A High-Fat-Diet-Induced Microbiota Imbalance Correlates with Oxidative Stress and the Inflammatory Response in the Gut of Freshwater Drum (Aplodinotus grunniens)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals and Experimental Design
2.3. Sample Collection
2.4. Biochemical Index Analysis
2.5. Enzyme-Linked Immunosorbent Assay Analysis
2.6. RNA Extraction and De Novo High-Throughput Sequencing
2.7. Microbial DNA Extraction and 16S Sequencing
2.8. Integrated Analysis between Key Genes and Bacteria
2.9. Transcriptional Expression Analysis by Real-Time PCR
2.10. Statistical Analysis
3. Results
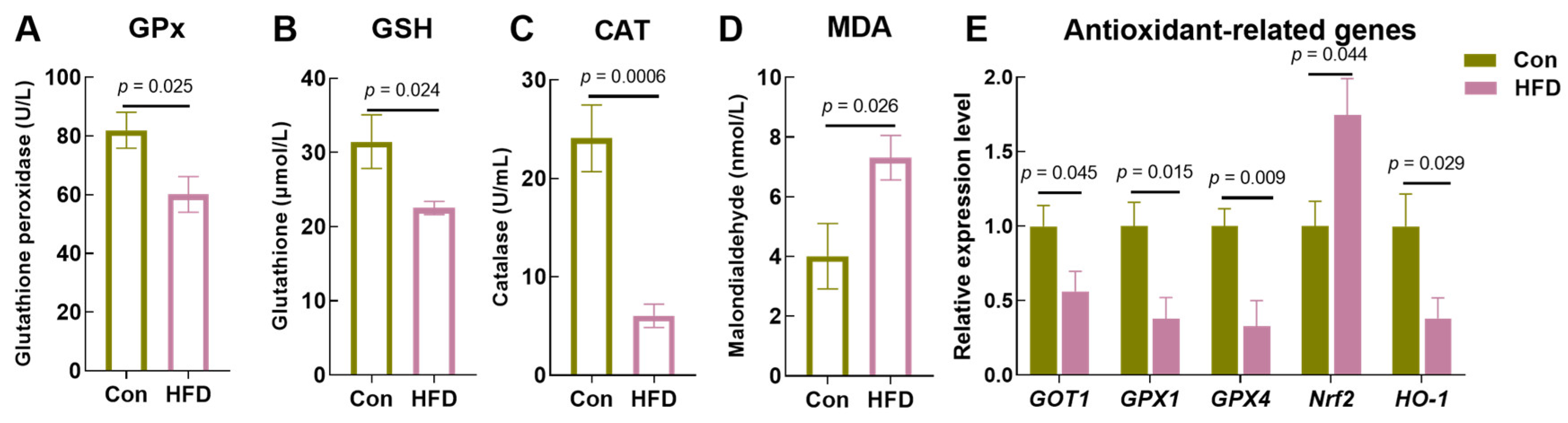
3.1. A High-Fat Diet Inhibits Antioxidant Capacity in the Gut of A. grunniens
3.2. A High-Fat Diet Suppresses Immunocompetence and Induces Cellular Inflammation, Apoptosis and Autophagy
3.3. Gut Transcriptome Analysis of A. grunniens on a High-Fat Diet
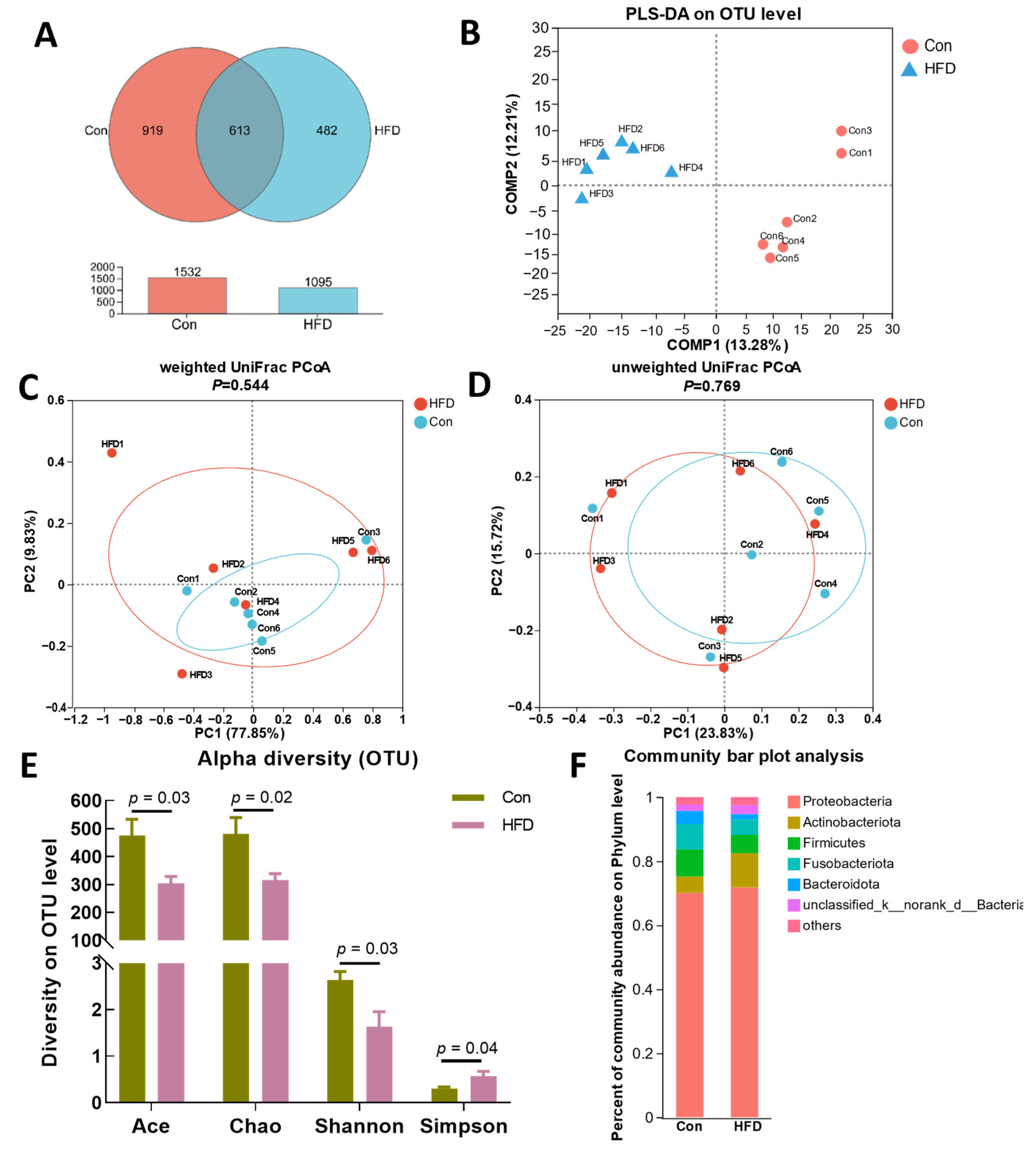
3.4. Gut Microbiota Alternation of A. grunniens Fed with a High-Fat Diet
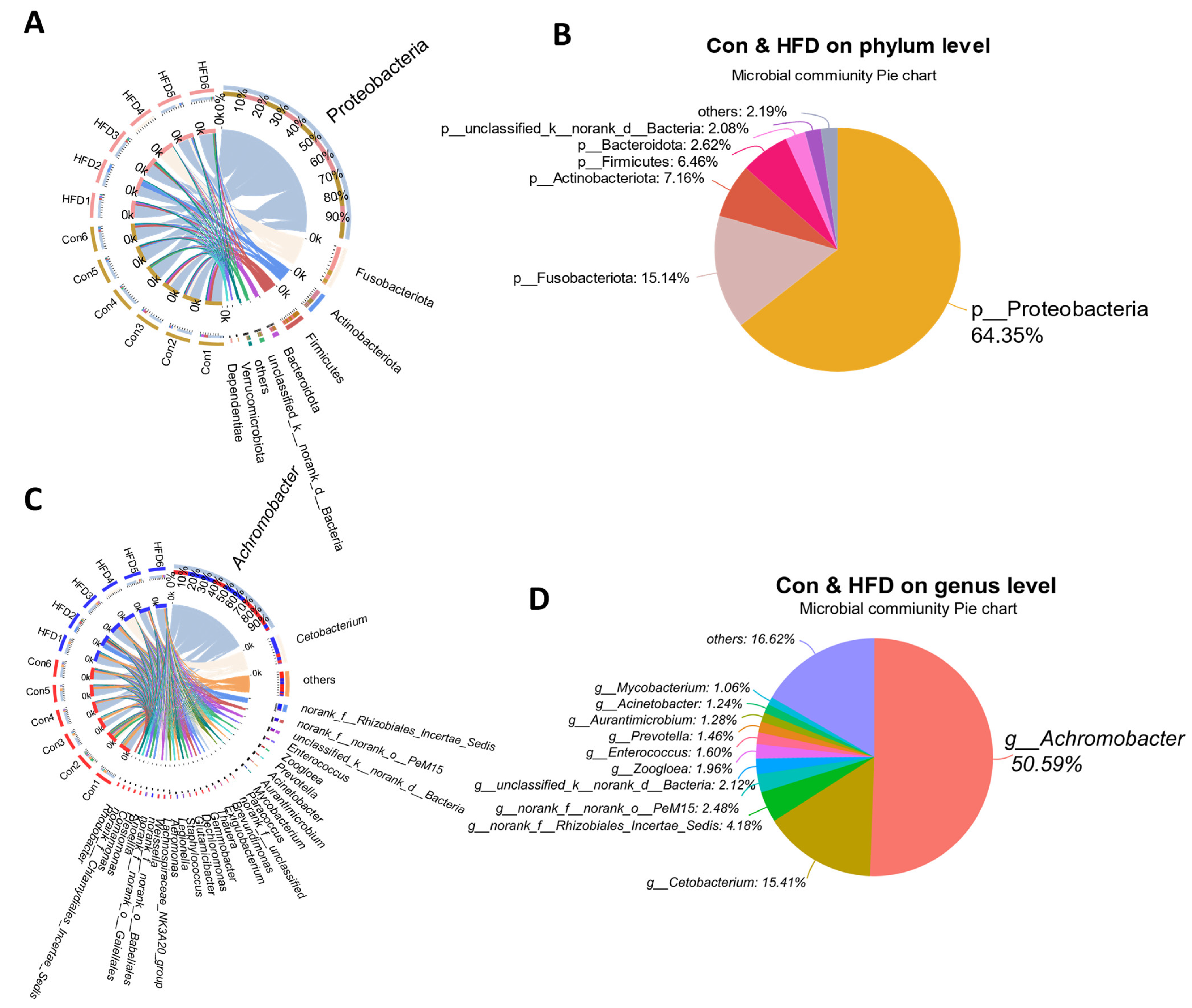
3.5. Dominant Microbe Distribution in the Gut of A. grunniens Fed with a High-Fat Diet
3.6. Microbial Comparison and Phylogenetic Tree Analysis
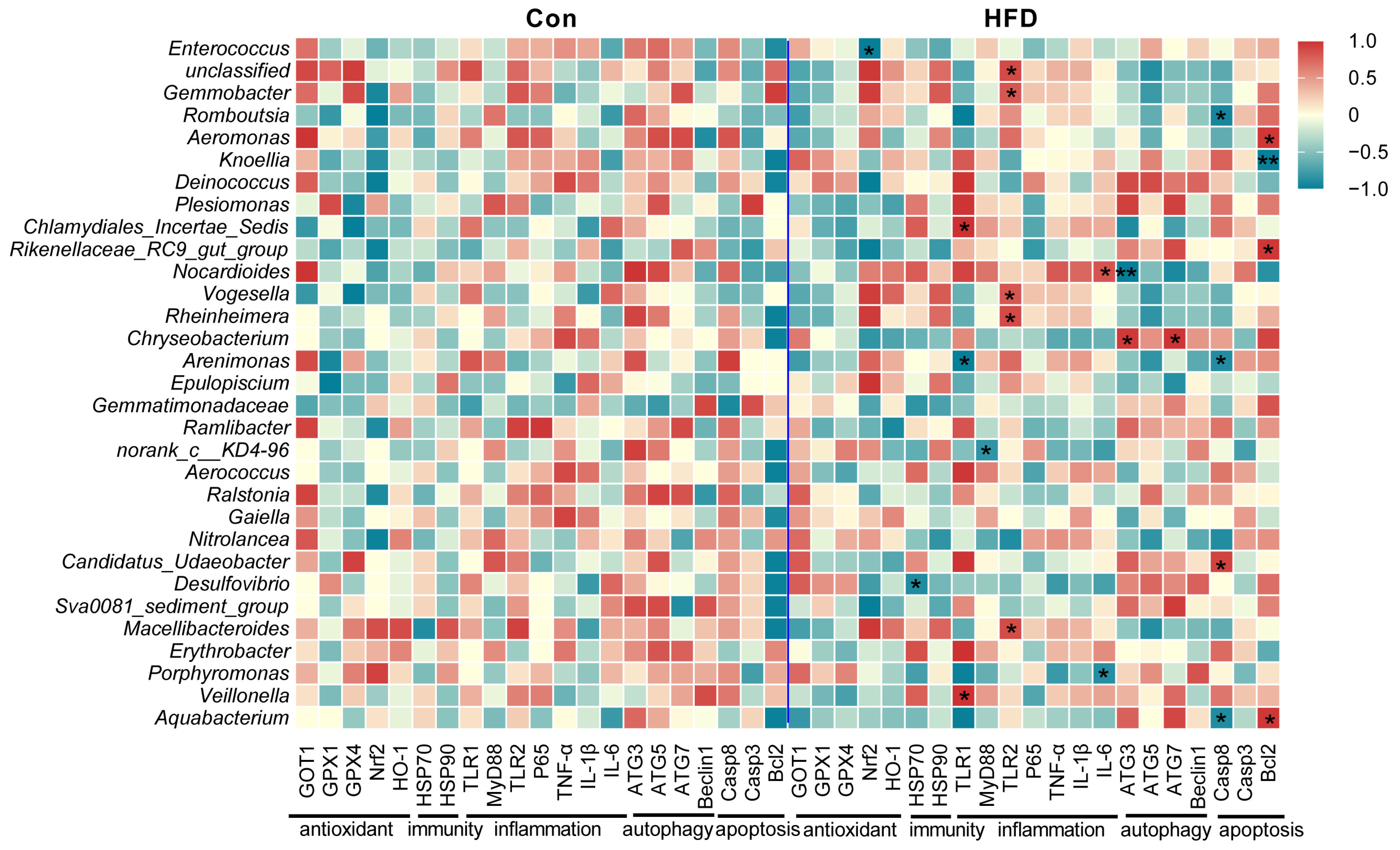
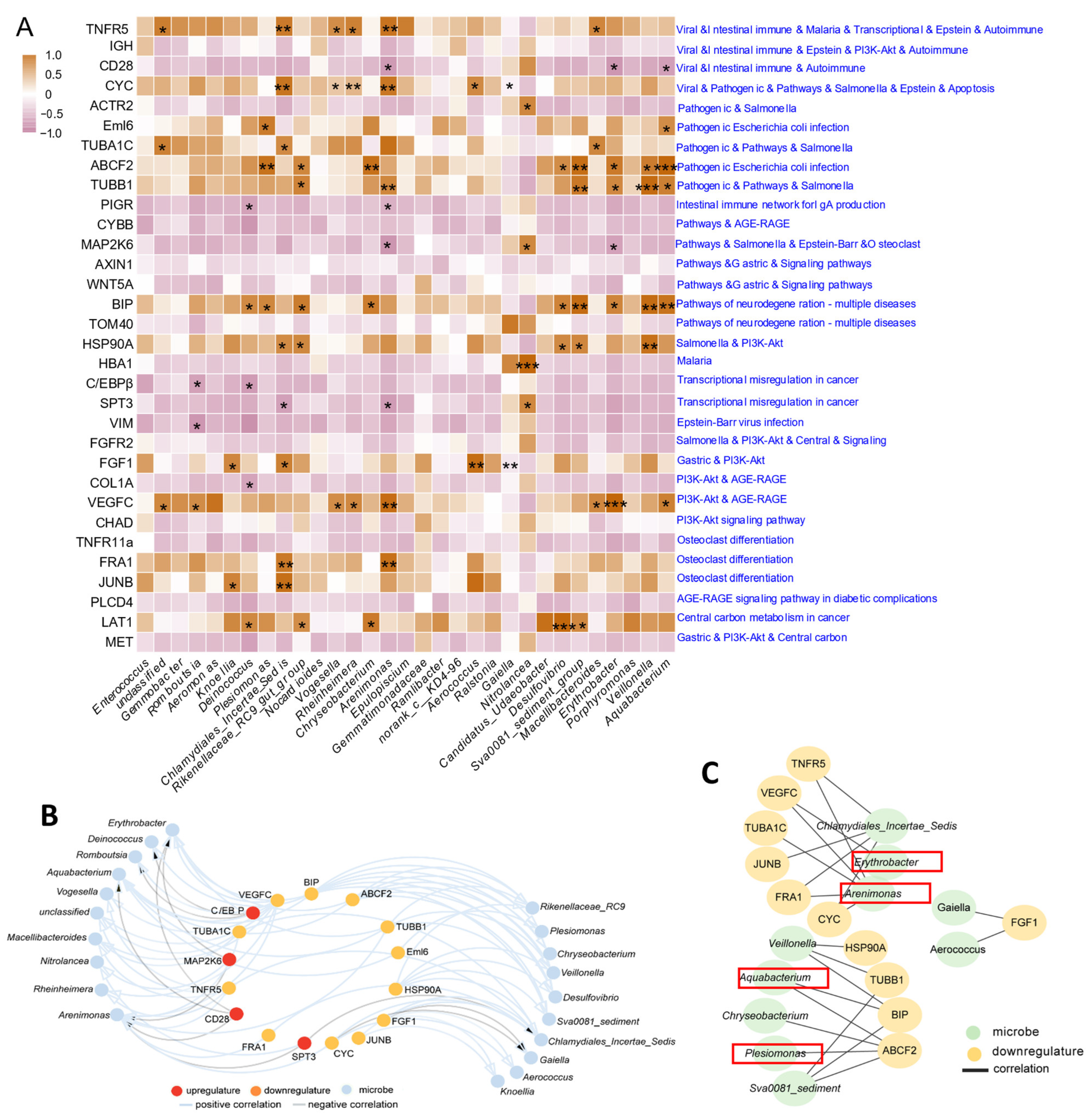
3.7. Correlation Analysis of Gut Antioxidant, Immune, Inflammatory, Autophagy and Apoptosis Genes with Gut Bacteria
3.8. Integrated Analysis between DEGs and Bacteria of A. grunniens on a High-Fat Diet
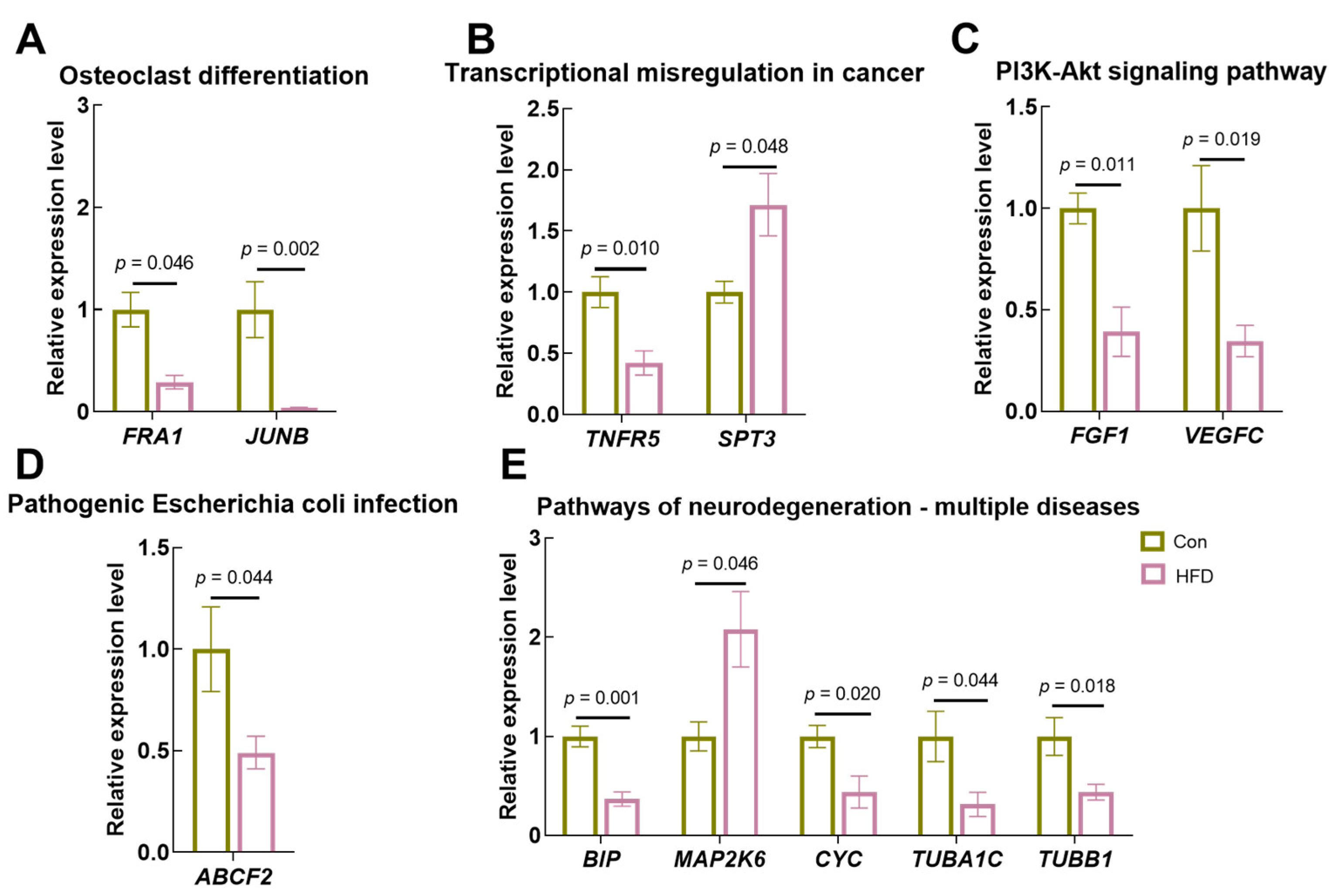
3.9. Expression Validation of Key Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, C.; Zhang, J.; Qin, Q.; Liu, J.; Xu, J.; Xu, W. Berberine Improved Intestinal Barrier Function by Modulating the Intestinal Microbiota in Blunt Snout Bream (Megalobrama amblycephala) under Dietary High-Fat and High-Carbohydrate Stress. Fish Shellfish Immunol. 2020, 102, 336–349. [Google Scholar] [CrossRef]
- Kamalam, B.S.; Medale, F.; Panserat, S. Utilisation of Dietary Carbohydrates in Farmed Fishes: New Insights on Influencing Factors, Biological Limitations and Future Strategies. Aquaculture 2017, 467, 3–27. [Google Scholar] [CrossRef]
- Abimorad, E.G.; Carneiro, D.J. Digestibility and Performance of Pacu (Piaractus mesopotamicus) Juveniles? Fed Diets Containing Different Protein, Lipid and Carbohydrate Levels. Aquac. Nutr. 2007, 13, 1–9. [Google Scholar] [CrossRef]
- Watanabe, T. Lipid Nutrition in Fish. Comp. Biochem. Physiol. Part B Comp. Biochem. 1982, 73, 3–15. [Google Scholar] [CrossRef]
- Xie, R.; Amenyogbe, E.; Chen, G.; Huang, J. Effects of Feed Fat Level on Growth Performance, Body Composition and Serum Biochemical Indices of Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus polyphekadion). Aquaculture 2021, 530, 735813. [Google Scholar] [CrossRef]
- Zhou, W.; Rahimnejad, S.; Lu, K.; Wang, L.; Liu, W. Effects of Berberine on Growth, Liver Histology, and Expression of Lipid-Related Genes in Blunt Snout Bream (Megalobrama amblycephala) Fed High-Fat Diets. Fish Physiol. Beachem. 2018, 45, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chatzifotis, S.; Panagiotidou, M.; Papaioannou, N.; Pavlidis, M.; Nengas, I.; Mylonas, C.C. Effect of Dietary Lipid Levels on Growth, Feed Utilization, Body Composition and Serum Metabolites of Meagre (Argyrosomus regius) Juveniles. Aquaculture 2010, 307, 65–70. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Clouet, P.; Zheng, W.-H.; Degrace, P.; Tian, L.-X.; Liu, Y.-J. Biochemical Hepatic Alterations and Body Lipid Composition in the Herbivorous Grass Carp (Ctenopharyngodon idella) Fed High-Fat Diets. Br. J. Nutr. 2006, 95, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Lin, Y.; Wu, T.; Tian, L.; Liang, J.; Tan, B. Dietary Lipid Levels Affected Growth Performance, Lipid Accumulation, Inflammatory Response and Apoptosis of Japanese Seabass (Lateolabrax japonicus). Aquac. Nutr. 2021, 27, 807–816. [Google Scholar] [CrossRef]
- Wang, A.; Meng, D.; Hao, Q.; Xia, R.; Zhang, Q.; Ran, C.; Yang, Y.; Li, D.; Liu, W.; Zhang, Z.; et al. Effect of Supplementation of Solid-State Fermentation Product of Bacillus Subtilis HGcc-1 to High-Fat Diet on Growth, Hepatic Lipid Metabolism, Epidermal Mucus, Gut and Liver Health and Gut Microbiota of Zebrafish. Aquaculture 2022, 560, 738542. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Abdelnour, S.A. The Risk Assessment of High-fat Diet in Farmed Fish and Its Mitigation Approaches: A Review. J. Anim. Physiol. Anim. Nutr. 2022, 107, 948–969. [Google Scholar] [CrossRef]
- Hamlin, H.J.; Von Herbing, I.H.; Kling, L.J. Histological and Morphological Evaluations of the Digestive Tract and Associated Organs of Haddock throughout Post-hatching Ontogeny. J. Fish Biol. 2000, 57, 716–732. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of Gastrointestinal Microbiota in Fish. Aquacult. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Ghanbari, M.; Kneifel, W.; Domig, K.J. A New View of the Fish Gut Microbiome: Advances from next-Generation Sequencing. Aquaculture 2015, 448, 464–475. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Zhuang, Z.; He, X.; Tang, X.; Tian, L.; Liu, Y.; Niu, J. Dietary Supplementation of Bile Acid Attenuate Adverse Effects of High-Fat Diet on Growth Performance, Antioxidant Ability, Lipid Accumulation and Intestinal Health in Juvenile Largemouth Bass (Micropterus salmoides). Aquaculture 2021, 531, 735864. [Google Scholar] [CrossRef]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and Ecological Factors That Shape the Gut Bacterial Communities of Fish: A Meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Pan, M.; Zan, Z.; Jakovlić, I.; Zhao, W.; Zou, H.; Ringø, E.; Wang, G. Regulation of Lipid Metabolism by Gut Microbiota in Aquatic Animals. Rev. Aquac. 2023, 16, 34–46. [Google Scholar] [CrossRef]
- Kortner, T.M.; Skugor, S.; Penn, M.H.; Mydland, L.; Djordjevic, B.; Hillestad, M.; Krasnov, A.; Krogdahl, Å. Dietary Soyasaponin Supplementation to Pea Protein Concentrate Reveals Nutrigenomic Interactions Underlying Enteropathy in Atlantic Salmon (Salmo salar). BMC Vet. Res. 2012, 8, 101. [Google Scholar] [CrossRef]
- Tacchi, L.; Bickerdike, R.; Douglas, A.; Secombes, C.J.; Martin, S.A.M. Transcriptomic Responses to Functional Feeds in Atlantic Salmon (Salmo salar). Fish Shellfish Immunol. 2011, 31, 704–715. [Google Scholar] [CrossRef]
- Tacchi, L.; Secombes, C.J.; Bickerdike, R.; Adler, M.A.; Venegas, C.; Takle, H.; Martin, S.A. Transcriptomic and Physiological Responses to Fishmeal Substitution with Plant Proteins in Formulated Feed in Farmed Atlantic Salmon (Salmo salar). BMC Genom. 2012, 13, 363. [Google Scholar] [CrossRef]
- Xu, W.-H.; Guo, H.-H.; Chen, S.-J.; Wang, Y.-Z.; Lin, Z.-H.; Huang, X.-D.; Tang, H.-J.; He, Y.-H.; Sun, J.-J.; Gan, L. Transcriptome Analysis Revealed Changes of Multiple Genes Involved in Muscle Hardness in Grass Carp (Ctenopharyngodon idellus) Fed with Faba Bean Meal. Food Chem. 2020, 314, 126205. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Y.; Dong, L.; Gan, J.; Mao, T.; Liu, T.; Li, X.; He, L. Effects of Moderate Exercise on Hepatic Amino Acid and Fatty Acid Composition, Liver Transcriptome, and Intestinal Microbiota in Channel Catfish (Ictalurus punctatus). Comp. Biochem. Phys. Part D Genom. Proteom. 2021, 40, 100921. [Google Scholar] [CrossRef]
- Hernández-Gómez, R.E.; Contreras-Sánchez, W.M.; Hernández-Franyutti, A.; Perera-García, M.A.; Torres-Martínez, A. Testicular Structure and Development of the Male Germinal Epithelium in the Freshwater Drum Aplodinotus grunniens (Perciformes: Sciaenidae) from the Usumacinta River, Southern Mexico. Acta Zool. 2021, 103, 414–432. [Google Scholar] [CrossRef]
- Chen, J.; Song, C.; Wen, H.; Liu, G.; Wu, N.; Li, H.; Xue, M.; Xu, P. miR-1/AMPK-Mediated Glucose and Lipid Metabolism under Chronic Hypothermia in the Liver of Freshwater Drum, Aplodinotus grunniens. Metabolites 2022, 12, 697. [Google Scholar] [CrossRef]
- Song, C.; Wen, H.; Liu, G.; Ma, X.; Lv, G.; Wu, N.; Chen, J.; Xue, M.; Li, H.; Xu, P. Gut Microbes Reveal Pseudomonas Medicates Ingestion Preference via Protein Utilization and Cellular Homeostasis Under Feed Domestication in Freshwater Drum, Aplodinotus grunniens. Front. Microbiol. 2022, 13, 861705. [Google Scholar] [CrossRef]
- Chen, J.; Xu, P.; Wen, H.; Xue, M.; Wang, Q.; He, J.; He, C.; Su, S.; Li, J.; Yu, F.; et al. Hypothermia-Mediated Oxidative Stress Induces Immunosuppression, Morphological Impairment and Cell Fate Disorder in the Intestine of Freshwater Drum, Aplodinotus grunniens. Aquaculture 2023, 575, 739805. [Google Scholar] [CrossRef]
- Wu, N.; Wen, H.; Xu, P.; Chen, J.; Xue, M.; Li, J.; Wang, M.; Song, C.; Li, H. PPAR Signaling Maintains Metabolic Homeostasis under Hypothermia in Freshwater Drum (Aplodinotus grunniens). Metabolites 2023, 13, 102. [Google Scholar] [CrossRef]
- Xue, M.; Xu, P.; Wen, H.; Chen, J.; Wang, Q.; He, J.; He, C.; Kong, C.; Song, C.; Li, H. Peroxisome Proliferator-Activated Receptor Signaling-Mediated 13-S-Hydroxyoctadecenoic Acid Is Involved in Lipid Metabolic Disorder and Oxidative Stress in the Liver of Freshwater Drum, Aplodinotus grunniens. Antioxidants 2023, 12, 1615. [Google Scholar] [CrossRef]
- Sun, Y.; Han, W.; Liu, J.; Huang, X.; Zhou, W.; Zhang, J.; Cheng, Y. Bacterial Community Compositions of Crab Intestine, Surrounding Water, and Sediment in Two Different Feeding Modes of Eriocheir Sinensis. Aquac. Rep. 2020, 16, 100236. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; He, Q.; Lin, F.; Wang, Q.; Xiao, S.; Dai, Y.; Zhang, Y.; Yang, H.; Zhao, H. Effect of Starvation and Refeeding on Growth, Gut Microbiota and Non-Specific Immunity in Hybrid Grouper (Epinephelus fuscoguttatus♀×E. Lanceolatus♂). Fish Shellfish Immunol. 2020, 97, 182–193. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and Antioxidants in Disease: Oxidative Stress in Farm Animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Feng, L.; Jiang, W.-D.; Kuang, S.-Y.; Jiang, J.; Li, S.-H.; Tang, L.; Zhou, X.-Q. Effects of Dietary Arginine Supplementation on Growth Performance, Flesh Quality, Muscle Antioxidant Capacity and Antioxidant-Related Signalling Molecule Expression in Young Grass Carp (Ctenopharyngodon idella). Food Chem. 2015, 167, 91–99. [Google Scholar] [CrossRef]
- Wang, B.; Sun, J.; Li, X.; Zhou, Q.; Bai, J.; Shi, Y.; Le, G. Resveratrol Prevents Suppression of Regulatory T-Cell Production, Oxidative Stress, and Inflammation of Mice Prone or Resistant to High-Fat Diet–Induced Obesity. Nutr. Res. 2013, 33, 971–981. [Google Scholar] [CrossRef]
- Imai, H.; Nakagawa, Y. Biological Significance of Phospholipid Hydroperoxide Glutathione Peroxidase (PHGPx, GPx4) in Mammalian Cells. Free Radical Biol. Med. 2003, 34, 145–169. [Google Scholar] [CrossRef]
- Kosower, N.S.; Kosower, E.M. The Glutathione Status of Cells. Int. Rev. Cytol. 1978, 54, 109–160. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ding, T.; Xu, N.; Liu, Y.; Du, J.; Xiang, X.; Xu, D.; Liu, Q.; Yin, Z.; Li, J.; Mai, K.; et al. Effect of Dietary Bile Acid (BA) on the Growth Performance, Body Composition, Antioxidant Responses and Expression of Lipid Metabolism-Related Genes of Juvenile Large Yellow Croaker (Larimichthys crocea) Fed High-Lipid Diets. Aquaculture 2020, 518, 734768. [Google Scholar] [CrossRef]
- Jia, Y.; Jing, Q.; Niu, H.; Huang, B. Ameliorative Effect of Vitamin E on Hepatic Oxidative Stress and Hypoimmunity Induced by High-Fat Diet in Turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2017, 67, 634–642. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Liu, B.; Gao, Q.; Sun, C.; Zhou, Q.; Zheng, X.; Liu, B. Effects of High Fat in the Diet on Growth, Antioxidant, Immunity and Fat Deposition of Macrobrachium rosenbergii Post-Larvae. Fish Shellfish Immunol. 2022, 129, 13–21. [Google Scholar] [CrossRef]
- Kremer, D.M.; Nelson, B.S.; Lin, L.; Yarosz, E.L.; Halbrook, C.J.; Kerk, S.A.; Sajjakulnukit, P.; Myers, A.; Thurston, G.; Hou, S.W.; et al. GOT1 Inhibition Promotes Pancreatic Cancer Cell Death by Ferroptosis. Nat. Commun. 2021, 12, 4860. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Chen, M.; Wang, Y.; Shi, J.; Hou, Y. Role of Ferroptosis on Tumor Progression and Immunotherapy. Cell Death Discov. 2022, 8, 427. [Google Scholar] [CrossRef]
- de Haan, J.B.; Crack, P.J.; Flentjar, N.; Iannello, R.C.; Hertzog, P.J.; Kola, I. An Imbalance in Antioxidant Defense Affects Cellular Function: The Pathophysiological Consequences of a Reduction in Antioxidant Defense in the Glutathione Peroxidase-1 (Gpx1) Knockout Mouse. Redox Rep. 2003, 8, 69–79. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription Factor Nrf2 Coordinately Regulates a Group of Oxidative Stress-Inducible Genes in Macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef]
- Itoh, K.; Mimura, J.; Yamamoto, M. Discovery of the Negative Regulator of Nrf2, Keap1: A Historical Overview. Antioxid. Redox Signaling 2010, 13, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The Role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef]
- Furfaro, A.L.; Traverso, N.; Domenicotti, C.; Piras, S.; Moretta, L.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. The Nrf2/HO-1 Axis in Cancer Cell Growth and Chemoresistance. Oxid. Med. Cell Longev. 2016, 2016, 1958174. [Google Scholar] [CrossRef]
- Rombout, J.H.W.M.; Abelli, L.; Picchietti, S.; Scapigliati, G.; Kiron, V. Teleost Intestinal Immunology. Fish Shellfish Immunol. 2011, 31, 616–626. [Google Scholar] [CrossRef]
- Castillo, J.; Teles, M.; Mackenzie, S.; Tort, L. Stress-Related Hormones Modulate Cytokine Expression in the Head Kidney of Gilthead Seabream (Sparus aurata). Fish Shellfish Immunol. 2009, 27, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Zou, J.; Savan, R.; Kono, T.; Sakai, M.; Woo, J.; Secombes, C. Characterisation and Expression Analysis of an Interleukin 6 Homologue in the Japanese Pufferfish. Dev. Comp. Immunol. 2005, 29, 775–789. [Google Scholar] [CrossRef]
- Xia, X.; Wang, X.; Qin, W.; Jiang, J.; Cheng, L. Emerging Regulatory Mechanisms and Functions of Autophagy in Fish. Aquaculture 2019, 511, 734212. [Google Scholar] [CrossRef]
- Zhang, D.-G.; Zhao, T.; Hogstrand, C.; Ye, H.-M.; Xu, X.-J.; Luo, Z. Oxidized Fish Oils Increased Lipid Deposition via Oxidative Stress-Mediated Mitochondrial Dysfunction and the CREB1-Bcl2-Beclin1 Pathway in the Liver Tissues and Hepatocytes of Yellow Catfish. Food Chem. 2021, 360, 129814. [Google Scholar] [CrossRef]
- Murakawa, M.; Jung, S.-K.; Iijima, K.; Yonehara, S. Apoptosis-Inducing Protein, AIP, from Parasite-Infected Fish Induces Apoptosis in Mammalian Cells by Two Different Molecular Mechanisms. Cell Death Differ. 2001, 8, 298–307. [Google Scholar] [CrossRef]
- Woo, S.; Park, I.-C.; Park, M.-J.; Lee, H.-C.; Lee, S.-J.; Chun, Y.-J.; Lee, S.-H.; Hong, S.-I.; Rhee, C. Arsenic Trioxide Induces Apoptosis through a Reactive Oxygen Species-Dependent Pathway and Loss of Mitochondrial Membrane Potential in HeLa Cells. Int. J. Oncol. 2002, 21, 57–63. [Google Scholar] [CrossRef]
- Tan, R.; Dong, H.; Chen, Z.; Jin, M.; Yin, J.; Li, H.; Shi, D.; Shao, Y.; Wang, H.; Chen, T.; et al. Intestinal Microbiota Mediates High-Fructose and High-Fat Diets to Induce Chronic Intestinal Inflammation. Front. Cell Infect. Microbiol. 2021, 11, 654074. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.-P.; Shen, Y.-Q. A Systematic Review of Advances in Intestinal Microflora of Fish. Fish Physiol. Biochem. 2021, 47, 2041–2053. [Google Scholar] [CrossRef]
- Fiedler, A.W.; Drågen, M.K.R.; Lorentsen, E.D.; Vadstein, O.; Bakke, I. The Stability and Composition of the Gut and Skin Microbiota of Atlantic Salmon throughout the Yolk Sac Stage. Front. Microbiol. 2023, 14, 1177972. [Google Scholar] [CrossRef]
- Borges, N.; Keller-Costa, T.; Sanches-Fernandes, G.M.M.; Louvado, A.; Gomes, N.C.M.; Costa, R. Bacteriome Structure, Function, and Probiotics in Fish Larviculture: The Good, the Bad, and the Gaps. Annu. Rev. Anim. Biosci. 2021, 9, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Xiong, F.; Hao, Y.-T.; Zhang, J.; Wu, S.-G.; Wang, G.-T. Starvation Influences the Microbiota Assembly and Expression of Immunity-Related Genes in the Intestine of Grass Carp (Ctenopharyngodon idellus). Aquaculture 2018, 489, 121–129. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.-F.; Ding, L.-G.; Wu, S.; Wu, Z.-B.; Cheng, G.-F.; Zhai, X.; Sun, R.-H.; Xu, Z. Interactions between Commensal Microbiota and Mucosal Immunity in Teleost Fish During Viral Infection With SVCV. Front. Immunol. 2021, 12, 654758. [Google Scholar] [CrossRef] [PubMed]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a Core Gut Microbiota in the Zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef]
- Santos, J.A.; Rodríguez-Calleja, J.-M.; Otero, A.; García-López, M.-L. Plesiomonas. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1111–1123. [Google Scholar] [CrossRef]
- Pękala-Safińska, A. Contemporary Threats of Bacterial Infections in Freshwater Fish. J. Vet. Res. 2018, 62, 261–267. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, D.; Liu, J.; Sun, X.; Gao, F.; Duan, H.; Dong, L.; Wang, X.; Wu, C. Pediococcus Pentosaceus PR-1 Modulates High-Fat-Died-Induced Alterations in Gut Microbiota, Inflammation, and Lipid Metabolism in Zebrafish. Front. Nutr. 2023, 10, 1087703. [Google Scholar] [CrossRef]
- Kalmbach, S.; Manz, W.; Wecke, J.; Szewzyk, U. Aquabacterium Gen. Nov., with Description of Aquabacterium citratiphilum sp. nov., Aquabacterium parvum sp. Nov. and Aquabacterium commune sp. nov., Three in Situ Dominant Bacterial Species from the Berlin Drinking Water System. Int. J. Syst. Evol. Microbiol. 1999, 49, 769–777. [Google Scholar] [CrossRef]
- Lin, M.-C.; Jiang, S.-R.; Chou, J.-H.; Arun, A.B.; Young, C.-C.; Chen, W.-M. Aquabacterium fontiphilum sp. nov., Isolated from Spring Water. Int. J. Syst. Evol. Microbiol. 2009, 59, 681–685. [Google Scholar] [CrossRef]
- Shelomi, M.; Lo, W.-S.; Kimsey, L.S.; Kuo, C.-H. Analysis of the Gut Microbiota of Walking Sticks (Phasmatodea). BMC Res. Notes 2013, 6, 368. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, Y.; Zhang, S.; Zhang, A.; Song, X.; Wang, L.; Li, H.; Chen, W. Effects of Culture Methods on the Nutrient Levels, Physiological Characteristics and Intestinal Microbiota of the Innkeeper Worm Urechis unicinctus. Aquac. Res. 2021, 52, 3843–3853. [Google Scholar] [CrossRef]
- Slinger, J.; Adams, M.B.; Stratford, C.N.; Rigby, M.; Wynne, J.W. The Effect of Antimicrobial Treatment upon the Gill Bacteriome of Atlantic Salmon (Salmo salar L.) and Progression of Amoebic Gill Disease (AGD) In Vivo. Microorganisms 2021, 9, 987. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ma, H.; Sun, M.; Wang, X.; Jin, S.; Yuan, X. Insufficient or Excessive Dietary Carbohydrates Affect Gut Health through Change in Gut Microbiota and Regulation of Gene Expression of Gut Epithelial Cells in Grass Carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2023, 132, 108442. [Google Scholar] [CrossRef]
- Rocha, S.D.C.; Lei, P.; Morales-Lange, B.; Mydland, L.T.; Øverland, M. From a Cell Model to a Fish Trial: Immunomodulatory Effects of Heat-Killed Lactiplantibacillus plantarum as a Functional Ingredient in Aquafeeds for Salmonids. Front. Immunol. 2023, 14, 1125702. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, C.; Zhang, C.; Li, P.; Zhang, J.; Ji, H.; Yu, H. Dietary Sea Buckthorn Polysaccharide Reduced Lipid Accumulation, Alleviated Inflammation and Oxidative Stress, and Normalized Imbalance of Intestinal Microbiota That Was Induced by High-Fat Diet in Zebrafish (Danio rerio). Fish Physiol. Biochem. 2022, 48, 1717–1735. [Google Scholar] [CrossRef]
- Zheng, Q.; Lin, W.; Liu, Y.; Chen, C.; Jiao, N. A Comparison of 14 Erythrobacter Genomes Provides Insights into the Genomic Divergence and Scattered Distribution of Phototrophs. Front. Microbiol. 2016, 7, 984. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Su, H.; Xu, W.; Wen, G.; Xu, C.; Yang, K.; Zhang, S.; Cao, Y. Effect of a Bacillus Probiotic Compound on Penaeus Vannamei Survival, Water Quality, and Microbial Communities. Fishes 2023, 8, 362. [Google Scholar] [CrossRef]
- Xie, S.; Wei, D.; Tan, B.; Liu, Y.; Tian, L.; Niu, J. Schizochytrium limacinum Supplementation in a Low Fish-Meal Diet Improved Immune Response and Intestinal Health of Juvenile Penaeus monodon. Front. Physiol. 2020, 11, 613. [Google Scholar] [CrossRef]
- Moya, A.; Ferrer, M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, M.; Xu, P.; Wen, H.; Chen, J.; Wang, Q.; He, J.; He, C.; Kong, C.; Li, X.; Li, H.; et al. A High-Fat-Diet-Induced Microbiota Imbalance Correlates with Oxidative Stress and the Inflammatory Response in the Gut of Freshwater Drum (Aplodinotus grunniens). Antioxidants 2024, 13, 363. https://doi.org/10.3390/antiox13030363
Xue M, Xu P, Wen H, Chen J, Wang Q, He J, He C, Kong C, Li X, Li H, et al. A High-Fat-Diet-Induced Microbiota Imbalance Correlates with Oxidative Stress and the Inflammatory Response in the Gut of Freshwater Drum (Aplodinotus grunniens). Antioxidants. 2024; 13(3):363. https://doi.org/10.3390/antiox13030363
Chicago/Turabian StyleXue, Miaomiao, Pao Xu, Haibo Wen, Jianxiang Chen, Qingyong Wang, Jiyan He, Changchang He, Changxin Kong, Xiaowei Li, Hongxia Li, and et al. 2024. "A High-Fat-Diet-Induced Microbiota Imbalance Correlates with Oxidative Stress and the Inflammatory Response in the Gut of Freshwater Drum (Aplodinotus grunniens)" Antioxidants 13, no. 3: 363. https://doi.org/10.3390/antiox13030363
APA StyleXue, M., Xu, P., Wen, H., Chen, J., Wang, Q., He, J., He, C., Kong, C., Li, X., Li, H., & Song, C. (2024). A High-Fat-Diet-Induced Microbiota Imbalance Correlates with Oxidative Stress and the Inflammatory Response in the Gut of Freshwater Drum (Aplodinotus grunniens). Antioxidants, 13(3), 363. https://doi.org/10.3390/antiox13030363







