The Oxidative Stability of Champagne Base Wines Aged on Lees in Barrels: A 2-Year Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Samples
2.2. Chemicals
2.3. Wines Antioxidant Capacity: DPPH Assay
2.4. Wine Phenolic Contents: Determinations of I280 and A420
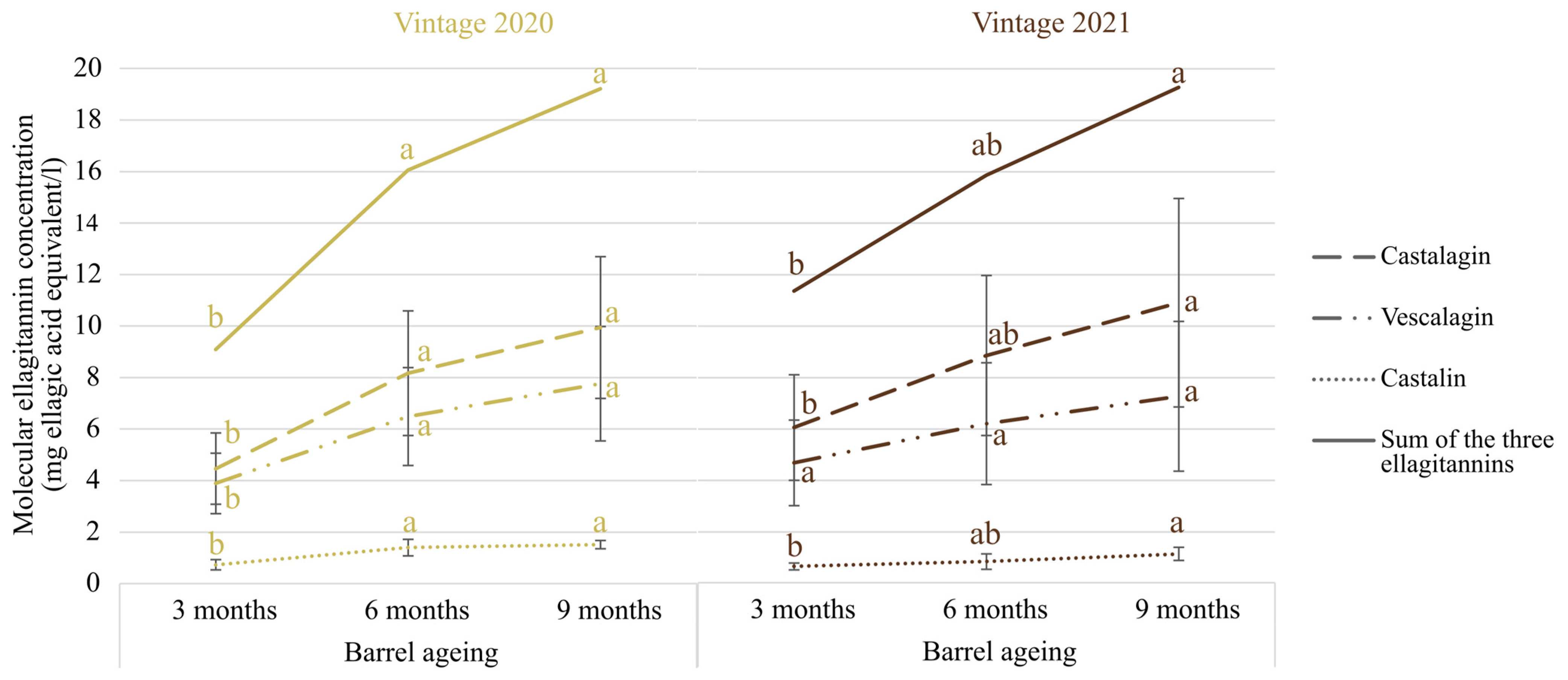
2.5. Quantification of Molecular Ellagitannins by UHPLC-Q-ToF-MS
2.6. Untargeted Analyses of Wines by UHPLC-Q-ToF-MS
2.7. Antioxidant Metabolome
2.8. Statistical Analyses
3. Results and Discussion
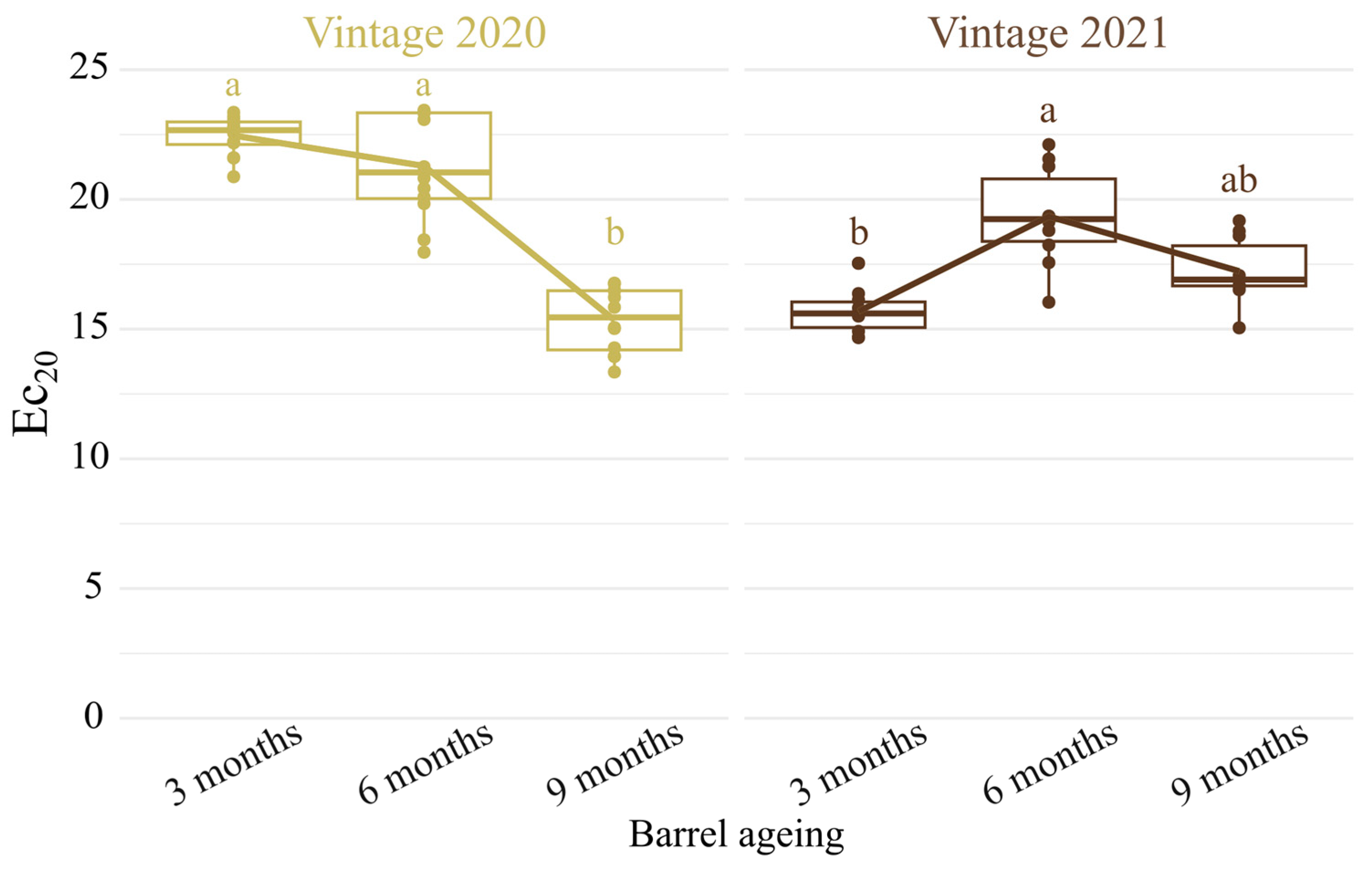
3.1. Wine Antioxidant Capacity of New Oak Barrel Ageing
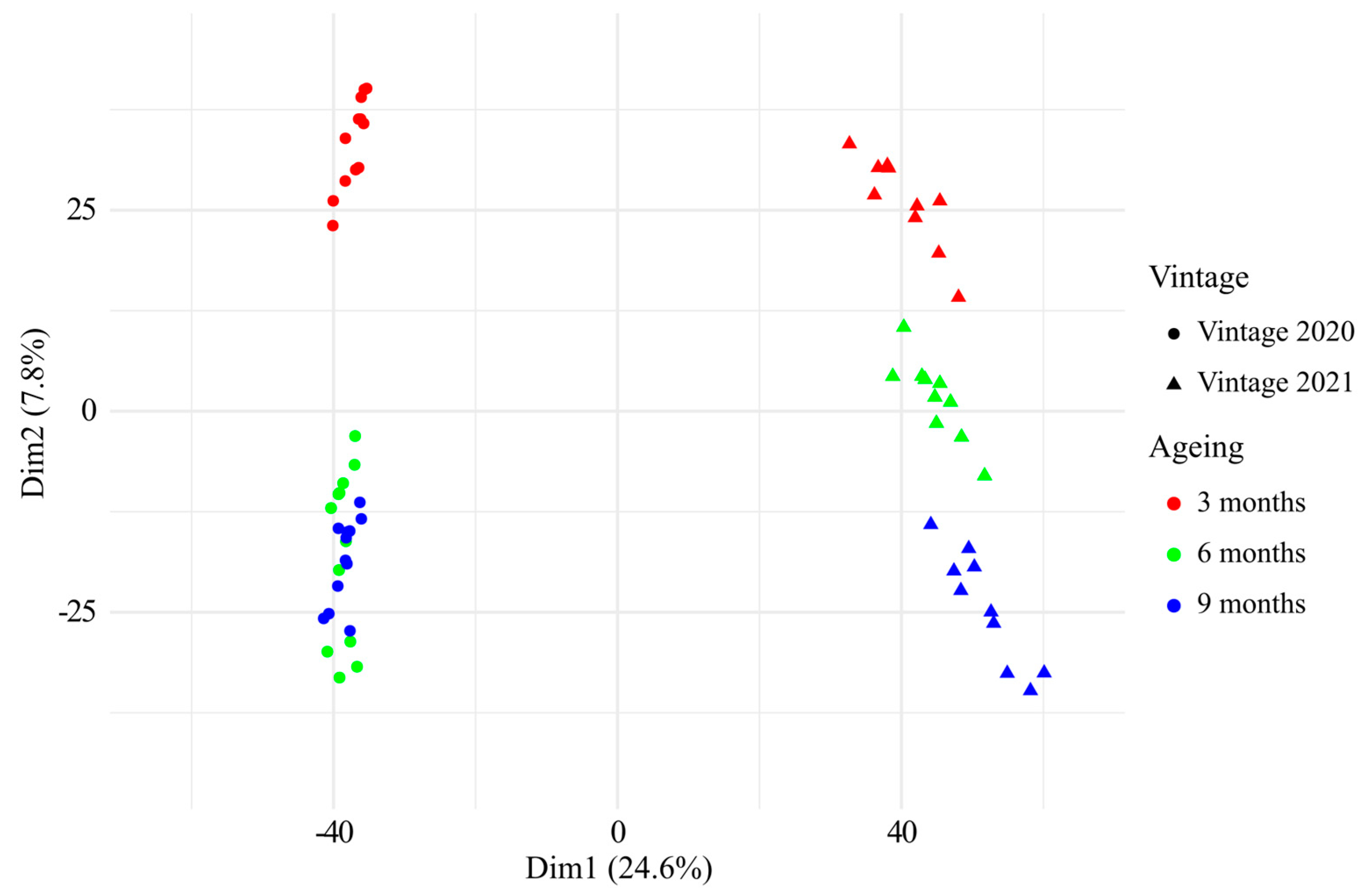
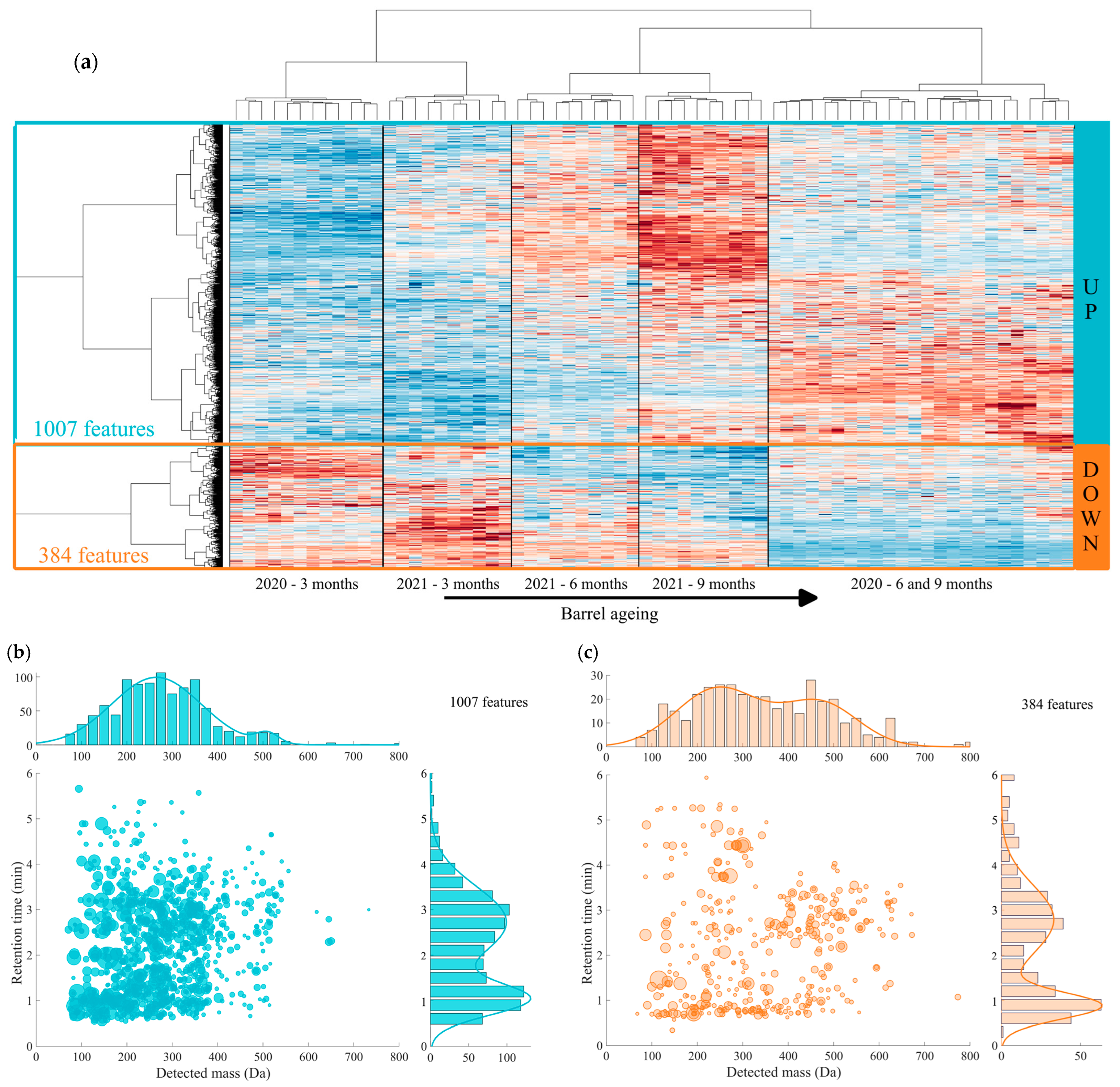
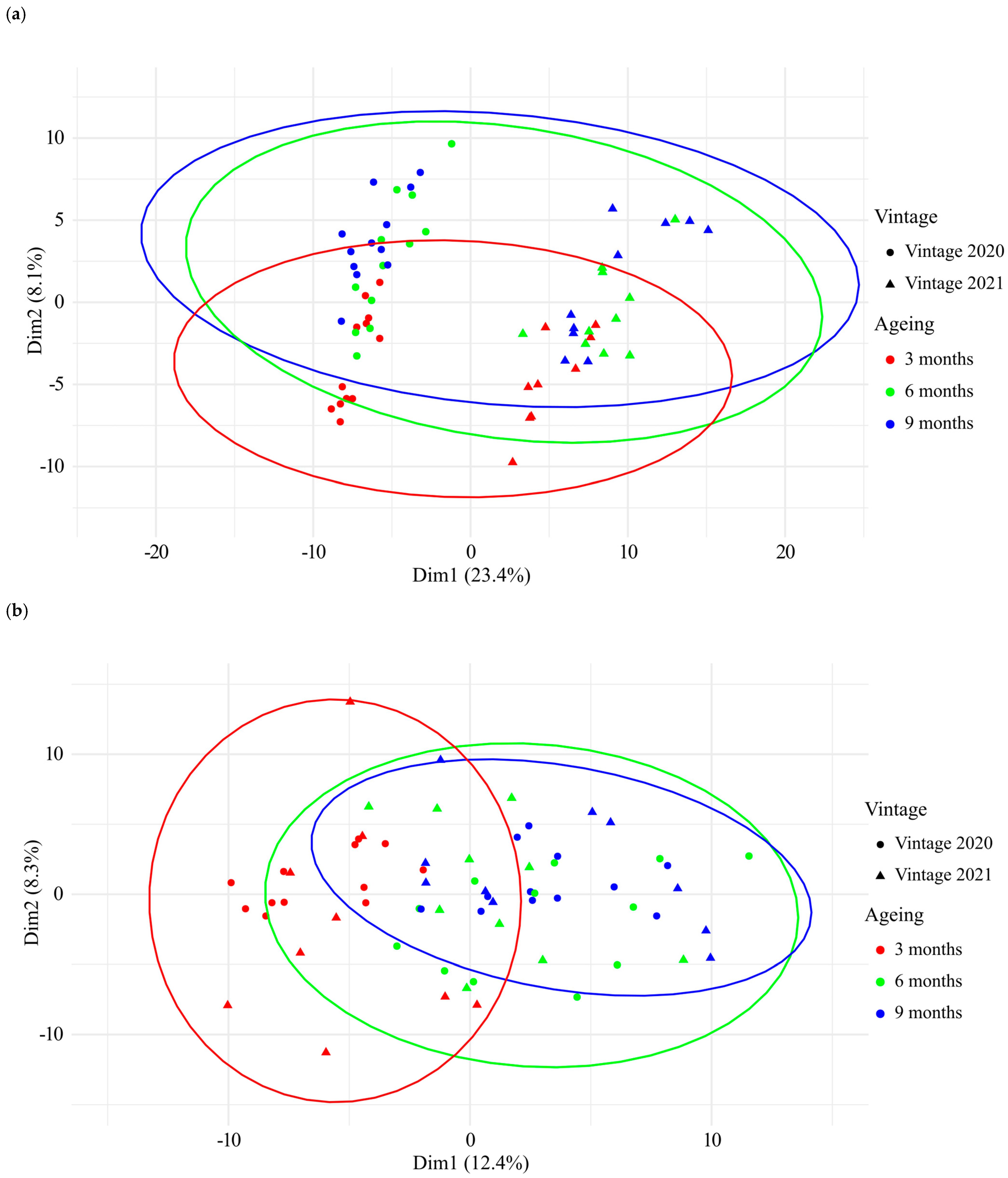
3.2. UHPLC-Q-ToF-MS Untargeted Analysis of Chardonnay Base Wines during Barrel Ageing
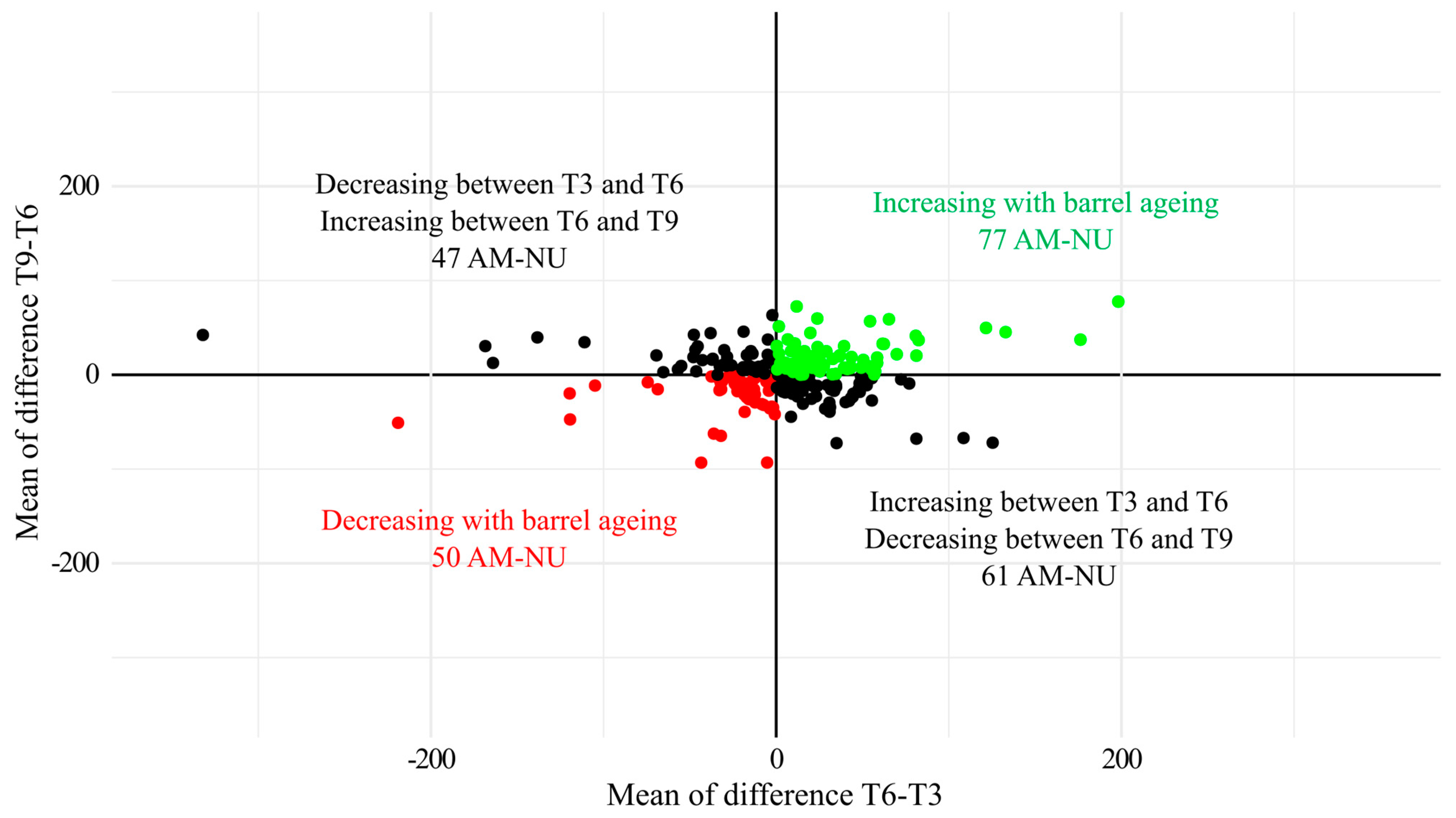
3.3. Chardonnay Base Wines Antioxidant Metabolome during Barrel Ageing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolantonaki, M.; Daoud, S.; Noret, L.; Coelho, C.; Badet-Murat, M.-L.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of Oak Wood Barrel Tannin Potential and Toasting on White Wine Antioxidant Stability. J. Agric. Food Chem. 2019, 67, 8402–8410. [Google Scholar] [CrossRef]
- Alexandre, H. Aging on Lees and Their Alternatives: Impact on Wine. In Managing Wine Quality Volume Two: Oenology and Wine Quality, 2nd ed.; Reynolds, A.G., Ed.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Cambridge, UK, 2022; pp. 213–244. ISBN 978-0-08-102065-4. [Google Scholar]
- Romanet, R. Contribution à l’étude Moléculaire de La Stabilité Oxydative Des Vins Blancs de Bourgogne; Université de Bourgogne: Dijon, France, 2019. [Google Scholar]
- Nikolantonaki, M.; Schmitt-Kopplin, P.; Gougeon, R.D. La Stabilité Oxydative Des Vins Blancs—Définition, Diagnostique, Pratiques Oenologiques, Oenologie Personnalisée. Rev. Des Oenologues Tech. Vitivinic. Oenologiques 2022, 184, 33–34. [Google Scholar]
- Romanet, R.; Gougeon, R.D.; Nikolantonaki, M. White Wine Antioxidant Metabolome: Definition and Dynamic Behavior during Aging on Lees in Oak Barrels. Antioxidants 2023, 12, 395. [Google Scholar] [CrossRef]
- Romanet, R.; Sarhane, Z.; Bahut, F.; Uhl, J.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Exploring the Chemical Space of White Wine Antioxidant Capacity: A Combined DPPH, EPR and FT-ICR-MS Study. Food Chem. 2021, 355, 129566. [Google Scholar] [CrossRef] [PubMed]
- Nikolantonaki, M.; Coelho, C.; Noret, L.; Zerbib, M.; Vileno, B.; Champion, D.; Gougeon, R.D. Measurement of White Wines Resistance against Oxidation by Electron Paramagnetic Resonance Spectroscopy. Food Chem. 2019, 270, 156–161. [Google Scholar] [CrossRef]
- Romanet, R.; Bahut, F.; Nikolantonaki, M.; Gougeon, R.D. Molecular Characterization of White Wines Antioxidant Metabolome by Ultra High Performance Liquid Chromatography High-Resolution Mass Spectrometry. Antioxidants 2020, 9, 115. [Google Scholar] [CrossRef]
- Romanet, R.; Coelho, C.; Liu, Y.; Bahut, F.; Ballester, J.; Nikolantonaki, M.; Gougeon, R.D. The Antioxidant Potential of White Wines Relies on the Chemistry of Sulfur-Containing Compounds: An Optimized DPPH Assay. Molecules 2019, 24, 1353. [Google Scholar] [CrossRef] [PubMed]
- Nikolantonaki, M.; Julien, P.; Coelho, C.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of Glutathione on Wines Oxidative Stability: A Combined Sensory and Metabolomic Study. Front. Chem. 2018, 6, 182. [Google Scholar] [CrossRef]
- Jordão, A.M.; Correia, A.C.; DelCampo, R.; González SanJosé, M.L. Antioxidant Capacity, Scavenger Activity, and Ellagitannins Content from Commercial Oak Pieces Used in Winemaking. Eur. Food Res. Technol. 2012, 235, 817–825. [Google Scholar] [CrossRef]
- Duteurtre, B. Elaboration Des Vins de Base. In Le Champagne de la Tradition à la Science; Lavoisier Tec & Doc.: Paris, France, 2016; pp. 61–104. ISBN 978-2-7430-2145-0. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Topics Related to Aging. In Understanding Wine Chemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; pp. 294–317. [Google Scholar]
- Pegram, Z.; Kwasniewski, M.T.; Sacks, G.L. Simplified Method for Free SO2 Measurement Using Gas Detection Tubes. Am. J. Enol. Vitic. 2013, 64, 405–410. [Google Scholar] [CrossRef]
- Mataix, E.; Luque de Castro, M.D. Simultaneous (or Sequential) Determination of the Total Polyphenol Index (or I280) and Density in Wines by Flow Injection. Analyst 2001, 126, 251–255. [Google Scholar] [CrossRef]
- Singleton, V.L.; Kramlinga, T.E. Browning of White Wines and an Accelerated Test for Browning Capacity. Am. J. Enol. Vitic. 1976, 27, 157–160. [Google Scholar] [CrossRef]
- Iland, P.; Bruer, N.; Edwards, G.; Caloghiris, S.; Wilkes, E.N. Chemical Analysis of Grapes and Wine: Techniques and Concepts, 2nd ed.; Patrick Iland Wine Promotion: Adelaide, Australia, 2004; ISBN 9780958160575. [Google Scholar]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Chapman and Hall, Ed.; Aspen Publishers Inc.: New York, NY, USA, 1995; ISBN 978-0834217010. [Google Scholar]
- Shrivastava, A.; Gupta, V.B. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Sumner, L.W.; Lei, Z.; Nikolau, B.J.; Saito, K.; Roessner, U.; Trengove, R.D. Proposed Quantitative and Alphanumeric Metabolite Identification Metrics. Metabolomics 2014, 10, 1047–1049. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Waterhouse, A.L. A Method to Quantify Quinone Reaction Rates with Wine Relevant Nucleophiles: A Key to the Understanding of Oxidative Loss of Varietal Thiols. J. Agric. Food Chem. 2012, 60, 8484–8491. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Šulc, M.; Faitová, K.; Pivec, V. Major Factors Influencing Antioxidant Contents and Antioxidant Activity in Grapes and Wines. Int. J. Wine Res. 2009, 1, 101–121. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Delso, C.; Maza, M.A.; Álvarez, I.; Raso, J. Pulsed Electric Fields Accelerate Release of Mannoproteins from Saccharomyces Cerevisiae during Aging on the Lees of Chardonnay Wine. Food Res. Int. 2019, 116, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Role of Lees in Wine Production: A Review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-Derived Peptidic Antioxidants: A Review of Their Production, Assessment, and Potential Applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Marinov, M.G.; Dimitrova, E.D.; Puech, J.-L. Kinetics of Ellagitannin Extraction from Oak Wood Using White Wine. J. Wine Res. 1997, 8, 29–40. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Chira, K.; Teissèdre, P.-L. Use of Oak Wood during Malolactic Fermentation and Ageing: Impact on Chardonnay Wine Character. Food Chem. 2019, 278, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Feldman, K.S. Ellagitannin Chemistry. Chem. Rev. 1996, 96, 475–503. [Google Scholar] [CrossRef] [PubMed]
- Mayer, W.; Seitz, H.; Jochims, J.C. Über Die Gerbstoffe Aus Dem Holz Der Edelkastanie Und Der Eiche, IV. Die Struktur Des Castalagins. Justus Liebigs Ann. Chem. 1969, 721, 186–193. [Google Scholar] [CrossRef]
- Mayer, W.; Gabler, W.; Riester, A.; Korger, H. Über Die Gerbstoffe Aus Dem Holz Der Edelkastanie Und Der Eiche, II. Die Isolierung von Castalagin, Vescalagin, Castalin Und Vescalin. Justus Liebigs Ann. Chem. 1967, 707, 177–181. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castro-Vázquez, L.I.; Díaz-Maroto, M.C.; Gordon, M.H.; Pérez-Coello, M.S. A Study of the Antioxidant Capacity of Oak Wood Used in Wine Ageing and the Correlation with Polyphenol Composition. Food Chem. 2011, 128, 997–1002. [Google Scholar] [CrossRef]
- Somers, T.C. The Polymeric Nature of Wine Pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Nuutila, A.M.; Kammiovirta, K.; Oksman-Caldentey, K.M. Comparison of Methods for the Hydrolysis of Flavonoids and Phenolic Acids from Onion and Spinach for HPLC Analysis. Food Chem. 2002, 76, 519–525. [Google Scholar] [CrossRef]
- Bozinou, E.; Palaiogiannis, D.; Athanasiadis, V.; Chatzilazarou, A.; Lalas, S.I.; Makris, D.P. Glycerol-Based Deep Eutectic Solvents for Simultaneous Organosolv Treatment/Extraction: High-Performance Recovery of Antioxidant Polyphenols from Onion Solid Wastes. Sustainability 2022, 14, 15715. [Google Scholar] [CrossRef]
- Natali, N.; Chinnici, F.; Riponi, C. Characterization of Volatiles in Extracts from Oak Chips Obtained by Accelerated Solvent Extraction (ASE). J. Agric. Food Chem. 2006, 54, 8190–8198. [Google Scholar] [CrossRef]
- Arapitsas, P.; Antonopoulos, A.; Stefanou, E.; Dourtoglou, V.G. Artificial Aging of Wines Using Oak Chips. Food Chem. 2004, 86, 563–570. [Google Scholar] [CrossRef]
- Li, R.; Liang, X.; Song, C.; Wang, H.; Liu, Y.; Ma, Y.; Sun, J.; Wang, J. Effects of Oak Chip Treatments on Quality of Dry White Wines during Aging. Am. J. Biochem. Biotechnol. 2020, 16, 76–86. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, J.; Duan, C.-Q.; Reeves, M.J.; He, F. A Review of Polyphenolics in Oak Woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef] [PubMed]
- Márquez, K.; Contreras, D.; Salgado, P.; Mardones, C. Production of Hydroxyl Radicals and Their Relationship with Phenolic Compounds in White Wines. Food Chem. 2019, 271, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Non-flavonoid Phenolics. In Understanding Wine Chemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; pp. 112–116. [Google Scholar]
- Magdas, D.A.; Pirnau, A.; Feher, I.; Guyon, F.; Cozar, B.I. Alternative Approach of Applying 1H NMR in Conjunction with Chemometrics for Wine Classification. LWT Food Sci. Technol. 2019, 109, 422–428. [Google Scholar] [CrossRef]
- Suarez, M.A.; Polo, M.C.; Llaguno, C. Etude de La Composition Des Vins Mousseux Pendant La Prise de Mousse Au Cours Du Vieillissement En Bouteilles. I. Etude Des Acides Aminés Libres, Du Glycérol, Des Sucres et Des Acides Organiques. Connaiss. Vigne Vin 1979, 13, 199–217. [Google Scholar] [CrossRef]
- Colagrande, O.; Silva, A. Considerazzioni Analitiche e Tecnologiche Sui Diversi Costituenti Azotati Dei Vini Spumanti. Ind. Delle Bevande 1981, 55, 349–363. [Google Scholar]
- Feuillat, M.; Charpentier, C. Autolysis of Yeasts in Champagne. Am. J. Enol. Vitic. 1982, 33, 6–13. [Google Scholar] [CrossRef]
- Marghery, G.; Versini, G.; della Serra, A.; Giannoti, L.; Pellegrini, R.; Mattarei, C. L’autolisi Dei Lievito in Enologia. Vignevini 1984, 11, 25–28. [Google Scholar]
- Kelly-Treadwell, P.H. Protease Activity in Yeast: Its Relationship to Autolysis and Champagne Character. Aust. Grapegrow. Winemak. 1988, 4, 58–66. [Google Scholar]
- Lurton, L.; Segain, J.; Feuillat, M. Etude de La Protéolyse Au Cours de l’autolyse de Levures En Milieu Acide. Sci. Aliment. 1989, 9, 111–124. [Google Scholar]
- Charpentier, C.; Feuillat, M. Yeast Autolysis. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 225–241. [Google Scholar]
- Guilloux-Bénatier, M.; Chassagne, D. Comparison of Components Released by Fermented or Active Dried Yeasts after Aging on Lees in a Model Wine. J. Agric. Food Chem. 2003, 51, 746–751. [Google Scholar] [CrossRef]
- Alexandre, H.; Heintz, D.; Chassagne, D.; Guilloux-Bénatier, M.; Charpentier, C.; Feuillat, M. Protease A Activity and Nitrogen Fractions Released during Alcoholic Fermentation and Autolysis in Enological Conditions. J. Ind. Microbiol. Biotechnol. 2001, 26, 235–240. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.J.; Carrascosa, A.V.; Polo, M.C. Release of Nitrogen Compounds to the Extracellular Medium by Three Strains of Saccharomyces Cerevisiae during Induced Autolysis in a Model Wine System. Int. J. Food Microbiol. 2001, 68, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Bretón, P.; Garde-Cerdán, T.; Martínez, J. Use of Oak Fragments during the Aging of Red Wines. Effect on the Phenolic, Aromatic, and Sensory Composition of Wines as a Function of the Contact Time with the Wood. Beverages 2018, 4, 102. [Google Scholar] [CrossRef]
- Ibern-Gómez, M.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M.; Buxaderas, S.; Singleton, V.L.; de la Torre-Boronat, M.C. Browning of Cava (Sparkling Wine) during Aging in Contact with Lees Due to the Phenolic Composition. Am. J. Enol. Vitic. 2000, 51, 29–36. [Google Scholar] [CrossRef]
- Sroka, Z. Antioxidative and Antiradical Properties of Plant Phenolics. Z. Für Naturforschung C 2005, 60, 833–843. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen Peroxide Scavenging, Antioxidant and Anti-Radical Activity of Some Phenolic Acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Vivas, N.; Vivas de Gaulejac, N.; Vitry, C.; Mouche, C.; Kahn, N.; Nonier-Bourden, M.-F.; Absalon, C. Impact of Ethanol Content on the Scavenging Activities of Oak Wood C-Glycosidic Ellagitannins. Application to the Evaluation of the Nutritional Status of Spirits. J. Inst. Brew. 2013, 119, 116–125. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Ito, H. Ellagitannins Renewed the Concept of Tannins. In Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; Quideau, S., Ed.; World Scientific: Singapore, 2009; pp. 1–54. [Google Scholar] [CrossRef]
- Kumar, A.; Kaushik, P.; Incerpi, S.; Pedersen, J.Z.; Goel, S.; Prasad, A.K.; Rohil, V.; Parmar, V.S.; Saso, L.; Len, C. Evaluation of the Free Radical Scavenging Activities of Ellagic Acid and Ellagic Acid Peracetate by EPR Spectrometry. Molecules 2021, 26, 4800. [Google Scholar] [CrossRef]
- Prakash, D.; Suri, S.; Upadhyay, G.; Singh, B.N. Total Phenol, Antioxidant and Free Radical Scavenging Activities of Some Medicinal Plants. Int. J. Food Sci. Nutr. 2007, 58, 18–28. [Google Scholar] [CrossRef]
- Kouno, Y.; Anraku, M.; Yamasaki, K.; Okayama, Y.; Iohara, D.; Nakamura, H.; Maruyama, T.; Hirayama, F.; Kragh-Hansen, U.; Otagiri, M. N-Acetyl-L-Methionine Is a Superior Protectant of Human Serum Albumin against Post-Translational Oxidation as Compared to N-Acetyl-L-Tryptophan. Biochem. Biophys. Rep. 2016, 6, 266–274. [Google Scholar] [CrossRef]
- Kertsch, A.-L.; Wagner, J.; Henle, T. Selected Maillard Reaction Products and Their Yeast Metabolites in Commercial Wines. J. Agric. Food Chem. 2023, 71, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.M.; Röder, A.; Modig, T.; Laadan, B.; Lidén, G.; Gorwa-Grauslund, M.F. NADH- vs NADPH-Coupled Reduction of 5-Hydroxymethyl Furfural (HMF) and Its Implications on Product Distribution in Saccharomyces Cerevisiae. Appl. Microbiol. Biotechnol. 2008, 78, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Akillioglu, H.G.; Mogol, B.A.; Gökmen, V. Degradation of 5-Hydroxymethylfurfural during Yeast Fermentation. Food Addit. Contam. 2011, 28, 1629–1635. [Google Scholar] [CrossRef]
- Jeandet, P.; Heinzmann, S.S.; Roullier-Gall, C.; Cilindre, C.; Aron, A.; Deville, M.A.; Moritz, F.; Karbowiak, T.; Demarville, D.; Brun, C.; et al. Chemical Messages in 170-Year-Old Champagne Bottles from the Baltic Sea: Revealing Tastes from the Past. Proc. Natl. Acad. Sci. USA 2015, 112, 5893–5898. [Google Scholar] [CrossRef]
- Serra-Cayuela, A.; Jourdes, M.; Riu-Aumatell, M.; Buxaderas, S.; Teissèdre, P.-L.; López-Tamames, E. Kinetics of Browning, Phenolics, and 5-Hydroxymethylfurfural in Commercial Sparkling Wines. J. Agric. Food Chem. 2014, 62, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Serra-Cayuela, A.; Castellari, M.; Bosch-Fusté, J.; Riu-Aumatell, M.; Buxaderas, S.; López-Tamames, E. Identification of 5-Hydroxymethyl-2-Furfural (5-HMF) in Cava Sparkling Wines by LC-DAD-MS/MS and NMR Spectrometry. Food Chem. 2013, 141, 3373–3380. [Google Scholar] [CrossRef]
- Masson, E.; Baumes, R.L.; Moutounet, M.; Puech, J.-L. The Effect of Kiln-Drying on the Levels of Ellagitannins and Volatile Compounds of European Oak (Quercus Petraea Liebl.) Stave Wood. Am. J. Enol. Vitic. 2000, 51, 201–214. [Google Scholar] [CrossRef]
- Prida, A. Aspects Sensoriels de l’usage Du Chêne En Oenologie: De l’élevage En Barrique Jusqu’au Vieillissement En Bouteilles; Seguin-Moreau: Merpins, France, 2018. [Google Scholar]
- Kredich, N.M. Biosynthesis of Cysteine. EcoSalPlus 2008, 3, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H.; Guilloux-Bénatier, M. Yeast Autolysis in Sparkling Wine—A Review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef]
- Duteurtre, B. Prise de Mousse et Séjour Sur Lies. In Le Champagne de la Tradition à la Science; Lavoisier Tec & Doc: Paris, France, 2016; pp. 160–181. ISBN 978-2-7430-2145-0. [Google Scholar]
- Winstel, D.; Marchal, A. Lignans in Spirits: Chemical Diversity, Quantification, and Sensory Impact of (±)-Lyoniresinol. Molecules 2019, 24, 117. [Google Scholar] [CrossRef] [PubMed]
- Gammacurta, M.; Waffo-Téguo, P.; Winstel, D.; Dubourdieu, D.; Marchal, A. Isolation of Taste-Active Triterpenoids from Quercus Robur: Sensory Assessment and Identification in Wines and Spirit. J. Nat. Prod. 2020, 83, 1611–1622. [Google Scholar] [CrossRef]
- Winstel, D.; Gautier, E.; Marchal, A. Role of Oak Coumarins in the Taste of Wines and Spirits: Identification, Quantitation, and Sensory Contribution through Perceptive Interactions. J. Agric. Food Chem. 2020, 68, 7434–7443. [Google Scholar] [CrossRef]
- Sindt, L.; Gammacurta, M.; Waffo-Téguo, P.; Dubourdieu, D.; Marchal, A. Taste-Guided Isolation of Bitter Lignans from Quercus Petraea and Their Identification in Wine. J. Nat. Prod. 2016, 79, 2432–2438. [Google Scholar] [CrossRef]
- Marchal, A.; Waffo-Téguo, P.; Gammacurta, M.; Prida, A.; Dubourdieu, D. Origins of the Sweetness Derived from the Aging of Dry Wines: The Role of Oak Triterpenoids. IVES Tech. Rev. Vine Wine 2020. [Google Scholar] [CrossRef]
- Filipsky, T.; Riha, M.; Macakova, K.; Anzenbacherova, E.; Karlickova, J.; Mladenka, P. Antioxidant Effects of Coumarins Include Direct Radical Scavenging, Metal Chelation and Inhibition of ROS-Producing Enzymes. Curr. Top. Med. Chem. 2015, 15, 415–431. [Google Scholar] [CrossRef]
- Foti, M.C.; Piattelli, M.; Baratta, M.T.; Ruberto, G. Flavonoids, Coumarins, and Cinnamic Acids as Antioxidants in a Micellar System. Structure-Activity Relationship. J. Agric. Food Chem. 1996, 44, 497–501. [Google Scholar] [CrossRef]
- Payá, M.; Halliwell, B.; Hoult, J.R.S. Peroxyl Radical Scavenging by a Series of Coumarins. Free Radic. Res. 1992, 17, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical Studies on Antioxidant Mechanisms and Free Radical Scavenging Properties of Lignans. Org. Biomol. Chem. 2005, 3, 3336–3347. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxe, C.; Romanet, R.; Parisot, M.; Gougeon, R.D.; Nikolantonaki, M. The Oxidative Stability of Champagne Base Wines Aged on Lees in Barrels: A 2-Year Study. Antioxidants 2024, 13, 364. https://doi.org/10.3390/antiox13030364
Maxe C, Romanet R, Parisot M, Gougeon RD, Nikolantonaki M. The Oxidative Stability of Champagne Base Wines Aged on Lees in Barrels: A 2-Year Study. Antioxidants. 2024; 13(3):364. https://doi.org/10.3390/antiox13030364
Chicago/Turabian StyleMaxe, Charlotte, Rémy Romanet, Michel Parisot, Régis D. Gougeon, and Maria Nikolantonaki. 2024. "The Oxidative Stability of Champagne Base Wines Aged on Lees in Barrels: A 2-Year Study" Antioxidants 13, no. 3: 364. https://doi.org/10.3390/antiox13030364





