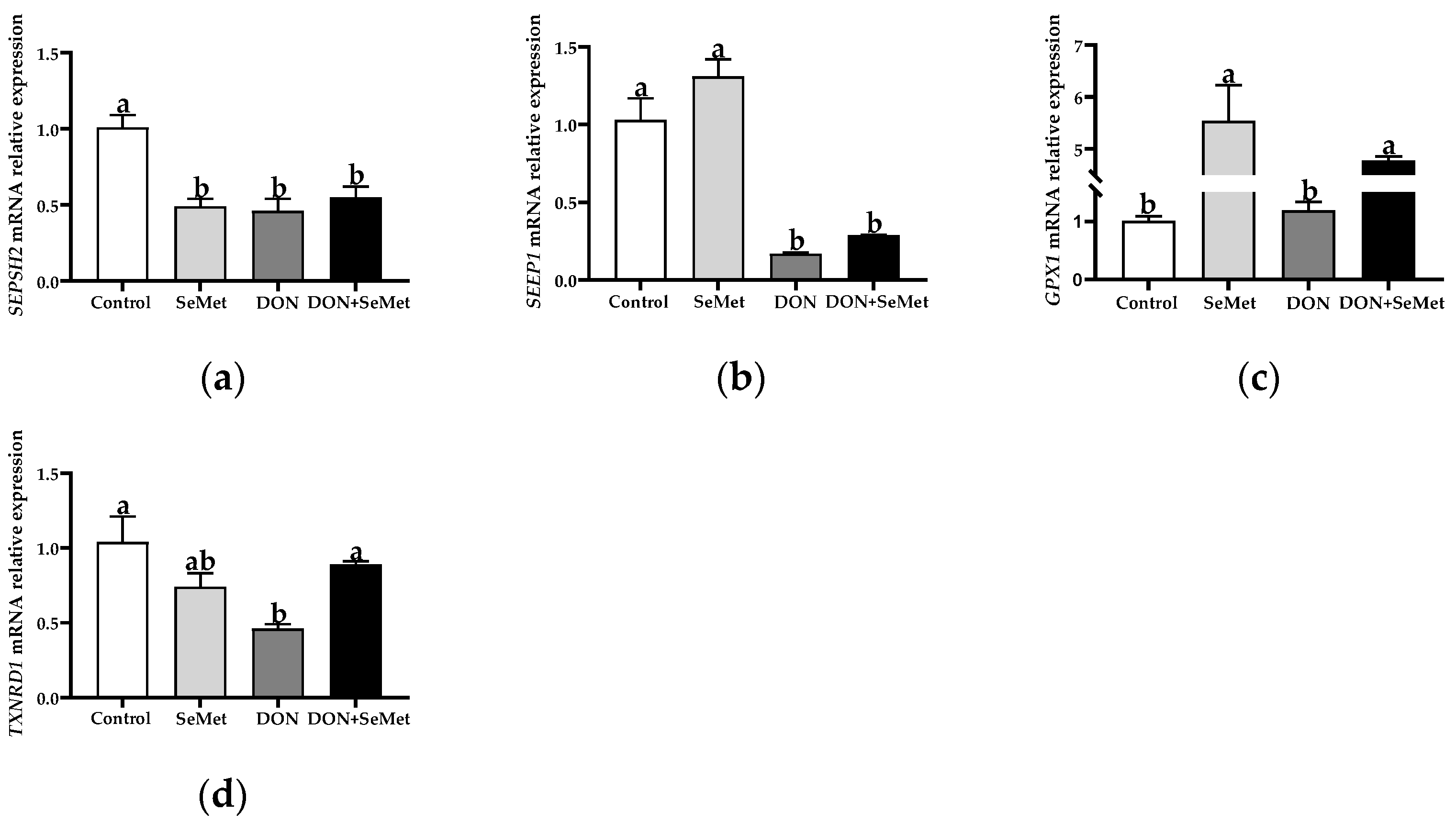
3.6. Effect of SeMet on the mRNA Relative Expression of Four Selenoproteins in IPEC-J2 Cells under DON Exposure
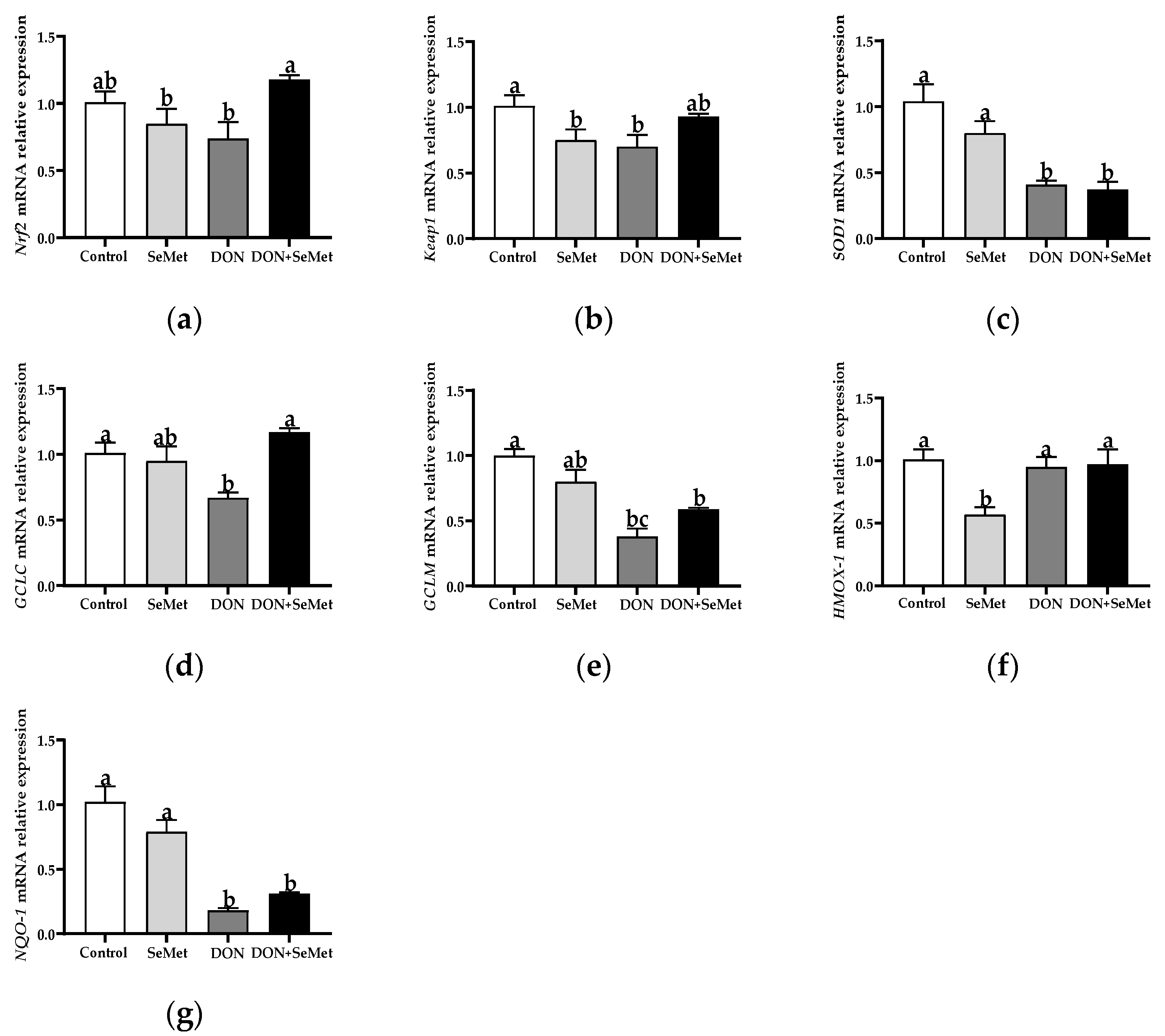
3.7. Effect of SeMet on the mRNA Relative Expression of Antioxidant-Related Genes in IPEC-J2 Cells under DON Exposure
3.8. Effect of SeMet on the mRNA and Protein Expression of Tight Junction Proteins in IPEC-J2 Cells under DON Exposure
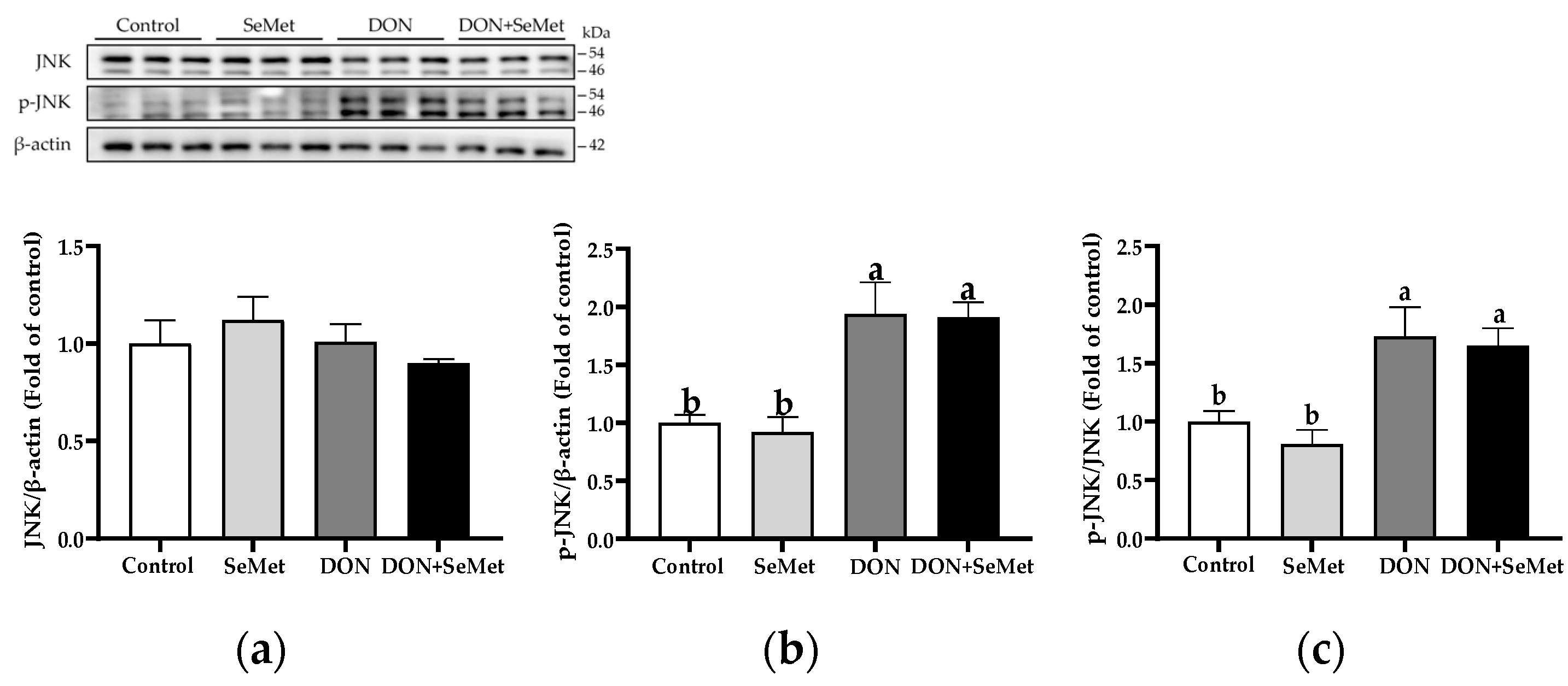
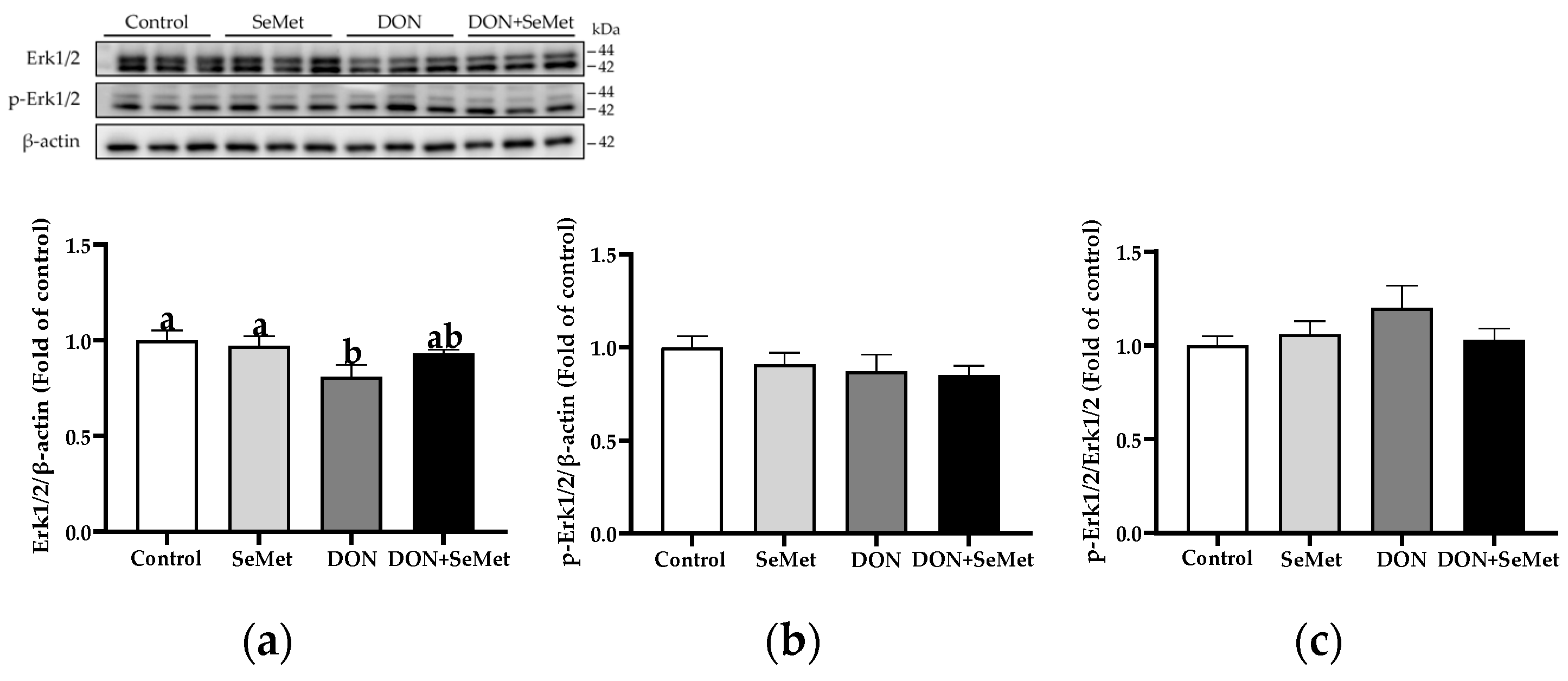
3.9. Effect of SeMet on Protein Expression of Key Proteins in MAPK Pathway in IPEC-J2 Cells under DON Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Huang, Z.; Cao, X.; Chen, X.; Zou, T.; You, J. Plant-derived polyphenols as Nrf2 activators to counteract oxidative stress and intestinal toxicity induced by deoxynivalenol in swine: An emerging research direction. Antioxidants 2022, 11, 2379. [Google Scholar] [CrossRef]
- Pronyk, C.; Cenkowski, S.; Abramson, D. Superheated steam reduction of deoxynivalenol in naturally contaminated wheat kernels. Food Control 2006, 17, 789–796. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.-T.; Xie, W.-M.; Zhang, N.-Y.; Dai, J.-F.; Wang, Y.; Rajput, S.; Qi, D.-S.; et al. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed. Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porcine Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Waché, Y.; Valat, C.; Postollec, G.; Bougeard, S.; Burel, C.; Oswald, I.; Fravalo, P. Impact of deoxynivalenol on the intestinal microflora of pigs. Int. J. Mol. Med. Sci. 2009, 10, 1–17. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Chu, J.-H.; Yan, Y.-X.; Gao, P.-C.; Chen, X.-W.; Fan, R.-F. Response of selenoproteins gene expression profile to mercuric chloride exposure in chicken kidney. Res. Vet. Sci. 2020, 133, 4–11. [Google Scholar] [CrossRef]
- Yang, J.; Shi, G.; Gong, Y.; Cai, J.; Zheng, Y.; Zhang, Z. LncRNA 0003250 accelerates heart autophagy and binds to miR-17-5p as a competitive endogenous RNA in chicken induced by selenium deficiency. J. Cell. Physiol. 2020, 236, 157–177. [Google Scholar] [CrossRef]
- Xiong, L.; Lin, T.; Yue, X.; Zhang, S.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal selenium-enriched yeast supplementation in sows enhances offspring growth and antioxidant status through the Nrf2/Keap1 pathway. Antioxidants 2023, 12, 2064. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xu, Y.; Ding, X.; Li, K.; Liang, S.; Li, D.; Wang, Y.; Fu, A.; Yu, W.; Zhan, X. Selenomethionine attenuated H2O2-induced oxidative stress and apoptosis by Nrf2 in chicken liver cells. Antioxidants 2023, 12, 1685. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, J.H.; Guan, W.T.; Chen, F.; Wang, C.X.; Zhang, Y.Z.; Lv, Y.T.; Lin, G. Selenium and vitamin E in sow diets: I. Effect on antioxidant status and reproductive performance in multiparous sows. Anim. Feed Sci. Technol. 2016, 221, 111–123. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, H.; Liu, Z.; Lei, L.; Feng, Z.; Zhang, D.; Ren, Y.; Zhao, S. Comparative study of yeast selenium vs. sodium selenite on growth performance, nutrient digestibility, anti-inflammatory and anti-oxidative activity in weaned piglets challenged by Salmonella typhimurium. Innate Immun. 2020, 26, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Wang, M.; Zhao, R.; Li, W.; Xu, Z. Effects of different selenium source on selenium distribution, loin quality and antioxidant status in finishing pigs. Anim. Feed Sci. Technol. 2007, 132, 202–211. [Google Scholar] [CrossRef]
- Wang, X.; Zuo, Z.; Zhao, C.; Zhang, Z.; Peng, G.; Cao, S.; Hu, Y.; Yu, S.; Zhong, Z.; Deng, J.; et al. Protective role of selenium in the activities of antioxidant enzymes in piglet splenic lymphocytes exposed to deoxynivalenol. Environ. Toxicol. Pharmacol. 2016, 47, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Fan, Y.; Zhang, Z.; Chen, C.; Chen, C.; Wang, X.; Deng, J.; Peng, G.; Hu, Y.; Cao, S.; et al. Sodium selenite inhibits deoxynivalenol-induced injury in GPX1-knockdown porcine splenic lymphocytes in culture. Sci. Rep. 2018, 8, 17676. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qiao, L.; Dou, X.; Chang, J.; Zhang, Y.; Xu, C. Selenium nanoparticles alleviate deoxynivalenol-induced intestinal epithelial barrier dysfunction by regulating endoplasmic reticulum stress in IPEC-J2 cells. Toxicology 2023, 494, 153593. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, M.; Li, J.; Shan, A. DL-Selenomethionine alleviates oxidative stress induced by zearalenone via Nrf2/Keap1 signaling pathway in IPEC-J2 cells. Toxins 2021, 13, 557. [Google Scholar] [CrossRef]
- Runchel, C.; Matsuzawa, A.; Ichijo, H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid. Redox Signal 2011, 15, 205–218. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zheng, W.; Yao, W. Protective role of hydrogen gas on oxidative damage and apoptosis in intestinal porcine epithelial cells (IPEC-J2) induced by deoxynivalenol: A preliminary study. Toxins 2020, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in deoxynivalenol induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Horn, N.; Ajuwon, K.M. Mechanisms of deoxynivalenol-induced endocytosis and degradation of tight junction proteins in jejunal IPEC-J2 cells involve selective activation of the MAPK pathways. Arch. Toxicol. 2021, 95, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Schierack, P.; Nordhoff, M.; Pollmann, M.; Weyrauch, K.D.; Amasheh, S.; Lodemann, U.; Jores, J.; Tachu, B.; Kleta, S.; Blikslager, A. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem. Cell Biol. 2006, 125, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, J.; Yang, J.; He, Q.; Luo, B.; Lu, Y.; Zou, T.; Wang, Z.; You, J. Seaweed polysaccharide mitigates intestinal barrier dysfunction induced by enterotoxigenic Escherichia coli through NF-κB pathway suppression in porcine intestinal epithelial cells. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Mahato, D.K.; Pandhi, S.; Kamle, M.; Gupta, A.; Sharma, B.; Panda, B.K.; Srivastava, S.; Kumar, M.; Selvakumar, R.; Pandey, A.K.; et al. Trichothecenes in food and feed: Occurrence, impact on human health and their detection and management strategies. Toxicon 2022, 208, 62–77. [Google Scholar] [CrossRef]
- Oswald, I.P. Role of intestinal epithelial cells in the innate immune defence of the pig intestine. Vet. Res. 2006, 37, 359–368. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Wang, S.-B.; Wang, R.-G.; Zhang, W.; Wang, P.-L.; Su, X.-O. Phosphoproteome analysis reveals the molecular mechanisms underlying deoxynivalenol-induced intestinal toxicity in IPEC-J2 cells. Toxins 2016, 8, 270. [Google Scholar] [CrossRef]
- Deng, C.; Ji, C.; Qin, W.; Cao, X.; Zhong, J.; Li, Y.; Srinivas, S.; Feng, Y.; Deng, X. Deoxynivalenol inhibits proliferation and induces apoptosis in human umbilical vein endothelial cells. Environ. Toxicol. Pharmacol. 2016, 43, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Springler, A.; Hessenberger, S.; Schatzmayr, G.; Mayer, E. Early activation of MAPK p44/42 is partially involved in DON-induced disruption of the intestinal barrier function and tight junction network. Toxins 2016, 8, 264. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol induces inflammation in IPEC-J2 cells by activating P38 Mapk and Erk1/2. Toxins 2020, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhao, J.; Cao, L.; Zhu, L.; Huang, Y.; Chen, X.; Rahman, S.U.; Feng, S.; Li, Y.; et al. Deoxynivalenol induces inflammatory injury in IPEC-J2 cells via NF-κB signaling pathway. Toxins 2019, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Moghadaszadeh, B.; Beggs, A.H. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology 2006, 21, 307–315. [Google Scholar] [CrossRef]
- Hu, H.; Wang, M.; Zhan, X.; Li, X.; Zhao, R. Effect of different selenium sources on productive performance, serum and milk Se concentrations, and antioxidant status of sows. Biol. Trace Elem. Res. 2011, 142, 471–480. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018, 2018, 465–468. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Cao, X.; Zou, T.; You, J.; Guan, W. Plant-derived polyphenols in sow nutrition: An update. Anim. Nutr. 2023, 12, 96–107. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Xin, Z.; Zhang, F.; Zhai, Z.; Ni, X.; Chen, J.; Yang, K.; Liao, P.; Zhang, L.; Xiao, Z.; et al. The cytoprotective effects of dihydromyricetin and associated metabolic pathway changes on deoxynivalenol treated IPEC-J2 cells. Food Chem. 2021, 338, 128116. [Google Scholar] [CrossRef]
- Liao, P.; Liao, M.; Li, L.; Tan, B.; Yin, Y. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells. Toxicol. Res. 2017, 6, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, S.; Ferreiro, A. Selenoproteins and protection against oxidative stress: Selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid. Redox Signal 2010, 12, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef]
- Touat-Hamici, Z.; Legrain, Y.; Bulteau, A.L.; Chavatte, L. Selective up-regulation of human selenoproteins in response to oxidative stress. J. Biol. Chem. 2014, 289, 14750–14761. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, F.P.; Raman, A.V.; Reeves, M.A.; Berry, M.J. Regulation and function of selenoproteins in human disease. Biochem. J. 2009, 422, 11–22. [Google Scholar] [CrossRef]
- Chen, X.D.; Zhao, Z.P.; Zhou, J.C.; Lei, X.G. Evolution, regulation, and function of porcine selenogenome. Free Radic. Biol. Med. 2019, 127, 116–123. [Google Scholar] [CrossRef]
- Tang, J.; Cao, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; Shang, H.; Zhao, H. The protective effect of selenium from heat stress-induced porcine small intestinal epithelial cell line (IPEC-J2) injury is associated with regulation expression of selenoproteins. Br. J. Nutr. 2019, 122, 1081–1090. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Wu, P.; Chu, Y.; Gui, S.; Zheng, Y.; Chen, X. Dietary selenium alleviated mouse liver oxidative stress and NAFLD induced by obesity by regulating the KEAP1/NRF2 pathway. Antioxidants 2022, 11, 349. [Google Scholar] [CrossRef]
- Arthur, J.R. The glutathione peroxidases. Cell Mol. Life Sci. 2001, 57, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, U.H.; Nagaraja, T.P.; Prabhu, K.S. Selenoproteins and their role in oxidative stress and inflammation. Curr. Chem. Biol. 2013, 7, 65–73. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, S.; Tang, J.; Liu, Y.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; Kang, B.; et al. Hydroxy selenomethionine improves meat quality through optimal skeletal metabolism and functions of selenoproteins of pigs under chronic heat stress. Antioxidants 2021, 10, 1558. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.J.; Kowalska, K.; Piastowska-Ciesielska, A.W. Nrf2: A main responsive element in cells to mycotoxin-induced toxicity. Arch. Toxicol. 2021, 95, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Matsushita, K.; Shindo, R.; Shimokawa, Y.; Sugiura, Y.; Yamashita, M. Selenoneine ameliorates hepatocellular injury and hepatic steatosis in a mouse model of NAFLD. Nutrients 2020, 12, 1898. [Google Scholar] [CrossRef]
- Eddie-Amadi, B.F.; Ezejiofor, A.N.; Orish, C.N.; Orisakwe, O.E. Zn and Se abrogate heavy metal mixture induced ovarian and thyroid oxido-inflammatory effects mediated by activation of NRF2-HMOX-1 in female albino rats. Curr. Res. Toxicol. 2023, 4, 100098. [Google Scholar] [CrossRef]
- Al-Brakati, A.; Alsharif, K.F.; Alzahrani, K.J.; Kabrah, S.; Al-Amer, O.; Oyouni, A.A.; Habotta, O.A.; Lokman, M.S.; Bauomy, A.A.; Kassab, R.B.; et al. Using green biosynthesized lycopene-coated selenium nanoparticles to rescue renal damage in glycerol-induced acute kidney injury in rats. Int. J. Nanomedicine 2021, 16, 4335–4349. [Google Scholar] [CrossRef]
- Ju, H.; Chen, S.; Xue, Y.; Zhang, X.; Wang, Y. The role of Nrf2 pathway in alleviating fluorine-induced apoptosis by different selenium sources in the chicken duodenum and jejunum. Ecotoxicol. Environ. Saf. 2021, 224, 112708. [Google Scholar] [CrossRef]
- Guan, Y.; Watson, A.J.M.; Marchiando, A.M.; Bradford, E.; Shen, L.; Turner, J.R.; Montrose, M.H. Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2011, 300, C1404–C1414. [Google Scholar] [CrossRef]
- Zhu, C.; Liang, S.; Zan, G.; Wang, X.; Gao, C.; Yan, H.; Wang, X.; Zhou, J. Selenomethionine alleviates DON-induced oxidative stress via modulating Keap1/Nrf2 signaling in the small intestinal epithelium. J. Agric. Food Chem. 2023, 71, 895–904. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lai, Y.-H.; Hsiao, F.S.-H.; Cheng, Y.-H. Effects of deoxynivalenol and mycotoxin adsorbent agents on mitogen-activated protein kinase signaling pathways and inflammation-associated gene expression in porcine intestinal epithelial cells. Toxins 2021, 13, 301. [Google Scholar] [CrossRef]




| Genes | Primer Sequence (5′-3′) | Accession Number | Product Size (bp) |
|---|---|---|---|
| TXNRD1 | F: CAAATCGAAGCAGGGATGC R: TCGTCACTGTTAGTGGCCTT | NM_214154.3 | 62 |
| SEPHS2 | F: CTGAGTGACCTCTACGCCAT R: GCATCCCGAAAGCCTTTGAT | NM_001093735.1 | 131 |
| SEPP1 | F: AGTCACCTGACAGTGTGGAG R: AATGACGTTCTCCTCTGCCA | EF113596.2 | 76 |
| Nrf2 | F: CCCATTCACAAAAGACAAACATTC R: GCTTTTGCCCTTAGCTCATCTC | XM_021075133.1 | 72 |
| Keap1 | F: GACGTGGAGACAGAAACGTG R: GTGACCATCATAGCCTCCGA | NM_001114671.1 | 114 |
| SOD1 | F: AAGGCCGTGTGTGTGCTGAA R: GATCACCTTCAGCCAGTCCTTT | NM_001190422.1 | 118 |
| GCLC | F: GACGACGCCAATGAGTCTGA R: AGCACCACAAACACCACGTA | XM_021098556.1 | 173 |
| GCLM | F: GATGCCGCCCGATTTAACTG R: ACAATGACCGAGTACCGCAG | XM_001926378.4 | 177 |
| HMOX-1 | F: TCAAGCAGAGGGTCCTCGAA R: CCTCTTGCGGATGTCGGATG | NM_001004027.1 | 134 |
| NQO-1 | F: GCCCAGATATTGTGGCCGAA R: AACTCCCCTATGAGCACACG | NM_001159613.1 | 130 |
| GPX1 | F: TGAATGGCGCAAATGCTCAC R: ATTGCGACACACTGGAGACC | NM_214201.1 | 125 |
| ZO-1 | F: AAGATCCGATAGGCGGTCTG R: CGGGATTTCACCAGTGTGAC | XM_021098827.1 | 72 |
| Claudin-1 | F: GGCAGATCCAGTGCAAAGTC R: CCCAGCAGGATGCCAATTAC | NM_001233539.1 | 94 |
| Occludin | F: CATTATGCACCCAGCAACGA R: GCACATCACGATAACGAGCA | XM_005672522.3 | 168 |
| β-actin | F: TCTGGCACCACACCTTCT R: TGATCTGGGTCATCTTCTCAC | XM_021086047.1 | 114 |
| Primary Antibody | Origin | Diluted Multiples | Brand |
|---|---|---|---|
| Erk1/2 | Rabbit | 1:1000 | Cell Signaling Technology |
| p-Erk1/2 | Rabbit | 1:1000 | Cell Signaling Technology |
| JNK | Rabbit | 1:1000 | Cell Signaling Technology |
| p-JNK | Rabbit | 1:1000 | Cell Signaling Technology |
| P38 | Rabbit | 1:1000 | Cell Signaling Technology |
| p-P38 | Rabbit | 1:1000 | Cell Signaling Technology |
| ZO-1 | Rabbit | 1:5000 | Proteintech |
| Claudin-1 | Rabbit | 1:5000 | Proteintech |
| Occludin | Rabbit | 1:5000 | Proteintech |
| β-actin | Mouse | 1:5000 | Proteintech |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Zhong, H.; Li, T.; Wang, Z.; Chen, X.; Zou, T.; You, J.; Chen, J. Selenomethionine Alleviates Deoxynivalenol-Induced Oxidative Injury in Porcine Intestinal Epithelial Cells Independent of MAPK Pathway Regulation. Antioxidants 2024, 13, 356. https://doi.org/10.3390/antiox13030356
Huang Z, Zhong H, Li T, Wang Z, Chen X, Zou T, You J, Chen J. Selenomethionine Alleviates Deoxynivalenol-Induced Oxidative Injury in Porcine Intestinal Epithelial Cells Independent of MAPK Pathway Regulation. Antioxidants. 2024; 13(3):356. https://doi.org/10.3390/antiox13030356
Chicago/Turabian StyleHuang, Zhouyin, Haopeng Zhong, Ting Li, Zirui Wang, Xingping Chen, Tiande Zou, Jinming You, and Jun Chen. 2024. "Selenomethionine Alleviates Deoxynivalenol-Induced Oxidative Injury in Porcine Intestinal Epithelial Cells Independent of MAPK Pathway Regulation" Antioxidants 13, no. 3: 356. https://doi.org/10.3390/antiox13030356
APA StyleHuang, Z., Zhong, H., Li, T., Wang, Z., Chen, X., Zou, T., You, J., & Chen, J. (2024). Selenomethionine Alleviates Deoxynivalenol-Induced Oxidative Injury in Porcine Intestinal Epithelial Cells Independent of MAPK Pathway Regulation. Antioxidants, 13(3), 356. https://doi.org/10.3390/antiox13030356






