Multivariate Analysis of Biochemical Properties Reveals Diversity among Yardlong Beans of Different Origins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials Collection, Cultivation, and Preparation
2.3. Determination of Nutritional Contents
2.4. Fatty Acid Analysis Using Gas Chromatography
2.5. Vitamin C Analysis Using High-Performance Liquid Chromatography
2.6. Determination of Total Metabolite Contents
2.7. Determination of Antioxidant Activities
2.8. Statistical Analysis
3. Results and Discussion
3.1. Field Performances and Characteristics of Yardlong Beans
3.2. Nutritional Components
3.3. Fatty Acid Contents
3.4. Vitamin C Content
3.5. Total Secondary Metabolite Contents
3.6. Antioxidant Activities
3.7. Effects of Genotype and Origin Differences
3.8. Multivariate Analysis
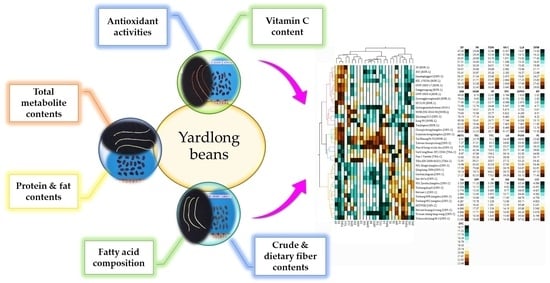
3.8.1. Hierarchical Cluster (HCA) and Principal Component (PCA) Analyses
3.8.2. Pearson’s Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, L.; Liu, M.; Kang, Y.; Mei, X.; Hu, G.; Bao, C.; Zheng, Y.; Zhao, H.; Chen, C.; Wang, N. Comprehensive genomic analyses of Vigna unguiculata provide insights into population differentiation and the genetic basis of key agricultural traits. Plant Biotechnol. J. 2023, 21, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, W.; Chen, H.; Chen, J.; Chen, X.; Yang, S. Evaluation and QTL Mapping of Salt Tolerance in Yardlong Bean [Vigna unguiculata (L.) Walp. Subsp. unguiculata Sesquipedalis Group] Seedlings. Plant Mol. Biol. Rep. 2020, 38, 294–304. [Google Scholar] [CrossRef]
- Quenum, A.J.C.; Pasquet, R.S.; Bodian, A.; Fonceka, D.; Djiboune, Y.R.; Cisse, N.; Mbaye, M.S.; Diouf, D. Molecular characterization of cowpea [Vigna unguiculata (L.) Walp.] subspecies with SSR markers. Genet. Resour. Crop Evol. 2023, 1–9. [Google Scholar] [CrossRef]
- Tantasawat, P.; Trongchuen, J.; Prajongjai, T.; Seehalak, W.; Jittayasothorn, Y. Variety identification and comparative analysis of genetic diversity in yardlong bean (Vigna unguiculata spp. sesquipedalis) using morphological characters, SSR and ISSR analysis. Sci. Hortic. 2010, 124, 204–216. [Google Scholar]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Pidigam, S.; Thuraga, V.; Pandravada, S.R.; Natarajan, S.; Adimulam, S.; Amarapalli, G.; Nimmarajula, S.; Venkateswaran, K. Genetic improvement of yardlong bean (Vigna unguiculata (L.) Walp ssp. sesquipedalis (L.) Verdc.). In Advances in Plant Breeding Strategies: Vegetable Crops; Springer: Berlin/Heidelberg, Germany, 2021; pp. 379–420. [Google Scholar]
- Abebe, B.K.; Alemayehu, M.T. A review of the nutritional use of cowpea (Vigna unguiculata L. Walp) for human and animal diets. J. Agric. Food Res. 2022, 10, 100383. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhang, Z.; Ren, S.; Wang, D.; Wang, X.; Wang, X.; Zhang, C.; Wang, M. Extraction and characterization of starch from Yard-long bean (Vigna unguiculata (L.) Walp. ssp. unguiculata cv.-gr. sesquipedalis). Int. J. Biol. Macromol. 2021, 181, 1023–1029. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, M.H.; Park, C.H.; Pae, S.B.; Shim, K.B.O.; Ko, J.M.; Shin, S.O.; Baek, I.N.Y.; Park, K.Y. Identification and characterization of anthocyanins in Yard-Long beans (Vigna unguiculata ssp. sesquipedalis L.) by High-Performance liquid chromatography with diode array detection and electrospray lonization/mass spectrometry (HPLC-DAD-ESI/MS) analysis. J. Agric. Food Chem. 2010, 58, 2571–2576. [Google Scholar]
- Watcharatpong, P.; Kaga, A.; Chen, X.; Somta, P. Narrowing Down a Major QTL Region Conferring Pod Fiber Contents in Yardlong Bean (Vigna unguiculata), a Vegetable Cowpea. Genes 2020, 11, 363. [Google Scholar] [CrossRef]
- Kongjaimun, A.; Somta, P.; Tomooka, N.; Kaga, A.; Vaughan, D.A.; Srinives, P. QTL mapping of pod tenderness and total soluble solid in yardlong bean [Vigna unguiculata (L.) Walp. subsp. unguiculata cv.-gr. sesquipedalis]. Euphytica 2013, 189, 217–223. [Google Scholar] [CrossRef]
- Quamruzzaman, A.K.M.; Islam, F.; Akter, L.; Khatun, A.; Mallick, S.R.; Gaber, A.; Laing, A.; Brestic, M.; Hossain, A. Evaluation of the Quality of Yard-Long Bean (Vigna unguiculata sub sp. sesquipedalis L.) Cultivars to Meet the Nutritional Security of Increasing Population. Agronomy 2022, 12, 2195. [Google Scholar]
- Machado, N.; Oppolzer, D.; Ramos, A.; Ferreira, L.; Rosa, E.A.S.; Rodrigues, M.; Domínguez-Perles, R.; Barros, A.I.R.N.A. Evaluating the freezing impact on the proximate composition of immature cowpea (Vigna unguiculata L.) pods: Classical versus spectroscopic approaches. J. Sci. Food Agric. 2017, 97, 4295–4305. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Goufo, P.; Barros, A.; Dominguez-Perles, R.; Trindade, H.; Rosa, E.A.S.; Ferreira, L.; Rodrigues, M. Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable agri-food system: Nutritional advantages and constraits. J. Sci. Food Agric. 2016; 96, 2941–2951. [Google Scholar]
- Shubha, K.; Choudhary, A.K.; Eram, A.; Mukherjee, A.; Kumar, U.; Dubey, A.K. Screening of Yardlong bean (Vigna unguiculata (L.) Walp. ssp. unguiculata cv.-gr. sesquipedalis) genotypes for seed, yield and disease resistance traits. Genet. Resour. Crop Evol. 2022, 69, 2307–2317. [Google Scholar]
- Zhang, Y.; Meenu, M.; Yu, H.; Xu, B. An investigation on phenolic and antioxidant capacity of under-utilized food legumes consumed in China. Foods 2020, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Menssen, M.; Linde, M.; Otunga Omondi, E.; Abukutsa-Onyango, M.; Dinssa, F.F.; Winkelmann, T. Genetic and morphological diversity of cowpea (Vigna unguiculata (L.) Walp.) entries from East Africa. Sci. Hortic. 2017, 226, 268–276. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Burlyaeva, M.O.; Zinchenko, Y.N.; Krylova, E.A.; Chunikhina, O.A.; Ivanova, N.M.; Zakharenko, A.M.; Golokhvast, K.S. Identification and Spatial Distribution of Bioactive Compounds in Seeds Vigna unguiculata (L.) Walp. by Laser Microscopy and Tandem Mass Spectrometry. Plants 2022, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Uribe, J.A.; Romo-Lopez, I.; Serna-Saldívar, S.O. Phenolic composition and mammary cancer cell inhibition of extracts of whole cowpeas (Vigna unguiculata) and its anatomical parts. J. Funct. Foods 2011, 3, 290–297. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kalpanadevi, V.; Mohan, V.R. Effect of processing on antinutrients and in vitro protein digestibility of the underutilized legume, Vigna unguiculata (L.) Walp subsp. unguiculata. LWT 2013, 51, 455–461. [Google Scholar] [CrossRef]
- Yao, T.; Xu, Y.; Jiang, H.; Chen, X.; Liu, X.; Chen, H.; Zhang, H.; Xing, G. Evaluating, Screening and Selecting Yardlong Bean [Vigna unguiculata subsp. sesquipedalis (L.) Verdc.] for Resistance to Common Cutworm (Spodoptera litura Fabricius). Agronomy 2023, 13, 502. [Google Scholar]
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef]
- Verma, S.K.; Singh, C.K.; Taunk, J.; Gayacharan; Chandra Joshi, D.; Kalia, S.; Dey, N.; Singh, A.K. Vignette of Vigna domestication: From archives to genomics. Front. Genet. 2022, 13, 1–23. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Choi, Y.M.; Yoon, H.; Shin, M.J.; Lee, S.; Yi, J.; Jeon, Y.A.; Wang, X.; Desta, K.T. Nutrient Levels, Bioactive Metabolite Contents, and Antioxidant Capacities of Faba Beans as Affected by Dehulling. Foods 2023, 12, 4063. [Google Scholar] [CrossRef] [PubMed]
- Vazquez Oderiz, M.L.; Vazquez Blanco, M.E.; Lopez Hernandez, J.; Simal Lozano, J.; Romero Rodriguez, M.A. Simultaneous determination of organic acids and vitamin C in green beans by liquid chromatography. J. AOAC Int. 1994, 77, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Pidigam, S.; Munnam, S.B.; Nimmarajula, S.; Gonela, N.; Adimulam, S.S.; Yadla, H.; Bandari, L.; Amarapalli, G. Assessment of genetic diversity in yardlong bean (Vigna unguiculata (L.) Walp subsp. sesquipedalis Verdc.) germplasm from India using RAPD markers. Genet. Resour. Crop Evol. 2019, 66, 1231–1242. [Google Scholar]
- Kongjaimun, A.; Kaga, A.; Tomooka, N.; Somta, P.; Vaughan, D.A.; Srinives, P. The genetics of domestication of yardlong bean, Vigna unguiculata (L.) Walp. ssp. unguiculata cv.-gr. sesquipedalis. Ann. Bot. 2012, 109, 1185–1200. [Google Scholar]
- Rambabu, E.; Reddy, K.R.; Kamala, V.; Saidaiah, P.; Pandravada, S.R. Correlation and path analysis for quality, yield and yield components in yardlong bean (Vigna unguiculata (L.) Walp. ssp. sesquipedalis Verdc.). Environ. Ecol. 2016, 34, 1655–1661. [Google Scholar]
- Bai, Z.; Huang, X.; Meng, J.; Kan, L.; Nie, S. A comparative study on nutritive peculiarities of 24 Chinese cowpea cultivars. Food Chem. Toxicol. 2020, 146, 111841. [Google Scholar] [CrossRef] [PubMed]
- Onwuliri, V.A.; Obu, J.A. Lipids and other constituents of Vigna unguiculata and Phaseolus vulgaris grown in northern Nigeria. Food Chem. 2002, 78, 1–7. [Google Scholar] [CrossRef]
- Perchuk, I.; Shelenga, T.; Gurkina, M.; Miroshnichenko, E.; Burlyaeva, M. composition of primary and secondary metabolite compounds in seeds and pods of asparagus bean (Vigna unguiculata (L.) Walp.) from China. Molecules 2020, 25, 1–16. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.H.; Lee, B.W.; Lee, Y.Y. Quality and physicochemical characterstics of Korean Cowpea cultivars grown in diffrenet seedling periods. Korean J. Food Nutr. 2018, 31, 502–510. [Google Scholar]
- Dakora, F.D.; Belane, A.K. Evaluation of Protein and Micronutrient Levels in Edible Cowpea (Vigna Unguiculata L. Walp.) Leaves and Seeds. Front. Sustain. Food Syst. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Baptista, A.; Pinho, O.; Pinto, E.; Casal, S.; Mota, C.; Ferreira, I.M.P.L.V.O. Characterization of protein and fat composition of seeds from common beans (Phaseolus vulgaris L.), cowpea (Vigna unguiculata L. Walp) and bambara groundnuts (Vigna subterranea L. Verdc) from Mozambique. J. Food Meas. Charact. 2017, 11, 442–450. [Google Scholar] [CrossRef]
- Lazzarin, T.; Martins, D.; Ballarin, R.S.; Monte, M.G.; Minicucci, M.F.; Polegato, B.F.; Zornoff, L. The Role of Omega-3 in Attenuating Cardiac Remodeling and Heart Failure through the Oxidative Stress and Inflammation Pathways. Antioxidants 2023, 12, 2067. [Google Scholar] [CrossRef] [PubMed]
- Morshedloo, M.R.; Fereydouni, S.; Ahmadi, H.; Hassanpouraghdam, M.B.; Aghaee, A.; Vojodi Mehrabani, L.; Maggi, F. Natural diversity in fatty acids profiles and antioxidant properties of sumac fruits (Rhus coriaria L.): Selection of preferable populations for food industries. Food Chem. 2022, 374, 131757. [Google Scholar] [CrossRef] [PubMed]
- Devi, C.B.; Kushwaha, A.; Kumar, A. Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata). J. Food Sci. Technol. 2015, 52, 6821–6827. [Google Scholar] [CrossRef] [PubMed]
- Wawire, M.; Oey, I.; Mathooko, F.; Njoroge, C.; Shitanda, D.; Hendrickx, M. Thermal stability of ascorbic acid and ascorbic acid oxidase in African cowpea leaves (Vigna unguiculata) of different maturities. J. Agric. Food Chem. 2011, 59, 1774–1783. [Google Scholar] [CrossRef]
- Affrifah, N.S.; Phillips, R.D.; Saalia, F.K. Cowpeas: Nutritional profile, processing methods and products—A review. Legume Sci. 2022, 4, 1–12. [Google Scholar] [CrossRef]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An overview on its nutritional facts and health benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Ahmad, S.; Amarowicz, R.; De Feo, V. Antioxidant activity of the extracts of some cowpea (Vigna unguiculata (L) Walp.) cultivars commonly consumed in Pakistan. Molecules 2013, 18, 2005–2017. [Google Scholar] [CrossRef]
- Tzanova, M.T.; Stoilova, T.D.; Todorova, M.H.; Memdueva, N.Y.; Gerdzhikova, M.A.; Grozeva, N.H. Antioxidant Potentials of Different Genotypes of Cowpea (Vigna unguiculata L. Walp.) Cultivated in Bulgaria, Southern Europe. Agronomy 2023, 13, 1684. [Google Scholar] [CrossRef]
- Kanner, J. Food Polyphenols as Preventive Medicine. Antioxidants 2023, 12, 2103. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Laurena, A.C.; Garcia, V.V.; Mae, E.; Mendoza, T. Effects of heat on the removal of polyphenols and in vitro protein digestibility of cowpea (Vigna unguiculata (L.) Walp.). Plant Food Hum. Nutr. 1987, 37, 183–192. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Queiroz, M.; Brites, C.; Rosa, E.A.S.; Barros, A.I.R.N.A. Nutrients, antinutrients, phenolic composition, and antioxidant activity of common bean cultivars and their potential for food applications. Antioxidants 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, S.; Wang, H.; Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014, 152, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, J.; Yang, Q. Effects of geographical origin, variety, harvest season, and their interactions on multi-elements in cereal, tuber, and legume crops for authenticity. J. Food Compos. Anal. 2021, 100, 103900. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, B.; Wei, Y.; Zhang, B. Effects of wheat origin, genotype, and their interaction on multielement fingerprints for geographical traceability. J. Agric. Food Chem. 2012, 60, 10957–10962. [Google Scholar] [CrossRef]
- Irakli, M.; Kargiotidou, A.; Tigka, E.; Beslemes, D.; Fournomiti, M.; Pankou, C.; Stavroula, K.; Tsivelika, N.; Vlachostergios, D.N. Genotypic and environmental effect on the concentration of phytochemical contents of lentil (Lens culinaris L.). Agronomy 2021, 11, 1154. [Google Scholar] [CrossRef]
- Meftahizadeh, H.; Hatami, M. Changes in agronomic characteristics and galactomannan content in twenty cluster bean genotypes of different origins affected by sowing dates. Acta Ecol. Sin. 2022, 42, 24–32. [Google Scholar] [CrossRef]
- Omomowo, O.I.; Babalola, O.O. Constraints and Prospects of Improving Cowpea Productivity to Ensure Food, Nutritional Security and Environmental Sustainability. Front. Plant Sci. 2021, 12, 751731. [Google Scholar] [CrossRef]
- Oluwatosin, O.B. Genetic and environmental variation for seed yield, protein, lipid and amino acid composition in cowpea (Vigna unguiculata (L.) Walp). J. Sci. Food Agric. 1997, 74, 107–116. [Google Scholar] [CrossRef]
- Flyman, M.V.; Afolayan, A.J. Effect of maturity on the mineral content of the leaves of Momordica balsamina L. and Vigna unguiculata subsp. sesquipedalis Verdc. J. Food Qual. 2008, 31, 661–671. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Loscos, J.; Coronado, M.J.; Ramos, J.; Sato, S.; Testillano, P.S.; Tabata, S.; Becana, M. Biosynthesis of ascorbic acid in legume root nodules. Plant Physiol. 2006, 141, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Torres-Franklin, M.L.; Repellin, A.; Huynh, V.B.; d’Arcy-Lameta, A.; Zuily-Fodil, Y.; Pham-Thi, A.T. Omega-3 fatty acid desaturase (FAD3, FAD7, FAD8) gene expression and linolenic acid content in cowpea leaves submitted to drought and after rehydration. Environ. Exp. Bot. 2009, 65, 162–169. [Google Scholar] [CrossRef]
- Grela, E.R.; Samolińska, W.; Kiczorowska, B.; Klebaniuk, R.; Kiczorowski, P. Content of minerals and fatty acids and their correlation with phytochemical compounds and antioxidant activity of leguminous seeds. Biol. Trace Elem. Res. 2017, 180, 338–348. [Google Scholar] [CrossRef]






| Accession Name | Individual Fatty Acid Contents (%) | DBI | TUFA: TSFA | ||||
|---|---|---|---|---|---|---|---|
| Palmitic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | Linolenic Acid | |||
| San chi lu | 29.58 ± 0.04 o | 4.72 ± 0.02 l | 14.59 ± 0.03 kl | 35.81 ± 0.02 a | 15.29 ± 0.04 ijk | 132.10 | 1.92 |
| 901 Qingjo jiangdou | 29.20 ± 0.04 mn | 4.95 ± 0.02 abc | 13.74 ± 0.03 ef | 37.16 ± 0.12 c–h | 14.95 ± 0.12 m | 132.92 | 1.93 |
| Qing jiang 2006 | 29.26 ± 0.05 mn | 5.16 ± 0.02 ab | 14.24 ± 0.08 de | 36.34 ± 0.06 f–l | 15.00 ± 0.08 m | 131.92 | 1.91 |
| Gaotian jinguan | 29.66 ± 0.04 ij | 5.29 ± 0.02 a | 13.62 ± 0.02 ef | 36.68 ± 0.04 d–k | 14.74 ± 0.04 mno | 131.22 | 1.86 |
| Gyeonggiyonginsujib | 32.42 ± 0.10 a | 4.01 ± 0.01 ghi | 11.09 ± 0.07 h–j | 37.03 ± 0.07 c–i | 15.45 ± 0.18 jkl | 131.51 | 1.75 |
| Gangwonpyeongchang-2003-4 | 32.45 ± 0.01 a | 4.05 ± 0.02 ghi | 11.32 ± 0.02 g–j | 36.20 ± 0.06 f–l | 15.99 ± 0.08 fg | 131.69 | 1.74 |
| Gangwonpyeongchang-2003-17 | 32.26 ± 0.08 abc | 4.01 ± 0.01 ghi | 10.95 ± 0.12 h–l | 37.18 ± 0.02 c–h | 15.60 ± 0.06 ij | 132.10 | 1.76 |
| Hang xin taikong wu jia dou | 29.39 ± 0.10 klm | 4.45 ± 0.00 d–g | 16.05 ± 0.08 ab | 34.71 ± 0.05 kl | 15.40 ± 0.08 jkl | 131.69 | 1.96 |
| Techang 908 jiangdou | 29.31 ± 0.01 lmn | 4.94 ± 0.00 abc | 14.54 ± 0.04 cde | 36.54 ± 0.07 e–k | 14.68 ± 0.08 nop | 131.64 | 1.92 |
| Taiwan chunqiu hong | 32.45 ± 0.03 a | 4.16 ± 0.02 fgh | 10.03 ± 0.04 jkl | 38.83 ± 0.12 bc | 14.53 ± 0.16 op | 131.28 | 1.73 |
| Techang 902 jiangdou | 29.55 ± 0.03 i–l | 4.21 ± 0.02 fgh | 15.58 ± 0.09 abc | 36.25 ± 0.05 f–l | 14.41 ± 0.06 p | 131.31 | 1.96 |
| Tai Htaung Pe Ni | 32.02 ± 0.07 c | 4.43 ± 0.04 d–g | 10.49 ± 0.04 i–l | 38.52 ± 0.05 bcd | 14.55 ± 0.05 op | 131.17 | 1.74 |
| THA-JSH-2008-81021 | 29.96 ± 0.04 h | 3.44 ± 0.04 kl | 11.87 ± 0.05 gh | 38.06 ± 0.09 b–f | 16.67 ± 0.11 c | 138.00 | 1.99 |
| Tanjingeun | 32.13 ± 0.06 bc | 3.89 ± 0.02 hij | 7.88 ± 0.09 m | 38.48 ± 0.17 b–e | 17.62 ± 0.16 b | 137.71 | 1.78 |
| Jasaekginggori | 32.09 ± 0.11 c | 4.27 ± 0.01 e–h | 11.66 ± 0.04 ghi | 35.19 ± 0.12 h–l | 16.79 ± 0.26 c | 132.41 | 1.75 |
| Gatggeungong | 32.01 ± 0.01 c | 4.16 ± 0.03 fgh | 11.26 ± 0.04 hij | 36.81 ± 0.12 d–j | 15.76 ± 0.11 ghi | 132.17 | 1.76 |
| MMR-JYH-2010-90 | 31.25 ± 0.07 e | 3.99 ± 0.01 ghi | 9.76 ± 0.04 l | 37.54 ± 0.08 b–g | 17.46 ± 0.05 b | 137.22 | 1.84 |
| Sung 99 | 30.80 ± 0.02 f | 3.48 ± 0.00 jkl | 11.90 ± 0.01 gh | 36.35 ± 0.12 f–l | 17.47 ± 0.14 b | 136.99 | 1.92 |
| Guamian hong jiangdou | 32.08 ± 0.13 c | 4.25 ± 0.01 e–h | 10.77 ± 0.06 h–l | 37.65 ± 0.03 b–g | 15.25 ± 0.05 l | 131.81 | 1.75 |
| Qiu jiang 512 | 29.93 ± 0.08 h | 3.61 ± 0.01 ijk | 14.05 ± 0.05 de | 34.39 ± 0.06 l | 18.02 ± 0.05 a | 136.89 | 1.98 |
| Chungbuk Geosan 2011-18 | 31.72 ± 0.20 d | 4.37 ± 0.02 d–g | 11.48 ± 0.12 ghi | 35.91 ± 0.09 g–l | 16.52 ± 0.15 cd | 132.87 | 1.77 |
| Chungbuk Geosan 2011-245 | 31.54 ± 0.03 d | 4.44 ± 0.02 d–g | 11.88 ± 0.12 gh | 35.92 ± 0.06 g–l | 16.22 ± 0.14 ed | 132.38 | 1.78 |
| 901 Zaoshu jiangdou | 29.32 ± 0.04l mn | 4.79 ± 0.01 bcd | 13.84 ± 0.12 def | 35.77 ± 0.12 g–l | 16.28 ± 0.22 de | 134.22 | 1.93 |
| Techang jinqili | 29.25 ± 0.08 mn | 4.71 ± 0.01 cde | 14.16 ± 0.05 de | 35.31 ± 0.10 h–l | 16.56 ± 0.09 c | 134.46 | 1.94 |
| Nan 1 Variety | 29.64 ± 0.04 ijk | 3.34 ± 0.02 kl | 13.27 ± 0.07 ef | 37.16 ± 0.07 c–h | 16.59 ± 0.18 c | 137.35 | 2.03 |
| Hei mei 1 | 29.14 ± 0.09 mn | 4.71 ± 0.01 cde | 14.24 ± 0.05 de | 36.00 ± 0.06 g–l | 15.92 ± 0.19 gh | 134.00 | 1.95 |
| Chunqiu hong jiangdou | 32.37 ± 0.04 ab | 4.29 ± 0.02 e–h | 10.35 ± 0.01 i–l | 37.50 ± 0.09 b–g | 15.49 ± 0.08 jkl | 131.82 | 1.73 |
| Man di hong wu jia dou | 29.75 ± 0.06 hi | 3.40 ± 0.02 kl | 11.86 ± 0.07 gh | 38.37 ± 0.05 b–e | 16.63 ± 0.07 c | 138.49 | 2.02 |
| Tichun zhi jiang 28-2 | 29.08 ± 0.05 n | 4.00 ± 0.01 ghi | 16.08 ± 0.04 ab | 34.82 ± 0.03 jkl | 16.01 ± 0.08 fg | 133.76 | 2.02 |
| Te xuan zhang tang wang | 29.41 ± 0.02 j–m | 4.31 ± 0.01 e–h | 16.36 ± 0.07 a | 35.05 ± 0.06 i–l | 14.88 ± 0.02 mn | 131.11 | 1.97 |
| Hei mei huang zi wang | 29.36 ± 0.09 lmn | 4.57 ± 0.01 c–f | 15.08 ± 0.03 bcd | 35.02 ± 0.09 jkl | 15.97 ± 0.06 fg | 133.04 | 1.95 |
| Yard long Bean 287/2556 | 29.14 ± 0.12 mn | 4.30 ± 0.03 e–h | 12.01 ± 0.18 gh | 39.26 ± 0.06 b | 15.28 ± 0.12 kl | 136.39 | 1.99 |
| SD 3135 | 32.04 ± 0.03 c | 4.22 ± 0.02 fgh | 11.84 ± 0.09 gh | 35.91 ± 0.05 g–l | 15.99 ± 0.09 fg | 131.64 | 1.76 |
| KSL 170256 | 31.27 ± 0.05 e | 4.60 ± 0.04 c–f | 12.62 ± 0.15 fg | 35.83 ± 0.08 g–l | 15.68 ± 0.09 hij | 131.31 | 1.79 |
| Gyeongnamhabcheon-2019-2 | 30.33 ± 0.02 g | 4.35 ± 0.06 d–h | 10.43 ± 0.12 i–l | 36.76 ± 0.11 d–j | 18.12 ± 0.05 a | 138.34 | 1.88 |
| Range (Min–Max) | 29.08–32.45 | 3.34–5.29 | 7.88–16.36 | 34.39–39.26 | 14.41–18.12 | 131.11–138.49 | 1.73–2.03 |
| Total mean | 30.60 | 4.28 | 12.60 | 36.58 | 15.94 | - | - |
| CV (%) | 4.18 | 11.02 | 16.06 | 3.36 | 6.20 | - | - |
| Accession Name | Total Metabolite Contents | Antioxidant Activities | ||||
|---|---|---|---|---|---|---|
| TPC (mg GAE/g) | TSC (mg DE/g) | TTC (mg CE/g) | DPPH (mg AAE/g) | ABTS (mg TE/g) | RP (mg AAE/g) | |
| San chi lu | 4.48 ± 0.07 opq | 50.58 ± 2.69 efg | 41.69 ± 2.19 k | 2.87 ± 0.13 j–m | 7.22 ± 0.23 mn | 3.19 ± 0.20 n–q |
| 901 Qingjo jiangdou | 6.18 ± 0.51 e–i | 53.22 ± 1.68 d–g | 55.85 ± 5.27 jk | 3.66 ± 0.28 f–k | 9.73 ± 0.64 g–k | 4.82 ± 0.12 j–m |
| Qing jiang 2006 | 5.12 ± 0.10 jp | 50.22 ± 1.62 efg | 46.16 ± 1.83 jk | 3.07 ± 0.11 h–m | 7.60 ± 0.12 k–n | 4.03 ± 0.06 l–o |
| Gaotian jinguan | 4.85 ± 0.22 m–p | 35.99 ± 0.66 hi | 48.32 ± 2.17 jk | 3.30 ± 0.26 g–l | 7.37 ± 0.64 lmn | 2.68 ± 0.27 pq |
| Gyeonggiyonginsujib | 5.29 ± 0.46 i–o | 47.65 ± 2.75 fg | 124.29 ± 9.90 fgh | 4.36 ± 0.14 efg | 10.55 ± 0.76 e–i | 7.64 ± 0.56 fg |
| Gangwonpyeongchang-2003-4 | 5.75 ± 0.43 g–m | 67.26 ± 2.69 bc | 141.41 ± 11.48 fg | 4.64 ± 0.34 def | 11.95 ± 1.18 ef | 8.20 ± 1.08 def |
| Gangwonpyeongchang-2003-17 | 5.71 ± 0.16 g–m | 56.17 ± 3.01 def | 107.37 ± 0.39 g–j | 5.38 ± 0.70 cd | 10.54 ± 0.59 e–i | 7.29 ± 1.01 fgh |
| Hang xin taikong wu jia dou | 5.07 ± 0.11 k–p | 48.70 ± 4.07 efg | 37.38 ± 2.31 k | 3.88 ± 0.16 f–j | 8.53 ± 0.26 i–n | 6.17 ± 0.20 hij |
| Techang 908 jiangdou | 5.56 ± 0.33 h–n | 53.22 ± 4.73 d–g | 53.99 ± 2.78 jk | 3.63 ± 0.09 f–k | 8.63 ± 0.83 i–n | 6.78 ± 0.32 ghi |
| Taiwan chunqiu hong | 8.15 ± 0.47 b | 76.06 ± 3.44 ab | 778.34 ± 78.94 a | 9.95 ± 0.88 a | 18.87 ± 1.21 b | 15.58 ± 0.68 a |
| Techang 902 jiangdou | 6.21 ± 0.09 e–i | 56.03 ± 6.16 def | 47.22 ± 2.41 jk | 5.17 ± 0.12 cde | 10.89 ± 0.11 efg | 7.54 ± 0.10 fg |
| Tai Htaung Pe Ni | 7.13 ± 0.45 cde | 75.77 ± 1.93 ab | 709.42 ± 57.87 b | 7.29 ± 0.63 b | 18.06 ± 0.54 b | 12.83 ± 0.68 b |
| THA-JSH-2008-81021 | 6.32 ± 0.13 e–h | 63.51 ± 2.79 cd | 590.93 ± 33.66 c | 4.16 ± 0.21 e–h | 14.06 ± 0.69 cd | 7.98 ± 0.27 efg |
| Tanjingeun | 6.39 ± 0.41 d–h | 59.18 ± 0.25 cde | 50.74 ± 5.40 jk | 3.04 ± 0.13 i–m | 10.77 ± 0.69 e–h | 5.72 ± 0.38 ijk |
| Jasaekginggori | 4.67 ± 0.14 n–q | 47.78 ± 1.69 fg | 140.20 ± 2.94 fg | 3.02 ± 0.06 i–m | 9.91 ± 0.36 f–j | 4.95 ± 0.21 j–m |
| Gatggeungong | 5.92 ± 0.33 g–k | 58.75 ± 4.30 cde | 147.23 ± 10.11 fg | 3.33 ± 0.19 g–l | 11.99 ± 1.38 ef | 6.19 ± 0.48 hij |
| MMR-JYH-2010-90 | 5.88 ± 0.29 g–l | 56.21 ± 2.40 def | 71.88 ± 1.96 h–k | 2.17 ± 0.18 mn | 8.31 ± 0.50 j–m | 4.84 ± 0.21 j–l |
| Sung 99 | 4.17 ± 0.02 pq | 51.01 ± 5.57 efg | 31.20 ± 4.61 k | 3.14 ± 0.03 h–m | 8.73 ± 0.62 h–m | 4.57 ± 0.78 k–m |
| Guamian hong jiangdou | 6.64 ± 0.44 c–g | 81.01 ± 4.03 a | 640.97 ± 52.94 c | 6.98 ± 0.48 b | 15.38 ± 1.07 c | 9.48 ± 0.53 cd |
| Qiu jiang 512 | 4.72 ± 0.21 n–q | 55.13 ± 5.88 def | 39.40 ± 2.64 k | 3.12 ± 0.35 h–m | 8.49 ± 0.17 i–n | 5.04 ± 0.09 j–m |
| Chungbuk Geosan 2011-18 | 4.55 ± 0.63 opq | 54.22 ± 6.38 def | 116.51 ± 15.06 ghi | 3.58 ± 0.19 f–k | 8.77 ± 1.25 h–m | 5.09 ± 0.51 j–m |
| Chungbuk Geosan 2011-245 | 3.78 ± 0.02 q | 53.83 ± 5.31 def | 90.15 ± 13.93 g–k | 2.36 ± 0.14l mn | 6.67 ± 0.26 mn | 4.19 ± 0.11 lmn |
| 901 Zaoshu jiangdou | 4.94 ± 0.17 l –p | 36.80 ± 1.13 hi | 48.50 ± 2.11 jk | 3.14 ± 0.39 h–m | 6.51 ± 0.15 n | 4.19 ± 0.27 lmn |
| Techang jinqili | 6.49 ± 1.03 c–h | 49.07 ± 3.90 efg | 52.79 ± 5.06 jk | 4.15 ± 1.56 e–h | 8.61 ± 1.47 i–n | 5.40 ± 0.85 jkl |
| Nan 1 Variety | 7.33 ± 0.26 bcd | 56.43 ± 8.92 def | 529.94 ± 17.07 d | 6.00 ± 0.98 c | 14.98 ± 1.09 c | 9.97 ± 0.68 c |
| Hei mei 1 | 7.00 ± 0.14 c–f | 53.66 ± 0.40 d–g | 60.25 ± 1.37 ijk | 4.14 ± 0.11 e–h | 9.43 ± 0.35 g–l | 6.02 ± 0.06 hij |
| Chunqiu hong jiangdou | 6.59 ± 0.80 c–g | 57.84 ± 7.49 c–f | 619.60 ± 92.35 c | 6.97 ± 0.72 b | 12.52 ± 1.65 de | 9.13 ± 1.54 cde |
| Man di hong wu jia dou | 9.13 ± 0.21 a | 82.55 ± 10.54 a | 334.63 ± 10.23 e | 9.27 ± 1.43 a | 21.21 ± 1.90 a | 14.69 ± 1.11 a |
| Tichun zhi jiang28-2 | 5.80 ± 0.26 g–m | 49.74 ± 3.15 efg | 33.41 ± 3.01 k | 4.10 ± 0.34 f–i | 8.43 ± 0.66 i–m | 5.31 ± 0.37 jkl |
| Te xuan zhang tang wang | 4.94 ± 0.49 l–p | 27.42 ± 3.86 ij | 31.98 ± 2.14 k | 1.66 ± 0.14 n | 6.78 ± 0.62 mn | 2.02 ± 0.24 q |
| Hei mei huang zi wang | 6.52 ± 0.88 c–h | 43.07 ± 5.77 gh | 52.23 ± 6.66 jk | 3.18 ± 0.52 h–m | 12.56 ± 1.94 de | 4.21 ± 0.37 lmn |
| Yard long Bean 287/2556 | 7.41 ± 0.41 bc | 56.95 ± 4.78 def | 326.49 ± 25.13 e | 5.62 ± 0.39 cd | 18.30 ± 0.80 b | 9.45 ± 1.19 cd |
| SD 3135 | 6.05 ± 0.28 f–j | 47.17 ± 1.41 fg | 179.53 ± 10.31 f | 3.95 ± 0.18 f–j | 12.32 ± 0.86 de | 6.07 ± 0.58 hij |
| KSL 170256 | 4.58 ± 0.15 opq | 30.20 ± 2.33 ij | 141.37 ± 15.83 fg | 2.67 ± 0.27 klm | 7.79 ± 0.70 j–n | 3.67 ± 0.35 m–p |
| Gyeongnamhabcheon-2019-2 | 4.47 ± 0.63 opq | 25.79 ± 4.69 j | 43.54 ± 5.08 k | 1.63 ± 0.13 n | 6.70 ± 0.92 mn | 2.76 ± 0.26 opq |
| Range (Min- Max) | 3.78–9.13 | 25.79–82.55 | 31.20–778.34 | 1.63–9.95 | 6.51–21.21 | 2.02–15.58 |
| Total mean | 5.82 | 53.38 | 187.57 | 4.25 | 10.83 | 6.51 |
| CV (%) | 20.01 | 24.31 | 118.36 | 44.92 | 34.86 | 48.17 |
| Category | Variable | Genotype | Origin | ||||
|---|---|---|---|---|---|---|---|
| Cultivar | Landrace | Thailand | Myanmar | China | Korea | ||
| Agronomic traits | DF (days) | 53.50 ± 3.04 b | 55.87 ± 2.73 a | 56.33 ± 2.05 a | 56.67 ± 2.05 a | 52.56 ± 2.89 b | 56.64 ± 1.55 a |
| DFM (days) | 21.60 ± 3.67 a | 20.47 ± 3.77 a | 25.67 ± 2.49 a | 23.33 ± 5.91 ab | 21.22 ± 3.26 ab | 19.09 ± 2.27 b | |
| DM (days) | 75.10 ± 4.68 a | 76.33 ± 4.83 a | 82.00 ± 4.24 a | 80.00 ± 7.87 ab | 73.78 ± 3.49 c | 75.73 ± 3.02 bc | |
| PL (cm) | 51.96 ± 11.63 a | 47.79 ± 9.96 a | 44.47 ± 11.45 ab | 50.53 ± 9.54 ab | 55.36 ± 11.38 a | 43.15 ± 4.48 b | |
| SPP (n) | 16.04 ± 1.98 a | 15.43 ± 1.86 a | 14.67 ± 2.50 a | 13.73 ± 1.54 a | 16.14 ± 1.59 a | 16.04 ± 1.97 a | |
| HSW (g) | 15.97 ± 2.60 a | 15.71 ± 2.63 a | 17.79 ± 3.93 a | 15.70 ± 2.48 a | 15.59 ± 2.11 a | 15.82 ± 2.73 a | |
| Nutritional components | TP (%) | 25.65 ± 1.20 a | 25.92 ± 0.89 a | 26.57 ± 1.11 a | 26.36 ± 0.94 a | 25.45 ± 1.09 a | 25.90 ± 0.87 a |
| TF (%) | 1.33 ± 0.34 a | 1.09 ± 0.30 b | 1.08 ± 0.40 b | 1.01 ± 0.07 b | 1.44 ± 0.31 a | 0.97 ± 0.14 b | |
| CFC (%) | 4.96 ± 0.51 a | 4.87 ± 0.59 a | 4.92 ± 0.67 a | 5.03 ± 0.04 a | 5.05 ± 0.42 a | 4.68 ± 0.66 a | |
| DFC (%) | 19.32 ± 1.33 a | 19.27 ± 1.57 a | 19.71 ± 0.65 a | 19.26 ± 1.12 a | 19.27 ± 1.42 a | 19.24 ± 1.67 a | |
| Vit C (mg/g) | 2.08 ± 0.60 a | 2.11 ± 0.65 a | 1.94 ± 0.10 a | 1.93 ± 0.27 a | 2.01 ± 0.76 a | 2.32 ± 0.44 a | |
| Fatty acids | PA (%) | 30.02 ± 1.16 b | 31.39 ± 0.98 a | 29.58 ± 0.34 b | 31.36 ± 0.50 a | 29.89 ± 1.10 b | 31.84 ± 0.59 a |
| SA (%) | 4.31 ± 0.56 a | 4.25 ± 0.32 a | 3.69 ± 0.43 b | 3.97 ± 0.39 ab | 4.47 ± 0.49 a | 4.21 ± 0.21 ab | |
| OA (%) | 13.42 ± 1.93 a | 11.50 ± 1.58 b | 12.39 ± 0.63 ab | 10.72 ± 0.89 b | 13.84 ± 0.1.86 a | 11.13 ± 1.16 b | |
| LA (%) | 36.60 ± 1.40 a | 36.55 ± 0.95 a | 38.16 ± 0.86 a | 37.47 ± 0.89 ab | 36.23 ± 1.25 b | 36.48 ± 0.86 b | |
| LLA (%) | 15.65 ± 0.91 b | 16.32 ± 0.96 a | 16.18 ± 0.64 a | 16.49 ± 1.37 a | 15.56 ± 0.89 a | 16.34 ± 0.82 a | |
| TSFA (%) | 34.33 ± 1.17 b | 35.63 ± 0.88 a | 33.27 ± 0.21 c | 35.32 ± 0.89 ab | 34.37 ± 1.06 bc | 36.06 ± 0.47 a | |
| TUFA (%) | 65.67 ± 1.17 a | 64.37 ± 0.88 b | 66.73 ± 0.21 a | 64.68 ± 0.89 bc | 65.63 ± 1.06 ab | 63.95 ± 0.47 c | |
| PUFA (%) | 52.25 ± 1.46 a | 52.87 ± 1.39 a | 54.34 ± 0.43 a | 53.96 ± 0.80 a | 51.79 ± 1.21 b | 52.82 ± 1.33 a | |
| Total secondary metabolites | TPC (mg GAE/g) | 6.18 ± 1.14 a | 5.34 ± 1.01 b | 7.02 ± 0.50 a | 5.73 ± 1.21 ab | 6.02 ± 1.21 ab | 5.20 ± 0.79 b |
| TTC (mg CE/g) | 223.78 ± 253.46 a | 139.30 ± 158.94 a | 482.45 ± 113.06 a | 270.84 ± 310.57 ab | 167.93 ± 39.55 b | 116.58 ± 3.22 b | |
| TSC (mg DE/g) | 54.46 ± 13.58 a | 51.94 ± 11.97 a | 58.96 ± 3.22 a | 61.00 ± 10.66 a | 53.35 ± 14.12 a | 49.82 ± 11.78 a | |
| Antioxidant activities | DPPH (mg AAE/g) | 4.82 ± 2.08 a | 3.48 ± 1.30 b | 5.26 ± 0.79 a | 4.20 ± 2.22 a | 4.57 ± 2.20 a | 3.45 ± 1.03 a |
| RP (mg AAE/g) | 7.09 ± 3.48 a | 5.73 ± 2.39 a | 9.13 ± 1.82 a | 7.41 ± 4.50 a | 6.46 ± 4.06 a | 5.62 ± 1.96 a | |
| ABTS (mg TE/g) | 11.504.23 a | 9.94 ± 2.83 a | 15.78 ± 0.84 a | 11.70 ± 3.83 ab | 10.49 ± 3.63 b | 9.81 ± 1.62 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-M.; Yoon, H.; Shin, M.-J.; Lee, S.; Yi, J.; Jeon, Y.-a.; Wang, X.; Desta, K.T. Multivariate Analysis of Biochemical Properties Reveals Diversity among Yardlong Beans of Different Origins. Antioxidants 2024, 13, 463. https://doi.org/10.3390/antiox13040463
Choi Y-M, Yoon H, Shin M-J, Lee S, Yi J, Jeon Y-a, Wang X, Desta KT. Multivariate Analysis of Biochemical Properties Reveals Diversity among Yardlong Beans of Different Origins. Antioxidants. 2024; 13(4):463. https://doi.org/10.3390/antiox13040463
Chicago/Turabian StyleChoi, Yu-Mi, Hyemyeong Yoon, Myoung-Jae Shin, Sukyeung Lee, Jungyoon Yi, Young-ah Jeon, Xiaohan Wang, and Kebede Taye Desta. 2024. "Multivariate Analysis of Biochemical Properties Reveals Diversity among Yardlong Beans of Different Origins" Antioxidants 13, no. 4: 463. https://doi.org/10.3390/antiox13040463